Abstract
Purpose
This study aimed to evaluate a possible association between the anti-tissue transglutaminase antibody (anti-tTG) titer and stage of duodenal mucosal damage and assess a possible cut-off value of anti-tTG at which celiac disease (CD) may be diagnosed in children in conjunction with clinical judgment.
Methods
This observational study was conducted at a gastroenterology clinic in a tertiary hospital from April 2012 to May 2013. Seventy children between 6-months and 18-years-old with suspected CD underwent celiac serology and duodenal biopsy. Statistical analyses were done using SPSS 16. Diagnostic test values were determined for comparing the anti-tTG titer with duodenal biopsy. An analysis of variance and Tukey-Kramer tests were performed for comparing the means between groups. A receiver operating characteristics curve was plotted to determine various cut-off values of anti-tTG.
Results
The mean antibody titer increased with severity of Marsh staging (p<0.001). An immunoglobulin (Ig) A-tTG value at 115 AU/mL had 76% sensitivity and 100% specificity with a 100% positive predictive value (PPV) and 17% negative predictive value (NPV) for diagnosis of CD (p<0.001, 95% confidence interval [CI], 0.75–1).
Conclusion
There is an association between the anti-tTG titer and stage of duodenal mucosal injury in children with CD. An anti-tTG value of 115 AU/mL (6.4 times the upper normal limit) had 76% sensitivity, 100% specificity, with a 100% PPV, and 17% NPV for diagnosing CD (95% CI, 0.75–1). This cut-off may be used in combination with clinical judgment to diagnose CD.
Keywords: Duodenitis, Celiac disease, Transglutaminase, Antibodies
INTRODUCTION
Celiac disease (CD) is a chronic small bowel disorder caused by an abnormal immune response to an array of epitopes of wheat gluten and related proteins of rye and barley in genetically susceptible individuals who express the HLA-DQ2/DQ8 haplotype. The diverse presentation of CD includes classical symptoms such as diarrhea, weight loss, failure to thrive, malabsorption, and anemia. Atypical manifestations include nonspecific abdominal pain, esophageal reflux, osteoporosis, and neurological symptoms.
CD occurs with a greater prevalence in patients with autoimmune diseases such as type-1 diabetes, autoimmune thyroiditis, Addison's disease, autoimmune cholangitis, autoimmune hepatitis, primary biliary cirrhosis, alopecia, and dilated cardiomyopathy. These associations have been interpreted to be a consequence of sharing an identical HLA haplotype.
CD is caused by a reaction to gliadin, a prolamine (gluten protein) found in wheat and similar proteins in crops of the tribe Triticeae (such as barley and rye). The tissue transglutaminase enzyme (tTG) is the target antigen of autoantibodies found in the serum of patients with CD. The most physiologically important function of tTG is to deamidate the glutamine residues of the gliadin peptides and convert them into glutamic acid; this modification makes the gliadin negatively charged, allowing it to bind with HLA-DQ2/DQ8 antigens, with the consequent exposure of the neopeptides for recognition by T cells. In people who are genetically predisposed to CD, the development of a T- and B-cell mediated immune response leads to the synthesis of anti-tTG immunoglobulin (Ig) A antibodies and proinflammatory cytokines, resulting in chronic inflammation and progressive destruction of the intestinal mucosa.
Small bowel biopsy is the gold standard in diagnosing CD. However, biopsy is an invasive procedure, and the diagnosis may be missed if mucosal involvement is patchy. Serology tests are sensitive, specific, and they are becoming the obligatory tool for correctly referring patients for biopsy. Immunoglobulin A against the tTG antigen, detected by the enzyme-linked immunosorbent assay (ELISA) method, is accepted as the best serologic screening tool. Testing for anti-tTG also can be used to monitor CD patients following a diet in which the autoantibodies gradually decline until they disappear. The anti-tTG IgA titer has been shown to correlate well with the staging of biopsy [1,2,3,4]. This association has raised the possibility of avoiding biopsies when antibody concentrations are especially high [5,6,7].
Though CD is quite common in North India, very few studies have investigated this disease, and most of them assessed adult participants. This study was undertaken to evaluate a possible association between the serum anti-tTG titer and the stage of duodenal mucosal damage in children with CD, to analyze sensitivity and specificity at various levels of anti-tTG, and to assess a possible cut-off level of anti-tTG at which CD could be diagnosed. This study of the association between tTG and biopsy in the diagnosis of CD, especially in a pediatric population, is probably the first in the state of Rajasthan where the disease burden is quite high.
MATERIALS AND METHODS
This observational study was conducted at a gastroenterology clinic at a tertiary care hospital in North India from April 2012 to May 2013. All children aged 6 months to 18 years suspected to have CD based on clinical signs and symptoms were included. The presence of one or more following features were used to identify suspected cases: i) chronic or recurrent diarrhea; ii) short stature (height for age below 5th percentile in the absence of any other identifiable cause); iii) failure to thrive (weight for age below 5th percentile or weight for height below 10th percentile); iv) unexplained anemia; v) abdominal symptoms (vomiting, abdominal bloating/discomfort); vi) diseases with a high association of CD (type-1 diabetes mellitus, Down syndrome, autoimmune cholangitis, autoimmune hepatitis, primary biliary cirrhosis, dilated cardiomyopathy, autoimmune thyroiditis; vii) family history of CD in a first-degree relative. The following cases were excluded from the study: i) a known case of CD, ii) age <6 months or >18 years, iii) any patient whose parents did not consent. A CD diagnosis was made in those children who presented with the clinical signs and symptoms and tested positive for serology and by duodenal biopsy screening.
The study protocol was approved by the Institutional Ethics Committee. Informed consent was obtained from the parents/guardians of all suspected children. These children were subjected to serology (anti-tTG) and small bowel biopsy.
Serum analysis
Serological testing was performed using a commercial kit (CHORUS tTG-A; DIESSE Diagnostica Senese, Monteriggioni, Italy) based on the ELISA approach. The test was considered negative when the result was <12 AU/mL, doubtful if between 12 and 18 AU/mL, and positive when >18 AU/mL, as per the manufacturer's recommended ani-tTG cut-off values. If the result was doubtful, the test was repeated with the same sample, and if it remained doubtful, then a new serum sample was tested. The serum IgA level was determined in patients in whom anti-tTG IgA levels were low to exclude the possibility of IgA deficiency. None of the patients was on any special diet prior to enrolment in the study.
Endoscopy and histopathology
All the children who tested positive for serology underwent an upper gastrointestinal (GI) endoscopy, which was performed with 4 forceps biopsies from D2 (2nd part of the duodenum) and 1 from D1 (1st part of the duodenum). Specimens were oriented before embedding by an experienced technician. Samples were fixed in buffered formalin and embedded in paraffin wax. Standard sections were obtained and stained with hematoxylin and eosin. All slides were examined in a blinded manner by senior experienced pathologists. No samples were excluded due to poor quality of the biopsy samples. Histologic staging was done using the Marsh-Oberhuber classification [8].
Statistical analyses
Statistical analyses were done using computer software (SPSS for Windows, Version 16.0; SPSS Inc., Chicago, IL, USA). Quantitative values are expressed as means and standard deviations, and qualitative values are expressed in percentages and proportions. Diagnostic test values were determined by comparing the anti-tTG titer with duodenal biopsy. An analysis of variance (ANOVA) test was used to observe an association between anti-tTG titers and Marsh grading. The Tukey-Kramer post-hoc test was performed to compare the mean of each Marsh staging. The receiver operated characteristics (ROC) curve was used to determine the anti-tTG titer cut-off for best sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) with a 95% confidence interval (CI) and 0.05 alpha error. The correlation coefficient and linear regression was used to determine the relationship between serology and age.
RESULTS
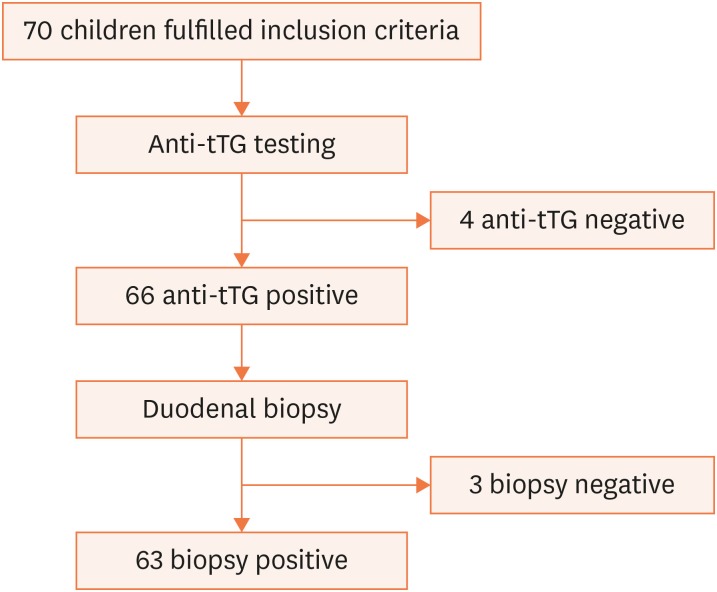
Seventy children fulfilled the inclusion criteria and were enrolled in the study (Fig. 1). These 70 children underwent serological testing, of which 66 subjects tested positive and 4 tested negative. Those with negative serologic tests underwent further serum-IgA measurements, of which all were normal and hence were excluded from the study as per the selection criteria. Of the 66 children included in the study, 53% were boys and 47% girls. The mean age of children at diagnosis was 7.05±4.4 years and the mean age at onset of symptoms was 5.4±3.8 years. The most common GI symptom was abdominal distension (38, 57.5%), followed by chronic diarrhea (31, 46.9%), abdominal pain (24, 36.3%), vomiting (14, 21.2%), and constipation (1, 1.5%) (Table 1). Three cases had CD in a first-degree relative, 1 case had type-1 diabetes mellitus, and 1 case had type-1 diabetes mellitus along with a family history of CD. The clinical profiles of these 5 cases were similar to the rest.
Fig. 1. Flow diagram.
tTG: tissue transglutaminase enzyme.
Table 1. Demographic characteristics of the study population.
| Demographic characteristics | No. (%) | |
|---|---|---|
| Age (y) | ||
| 0.5–2 | 6 (9.1) | |
| 2–5 | 23 (34.8) | |
| 6–10 | 24 (36.3) | |
| 10–15 | 11 (16.6) | |
| 15–18 | 2 (3) | |
| Sex | ||
| Male | 35 (53) | |
| Female | 31 (47) | |
| GI symptoms | ||
| Abdominal distension | 38 (57.5) | |
| Chronic diarrhea | 31 (46.9) | |
| Abdominal pain | 24 (36.3) | |
| Anorexia | 20 (30.3) | |
| Vomiting | 14 (21.2) | |
| Constipation | 1 (1.5) | |
| Non-GI symptoms | ||
| Pallor | 45 (68.1) | |
| Weight loss/not gaining weight | 43 (65.1) | |
| Weakness | 22 (33.3) | |
| Pedal edema | 10 (15.1) | |
| Clubbing | 3 (4.5) | |
| Others | 8 (12.1) | |
GI: gastrointestinal.
Of the 66 cases, 3 were negative by duodenal biopsy. Of the 63 biopsy-positive cases, 1 (1.5%) belonged to Marsh stage II, and 14 (21.2%), 17 (25.7%), and 31 (46.9%) were IIIA, IIIB, and IIIC, respectively. The mean anti-tTG level was 218.9. The mean anti-tTG titer increased with severity of Marsh staging, and the result was statistically significant (p<0.001) (Table 2). Post hoc tests showed that the mean anti-tTG titers differed significantly between stages IIIA and IIIB, IIIB and IIIC, and IIIA and IIIC, suggesting that the increase in titer with disease progression is not merely by chance.
Table 2. Relation between stage of duodenal biopsy and anti-tTG titer.
| Marsh grading | Mean anti-tTG titer (SD) | No. |
|---|---|---|
| 0 & I | 54.5 (42.7)* | 3 |
| II | 136 (NA)* | 1 |
| IIIA | 88.5 (78.1)* | 14 |
| IIIB | 203.9 (114.9)* | 17 |
| IIIC | 304.7 (101.9)* | 31 |
| Total | 218.9 (133.8)* | 66 |
SD: standard deviation, tTG: tissue transglutaminase enzyme, NA: not applicable.
*Analysis of variance (p<0.001).
The cases with negative biopsy or serology were excluded from the analysis as per the study protocol, and only those children who were ultimately diagnosed with CD as per the defined diagnostic criteria were offered treatment. Although the clinical suspicion of CD was still high in the other subset, it was thought inappropriate to subject them to the lifelong treatment for CD without confirmation; hence they were advised for review after 3 months for further testing, including repeat biopsy, genetic analysis, and anti-endomysial antibody (EMA) testing. However, follow-up of these patients was not a part of the study, and the facilities to test for IgG deamidated gliadin peptide and EMA were not available at our institute.
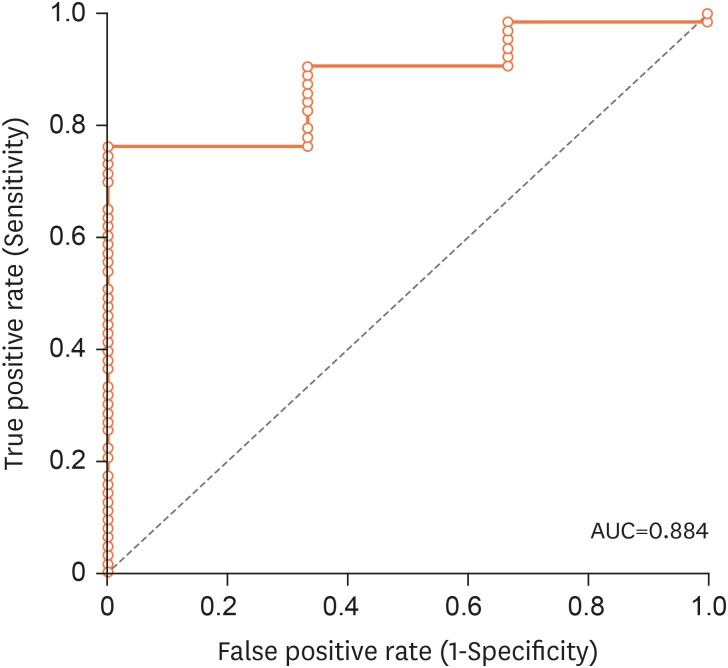
In order to establish the optimal cut-off values of anti-tTG, the ROC curve was analyzed by plotting sensitivity against 1-specificity at different values of anti-tTG. The area under the ROC curve (AUROC) was 0.884 with 0.068 standard error, p<0.0001, and 95% CI, 0.75–1. An anti-tTG value of 115 AU/mL at 6.4 times the upper normal limit (UNL) had 76% sensitivity and 100% specificity, with a 100% PPV and 17% NPV (Fig. 2).
Fig. 2. ROC curve for anti-tTG titers and prediction of CD.
ROC: receiver operated characteristics, tTG: tissue transglutaminase enzyme, AUC: area under the curve, CD: celiac disease.
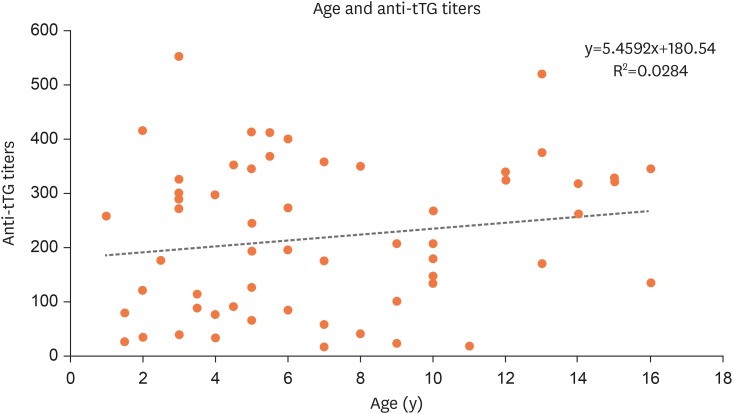
To determine if serology becomes a better predictor with rising age, the correlation coefficient and linear regression equation were calculated, and R was found to be 0.16 (p=0.17, 95% CI, −0.32–0.51) (Fig. 3). As the value of R is close to 0 in our study, there is no significant correlation between serology and advancing age.
Fig. 3. Correlation between age and anti-tTG titers.
tTG: tissue transglutaminase enzyme.
DISCUSSION
Small intestinal biopsy is the gold standard for diagnosing CD. However, besides being an invasive procedure, biopsies have other limitations. In the initial phase of the disease, the mucosal involvement is patchy, requiring at least 4 biopsies to be taken from different parts of the duodenum. Improper processing of the sample can result in pseudo shortening of villi, leading to false-positive diagnosis of CD. In pediatric populations, the sample obtained may be insufficient for diagnosis. Anti-gliadin and anti-EMA antibodies have been used as serologic tests for diagnosis and screening of CD. Although the specificity of EMA is very high, inadequate sensitivity of these tests made them undesirable as a screening tool. Anti-tTG (IgG, IgA) is a valuable screening test with high sensitivity and specificity, and it can be used to monitor the success of therapy with a gluten free diet. The anti-tTG IgA titer also has been shown to correlate well with severity of biopsy staging.
In this study, the mean anti-tTG titer increased with the severity of mucosal damage, and an anti-tTG value of 115 AU/mL, which is 6.4 times the UNL, had 76% sensitivity, 100% specificity, with a 100% PPV and 17% NPV for CD (95% CI, 0.75–1). As the titer increased, specificity increased but the sensitivity decreased.
Our results are comparable with those of Zanini et al. [8], who showed that anti-tTG levels >5-fold UNL is 100% specific for duodenal atrophy, and using this cut-off, duodenal biopsy could be avoided in one third of patients. Alessio et al. [9] showed that patients with anti-tTG ≥7-fold UNL and positive anti-EMA have a high probability of duodenal damage. Rahmati et al. [10] showed that duodenal biopsy is not always necessary when the anti-tTG level is >9-fold UNL. The difference in various cut-off points depends on the kit used for serology [11]. The new European Society for Paediatric Gastroenterology Hepatology and Nutrition guidelines for diagnosis of CD in pediatric populations also suggest that, in symptomatic individuals whose anti-tTG levels are 10 times above UNL, anti-EMA antibody is positive, and HLA-DQ2/DQ8 typing is suggestive of CD, histological confirmation is not necessary [12]. Our study cut-off is 115 AU/mL, which is 6.4 times UNL. The lower cut-off in this study could be due to factors such as race, genetics, and ethnicity in different geographical regions of the world.
A limitation of this study is that it is an observational study, done in a single center, and a small number of cases was recruited. The siblings of the 66 children were not screened, and follow-up of the other subset could not be done.
To conclude, even in a pediatric population with CD, there is an association between anti-tTG titer and the stage of duodenal mucosal injury (p<0.001), and intestinal biopsy may be avoided in symptomatic patients with very high anti-tTG titers. Because there is variation amongst the cut-offs between various anti-tTG kits as seen in numerous studies, generalization of any cut-off value or multiples of UNL based on a single kit would be inappropriate. Presently, this cut-off may be used in combination with clinical judgment to diagnose CD. Further studies involving more numbers of children and at different centers are required to validate these findings.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
References
- 1.Donaldson MR, Book LS, Leiferman KM, Zone JJ, Neuhausen SL. Strongly positive tissue transglutaminase antibodies are associated with Marsh 3 histopathology in adult and pediatric celiac disease. J Clin Gastroenterol. 2008;42:256–260. doi: 10.1097/MCG.0b013e31802e70b1. [DOI] [PubMed] [Google Scholar]
- 2.Kalhan S, Joseph P, Sharma S, Dubey S, Dudani S, Dixit M. Comparative study of histopathological Marsh grading with clinical and serological parameters in celiac iceberg of north India. Indian J Pathol Microbiol. 2011;54:279–283. doi: 10.4103/0377-4929.81593. [DOI] [PubMed] [Google Scholar]
- 3.Parizade M, Bujanover Y, Weiss B, Nachmias V, Shainberg B. Performance of serology assays for diagnosing celiac disease in a clinical setting. Clin Vaccine Immunol. 2009;16:1576–1582. doi: 10.1128/CVI.00205-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker CC, Mitton C, Jevon G, Mock T. Can tissue transglutaminase antibody titers replace small-bowel biopsy to diagnose celiac disease in select pediatric populations? Pediatrics. 2005;115:1341–1346. doi: 10.1542/peds.2004-1392. [DOI] [PubMed] [Google Scholar]
- 5.Sugai E, Vázquez H, Nachman F, Moreno ML, Mazure R, Smecuol E, et al. Accuracy of testing for antibodies to synthetic gliadin-related peptides in celiac disease. Clin Gastroenterol Hepatol. 2006;4:1112–1117. doi: 10.1016/j.cgh.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Vivas S, Ruiz de Morales JG, Riestra S, Arias L, Fuentes D, Alvarez N, et al. Duodenal biopsy may be avoided when high transglutaminase antibody titers are present. World J Gastroenterol. 2009;15:4775–4780. doi: 10.3748/wjg.15.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185–1194. doi: 10.1097/00042737-199910000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Zanini B, Magni A, Caselani F, Lanzarotto F, Carabellese N, Villanacci V, et al. High tissue-transglutaminase antibody level predicts small intestinal villous atrophy in adult patients at high risk of celiac disease. Dig Liver Dis. 2012;44:280–285. doi: 10.1016/j.dld.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Alessio MG, Tonutti E, Brusca I, Radice A, Licini L, Sonzogni A, et al. Correlation between IgA tissue transglutaminase antibody ratio and histological finding in celiac disease. J Pediatr Gastroenterol Nutr. 2012;55:44–49. doi: 10.1097/MPG.0b013e3182470249. [DOI] [PubMed] [Google Scholar]
- 10.Rahmati A, Shakeri R, Sohrabi M, Alipour A, Boghratian A, Setareh M, et al. Correlation of tissue transglutaminase antibody with duodenal histologic marsh grading. Middle East J Dig Dis. 2014;6:131–136. [PMC free article] [PubMed] [Google Scholar]
- 11.Paul SP, Harries SL, Basude D. Barriers to implementing the revised ESPGHAN guidelines for coeliac disease in children: a cross-sectional survey of coeliac screen reporting in laboratories in England. Arch Dis Child. 2017;102:942–946. doi: 10.1136/archdischild-2016-312027. [DOI] [PubMed] [Google Scholar]
- 12.Husby S, Koletzko S, Korponay-Szabó IR, Mearin ML, Phillips A, Shamir R, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–160. doi: 10.1097/MPG.0b013e31821a23d0. [DOI] [PubMed] [Google Scholar]