Abstract
Background
A self-assessment questionnaire, the GERD-Questionnaire (GERD-Q) was used to determine the prevalence of GERD in adolescents, describe the related factors, and determine the impact on quality of life (QoL).
Methods
The incidence of GERD was evaluated using the GERD-Q in adolescents aged 12–18 years. The Pediatric Gastroesophageal Reflux Disease Symptom Questionnaire and Quality of Life Questionnaire (PGSQ-A) for adolescents were additionally administered. Some factors considered related to GERD were also evaluated.
Results
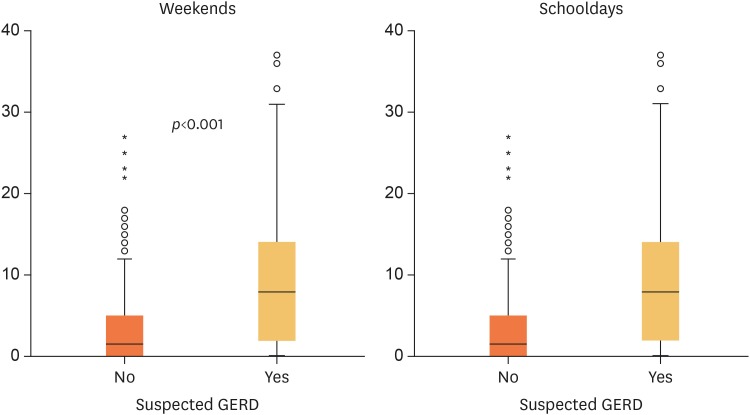
The 520 adolescents were included. The prevalence of suspected GERD, according to a GERD-Q cutoff score of ≥7 was 32.9%, and those drinking soda were 1.7 times more likely to have GERD (95% confidence interval, 1.3–2.2; p<0.001). However, soda consumption was not a risk factor for development of GERD symptoms. Applying a cutoff score of ≥8, only 10.9% of the participants had a positive GERD score, but the association with soda consumption persisted. The median PGSQ-A score in subjects suspected of GERD was 8 (range 0–37) on weekends and 1 (range 0–17) during weekdays (p<0.001) compared to those not suspected of GERD, with a median of 2 (range 0–27) during weekends and 0 (range 0–10) during weekdays. Heartburn, regurgitation, and extraesophageal symptoms correlated significantly with QoL (p<0.001).
Conclusion
The prevalence of suspected GERD in adolescents was 32.9% or 10.9%, depending on the cutoff score used. There was a statistically significant difference in PGSQ-A scores between the subjects suspected or not of GERD, indicating an impaired QoL.
Keywords: Gastroesophageal reflux disease, Questionnaire, Quality of life, Adolescent
INTRODUCTION
Gastroesophageal reflux disease (GERD) is a condition in which reflux of gastric content into the esophagus leads to mucosal damage and clinical problems. Symptoms suggestive of GERD are estimated to occur in 10% of adolescents, and can persist until adulthood [1,2]. Diagnostic tools such as endoscopy, biopsy, pH-metry and impedancemetry are invasive, and/or expensive, and have limited accessibility as they can only be performed in some referral hospitals [3,4,5]. Self-assessment questionnaires for GERD have been developed and were shown to be a useful tool for health care professionals in diagnosing and managing suspected GERD cases. The GERD-Questionnaire (GERD-Q) was developed for adults and was shown to have a sensitivity of 65%, and specificity of 71% [6]. A positive association was demonstrated between the GERD-Q and erosive esophagitis in adolescents [7].
Several studies have reported a significant quality of life (QoL) impairment in GERD patients compared to the healthy population, similar to other chronic conditions [8]. Different generic and disease specific QoL instruments were evaluated in patients with GERD. The GERD-Q and QoL questionnaire for adolescents (Pediatric Gastroesophageal Reflux Disease Symptom Questionnaire and Quality of Life Questionnaire [PGSQ-A]) is a disease specific questionnaire developed for 9–17 years old children [9]. We previously validated the GERD-Q in an Indonesian population of unselected adolescents (data not published). The goal of this study was to collect information on the prevalence of GERD symptoms in Indonesian adolescents using the GERD-Q, and the impact of these symptoms on QoL. Additional information was collected regarding the prevalence of symptoms during schooldays and weekends. We evaluated whether the prevalence of symptoms could be associated with some dietary risk factors.
MATERIALS AND METHODS
The study was conducted from May until November 2017 in high schools in Jakarta. Subjects aged 12–18 years old were recruited through convenience sampling. Subjects who were known to have a chronic disease, had experienced major surgery, or were treated with anti-epileptic drugs, steroids, or non-steroid anti-inflammatory drugs in the past 4 weeks, were excluded. All included subjects were asked to complete self-reporting questionnaires which consisted of the GERD-Q, PGSQ-A and additional questions about lifestyle and selected extraesophageal symptoms possibly related to GERD. Demographic and anthropometric data were also collected. This study was approved by the Health Research Ethics Committee, University of Indonesia Medical School/Cipto Mangunkusumo General Hospital, Jakarta.
The GERD-Q and PGSQ-A were previously translated to the Indonesian language and validated in a comparable population in Jakarta. They are also back-translated from Indonesian to English. The GERD-Q consists of six questions about frequency of GERD-related symptoms during the past seven days (Table 1) [10]. Questions numbered 1, 2, 5, and 6 are scaled 0 for “0 days”, 1 for “1 day”, 2 for “2–3 days”, and 3 for “4–7 days.” Questions numbered 3 and 4 are scaled 3 for “0 days”, 2 for “1 day”, 1 for “2–3 days”, and 0 for “4–7 days,” resulting in a GERD-Q score ranging from 0 to 18 [6,10]. While a score of ≥8 was reported to be the best cutoff score in adults, a score ≥7 has been proposed for adolescents [7]. Therefore, we used both cutoff scores for our data analysis. The PGSQ-A consists of three sections and 35 items (questionnaire can be found at: http://links.lww.com/MPG/A36). Section scores consisted of the sum of item scores divided by the number of items [9].
Table 1. Patient characteristics.
| Variables | Suspected GERD | Non-suspected GERD | p-value | |
|---|---|---|---|---|
| Gender | 0.173 | |||
| Boys | 69 (45.7) | 121 (39.0) | ||
| Girl | 82 (54.3) | 189 (61.0) | ||
| Nutritional status | 0.897 | |||
| Undernourished | 19 (12.6) | 43 (13.9) | ||
| Well-nourished | 94 (62.3) | 191 (61.6) | ||
| Overweight | 23 (15.2) | 41 (13.2) | ||
| Obesity | 15 (9.9) | 35 (11.3) | ||
| Age (y) | 0.131 | |||
| 12 | 34 (22.5) | 76 (24.5) | ||
| 13 | 40 (26.5) | 82 (26.5) | ||
| 14 | 28 (18.5) | 40 (12.9) | ||
| 15 | 30 (19.9) | 68 (21.9) | ||
| 16 | 8 (5.3) | 29 (9.4) | ||
| 17 | 11 (7.3) | 13 (4.2) | ||
| 18 | 0 (0.0) | 2 (0.6) | ||
Values are presented as number (%).
GERD: gastroesophageal reflux disease.
Statistical analysis was performed using Stata version 12 and IBM SPSS software version 24 (SPSS Inc., Chicago, IL, USA). The association between risk factors and GERD was analyzed by univariate and multiple logistic regression model. Variables with an odds ratio (OR) and a confidence interval (CI) greater than 1, and p-value <0.05, were considered as significant risk factors for GERD.
RESULTS
We included 520 adolescents attending seven junior high schools (SMPN 1, SMPN 3, SMPN 7, SMPN 18, SMPN 88, SMPN 116, SMPN 216) and five senior high schools (SMAN 2, SMAN 8, SMAN 22, SMAN 45, SMAN 68) in Jakarta. However, 59 subjects were excluded from data analysis because of incomplete or inconsistent answers. Most subjects were female (58.8%) and well-nourished (61.8%) (Table 1). More than 90% of the adolescents reported to have stomach pain and nausea, and about 25% said to suffer from tooth erosions and/or sinusitis (Table 2).
Table 2. Associations between symptoms related to GERD and suspected GERD.
| Symptoms | Suspected GERD | Non-suspected GERD | p-value | |
|---|---|---|---|---|
| Esophageal symptoms | ||||
| Heartburn | 61 (40.4) | 14 (4.5) | <0.001 | |
| Regurgitation | 139 (92.1) | 118 (38.1) | <0.001 | |
| Pain in the center or upper abdomen | 147 (97.4) | 307 (99.0) | 0.166 | |
| Nausea | 149 (98.7) | 303 (97.7) | 0.497 | |
| Sleeping difficulty because of heartburn or regurgitation | 5 (1.6) | 37 (24.5) | <0.001 | |
| Consuming drugs for heartburn or regurgitation | 20 (6.5) | 69 (45.7) | <0.001 | |
| Extraesophageal symptoms | ||||
| Tooth erosion | 45 (30.4) | 82 (27.5) | 0.524 | |
| Chronic cough | 6 (4.0) | 1 (0.3) | 0.003 | |
| Pneumonia | 5 (3.3) | 11 (3.5) | 0.896 | |
| Sinusitis | 49 (32.5) | 60 (19.4) | 0.002 | |
| Otitis media | 21 (13.9) | 23 (7.4) | 0.026 | |
Values are presented as number (%).
GERD: gastroesophageal reflux disease.
The prevalence of adolescents with a GERD-Q score of ≥7 was 32.9%, with a median score of 7 (ranged 7–11). Subjects with suspected GERD were female (54.3%), well-nourished (62.3%), and under 16 years old (87.4%). However, when a cutoff score of ≥8 was applied only 10.9% (33/461) of adolescents were suspected to suffer from GERD. There were no significant correlations between a GERD-Q score of ≥7 and gender (p=0.173), nutritional status (p=0.897), or age (p=0.131). Heartburn and regurgitation were significantly correlated with a positive GERD-Q score (p<0.001). Some extraesophageal symptoms were also significantly correlated with a positive score: chronic cough (p=0.003), sinusitis (p=0.002) or otitis media (p=0.026) (Table 2). There was a significant correlation (p=0.001) between a positive score (≥7) and soda consumption, with an OR of 1.7 (95% CI, 1.3–2.2), but not for chocolate or coffee. When the data were recalculated using a cutoff score of ≥8, the significant correlation between the score and soda consumption persisted (OR, 2.5; 95% CI, 0.97–6.65; 89.8%) and became positive for chocolate consumption (OR, 1.1; 95% CI, 1.1–1.2). Data on the influence of consumption of coffee, chocolate, soda, spicy foods, or sour foods, and smoking as risk factors for GERD symptoms are listed in Table 3. Univariate logistic regression analysis showed that neither the consumption of coffee, chocolate, soda, spicy foods, or sour foods, nor smoking were significant risk factors for GERD. However, consumption of soda showed the strongest trend as a risk factor, and was almost statistically significant. Multivariate logistic regression analysis also confirmed that none of those foods or beverages constituted risk factors.
Table 3. Eating, drinking, and smoking habits as potential risk factors for GERD symptoms.
| Risk factors | OR | 95% CI | p-value | |
|---|---|---|---|---|
| Univariate logistic regression analysis | ||||
| Drinking coffee in the past 1 wk | 0.83 | 0.38–1.80 | 0.641 | |
| Eating chocolate in the past 1 wk | 1.00 | Cannot be calculated* | ||
| Drinking soda in the past 1 wk | 2.53 | 0.97–6.56 | 0.056 | |
| Smoking in the past 1 wk | 1.70 | 0.19–14.82 | 0.633 | |
| Eating spicy foods in the past 1 wk | 1.20 | 0.27–5.29 | 0.811 | |
| Eating sour foods in the past 1 wk | 2.11 | 0.73–6.06 | 0.167 | |
| Multivariate logistic regression analysis | ||||
| Drinking soda in the past 1 wk | 2.29 | 0.87–6.03 | 0.094 | |
| Eating sour foods in the past 1 wk | 1.74 | 0.59–5.09 | 0.315 | |
GERD: gastroesophageal reflux disease, OR: odds ratio, CI: confidence interval.
*Cannot be calculated because all of the subjects with GERD had homogenous characteristics.
There was a statistically significant difference (p<0.001) between PGSQ-A scores between subjects with a GERD-Q score ≥7 and <7 (Fig. 1). Based on the PGSQ-A, 61.6% subjects with a positive score worried about having stomach, chest, and/or throat problems during the past 7 days, and 52.9% felt their school activities were disrupted because of stomach, chest, and/or throat problems (Table 4). In subjects with a GERD-Q ≥7, only heartburn, regurgitation and extraesophageal symptoms were weakly correlated with QoL. Heartburn had a weak correlation with weekend (R=0.448) and weekday (R=0.393) QoL scores, whereas regurgitation had a weak correlation with weekday QoL scores only (R=0.317) (Table 5). Heartburn (R=0.46) and extraesophageal symptoms (R=0.45) were moderately correlated with weekend and weekday QoL scores.
Fig. 1. Comparison of PGSQ-A score between subjects suspected and not suspected of having GERD. (A) Weekends. (B) Schooldays.
PGSQ-A: Pediatric Gastroesophageal Reflux Disease Symptom Questionnaire and Quality of Life Questionnaire, GERD: gastroesophageal reflux disease.
Table 4. Effect of GERD symptoms on weekdays and weekends based on the PGSQ-A.
| GERD symptoms effect | Never | Almost never | Sometimes | Almost always | Always |
|---|---|---|---|---|---|
| Didn't feel like doing anything | 73 (48.3) | 43 (28.5) | 26 (17.2) | 9 (6.0) | 0 (0.0) |
| Missed out on doing things with friends | 92 (60.9) | 30 (19.9) | 23 (15.2) | 4 (2.6) | 2 (1.3) |
| Disrupted sport activities | 86 (57.0) | 35 (23.2) | 22 (14.6) | 5 (3.3) | 3 (2.0) |
| Had to lay down | 79 (52.3) | 37 (24.5) | 25 (16.6) | 5 (3.3) | 5 (3.3) |
| Couldn't eat what they wanted | 71 (47.0) | 40 (26.5) | 23 (15.2) | 12 (7.9) | 5 (3.3) |
| Couldn't drink what they wanted | 73 (48,3) | 37 (24.5) | 25 (16.6) | 11 (7.3) | 5 (3.3) |
| Changed family plans | 106 (70.2) | 27 (17.9) | 15 (9.9) | 1 (0.7) | 2 (1.3) |
| Eat different meals | 70 (46.3) | 42 (27.8) | 24 (15.8) | 10 (6.6) | 5 (3.3) |
| Felt tired during the day | 69 (45.7) | 46 (30.5) | 26 (17.2) | 6 (4.0) | 4 (2.6) |
| Felt frustated | 115 (76.2) | 18 (11.9) | 12 (7.9) | 3 (2.0) | 3 (2.0) |
| Bad mood | 87 (57.6) | 30 (19.9) | 21 (13.9) | 10 (6.6) | 3 (2.0) |
| Worried about having stomach/chest/throat problems | 58 (38.4) | 38 (25.2) | 34 (22.5) | 13 (8.6) | 8 (5.3) |
| Felt upset | 80 (53.0) | 37 (24.5) | 16 (10.6) | 9 (6.0) | 9 (6.0) |
| Disrupted school activities | 71 (47.0) | 41 (27.2) | 28 (18.5) | 7 (4.6) | 4 (2.6) |
| Had to go to health room during school | 107 (70.9) | 25 (16.6) | 15 (9.9) | 3 (2.0) | 1 (0.7) |
| Hard time paying attention at school | 88 (58.3) | 32 (21.2) | 21 (13.9) | 8 (5.3) | 2 (1.3) |
| Absent from school | 106 (70.2) | 25 (16.6) | 18 (11.9) | 2 (1.3) | 0 (0.0) |
| Late to school | 125 (85.4) | 15 (9.9) | 5 (3.3) | 1 (0.7) | 1 (0.7) |
Values are presented as number (%).
GERD: gastroesophageal reflux disease, PGSQ-A: Pediatric Gastroesophageal Reflux Disease Symptom Questionnaire and Quality of Life Questionnaire.
Table 5. Correlations between GERD symptoms (based on PGSQ-A) and quality of life.
| Symptoms | Weekends score | Schooldays score | ||
|---|---|---|---|---|
| R | p-value | R | p-value | |
| Heartburn (PGSQ-A 1, 2, 3) | 0.448 | <0.001 | 0.393 | <0.001 |
| Regurgitation (PGSQ-A 4, 5, 6, 8) | 0.285 | <0.001 | 0.317 | <0.001 |
| Hoarseness (PGSQ-A 11) | 0.192 | 0.018 | 0.166 | 0.041 |
| Extraesophageal symptoms (PGSQ-A 7, 9, 10, 12) | 0.388 | <0.001 | 0.364 | <0.001 |
| Lack of appetite (PGSQ-A 13) | 0.215 | 0.008 | 0.149 | 0.069 |
| Sleep disturbances (PGSQ-A 14, 15) | 0.296 | <0.001 | 0.229 | <0.005 |
GERD: gastroesophageal reflux disease, PGSQ-A: Pediatric Gastroesophageal Reflux Disease Symptom Questionnaire and Quality of Life Questionnaire.
DISCUSSION
The prevalence of a GERD-Q score of ≥7 in adolescents aged 12–18 years old in this study was 32.9%. Syam et al. [11] showed that based on a GERD-Q cutoff score of ≥7, the GERD prevalence in an Indonesian adult population was as high as 57.6%. This is much higher than other studies in adults.
Data from Japan and France report a prevalence of GERD of 4% and 8% based on the GERD-Q [11,12]. A major difference is the definition of GERD and the diagnostic tool used. In Japan, Okimoto et al. [12] reported a prevalence of GERD in 4.4% of children aged 6–19 years old based on the GERD-Q using a cutoff score of ≥8. Using the same cutoff score, we found a prevalence of 10.9%. The majority of adolescents in our study had a GERD-Q score between 7 and 9, which is in the intermediate zone between ≥7, as proposed in adolescents, and ≥9, as proposed in adults. Since the age of our study population was 12–18 years old, with the majority younger than 15 years (398/459; 87%) (Table 1), we did not use the cutoff score of ≥9 because it has only been proposed in adults. In France, Martigne et al. [13] defined GERD as QoL impairment due to GERD symptoms, and found that 7.6% of the adolescents aged 12–18 years old suffer from GERD.
There were statistically significant associations between GERD and heartburn, regurgitation, and sleep disturbances. Regurgitation is the most common symptom of GERD in infants, but its frequency decreases with age. On the other hand, heartburn is a typical symptom of GERD that increases n frequency with increasing age. Adolescents have a better ability to recognize and describe heartburn compared to younger children [13]. Some extraesophageal symptoms (chronic cough, sinusitis, otitis media) also showed statistically significant associations with a positive GERD-Q score. Reflex responses between the esophagus and naso-pharyngo-bronchial airways, microaspiration, and direct reflux into the nasopharynx are considered mechanisms of GERD related to cough and sinusitis [14,15,16,17]. Direct gastroesophageal reflux into the middle ear may cause mucosal edema in the tuba eustachius area, and obstruction will lead to otitis media. A study by Sone et al. [18] showed that the pepsinogen titer in the middle ear liquid was higher in patients with GERD, and it decreased after administration of proton pump inhibitors (PPIs). However, these findings are open for debate as PPIs do decrease acid reflux, but not overall reflux [19]. This would mean that only acid reflux would be relevant to explain these associations. Moreover, although associations have been shown, causality has not.
We did not evaluate whether the cause of chronic cough was due to asthma, so there might be overlap in the association assessment between GERD and chronic cough or asthma. A systematic review described that there was a possible association between GERD and asthma in children, but causality could not be defined [20]. Asthmatic patients are more likely to have GERD because the increased intrathoracic negative pressure and greater descent of the diaphragm may cause more reflux [16,21]. Reflux was reported to precede cough [22]. Thus, reflux seems to be a possible cause of cough, but cough seems not to induce reflux [22].
The current study also showed a statistically significant association between suspected GERD and soda consumption. A systematic review by Johnson et al. [23] showed that there is no consistent evidence that soda drinks directly damage the esophagus or increase the prevalence of GERD. However, soda drinks decrease intraesophageal pH, reduce lower esophageal sphincter pressure, and increase gastric load. Associations have been shown between GERD and smoking cigarettes [24], and between GERD and alcohol consumption [25]. However, in our study very few adolescents reported smoking cigarettes (6/461) or consuming alcohol (4/461). Although soda consumption was higher in adolescents with a positive GERD-Q score, it was not a risk factor for GERD although it nearly reached statistical significance.
Because of the research method applied, we have no information about lactose intolerance, Helicobacter pylori infection, or other gastrointestinal infections such as those caused by parasites which may be confounding variables to our findings. These conditions may cause similar symptoms to those in GERD.
Subjects suspected of having GERD had a lower QoL compared to subjects not suspected of having GERD, and PGSQ-A scores differed significantly between the groups. This finding is similar with other studies conducted in pediatric or adult populations using different QoL assessment tools [26,27,28]. The most common QoL disturbances were feeling worried about stomach, chest, and/or throat problems (60% of subjects) and feeling tired during the day. These two complaints can lead to anxiety disorders or depression, which are the two most common emotional problems in children with chronic diseases [29,30]. Almost 15% of the adolescents in our study reported being “always anxious.” Further evaluation to detect anxiety disorders seems indicated in these adolescents. Additionally, 50% of subjects suspected of having GERD felt their school activities were disrupted because of GERD-related symptoms. Children and adolescents with chronic diseases that are not related to cognitive functions can also have academic problems because of absences from school, emotional problems, or disease or treatment effects of the general condition.
Many studies have shown that severity and frequency of symptoms are correlated with QoL. In this study we found positive correlations between QoL and heartburn, regurgitation, and extraesophageal GERD symptoms, although the correlation coefficient showed a weak relationship. We recognize that association does not mean causality. However, these results indirectly indicate that optimal management of GERD to decrease these symptoms could increase the QoL of these patients.
In conclusions, the prevalence of suspected GERD in Indonesian adolescents aged 12–18 years old using the GERD-Q with a cutoff score of ≥7 was 32.9%. However, applying a cutoff score of ≥8 decreased the prevalence to 10.9%. However, since applying a cutoff score of ≥7 or ≥8 decreases the prevalence of suspected GERD by more than 20%, the majority of adolescents do not present with severe manifestations. Using the cutoff score of ≥7, statistically significant associations were found between GERD and soda consumption, heartburn, regurgitation, sleep disturbances, and extraesophageal GERD related symptoms (chronic cough, sinusitis, and otitis media). Subjects suspected of having GERD had a lower QoL compared to subjects not suspected of having GERD subjects, and PGSQ-A scores differed statistically between the two groups. The diagnosis of GERD in adolescents remains a challenge since objective measurements such as impedancemetry are often impossible to perform, and many adolescents have a GERD-Q score “around” the cutoff scores proposed in literature.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest related to this paper.
References
- 1.Nelson SP, Chen EH, Syniar GM, Christoffel KK Pediatric Practice Research Group. Prevalence of symptoms of gastroesophageal reflux during childhood: a pediatric practice-based survey. Arch Pediatr Adolesc Med. 2000;154:150–154. doi: 10.1001/archpedi.154.2.150. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Sweet S, Winchester CC, Dent J. Systematic review: update of gastro-oesophageal reflux disease. Gut. 2014;63:871–880. doi: 10.1136/gutjnl-2012-304269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hegar B, Vandenplas Y. Evaluation and management of the pediatric patients with suspected gastroesophageal reflux disease. Indones J Gastroenterol Hepatol Dig Endosc. 2011;12:171–178. [Google Scholar]
- 4.Hegar B, Mulyani L. Esofagitis refluks pada anak. Sari Pediatri. 2006;8:43–52. [Google Scholar]
- 5.Vandenplas Y, Rudolph CD, Di Lorenzo C, Hassall E, Liptak G, Mazur L, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) J Pediatr Gastroenterol Nutr. 2009;49:498–547. doi: 10.1097/MPG.0b013e3181b7f563. [DOI] [PubMed] [Google Scholar]
- 6.Jones R, Junghard O, Dent J, Vakil N, Halling K, Wernersson B, et al. Development of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther. 2009;30:1030–1038. doi: 10.1111/j.1365-2036.2009.04142.x. [DOI] [PubMed] [Google Scholar]
- 7.Chiu JY, Wu JF, Ni YH. Correlation between gastroesophageal reflux disease questionnaire and erosive esophagitis in school-aged children receiving endoscopy. Pediatr Neonatol. 2014;55:439–443. doi: 10.1016/j.pedneo.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Irvine EJ. Quality of life assessment in gastro-oesophageal reflux disease. Gut. 2004;53(Suppl 4):iv35–iv39. doi: 10.1136/gut.2003.034314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kleinman L, Nelson S, Kothari-Talwar S, Roberts L, Orenstein SR, Mody RR, et al. Development and psychometric evaluation of 2 age-stratified versions of the Pediatric GERD Symptom and Quality of Life Questionnaire. J Pediatr Gastroenterol Nutr. 2011;52:514–522. doi: 10.1097/MPG.0b013e318205970e. [DOI] [PubMed] [Google Scholar]
- 10.Jonasson C, Wernersson B, Hoff DA, Hatlebakk JG. Validation of the GerdQ questionnaire for the diagnosis of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2013;37:564–572. doi: 10.1111/apt.12204. [DOI] [PubMed] [Google Scholar]
- 11.Syam AF, Sobur CS, Hapsari FC, Abdullah M, Makmun D. Prevalence and risk factors of GERD in Indonesian population - an internet-based study. Adv Sci Lett. 2017;23:6734–6738. [Google Scholar]
- 12.Okimoto E, Ishimura N, Morito Y, Mikami H, Shimura S, Uno G, et al. Prevalence of gastroesophageal reflux disease in children, adults, and elderly in the same community. J Gastroenterol Hepatol. 2015;30:1140–1146. doi: 10.1111/jgh.12899. [DOI] [PubMed] [Google Scholar]
- 13.Martigne L, Delaage PH, Thomas-Delecourt F, Bonnelye G, Barthélémy P, Gottrand F. Prevalence and management of gastroesophageal reflux disease in children and adolescents: a nationwide cross-sectional observational study. Eur J Pediatr. 2012;171:1767–1773. doi: 10.1007/s00431-012-1807-4. [DOI] [PubMed] [Google Scholar]
- 14.Borrelli O, Marabotto C, Mancini V, Aloi M, Macrì F, Falconieri P, et al. Role of gastroesophageal reflux in children with unexplained chronic cough. J Pediatr Gastroenterol Nutr. 2011;53:287–292. doi: 10.1097/MPG.0b013e318216e1ad. [DOI] [PubMed] [Google Scholar]
- 15.Madanick RD. Management of GERD-related chronic cough. Gastroenterol Hepatol (N Y) 2013;9:311–313. [PMC free article] [PubMed] [Google Scholar]
- 16.Phipps CD, Wood WE, Gibson WS, Cochran WJ. Gastroesophageal reflux contributing to chronic sinus disease in children: a prospective analysis. Arch Otolaryngol Head Neck Surg. 2000;126:831–836. doi: 10.1001/archotol.126.7.831. [DOI] [PubMed] [Google Scholar]
- 17.Hanna BC, Wormald PJ. Gastroesophageal reflux and chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2012;20:15–18. doi: 10.1097/MOO.0b013e32834e8f11. [DOI] [PubMed] [Google Scholar]
- 18.Sone M, Yamamuro Y, Hayashi H, Niwa Y, Nakashima T. Otitis media in adults as a symptom of gastroesophageal reflux. Otolaryngol Head Neck Surg. 2007;136:19–22. doi: 10.1016/j.otohns.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 19.Turk H, Hauser B, Brecelj J, Vandenplas Y, Orel R. Effect of proton pump inhibition on acid, weakly acid and weakly alkaline gastro-esophageal reflux in children. World J Pediatr. 2013;9:36–41. doi: 10.1007/s12519-013-0405-5. [DOI] [PubMed] [Google Scholar]
- 20.Thakkar K, Boatright RO, Gilger MA, El-Serag HB. Gastroesophageal reflux and asthma in children: a systematic review. Pediatrics. 2010;125:e925–30. doi: 10.1542/peds.2009-2382. [DOI] [PubMed] [Google Scholar]
- 21.McCallister JW, Parsons JP, Mastronarde JG. The relationship between gastroesophageal reflux and asthma: an update. Ther Adv Respir Dis. 2011;5:143–150. doi: 10.1177/1753465810384606. [DOI] [PubMed] [Google Scholar]
- 22.Blondeau K, Mertens V, Dupont L, Pauwels A, Farré R, Malfroot A, et al. The relationship between gastroesophageal reflux and cough in children with chronic unexplained cough using combined impedance-pH-manometry recordings. Pediatr Pulmonol. 2011;46:286–294. doi: 10.1002/ppul.21365. [DOI] [PubMed] [Google Scholar]
- 23.Johnson T, Gerson L, Hershcovici T, Stave C, Fass R. Systematic review: the effects of carbonated beverages on gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2010;31:607–614. doi: 10.1111/j.1365-2036.2010.04232.x. [DOI] [PubMed] [Google Scholar]
- 24.Kohata Y, Fujiwara Y, Watanabe T, Kobayashi M, Takemoto Y, Kamata N, et al. Long-term benefits of smoking cessation on gastroesophageal reflux disease and health-related quality of life. PLoS One. 2016;11:e0147860. doi: 10.1371/journal.pone.0147860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang CH, Wu CP, Wang JD, Lee SW, Chang CS, Yeh HZ, et al. Alcohol and tea consumption are associated with asymptomatic erosive esophagitis in Taiwanese men. PLoS One. 2017;12:e0173230. doi: 10.1371/journal.pone.0173230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Acierno SP, Chilcote HC, Edwards TC, Goldin AB. Development of a quality of life instrument for pediatric gastroesophageal reflux disease: qualitative interviews. J Pediatr Gastroenterol Nutr. 2010;50:486–492. doi: 10.1097/MPG.0b013e3181b99ca6. [DOI] [PubMed] [Google Scholar]
- 27.Gisbert JP, Cooper A, Karagiannis D, Hatlebakk J, Agréus L, Jablonowski H, et al. Impact of gastroesophageal reflux disease on patients' daily lives: a European observational study in the primary care setting. Health Qual Life Outcomes. 2009;7:60. doi: 10.1186/1477-7525-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gong EJ, Choi KD, Jung HK, Youn YH, Min BH, Song KH, et al. Quality of life, patient satisfaction, and disease burden in patients with gastroesophageal reflux disease with or without laryngopharyngeal reflux symptoms. J Gastroenterol Hepatol. 2017;32:1336–1340. doi: 10.1111/jgh.13716. [DOI] [PubMed] [Google Scholar]
- 29.Pao M, Bosk A. Anxiety in medically ill children/adolescents. Depress Anxiety. 2011;28:40–49. doi: 10.1002/da.20727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thapar A, Collishaw S, Pine DS, Thapar AK. Depression in adolescence. Lancet. 2012;379:1056–1067. doi: 10.1016/S0140-6736(11)60871-4. [DOI] [PMC free article] [PubMed] [Google Scholar]