Abstract
Background
Psoriasis is an inflammatory skin disease that presents with itching, red, scaling plaques; its worsening has been associated with obesity, drinking, smoking, lack of sleep, and a sedentary lifestyle. Lifestyle changes may improve psoriasis.
Objectives
To assess the effects of lifestyle changes for psoriasis, including weight reduction, alcohol abstinence, smoking cessation, dietary modification, exercise, and other lifestyle change interventions.
Search methods
We searched the following databases up to July 2018: the Cochrane Skin Specialised Register, CENTRAL, MEDLINE, Embase, and LILACS. We also searched the China National Knowledge Infrastructure, the Airiti Library, and five trials registers up to July 2018. We checked the references of included trials for further relevant trials, and we asked the authors of the included trials if they were aware of any relevant unpublished data.
Selection criteria
We included randomised controlled trials (RCTs) of lifestyle changes (either alone or in combination) for treating psoriasis in people diagnosed by a healthcare professional. Treatment had to be given for at least 12 weeks. Eligible comparisons were no lifestyle changes or another active intervention.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. The primary outcome measures were 'Severity of psoriasis' and 'Adherence to the intervention'. Secondary outcomes were 'Quality of life', 'Time to relapse', and 'Reduction in comorbidities'. We used GRADE to assess the quality of the evidence for each outcome.
Main results
We included 10 RCTs with 1163 participants (mean age: 43 to 61 years; 656 men and 478 women were reported). Six trials examined the effects of dietary intervention (low‐calorie diet) in 499 obese participants (mean age: 44.3 to 61 years; where reported, 395 had moderate‐to‐severe psoriasis). One trial assessed a combined dietary intervention and exercise programme in 303 obese participants with moderate‐to‐severe psoriasis who had started a systemic therapy for psoriasis and had not achieved clearance after four weeks of continuous treatment (median age: 53 years). Another trial assessed a walking exercise and continuous health education in 200 participants (mean age: 43.1 years, severity not reported). Finally, two trials included education programmes promoting a healthy lifestyle in 161 participants (aged 18 to 78 years), with one trial on mild psoriasis and the other trial not reporting severity.
Comparisons included information only; no intervention; medical therapy alone; and usual care (such as continuing healthy eating).
All trials were conducted in hospitals and treated participants for between 12 weeks and three years. One trial did not report the treatment period. Seven trials measured the outcomes at the end of treatment and there was no additional follow‐up. In two trials, there was follow‐up after the treatment ended. Five trials had a high risk of performance bias, and four trials had a high risk of attrition bias.
We found no trials assessing interventions for alcohol abstinence or smoking cessation. No trials assessed time to relapse. Only two trials assessed adverse events; in one trial these were caused by the add‐on therapy ciclosporin (given in both groups). The trial comparing two dietary interventions to a no‐treatment group observed no adverse events.
The results presented in this abstract are based on trials of obese participants.
Outcomes for dietary interventions versus usual care were measured 24 weeks to six months from baseline. Compared to usual care, dietary intervention (strict caloric restriction) may lead to 75% or greater improvement from baseline in the Psoriasis Area and Severity Index (PASI 75) (risk ratio (RR) 1.66, 95% confidence interval (CI) 1.07 to 2.58; 2 trials, 323 participants; low‐quality evidence). Adherence to the intervention may be greater with the dietary intervention than usual care, but the 95% CI indicates that the dietary intervention might also make little or no difference (RR 1.26, 95% CI 0.76 to 2.09; 2 trials, 105 participants; low‐quality evidence). Dietary intervention probably achieves a greater improvement in dermatology quality‐of‐life index (DLQI) score compared to usual care (MD −12.20, 95% CI −13.92 to −10.48; 1 trial, 36 participants; moderate‐quality evidence), and probably reduces the BMI compared to usual care (MD −4.65, 95% CI −5.93 to −3.36; 2 trials, 78 participants; moderate‐quality evidence).
Outcomes for dietary interventions plus exercise programme were measured 16 weeks from baseline and are based on one trial (303 participants). Compared to information only (on reducing weight to improve psoriasis), combined dietary intervention and exercise programme (dietetic plan and physical activities) probably improves psoriasis severity, but the 95% CI indicates that the intervention might make little or no difference (PASI 75: RR 1.28, 95% CI 0.83 to 1.98). This combined intervention probably results in a greater reduction in BMI (median change −1.10 kg/m², P = 0.002), but there is probably no difference in adherence (RR 0.95, 95% CI 0.89 to 1.01; 137/151 and 145/152 participants adhered in the treatment and control group, respectively). There were no data on quality of life. These outcomes are based on moderate‐quality evidence.
Authors' conclusions
Dietary intervention may reduce the severity of psoriasis (low‐quality evidence) and probably improves quality of life and reduces BMI (moderate‐quality evidence) in obese people when compared with usual care, while combined dietary intervention and exercise programme probably improves psoriasis severity and BMI when compared with information only (moderate‐quality evidence). None of the trials measured quality of life.
We did not detect a clear difference in treatment adherence between those in the combined dietary intervention and exercise programme group and those given information only (moderate‐quality evidence). Adherence may be improved through dietary intervention compared with usual care (low‐quality evidence). Participants generally adhered well to the lifestyle interventions assessed in the review.
No trials assessed the time to relapse. Trial limitations included unblinded participants and high dropout rate.
Future trials should reduce dropouts and include comprehensive outcome measures; they should examine whether dietary intervention with or without an exercise programme is effective in non‐obese people with psoriasis, whether an additional exercise programme is more effective than dietary intervention alone, whether the time to relapse prolongs in people who receive dietary intervention with or without exercise programme, and whether smoking cessation and alcohol abstinence are effective in treating psoriasis.
Plain language summary
Lifestyle changes for treating psoriasis
Review question
We wanted to see whether lifestyle changes (e.g. changing diet, exercising, and avoiding smoking and drinking alcohol), alone or combined, were useful in treating psoriasis when compared to no such changes or another psoriasis treatment.
Background
Psoriasis is a long‐lasting, inflammatory skin disease; it causes thick, red, itching, and scaling patches. Obesity, drinking, smoking, and an inactive lifestyle can worsen psoriasis. We intended to find out if lifestyle changes can improve psoriasis severity and quality of life, and reduce comorbidities (other conditions occurring alongside a primary condition).
Trial characteristics
We included 10 trials, with 1163 participants, which assessed the effects of low‐calorie diet alone; low‐calorie diet combined with an exercise programme; a combination of walking exercise and continuous health education; and educational instructions to promote a healthy lifestyle (diet, smoking, and alcohol abstinence). We examined the research evidence up to July 2018.
Non‐profit organisations funded four trials, one trial received funding for the education programme from pharmaceutical companies, and the other five trials had no funding or did not report the funding source. All participants were aged at least 18 years (mean age: 43 to 61 years). Where reported, the trials included 656 men and 478 women; all were set in a hospital. In four trials, the participants were limited to people with moderate‐to‐severe psoriasis. One trial included participants who had initially been treated with oral medicines for moderate‐to‐severe psoriasis but whose psoriasis had not cleared after four weeks. In four trials, all severities of psoriasis were eligible, but these trials either did not report the participants’ psoriasis severity or only provided average severity scores. One trial included participants with mild psoriasis. Trials compared lifestyle change interventions with usual care (including to continue healthy eating), information only, no treatment, or medical treatment alone. Treatment was given for between 12 weeks to three years.
Key results
The following results are based on obese participants and compare lifestyle change interventions (low‐calorie diet) to usual care. A low‐calorie diet may reduce the severity of psoriasis (when assessed as the proportion of participants achieving at least 75% improvement from the start of treatment in the Psoriasis Area and Severity Index (PASI 75), a widely used tool for the measurement of psoriasis severity) (low‐quality evidence) and probably improves quality of life (moderate‐quality evidence). Participants on a low‐calorie diet may be more likely to stick to treatment (treatment adherence), but treatment effects vary so it is possible that it may make little or no difference (low‐quality evidence). A low‐calorie diet probably improves BMI (body mass index: a healthy weight calculator) (moderate‐quality evidence). The trials did not say how long they treated participants before they stopped dieting (time to relapse).
The following results are based on obese participants and compare lifestyle change interventions (low‐calorie diet plus an exercise programme) to weight‐loss information aimed at improving psoriasis. A low‐calorie diet plus an exercise programme probably results in a greater reduction in the severity of psoriasis (based on PASI 75), but the effects of this treatment vary, so it is possible that it may make little or no difference. There is probably no difference in treatment adherence between the two groups; however, a low‐calorie diet plus exercise programme probably improves BMI reduction (all outcomes based on moderate‐quality evidence). Trials did not report quality of life or time to relapse.
Only two trials in this review assessed side effects. In one trial side effects were caused by an additional therapy given to both groups of participants (they were not caused by the dietary treatment). The other trial, which compared two dietary treatments to no treatment, did not observe any side effects. Generally, participants complied with the assessed lifestyle changes successfully.
We found no trials on alcohol abstinence or smoking cessation.
Quality of the evidence
The quality of evidence was moderate to low for the outcomes 'Severity of psoriasis' and 'Adherence to the intervention' and moderate for 'Reduction in comorbidities: change in Body Mass Index (BMI)'. Quality of life, measured in only one of the two key comparisons, was based on moderate‐quality evidence. Trial limitations included participants knowing which treatment they were receiving and large number of participants withdrawing from trials.
Summary of findings
Background
Description of the condition
Psoriasis is a chronic inflammatory skin disease affecting about 2% of the population worldwide (Parisi 2013). Psoriasis most frequently presents with red scaly plaques involving the scalp, trunk and extensor parts of the limbs (backs of elbows and front of knees; Treloar 2010). About 50% of people with psoriasis have nail involvement that may deform their nails (De Vries 2013). Psoriasis may also present with pustular lesions or with erythroderma, that is, extensive red lesions that involve the entire or almost entire skin (Camp 1992). Guttate psoriasis is a specific form that primarily occurs in children and young adults following a streptococcal sore throat or tonsillitis (Owen 2000). The characteristic lesions are usually adequate for a diagnosis of psoriasis to be made, though a skin biopsy may sometimes be needed for confirmation when doubt arises (Camp 1992). The Psoriasis Area and Severity Index (PASI) score is a weighted scoring system of the severity of psoriasis that ranges from 0 to 72 and is calculated based on the proportion of involved skin, degree of erythema, scaling, and induration (Feldman 2005). Mild psoriasis is usually defined as a PASI score of under 10, while moderate to severe is defined as a PASI score of 10 or above (Mrowietz 2011; Tsai 2017). Psoriasis has a huge impact on quality of life (Ko 2016), especially of people with skin lesions involving the hands and face (Yang 2015). Psoriasis places a heavy economic burden on healthcare systems (Chi 2014). For example, the annual costs of adalimumab treatment per patient is around GBP 10,000 in the UK (NICE 2008).
The pathogenesis of psoriasis involves the activation of T lymphocytes (a type of white blood cell) with resultant production of proinflammatory cytokines (a category of small proteins that carry signals), for example, tumour necrosis factor (TNF) and interleukin (IL)‐17, leading to inflammation and proliferation of the skin (Nestle 2009). Chronic inflammation is considered the link between psoriasis and associated comorbidities (i.e. other diseases co‐occurring with psoriasis), such as metabolic syndrome, cardiovascular disease, stroke, renal diseases, and uveitis (Wang 2014; Chi 2015; Chi 2017). Psoriasis has also been associated with hyperlipidaemia, which is a broad term describing abnormal elevation of blood lipids, including hypercholesterolaemia and hypertriglyceridaemia that refers to abnormal elevation of the blood levels of cholesterol and triglyceride, respectively (Ma 2013; Tsai 2017).
Previous epidemiological trials have found that compared to the general population, people with mild psoriasis had a 1.08‐fold to 1.29‐fold risk of incident (i.e. newly developed) myocardial infarction while those with severe psoriasis had a 1.36‐fold to 3.10‐fold risk of incident myocardial infarction (Gelfand 2006). Also, people with mild psoriasis had an 1.06‐fold risk of incident stroke while those with severe psoriasis had an 1.43‐fold risk of incident stroke compared to the general population (Gelfand 2009). However, the causality between these risks and psoriasis is not clear. Inflammation may play a role comorbid cardiovascular diseases; however, other factors might also play a role. For example, genetic trials have found that people with psoriasis frequently carry certain common genes predisposing to increased risk for hyperlipidaemia, hypertension, and cardiovascular disease (Lu 2013).
Obesity has been associated with the development of psoriasis and psoriasis of increased severity (Debbaneh 2014). It has been proposed that the fat tissue acts like an endocrine organ (i.e. an organ that directly secretes hormones into the blood stream) and secretes proteins, such as adiponectin and leptin, which are involved in inflammation, altered glucose metabolism, and alterations in the inner lining of blood vessels (Gerdes 2011).
In addition to obesity, unhealthy lifestyles, such as excessive alcohol consumption, smoking, and a sedentary lifestyle, have been associated with onset and worsening of psoriasis (Gerdes 2010; Frankel 2012; Keyworth 2014). Stress (Naldi 2005; Altunay 2013), and lack of sleep (Treloar 2010), are also associated with worsening of psoriasis. The mechanism underlying the link between stress and exacerbation of psoriasis is unclear, but may involve the promotion of neurogenic inflammation, change in the neuroendocrine system, and redirecting leukocytes to the skin (Hunter 2013; Ryan 2014).
Description of the intervention
Psoriasis usually follows a relapsing‐remitting course, but may evolve into persistent severe disease (Treloar 2010); there is no cure for psoriasis (Ryan 2014). The current available medical interventions include topical drugs (Mason 2013), phototherapy (Chen 2013b), and systemic drugs (Wang 2014). The clinical decision for using these interventions either alone or in combination is made by physicians after considering the evidence for efficacy and safety, disease severity, and the person's preferences and circumstances (Chi 2013).
Psoriasis is associated with metabolic and cardiovascular comorbidities that lifestyle changes, such as weight reduction, may modify (Chi 2015). The lifestyle change interventions that are potentially effective in treating psoriasis include diet, exercise, weight reduction, smoking cessation, and alcohol abstinence.
How the intervention might work
The potential mechanism of lifestyle interventions in treating psoriasis is shown in Table 3.
1. Potential mechanism of lifestyle change interventions in treating psoriasis.
| Lifestyle change intervention | Potential mechanism in treating psoriasis |
| Weight reduction | Reduction of the amounts of adipose tissue that causes inflammation Decrease in the volume of drug distribution |
| Alcohol abstinence | Reduction of inflammation associated with alcohol consumption |
| Smoking cessation | Reduction of the production of free radicals induced by smoking |
| Exercise | Improvement of the body composition Reduction of inflammation |
Adipose (fat) tissue in obese people with psoriasis produces inflammatory adipokines (bioactive products) and propagates inflammation, which plays a major role in both psoriasis and its associated comorbidities (Gerdes 2011). The excessive adipose tissue in obesity may also increase the volume of drug distribution and diminish the response to medical treatments (Toussirot 2014). Two biological therapies, infliximab and ustekinumab, adopt weight‐adjusted regimens to overcome the decrease in clinical response in obese people (Chi 2014). Weight reduction decreases the amounts of adipose tissue, and therefore could reduce inflammation and improve the severity of psoriasis and the response to medical treatments (Al‐Mutairi 2014). In addition, weight reduction may lead to increased exercise tolerance (Foss 1980), and a positive psychological impact (Essayli 2017).
Excessive alcohol consumption and smoking have been associated with psoriasis of increased severity (Gerdes 2010). TNF‐α plays a major role in inflammation, which is a key feature in psoriasis (Serwin 2007). Excessive alcohol consumption increases the expression of TNF‐α‐converting enzyme (TACE) and plasma levels of tumour necrosis factor‐α receptor (sTNF‐α‐R1; Serwin 2008). Smoking induces the production of free radicals that trigger inflammation and thus may promote the development of psoriasis (Armstrong 2014). Furthermore, smoking may increase the already increased risk of comorbid cardiovascular disease in people with psoriasis (Armstrong 2014). Thus, alcohol abstinence and smoking cessation may help reduce inflammation and the severity of psoriasis (Treloar 2010).
Previous trials have found that exercise improves body composition (i.e. lowering of the percentage of body fat), reduces stress, and lessens chronic inflammation and the levels of pro‐inflammatory cytokines (small proteins released by cells that promote inflammation; Treloar 2010; Frankel 2012). Therefore, exercise may improve both the severity and comorbidities of psoriasis (Treloar 2010).
Why it is important to do this review
Although there is a large body of evidence on the associations between unhealthy lifestyles and worsening of psoriasis, it is unclear if lifestyle changes can effectively reduce the severity of psoriasis or prolong the remission of psoriasis (Ryan 2014). We conducted a systematic review to evaluate the evidence of the effects of lifestyle changes in treating psoriasis.
The plans for this review were published as a protocol 'Lifestyle changes for treating psoriasis' (Chi 2015b).
Objectives
To assess the effects of lifestyle changes for psoriasis, including weight reduction, alcohol abstinence, smoking cessation, dietary modification, exercise, and other lifestyle change interventions.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) that assessed the effects of lifestyle changes for treating psoriasis. Interventions had to be given for at least 12 weeks, which is the current gold standard, short‐term endpoint of clinical trials on interventions for treating psoriasis (Ryan 2014).
Types of participants
People with psoriasis diagnosed by a healthcare professional. We did not impose any limitations on the severity of psoriasis or the age of the participants.
Types of interventions
We included all lifestyle change interventions, including the following:
weight reduction;
alcohol abstinence;
smoking cessation;
dietary modification;
exercise; and
other lifestyle change interventions.
We compared all of the listed lifestyle change interventions either alone or in combination against no lifestyle changes or another active intervention.
Types of outcome measures
Except for the outcome 'time to relapse', we assessed the following outcomes at week 12, week 24, and year 1. If there were no data at these time points, we assessed data at other available time points.
Primary outcomes
Severity of psoriasis: the proportion of participants achieving at least 75% improvement from baseline in the Psoriasis Area and Severity Index (PASI 75). A European consensus proposed PASI 75 as a treatment goal for psoriasis (Mrowietz 2011). We also reported the proportion of participants achieving at least 50%, 90%, or 100% improvement from baseline in PASI (i.e. PASI 50, PASI 90, and PASI 100, respectively) when relevant data were available. If none of these were available, we would use other validated assessment tools for psoriasis, including Body Surface Area (BSA), Physician Global Assessment (PGA), Lattice System Physician's Global Assessment (LS‐PGA), Self‐Administered Psoriasis Area Severity Index (SAPASI), Salford Psoriasis Index (SPI), Copenhagen Psoriasis Severity Index (CoPSI), and other validated assessment tools for psoriasis (Puzenat 2010).
Adherence to the intervention (i.e. following the assigned intervention): the proportion of participants adhering to their allocated treatment.
Secondary outcomes
Quality of life: as measured by validated tools, including Dermatology Life Quality Index (DLQI), 36‐item Short Form (SF‐36), Skindex 29, Skindex 17, Dermatology Quality of life Scale (DQOLS), Psoriasis Disability Index (PDI), Impact of Psoriasis Questionnaire (IPSO), Psoriasis Index of Quality of Life (PSORIQOL), and other validated quality‐of‐life assessment tools for psoriasis (Bronsard 2010). Regarding the DLQI, we considered a DLQI score change of at least 5 as a minimally important difference (Khilji 2001).
Time to relapse
Reduction in comorbidities (i.e. diseases associated with psoriasis, for example, reduction in obesity, hypertension, diabetes mellitus, and metabolic syndrome).
Search methods for identification of studies
We aimed to identify all relevant randomised controlled trials (RCTs) regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
The Cochrane Skin Information Specialist searched the following databases up to 17 July 2018 using strategies based on the draft strategy for MEDLINE in our published protocol (Chi 2015b):
the Cochrane Skin Group's Specialised Registers using the search strategy in Appendix 1;
the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 6) in the Cochrane Library using the strategy in Appendix 2;
MEDLINE via Ovid (from 1946 to 17 July 2018) using the strategy in Appendix 3;
Embase via Ovid (from 1974 to 17 July 2018) using the strategy in Appendix 4; and
LILACS (Latin American and Caribbean Health Science Information database; from 1982 to 17 July 2018) using the strategy in Appendix 5.
Two review authors (CC and YT) searched the following databases up to 6 August 2018:
China National Knowledge Infrastructure (CNKI; from 1994) using the strategy in Appendix 6; and
Airiti Library (publications and theses from Taiwan; from 1991) using the strategy in Appendix 7.
Trials registers
Two review authors (CC and YT) searched the following trials registers up to 6 August 2018:
the ISRCTN registry (www.isrctn.com) using the strategy in Appendix 8;
ClinicalTrials.gov (www.clinicaltrials.gov) using the strategy in Appendix 8;
the Australian New Zealand Clinical Trials Registry (ANZCTR) (www.anzctr.org.au) using the search term "psoriasis";
the World Health Organization International Clinical Trials Registry platform (ICTRP) (apps.who.int/trialsearch/ ) using the strategy in Appendix 8; and
the EU Clinical Trials Register (www.clinicaltrialsregister.eu) using the strategy in Appendix 9.
Searching other resources
References from included trials
We examined the reference lists of included RCTs to identify further references to relevant trials on lifestyle changes for treating psoriasis.
Unpublished literature
We contacted the authors of the included RCTs to ask if they were aware of any relevant unpublished data (Table 4).
2. Contact with authors of included trials.
| Author of included trials | Contact date | Reply |
| Al‐Mutairi 2014 | 2 November 2016 | No reply received |
| Bostoen 2012 | 14 February 2017 | No reply received |
| Chen 2013a | Not contacted because no email address was available | ‐ |
| Del Giglio 2012 | 2 November 2016 | No reply received |
| Gisondi 2008 | 2 November 2016 | "thank you for the email and considering me and my study. I would add that reducing body weight in obese patients is helpful in increasing the response to biologics as well." |
| Guida 2014 | 2 November 2016 | No reply received |
| Jensen 2013 | 2 November 2016 | "Thanks for the e‐mail. We recently published a one‐year extension study of the study mentioned in your e‐mail. Other than that I do not know of any unpublished data." |
| Kimball 2012 | 2 November 2016 | No reply received |
| Li 2015 | 14 February 2017 | No reply received |
| Naldi 2014 | 2 November 2016 | "No, I am not aware of any unpublished data on the topics." |
We sent a standard e‐mail to all trial authors enquiring if they were aware of any unpublished data as follows:
Dear XXX, We are conducting a Cochrane review on lifestyle changes for treating psoriasis (http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD011972/full), and have included your trial (XXX). As you are an expert in this field, I was wondering if you are aware of any other relevant unpublished data. Your assistance would be gratefully appreciated. Best wishes, Ching‐Chi ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Prof Ching‐Chi Chi, MD, MMS, DPhil (Oxford) Department of Dermatology Chang Gung Memorial Hospital, Linkou 5, Fuxing St Guishan Dist Taoyuan 33305 Taiwan Tel: +886‐3‐328‐1200 ext 3556 E‐mail: chingchi@cgmh.org.tw; chingchichi@gmail.com ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Adverse effects
We did not perform a separate search for adverse effects of the lifestyle change interventions. We only examined data on adverse events from the included RCTs.
Data collection and analysis
Some parts of this section uses text that was originally published in another Cochrane protocol (Chi 2012), and the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Two review authors (CC and SK) independently checked the titles and abstracts from the searches. They were not blinded to the names of the trial authors and their institutions. If we judged from the title and abstract that a trial did not relate to an RCT on lifestyle change interventions for treating psoriasis, we excluded it straight away. The same two review authors independently examined the full text of each remaining trial and judged if it met our inclusion criteria. If the two review authors disagreed on whether they should have included a trial, they achieved unanimity through discussion with a third review author (MY). We listed the trials that we excluded after examining the full text and the reasons for exclusion in the 'Characteristics of excluded studies' tables.
Data extraction and management
Two review authors (CC and SK) independently extracted data (including methods, participants, interventions, outcomes, funding source, country, and setting) from the included RCTs using a data extraction form. We pilot tested the data extraction form (Appendix 10). If CC and SK disagreed about the data, they consulted a third review author (MY) to achieve unanimity. One review author (CC) entered the data into Review Manager 5 (Review Manager 2014).
Assessment of risk of bias in included studies
We used Cochrane's tool for assessing risk of bias in RCTs in evaluating the following domains (Higgins 2017):
Random sequence generation (selection bias): adequacy of the method of random sequence generation to produce comparable groups in every aspect except for the intervention.
Allocation concealment (selection bias): adequacy of the method used to conceal the allocation sequence to prevent anyone foreseeing the allocation sequence in advance of, or during, enrolment.
Blinding of participants and personnel (performance bias): adequacy of blinding participants and investigators from knowledge of which intervention a participant received.
Blinding of outcome assessment (detection bias): adequacy of blinding outcome assessors from knowledge of which intervention a participant received.
Incomplete outcome data (attrition bias): the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis, whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomised participants), reasons for attrition or exclusions where reported, and any re‐inclusions in our analyses.
Selective reporting (reporting bias). When the trial protocol was available, we examined if all pre‐specified outcomes were reported. When the trial protocol was unavailable, we examined if the published reports included all expected outcomes, including those that were prespecified.
Other bias: any important concerns about bias not addressed in the other domains.
Two review authors (CC and SK) independently assessed the risk of bias of included RCTs. We discussed any disagreements in our assessment with a third review author (MY) to resolve them. We judged risk of bias low, unclear or high for each individual trial.
Measures of treatment effect
Dichotomous data
We expressed dichotomous data as risk ratios (RR) with 95% confidence intervals (CI). When the RR was statistically significant, we also presented the number needed to treat for an additional beneficial outcome (NNTB) with 95% CI and the baseline risk to which it applies.
Continuous data
We expressed continuous data as difference in means (MD) with 95% CI. If we pooled different outcome scales, we would express continuous data as standardised mean differences (SMD) with 95% CI.
Time‐to‐event data
We planned to express time‐to‐event data as hazard ratios (HRs) with 95% CI.
Unit of analysis issues
We did not pool trials of different designs. For trials of the following types of design, we planned to analyse them separately using appropriate techniques described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017).
Cluster‐randomised trials
For cluster‐randomised trials, we planned to employ the methods described in Chapter 16.3 in theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b), and estimate the intervention effect assuming an intracluster correlation coefficient (ICC) of 0.072 (Adams 2004). We based this assumption on an analogy to a cluster‐RCT assessing the efficacy of adherence to the Adult Treatment Panel (ATP III) guidelines for cholesterol management (Parker 2005).
Cross‐over trials
As carry‐over effect of lifestyle changes could not be excluded in cross‐over trials, we planned to include only data from the first period for analysis.
Trials with multiple treatment groups
For trials with multiple intervention groups, we would make pair‐wise comparisons of one intervention versus another.
Dealing with missing data
We contacted the authors of trial less than 10 years old for missing data. When missing data were not available, we planned to conduct an intention‐to‐treat (ITT) analysis to recalculate the intervention effect estimates. That is, we would include all randomised participants in the analysis and assume those with missing dichotomous outcome data were treatment failures. For missing continuous outcome data, we would adopt the last observation carried forward (LOCF) approach in analysis. When SDs for changes from baseline in continuous outcomes were missing, we followed the methods stated in section 16.1.3.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). When there were no adequate data for obtaining correlation coefficients, we used an imputed correlation coefficient of 0.5 (Follman 1982). A sensitivity test by using imputed correlation coefficients of 0.25 and 0.75 did not show substantial variations between the three imputations.
Assessment of heterogeneity
We assessed the clinical diversity (i.e. variations in the participants, interventions, and outcomes) and methodological diversity (i.e. variations in the trial design and risk of bias) to determine whether a meta‐analysis was appropriate. We anticipated clinical heterogeneity would include baseline severity of psoriasis and various regimens of the same intervention.
We calculated the I² statistic to assess statistical heterogeneity across the included trials. The Cochrane Handbook for Systematic Reviews of Interventions provides a rule of thumb as follows (Deeks 2017):
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity; and
75% to 100%: considerable heterogeneity.
Assessment of reporting biases
When there were at least 10 trials that reported useable data on primary outcomes for an intervention, we planned to use a funnel plot to examine the publication bias.
Data synthesis
We planned to provide a narrative description on all outcomes when data were available. We would only pool trials that were clinically homogeneous for participants, interventions, and outcomes. We performed a meta‐analysis employing the random‐effects model to obtain a pooled intervention effect. When a meta‐analysis was not feasible, we would summarise the data narratively instead. When relevant data were available, we would perform meta‐regression to see whether amount of weight loss reduction was associated with an outcome.
Where we estimated results for individual trials with low numbers of outcomes (fewer than 10 in total) or where the total sample size was fewer than 30 participants and a RR was used, we would report the proportion of outcomes in each group together with a P value from a Fisher's exact test.
Subgroup analysis and investigation of heterogeneity
We planned to conduct the following subgroup analyses but no relevant data were available.
Paediatric and adult participants
Regarding lifestyle interventions that involved weight reduction, we planned to conduct a subgroup analysis on overweight and non‐overweight participants.
Sensitivity analysis
We planned to conduct a sensitivity analysis to examine the intervention effects after excluding trials with high risk of bias for one or more key domains. However, we did not conduct such a sensitivity analysis because only two trials (Al‐Mutairi 2014; Del Giglio 2012), had no domains with a high risk of bias.
'Summary of findings' table
We presented two 'Summary of findings' tables in our review summarising the main outcome data for the most important comparisons (Schünemann 2017), and assessed the quality of the body of evidence using the five GRADE considerations (trial limitations, consistency of effect, imprecision, indirectness, and publication bias), where evidence could be rated as high, moderate, low or very low quality (Schünemann 2013). We used the optimal information size (OIS) calculated in Appendix 11 as a reference for assessing imprecision.
Results
Description of studies
Results of the search
The searches of the electronic databases (see Electronic searches) retrieved 1798 records. Once duplicates had been removed, we had a total of 1770 records. We excluded 1742 records based on titles and abstracts, and identified one ongoing trial (see Characteristics of ongoing studies). We obtained the full text of the remaining 27 records. We excluded nine trials (see Characteristics of excluded studies). We included 10 parallel RCTs (reported in 18 records) with 1163 participants (see Characteristics of included studies). For a further description of our screening process, see the study flow diagram Figure 1.
1.
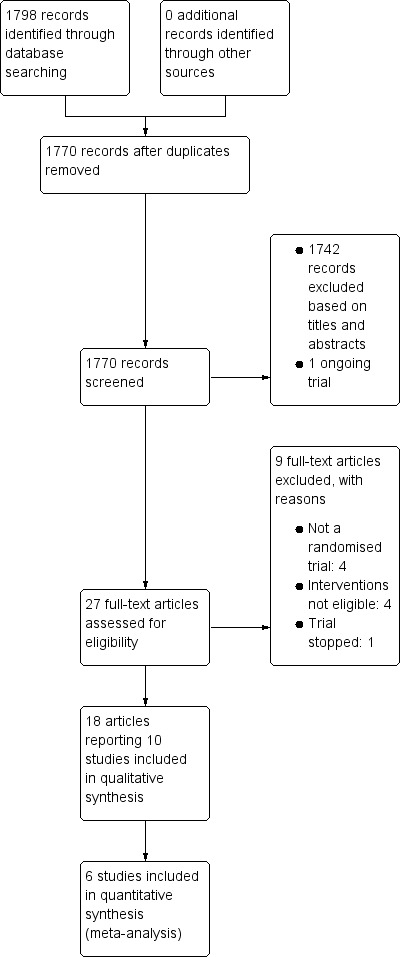
Study flow diagram
Included studies
Participants
We have presented details of the included trials in the Characteristics of included studies tables. We included 10 RCTs with 1163 participants (mean age from 43 to 61 years, 656 men and 478 women where data were available (Bostoen 2012 did not specify the number of men and women included). The type of psoriasis of included participants was chronic plaque psoriasis in eight trials (Al‐Mutairi 2014; Bostoen 2012; Del Giglio 2012; Gisondi 2008; Guida 2014; Jensen 2013; Kimball 2012; Naldi 2014) and not specified in the Chen 2013a trial, while chronic plaque psoriasis, erythrodermic psoriasis, and pustular psoriasis were eligible for inclusion in Li 2015.
Al‐Mutairi 2014, Del Giglio 2012, Gisondi 2008, and Kimball 2012 only included participants with moderate to severe psoriasis. Naldi 2014 included participants with moderate to severe psoriasis who then did not achieve clearance after four weeks' systemic therapy. Bostoen 2012 only included participants with mild psoriasis. Chen 2013a, Guida 2014, Jensen 2013 and Li 2015 did not limit the severity of psoriasis of their included participants, but did not document the proportion with each severity.
Only four included RCTs reported the number of participants with moderate to severe psoriasis (Al‐Mutairi 2014, Del Giglio 2012, Gisondi 2008, Kimball 2012), with 395 participants with documented moderate to severe psoriasis. Three trials (Al‐Mutairi 2014; Del Giglio 2012; Gisondi 2008), reported participants' psoriasis duration, which ranged from 13 to 20 years. The sample size ranged from 29 to 303.
Except for Bostoen 2012, Chen 2013a and Li 2015, all the participants in the other seven trials had a BMI of at least 25. The inclusion criteria regarding BMI was 25 or over but under 35 in Al‐Mutairi 2014, 25 or over in Kimball 2012 and Naldi 2014, over 27 in Jensen 2013, 30 or over in Del Giglio 2012, over 30 in Guida 2014, and over 30 but under 45 in Gisondi 2008.
Design
All 10 trials were parallel RCTs and all trials had two arms, except Kimball 2012, which had three.
Trials provided treatment for between 12 weeks and three years. Participants in half of the trials were treated for 20 to 24 weeks; two trials treated participants for 12 weeks, one for 16 weeks, and another for three years. One trial did not report the treatment period (Chen 2013a).
Seven trials measured outcomes at the end of treatment and there was no additional follow‐up (Al‐Mutairi 2014; Gisondi 2008; Guida 2014; Jensen 2013; Kimball 2012; Li 2015; Naldi 2014). Bostoen 2012 treated participants for 12 weeks and measured outcomes at three, six, and nine months' follow‐up from baseline. Del Giglio 2012 treated participants for 24 weeks, and measured them at this time point as well as at 36 weeks from baseline.
Non‐profit organisations funded four trials (Gisondi 2008; Jensen 2013; Li 2015; Naldi 2014), one trial received funding for the education programme from pharmaceutical companies (Bostoen 2012), and the other five trials had no funding (Al‐Mutairi 2014), or did not report the funding source (Chen 2013a; Del Giglio 2012; Guida 2014; Kimball 2012).
Interventions and comparators
Interventions
We included 10 trials.
Six trials (499 participants, mean age from 44.3 to 61 years, 232 men and 267 women, 395 of these participants with documented moderate to severe psoriasis, where reported) examined the effects of dietary intervention, where low‐calorie diet was given, versus usual care, which included drug therapy alone; no dietary interventions; no dietetic recommendations; or instruction to continue eating ordinary, healthy foods (Al‐Mutairi 2014; Del Giglio 2012; Gisondi 2008; Guida 2014; Jensen 2013; Kimball 2012). Specifically, Gisondi 2008 and Guida 2014 compared the effects of combined dietary intervention and drug therapy against drug therapy alone.
One trial (303 participants, median age 53 years, 215 men and 88 women, all with moderate to severe psoriasis) assessed combined dietary intervention and exercise versus information only, about the utility of reducing weight for improving the clinical control of psoriasis (Naldi 2014).
One trial (200 participants, mean age 43.1 years, 136 men and 64 women, number of participants with moderate to severe psoriasis not reported) assessed walking exercise and continuous health education versus no interventions (Li 2015).
-
Two trials assessed the effects of education programmes promoting healthy lifestyle in 161 participants:
Chen 2013a (77 participants aged 18‐49 years and 55 aged 50‐78 years, mean age not reported, 73 men and 59 women; number of participants with moderate to severe psoriasis not reported) assessed the effects of general instructions on diet, smoking cessation and alcohol abstinence against usual nursing care, including psychological guidance and care, explanation and education of disease‐related knowledge, planning of activities and rest, and skin care;
Bostoen 2012 (29 participants, age and sex distribution of the participants not specified, with mild psoriasis (mean PASI score of 7.7 ± 3.9)) compared education programme, including healthy lifestyle and stress‐reducing techniques (involving physical training, yoga, and meditation) with medical therapy against medical therapy alone.
Co‐interventions
Al‐Mutairi 2014: all the participants received co‐treatments with biologics including infliximab, etanercept, adalimumab, and ustekinumab with no significant differences between the dietary intervention and control groups. The trial authors did not report whether the co‐interventions could be changed.
Bostoen 2012: both the intervention and control groups received medical therapy but the trial authors did not report which medications they administered.
Chen 2013a: both the experimental and control groups were encouraged to adhere to medical treatments prescribed.
Del Giglio 2012: intramuscular methotrexate was stopped before enrolment, but trial authors did not report whether co‐interventions were allowed in the trial period.
Gisondi 2008: both the experimental and control groups received a fixed‐dose regimen of ciclosporin 2.5 mg per kg per day.
Guida 2014: all participants received daily therapy including one of five prescription medications: adalimumab, infliximab, etanercept, cyclosporine, or methotrexate, with no differences in the proportion of participants assuming any specific drug between two groups. The trial authors did not report whether the co‐interventions could be changed.
Jensen 2013: antipsoriatic treatments, if any, had to be stable and unchanged during the trial.
Kimball 2012: all the participants received concurrent NB‐UVB (narrowband ultraviolet B) phototherapy using TL‐01 lamps (310 to 312 nm) three times a week for 12 weeks.
Li 2015: trial authors did not state whether co‐interventions were allowed.
Naldi 2014: the dosage of weight‐adjusted therapies could be modified when there was a body weight change of more than 5 kg at a scheduled follow‐up visit. Stopping of the systemic treatment or moving to another treatment was allowed only when a participant experienced adverse events or intolerance to the prescribed treatment. The dietary intervention and control groups did not significantly differ in the proportion of participants whose co‐intervention was stopped or shifted to another (7 and 11 in the dietary intervention and control groups, respectively; P = 0.34), dose adjustment (14 and 6 in the dietary intervention and control groups, respectively; P = 0.06), and the proportion of participants who regularly applied topical drugs (12 and 16 in the dietary intervention and control groups, respectively; P = 0.44).
Setting
The settings of all included trials were hospitals. Half of the trials were conducted in Europe: four trials were conducted in Italy (Del Giglio 2012; Gisondi 2008; Guida 2014; Naldi 2014), two in China (Chen 2013a; Li 2015), one in Kuwait (Al‐Mutairi 2014), one in Belgium (Bostoen 2012), one in Denmark (Jensen 2013), and one in the USA (Kimball 2012).
Outcomes
The outcomes included in this systematic review that the included trials measured are listed in Table 5.
3. Outcomes reported by included trials.
| Severity of psoriasis | Adherence to the intervention | Quality of life | Time to relapse | Reduction in comorbidities | |
| Al‐Mutairi 2014 | + | ‐ | ‐ | ‐ | + |
| Bostoen 2012 | + | + | + | ‐ | ‐ |
| Chen 2013a | + | ‐ | ‐ | ‐ | ‐ |
| Del Giglio 2012 | + | ‐ | ‐ | ‐ | + |
| Gisondi 2008 | + | + | ‐ | ‐ | + |
| Guida 2014 | + | + | + | ‐ | + |
| Jensen 2013 | + | + | + | ‐ | + |
| Kimball 2012 | + | ‐ | ‐ | ‐ | + |
| Li 2015 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Naldi 2014 | + | ‐ | ‐ | ‐ | + |
| + outcome was reported ‐ outcome was not reported | |||||
Excluded studies
The reasons for exclusion are listed in the Characteristics of excluded studies tables. We identified a trial from the ANZCTR (ACTRN12613001031752), and contacted the trial authors for further data, but they replied to say that they had stopped the trial because very few participants were eligible. In addition, after assessing the full text, we excluded eight trials, with five excluded for not being a RCT (Chang 2016; Fortes 2006; Hsiao 2001; Rucevic 2003; Zackheim 1971), and three excluded because they examined interventions that were not of interest for this review (Balato 2013; He 2016; Keyworth 2014).
Studies awaiting classification
We did not find any trials awaiting classification.
Ongoing studies
We found one ongoing trial (NCT03440736), which is an industry‐sponsored German multicentre trial, expected to be completed in September 2020 (see Characteristics of ongoing studies). The experimental group will receive secukinumab treatment and lifestyle intervention consisting of a structured programme to guide weight loss and increased physical activity, while the control group received secukinumab treatment alone.
Risk of bias in included studies
The percentages of risk of bias across the included trials are presented in Figure 2. The most common items that we judged at high risk of bias were blinding of participants and personnel, and incomplete outcome data. We have presented our judgement of each 'Risk of bias' item for each of the included trials in Figure 3 and Characteristics of included studies.
2.
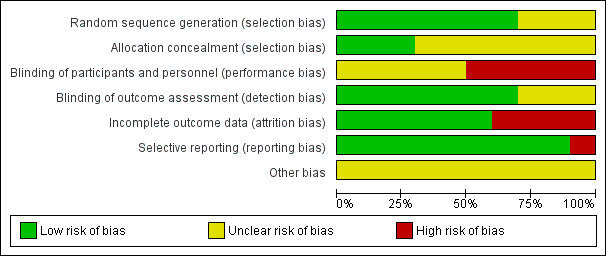
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included trials
3.
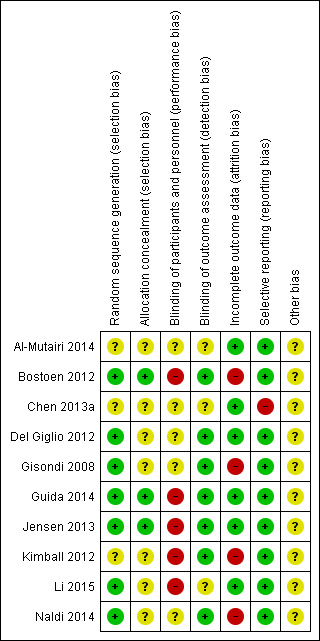
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included trial
Allocation
A total of seven trials employed an adequate method of generating the randomisation sequence, such as computer‐generated randomisation, random number table, and chit pick box method, so we judged them to be at low risk of bias for this domain (Bostoen 2012; Del Giglio 2012; Gisondi 2008; Guida 2014; Jensen 2013; Li 2015; Naldi 2014), while the three remaining trials did not describe their methods of randomisation, so we deemed them at unclear risk (Al‐Mutairi 2014; Chen 2013a; Kimball 2012).
In three trials (Bostoen 2012; Guida 2014; Jensen 2013), allocation could not be foreseen, so they were at low risk of bias. In seven other trials (Al‐Mutairi 2014; Chen 2013a; Del Giglio 2012; Gisondi 2008; Kimball 2012; Li 2015; Naldi 2014), it was unclear if allocation was concealed.
Blinding
A total of five trials had a high risk of performance bias because the lack of blinding of participants might have affected subjective outcomes such as DLQI and adverse effects (Bostoen 2012; Guida 2014; Jensen 2013; Kimball 2012; Li 2015). Although blinding of participants was impossible due to the nature of dietary intervention in five other trials (Al‐Mutairi 2014; Chen 2013a; Del Giglio 2012; Gisondi 2008; Naldi 2014), objective outcomes, for example, body weight and laboratory data, were likely not affected; hence, we considered them at unclear risk of performance bias.
Regarding the blinding of outcome assessment, we rated seven trials at low risk of bias because the outcome assessors were blinded or the outcomes were objective (Bostoen 2012; Del Giglio 2012; Gisondi 2008; Guida 2014; Jensen 2013; Kimball 2012; Naldi 2014). In three trials (Al‐Mutairi 2014; Chen 2013a; Li 2015), it was unclear whether the outcome assessors were blinded.
Incomplete outcome data
We rated Bostoen 2012, Gisondi 2008 and Kimball 2012 as high risk of bias for incomplete outcome data because of a high loss‐to‐follow‐up rate. We also rated Gisondi 2008 and Naldi 2014 as high risk of attrition bias because the dropout rates differed between the experimental and control groups. We rated six trials at low risk of attrition bias because there were no dropouts or withdrawals (Al‐Mutairi 2014; Chen 2013a; Del Giglio 2012; Guida 2014; Jensen 2013; Li 2015).
Selective reporting
Chen 2013a only reported one outcome, 'severity of psoriasis' and we rated it at high risk of selective reporting bias. All other nine trials reported all planned outcomes and we rated them at low risk of reporting bias.
Other potential sources of bias
There was insufficient information to assess whether an important risk of other bias existed in the included trials.
Effects of interventions
Summary of findings for the main comparison. Dietary intervention compared to usual care for treating psoriasis.
| Dietary intervention compared to usual care for treating psoriasis | ||||||
| Patient or population: people with psoriasis Setting: hospital Intervention: dietary intervention Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with dietary intervention | |||||
|
Severity of psoriasis PASI 75 (the proportion of participants achieving at least 75% improvement from baseline in the Psoriasis Area and Severity Index) Follow‐up: 24 weeks |
Trial population | RR 1.66 (1.07 to 2.58) | 323 (2 RCTs) | ⊕⊕⊝⊝ Lowa | ‐ | |
| 537 per 1000 | 891 per 1000 (575 to 1000) | |||||
| Adherence to the intervention Follow‐up: 24 weeks to 6 months | Trial population | RR 1.26 (0.76 to 2.09) | 105 (2 RCTs) | ⊕⊕⊝⊝ Lowa | ‐ | |
| 654 per 1000 | 824 per 1000 (497 to 1000) | |||||
|
Quality of life Change in DLQI (scale from 0 to 30; lower DLQI scores represent improvement in quality of life) Follow‐up: 6 months |
The mean quality of life assessed by change in DLQI was −2.2 | MD 12.20 lower (13.92 lower to 10.48 lower) | ‐ | 36 (1 RCT) | ⊕⊕⊕⊝ Moderateb | ‐ |
|
Time to relapse Not measured |
‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
|
Reduction in comorbidities Change in BMI (lower BMI is beneficial) Follow‐up: 24 weeks to 6 months |
The mean reduction in comorbidities assessed by change in BMI was 0.1 kg/m² | MD 4.65 kg/m² lower (5.93 lower to 3.36 lower) | ‐ | 78 (2 RCTs) | ⊕⊕⊕⊝ Moderateb | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMI: body‐mass index; CI: confidence interval; DLQI: Dermatology Life Quality Index; MD: mean difference; PASI: Psoriasis Area and Severity Index; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by two levels due to a high risk of attrition bias or performance bias, and inconsistency. bDowngraded by one level due to a high risk of performance bias.
None of the outcomes reported here were downgraded for imprecision as the optimal information size was met (Appendix 11).
Summary of findings 2. Dietary intervention and exercise programme compared to information only for treating psoriasis.
| Dietary intervention and exercise programme compared to information only for treating psoriasis | ||||||
| Patient or population: people with psoriasis Setting: hospital Intervention: dietary intervention and exercise programme Comparison: information only | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Risk with information only | Risk with dietary intervention and exercise programme | |||||
|
Severity of psoriasis PASI 75 (the proportion of participants achieving at least 75% improvement from baseline in the Psoriasis Area and Severity Index) Follow‐up: 16 weeks |
Trial population | RR 1.28 (0.83 to 1.98) | 303 (1 RCT) | ⊕⊕⊕⊝ Moderatea | ‐ | |
| 191 per 1000 | 244 per 1000 (158 to 378) | |||||
| Adherence to the intervention Follow‐up: 16 weeks | Trial population | RR 0.95 (0.89 to 1.01) | 303 (1 RCT) | ⊕⊕⊕⊝ Moderatea | ‐ | |
| 954 per 1000 | 906 per 1000 (849 to 963) | |||||
|
Quality of life Not measured |
‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
|
Time to relapse Not measured |
‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
|
Reduction in comorbidities Change in BMI (lower BMI is beneficial) Follow‐up: 16 weeks |
Median change in BMI was 1.9 kg/m² | Median 1.10 kg/m² lower | ‐ | 303 (1 RCT) | ⊕⊕⊕⊝ Moderatea | The diet and exercise group had a significantly greater reduction in BMI (median 3.0, IQR 5.2) than the information only group (median 1.9, IQR 3.6; P = 0.002, Mann‐Whitney U test) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMI: body‐mass index; CI: confidence interval; IQR: interquartile range; PASI: Psoriasis Area and Severity Index; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by one level due to a high risk of attrition bias.
None of the outcomes reported here were downgraded for imprecision as the optimal information size was met (Appendix 11).
Our prespecified outcomes were as follows:
-
Primary outcomes
Severity of psoriasis
Adherence to the intervention
-
Secondary outcomes
Quality of life
Time to relapse
Reduction in comorbidities
As shown in Table 5, the included trials did not report all the review's prespecified primary and secondary outcomes.
Dietary intervention (strict caloric restriction) versus usual care
See Table 1.
Primary outcome 1. Severity of psoriasis
Six included trials on dietary intervention (strict caloric restriction) reported the outcome 'severity of psoriasis' (Al‐Mutairi 2014; Del Giglio 2012; Gisondi 2008; Guida 2014; Jensen 2013; Kimball 2012). We merged data from the Ornish Diet and South Beach Diet groups in Kimball 2012.
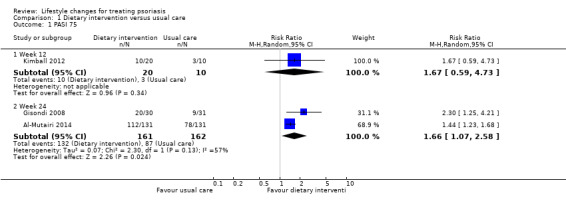
Compared to the control group, the dietary intervention group did not have a significantly greater chance of achieving PASI 75 at week 12 (RR 1.67, 95% CI 0.59 to 4.73; 1 trial, 30 participants), but did at week 24 (RR 1.66, 95% CI 1.07 to 2.58; I² = 57%; 2 trials, 323 participants; low‐quality evidence; Analysis 1.1). The NNTB was 3 (95% CI 3 to 6).
1.1. Analysis.
Comparison 1 Dietary intervention versus usual care, Outcome 1 PASI 75.
The dietary intervention group had a significantly greater reduction in the PASI score than the control group at week 16 (MD −2.00, 95% CI −3.94 to −0.06; 1 trial, 60 participants) and week 24 (MD −3.71, 95% CI −6.80 to −0.62; I² = 82%; 3 trials, 139 participants; very low‐quality evidence; Analysis 1.2).
1.2. Analysis.
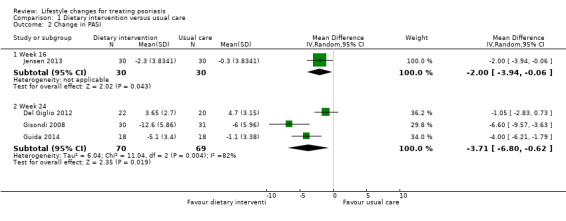
Comparison 1 Dietary intervention versus usual care, Outcome 2 Change in PASI.
Compared to the control group, the dietary intervention group also had a significantly greater reduction in BSA at week 24 (MD −6.06, 95% CI −8.56 to −3.56; 1 trial, 262 participants; moderate‐quality evidence; Analysis 1.3).
1.3. Analysis.
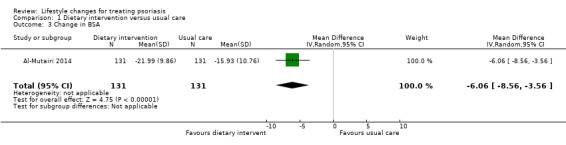
Comparison 1 Dietary intervention versus usual care, Outcome 3 Change in BSA.
Primary outcome 2. Adherence to the intervention
As shown in Analysis 1.4, four included trials on dietary intervention provided data on the number of participants who completed the trials, which were used for calculating the outcome 'adherence to the intervention' (Kimball 2012; Gisondi 2008; Guida 2014; Jensen 2013). We found no significant differences in adherence to the trial intervention between the dietary intervention and control groups at week 12 (RR 1.17, 95% CI 0.65 to 2.09; 1 trial, 30 participants; moderate‐quality evidence), week 16 (RR 1.04, 95% CI 0.86 to 1.25; 1 trial, 60 participants; moderate‐quality evidence), and week 24 (RR 1.26, 95% CI 0.76 to 2.09; I² = 80%; 2 trials, 60 participants; low‐quality evidence). A high statistical heterogeneity existed across the two trials that followed their participants up to 24 weeks (Gisondi 2008; Guida 2014), but we did not carry out a subgroup analysis due to the low number of trials. In Gisondi 2008, four out of 31 participants in the dietary group dropped out due to the adverse effects caused by ciclosporin, compared to 14 out of 30 in the control group, with 10 due to unsatisfactory efficacy and four due to the adverse effects of ciclosporin. Guida 2014 lost four out of 22 participants per group to follow‐up for inadherence to drug therapy.
1.4. Analysis.
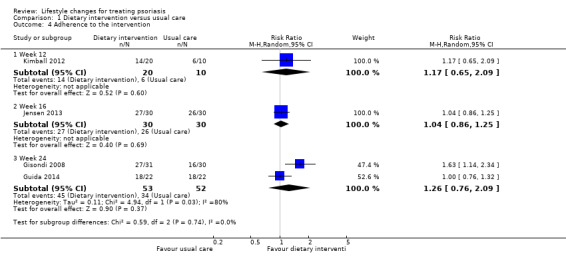
Comparison 1 Dietary intervention versus usual care, Outcome 4 Adherence to the intervention.
Kimball 2012 reported no adverse events in any of the three groups. No other trial apart from Gisondi 2008 assessed adverse events.
Secondary outcome 1. Quality of life
Two trials on dietary intervention provided data on the outcome 'quality of life' (Guida 2014; Jensen 2013). As shown in Analysis 1.5, the dietary intervention group achieved a significantly greater reduction (improvement) in DLQI score than the control group at week 16 (MD −2.00, 95% CI −3.66 to −0.34; 1 trial, 60 participants) and month 6 (MD −12.20, 95% CI −13.92 to −10.48; 1 trial, 36 participants; moderate‐quality evidence). As shown in Analysis 1.5, only in the dietary group at month 6 did the mean reduction in DLQI score (MD −14.4 ± 1.9) achieve a minimally important difference of 5.
1.5. Analysis.
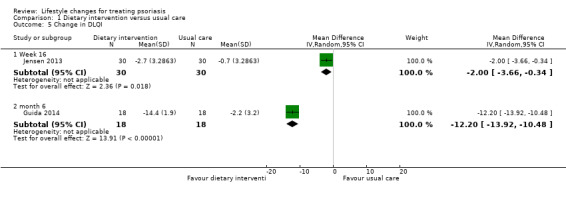
Comparison 1 Dietary intervention versus usual care, Outcome 5 Change in DLQI.
Secondary outcome 2. Time to relapse
None of the included trials assessed this outcome.
Secondary outcome 3. Reduction in comorbidities
Reduction in obesity
Five included trials on dietary intervention reported the change in body weight (Al‐Mutairi 2014; Del Giglio 2012; Gisondi 2008; Guida 2014; Jensen 2013). As shown in Analysis 1.6, the dietary intervention group achieved a significantly greater reduction in body weight than the control group at week 16 (MD −15.40, 95% CI −18.45 to −12.35; 1 trial, 60 participants) and week 24 (MD −10.04, 95% CI −15.61 to −4.48; I² = 98%; 4 trials, 401 participants; very low‐quality evidence). As to the latter comparison, the high statistical heterogeneity arose from the Kuwaiti Al‐Mutairi 2014 trial, associated with a greater reduction in body weight than the other three Italian trials (Del Giglio 2012; Gisondi 2008; Guida 2014).
1.6. Analysis.
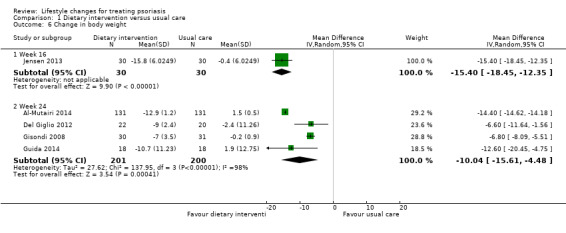
Comparison 1 Dietary intervention versus usual care, Outcome 6 Change in body weight.
Three included trials on dietary intervention assessed the change in BMI (Del Giglio 2012; Guida 2014; Jensen 2013). As illustrated in Analysis 1.7, the dietary intervention group showed a significantly greater reduction in BMI than the control group at week 16 (MD −5.00, 95% CI −5.83 to −4.17; 1 trial, 60 participants) and week 24 (MD −4.65, 95% CI −5.93 to −3.36; I² = 7%; 2 trials, 78 participants; moderate‐quality evidence).
1.7. Analysis.
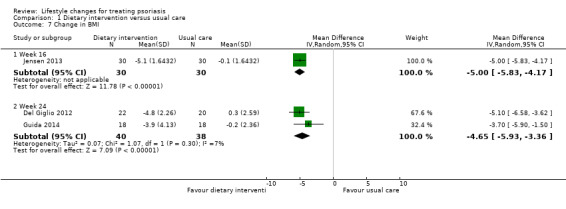
Comparison 1 Dietary intervention versus usual care, Outcome 7 Change in BMI.
Four included trials on dietary intervention examined the change in waist circumference (Al‐Mutairi 2014; Gisondi 2008; Guida 2014; Jensen 2013). Due to zero values in the control group, we did not include Al‐Mutairi 2014 and Gisondi 2008 in the meta‐analysis as the mean difference was not estimable for these two trials. As demonstrated in Analysis 1.8, the dietary intervention group obtained a significantly greater reduction in waist circumference at week 16 (MD −11.50, 95% CI −13.99 to −9.01; 1 trial, 60 participants) and week 24 (MD −12.00, 95% CI −17.27 to −6.73; 1 trial, 36 participants; low‐quality evidence).
1.8. Analysis.
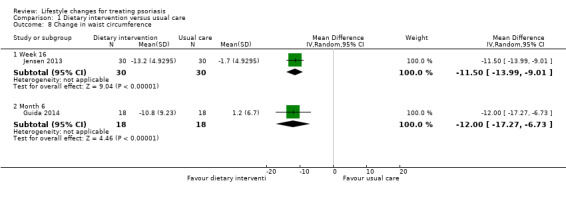
Comparison 1 Dietary intervention versus usual care, Outcome 8 Change in waist circumference.
Reduction in blood lipids
As shown in Analysis 1.9, four included trials assessed the change in the serum levels of total cholesterol (Al‐Mutairi 2014; Gisondi 2008; Guida 2014; Jensen 2013). The dietary intervention group achieved a significantly greater reduction in total cholesterol than the control group at week 16 (MD −0.44, 95% CI −0.49 to −0.39; 1 trial, 60 participants; low‐quality evidence) and at week 24 (MD −0.55, 95% CI −1.08 to −0.01; I² = 78%; 3 trials, 359 participants; low‐quality evidence). The high statistical heterogeneity in the latter arose from the Kuwaiti Al‐Mutairi 2014 trial with a greater reduction in total cholesterol levels than the other two Italian trials (Gisondi 2008; Guida 2014).
1.9. Analysis.
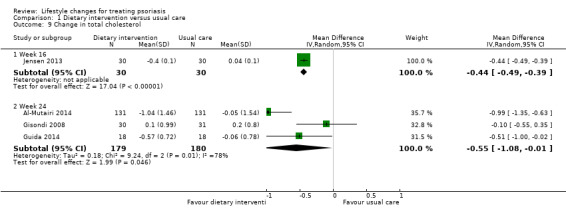
Comparison 1 Dietary intervention versus usual care, Outcome 9 Change in total cholesterol.
The same four included trials (Al‐Mutairi 2014; Gisondi 2008; Guida 2014; Jensen 2013), examined the change in the serum levels of triglyceride (Analysis 1.10). Similar to the change in total cholesterol, the dietary intervention group achieved a significantly greater reduction in triglyceride than the control group at week 16 (MD −0.34, 95% CI −0.38 to −0.30; 1 trial, 60 participants; low‐quality evidence). However, there were no significant differences in the change in the serum levels of triglyceride between the two groups at week 24 (MD −0.32, 95% CI −1.09 to 0.44; I² = 82%; 3 trials, 359 participants; low‐quality evidence). The high statistical heterogeneity in the latter arose from the Guida 2014 trial with a significant reduction in triglyceride levels, compared to no significant change in the other two trials (Al‐Mutairi 2014; Gisondi 2008).
1.10. Analysis.
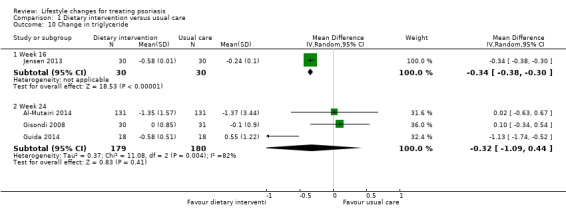
Comparison 1 Dietary intervention versus usual care, Outcome 10 Change in triglyceride.
Dietary intervention and exercise programme versus information only
Only one trial compared combined dietary intervention and exercise programme against only giving information about the utility of reducing weight in improving psoriasis (Naldi 2014). See Table 2.
Primary outcome 1. Severity of psoriasis
As shown in Analysis 2.1, when compared to information only, the combination of dietary intervention and exercise programme might not have increased PASI 75 response at week 16 (RR 1.28, 95% CI 0.83 to 1.98; 1 trial, 303 participants; moderate‐quality evidence). As to two other unprespecified outcomes, combined dietary intervention and exercise programme might have increased PASI 50 response (RR 1.45, 95% CI 1.11 to 1.91; 1 trial, 303 participants; low‐quality evidence), with NNTB of 7 (95% CI 4 to 25); but did not significantly increase PASI 100 response (RR 1.57, 95% CI 0.88 to 2.83; 1 trial, 303 participants; low‐quality evidence) at week 16.
2.1. Analysis.
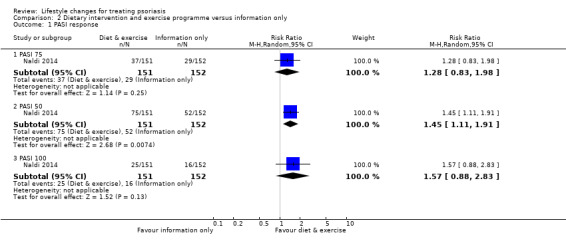
Comparison 2 Dietary intervention and exercise programme versus information only, Outcome 1 PASI response.
Primary outcome 2. Adherence to the intervention
The number of participants who completed the trial was used for calculating adherence to the intervention. No significant differences in adherence existed between the diet and exercise group and the information only group at week 16 (RR 0.95, 95% CI 0.89 to 1.01; 1 trial, 303 participants; moderate‐quality evidence: Analysis 2.2).
2.2. Analysis.
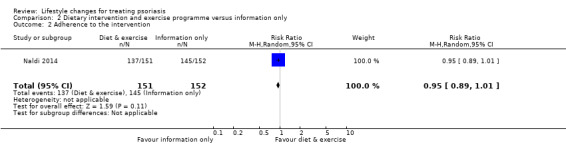
Comparison 2 Dietary intervention and exercise programme versus information only, Outcome 2 Adherence to the intervention.
Secondary outcome 1. Quality of life
None of the included trials assessed this outcome.
Secondary outcome 2. Time to relapse
None of the included trials assessed this outcome.
Secondary outcome 3. Reduction in comorbidities
Reduction in obesity
The diet and exercise group probably had a significantly greater reduction in BMI (median 3.0, interquartile range (IQR) 5.2) than the information only group (median 1.9, IQR 3.6) (P = 0.002, Mann‐Whitney U test; moderate‐quality evidence). Also, the reduction in body weight was probably greater in the diet and exercise group (median 3.0, IQR 4.5) than in the information only group (median 1.7, IQR 3.0) (P < 0.001, Mann‐Whitney U test; moderate‐quality evidence). Moreover, the reduction in waist circumference was probably greater in the diet and exercise group (median 3.0, IQR 5.0) than in the information only group (median 2.0, IQR 3.5; moderate‐quality evidence).
Walking exercise combined with continuous health education versus control
Primary outcome 1. Severity of psoriasis
None of the included trials assessed this outcome.
Primary outcome 2. Adherence to the intervention
None of the included trials assessed this outcome.
Secondary outcome 1. Quality of life
None of the included trials assessed this outcome.
Secondary outcome 2. Time to relapse
One trial (Li 2015), assessed the effect of walking exercise with continuous health education in reducing the flares of psoriasis. Li 2015 did not directly report the outcome 'time to relapse', but reported the proportion of participants whose psoriasis did not recur. The intervention group was probably less likely to flare than the control group over a three‐year period (88% versus 34%; P < 0.0001; moderate‐quality evidence). We downgraded our quality judgement for this trial by one level due to trial limitations, as participants were not blinded and it was unclear if outcome assessment was blinded and if allocation was concealed.
Secondary outcome 3. Reduction in comorbidities
None of the included trials assessed this outcome.
Educational programme versus control (no education programme)
Two trials (Bostoen 2012; Chen 2013a), examined the effects of an education programme promoting a healthy lifestyle. One trial (Bostoen 2012), compared an education programme combined with medical therapy with medical therapy only. Another trial assessed the effect on psoriasis of general instructions on diet, smoking cessation and alcohol abstinence against usual nursing care (Chen 2013a).
Primary outcome 1. Severity of psoriasis
We are uncertain whether the intervention and control groups in Bostoen 2012 differed in the reduction in the PASI score at three, six, and nine months into the trial. At six months: MD −3.4 (95% CI −7.34 to 0.54; 1 trial, 21 participants; downgraded to very low‐quality evidence due to high risk of performance and attrition biases and imprecision; Analysis 3.1). The experimental and control groups in Chen 2013a may not differ in the proportion of participants achieving at least 60% reduction in BSA (RR 1.17, 95% CI 0.97 to 1.40; 1 trial, 132 participants; downgraded to low‐quality evidence due to high risk of reporting bias and imprecision; Analysis 3.2).
3.1. Analysis.
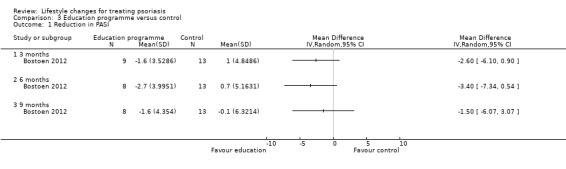
Comparison 3 Education programme versus control, Outcome 1 Reduction in PASI.
3.2. Analysis.
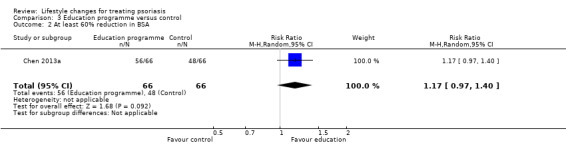
Comparison 3 Education programme versus control, Outcome 2 At least 60% reduction in BSA.
Primary outcome 2. Adherence to the intervention
In Bostoen 2012, the number of participants who completed the trial was used for calculating adherence to the intervention. At three months when the 12‐week education programme ended, there were six (40%) dropouts in the intervention group and one (7%) dropout in the control group (RR 0.65, 95% CI 0.42 to 1.00; P = 0.080 from Fisher's exact test; 1 trial, 29 participants; downgraded to very low‐quality evidence due to high risk of performance and attrition biases and imprecision; Analysis 3.3).
3.3. Analysis.
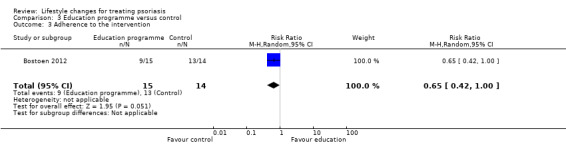
Comparison 3 Education programme versus control, Outcome 3 Adherence to the intervention.
Secondary outcome 1. Quality of life
The intervention and control groups in Bostoen 2012 might not have differed in the reduction in the DLQI score at three, six, and nine months into the trial (1 trial, 22 participants; downgraded to low‐quality evidence due to high risk of performance and attrition biases; Analysis 3.4). Similarly, the two groups might have not differed in the reduction in the PDI score at three, six, and nine months into the trial (1 trial, 22 participants; downgraded to low‐quality evidence due to high risk of performance and attrition biases; Analysis 3.5). The two groups might not have differed in the reduction in the Skindex 29 score, but the trial authors did not provide relevant numerical data (downgraded to low‐quality evidence due to high risk of performance and attrition biases).
3.4. Analysis.
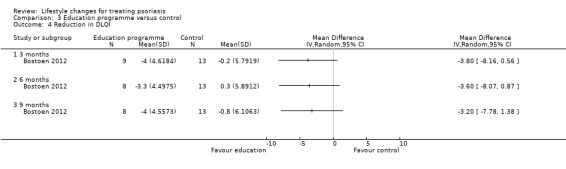
Comparison 3 Education programme versus control, Outcome 4 Reduction in DLQI.
3.5. Analysis.
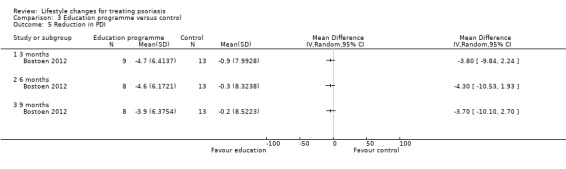
Comparison 3 Education programme versus control, Outcome 5 Reduction in PDI.
Secondary outcome 2. Time to relapse
None of the included trials assessed this outcome.
Secondary outcome 3. Reduction in comorbidities
None of the included trials assessed this outcome.
Discussion
Summary of main results
In this systematic review we included 10 RCTs with 1163 participants. All trials except for Bostoen 2012, Chen 2013a and Li 2015 were limited to obese participants.
Six trials (Al‐Mutairi 2014; Del Giglio 2012; Gisondi 2008; Guida 2014; Jensen 2013; Kimball 2012), examined the effects of dietary intervention (low‐calorie diet, with or without drug therapy) versus usual care (drug therapy alone, no dietary interventions, no dietetic recommendations, or instruction to continue eating ordinary, healthy foods).
One trial (Naldi 2014), assessed a combined dietary intervention and exercise programme versus information only (about the utility of reducing weight for improving the clinical control of psoriasis).
Another trial (Li 2015), assessed a walking exercise and continuous health education programme versus a control group receiving no intervention.
Finally, two trials (Bostoen 2012; Chen 2013a), included education programmes promoting healthy lifestyle versus usual care (usual nursing care including psychological guidance and care, explanation and education of disease‐related knowledge, planning of activities and rest, and skin care; Chen 2013a) or medical therapy alone; Bostoen 2012).
Evidence showed that when compared against usual care, dietary intervention (strict caloric restriction) may reduce the severity of psoriasis (based on a 75% or greater improvement from baseline in the PASI 75 (low‐quality evidence from two trials in 323 participants, assessment at 24 weeks)). With assessment between 24 weeks and six months, dietary intervention probably both improves quality of life (based on the DLQI score; moderate‐quality evidence from one trial in 36 participants) and reduces the BMI (moderate‐quality evidence from two trials in 78 participants). See Table 1.
Moderate‐quality evidence from one trial in 303 participants, assessed at 16 weeks, showed that when compared with information only, combined dietary intervention and exercise probably improves psoriasis severity (assessed using PASI 75), but the 95% confidence interval indicates that the combined intervention may also make little or no difference, and probably leads to a greater reduction in BMI. For this comparison, there were no data on quality of life. See Table 2.
None of the included trials measured time to relapse. We found no trials assessing interventions for alcohol abstinence or smoking cessation. The evidence reviewed is generally limited to obese people; only three trials assessed the general population.
Adherence to the trial intervention may be greater with dietary intervention than usual care, but the 95% confidence interval indicates that dietary intervention may also make little or no difference (low‐quality evidence from two trials in 105 participants; outcome assessed between 24 weeks and six months). Adherence probably does not differ between those receiving information only or those on a combined dietary intervention and exercise programme (moderate‐quality evidence from one trial in 303 participants; outcome assessed at 16 weeks). Participants generally adhered well to the lifestyle interventions assessed in the review.
Overall completeness and applicability of evidence
We did not find enough evidence to address fully all of the objectives of the review: to assess the effects of lifestyle changes for psoriasis, including weight reduction, alcohol abstinence, smoking cessation, dietary modification, exercise, and other lifestyle change interventions.
The available evidence (10 trials) is limited to dietary intervention with (one trial) or without (six trials) an exercise programme, walking exercise combined with continuous health education, and the effects of an education programme promoting a healthy lifestyle. There were no trials that directly compared diet intervention alone versus a combined dietary intervention and exercise programme. Only one trial (Kimball 2012), compared the effects of two dietary interventions against no dietary intervention. There is a lack of evidence comparing the effects of different dietary interventions. Also, there is a lack of trials comparing the effects of different exercise programmes (for example, walking and jogging) in treating psoriasis.
Only one trial evaluated the effects of walking exercise combined with continuous health education, but it did not assess any of our outcomes of interest. Just two trials examined the effects of an education programme promoting a healthy lifestyle, and they provided mainly very low‐quality evidence for the outcomes assessed, meaning we are uncertain of their results. We found no trials on a number of lifestyle changes, including alcohol abstinence, smoking cessation, coping with stress, and non‐sedentary lifestyle.
None of the included trials assessed the outcome 'Time to relapse'. Psoriasis severity and treatment adherence were the most reported outcomes (reported by three quarters of the trials), but the quality of the evidence underlying these outcomes was mixed (very low to moderate). Thus, we could not be conclusive. Participants' BMI was the most commonly assessed comorbidity (reported in two trials), but one trial also reported change in body weight, waist circumference, and serum levels in cholesterol and triglyceride. Half of the trials assessed quality of life but evidence quality was low to moderate.
All of the trials assessing dietary intervention with or without exercise included obese participants only, which means most of the evidence is limited to this population (only three trials included the general population). It is unclear if dietary intervention with or without an exercise programme is effective in non‐obese people with psoriasis.
Furthermore, most trials assessing dietary interventions with or without an exercise programme only included participants with moderate to severe psoriasis (Al‐Mutairi 2014; Del Giglio 2012; Gisondi 2008; Kimball 2012; Naldi 2014). (The Jensen 2013 trial included participants with mild to moderate psoriasis (median PASI score 5.4; IQR 3.8 to 7.6) and showed that dietary intervention was effective in this population as well.) The severity of psoriasis was mild among participants included in Bostoen 2012. There were no data regarding the severity of psoriasis in Chen 2013a and Li 2015. The available evidence is mainly applicable to chronic plaque psoriasis as most trials only included participants with this type of psoriasis.
Except for two trials (Jensen 2013; Li 2015), there was a lack of data post‐24 weeks; hence, we are uncertain if the benefits and harms of the interventions found will remain.
Quality of the evidence
We rated the quality of the body of evidence moderate to low for most outcomes, but there were a few results which we rated very low quality.
Trial limitations
The risk of bias of the included trials varied from low to high (Figure 3). As illustrated in Figure 2, a high risk of bias most frequently appeared in the 'blinding of participants and personnel' and 'incomplete outcome data'. Based on the intrinsic limitation of dietary instructions with or without an exercise programme, blinding of participants was impossible and might have affected subjective outcome assessment, for example, DLQI in four trials (Bostoen 2012; Guida 2014; Jensen 2013; Kimball 2012). Loss to follow‐up exceeded 20% in Bostoen 2012, Gisondi 2008 and Kimball 2012. The loss to follow‐up rate differed between the experimental and control groups in Gisondi 2008 and Naldi 2014. Chen 2013a reported only one outcome 'severity of psoriasis' and thus we rated it at high risk of selective reporting bias. These high risks of bias led to downgrading for many outcomes (Table 1; Table 2).
Imprecision
The sample size of the included trials was mostly small and the number of events was limited, but we did not downgrade for imprecision as the trials met the optimal information size.
Inconsistency of the results
The direction of intervention effects was consistent across the outcomes except for the change in the levels of triglyceride. Jensen 2013 showed a significant reduction in the levels of triglyceride in the dietary intervention group at week 16, but the data from three other trials (Al‐Mutairi 2014; Gisondi 2008; Guida 2014), found no significant differences in the change of triglyceride levels between the dietary intervention group and control group at week 24. We downgraded two outcomes for inconsistency (PASI 75 and adherence) in Table 1 because the I² statistic values were high.
Indirectness of evidence
We found no indirect evidence for this review. The participants were allowed to receive concomitant systemic treatments and phototherapy, which met the clinical practice setting where patients may receive various treatments at the same time.
Publication bias
No primary outcome had data from 10 trials or more. Thus, we did not use funnel plots to detect publication bias.
Potential biases in the review process
We conducted this review following the a priori protocol (Chi 2015b). As shown in Electronic searches, we conducted a systematic search of databases including Chinese and a Taiwanese databases. We also scanned the references of included trials and enquired if the trial authors were aware of any relevant unpublished data. We did not impose any limitation on language or publication status. Therefore, our search is unlikely to have missed relevant important trials. Although we made some departures from the protocol (see Differences between protocol and review), these changes are unlikely to introduce biases. We did not make a posteriori decisions about the analysis or investigation of heterogeneity after obtaining the data.
Chen 2013a only reported one outcome. However, the authors did not provide a correspondence email address and thus we were unable to contact them for further unreported data. As shown in Table 4, we contacted other trial authors but only a few of them replied. If the trial authors had provided unreported data and imprecision was thus mitigated, we could have upgraded the quality of the body of evidence.
Agreements and disagreements with other studies or reviews
We have only found two other reviews on a similar topic for this review (Upala 2015; Ford 2018). One systematic review assessed the effect of weight loss intervention on the severity of psoriasis (Upala 2015). The conclusion agrees with our review, indicating that weight loss interventions are effective in reducing the severity of psoriasis in obese people. However, the review included a trial on people with psoriatic arthritis (Di Minno 2014), and did not separate combined diet intervention and exercise programme (Naldi 2014) from the meta‐analysis. The other systematic review aimed to provide dietary recommendations for adults with psoriasis or psoriatic arthritis, or both, from the Medical Board of the US National Psoriasis Foundation (Ford 2018). They strongly recommend dietary weight reduction with a hypocaloric diet in overweight and obese people with psoriasis, which is consistent with our conclusions.
The clinical benefits of dietary intervention with or without an exercise programme is compatible with the theory that weight reduction and exercise improve body composition and reduce the amounts of adipose tissue that potentiates inflammation (Al‐Mutairi 2014). The lack of consistent evidence in the reduction in triglycerides associated with dietary intervention suggests that hypertriglyceridaemia in people with psoriasis may be associated with factors other than inflammation, for example, congenital defect in lipid metabolism. A genetic trial has found that people with psoriasis frequently had common genetic variants predisposing to hyperlipidaemia (Lu 2013).
Authors' conclusions
Implications for practice.
The body of evidence regarding the effects of lifestyle changes for treating psoriasis is limited, with a number of key interventions not assessed by any included trial, such as alcohol abstinence or smoking cessation. Most trials reported our primary outcomes, but evidence quality was variable, ranging from very low to moderate.
The evidence in our review demonstrates that compared with usual care, dietary intervention (strict caloric restriction) may reduce psoriasis severity in obese people (low‐quality evidence) and probably improves quality of life and reduces body‐mass index (BMI) (moderate‐quality evidence).
When compared with information only, combined dietary intervention and exercise probably leads to a greater reduction in BMI, but with no difference in treatment adherence (moderate‐quality evidence). For this comparison, there were no data on quality of life. The combined treatment probably improves psoriasis severity (moderate‐quality evidence), but the 95% confidence interval indicates that the combined intervention may also make little or no difference. Similarly, treatment adherence may be greater with dietary intervention than usual care, but for the same reason it may also make little or no difference (low‐quality evidence). The lifestyle interventions assessed in the review were largely well‐adhered to by the participants.
Implications for research.
Interventions and comparators
More trials are needed to provide evidence for the following questions.
Whether dietary intervention with or without an exercise programme versus usual care is effective in non‐obese people with psoriasis?
Whether additive exercise programme is better than dietary intervention alone in treating psoriasis?
What are the effects of different dietary interventions (for example, vegetarian diet versus ketogenic diet) in treating psoriasis?
What are the effects of different exercise programmes (for example, walking and jogging) in treating psoriasis? The comparisons could be one exercise programme versus another or sedentary lifestyle.
Whether the time to relapse prolongs in people who receive dietary intervention with or without exercise programme when compared to usual care?
Whether other lifestyle changes (for example, strict smoking cessation, alcohol abstinence programme, coping with stress, and non‐sedentary lifestyle) are effective in treating psoriasis when compared to usual care?
Participants
To date only one trial (Jensen 2013), has examined the effects of dietary intervention in people with mild to moderate psoriasis. More trials including participants with mild psoriasis are warranted to confirm the benefits of dietary intervention with or without an exercise programme in this population. Moreover, inclusion of non‐obese people with psoriasis in future trials will answer whether dietary intervention with or without exercise programme is effective in this population with psoriasis.
Outcomes
The length of follow‐up in future trials should be at least 24 weeks and may be extended to one year or longer to examine the outcome 'Time to relapse'. Future trials should report participants' severity of psoriasis and adherence and trial authors should consult the Cochrane Skin Core Outcomes Set Initiative (CS‐COUSIN 2019) to check for any core outcome measures.
Loss to follow‐up exceeded 20% in three trials; future trials should routinely incorporate various measures to minimise dropouts. Where practical, trials should be double‐blinded to reduce performance and detection bias.
In addition, the effects of lifestyle changes for treating other skin conditions, not just psoriasis, should be assessed. For example, obesity has been associated with an increased prevalence of atopic dermatitis (Zhang 2015); therefore, the effects of weight reduction on the severity of atopic dermatitis should be examined.
Acknowledgements
The authors thank the Cochrane Skin Editorial Base for assistance in preparing this review.
The Cochrane Skin editorial base wishes to thank Laurence Le Cleach, who was the Cochrane Dermatology and Methods Editor for this review; Ben Carter, who was the Statistical Editor; external content experts Luigi Naldi and Robert Chalmers; and the consumer referee, Carolyn Hughes. We would also like to thank Denise Mitchell for copy‐editing the review.
Appendices
Appendix 1. Skin Group Specialised Register/CRSW search strategy
(psoria* OR (palmoplantar* pustulosis) OR (pustulosis palmaris et plantaris) OR (pustulosis and palms and soles)) and (Diet* or food habit* or meal? or nutrition or weight loss or body mass index or bmi or obesity or overweight or lifestyle* or life style* or (health near (behaviour* or behavior*)) or smoking cessation or ((quit* or stop*) near smok*) or alcohol or exercis* or sport* or walk* or swim* or cycl* or run* or jog* or (physical* near (fit* or exer* or activ*)))
Appendix 2. CENTRAL (Cochrane Library) search strategy
#1 MeSH descriptor: [Psoriasis] this term only #2 psoria*:ti,ab,kw #3 palmoplantar* pustulosis:ti,ab,kw #4 pustulosis palmaris et plantaris:ti,ab,kw #5 pustulosis and palms and soles:ti,ab,kw #6 #1 or #2 or #3 or #4 or #5 #7 MeSH descriptor: [Diet] explode all trees #8 MeSH descriptor: [Dietary Supplements] explode all trees #9 MeSH descriptor: [Food Habits] explode all trees #10 MeSH descriptor: [Meals] explode all trees #11 MeSH descriptor: [Diet Therapy] explode all trees #12 MeSH descriptor: [Nutrition Therapy] explode all trees #13 MeSH descriptor: [Caloric Restriction] explode all trees #14 MeSH descriptor: [Diet, Reducing] explode all trees #15 diet*:ti,ab,kw #16 MeSH descriptor: [Weight Reduction Programs] explode all trees #17 MeSH descriptor: [Weight Loss] explode all trees #18 (weight near/2 (loss or lost or reduc* or eliminat*)):ti,ab,kw #19 ((body mass index or bmi) near/3 (reduc* or decreas* or low*)):ti,ab,kw #20 MeSH descriptor: [Body Mass Index] explode all trees #21 MeSH descriptor: [Obesity] explode all trees #22 MeSH descriptor: [Overweight] explode all trees #23 MeSH descriptor: [Life Style] explode all trees #24 (lifestyle* or life style*):ti,ab,kw #25 MeSH descriptor: [Health Behavior] explode all trees #26 (health and (behaviour* or behavior*)):ti,ab,kw #27 MeSH descriptor: [Smoking Cessation] explode all trees #28 ((quit* or stop) and smok*):ti,ab,kw #29 MeSH descriptor: [Tobacco Use Cessation] explode all trees #30 (smoking near/2 cessation):ti,ab,kw #31 MeSH descriptor: [Alcohol Abstinence] explode all trees #32 alcohol abstinence:ti,ab,kw #33 MeSH descriptor: [Alcohol Drinking] explode all trees #34 ((quit* or stop) and (drinking or alcohol)):ti,ab,kw #35 exercis*:ti,ab,kw #36 MeSH descriptor: [Exercise] explode all trees #37 MeSH descriptor: [Exercise Therapy] explode all trees #38 MeSH descriptor: [Walking] explode all trees #39 MeSH descriptor: [Sports] explode all trees #40 MeSH descriptor: [Bicycling] explode all trees #41 MeSH descriptor: [Running] explode all trees #42 MeSH descriptor: [Swimming] explode all trees #43 MeSH descriptor: [Physical Fitness] explode all trees #44 (sport* or walk* or swim* or cycl* or run* or jog*):ti,ab,kw #45 (physical* near/2 (fit* or exer* or activ*)):ti,ab,kw #46 MeSH descriptor: [Habits] explode all trees #47 MeSH descriptor: [Risk Reduction Behavior] explode all trees #48 {or #7‐#47} #49 #6 and #48
Appendix 3. MEDLINE (Ovid) search strategy
exp Psoriasis/ or psoria$.mp.
palmoplantar$ pustulosis.mp.
pustulosis palmaris et plantaris.mp.
(pustulosis and palms and soles).mp.
1 or 2 or 3 or 4
Diet/
exp Dietary Supplements/
exp food habits/ or meals/
exp Diet Therapy/
exp Nutrition Therapy/
Caloric Restriction/
Diet, Reducing/
diet$.mp.
Weight Reduction Programs/
Weight Loss/
(weight adj2 (loss or lost or reduc$ or eliminat$)).mp.
((body mass index or bmi) adj3 (reduc$ or decreas$ or low$)).mp.
body mass index/
Obesity/
Overweight/
exp Life Style/
(lifestyle$ or life style$).mp.
exp Health Behavior/
(health and (behaviour$ or behavior$)).mp.
Smoking Cessation/
((quit$ or stop) and smok$).mp.
"Tobacco Use Cessation"/
(smoking adj2 cessation).mp.
Alcohol Abstinence/
alcohol abstinence.mp.
exp Alcohol Drinking/
((quit$ or stop) and (drinking or alcohol)).mp.
exercis$.mp.
exp Exercise/
exp Exercise Therapy/
Walking/ or sports/ or bicycling/ or running/ or swimming/
Physical Fitness/
(sport$ or walk$ or swim$ or cycl$3 or run$3 or jog$3).mp.
(physical$ adj (fit$ or exer$ or activ$)).mp.
Habits/
risk reduction behavior/
or/6‐41
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
clinical trials as topic.sh.
randomly.ab.
trial.ti.
43 or 44 or 45 or 46 or 47 or 48 or 49
exp animals/ not humans.sh.
50 not 51
5 and 42 and 52
[Lines 43‐52: Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision)]
Appendix 4. Embase (Ovid) search strategy
1. exp PSORIASIS/ 2. psoria$.mp. 3. palmoplantar$ pustulosis.mp. 4. pustulosis palmaris et plantaris.mp. 5. (pustulosis and palms and soles).mp. 6. 1 or 2 or 3 or 4 or 5 7. exp diet/ 8. exp feeding behavior/ 9. exp diet supplementation/ 10. exp meal/ 11. exp diet therapy/ 12. exp caloric restriction/ 13. exp low calory diet/ 14. diet$.mp. 15. exp weight reduction/ 16. (weight adj2 (loss or lost or reduc$ or eliminat$)).mp. 17. ((body mass index or bmi) adj3 (reduc$ or decreas$ or low$)).mp. 18. exp body mass/ 19. exp obesity/ 20. exp lifestyle/ 21. (lifestyle$ or life style$).mp. 22. exp health behavior/ 23. (health and (behaviour$ or behavior$)).mp. 24. exp smoking cessation/ 25. ((quit$ or stop) and smok$).mp. 26. (smoking adj2 cessation).mp. 27. exp alcohol abstinence/ 28. alcohol abstinence.mp. 29. exp drinking behavior/ 30. ((quit$ or stop) and (drinking or alcohol)).mp. 31. exercis$.mp. 32. exp exercise/ 33. exp kinesiotherapy/ 34. exp walking/ 35. exp sport/ 36. exp fitness/ 37. (sport$ or walk$ or swim$ or cycl$3 or run$3 or jog$3).mp. 38. (physical$ adj (fit$ or exer$ or activ$)).mp. 39. exp habit/ 40. exp risk reduction/ 41. or/7‐40 42. crossover procedure.sh. 43. double‐blind procedure.sh. 44. single‐blind procedure.sh. 45. (crossover$ or cross over$).tw. 46. placebo$.tw. 47. (doubl$ adj blind$).tw. 48. allocat$.tw. 49. trial.ti. 50. randomized controlled trial.sh. 51. random$.tw. 52. or/42‐51 53. exp animal/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 54. human/ or normal human/ 55. 53 and 54 56. 53 not 55 57. 52 not 56 58. 6 and 41 and 57
Appendix 5. LILACS search strategy
In LILACS we searched using the Controlled clinical trials topic‐specific query filter and these terms:
(psoria$ OR (palmoplantar$ pustulosis) OR (pustulosis palmaris et plantaris) OR (pustulosis and palms and soles)) and (diet$ or food habit$ or meal$ or nutrition or weight loss or body mass index or bmi or obesity or overweight or lifestyle$ or life style$ or (health and (behaviour$ or behavior$)) or smok$ or alcohol or exercis$ or sport$ or walk$ or swim$ or cycl$ or run$ or jog$ or fitness)
Appendix 6. CNKI search strategy
干癣 and 生活型态
干癣 and 减重
干癣 and 运动
干癣 and 戒烟
干癣 and 戒酒
牛皮癣 and 生活型态
牛皮癣 and 减重
牛皮癣 and 运动
牛皮癣 and 戒烟
牛皮癣 and 戒酒
银屑病 and 生活型态
银屑病and 减重
银屑病and 运动
银屑病 and 戒烟
银屑病and 戒酒
Appendix 7. Airiti Library search strategy
乾癬 and 生活型態
乾癬 and 減重
乾癬 and 運動
乾癬 and 戒菸
乾癬 and 戒酒
牛皮癬 and 生活型態
牛皮癬 and 減重
牛皮癬 and 運動
牛皮癬 and 戒菸
牛皮癬 and 戒酒
銀屑病 and 生活型態
銀屑病 and 減重
銀屑病 and 運動
銀屑病 and 戒菸
銀屑病 and 戒酒
Appendix 8. Search strategy for ISRCTN registry, US National Institutes of Health Ongoing Trials Register, and WHOICTRP
Condition: psoriasis AND Interventions: lifestyle
Condition: psoriasis AND Interventions: diet
Condition: psoriasis AND Interventions: weight reduction
Condition: psoriasis AND Interventions: smoking cessation
Condition: psoriasis AND Interventions: alcohol abstinence
Condition: psoriasis AND Interventions: exercise
Appendix 9. Search strategy for EU Clinical Trials Register
psoriasis AND lifestyle
psoriasis AND diet
psoriasis AND weight reduction
psoriasis AND smoking cessation
psoriasis AND alcohol abstinence
psoriasis AND exercise
Appendix 10. Data extraction form
Study ID (first author, year of publication):
Reviewer:
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Funding source | |
| Country | |
| Setting |
Risk of bias
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | ||
| Allocation concealment (selection bias) | ||
| Blinding of participants and personnel (performance bias) All outcomes |
||
| Blinding of outcome assessment (detection bias) All outcomes |
||
| Incomplete outcome data (attrition bias) All outcomes |
||
| Selective reporting (reporting bias) | ||
| Other bias |
Appendix 11. Optimal information size calculation
For dichotomous outcomes, we calculated the optimal information size (OIS) by using the following website: www.stat.ubc.ca/˜rollin/stats/ssize/b2.html. For continuous outcomes, we calculated the OIS by using Stata SE 11.2 (Stata 2009). The OIS is listed as follows:
PASI 75 (dichotomous outcome): The OIS was 93 for each group when assuming P1 = 0.5, P2 = 0.7, a = 0.05, power = 0.80.
Change in the PASI score or BSA: The OIS was 120 for each group when assuming mean1 = ‐12, SD1 = 6, mean2 = ‐10, SD2 = 5.
Adherence (dichotomous outcome): The OIS was 199 for each group when assuming P1 = 0.9, P2 = 0.8, a = 0.05, power = 0.80.
Change in the DLQI score (continuous outcome): The OIS was 6 for each group when assuming mean1 = ‐7, SD1 = 3, mean2 = ‐2, SD2 = 3.
Change in the BMI (continuous outcome): The OIS was 8 for each group when assuming mean1 = ‐5, SD1 = 4, mean2 = 0, SD2 = 3.
Change in body weight or waist circumference (continuous outcome): The OIS was 5 for each group when assuming mean1 = ‐5, SD1 = 3, mean2 = 0, SD2 = 2.
Data and analyses
Comparison 1. Dietary intervention versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PASI 75 | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Week 12 | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.59, 4.73] |
| 1.2 Week 24 | 2 | 323 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.07, 2.58] |
| 2 Change in PASI | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Week 16 | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐3.94, ‐0.06] |
| 2.2 Week 24 | 3 | 139 | Mean Difference (IV, Random, 95% CI) | ‐3.71 [‐6.80, ‐0.62] |
| 3 Change in BSA | 1 | 262 | Mean Difference (IV, Random, 95% CI) | ‐6.06 [‐8.56, ‐3.56] |
| 4 Adherence to the intervention | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Week 12 | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.65, 2.09] |
| 4.2 Week 16 | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.86, 1.25] |
| 4.3 Week 24 | 2 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.76, 2.09] |
| 5 Change in DLQI | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Week 16 | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐3.66, ‐0.34] |
| 5.2 month 6 | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐12.2 [‐13.92, ‐10.48] |
| 6 Change in body weight | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Week 16 | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐15.4 [‐18.45, ‐12.35] |
| 6.2 Week 24 | 4 | 401 | Mean Difference (IV, Random, 95% CI) | ‐10.04 [‐15.61, ‐4.48] |
| 7 Change in BMI | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Week 16 | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐5.0 [‐5.83, ‐4.17] |
| 7.2 Week 24 | 2 | 78 | Mean Difference (IV, Random, 95% CI) | ‐4.65 [‐5.93, ‐3.36] |
| 8 Change in waist circumference | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Week 16 | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐11.5 [‐13.99, ‐9.01] |
| 8.2 Month 6 | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐12.0 [‐17.27, ‐6.73] |
| 9 Change in total cholesterol | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Week 16 | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.49, ‐0.39] |
| 9.2 Week 24 | 3 | 359 | Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐1.08, ‐0.01] |
| 10 Change in triglyceride | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 Week 16 | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.38, ‐0.30] |
| 10.2 Week 24 | 3 | 359 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐1.09, 0.44] |
Comparison 2. Dietary intervention and exercise programme versus information only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PASI response | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 PASI 75 | 1 | 303 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.83, 1.98] |
| 1.2 PASI 50 | 1 | 303 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.11, 1.91] |
| 1.3 PASI 100 | 1 | 303 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.88, 2.83] |
| 2 Adherence to the intervention | 1 | 303 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.89, 1.01] |
Comparison 3. Education programme versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reduction in PASI | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 3 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 9 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 At least 60% reduction in BSA | 1 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.97, 1.40] |
| 3 Adherence to the intervention | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.42, 1.00] |
| 4 Reduction in DLQI | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 3 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 9 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Reduction in PDI | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 3 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 6 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 9 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Al‐Mutairi 2014.
| Methods | A parallel RCT on the effects of dietary intervention in treating psoriasis, conducted from February 2012‐Feburary 2013 | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 262 participants (93 men and 169 women) were randomised in 1:1 ratio, with 131 allocated to the diet intervention group and 131 to control group. The mean age of participants was 46.9 ± 6.4 years. The baseline BMI was 29.3 ± 4.2 and 29.5 ± 5.2 in the dietary intervention and control groups, respectively. The baseline PASI score was 32.7 ± 5.5 and 31.1 ± 6.3 kg/m² in the dietary intervention and control groups, respectively. The participants' psoriasis duration was 19.2 ± 9.2 years in the diet intervention group and 18.9 ± 8.9 years in the control group. |
|
| Interventions |
Intervention group
Control group
All participants were advised not to make any changes in their smoking habits or daily workout during the period of trial. All participants were treated for 24 weeks. Co‐interventions included infliximab, etanercept, adalimumab, and ustekinumab. |
|
| Outcomes |
Outcomes assessed every 4 weeks from baseline‐week 24 |
|
| Notes | Setting: a general hospital Country: Kuwait Funding source: none Declaration of interest: none Trial registration: not known; probably not done |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "They were randomised in 1:1 ratio into two groups". Comment: did not report methods of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Did not report relevant details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: blinding of participants was impossible due to the intrinsic limitation of dietary intervention, but this did not affect objective outcome measurement. Did not report whether the investigators were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was unknown whether the outcome assessors for PASI and BSA were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts or withdrawals |
| Selective reporting (reporting bias) | Low risk | All the outcomes planned in the methods section of the trial report were reported. |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed. |
Bostoen 2012.
| Methods | A parallel RCT on the effect of an educational programme promoting healthy lifestyle in treating psoriasis or atopic dermatitis, conducted from Feburary 2010‐2011 | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 50 participants, including 29 with psoriasis and 21 with atopic dermatitis were recruited. Of 29 participants with psoriasis, 15 were randomised to the intervention group and 14 to the control group. The age and sex distribution as well as the baseline BMI of the 29 participants with psoriasis was not specified. At baseline the mean PASI score was 7.7 ± 3.9, indicating mild psoriasis. The baseline PASI score was 8.9 ± 4.3 and 7.1 ± 3.8 in the intervention and control groups, respectively. |
|
| Interventions |
Intervention group
Control group
All participants were treated for 12 weeks. |
|
| Outcomes |
There was a follow‐up period of 9 months. |
|
| Notes | Setting: a university hospital Country: Belgium Funding source: the organisation of the educational programme was supported by unrestricted grants from Pierre Fabre SA, Schering‐Plough, Abbott, LEO Pharma and Johnson & Johnson Declaration of interest: none. Trial registration: NCT01077882 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomised (1:1) to the intervention or control group. This procedure involved computer‐generated randomisation of lists in which allocation was indicated, and stratified by diagnosis using a block size of two." Comment: we considered the randomisation method adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "Sequentially numbered envelopes were used by the investigator to assign patients to the intervention or control group." Comment: allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Based on the intrinsic limitation of educational programme, blinding of participants was impossible. Quality‐of‐life measure might have been affected. Personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Two clinicians performed the assessments of disease severity at the four study visits and were blinded for randomisation." Comment: outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were 6 (40%) dropouts in the intervention group and 1 (7%) dropout in the control group. In the intervention group, 1 (7%) was excluded from analysis because the condition deteriorated severely due to stress caused by workplace bullying. |
| Selective reporting (reporting bias) | Low risk | All the outcomes planned in the protocol (clinicaltrials.gov/ct2/show/NCT01077882) were reported |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed. |
Chen 2013a.
| Methods | A parallel RCT on the effect of an educational programme promoting healthy lifestyle in treating psoriasis, conducted from March 2010‐Feburary 2012 | |
| Participants |
Inclusion criteria
A total of 132 participants (including 73 men and 59 women) were randomised in 1:1 ratio, with 66 allocated to the experimental group and 66 to the control group. There were 77 participants aged 18‐49 years and 55 aged 50‐78 years. No data on the psoriasis severity and BMI were reported. |
|
| Interventions |
Intervention group
Control group
|
|
| Outcomes | The response was evaluated by using the following criteria: cleared (clearance > 90%), very effective (clearance > 60% to < 90%), effective (clearance > 30% to < 60%), and ineffective (clearance < 30%, no change, or worsened). However, the trial authors did not specify when the outcome assessment was done. | |
| Notes | Setting: a university hospital Country: China Funding source: not reported Declaration of interest: not reported Trial registration: not known; probably not done |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly allocated to two groups". Comment: did not report methods of generating random sequence |
| Allocation concealment (selection bias) | Unclear risk | Did not report relevant details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: based on the intrinsic limitation of dietary instructions, blinding of participants was impossible but objective outcome assessment would not have been influenced. Did not report whether the investigators were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unknown whether the outcome assessors for PASI and BSA were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts or withdrawals |
| Selective reporting (reporting bias) | High risk | Only one outcome 'severity of psoriasis' was reported |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed. |
Del Giglio 2012.
| Methods | A parallel RCT on the effects of dietary intervention in treating psoriasis | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 42 participants (26 men and 16 women) were randomised, with 22 in the dietary intervention group and 20 in the control group. The mean age in the intervention and control group was 61.0 ± 10.0 and 58.0 ± 9.4 years. The PASI score at enrolment was 1.1 ± 1.3 and 0.8 ± 0.9 in the dietary intervention and control groups, respectively. The baseline BMI was 31.9 ± 0.6 and 30.9 ± 2.3 kg/m² respectively. The participants' psoriasis duration was 15.0 ± 3.0 years in the diet intervention group and 13.0 ± 5.0 years in the control group. |
|
| Interventions |
Intervention group
Control group
All participants were treated for 24 weeks. |
|
| Outcomes |
These outcomes were measured at week 24 (primary end point) and week 36 (final analysis) |
|
| Notes | Setting: a university hospital Country: Italy Funding source: not reported Declaration of interest: none Trial registration: registered at clinicaltrials.gov as NCT01439425 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed with the use of computer generated random numbers and block size of 4 subjects.” Comment: we considered randomisation method adequate |
| Allocation concealment (selection bias) | Unclear risk | Did not report relevant details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: blinding of participants was impossible due to the intrinsic limitation of dietary instructions, but this would not affect objective outcome assessment. Did not report whether the investigators were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The dermatologist who performed the PASI scoring was unaware of the randomisation assignment." Comment: outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts or withdrawals |
| Selective reporting (reporting bias) | Low risk | All the outcomes planned in the protocol (clinicaltrials.gov/ct2/show/NCT01439425) were reported. |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed. |
Gisondi 2008.
| Methods | A parallel RCT on the effects of dietary intervention in treating psoriasis | |
| Participants |
Inclusion criteria
Exclusion criteria
61 participants (30 men and 31 women) were enrolled, with 30 randomised to the dietary intervention group and 31 to the control group. The mean age was 52.3 ± 14.5 years in the intervention group and 50.9 ± 10.5 years in the control group. The baseline PASI score was 15.1 ± 5.3 and 14.1 ± 6.4 in the dietary intervention and control groups, respectively. The baseline BMI was 34.0 ± 4.9 and 33.4 ± 3.6 kg/m² in the dietary intervention and control groups, respectively. The participants' psoriasis duration was 19 ± 12 years in the diet intervention group and 20 ± 12.5 years in the control group. |
|
| Interventions |
Intervention group
Control group
All of the participants were also encouraged to perform moderate physical exercise for 40 min, 4 times/week The treatment period was 24 weeks. |
|
| Outcomes |
The above outcomes were measured at baseline and week 24. |
|
| Notes | Setting: a university hospital Country: Italy Funding source: the Ministero della Salute and the Ministero dell' Istruzione, Universita' e Ricerca Scientifica (Programmi di Ricerca Scientifica di Rilevante Interesse Nazionale) Declaration of interest: not reported Trial registration: registered at Clinicaltrials.gov as NCT00512187 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed with the use of computer‐generated random numbers and block size of 4 patients." Comment: we considered randomisation method adequate |
| Allocation concealment (selection bias) | Unclear risk | Did not report relevant details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The study was investigator blinded, i.e. the dermatologist who performed the PASI scoring was unaware of the randomisation assignment." Comment: blinding of participants was impossible due to the intrinsic limitation of dietary instructions, but it was unclear if premature withdrawal was affected. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The study was investigator blinded, i.e. the dermatologist who performed the PASI scoring was unaware of the randomisation assignment." Comment: outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Withdrawal from the study rate was also significantly different between the groups. In particular, 4 patients in the intervention group (13.3%) dropped out of the study...compared with 14 patients (45.1%) in the control group...(P < 0.001)." Comment: significant differences in withdrawal between groups |
| Selective reporting (reporting bias) | Low risk | All the outcomes planned in the protocol (clinicaltrials.gov/ct2/show/NCT00512187) were reported. |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed. |
Guida 2014.
| Methods | A parallel RCT on the effects of dietary intervention in treating psoriasis, enrolling participants from April 2007‐March 2008 | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 44 participants (32 men and 12 women) were randomised in 1:1 ratio, with 22 to each group. A total of 18 participants per group completed the trial. Four participants per group were lost at the 6‐month follow‐up for poor compliance to the drug therapy. The mean age of the participants was 52.18 ± 11.12 years. The mean baseline PASI score was 7.7 ± 3.7 and 8.9 ± 3.9 for the intervention and control group, respectively. The mean BMI was 33.4 ± 3.4 and 31.0 ± 2.6 kg/m² for the two groups, respectively. |
|
| Interventions |
Intervention group
Control group
During a 6‐month trial period, all 44 participants received daily therapy, including 1 of 5 prescription medications: adalimumab, infliximab, etanercept, cyclosporine, or methotrexate. |
|
| Outcomes |
In all participants, anthropometric measurements, metabolic markers, and clinical assessments were recorded at baseline, 3 months, and 6 months. |
|
| Notes | Setting: A university hospital Country: Italy Funding source: not reported Declaration of interest: none Trial registration: ClinicalTrials.gov identifier: NCT01876875 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "This was an open label randomised parallel group clinical trial"; "simple randomisation with a 1:1 allocation ratio using the chit pick box method (Fig. 1). Briefly, 22 chits labeled "group‐A" and 22 chits labeled "group‐B" were put into a single box. Whenever patient was selected for study, a chit was picked from the box, and whatever chit was picked, the patient was assigned to that group." Comment: we considered randomisation method adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "Whenever patient was selected for study, a chit was picked from the box, and whatever chit was picked, the patient was assigned to that group." Comment: allocation was likely concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The medical doctor performing the first visit was responsible for enrolment and randomised group assignment." Comment: based on the intrinsic limitation of dietary instructions, blinding of participants was impossible and might have affected subjective outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "To prevent rater biases the dermatologists who evaluated the PASI scores were blinded to the treatment." Comment: other outcome measures, for example anthropometric measures, were objective; hence, low risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Four participants (18.2%) per group dropped out. |
| Selective reporting (reporting bias) | Low risk | All the outcomes planned in the protocol (clinicaltrials.gov/ct2/show/NCT01876875) were reported. |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed. |
Jensen 2013.
| Methods | A parallel RCT on the effects of dietary intervention in treating psoriasis, conducted from 1 June 2010‐1 June 2011 | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 60 participants (32 men and 28 women) were randomised in a 1:1 ratio to either 16 weeks of intensive weight loss therapy (the LED group) or 16 weeks of standard routine dietary guidance (the control group). The mean age of participants was 50.8 ± 10.3 years. The median PASI score at baseline was 5.4 (IQR 3.8‐7.6). The mean BMI at baseline was 34.2 ± 5.2 kg/m². |
|
| Interventions |
Intervention group
Control group
The treatment period was 16 weeks. Antipsoriatic treatment, if any, had to be stable and unchanged for at least 3 months before inclusion; during the trial, participants were instructed not to change their antipsoriatic treatment, tobacco use, or physical exercise levels in any way. Medications for other medical conditions (e.g. hypertension) could be changed as necessary |
|
| Outcomes |
All the outcomes were measured at baseline and at weeks 4, 8, 12, and 16 |
|
| Notes | Setting: a university hospital Country: Denmark Funding source: this trial was supported in part by Cambridge Manufacturing Company Limited, the Michaelsen Foundation, the Aase and Ejnar Danielsen Foundation, the Research Foundation of the Danish Academy of Dermatology, the Danish Agriculture and Food Council, the Jacob Madsen and Olga Madsen Foundation, the Danish Psoriasis Research Foundation, and the Medical Research Foundation of the Capital Region of Denmark. The Parker Institute is supported by unrestricted grants from the Oak Foundation. Declaration of interest: none Trial Registration: Clinicaltrials.gov Identifier: NCT01137188 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The 60 participants who met the inclusion criteria were randomised in a 1:1 ratio to either 16 weeks of intensive weight loss therapy (the LED group) or 16 weeks of standard routine dietary guidance (the control group). To compensate for seasonal variations in sunlight exposure, we divided the patients into 4 pairs of LED and control groups that started the study at 2‐month intervals. Randomization was stratified according to sex to ensure an equal distribution into the LED and control groups." Comment: we considered randomisation method adequate |
| Allocation concealment (selection bias) | Low risk | Quote: "One of us (P.J.) placed the names of the participating men in sealed opaque envelopes and repeated this procedure for the women. An independent colleague (with P.J. absent) then shuffled and randomly divided the envelopes containing the men's names into 2 groups and repeated the procedure for the women. Finally, group allocation was decided by coin toss by the independent colleague. We informed the participants of their allocations when they came on the first day of the study." Comment: allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Based on the intrinsic limitation of dietary instructions, blinding of participants was impossible and might have affected DLQI assessment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Treatment efficacy was assessed by the primary investigator (P.J.) at baseline and after 4, 8, 12, and 16 weeks."“Body weight was measured to the nearest 0.1 kg with a digital scale (HD‐351; Tanita). Standing height was measured with a wall‐mounted stadiometer to the nearest 0.01 m. Lean body mass and fat mass were measured to the nearest 0.1 kg with dual energy x‐ray absorptiometry (Lunar iDXA; GEHealthcare). Waist and hip circumferences were measured to the nearest 0.1 cm with a standard tape measure." Comment: outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "there was no significant difference between groups in the number of patients who withdrew from the study after randomisation (3 of 30 in the LED group vs 4 of 30 in the control group; Fisher exact test, P > .99)." The loss to follow‐up was 11.7%." Comment: low risk of attrition bias |
| Selective reporting (reporting bias) | Low risk | All the outcomes planned in the protocol (clinicaltrials.gov/ct2/show/NCT01137188) were reported. |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed. |
Kimball 2012.
| Methods | A parallel RCT on the effects of dietary intervention in treating psoriasis | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 30 (19 men and 11 women) were enrolled, with 10 randomised to the Ornish Diet, South Beach Diet, and non‐intervention groups, respectively. Only 20 participants completed the trial, with 6, 7, and 7 in the Ornish Diet, South Beach Diet, and non‐intervention groups, respectively. The mean age of the Ornish Diet, South Beach Diet, and non‐intervention groups was 49.1 ± 8.8, 44.3 ± 14.4, and 48.1 ± 17.7 years. The baseline PASI score was 18.0 ± 5.57, 15.0 ± 5.35, and 13.2 ± 6.84, respectively. The baseline weight was 97.2, 98.1, and 109.0 kg, respectively. |
|
| Interventions |
Intervention group 1: Ornish Diet
Intervention group 2: South Beach Diet
Control group
The treatment period was 12 weeks. All participants received concurrent NB‐UVB phototherapy using TL‐01 lamps (310‐312 nm) 3 times/week for 12 weeks |
|
| Outcomes |
PASI assessments were performed by a blinded physician investigator at baseline, weeks 4, 8 and 12 |
|
| Notes | Setting: a teaching hospital Country: USA Funding source: not reported Declaration of interest: not reported Trial registration: Clinicaltrials.gov identifier NCT00537212 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Did not report methods of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Did not report relevant details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The intrinsic limitation of dietary instructions made blinding of participants impossible. It is unknown if the outcome 'adverse effects' might have been affected |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "PASI assessments were performed by a blinded physician investigator at baseline, weeks 4, 8 and 12." Comment: outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Twenty patients completed all study visits (Fig. 1 and Table 2). Three patients who had at least one follow‐up visit were also included in a modified intent‐to‐treat analysis (mITT)." Comment: 33.3% of the participants were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All the outcomes planned in the protocol (clinicaltrials.gov/ct2/show/NCT00537212) were reported. |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed. |
Li 2015.
| Methods | A parallel RCT on the effects of walking exercise combined with continuous health education on the prevention of psoriasis flares, enrolling participants from June 2009‐October 2014 | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 200 participants (136 men and 64 women, aged 18‐70 years (mean 43.1 ± 8.9 years)) were enrolled, with 100 randomised to the intervention group and control group, respectively. The severity of psoriasis and BMI were not reported. |
|
| Interventions |
Intervention group
Control group
The treatment period was 3 years. |
|
| Outcomes |
The outcomes were measured before intervention as well as 12, 24, and 36 months into intervention. |
|
| Notes | Setting: a university hospital Country: China Funding source: the Administration of Health, Guangxi Zhuang Region, China (Z2012442) Declaration of interest: not reported Trial registration: not reported, probably not done |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Trial used a random number table |
| Allocation concealment (selection bias) | Unclear risk | Did not report relevant details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The intrinsic limitation of walking exercise and health education made blinding of participants impossible. Did not report whether the personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Did not report relevant details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts or withdrawals |
| Selective reporting (reporting bias) | Low risk | All the outcomes planned in the methods section of the trial report were reported. |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed. |
Naldi 2014.
| Methods | A parallel RCT on the effects of dietary intervention and exercise programme on the severity of psoriasis and obesity, enrolling participants between Feburary 2011‐July 2011 | |
| Participants |
Inclusion criteria
Exclusion criteria
A total of 303 participants (215 men and 88 women; median age 53 years; median PASI score 4.0 (IQR 10.7); median BMI 30.8 (IQR 6.2) kg/m²) were enrolled, with 151 randomised to the dietary intervention group and 152 to the control group. There were 14 (9.3%) in the dietary intervention group and 7 (4.6%) in the control group that were lost to follow‐up. |
|
| Interventions |
Intervention group
Control group
Participating dermatologists were instructed to review the participants' treatment periodically according to local guidelines. The dosage of weight‐adjusted therapies could be modified in the event that a body weight change of > 5 kg was documented at a scheduled follow‐up visit. It was also permissible to stop the systemic treatment and/or to shift participants to a different therapy, but only if a participant experienced adverse events or intolerance to the prescribed treatment. The trial period was 20 weeks. |
|
| Outcomes |
All outcome variables were also evaluated as overall changes, considering the slope of the curve constructed with data from baseline and weeks 8, 16 and 20 |
|
| Notes | Setting: 9 hospitals located in the Emilia Romagna region Country: Italy Funding source: a research grant from the Emilia Romagna region (Programma di Ricerca Regione Universita 2007–2009 AREA 2 Ricerca per il governo clinico). Declaration of interest: none Trial Registration: NCT01714284 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised on a 1 : 1 basis to the two intervention arms, using a centralized telephone randomisation procedure with stratification for age and BMI. The random allocation sequence was generated by using a pseudorandom number generator algorithm." Comment: we considered randomisation method adequate |
| Allocation concealment (selection bias) | Unclear risk | Did not report relevant details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The the intrinsic limitation of dietetic intervention with exercise programme made blinding of participants impossible, but measurement of objective outcomes was not affected. Whether the personnel was blinded was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An independent assessor (not the treating physician) who was blind to the treatment arm allocation calculated the PASI score at the different time points. Height (to the nearest mm), body weight (to the nearest 01 kg) and waist circumference (in cm) were measured by trained research staff." Comment: outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Twice as many participants in the dietary intervention group (9.3%) than the control group (4.6%) were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the outcome planned in the protocol (clinicaltrials.gov/ct2/show/NCT01714284) were reported. |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias existed. |
BDI: Beck Depression Inventory; BMI: body‐mass index; BSA: body surface area; DLQI: Dermatology Life Quality Index; EQ‐5D: EuroQol‐5D; HDL‐C: high‐density lipoprotein‐cholesterol; IQR: interquartile range; LDL‐C: low‐density lipoprotein‐cholesterol; NB‐UVB: narrowband ultraviolet B; PASI: Psoriasis Area and Severity Index PDI: Psoriasis Disability Index; RCT: randomised controlled trial; RMR: resting metabolic rate; TB: tuberculosis; VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12613001031752 | The authors stopped the trial and replied to our query: "Unfortunately we did not proceed with the trial as there were very few patients in our trials catchment that were being prescribed Humira for psoriasis." |
| Balato 2013 | A randomised trial on the efficacy of text messages use in improving treatment adherence. |
| Chang 2016 | Not a randomised trial, but an introduction to the application of sweating exercise in treating psoriasis vulgaris from the viewpoint of traditional Chinese medicine. |
| Fortes 2006 | Not a randomised trial, but a cross‐sectional trial on the relationship between smoking and the severity of psoriasis. |
| He 2016 | A randomised trial on the effects of 8‐section brocade (an intervention of complementary and alternative medicine) in treating psoriasis. |
| Hsiao 2001 | Not a randomised trial, but an observational trial on the severity of psoriasis in participants with different dietary habits. |
| Keyworth 2014 | A randomised trial comparing the effects of four different message frames in prompting changes in behaviours, but not examining whether behavioural changes could improve psoriasis. |
| Rucevic 2003 | Not a randomised trial, but a controlled clinical trial on the effects of low‐energy diet on psoriasis vulgaris. |
| Zackheim 1971 | There were no statements of random allocation. The trial was probably a controlled clinical trial. |
Characteristics of ongoing studies [ordered by study ID]
NCT03440736.
| Trial name or title | Comparison of secukinumab 300 mg combined with a lifestyle intervention to secukinumab alone for the treatment of moderate to severe psoriasis patients with concomitant metabolic syndrome (METABOLYX) |
| Methods | A randomised, multicentre, open‐label, parallel‐group, active comparator‐controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria Patients fulfilling any of the following criteria are not eligible for inclusion in this trial. No additional exclusions may be applied by the investigator, in order to ensure that the trial population will be representative of all eligible patients.
|
| Interventions |
Intervention group
Control group
The trial period is 28 weeks, followed by a 28‐week extension period. The secukinumab treatment is administered as secukinumab 300 mg subcutaneously, which consists of 2 injections with 150 mg prefilled syringes at weeks 0, 1, 2, 3, 4, 8, 12, 16, 20, and 24 (last injection is performed at week 24) |
| Outcomes |
|
| Starting date | 28 February 2018 |
| Contact information | Novartis Pharmaceuticals, Tel: +41 613 241111. E‐mail: novartis.email@novartis.com |
| Notes | Setting: 65 sites in Germany Country: Germany Funding source: Novartis Pharmaceuticals Declaration of interest: not available yet Trial registration: NCT03440736 (clinicaltrials.gov/ct2/show/NCT03440736) |
BMI: body‐mass index; BSA: body surface area; DLQI: Dermatology Life Quality Index; HDL‐C: high‐density lipoprotein‐cholesterol; hsCRP: high‐sensitivity C‐reactive protein; LDL‐C: low‐density lipoprotein‐cholesterol; NRS: numeric rating scale; TB: tuberculosis; PASI: Psoriasis Area and Severity Index; WHO‐5: 5‐item World Health Organization Well‐Being Index
Differences between protocol and review
The table showing the potential mechanism of lifestyle intervention has been relocated to Table 3.
We added a description of lifestyle changes to the Objectives of the review, in response to a peer reviewer comment.
In the protocol (Chi 2015b), we did not report the time points at which we assessed outcome data. In this review, except for the outcome 'time to relapse', we decided to assess outcome data at week 12, week 24, and year 1. If there were no data at these time points, we assessed data at other available time points.
The current gold standard of Phase II and III trials on interventions for psoriasis use a control period of 12 weeks as short‐term primary endpoint (see our Additional references Ryan 2014). Therefore, we consider our included trials on lifestyle changes should also be at least 12 weeks for comparison. We have revised Types of studies as follows: “Randomised controlled trials (RCTs) that assessed the effects of lifestyle changes for treating psoriasis. Interventions had to be given for at least 12 weeks which is the current gold standard, short‐term endpoint of clinical trials on interventions for treating psoriasis (Ryan 2014).”
For the first primary outcome 'Severity of psoriasis', we initially planned to include PASI 75, and included PASI 50 and PASI 90 when data on PASI 75 were unavailable (Chi 2015b). However, as PASI 50, PASI 90, and PASI 100 (clearing of psoriatic lesions) are different and important measures of the efficacy of an intervention, we decided to also report these outcomes when they were available.
We originally planned to conduct subgroup analysis based on age groups and overweight status when high statistical heterogeneity was present across the included trials (Chi 2015b). However, the low number of included trials made this impossible. We decided to describe narratively the potential cause for statistical heterogeneity.
We planned to use a funnel plot to examine the presence of publication bias on primary outcomes in the protocol (Chi 2015b), but did not do so due to the low number of included trials that provided useable data.
We added an optimal information size (OIS) calculation (Appendix 11), as a reference for judging imprecision.
In the protocol we did not specify how we would calculate missing SDs for change from baseline data; hence, we added this methods to the 'Dealing with missing data' section.
Contributions of authors
CC was the contact person with the Editorial Base. CC co‐ordinated contributions from MY, SW, and MH. SK and CC wrote the final draft of the review. CC and YT searched the China National Knowledge Infrastructure (CNKI), Airiti Library, and trials registers. SK and CC screened papers against eligibility criteria. CC obtained data on ongoing and unpublished trials. SK and CC appraised the quality of papers. SK and CC extracted data for the review and CC sought additional information about papers. CC entered data into Review Manager 5 (Review Manager 2014). SK and CC analysed and interpreted data. CC worked on the methods sections. CC drafted the clinical sections of the background and responded to the clinical comments of the referees. CC responded to the methodology and statistics comments of the referees. SW was the consumer co‐author and checked the review for readability and clarity, as well as ensuring outcomes are relevant to consumers. CC is the guarantor of the update.
Disclaimer
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Skin. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health.
Sources of support
Internal sources
-
Chang Gung Memorial Hospital, Chiayi, Taiwan.
Provided research grant (CMRPG6F0401) and support for CC and YT.
External sources
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of Cochrane Skin.
Declarations of interest
Shu‐Hua Ko: nothing to declare. Ching‐Chi Chi: I received fees for speaking from AbbVie Taiwan (Aug 2017), Ego Pharmaceuticals Taiwan (Jan 2017), Janssen‐Cilag Taiwan (April 2017), Novartis Taiwan (May 2017), and Pfizer Taiwan (Sept 2017). These companies distribute biologics for treating psoriasis in Taiwan. Mei‐Ling Yeh: nothing to declare. Shu‐Hui Wang: nothing to declare. Yu‐Shiun Tsai: nothing to declare. Mei‐Ya Hsu: nothing to declare.
Ching‐Chi Chi, lead author on the protocol of this Cochrane Review, became conflicted in terms of Cochrane’s commercial sponsorship policy and has been replaced by Shu‐Hua Ko, as lead author. Shu‐Hua Ko has no relevant financial conflict of interest, and in the judgement of Cochrane Skin’s editorial team, she has made a contribution equal to Ching‐Chi Chi, which justifies her position as first author. This change has been discussed with and approved by the Funding Arbiters.
Content referee Luigi Naldi was an author of one of the included studies (Naldi 2014).
New
References
References to studies included in this review
Al‐Mutairi 2014 {published data only}
- Al‐Mutairi N, Manchanda Y. The effect of weight reduction on treatment outcomes in obese patients of psoriasis on biologic therapy. Journal of the American Academy of Dermatology 2015;72(5 Suppl 1):AB254. [Google Scholar]
- Al‐Mutairi N, Nour T. The effect of weight reduction on treatment outcomes in obese patients with psoriasis on biologic therapy: a randomized controlled prospective trial. Expert Opinion on Biological Therapy 2014;14(6):749‐56. [CENTRAL: CN‐00993130; PUBMED: 24661040] [DOI] [PubMed] [Google Scholar]
Bostoen 2012 {published data only}
- Bostoen J, Bracke S, Keyser S, Lambert J. An educational programme for patients with psoriasis and atopic dermatitis: a prospective randomized controlled trial. British Journal of Dermatology 2012;167(5):1025‐31. [CENTRAL: CN‐00856431; PUBMED: 22709422] [DOI] [PubMed] [Google Scholar]
- Bostoen J, Geusens B, Lambert J, Bracke S, Dekeyser S. An educational program for patients with psoriasis and atopic dermatitis: a prospective randomized, controlled trial. British Journal of Dermatology 2012; Vol. 66, issue 4 Suppl 1:Ab84. [CENTRAL: CN‐01005670] [DOI] [PubMed]
Chen 2013a {published data only}
- Chen GL. Sensible diet and nursing strategy for psoriasis [銀屑病的合理飲食和護理對策]. Laboratory Medicine and Clinic 2013;10(16):2186‐7. [Google Scholar]
Del Giglio 2012 {published data only}
- Giglio M, Gisondi P, Tessari G, Girolomoni G. Weight reduction alone may not be sufficient to maintain disease remission in obese patients with psoriasis: a randomized, investigator‐blinded study. Dermatology 2012;224(1):31‐7. [CENTRAL: CN‐01017641; PUBMED: 22456343] [DOI] [PubMed] [Google Scholar]
Gisondi 2008 {published data only}
- Gisondi P, Giglio M, Francesco V, Zamboni M, Girolomoni G. Weight loss improves the response of obese patients with moderate‐to‐severe chronic plaque psoriasis to low‐dose cyclosporine therapy: a randomized, controlled, investigator‐blinded clinical trial. American Journal of Clinical Nutrition 2008;88(5):1242‐7. [CENTRAL: CN‐00665491] [DOI] [PubMed] [Google Scholar]
Guida 2014 {published data only}
- Guida B, Napoleone A, Trio R, Nastasi A, Balato N, Laccetti R, et al. Energy‐restricted, n‐3 polyunsaturated fatty acids‐rich diet improves the clinical response to immuno‐modulating drugs in obese patients with plaque‐type psoriasis: a randomized control clinical trial. Clinical Nutrition (Edinburgh, Scotland) 2014;33(3):399‐405. [CENTRAL: CN‐00988461] [DOI] [PubMed] [Google Scholar]
Jensen 2013 {published data only}
- Geiker N, Jensen P, Zachariae C, Christensen R, Schaadt B, Stender S, et al. Weight loss improves the cardiovascular risk profile of obese patients with psoriasis. Obesity Reviews 2014;15(S2):175. [CENTRAL: CN‐01066070] [DOI] [PubMed] [Google Scholar]
- Jensen P. Erratum: Effect of weight loss on the severity of psoriasis: a randomized clinical study (JAMA Dermatology (2013) 149:7 (795‐801) doi:10.1001/ jamadermatol.2013.722). JAMA Dermatology 2013;149(8):997. [PUBMED: 23752669] [DOI] [PubMed] [Google Scholar]
- Jensen P, Christensen R, Zachariae C, Geiker NR, Schaadt BK, Stender S, et al. Long‐term effects of weight reduction on the severity of psoriasis in a cohort derived from a randomized trial: a prospective observational follow‐up study. American Journal of Clinical Nutrition 2016;104(2):259‐65. [PUBMED: 27334236] [DOI] [PubMed] [Google Scholar]
- Jensen P, Zachariae C, Christensen R, Geiker N, Schaadt B, Stender S, et al. Effect of weight loss on the severity of psoriasis: a randomised controlled trial. Journal of Investigative Dermatology 2013;133(Supp 1):S182. [EMBASE: 71083543] [DOI] [PubMed] [Google Scholar]
- Jensen P, Zachariae C, Christensen R, Geiker NR, Schaadt BK, Stender S, et al. Effect of weight loss on the cardiovascular risk profile of obese patients with psoriasis. Acta Dermato‐Venereologica 2014;94(6):691‐4. [CENTRAL: CN‐01022975] [DOI] [PubMed] [Google Scholar]
- Jensen P, Zachariae C, Christensen R, Geiker NR, Schaadt BK, Stender S, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatology 2013;149(7):795‐801. [PUBMED: 23752669] [DOI] [PubMed] [Google Scholar]
- Larsson LK, Geiker NR, Jensen P, Zachariae C, Astrup A, Skov L. Weight loss and skin manifestations in obese patients with psoriasis ‐ a controlled randomized cross‐over study. Obesity Reviews 2011;12(S1):205. [EMBASE: 70605096] [Google Scholar]
Kimball 2012 {published data only}
- Kimball AB, Alavian C, Alora‐Palli M, Bagel J. Weight loss in obese patients with psoriasis can be successfully achieved during a course of phototherapy. Journal of the European Academy of Dermatology and Venereology : JEADV 2012;26(12):1582‐4. [CENTRAL: CN‐00899892] [DOI] [PubMed] [Google Scholar]
Li 2015 {published data only}
- Li YF, Jiang LJ, Tao Y, Qin GL, Li Z, He W. The effects of walking exercise combined with continuous health education on prevention of psoriasis recurrence [慢走運動與持續健康教育對預防銀屑病復發的效果研究]. Journal of Nursing Adminstration 2015;15(4):294‐6. [Google Scholar]
Naldi 2014 {published data only}
- Naldi L, Conti A, Cazzaniga S, Patrizi A, Pazzaglia M, Lanzoni A, et al. Diet and physical exercise in psoriasis: a randomized controlled trial. British Journal of Dermatology 2014;170(3):634‐42. [CENTRAL: CN‐00981223] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12613001031752 {unpublished data only}
- ACTRN12613001031752. Exercise and nutrition intervention for patients with psoriasis [The effect of an exercise and nutrition intervention on body composition in patients with psoriasis]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=364970 (first received 12 September 2013). [ACTRN12613001031752]
Balato 2013 {published data only}
Chang 2016 {published data only}
- Chang YH, Wang CF. Application of sweating exercise in treating psoriasis vulgaris [運動汗法在尋常型銀屑病治療中的運用]. Asia‐Pacific Traditional Medicine 2016;12(4):97‐8. [DOI: 10.11954/ytctyy.201604042] [DOI] [Google Scholar]
Fortes 2006 {published data only}
- Fortes C, Mastroeni S, Leffondré K. Relationship between smoking and the clinical severity of psoriasis [吸煙与銀屑病臨床嚴重度的關系]. Digest of the World Core Medical Journals ‐ Dermatology 2006;2(3):28‐9. [Google Scholar]
He 2016 {published data only}
- He H, Dong D, Jiang G. Influence of eight‐section brocade on quality of life of psoriasis patients with qiyu stagnation [八段錦對氣郁質銀屑病病人生活質量的影響]. Chinese Nursing Research 2016;30(6):2251‐2. [DOI: 10.3969/j.issn.1009-6493.2016.18.025] [DOI] [Google Scholar]
Hsiao 2001 {published data only}
- Hsaio CL, Yen DC, He XC, Liu SC, Fang YL. Effects of various diets on the flares of psoriasis [不同飲食結構對銀屑病患者病情轉歸的影響]. Chinese Journal of Dermatology 2001;34(2):132. [Google Scholar]
Keyworth 2014 {published data only}
- Keyworth C, Nelson PA, Bundy C, Griffiths CE, Cordingley L. Does health message framing affect behavioural intentions in patients with psoriasis? An experimental study. British Journal of Dermatology 2014;171(6):e143‐4. [CENTRAL: CN‐01088032] [Google Scholar]
Rucevic 2003 {published data only}
- Barisic‐Drusko V, Rucevic I. Psoriasis vulgaris and low energy diet. Journal of the European Academy of Dermatology and Venereology: JEADV 2003;17(Suppl 3):378. [CENTRAL: CN‐00478458] [Google Scholar]
- Rucevic I, Perl A, Barisic‐Drusko V, Adam‐Perl M. The role of the low energy diet in psoriasis vulgaris treatment. Collegium Antropologicum 2003;27(Suppl 1):41‐8. [PUBMED: 12955890] [PubMed] [Google Scholar]
Zackheim 1971 {published data only}
- Zackheim HS, Farber EM. Rapid weight reduction and psoriasis. Archives of Dermatology 1971;103(2):136‐40. [PUBMED: 4928232] [PubMed] [Google Scholar]
References to ongoing studies
NCT03440736 {published data only}
- Comparison of secukinumab 300 mg combined with a lifestyle intervention to secukinumab alone for the treatment of moderate to severe psoriasis patients with concomitant metabolic syndrome (METABOLYX). clinicaltrials.gov/ct2/show/NCT03440736 (first received 21 February 2018).
Additional references
Adams 2004
- Adams G, Gulliford MC, Ukoumunne OC, Eldridge S, Chinn S, Campbell MJ. Patterns of intra‐cluster correlation from primary care research to inform study design and analysis. Journal of Clinical Epidemiology 2004;57(8):785‐94. [PUBMED: 15485730] [DOI] [PubMed] [Google Scholar]
Alberti 2009
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120(16):1640‐5. [PUBMED: 19805654] [DOI] [PubMed] [Google Scholar]
Altunay 2013
- Altunay I, Doner N, Mercan S, Demirci GT. Stress coping mechanisms in smoking psoriatics. Dermatologica Sinica 2013;31(3):130‐3. [DOI: 10.1016/j.dsi.2013.02.001] [DOI] [Google Scholar]
Armstrong 2014
- Armstrong AW, Harskamp CT, Dhillon JS, Armstrong EJ. Psoriasis and smoking: a systematic review and meta‐analysis. British Journal of Dermatology 2014;170(2):304‐14. [PUBMED: 24117435] [DOI] [PubMed] [Google Scholar]
Bronsard 2010
- Bronsard V, Paul C, Prey S, Puzenat E, Gourraud PA, Aractingi S, et al. What are the best outcome measures for assessing quality of life in plaque type psoriasis? A systematic review of the literature. Journal of the European Academy of Dermatology and Venereology: JEADV 2010;24 Suppl 2:17‐22. [PUBMED: 20443996] [DOI] [PubMed] [Google Scholar]
Camp 1992
- Camp RD. Psoriasis. In: Champion RH, Burton JL, Ebling EJG editor(s). Textbook of Dermatology. 5th Edition. Vol. 2, Oxford: Blackwell Scientific Publications, 1992:1391‐1457. [Google Scholar]
Chen 2013b
- Chen X, Yang M, Cheng Y, Liu GJ, Zhang M. Narrow‐band ultraviolet B phototherapy versus broad‐band ultraviolet B or psoralen‐ultraviolet A photochemotherapy for psoriasis. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD009481.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chi 2012
- Chi CC, Wang SH, Peters MC, Kanjirath PP, Delamere FM, Wojnarowska F. Interventions for prevention of herpes simplex labialis (cold sores on the lips). Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD010095] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chi 2013
- Chi CC. Evidence‐based dermatology. Dermatologica Sinica 2013;31(1):2‐6. [DOI: 10.1016/j.dsi.2012.06.002] [DOI] [Google Scholar]
Chi 2014
- Chi CC, Wang SH. Efficacy and cost‐efficacy of biologic therapies for moderate to severe psoriasis: a meta‐analysis and cost‐efficacy analysis using the intention‐to‐treat principle. BioMed Research International 2014; Vol. 2014:862851. [DOI: 10.1155/2014/862851; PUBMED: 24605338] [DOI] [PMC free article] [PubMed]
Chi 2015
- Chi CC, Wang J, Chen YF, Wang SH, Chen FL, Tung TH. Risk of incident chronic kidney disease and end‐stage renal disease in patients with psoriasis: a nationwide population‐based cohort study. Journal of Dermatological Science 2015;78(3):232‐8. [DOI: 10.1016/j.jdermsci.2015.03.012; PUBMED: 25862150] [DOI] [PubMed] [Google Scholar]
Chi 2017
- Chi CC, Tung TH, Wang J, Lin YS, Chen YF, Hsu TK, et al. Risk of uveitis among people with psoriasis: a nationwide cohort study. JAMA Ophthalmology 2017;135(5):415‐22. [PUBMED: 28418500] [DOI] [PMC free article] [PubMed] [Google Scholar]
CS‐COUSIN 2019
- Cochrane Skin Core Outcome Set Initiative. cs‐cousin.org/ (accessed prior to 11 July 2019).
De Vries 2013
- Vries AC, Bogaards NA, Hooft L, Velema M, Pasch M, Lebwohl M, et al. Interventions for nail psoriasis. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD007633.pub2] [DOI] [PubMed] [Google Scholar]
Debbaneh 2014
- Debbaneh M, Millsop JW, Bhatia BK, Koo J, Liao W. Diet and psoriasis, part I: Impact of weight loss interventions. Journal of the American Academy of Dermatology 2014;71(1):133‐40. [PUBMED: 24709272] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG (editors), on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Di Minno 2014
- Minno MN, Peluso R, Iervolino S, Russolillo A, Lupoli R, Scarpa R. Weight loss and achievement of minimal disease activity in patients with psoriatic arthritis starting treatment with tumour necrosis factor alpha blockers. Annals of the Rheumatic Diseases 2014;73(6):1157‐62. [PUBMED: 23771989] [DOI] [PMC free article] [PubMed] [Google Scholar]
Essayli 2017
- Essayli JH, Murakami JM, Wilson RE, Latner JD. The impact of weight labels on body image, internalized weight stigma, affect, perceived health, and intended weight loss behaviors in normal‐weight and overweight college women. American Journal of Health Promotion 2017;31(6):484‐90. [PUBMED: 27553058] [DOI] [PubMed] [Google Scholar]
Feldman 2005
- Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Annals of the Rheumatic Diseases 2005;64(Suppl 2):ii65‐8; discussion ii69‐73. [PUBMED: 15708941] [DOI] [PMC free article] [PubMed] [Google Scholar]
Follman 1982
- Follmann D, Elliot P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45(7):769‐73. [DOI] [PubMed] [Google Scholar]
Ford 2018
- Ford AR, Siegel M, Bagel J, Cordoro KM, Garg A, Gottlieb A, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the National Psoriasis Foundation: a systematic review. JAMA dermatology 2018;154(8):934‐50. [PUBMED: 29926091] [DOI] [PubMed] [Google Scholar]
Foss 1980
- Foss ML, Lampman RM, Schteingart DE. Extremely obese patients: improvements in exercise tolerance with physical training and weight loss. Archives of Physical Medicine and Rehabilitation 1980;61(3):119‐24. [PUBMED: 7369849] [PubMed] [Google Scholar]
Frankel 2012
- Frankel HC, Han J, Li T, Qureshi AA. The association between physical activity and the risk of incident psoriasis. Archives of Dermatology 2012;148(8):918‐24. [PUBMED: 22911187] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gelfand 2006
- Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA 2006;296(14):1735‐41. [PUBMED: 17032986] [DOI] [PubMed] [Google Scholar]
Gelfand 2009
- Gelfand JM, Dommasch ED, Shin DB, Azfar RS, Kurd SK, Wang X, et al. The risk of stroke in patients with psoriasis. Journal of Investigative Dermatology 2009;129(10):2411‐8. [PUBMED: 19458634] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gerdes 2010
- Gerdes S, Zahl VA, Weichenthal M, Mrowietz U. Smoking and alcohol intake in severely affected patients with psoriasis in Germany. Dermatology 2010;220(1):38‐43. [PUBMED: 19996578] [DOI] [PubMed] [Google Scholar]
Gerdes 2011
- Gerdes S, Rostami‐Yazdi M, Mrowietz U. Adipokines and psoriasis. Experimental Dermatology 2011;20(2):81‐7. [PUBMED: 21255085] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Hunter 2013
- Hunter HJ, Griffiths CE, Kleyn CE. Does psychosocial stress play a role in the exacerbation of psoriasis?. British Journal of Dermatology 2013;169(5):965‐74. [PUBMED: 23796214] [DOI] [PubMed] [Google Scholar]
Khilji 2001
- Khilji FA, Gonzalez M, Finlay AY. Clinical meaning of change in Dermatology Life Quality Index scores. British Journal of Dermatology 2001;147(Suppl 62):25‐54. [Google Scholar]
Ko 2016
- Ko W‐C, Tsai T‐F, Tang C‐H. Health state utility, willingness to pay, and quality of life among Taiwanese patients with psoriasis. Dermatologica Sinica 2016;34(4):185‐91. [EMBASE: 20160830045] [Google Scholar]
Lu 2013
- Lu Y, Chen H, Nikamo P, Qi Low H, Helms C, Seielstad M, et al. Association of cardiovascular and metabolic disease genes with psoriasis. Journal of Investigative Dermatology 2013; Vol. 133, issue 3:836‐9. [PUBMED: 23190900] [DOI] [PMC free article] [PubMed]
Ma 2013
- Ma C, Harskamp CT, Armstrong EJ, Armstrong AW. The association between psoriasis and dyslipidaemia: a systematic review. British Journal of Dermatology 2013;168(3):486‐95. [PUBMED: 23106411] [DOI] [PubMed] [Google Scholar]
Mason 2013
- Mason AR, Mason J, Cork M, Dooley G, Hancock H. Topical treatments for chronic plaque psoriasis. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD005028.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mrowietz 2011
- Mrowietz U, Kragballe K, Reich K, Spuls P, Griffiths CE, Nast A, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Archives of Dermatological Research 2011;303(1):1‐10. [PUBMED: 20857129] [DOI] [PMC free article] [PubMed] [Google Scholar]
Naldi 2005
- Naldi L, Chatenoud L, Linder D, Belloni Fortina A, Peserico A, Virgili AR, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case‐control study. Journal of Investigative Dermatology 2005;125(1):61‐7. [PUBMED: 15982303] [DOI] [PubMed] [Google Scholar]
Nestle 2009
- Nestle FO, Kaplan DH, Barker J. Psoriasis. New England Journal of Medicine 2009;361(5):496‐509. [PUBMED: 19641206] [DOI] [PubMed] [Google Scholar]
NICE 2008
- National Institute for Health and Care Excellence. Adalimumab for the treatment of adults with psoriasis. Technology appraisal guidance [TA146] 2008. www.nice.org.uk/guidance/ta146. London, (accessed 17 October 2017).
Owen 2000
- Owen CM, Chalmers R, O'Sullivan T, Griffiths CE. Antistreptococcal interventions for guttate and chronic plaque psoriasis. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD001976] [DOI] [PubMed] [Google Scholar]
Parisi 2013
- Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. Journal of Investigative Dermatology 2013;133(2):377‐85. [PUBMED: 23014338] [DOI] [PubMed] [Google Scholar]
Parker 2005
- Parker DR, Evangelou E, Eaton CB. Intraclass correlation coefficients for cluster randomized trials in primary care: the cholesterol education and research trial (CEART). Contemporary Clinical Trials 2005;26(2):260‐7. [PUBMED: 15837446] [DOI] [PubMed] [Google Scholar]
Puzenat 2010
- Puzenat E, Bronsard V, Prey S, Gourraud PA, Aractingi S, Bagot M, et al. What are the best outcome measures for assessing plaque psoriasis severity? A systematic review of the literature. Journal of the European Academy of Dermatology and Venereology: JEADV 2010;24 Suppl 2:10‐6. [PUBMED: 20443995] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ryan 2014
- Ryan C, Korman NJ, Gelfand JM, Lim HW, Elmets CA, Feldman SR, et al. Research gaps in psoriasis: opportunities for future studies. Journal of the American Academy of Dermatology 2014;70(1):146‐67. [PUBMED: 24126079] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Schünemann 2017
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Akl E, et al. on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Serwin 2007
- Serwin AB, Sokolowska M, Chodynicka B. Tumour necrosis factor alpha (TNF‐alpha)‐converting enzyme (TACE) and soluble TNF‐alpha receptor type 1 in psoriasis patients treated with narrowband ultraviolet B. Photodermatology, Photoimmunology & Photomedicine 2007;23(4):130‐4. [PUBMED: 17598866] [DOI] [PubMed] [Google Scholar]
Serwin 2008
- Serwin AB, Sokolowska M, Dylejko E, Chodynicka B. Tumour necrosis factor (TNF‐alpha) alpha converting enzyme and soluble TNF‐alpha receptor type 1 in psoriasis patients in relation to the chronic alcohol consumption. Journal of the European Academy of Dermatology and Venereology: JEADV 2008;22(6):712‐7. [PUBMED: 18312327] [DOI] [PubMed] [Google Scholar]
Stata 2009 [Computer program]
- StataCorp. Stata. Version SE 11.2. College Station, TX, USA: StataCorp, 2009.
Toussirot 2014
- Toussirot E, Aubin F, Dumoulin G. Relationships between adipose tissue and psoriasis, with or without arthritis. Frontiers in Immunology 2014;5:368. [PUBMED: 25161652] [DOI] [PMC free article] [PubMed] [Google Scholar]
Treloar 2010
- Treloar V. Integrative dermatology for psoriasis: facts and controversies. Clinics in Dermatology 2010;28(1):93‐9. [PUBMED: 20082958] [DOI] [PubMed] [Google Scholar]
Tsai 2017
- Tsai TF, Lee CH, Huang YH, Chi CC, Chang YT, Wong TW, et al. Taiwanese Dermatological Association consensus statement on management of psoriasis. Dermatologica Sinica 2017;35(2):66‐77. [DOI: 10.1016/j.dsi.2017.01.002] [DOI] [Google Scholar]
Upala 2015
- Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta‐analysis. International Journal of Obesity (2005) 2015;39(8):1197‐202. [PUBMED: 25920774] [DOI] [PubMed] [Google Scholar]
Wang 2014
- Wang SH, Chi CC, Hu S. Cost‐efficacy of biologic therapies for moderate to severe psoriasis from the perspective of the Taiwanese healthcare system. International Journal of Dermatology 2014;53(9):1151‐6. [PUBMED: 24738910] [DOI] [PubMed] [Google Scholar]
Yang 2015
- Yang HJ, Yang KC. Impact of psoriasis on quality of life in Taiwan. Dermatologica Sinica 2015;33(3):146‐50. [DOI: 10.1016/j.dsi.2015.02.001] [DOI] [Google Scholar]
Zhang 2015
- Zhang A, Silverberg JI. Association of atopic dermatitis with being overweight and obese: a systematic review and metaanalysis. Journal of the American Academy of Dermatology 2015;72(4):606‐16.e4. [PUBMED: 25773409] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Chi 2015b
- Chi CC, Ko SH, Yeh ML, Wang SH, Tsai YS, Hsu MY. Lifestyle changes for treating psoriasis. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD011972] [DOI] [PMC free article] [PubMed] [Google Scholar]


