Abstract
Introduction
Mucopolysaccharidosis (MPS) type IVA is a rare, autosomal recessive lysosomal storage disease causing substrate accumulation in various organs and tissues. MPS IVA is associated with both obstructive and restrictive airway disease, with the former often resulting in sleep disordered breathing (SDB). Respiratory failure is a primary cause of death in this condition. The aim of this study was to characterise and catalogue the long-term respiratory changes in patients with MPS IVA treated with, or without, enzyme replacement therapy (ERT).
Methods
In this retrospective, longitudinal, repeated-measures cohort study, descriptive statistics and non-parametric correlation were performed for demographic, respiratory function and oximetry variables over a study period from January 2009 to December 2018. Composite clinical endpoints used in this study for evaluating pulmonary function included spirometry variables (FEV1, FEV1 [%Pred] FVC, FVC [%Pred] and FEV1/FVC), oximetry variables (median %Spo2, ODI 3%, mean nadir 3%, ODI 4%, mean nadir 4% and min dip SpO2 [%]) and 6MWT to assess functional exercise capacity and thus integrated cardiopulmonary function.
Results
Sequential spirometry and oximetry values were collected from 16 patients, of which 13/16 were ERT treated. In general, during the study period there was a global reduction in static spirometry values in all subjects, as well as cardiorespiratory function as assessed by the 6MWT, with the decline being delayed in the ERT group. Oximetry changed to a minor degree over time in the ERT group, whereas it declined in the non-ERT group. FEV1, FVC [%predicted] and ODI 3% exhibited a strong, combined positive correlation (r 0.74–95% CI 0.61 to 0.83; p < .0001). Non-invasive ventilation (NIV) and adenotonsillectomy appeared more effective in the ERT group, either improving pulmonary function or attenuating deterioration.
Conclusions
Whilst spirometry values showed a gradual decline across all groups, oximetry showed modest improvement in respiratory function. The amalgamation of FEV1, FVC [%predicted] and ODI 3% appeared predictive of changes in respiratory function in this study, suggestive as being composite endpoints for monitoring disease progression as well as guiding response to ERT in MPS IVA patients.
Keywords: Morquio syndrome, Mucopolysaccharidosis IVA, MPS, Respiratory changes, Enzyme replacement therapy, Sleep disordered breathing
Abbreviations: 6MWT, 6-minute walk test; AASM, American Academy of Sleep Medicine; ADLs, Activities of daily living; AEs, Adverse Events; ATS, American Thoracic Society; BiPAP, Bi-level non-invasive ventilation; BTS, British Thoracic Society; C6S, Chondroitin Sulphate; CPET, Cardiopulmonary exercises testing; ERT, Enzyme replacement therapy; ECM, Extracellular matrix; FDA, Food and Drug Administration; FEV1, Forced expiratory volume in one second; FEV1 [%Pred], FEV1 as a percentage of predicted; FVC, Forced vital capacity; FVC: [%Pred], FVC as a percentage of predicted; GAG, Glycosaminoglycan; GALNS, Acetylgalactosamine-6-sulfatase; KS, Keratan sulfate; LSD, Lysosomal storage disease.; MPS, Mucopolysaccharidosis; MPS IVA, Mucopolysaccharidosis Type IVA; Med nadir 3%, Median nadir of arterial oxygen saturations 3% from baseline; Min dip Spo2, Minimum dips in arterial oxygen saturations [%]; ODI 3%, Oxygen desaturation index; ≥ 3% arterial oxygen desaturations per hour; OSA, Obstructive Sleep Apnea; Spo2, Arterial saturations; T&A, Adenotonsillectomy; uKS, Urinary keratan sulfate
1. Introduction
1.1. Background
There are 500 known metabolic disorders (approximately 10% of all known genetic diseases), of which 50 are lysosomal storage diseases (LSD) – comprising of mucopolysaccharidosis, sphingolipidosis and oligosaccharidosis. Mucopolysaccharidosis type IVA (MPS IVA; Morquio Syndrome [OMIM #253000]) is a rare, autosomal recessive LSD that was first described in 1929 (independently) by both Morquio and Brailsford [1]. The disorder is caused by an enzyme deficiency of N-acetyl-galactosamine-6-sulfatase (GALNS), manifesting in the accumulation of the partially-degraded glycosaminoglycans (GAGs) keratan sulfate (KS) and chondroitin-6-sulfate (C6S) within lysosomes, as well as the extracellular matrix (ECM) of various tissues [2,3]. MPS IVA is characterized by progressive multi-level airway obstruction, additionally, there are features of gross skeletal abnormalities, small stature, coarse features, neurological, ocular, dental, auditory and valvular cardiac corollaries [[4], [5], [6]]. Musculoskeletal abnormalities are often the primary reason for initial presentation and include short trunk stature, Genu valgum, pectus carinatum, kyphoscoliosis (especially Gibbus deformity), hypermobile joints, cervical spinal cord compression and gait abnormalities that increase the propensity for falls [3,5,7].
Whilst there remains heterogeneity in the context of severity and phenotype, patients with MPS IVA rarely survive past their second decade of life, with the exception being those that have the attenuated phenotype and thus, milder disease [2,7]. The majority of children cease growing in height by the age of 8 years, are wheelchair bound by their teenage years and often require multiple surgical interventions throughout their lifetime. Airway compromise and spinal cord compression remain the leading cause of death in this cohort [2,5,7].
1.2. Pathophysiology of airway and respiratory disease
The pathophysiology of MPS IVA has already been well described in the literature [[8], [9], [10], [11], [12], [13], [14]]. It is important to emphasise that GAGs are far from being inert molecules, where accumulation simply leads to mass effect and compression of proximally located structures. Rather, there is an intricate and multifarious interchange of storage of substrate in lysosomes and the ECM that leads to an impairment of substrate catalysis and cellular autophagy, the accumulation of precursors, as well as mitochondrial dysfunction, cellular distress and a chronic inflammatory response [2,12,14]. The latter being triggered by the release of proinflammatory cytokines, transcription factors and other mediators of inflammation. This eventually culminates in the degradation of the ECM in bone, cartilage and joints.
The presence of airway and respiratory disease is common in patients with MPS IVA, with the cause being multifaceted, albeit having not been well defined, characterized and studied [5,14]. There is often a multilevel involvement of GAG deposition that causes both a restrictive and obstructive disease (see Fig. 1). Airway and respiratory disease in MPS IVA involves both the upper and lower airways, resulting in a mixed obstructive and restrictive picture [7,[15], [16], [17]]. Multilevel upper airway pathology includes nasal mucosal hypertrophy, macroglossia, decreased mouth opening capacity (MOC), adenotonsillar hypertrophy, and less commonly enlarged and redundant supraglottic tissues. Upper airway obstruction is frequently progressive resulting in sleep disordered breathing (SDB), and more specifically obstructive sleep apnoea (OSA) [15,18,19]. Lower airway pathology includes a combination of tracheomalacia, bronchomalacia, a reduction in the radial calibre of the trachea; with the trachea macroscopically appearing torturous and ‘buckling’ at certain (and often at multiple) positions (Fig. 1B). Tracheal narrowing is principally thought to occur due to tracheomalacia, with GAG accumulation in the submucosa of the tracheal cartilage having a less significant contribution. Moreover, buckling of the trachea is thought to result from abnormal cartilage metabolism and an imbalance between the relatively ‘normal’ longitudinal growth of the trachea and abnormal thoracic cage morphology resulting from chest wall deformity and kyphoscoliosis [[15], [16], [17], [18], [19]]. Of special note is that in MPS IVA, the relative short stature, wider antero-posterior diameter of the thorax, and stiffer ribs compound the restrictive component of the pulmonary and repository dysfunction. In a recent review article, Broomfield and Kenth et al. (2019) expounded upon the inimitable nuances of respiratory pathology in MPS IVA, explicating how lower airway disease is pathognomonic of MPS IVA and often severe, whilst upper airway pathology may also be present (e.g. SDB), this is often less prevalent and significant than in other types of MPS [14].
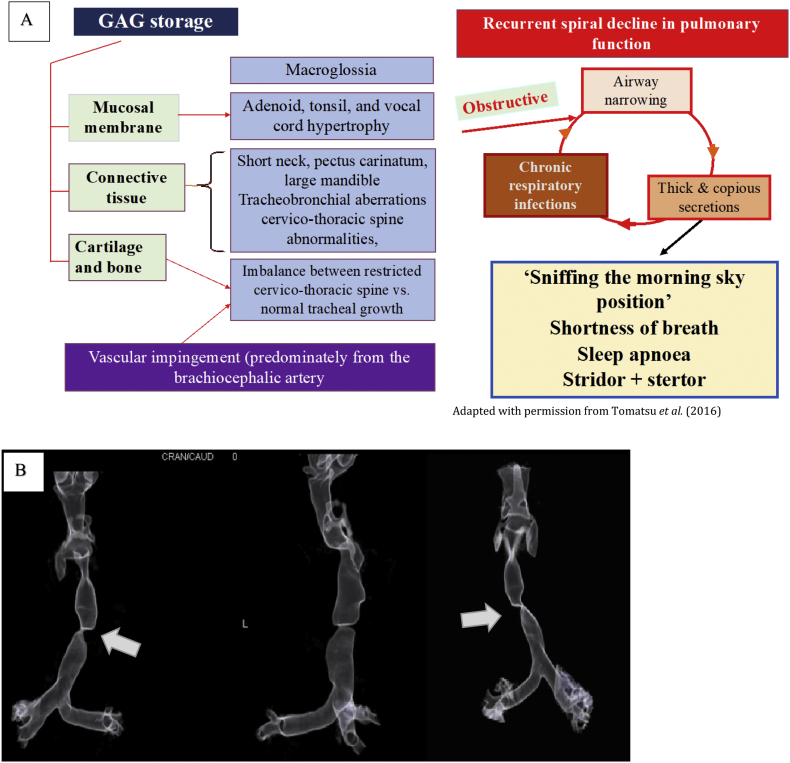
Fig. 1.
Pathophysiology of airway disease in MPS IVA.
(A) Diagram to illustrate the pathophysiology for airway and respiratory disease in MPS IVA. Note the combination of GAG accumulation into tissues organs results in both obstructive and restrictive disease effecting both the lower and upper airways. A positive feedback loops develops from thick, copious secretions, airway narrowing and chronic respiratory infections. Image kindly reproduced with permission from Tomatsu et al. (2016). (B) The second image depicts a severely diseased traches in a 19 year patient with MPS IVA who had not received ERT treatment. Note the profound tortuosity and tracheal buckling (solid white arrow) that is pathognomic of MPS IVA; in this case the narrowest point of the trachea is 2 mm.
Microscopically, tracheal chondrocytes in hyaline cartilage (which comprises the anterior ring of the trachea), are abundant in number with vacuoles appearing enlarged due to GAG storage, particularly C6S [11,13]. Interestingly, Tomatsu et al. (2016) have recently suggested in a study involving sequential MRI imaging, that an additional component may be due to direct compression of the trachea from the overlying brachiocephalic artery [5]. Furthermore, it is believed that there is a positive feedback cycle of SDB, hypoxia and hypercapnia, leading to hypoventilation, reduction in exercise capacity, secretion retention and recurrent respiratory infections; eventually leading to right heart failure and critical airway obstruction [19,21].
1.3. Current management strategies of MPS IVA
Patients with this condition require extensive medical and supportive therapies that require constant specialist care. Airway obstruction may require early tracheostomy with subsequent long term ventilation. Furthermore, patients with MPS IVA are at very high risk for anaesthesia, particularly in the context of acute airway management – including endotracheal intubation and bag-mask-ventilation [19]. Delivery of care is complex and expensive, with enzyme replacement therapy (ERT) incurring a significant financial burden. ERT (Elosulfase alfa / Vimizim®), BioMarin) remains the mainstay of treatment, with other treatments including gene therapy (currently in development). Care for individuals with MPS IVA in England is via the highly specialised service for LSD, with only 3 designated paediatric centres and 5 adult centres. There are around 90 individuals with MPS IVA in England 60 of which are currently treated with ERT. In the UK, the annual average cost of ERT is £394,680 per person [22]; with the total annual expenditures of this medication alone costing the NHS over £23 million. The National Institute for Health and Care Excellence (NICE) approved the use of this medication in 2016 only with the caveat that all patients must be enrolled in a managed access programme and show individual annual patient benefit [22].
Current evidence has suggested that whilst ERT with elosulfase alfa may slow disease progression in obstructive disease, there appears to be a less pronounced improvement in tracheal pathology, such as calibre and tortuosity, without normalisation of the tracheal architecture. The studies of Hendriksz et al. (2014, 2015, 2016) demonstrated that ERT appeared to have improved both static pulmonary function (FRC, FVC) and dynamic exercise tolerance (6MWT). What remains unclear is whether the improvement was solely due to the ERT therapy, growth and whether there were nuanced changes due to different phenotypes [7,20,21].
NICE has critically evaluated the efficacy of elosulfase alfa at 2 mg/kg/week for the attenuation of symptoms and disease progression in MPS IVA. They identified seven clinical studies involving over 255 patients. These studies included: MOR-004, a randomised, placebo-controlled phase III trial, its long-term extension study (MOR-005), MOR-002 -an open-label ascending dose study, with MOR-100 being its long-term extension, MOR-008 -a randomised phase II trial and MOR-006 and -007 single-arm studies in specific population groups. NICE reported that MOR-004 and -005 were the axial clinical effectiveness trials used to guide their recommendations, MOR-004 reported outcomes measured over 24 weeks, followed by 96-week long-term follow-up in MOR-005, with MOR-002, -100, -007 and -008 studies also providing corroboratory evidence and validation. Primary outcomes from these studies included the 6MWT that examined endurance and integrated cardiopulmonary function, as well as urinary keratan sulphate (uKS) and metrics to assess pulmonary function. The baseline and disease characteristics were broadly comparable between treatment groups in MOR-004 and -005. The results from the preliminary studies showed that by week 24, elosulfase alfa (at 2 mg/kg/week) was associated with a statistically significant improvement in the 6MWT and global pulmonary function, when compared to placebo [23]. The results of MOR-004 suggested that elosulfase alfa 2 mg/kg/week provided improvements in cardiorespiratory endurance, pulmonary function and disability with an overall improvement of quality of life. It should be noted although statistical significance was not reached, this was attributed to the study design not being powered to detect differences in secondary or tertiary outcomes [24]. The outcome measures used in these clinical trials were identified and selected due to various factors but there was little prior data supporting the validity of these measures in predicting important meaningful outcomes such as survival or quality of life indices. The selection was based on previous trials in MPS diseases, the familiarity of the Food and Drug Administration (FDA) with these endpoints, measures likely to show a change within a 6 month time period and available biochemical tests (e.g. uKS). Studies have been conflicting on the relevance of uKS as a biomarker in this disease, although it is currently used by National Health Services England (NHSE) and the NICE in the ongoing Managed access agreement as a marker of efficacy. No studies have so far have looked at individual or variable response to therapy or long-term prediction of important and meaningful outcomes in order to assess early treatment failure and modulate therapy. Significant uncertainty still exists however around the most useful and meaningful outcome measures to use in this cohort. The aim of this study was to undertake a longitudinal evaluation and characterisation of the long-term respiratory changes in patients treated with ERT.
2. Materials and methods
2.1. Study subjects
MPS IVA patients treated at the Royal Manchester Children's Hospital, Manchester (UK) between 2009 and 2018, were identified from an existing patient database and medical records. This yielded a study group of 16 subjects for whom long-term follow-up was available at a single centre. A retrospective review was undertaken of baseline demographics, spirometry and (overnight) oximetry, ERT and other therapeutic interventions – including both medical and surgical measures. The data for each subject was tabulated and examined sequentially over the study period to provide a nuanced characterisation of the changes in pulmonary function, evolution and natural history of disease progression. The subjects in this study included those from the MOR 100 phase 1 study (n = 5), MOR 004 phase III placebo-controlled (n = 5) and the MOR 007 trial (n = 6).
2.2. Evaluation of respiratory function
The methodology employed to assess pulmonary function in this study is validated by and aligned to The American Thoracic Society (ATS) and the British Thoracic Society (BTS) guidance; with the methodology used for oximetry as previously described by Pal et al. (2015) [25]. Composite clinical endpoints used in this study for evaluating pulmonary function included spirometry variables (FEV1, FEV1 [%Pred] FVC, FVC [%Pred], FEV1/FVC); oximetry variables (median %Spo2, ODI 3%, mean nadir 3%, ODI 4%, mean nadir 4% and min dip SpO2 (%)) and 6MWT for cardiorespiratory reserve. As the study was a retrospective analysis, the time interval between each measurement was varied and could not be standardised or controlled for. The mean time interval between each measurement was 6.0 months for the ERT group and 6.6 months for the non-ERT cohort.
2.2.1. Spirometry
Both the ATS and the BTS advise that spirometry gives three important measures of pulmonary function, namely FEV1, FVC and FEV1/FVC. FEV1: is the volume of air that the patient is able to exhale in the first second of forced expiration; FVC: the total volume of air that the patient can forcibly exhale in one breath; FEV1/FVC: the ratio of FEV1 to FVC expressed as a percentage [26,27]. For diseases associated with known growth abnormalities such as MPS IVA, FEV1 and FVC may be reduced. Thus, FEV1 [% Pred] defined as the actual FEV1 as a percentage of the predicted FEV1 based on age, sex and height was used. This was also the case for FVC [% Pred]. Obstructive disease is defined by the BTS as an FEV1 < 80% predicted and FEV1/FVC <0.7 or 70% [20,27]. Furthermore, the severity of the airflow obstruction is indicated by the extent of FEV1 reduction, with FEV1. All respiratory function tests conducted in this study were undertaken in accordance with the ATS standards (1995) at a single centre [26,28]. The undertaking of the study at a single centre using standardised algorithms conferred methodological rigour by reducing the variability in practice that may arise if multiple centres are used, notwithstanding of the ATS and BTS standards. Moreover, one should be mindful of the quandary for what is the optimal test for monitoring pulmonary function in MPS IVA, especially when contextualising to the reliability of age and height normalized curves in this cohort, particularly as these children commonly have a small stature and progressive joint and spine abnormalities. Nonetheless, the predicted FEV1 and predicted FVC remain the mainstay of monitoring pulmonary function in this cohort [7,14,21,29].
2.2.2. Overnight oximetry
Overnight oximetry has been validated by numerous studies as an effective tool to quantify the degree of disease burden in SDB, which is frequently present in LSD such as MPS IVA [20,29,30]. In this study, we adopted the methodology for overnight oximetry used by Pal et al. (2015), based on the American Academy of Sleep Medicine (AASM) manual for the scoring of sleep and associated events [25,31]. The sleep studies were undertaken in un-sedated natural sleep, using sleep oximetry equipment (Pulsox 300i, Konica Minolta) quantifying transcutaneous arterial oxygen saturations and heart rate using a finger probe. The severity of SDB was assessed using the recent update of the AASM guidance and were also aligned to the recommendations made by the ‘Working Party on Sleep Physiology and Respiratory Control Disorders in Childhood’, Royal College of Paediatrics and Child Health (2009) [32]. The ODI 3% (3% oxygen desaturation index) was defined as the number of ≥3% arterial oxygen (SpO2) desaturations per hour of sleep, median SpO2 and mean nadir 3% – the latter pertaining to the mean of the SpO2 nadir that was ≥3% from baseline. A common scoring system for severity of SDB based on oximetry recording has a median SpO2 level >95% with no >4 desaturations of 3% or greater per hour; 5–10 would constitute moderate SDB and > 10 would indicate the presence of severe disease [[32], [33], [34], [35]].
2.3. Treatment
Patients that were included in the ERT group were treated with recombinant human GALNS ERT (elosulfase alfa (Vimizim™)). A standardised dosing regimen of 2 mg/kg/week intravenous was used. Therapeutic interventions can affect pulmonary function as well as modulate the disease burden from SDB. The interventions used to ameliorate respiratory symptoms may be surgical (i.e. adenotonsillectomy) or non-surgical (i.e. non-invasive ventilation, NIV). It should be noted that these interventions will confound the results of the overnight oximetry studies. To account for this, we made clear and specific markers of when such interventions were undertaken (see supplementary material).
2.4. Statistical methods
Descriptive statistics and non-parametric correlation were performed for demographic, oximetry, and respiratory function variables. Spirometry variables included – FEV1, FEV1 [%Pred] FVC, FVC [%Pred] and FEV1/FVC]; oximetry variables – median %Spo2, ODI3%, mean nadir 3%, ODI4%, mean nadir 4% and min dip SpO2 (%); 6MWT- as a measure of cardiorespiratory function. For non-parametrically distributed variables, data were presented as the median and range, whereas the mean and standard deviation (SD) were used for data with a normal (Gaussian) distribution. The study null hypotheses (H0) were that throughout the duration of the study, there would be no change in pulmonary function and that there was no correlation between the variables that were predictive for any changes.
For each subject, sequential spirometry and oximetry values were individually plotted to depict the longitudinal trends and overall changes for each variable. Each of the plots was then manually inspected in order to undertake both quantitative and qualitative analysis of changes. This included any perceived changes of an intervention such as adenotonsillectomy or the institution of NIV. Summary plots were then constructed to illustrate how the ERT and no-ERT groups evolved over the study period. For each of the continuous spirometry and oximetry variables, linear regression was then undertaken, and lines of best fit plotted against time (the latter being the independent variable). Significance was assumed where p-values <.05. Finally, correlation testing was undertaken using Pearson's r for normally distributed data, and Spearman's rho for non-normally distributed data.
3. Results
3.1. Study demographics
Clinical data were collected from a total of 16 individuals; 13 patients had received ERT whilst three subjects were not ERT treated, the very small sample size in the non-ERT group (n = 3) meant that direct statistical comparison of ERT versus non-ERT could not faithfully be undertaken. Instead, descriptive statistics, individualised and group trajectories of changes over time and non-parametric correlation tests were performed for demographic, respiratory function and oximetry variables.
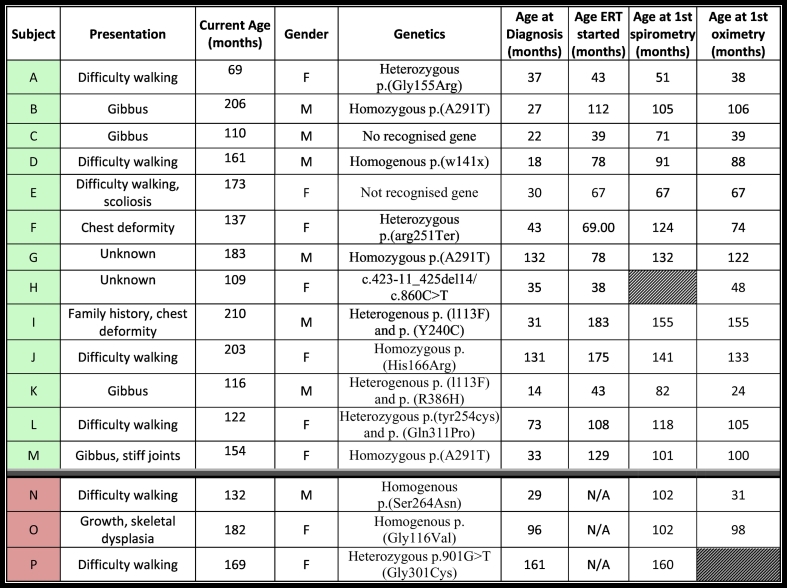
Table 1 outlines the baseline demographics, including genetics, age at diagnosis, age when ERT was commenced (if applicable), age when the first spirometry and oximetry values were recorded. The median age at diagnosis was 32 months (range 14–131). The median age commencing ERT was 73.5 months (range: 43–183 months), and the median duration of follow-up was 90 months (range 3–179 months) with the median time interval between each test was 6 months (range 1–10 months). 43.8% (7/16) of the subjects were males and 56.3% females (9/16). Initial presenting symptoms that led to disease diagnosis included: difficulty walking (n = 7), gibbus deformity (n = 4), kyphoscoliosis (n = 1), chest-wall deformity (n = 1).
Table 1.
Baseline demographics.
The table above shows the baseline demographics of the 16 subjects included in this study. Subjects A – M (highlighted in green) were ERT treated, whilst N, O, P were not ERT treated (pink). We have included the presenting symptoms, age when study commenced, gender. For comparison and further clarity we have included the age at diagnosis, the age when enzyme replacement therapy (ERT) commenced, as well as the age of the subject when the first spirometry and oximetry tests were undertaken.
A physician diagnosis of OSA was present in 68.6% (11/16) of patients, with a difficult airway (defined as a Cormack and Lehane score of >2b) was present in 37.5% of patients (6/16) – with video laryngoscopy having been utilised in 68.8% (11/16) of cases, macroglossia was present in 43.8% of patients (7/16). We ascertained that 66.7% (10/15) of patients in the study had undergone an adenotonsillectomy, with a median age at adenotonsillectomy of 72 months (range: 32–147 months). Therapeutic NIV (BIPAP) was used in 31.3% (5/16) of patients, with the median age of commencing NIV being 153 months (range: 73–168 months).
Orthopaedic surgical interventions were very common, with 75.0% (12/16) having undergone C-spine fixation and halo brace, 12.5% (2/16) had a hip replacement and 31.3% (5/16) underwent Epiphyseodesis (8-plate). Cardiac disease, as assessed by echocardiography was normal in 62.5% (10/16) of patients. There was a single incidence – 1/16 (6.3%) of the following cardiac abnormalities: small patent ductus arteriosus (PDA), small PDA and physiological mild tricuspid regurgitation, mitral regurgitation, pulmonary and mitral regurgitation, tricuspid and mitral regurgitation and dysplastic mitral and aortic valvular disease. Table 1 exhibits the genetic abnormalities present; genetic analysis of the MPS IVA cohort demonstrated significant heterogeneity of the disease, as is anticipated with many conditions within the LSD family. We found that there was poor genotype-phenotype correlation in terms of severity of the disease, however this may have been confounded by limitations in sample size. Interestingly, three subjects with homozygous p.(A291T) mutation were found to have less significant decline in pulmonary function, although this was not statistically significant.
3.2. Characterisation of changes in respiratory function & natural history
3.2.1. Spirometry
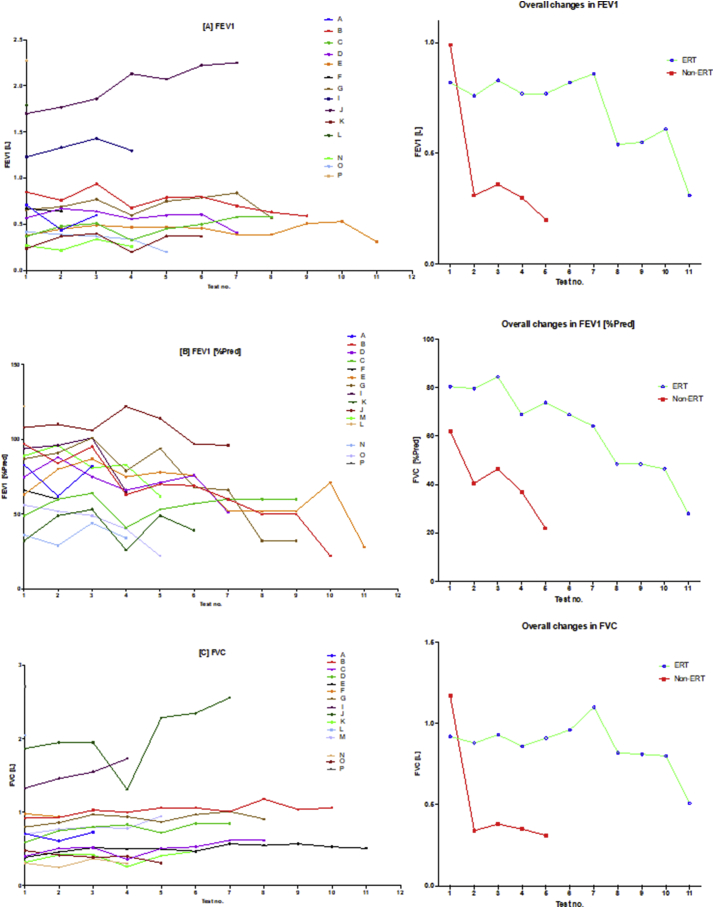
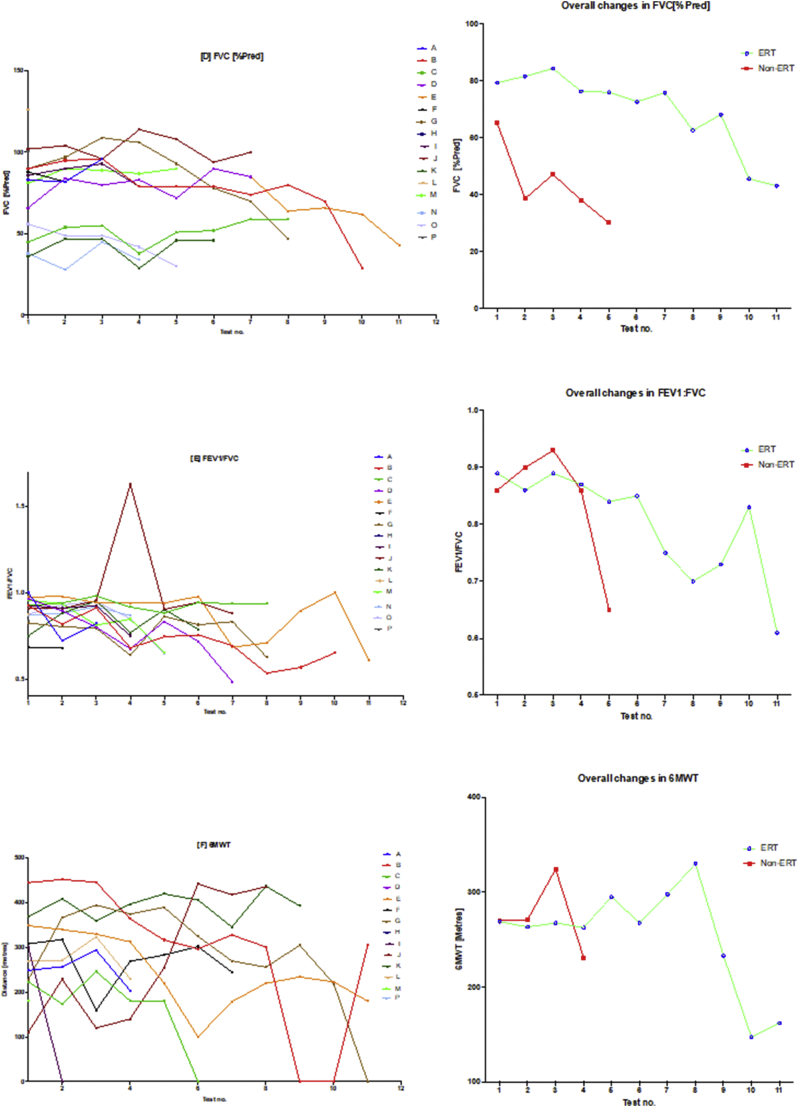
There was in general, global reduction in static spirometry values in all subjects (Fig. 2, Fig. 7), as well as cardiorespiratory function, as assessed by the 6MWT, with the decline being delayed in the ERT group. Table 2 and Fig. 2, Fig. 4, Fig. 7(A) illustrate the changes in the spirometry variables over the study period. Fig. 2 illustrates that when values are averaged out for both the ERT and non-ERT group; both individual changes and mean trends (as an average of each test) in the ERT and non-ERT groups after each successive study; one should note that the interval between each study was not fixed. Generally, there was a global decline in spirometry values (generally for all of the metrics used) over the course of the study. For FEV1 in the ERT group, the majority of induvial plots decreased, with two subjects (D and J) demonstrating individual plots that increased. This suggests that subjects D and J may have had a less severe phenotype, further corroborated by the FEV1 data points being universal of higher values, including at baseline. This was in comparison to the non-ERT values where all FEV1 values decreased. Fig. 2(B) shows the FEV1 [%pred] which depicts a more precise picture of the changes in FEV1 – as FEV1 has been corrected for age and height. The decline in both the ERT and the non-ERT subjects in FEV1 [%pred] was more explicit, with the former (ERT) subjects exhibiting a lag in the decline of FEV1 [%pred]. FVC, FVC [%pred] and FEV1/FVC similarly showed a global decline in spirometry values over the course of the studies in both the groups, with subject D and J being relative outliers as some values demonstrated improvement post commencing ERT. It should be noted that subject H had no spirometry data available, whilst subject P had no overnight oximetry data.
Fig. 2.
Spirometry changes over the course of the study.
The diagram illustrates individual plots and natural progression in spirometry for each subject, along with summary plots, divided into ERT and non-ERT groups. This was conducted for each of the following spirometry variables: (A) FEV1, (B) FEV1[%Pred], (C) FVC, (D) FVC [%Pred], (E) FEV1/FVC) and (F) 6MWT. Note, when values are averaged out for both the ERT and non-ERT group, there is global decline in spirometry values over the course of the study.
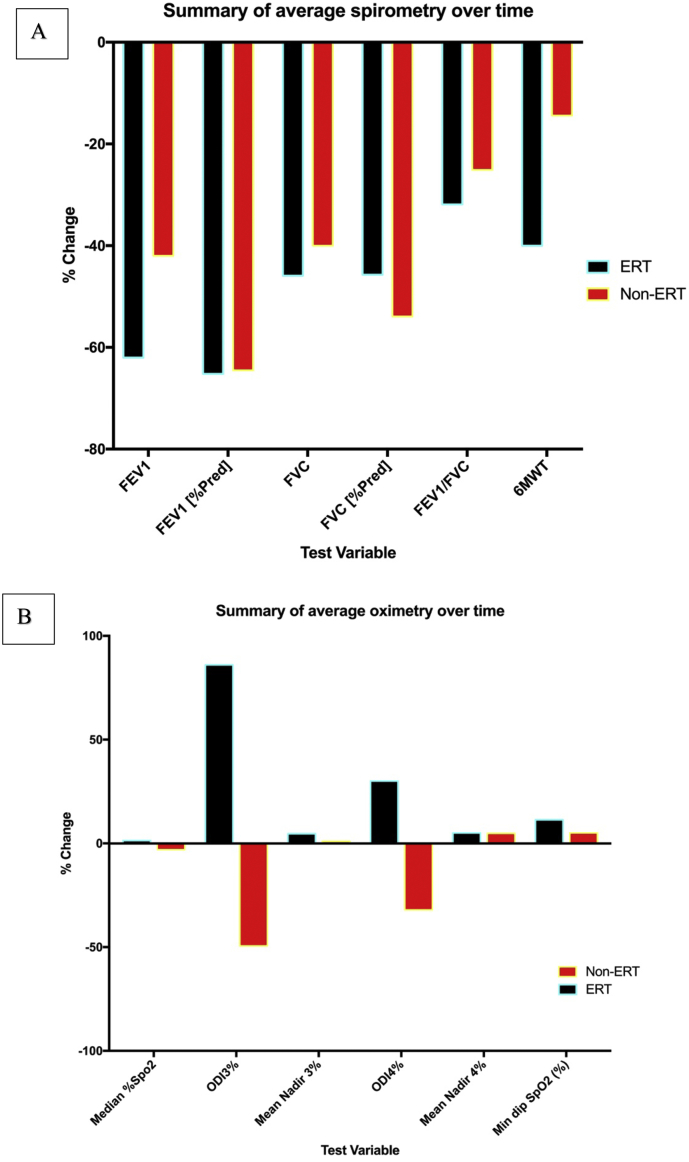
Fig. 7.
Summary of spirometry and oximetry changes.
The figure above depicts the overall changes in spirometry and oximetry from baseline over the course of the study, grouped by each of the variables of interest. There was a general decline in spirometry variables over the course of the study, which appeared to be more significant in the ERT group. The decline in 6MWT was also more appreciable in the ERT group. The oximetry data showed that function remained more static with either small positive changes suggesting improvement.
Table 2.
Summary of spirometry results.
| Subject | Spirometry variable |
|||||
|---|---|---|---|---|---|---|
| FEV1 [Litres] | FEV1 [%pred.] | FVC [Litres] | FVC [%pred.] | FEV1:FVC | 6MWT [Metres] | |
| A | 0.60 [0.44–0.71] {↓} | 82 [61–83] {→} | 0.71 [0.61–0.73] {↑} | 83 [82–96] {↑} | 0.82 [0.72–1.0] {→} | 253 [204–294 {↓} |
| B | 0.76 [0.59–0.04] {↓} | 66 [22–97] {↓} | 1.04 [0.92–1.18] {↑} | 79 [29–96] {↑} | 0.72 [0.53–0.92] {↓} | 317 [0–452] {↓} |
| C | 0.48 [0.33–0.58] {↑} | 60 [41–64] {→} | 0.52 [0.36–0.62]{↑} | 53 [38–59] {↑} | 0.94 [0.88–0.98] {→} | 180.5 [0–246] {↓} |
| D | 0.60 [0.41–0.67] {↓} | 75 [51–88] {↓} | 0.8 [0.59–0.85] {↑} | 83 [66–90] {↑} | 0.8 [0.4–0.97] {↓} | N/A |
| E | 0.46 [0.31–0.64] {↓} | 71 [28–87] {↓} | 0.51 [0.39–0.57 {↑} | 72 [43–90] {↑} | 0.94 [0.61–0.98] {↓} | 223 [100–349] {↓} |
| F | 0.66 [0.64–0.67] {→} | 63 [60–66] {↓} | 0.96 [0.94–0.98] {↓} | 85 [82–88] {↓} | 0.682 [0.68–0.684] {↓} | 283 [159–318] {→} |
| G | 0.72 [0.57–0.84] {→} | 79 [32−101] {↓} | 0.93 [0.8–1.01] {↑} | 91.5 [47–109] {↑} | 0.68 [0.63–0.86] {→} | 305 [0–395] {↓} |
| I | 1.32 [1.23–1.43]{↑} | 95 [65–101] {↓} | 1.51 [1.33–1.73] {↑} | 88 [79–93 {↑} | 0.91 [0.75–0.92] {↓} | 150 [0−300] {↓} |
| J | 2.07 [1.7–2.25] {↑} | 108 [96–122] {→} | 1.95 [1.31–2.56] {↑} | 102 [94–114] {↑} | 0.91 [0.88–1.63] {→} | 241.5 [110–442] {↑} |
| K | 0.37 [0.2–0.4] {↑} | 44 [26–53] {→} | 0.42 [0.26–0.47] {↑} | 46 [29–47] {↑} | 0.83 [0.75–0.95] {→} | 397 [346–437] {→} |
| M | 0.66 [0.62–0.72] {↓} | 83 [62–96] {↓} | 0.78 [0.7–0.96 {↑} | 89 [81–90] {↑} | 0.85 [0.65–0.96] {↑} | NA |
| N | 0.27 [0.22–0.34] {↑} | 35 [29–44 {↑} | 0.31 [0.25–0.37] {↑} | 36 [28–45] {↑} | 0.88 [0.87–0.92] {↑} | NA |
| O | 0.37 [0.2–0.42] {↓} | 49 [22–56] {↓} | 0.4 [0.31–0.48] {↓} | 49 [30–56] {↓} | 0.88 [0.65–0.95] {↓} | NA |
The table above illustrates the values for the 5 spirometry variables for each subject, (FEV1, FEV1 [%Pred] FVC, FVC [%Pred] and FEV1/FVC) as well as the six-minute walking test (6MWT).
Median values are displayed in bold, minimum and maximum range in square brackets. Curly braces {} denote the overall trend of the variable throughout the study: ↑, trend increased; ↓ trend decreased; →, no change in trend. FEV1, Forced Expiratory Volume in the first second (Litres); FVC, Forced Vital Capacity, (Litres); NA, result not available.
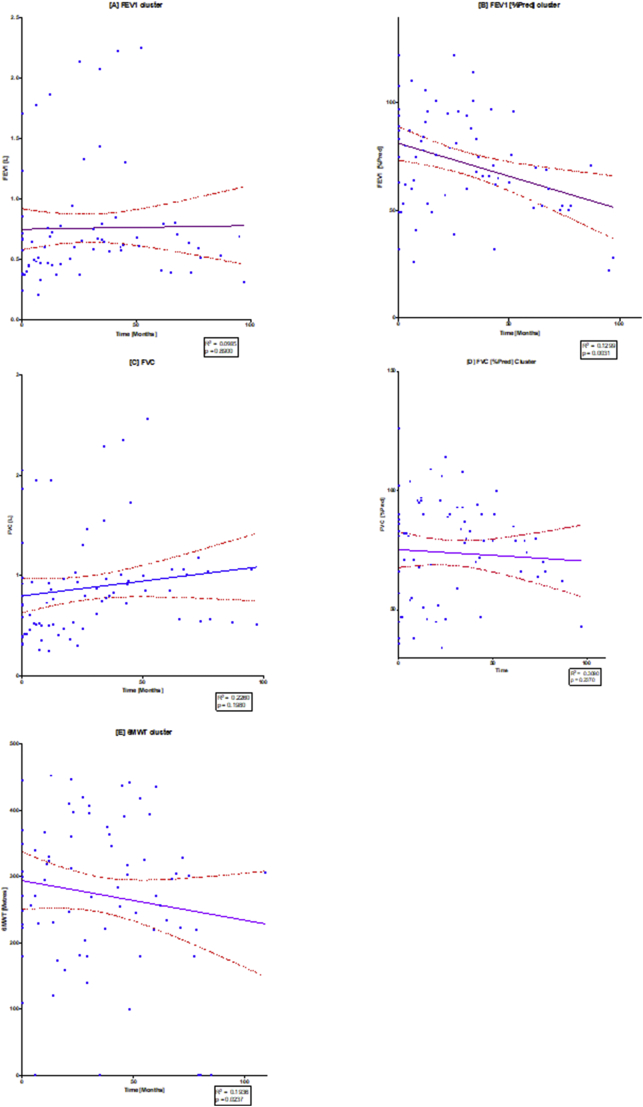
Fig. 4.
Linear regression of spirometry changes over time.
The diagram illustrates individual scatterplots for spirometry changes over time: (A) FEV1, (B) FEV1[%Pred], (C) FVC, (D) FVC [%Pred], (E) FEV1/FVC) and (F) 6MWT. Note the solid purple line is the linear regression line with the red dashed lines providing the 95% confidence lines, where there can be 95% confidence that the population mean would lie between these lines. The widening of the 95% confidence band towards the end of the study demonstrates that there were fewer results towards the end of the study. R2 and p values are shown in each of the regression plots. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 4 shows that whilst the FEV1 changes over time may have been static, the more meaningful FEV1 [%pred] decreased significantly (p = .0031). Similarly, in Fig. 2(B), FVC appeared to increase over time, however FVC [%pred] showed an overall non-significant decline (p 0.2302). There was also a significant decline in 6MWT over time as shown by the negative regression line (p = .0237). It should be made clear that Fig. 4 Illustrates a quantitative change in the spirometry over time (in months) for all ERT treated subjects, presented as a scatter plot and a regression line. This was in comparison to the individual and summary plots presented in Fig. 2 that did not have ratio interval on the y-axis, it was simply labelled by test number. Consequently, Fig. 4. allows statistical analysis and shows changes for the ERT treated subjects over time, whilst Fig. 2 better illustrates the individual trends and overall group changes over the course of the study.
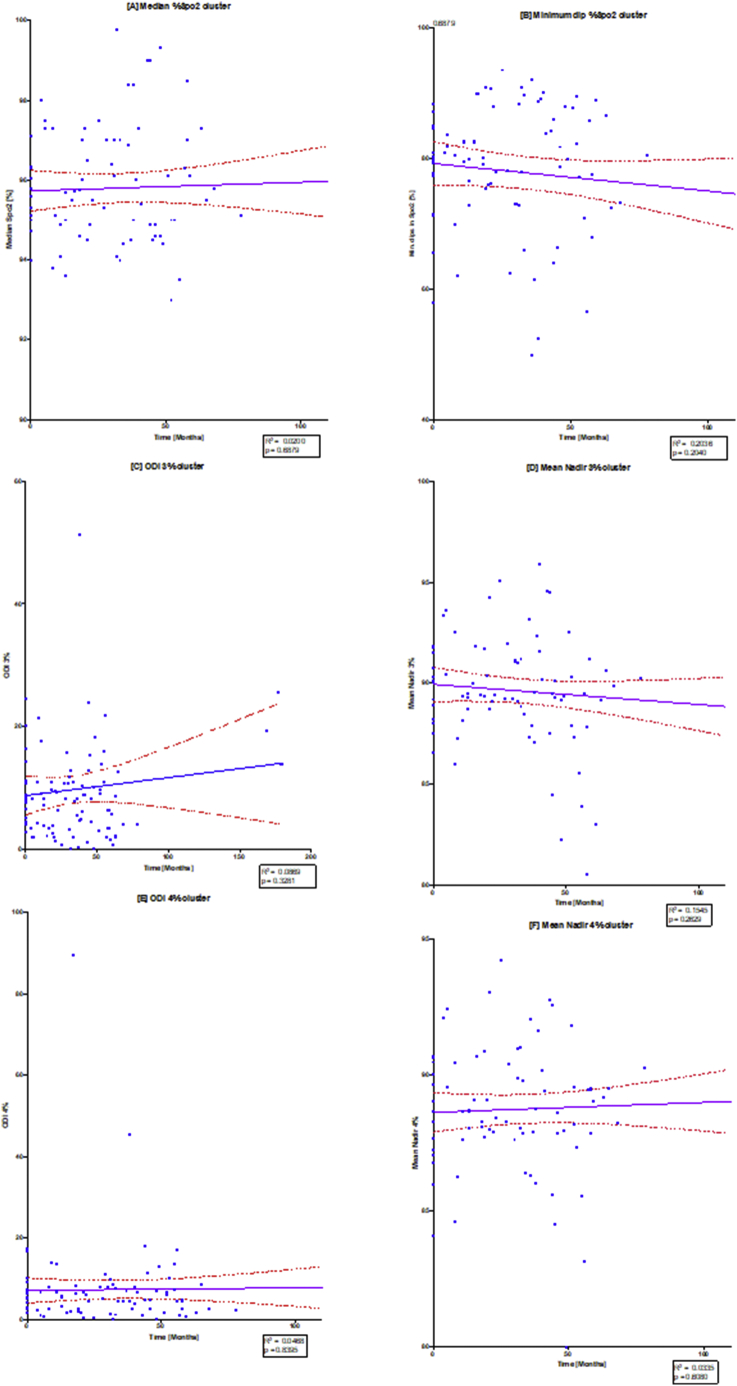
3.2.2. Overnight oximetry
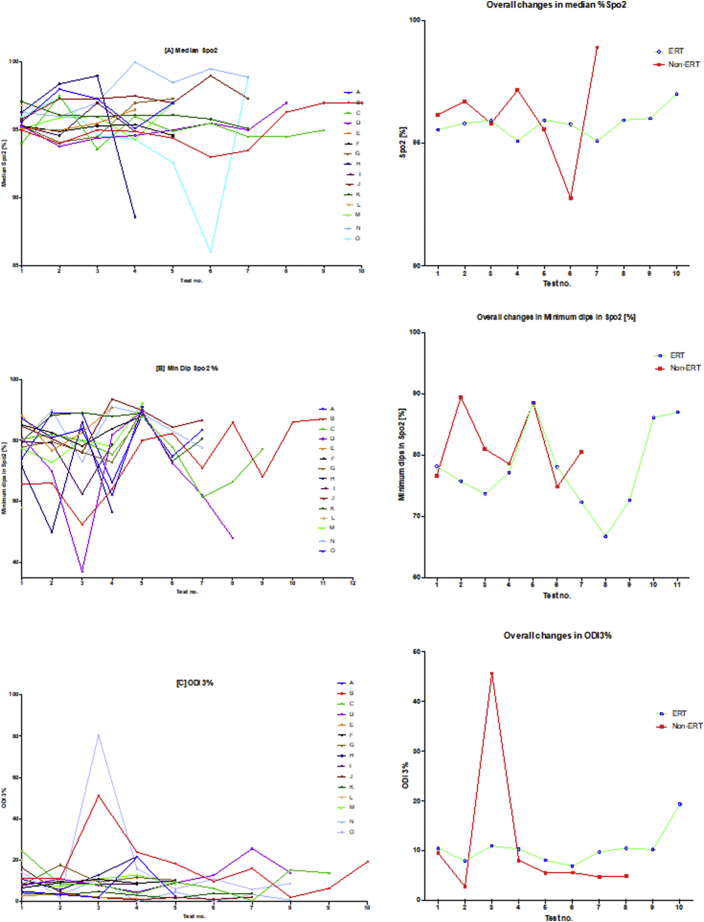
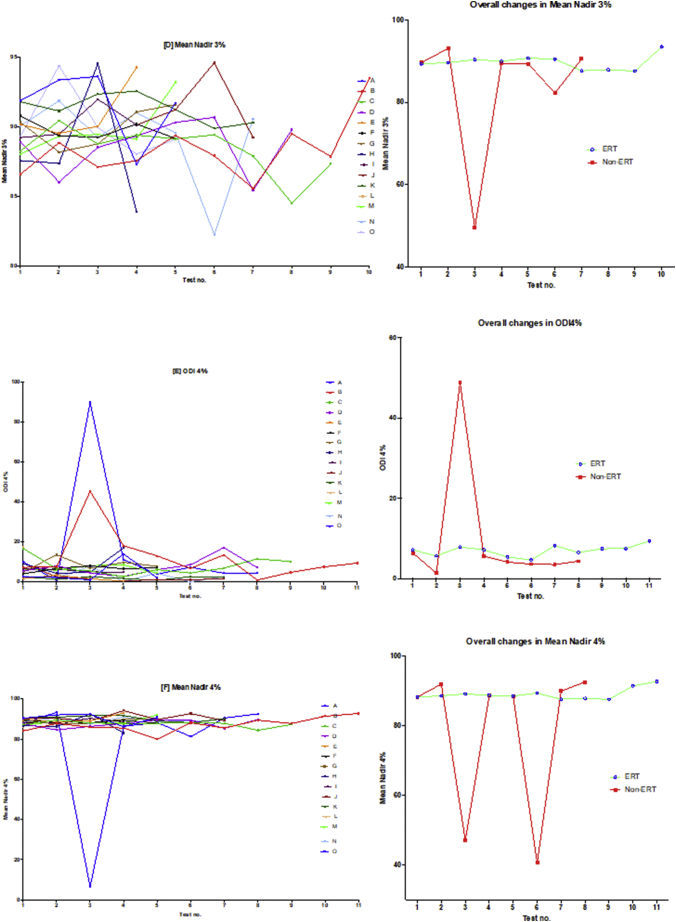
Table 3 and Fig. 3, Fig. 5 illustrate the changes in the overnight oximetry variables over the study period. Similar to the spirometry tests results described above, Fig. 5 is a scatter plot of each oximetry variable over time whereas Fig. 3 shows the individual change and the overall trends qualifying the differences in the ERT and non-ERT groups. Fig. 5 illustrates that median % SpO2 shows a mild (non-significant; p = .6879) increase over time, with Fig. 3(A) illustrating mildly increasing changes in individual subjects as well as the average trends for the ERT treated and non-ERT treated cohorts. Fig. 3(B) and 5(B) depicted changes in the Min. dips in Spo2 that overall decreased throughout the study, although there was a mid-study spike in both the ERT and non-ERT groups. Fig. 5(C) showed that there was a non-significant increase in ODI 3% over time (p = .3281), with Fig. 3(C) qualifying that there was a far greater spike in ODI 3% in the non-ERT that occurred early (although this was a very small sample), whereas the ERT treated cohort showed little change until the last oximetry study. Likewise, in Fig. 3(D) mean nadir 3% showed little overall change in the ERT-treated group, whilst in contrast, the non-ERT group exhibited a decrease coinciding with the increase in ODI 3%, suggesting deterioration in the disease burden of SDB; Fig. 5(D) shows that the decrease in mean nadir 3% was non-significant (p = .2629). ODI 4% and mean nadir 4% exhibited changes that were in parallel with ODI 3% and mean nadir 3%, with the exception of the non-ERT treated group, exhibiting a double dip in the mean nadir 4% before returning to the baseline value. Finally, we found that Spearman rank correlation of FEV1, FVC [%predicted] & ODI 3% together, exhibited a strong positive relationship (r 0.74–95% CI 0.61 to 0.83; p < .0001).
Table 3.
Summary of oximetry results.
| Subject | Oximetry variable |
|||||
|---|---|---|---|---|---|---|
| Median %Spo2 | Median ODI 3% | Mean Nadir 3% | Median ODI 4% | Mean Nadir 4% | Min dip. SpO2 (%) | |
| A | 97 [95.1–98] {→} | 3.5 [2–21.5] {→} | 91.9 [87.3–93.6] {↓} | 2.2 [1–13.9] {→} | 90.9 [86.2–92.4] {↓} | 83.6 [62–90.9] {→} |
| B | 95 [93–97] {↑} | 13.6 [2–51.2] {→} | 87.8 [85.6–93.5] {↑} | 7.5 [0.9–45.4] {→} | 87.8 [80–92.8] {↑} | 71 [52.5–87] {↑} |
| C | 94.9 [93.6–97.5] {→} | 8.3 [0.4–24.6] {↓} | 88.8 [84.5–90.4] {↓} | 6.5 [2.6–16.7] {→} | 87.9 [84.5–89.6] {↓} | 78 [61.4–87.9] {↓} |
| D | 95 [93.8–97] {↑} | 10 [4.6–25.7] {↑} | 89.1 [85.4–90.7] {→} | 6.6 [2.7–17.2]{↑} | 87.8 [84.6–89.8] {↑} | 71.3 [37.1–89.6] {↓} |
| E | 95.3 [95–96.5] {↑} | 2.5 [1.5–3.8] {↓} | 90.8 [89.5–94.3]] {↑} | 1.6 [0.4–3.3] {↓} | 89.6 [87.6–90.3] {↑} | 85.5 [76.6–90.8] {→} |
| F | 95.3 [94.6–95.4] {→} | 9.2 [6.7–10.9] {↑} | 89.3 [89.2–90.8] {↓} | 6.8 [4–8] {↑} | 88.6 [87.9–89.6] {↓} | 83.9 [78.2–87.9] {↑} |
| G | 95.3 [94.1–97.3] {↑} | 10.5 [8–17.8] {↓} | 90.3 [88.2–91.6] {↑} | 7.8 [5–13.5] {→} | 89.1 [87.6–90.2] {↑} | 77.8 [82.9–90.2] {↑} |
| H | 97.4 [88.6–99] {↓} | 11.9 [5.7–21.8] {↑} | 87.5 [83.9–94.5] {→} | 6.9 [4.3–17] {↑} | 86.6 [83.1–92.6] {→} | 64 [49.9–86] {→} |
| I | 95.1 [94.6–97] {→} | 8.4 [8.3–9.8] {↓} | 89.8 [89.2–92] {↑} | 5.6 [4.6–6.6] {→} | 88.5 [88.1–90.4] {→} | 79 [62.5–79.5] {→} |
| J | 97.3 [95.8–99] {↑} | 2 [0.9–16.4] {↓} | 90.1 [89.2–94.6] {→} | 1.5 [0.4–9.3] {↓} | 90.4 [89.1–94.3] {→} | 84.6 [76–93.6] {↑} |
| K | 96.1 [95.1–97.1] {↓} | 4 [1.7–4.9] {↓} | 91.2 [89.9–92.5]{↓} | 2.2 [0.8–2.6] {→} | 90.7 [88.2–91.8] {↓} | 87.8 [73.2–89.1] {→} |
| M | 95.9 [94.3–97] {→} | 8.8 [7.2–12.9] {→} | 89.3 [88–93.2] {↑} | 6.4 [4.4–8.4] {→} | 88.3 [87–92.1] {↑} | 78[72.8–92.1]] {↑} |
| N | 98.6 [96–100] {→} | 3.5 [0.2–10.8] {↓} | 90.4 [82.3–91.9] {↓} | 2.7 [0–7.9] {→} | 89.1 [87.6–91] {↓} | 84.1 [73–91] {→} |
| O | 94.6 [86–98.9] {↓} | 9.9 [2–80.5] {↓} | 89.3 [88–94.4] →} | 5.8 [0.8–89.6] {↓} | 88.1 [6.58–93.4] {↑} | 83.5 [66–89.2] {→} |
The table above illustrates the values for the 6 oximetry variables for each subject.
Median values are displayed in bold, minimum and maximum range in square brackets. Curly braces {} denote the overall trend of the variable throughout the study: ↑, trend increased; ↓ trend decreased; →, no change in trend. ODI 3%, ≥3% arterial oxygen desaturations/h; ODI 4%, ≥4% arterial oxygen desaturations/h; min dip SpO2, minimum dips in oxygen saturations.
Fig. 3.
Oximetry changes over the course of the study.
The diagram illustrates individual plots and natural progression in oximetry for each subject, along with summary plots, divided into ERT and non-ERT groups. This was conducted for: (A) median %Spo2, (B) ODI3%, (C) mean nadir 3%, (D) ODI4%, (E) mean nadir 4% and (F) min dip SpO2 (%). Note also that the graph on the right of the induvial plots is a summary that describes the mean changes of all subjects in the ERT and non-ERT of the study period.
Fig. 5.
Linear regression of oximetry changes over time.
The diagram illustrates individual scatterplots for oximetry changes over time: (A) median %Spo2, (B) ODI3%, (C) mean nadir 3%, (D) ODI4%, (E) mean nadir 4% and (F) min dip SpO2 (%). Note the solid purple line is the linear regression line with the red dashed lines providing the 95% confidence lines. R2 and p values are shown in each of the regression plots. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2.3. 6MWT
6MWT, as previously indicated is a validated metric of assessing cardiorespiratory function. In our study, we found that there was a global decline in both the ERT and non-ERT treated subjects (see Fig. 2F), with the decline in the ERT subjects appearing to have a phased delay (i.e. lagging behind that of the non-ERT treated subjects). One must be cautious with these results, however as there was either incomplete or missing data from subjects D, H, I, N, O and P; subjects I and D were unable to walk – being confined to mobilising with a wheelchair. Furthermore, subjects B, C, E, F and G had orthopaedic interventions that would have confounded the changes in 6MWT, we addressed this by noting on the induvial plots when such orthopaedic interventions were undertaken in order to obtain a more holistic picture in Fig. 2F.
3.2.4. Adenotonsillectomy and non-invasive ventilation
3.2.4.1. Adenotonsillectomy
In our study, 8 subjects (B, C, F, G, I, K, M and O) underwent adenotonsillectomy (T&A), of which 7 where ERT treated. Overall, there appeared to be a global decline in spirometry variables, besides FVC, this could be confounded by the fact the growth of the child. We thus inspected individual plots (see Fig. 7 and supplementary data) and generally found that there was improvement immediately post T&A, albeit the overall result being a decline in function. Results from oximetry data were far more favourable, with ODI 3% having decreased in 6/7 ERT treated subjects – post T&A, this effect was not seen in the non-ERT subjects. We also found that the ODI4% had less significant decrease compared to ODI 3%, the mean nadir 3% generally increased in 4/7 of the subjects and the Min. Dips Spo2% showed no overall change post intervention.
3.2.4.2. Non-invasive ventilation
We found that subjects B, D, G, N and O had been established on bi-level non-invasive ventilation (BiPAP); of which 3 were ERT treated. We ascertained that the effect of on oximetry and spirometry was an increase in FVC, median Spo2% and mean nadir 3&4%, with a decrease in ODI 3% and ODI 4%. The improvements in oximetry was either diminished or reduced in the non-ERT treated subjects.
4. Discussion
This is one of the first studies to report longitudinal post-therapeutic outcomes and changes of pulmonary function for patients with MPS IVA using spirometry and oximetry variables as composite clinical endpoints, as well as the longitudinal effects of adenotonsillectomy and NIV. The study not only quantifies the trends for these endpoints, but also qualifies the nuances of individual patterns, variation and response to therapeutics and intervention. We undertook rigorous longitudinal analysis of each variable individually (i.e. for each subject), along with a series of statistical tests and summary plots to construct a more comprehensive understanding of the data. Moreover, the results describe the overall changes in pulmonary function and how the patterns of changes differed for ERT and non-ERT treated. By constructing individualised and then summary plots over time, a more nuanced and precise assessment can be made of these changes rather than simply comparing the baseline values to the final values in order to ascertain change. Thus, data triangulation from the individual trends, grouped summary plots and linear regression ensured a more rigorous methodology for interrogating the data accrued.
We found that there was a general decrease in pulmonary function over time. Whilst FEV1 and FVC did increase over time (Fig. 4), FEV1 [%Pred] and FVC [%Pred] decreased, with the latter two variables being superior predictors of disease progression as they are adjusted for age, sex and height. Furthermore, the increase in FEV1 and FVC may be explained by the growth of the child and thus this could have confounded interpretation. The study established that the decline in pulmonary function was more pronounced in the non-ERT group, with the ERT group having a more attenuated decline with no evidence of reversal of disease. The results inferred that as the mean age of commencing ERT was relatively late (median age commencing ERT 73.5 months – range 43–183 months), significant child growth and GAG deposition had already occurred. Previous studies that have demonstrated the greatest improvement in pulmonary function, reported that early commencement of ERT conferred a preventive rather than a curative role of GAG accumulation, along with improved bone cell health, nutrition, neuroendocrine function and reduced inflammation [15,21,36]. As previously described, the 6MWT has already been validated as an effective tool to investigate cardiorespiratory reserve in children with MPS IVA [7,23,24]. Unlike previous studies, our results showed reduction in 6MWT to be aligned with the reduction of spirometry values and supported the notion that the children in this study deteriorated over the time period studied. It should also be noted that the 6MWT has certain limitations such as mobility, and thus orthopaedic symptoms may have been the limiting factor in the reduction of the 6MWT in certain patients rather than a true decline in cardiorespiratory reserve.
The changes in oximetry showed a general, overall improvement in SDB, with the delineation between the ERT and the non-ERT group being far more tangible. Fig. 3. illustrates how (in general) the non-ERT had greater erraticism in temporal changes of the variable with overall more significant evidence of deterioration, using the clinical endpoints of median % SpO2, ODI 3%, mean nadir 3% and minimum dups in SPO2.
Overall, both adenotonsillectomy and NIV was more effective in the ERT cohort, as it either improved respiratory function or attenuated the decline. Fig. 6 showed how each manual plot was marked with distinct markers to identify how the variable changed post-intervention (see supplementary material for each individual plot). Eight patients underwent T&A in the study, of which seven were in the ERT treated group. ODI 3% and 4% decreased in 6/7 subjects after adenotonsillectomy in the ERT cohort only, mean nadir 3% generally increased in 4/7 subjects and min dips Spo2% showed no overall change post-intervention. Five subjects received NIV treatment, of which three were ERT treated and two were untreated. The effect of NIV on oximetry and spirometry was an increase in FVC, median Spo2%, mean nadir 3&4%. ODI 3% decreased, ODI4% demonstrated a less significant decrease. The improvement in oximetry was less pronounced or reduced in the non-ERT treated cohort.
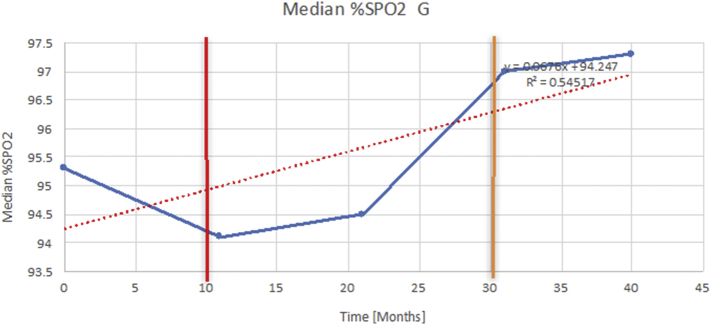
Fig. 6.
The effects of adenotonsillectomy and NIV.
The above graph demonstrates an example of how each of the individual plots where constructed to ascertain the changes in pulmonary function over time. A line of best fit creating a regression line was created to ascertain the overall trend of whether there was a decline or improvement. Note also, the solid red and orange bars, that mark when a therapeutic intervention was undertaken. The solid red line (−) indicates when adenotonsillectomy was undertaken and the orange line (−) illustrates when NIV was instituted. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The main strength of this study is the amount of data analysed and candour employed in assessing change in pulmonary function using the aforementioned clinical endpoints, where data analysis was triangulated from individual subject analysis, group analysis (ERT treated and non-ERT) and scatter plots with regression lines of best fit. Utilising the described methodology enabled characterisation of overall changes in pulmonary function, whilst facilitating meaningful analysis of the effect of therapeutic interventions on the clinical endpoints. The limitations of this study was firstly that it was a retrospective analysis, with a relatively small sample size (although reasonable numbers for this very rare disease) and the lack of a true control arm. Moreover, the non-ERT only consisted of only three individuals. Due to the rarity of the disease, a randomised control trial may be difficult to undertake to truly ascertain: 1. the effect of ERT on pulmonary function over time; 2. whether certain phenotypes could be targeted earlier with a more aggressive treatment regimen, and 3. the effect of treatment modulated with the use of novel therapies such as gene therapy. What may be prudent is large scale, multi-site study involving more patients in the control arm to further elaborate and compare changes in pulmonary function compared to those treated with ERT. However, due the rarity of disease and the early institutions of ERT across all centres in the UK, recruitment to the non-ERT could be taken from international centres that currently do not offer ERT to all patients. Additionally, future studies may benefit from using modified cardiopulmonary exercises testing (CPET) that arguably provide a more rigorous and objective assessment of pulmonary, cardiovascular, haematopoietic, neuropsychological, and skeletal muscle systems.
5. Conclusions
In patients with MPS IVA, respiratory disease is a leading cause of morbidity and mortality. This In this retrospective, longitudinal, repeated-measures cohort study, we found that treatment ERT with elosulfase alfa may attenuate, but does not reverse, the natural progression of respiratory dysfunction, with the magnitude of effects being greater in oximetry tests. In addition, this study demonstrated that existing adjunctive therapies, such as T&A and NIV, appear to be more effective in ERT treated subjects. Since MPS IVA is a phenotypically heterogenous disease, it remains unclear which cohort of patients will benefit most from ERT therapy to prevent or limit decline in pulmonary function. Further research is required to ascertain how therapy (medical, surgical and general supportive measures) can be tailored to maximise positive outcomes. Further work is needed to determine the best outcome measure and outcome measurement instruments for patients with MPS IVA, both to aid clinicians to monitor performance over the long-term using existing therapies, and to facilitate meaningful clinical evaluation of novel therapies. Finally, the use of a precision medicine ontological approach can allow early identification of treatment failure, thereby enabling treatment modulation with individualised tailored therapy – particularly in the context of novel and emerging therapies (e.g. bone marrow transplant or gene therapy). This would allow for the selection of an alternative therapeutic strategy to ERT in non-responders, prior to the development of irreversible airway changes.
Acknowledgments
Acknowledgements
We would like to thank Ms. Pauline Hensman, Highly Specialised Physiotherapist in Lysosomal Storage Disorders and Ms. Jean Mercer, Senior Clinical Nurse Specialist, Willink Biochemical Genetics Unit, Royal Manchester Children's Hospital, Manchester, UK for their help with data collection and triangulation.
Competing interests
The authors IAB has received an unrestricted research grant and travel grants from Shire PLC. SAJ has received speaker and consulting fees as well as research grants and has been an investigator on sponsored trials for Genzyme Sanofi, Biomarin and Shire.
Funding
This research project did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ymgmr.2019.100487.
Appendix A. Supplementary data
Supplementary material
References
- 1.Morquio L. Sur une forme de dystrophie osseuse familiale. Bull. Soc. Pediatr. Paris. 1929;27:145–152. [Google Scholar]
- 2.Jones S., Wynn R. Mucopolysaccharidoses: Clinical Features and Diagnosis – UpToDate. 2019. https://www.uptodate.com/contents/mucopolysaccharidoses-clinical-features-and-diagnosis?search=MPS%20IV&source=search_result&selectedTitle=1~15&usage_type=default&display_rank=1#H10
- 3.Jones S., Wijburg F. Inborn Metab. Dis. Diagn. Treat. 6th ed. Springer; Berlin: 2016. Mucopolysaccharidoses, oligosaccharidoses and sialic acid disorders; p. 577. [Google Scholar]
- 4.Tomatsu S., Montaño A.M., Oikawa H., Smith M., Barrera L., Chinen Y., Thacker M.M., Mackenzie W.G., Suzuki Y., Orii T. Mucopolysaccharidosis type IVA (Morquio A disease): clinical review and current treatment. Curr. Pharm. Biotechnol. 2011;12:931–945. doi: 10.2174/138920111795542615. [DOI] [PubMed] [Google Scholar]
- 5.Tomatsu S., Averill L.W., Sawamoto K., Mackenzie W.G., Bober M.B., Pizarro C., Goff C.J., Xie L., Orii T., Theroux M. Obstructive airway in Morquio A syndrome, the past, the present and the future. Mol. Genet. Metab. 2016;117:150–156. doi: 10.1016/j.ymgme.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wraith J.E. The mucopolysaccharidoses: a clinical review and guide to management. Arch. Dis. Child. 1995;72:263–267. doi: 10.1136/adc.72.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harmatz P., Mengel K.E., Giugliani R., Valayannopoulos V., Lin S.-P., Parini R., Guffon N., Burton B.K., Hendriksz C.J., Mitchell J., Martins A., Jones S., Guelbert N., Vellodi A., Hollak C., Slasor P., Decker C. The Morquio A clinical assessment program: baseline results illustrating progressive, multisystemic clinical impairments in Morquio A subjects. Mol. Genet. Metab. 2013;109:54–61. doi: 10.1016/j.ymgme.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 8.McMillan J.A., Feigin R.D., DeAngelis C., Jones M.D. Oskis Pediatr. Princ. Pract. 4th ed. Lippincott Williams & Wilkins; Philadelphia: 2006. Lysosomal storage disorders; p. 2199. [Google Scholar]
- 9.Meyer K. Biochemistry and biology of mucopolysaccharides. Am. J. Med. 1969;47:664–672. doi: 10.1016/0002-9343(69)90162-4. [DOI] [PubMed] [Google Scholar]
- 10.Di Ferrante N., Ginsberg L.C., Donnelly P.V., Di Ferrante D.T., Caskey C.T. Deficiencies of glucosamine-6-sulfate or galactosamine-6-sulfate sulfatases are responsible for different mucopolysaccharidoses. Science. 1978;199:79–81. doi: 10.1126/science.199.4324.79. [DOI] [PubMed] [Google Scholar]
- 11.Doherty C., Averill L.W., Theroux M., Mackenzie W.G., Pizarro C., Mason R.W., Tomatsu S. Natural history of Morquio A patient with tracheal obstruction from birth to death. Mol. Genet. Metab. Rep. 2018;14:59–67. doi: 10.1016/j.ymgmr.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tylki-Szymańska A., Czartoryska B., Bunge S., van Diggelen O.P., Kleijer W.J., Poorthuis B.J., Huijmans J.G., Górska D. Clinical, biochemical and molecular findings in a two-generation Morquio A family. Clin. Genet. 1998;53:369–374. doi: 10.1111/j.1399-0004.1998.tb02747.x. [DOI] [PubMed] [Google Scholar]
- 13.Fujimoto A., Horwitz A.L. Biochemical defect of non-keratan-sulfate-excreting Morquio syndrome. Am. J. Med. Genet. 1983;15:265–273. doi: 10.1002/ajmg.1320150210. [DOI] [PubMed] [Google Scholar]
- 14.Broomfield A., Kenth J., Bruce I.A., Hl T., S W. Respiratory complications of metabolic disease in the paediatric population: a review of presentation, diagnosis and therapeutic options. Paediatr. Respir. Rev. 2019 doi: 10.1016/j.prrv.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Muenzer J. Early initiation of enzyme replacement therapy for the mucopolysaccharidoses. Mol. Genet. Metab. 2014;111:63–72. doi: 10.1016/j.ymgme.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Peters M.E., Arya S., Langer L.O., Gilbert E.F., Carlson R., Adkins W. Narrow trachea in mucopolysaccharidoses. Pediatr. Radiol. 1985;15:225–228. doi: 10.1007/BF02388760. [DOI] [PubMed] [Google Scholar]
- 17.Semenza G.L., Pyeritz R.E. Respiratory complications of mucopolysaccharide storage disorders. Medicine (Baltimore) 1988;67:209–219. doi: 10.1097/00005792-198807000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Santamaria F., Andreucci M.V., Parenti G., Polverino M., Viggiano D., Montella S., Cesaro A., Ciccarelli R., Capaldo B., Andria G. Upper airway obstructive disease in mucopolysaccharidoses: polysomnography, computed tomography and nasal endoscopy findings. J. Inherit. Metab. Dis. 2007;30:743–749. doi: 10.1007/s10545-007-0555-5. [DOI] [PubMed] [Google Scholar]
- 19.Walker P.P., Rose E., Williams J.G. Upper airways abnormalities and tracheal problems in Morquio's disease. Thorax. 2003;58:458–459. doi: 10.1136/thorax.58.5.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muhlebach M.S., Wooten W., Muenzer J. Respiratory manifestations in mucopolysaccharidoses. Paediatr. Respir. Rev. 2011;12:133–138. doi: 10.1016/j.prrv.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Hendriksz C.J., Burton B.K., AlSayed M.D., Giugliani R., Guelbert N., Hughes D., Mealiffe M., Shaywitz A.J., Slasor P., Mitchell J.J., Parini R., Raiman J., Solano Villarreal M.L., Stewart F., Berger K.I., Harmatz P.R. Impact of long-term elosulfase alfa treatment on pulmonary function in patients with Morquio syndrome type A. Mol. Genet. Metab. 2016;117 [Google Scholar]
- 22.Elosulfase Alfa for Treating Mucopolysaccharidosis Type IVa | Guidance and Guidelines | NICE. 2015. https://www.nice.org.uk/guidance/hst2 accessed December 16, 2018.
- 23.Hendriksz C.J., Burton B., Fleming T.R., Harmatz P., Hughes D., Jones S.A., Lin S.-P., Mengel E., Scarpa M., Valayannopoulos V., Giugliani R., STRIVE Investigators, Slasor P., Lounsbury D., Dummer W. Efficacy and safety of enzyme replacement therapy with BMN 110 (elosulfase alfa) for Morquio A syndrome (mucopolysaccharidosis IVA): a phase 3 randomised placebo-controlled study. J. Inherit. Metab. Dis. 2014;37:979–990. doi: 10.1007/s10545-014-9715-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hendriksz C.J. Elosulfase alfa (BMN 110) for the treatment of mucopolysaccharidosis IVA (Morquio A syndrome) Expert. Rev. Clin. Pharmacol. 2016;9:1521–1532. doi: 10.1080/17512433.2017.1260000. [DOI] [PubMed] [Google Scholar]
- 25.Pal A.R., Langereis E.J., Saif M.A., Mercer J., Church H.J., Tylee K.L., Wynn R.F., Wijburg F.A., Jones S.A., Bruce I.A., Bigger B.W. Sleep disordered breathing in mucopolysaccharidosis I: a multivariate analysis of patient, therapeutic and metabolic correlators modifying long term clinical outcome. Orphanet J. Rare Dis. 2015;10:42. doi: 10.1186/s13023-015-0255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Standardization of spirometry, 1994 update. American thoracic society. Am. J. Respir. Crit. Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 27.Bellamy D., Booker R. Direct Pub. Solutions; 2000. Spirometry in Practice: A Practical Guide to Using Spirometry in Primary Care. [Google Scholar]
- 28.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 29.Harmatz P.R., Mengel K.E., Giugliani R., Valayannopoulos V., Lin S.-P., Parini R., Guffon N., Burton B.K., Hendriksz C.J., Mitchell J.J., Martins A.M., Jones S.A., Guelbert N., Vellodi A., Wijburg F.A., Yang K., Slasor P., Decker C. Longitudinal analysis of endurance and respiratory function from a natural history study of Morquio A syndrome. Mol. Genet. Metab. 2015;114:186–194. doi: 10.1016/j.ymgme.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 30.Semenza G.L., Pyeritz R.E. Respiratory complications of mucopolysaccharide storage disorders. Medicine (Baltimore) 1988;67:209–219. doi: 10.1097/00005792-198807000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Berry R.B., Budhiraja R., Gottlieb D.J., Gozal D., Iber C., Kapur V.K., Marcus C.L., Mehra R., Parthasarathy S., Quan S.F., Redline S., Strohl K.P., Ward S.L.D., Tangredi M.M. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Work. Party Sleep Physiol. Respir. Control Disord. Child. Royal College of Paediatrics and Child Health; London: 2009. Standards for services for children with disorders of sleep physiology. [Google Scholar]
- 33.Berry R.B., Brooks R., Gamaldo C., Harding S.M., Lloyd R.M., Quan S.F., Troester M.T., Vaughn B.V. AASM scoring manual updates for 2017 (Version 2.4) J Clin Sleep Med. 2017;13(5):665–666. doi: 10.5664/jcsm.6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brouillette R.T., Morielli A., Leimanis A., Waters K.A., Luciano R., Ducharme F.M. Nocturnal pulse oximetry as an abbreviated testing modality for pediatric obstructive sleep apnea. Pediatrics. 2000;105:405–412. doi: 10.1542/peds.105.2.405. [DOI] [PubMed] [Google Scholar]
- 35.Urschitz M.S., Wolff J., Von Einem V., Urschitz-Duprat P.M., Schlaud M., Poets C.F. Reference values for nocturnal home pulse oximetry during sleep in primary school children. Chest. 2003;123:96–101. doi: 10.1378/chest.123.1.96. [DOI] [PubMed] [Google Scholar]
- 36.Hughes D., Giugliani R., Guffon N., Jones S.A., Mengel K.-E., Parini R., Matousek R., Jurecki E., Quartel A. Sub-analysis of long-term elosulfase alfa treatment outcomes in adults with Morquio syndrome type A. Mol. Genet. Metab. 2017;120 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material