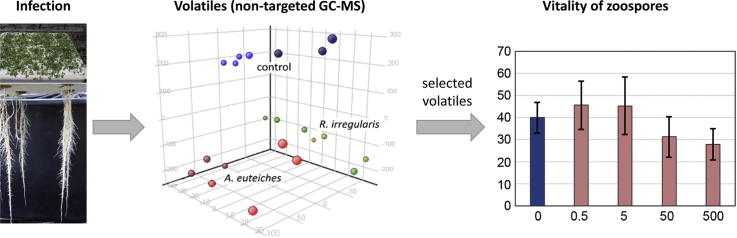
Graphical abstract
Using non-targeted metabolite profiling, we show that Medicago truncatula roots grown in an aeroponic cultivation system emit specific volatiles in response to two soil-born microorganisms, the symbiont Rhizophagus irregularis and the pathogen Aphanomyces euteiches.
Keywords: Arbuscular mycorrhiza, Oomycetous pathogen, Volatile organic compound (VOC), Aeroponic cultivation, Gas chromatography–mass spectrometry (GC–MS)
Highlights
-
•
Medicago roots emit volatiles as an early response to microorganisms (MOs).
-
•
More than 90 compounds were released from roots as detected by untargeted GC–MS.
-
•
Principal component analyses clearly distinguished untreated roots from treated roots.
-
•
Several volatiles were found to be emitted specifically in response to symbiotic or pathogenic MOs.
-
•
Plants discriminate between friend and foe at very early stages of their interaction.
Abstract
Plants are in permanent contact with various microorganisms and are always impacted by them. To better understand the first steps of a plant’s recognition of soil-borne microorganisms, the early release of volatile organic compounds (VOCs) emitted from roots of Medicago truncatula in response to the symbiont Rhizophagus irregularis or the pathogenic oomycete Aphanomyces euteiches was analysed. More than 90 compounds were released from roots as detected by an untargeted gas chromatography-mass spectrometry approach. Principal component analyses clearly distinguished untreated roots from roots treated with either R. irregularis or A. euteiches. Several VOCs were found to be emitted specifically in response to each of the microorganisms. Limonene was specifically emitted from wild-type roots after contact with R. irregularis spores but not from roots of the mycorrhiza-deficient mutant does not make infections3. The application of limonene to mycorrhizal roots, however, did not affect the mycorrhization rate. Inoculation of roots with A. euteiches zoospores resulted in the specific emission of several sesquiterpenes, such as nerolidol, viridiflorol and nerolidol-epoxyacetate but application of nerolidol to zoospores of A. euteiches did not affect their vitality. Therefore, plants discriminate between different microorganisms at early stages of their interaction and respond differently to the level of root-emitted volatiles.
Introduction
As sessile organisms, plants cannot avoid attacks by invaders via rapid relocation. To cope with microorganisms, plants produce diverse metabolites, which have a wide range of toxicological characteristics and may act as repellents and deterrents [1]. Therefore, the so-called secondary metabolites are highly beneficial for plants since they mediate interactions with other organisms [2]. Even roots may affect the microbes in the rhizosphere and the soil via the exudation of secondary compounds, leading to either repelling herbivores and pathogens or attracting symbionts, such as arbuscular mycorrhizal (AM) fungi [2].
Plants are able to produce more than 100,000 chemical products, nearly two percent of which are volatile [3]. Volatile organic compounds (VOCs) are defined as compounds with vapour pressure leading to vaporization under normal environmental conditions. VOCs are also involved in the plant’s reaction to a wide range of biotic or abiotic factors [4]. Most reported VOCs are lipophilic products with molecular masses below 300 Da and are mainly assigned to different compound classes, such as various nitrogen- and sulphur-containing compounds as well as terpenoids, fatty acid derivatives, benzenoids and phenylpropanoids [5]. VOCs emitted by plant shoots and flowers have been extensively studied [3]. However, the VOCs released from root systems in response to environmental stresses are expected to be as common as above-ground VOCs [6].
The model plant Medicago truncatula belongs to the family Fabaceae, which is the third largest higher plant family and consists of more than 700 genera including 20,000 species [7]. As a legume, M. truncatula is a model plant for research on plant-microbe interactions since it can establish mutualistic interactions with nitrogen-fixing rhizobacteria and AM fungi, such as Rhizophagus irregularis, but can also be attacked by pathogens, such as the oomycete Aphanomyces euteiches. As a mutualistic symbiosis, the interaction of M. truncatula with AM fungi leads to improved fitness for both interacting partners [8]. The host plant supplies the obligate biotrophic fungus with photoassimilates, such as hexoses and fatty acids [9]. In return, the AM fungus supports the plant by mobilizing nutrients, mainly phosphate and nitrogen, and water from the soil [10]. There are three distinct growth phases of AM fungi: (i) the asymbiotic phase, where AM fungal spores germinate in absence of a plant; (ii) the pre-symbiotic phase characterized by hyphae branching under the influence of plant-derived signals; and (iii) the symbiotic phase, which starts with the formation of hyphopodia on epidermal root cells and is followed by the plant-facilitated penetration of the root by fungal hyphae, the formation of arbuscules and vesicles, and ends in the formation of secondary spores [11].
The oomycete A. euteiches is a pathogen of legumes and several weed species [12]. It causes root rot diseases and is the most limiting factor for pea production in the USA and Europe [13]. The life cycle of A. euteiches includes asexual and sexual stages [13]. Adjacent to a host root, oospores form a germ tube and a terminal zoosporangium, which releases hundreds of primary zoospores. These oospores are motile and adhere to the host tissue, where they develop coenocytic hyphae. These hyphae then penetrate the root and differentiate into haploid antheridia and oogonia, later producing diploid oospores [13].
Roots respond to the early contact of microorganisms, as shown by the common symbiotic signalling pathway (CSSP) for symbiotic interactions [11] and the recognition of pathogen-associated molecular patterns (PAMPs) by specific receptors for pathogenic interactions [14]. However, knowledge of the volatiles emitted by M. truncatula roots as an early response to contact with a symbiont or pathogen is lacking. In the present work, an untargeted approach was applied to monitor the volatiles released by M. truncatula roots after treatment with spores of R. irregularis or zoospores of A. euteiches. We expected that M. truncatula roots specifically release volatiles upon contact with each of the microorganisms, suggesting a microorganism-specific VOC response.
Material and methods
Plant material and growth conditions
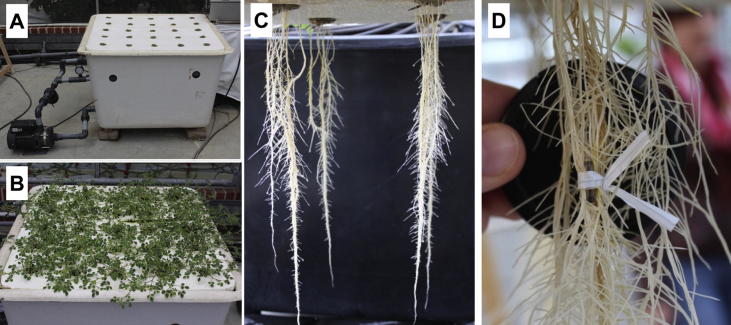
The M. truncatula cv. Jemalong A17 wild type and the isogenic mycorrhiza-deficient mutant does not make infections3 (dmi3) [15] were used for all the experiments. Seeds were scarified using concentrated anhydrous sulphuric acid for 5 min, followed by intensive washing steps with distilled water. The seeds were then placed in sterile petri dishes containing filter paper and 4 mL sterile H2O and incubated at 12 °C for 2 days. For aeroponic cultivation, seedlings were inserted into wetted rock wool located in holes within a Styrofoam plate (Fig. 1A). The plate was placed over an aeroponic cultivation system filled with modified Strullu-Romand (MSR) medium [16] without vitamins and sucrose. The aeroponic container provided a plant growth system in which plant roots grew in the air and were frequently sprayed with MSR medium pumped through nozzles. Plants were cultivated in a greenhouse under long-day conditions with a light period of 16 h at 26 °C and a dark period of 8 h at 20 °C, both with 40% humidity. Three to four weeks after seedling transfer, the nutrient solution was exchanged. Plants were grown for 6 weeks until well-grown shoots and root systems developed (Fig. 1B, C). After collection of volatiles (see below), roots were harvested and checked for infections by staining with wheat-germ-agglutinin coupled to AlexaFluor488 (Life Technologies, Darmstadt, Germany) followed by microscopic analysis according to [17] and revealed no further infection except the applied microorganisms. To establish mycorrhiza, seedlings were transferred into expanded clay (Original Lamstedt Ton; Fibo ExClay, Lamstedt, Germany), inoculated and cultivated as described previously [18].
Fig. 1.
The aeroponic growth system. (A) Container coupled with a pump and equipped with a plate for insertion of plants. (B) M. truncatula shoots in the container after 6 weeks of cultivation. (C) Well-developed root systems after 6 weeks of cultivation. (D) Insertion of absorbing sticks to collect volatiles.
Cultivation of R. irregularis and A. euteiches
R. irregularis DAOM197198 was cultivated with Aphanomyces rhizogenes-transformed carrot roots in a two-compartment petri-dish system as described previously [19]. After 6–10 months of growth, spores were collected by dissolving agar pieces in 0.01 M citrate buffer (pH of 6) at room temperature and with slow shaking in the dark overnight. Spores were washed twice with water and used directly for inoculation. For mycorrhization in expanded clay, R. irregularis was propagated on Allium porrum plants grown in expanded clay for several months. The substrate containing spores and hyphae was then used for inoculation as described previously [20].
A. euteiches strain GBI1 was routinely sub-cultured on 1.7% (w/v) corn meal agar (CMA) (Sigma-Aldrich, Taufkirchen, Germany) in the dark at 24 °C. To produce zoospores, 15–20 mycelial explants from 10 to 15-day-old CMA plates were immersed in a 1:3 mixture of yeast tryptone medium (0.3% w/v yeast extract, 0.5% w/v tryptone) and sterile swamp water in the dark at 24 °C. After 3 days of growth, mycelial mats were washed several times with sterile tap water, re-suspended in fresh tap water and incubated overnight for the release of zoospores, which were then counted in a counting chamber. To determine the vitality of A. euteiches zoospores after the application of nerolidol (Sigma-Aldrich, Taufkirchen, Germany), freshly propagated primary zoospores were suspended in water containing different concentrations of nerolidol (tenfold dilutions from 0.5 µM to 0.5 nM) and incubated for 15 min. Incubations without nerolidol (0 nM) served as a control. The number of vital (mobile) zoospores was then determined using the counting chamber as described above.
Inoculation of aeroponically grown plants and collection of volatiles
Three different aeroponic containers, each containing 25 plants, were used. Plants of one container served as a control and remained untreated, whereas roots of the second and third containers were treated with R. irregularis spores and A. euteiches zoospores, respectively. One-third of the R. irregularis spores in a mature hairy root culture plate harvested as described above (approx. 1500 spores) were used to inoculate one root system. To inoculate with A. euteiches, approximately 50,000 zoospores were applied per root system. Before inoculation, Twisters (GERSTEL Twisters™, polydimethylsiloxane [PDMS] phase [0.5 mm film thickness, 1 cm length], Gerstel GmbH & Co. KG, Mühlheim a.d.R., Germany) were fixed in each root system to collect volatiles emitted from roots [21] (Fig. 1D). One Twister was used per plant root system, and three were combined for one biological replicate, leading to a total of three and four biological replicates per treatment of wild-type and dmi3 plants, respectively. All inoculations were performed at 8 pm. Twisters were collected for gas chromatography-mass spectrometry (GC–MS) analysis 12 h after inoculation.
Analysis of volatiles by GC–MS
The analysis of volatiles collected by Twister sorptive extraction bars was performed with an Agilent 6890 Series gas chromatograph (Agilent Technologies, Santa Clara, CA, USA) equipped with an Agilent 5973 Network mass detector controlled by a GC-MSD ChemStation (G1701EA). Volatiles were separated on a BP5MS column (30 m × 250 μm i.d., 0.25 μm; SGE Analytical Sciences by Trajan Scientific and Medical, Ringwood, Victoria, Australia) using the following oven temperature programme: a 40 °C isotherm for 3 min, an increase of 2 °C/min until 60 °C, holding for 2 min, an increase of 3 °C/min until 180 °C, and holding for 10 min. Helium was used as a carrier gas at a constant flow rate of 1.2 mL/min. The gas chromatograph was equipped with a Gerstel multiple purpose sampler (MPS) 2 injection system and a Gerstel thermal desorption unit (TDU). The cryofocusing was performed at −100 °C, and the temperature was then increased by 12 °C/min to 280 °C and held for 3 min. Thermodesorption was performed using the following temperature programme: a starting temperature of 25 °C, an increase of 100 °C/min to 260 °C, and holding for 4 min. The scan range was set to 50–300 m/z at 70 eV. The source temperature was set to 230 °C, and the quadrupole temperature was set to 150 °C.
The raw data from the GC analysis were de-convoluted (GC–MS Translator, Agilent Technologies, Santa Clara, CA, USA,) and processed by Mass Profiler Professional (MPP; Version 12.1, Agilent Technologies, Santa Clara, CA, USA). Compounds with a minimum of 70% abundance in all samples of one treatment were subjected to statistical analysis, which included one-way ANOVA followed by Tukey’s honestly significant difference (HSD) post hoc test (P ≤ 0.01; fold change ≥2). Putative identification of compounds with significantly different levels was performed by comparing the mass spectra with the Wiley 6 L and NIST 98 L libraries. The identity of the following compounds was verified by co-injection with authentic reference compounds (all with a purity of >98%): hexanal, nerolidol, limonene (Sigma-Aldrich, Taufenkirchen, Germany), and β-pinene (Lancaster Synthesis, Ward Hill, MA, USA). For statistical evaluation of differences in the levels of identified/annotated compounds, a two-factorial ANOVA followed by Tukey’s HSD post hoc test (P ≤ 0.05) was applied.
Treatment of plants with limonene and determination of mycorrhization rate by qRT-PCR
Plants inoculated with R. irregularis and grown in expanded clay as described above were treated with 10 mL 1 nM limonene (purity of >98%, Sigma-Aldrich, Taufkirchen, Germany) solution twice per week. Control plants remained untreated. Three weeks post inoculation, roots were harvested and subjected to RNA isolation using an RNeasy Plant Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. To determine the mycorrhization rate, transcript levels of the R. irregularis housekeeping gene β-tubulin (Riβ-TUB) were determined in relation to the plant housekeeping gene encoding Elongation Factor 1α (MtEF1α) as previously described [18].
Results and discussion
Secondary metabolites emitted by roots are important and may influence the associations between plants and different species of soil-borne microorganisms [3]. Plants might be dependent on the interaction with such microbes or have to repel them and therefore secrete specific compounds to communicate with their environment [22]. However, the secondary metabolites that are exuded or emitted by M. truncatula as a specific reaction to contact with different types of microbes, such as symbionts or pathogens, are still unknown. The experiments of this study provide the first insight into the composition of VOCs emitted by M. truncatula roots 12 h after treatment with spores of the symbiont R. irregularis or zoospores of the pathogen A. euteiches. To relate the differences in volatile emission to the specific contact with spores of the AM fungus, a mycorrhiza-deficient mutant was included in the study. The mutant dmi3 is deficient in the CSSP due to a defect in a Ca2+/calmodulin-dependent protein kinase, which functions in the Nod- or Myc-factor-specific signal transduction pathway downstream of calcium spiking [15]. Therefore, dmi3 mutant plants fail to respond to inoculation with AM fungi, and VOCs that are specifically emitted after contact with spores of the AM fungus should be missing in the volatile blend emitted by roots of dmi3 plants.
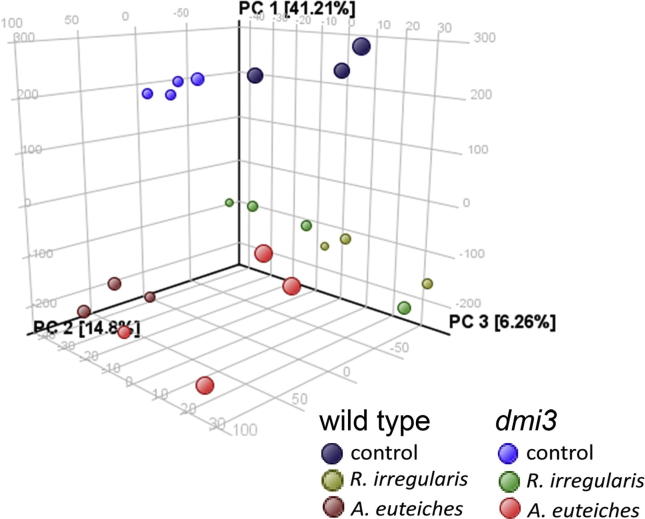
Volatiles emitted from roots of aeroponically grown M. truncatula plants (both genotypes) after inoculation with either R. irregularis or A. euteiches were analysed using GC–MS. Differentially occurring compounds were those showing a minimum abundance of 70% per sample group and a two-fold change with a P-value of 0.05 according to one-way ANOVA. Applying these criteria, 93 compounds were detected that showed differential appearance upon treatment of roots with either R. irregularis or A. euteiches. A principal component analysis (PCA) of all samples showed that genotype had only minor effects on the root volatile composition, in contrast to the treatment (Fig. 2). The PCA revealed that component one contributed 41.2% to the sample distribution, whereas components 2 and 3 contributed 14.8 and 6.26%, respectively. Among the detected compounds, 10 compounds could be identified, either by comparison of MS spectra in available library databases or to identical standards.
Fig. 2.
Principal component analysis (PCA) of volatiles released by roots of M. truncatula. Roots of 6-week-old plants (wild type and dmi3) were inoculated with either spores of R. irregularis or zoospores of A. euteiches or left untreated (control) for 12 h. For PCA, compounds showing at least 70% abundance per treatment were included. Statistical analysis was performed by one-way ANOVA with P = 0.05 and a fold change >2.
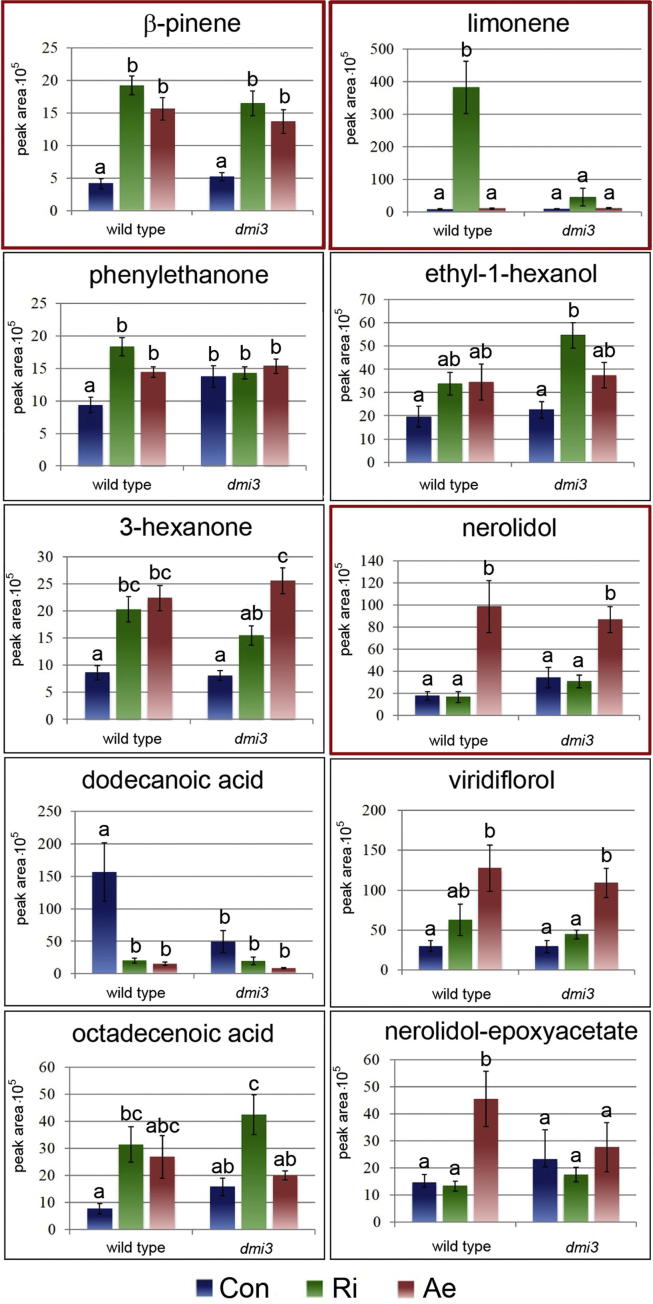
Relative quantification of the identified compounds by calculation of peak areas revealed 10 volatiles with significant differences in at least one of the two treatments of wild-type plants (Fig. 3). Due to the experimental set-up it was not possible to determine, however, whether R. irregularis spores and A. euteiches zoospores also emit compounds that might be measured as compounds released by treated roots. Although most compounds exhibited higher emission from roots treated with microorganisms than from untreated roots, there are also several compounds that decreased after treatment or were emitted from roots of only one of the plant genotypes used. Moreover, the relation between root biomass and spore biomass may also speak against detection of compounds released by (zoo)spores. All these facts point to the fact that the measured compounds are released by M. truncatula roots.
Fig. 3.
Mean peak area of gas chromatography-mass spectrometry (GC–MS)-measured volatile compounds. Volatiles were collected for 12 h after the application of spores of R. irregularis or zoospores of A. euteiches to roots of M. truncatula grown in an aeroponic system for six weeks. Annotation was performed using a library, and the identity of the substances framed in red was verified using standard compounds. Bars indicate means ± SDs; n = 9–12. Different letters show significant differences determined by two-factorial ANOVA followed by Tukey’s HSD post hoc test (P ≤ 0.05).
The exudation of β-pinene, phenylethanone, 3-hexanone and octadecanoid acid was strongly induced after both treatments (Fig. 3). The emission of the monoterpene β-pinene might be a general response of plants to the presence of microorganisms since a potential antimicrobial effect of β-pinene was shown [23]. In addition, β-pinene might be a direct signal for intra- and inter-plant communication and activate defense responses within a plant or between different plants [24]. The emission of dodecanoic acid was, however, drastically reduced upon treatment with (zoo)spores of the two microbes (Fig. 3). Moreover, the emission of dodecanoic acid from non-treated wild-type roots was lower than that from untreated roots of dmi3. This low level was even lower in dmi3 roots after contact with pathogenic zoospores. This finding indicated a strong regulatory role of DMI3 in the emission of dodecanoic acid in non-inoculated roots. However, when roots were inoculated with one or the other microbe, its role appears to be rather weak.
In addition to the compounds that were regulated by contact with both microorganisms, there were several compounds that showed increased emission specifically upon treatment with either R. irregularis (limonene) or A. euteiches (nerolidol, nerolidol-epoxyacetate, and viridiflorol) (Fig. 3). Interestingly, the monoterpene limonene increased after treatment with R. irregularis spores but did not show increased emission from dmi3 roots after treatment with R. irregularis. This result indicates the DMI3-dependent regulation of limonene biosynthesis and/or emission. In contrast, enhanced emission of the primary alcohol ethyl-1-hexanol was detectable from only dmi3 plants treated with spores of R. irregularis, suggesting that the CSSP might be involved in the suppression of ethyl-1-hexanol synthesis. The level of ethyl-1-hexanol emission after pathogen attack was, however, independent of genotype. It is tempting to speculate that ethyl-1-hexanol exhibits overall antimicrobial activity and that its production is specifically suppressed by the AM fungus to promote mycorrhizal interaction.
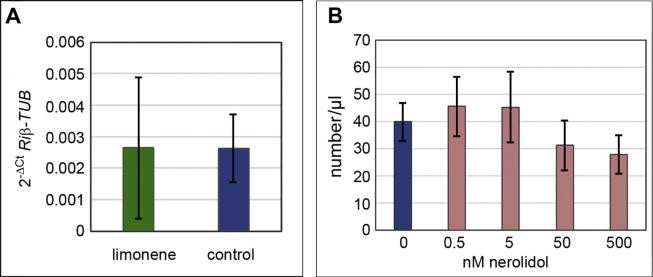
The mycorrhiza-dependent regulation of limonene biosynthesis led to the hypothesis that limonene might act as an attractant for AM fungi. To determine whether limonene affects the mycorrhization of M. truncatula, limonene was repeatedly applied to mycorrhizal M. truncatula plants. As quantified by Riß-Tub, no differences in mycorrhizal colonization were detectable (Fig. 4A). Therefore, treatment of mycorrhizal roots with limonene did not increase the rate of root colonization, at least under the conditions used in this study. Whether a different concentration of limonene could promote mycorrhization remains to be clarified. Moreover, these findings do not exclude the fact that upon interaction with an AM fungus, roots synthesize and exude the optimal level of limonene, and an additional supply does not affect its impact. Nevertheless, a possible effect of limonene via the alteration of fungal hyphae growth or branching should be analysed in detail after limonene application to in vitro-grown R. irregularis.
Fig. 4.
Application of limonene and nerolidol to mycorrhizal M. truncatula and A. euteiches zoospores, respectively. (A). Mycorrhizal M. truncatula plants were treated with 1 nM limonene twice per week, whereas control plants remained untreated. After 3 weeks, mycorrhizal colonization was determined by quantification of transcript levels of Riβ-Tub using qRT-PCR. (B). Number of vital A. euteiches zoospores treated for 15 min with different concentrations of nerolidol. Bars indicate means ± SDs; n = 9 in A, and n = 3 in B. Student’s t-test revealed no significant differences among the treatments.
The sesquiterpenes nerolidol and viridiflorol as well as nerolidol-epoxyacetate accumulated specifically after treatment of roots with A. euteiches independently of DMI3 (Fig. 3). This finding points to an involvement in the general response of M. truncatula roots to contact with pathogenic microorganisms. To test whether these sesquiterpenes may have antimicrobial activity, freshly harvested A. euteiches zoospores were incubated with nerolidol, and their vitality was determined (Fig. 4B). However, the numbers of vital zoospores after application of various concentrations of nerolidol did not differ. Nerolidol is known to be emitted by plants that are under herbivore attack [25], but an antimicrobial effect has not yet been described and could also not be shown here regarding zoospores of A. euteiches. However, the mode of action of nerolidol may be indirect, e.g., nerolidol might affect zoospore germination or might disrupt quorum sensing, a communication tool that was originally described in bacteria that coordinates growth processes, such as biofilm formation [26]. Quorum sensing has also been described in fungi, such as Candida albicans, where filamentation and biofilm formation are controlled by the sesquiterpene alcohol farnesol [27].
Conclusions
Plants reacted differently to contact with different types of microorganisms. By analysing the level of volatiles emitted from roots, different compositions of VOCs were found to be released in response to either spores of an AM fungus or zoospores of a pathogen. Several substances were induced by both microorganisms, but few volatiles were found to be specifically emitted in response to the symbiont or pathogen. This on the one hand general and on the other hand specific VOC emission in response to different organisms could be based on the action of different receptors at the plasma membrane, such as receptors for specific factors of AM fungi leading to CSSP activation and receptors that recognize general fungal presence inducing a PAMP-induced defence response. The resulting crosstalk of the signalling pathways might help the plant to discriminate between friend (symbiont) and foe (pathogen) and enforce either the mutualistic interaction or defence reactions.
Conflicts of interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Acknowledgements
Natalia Requena (KIT Karlsruhe, Germany) and Philipp Franken (IGZ Großbeeren, Germany) are acknowledged for providing Rhizophagus irregularis DAOM197198 and Aphanomyces euteiches strain GBI1, respectively. We thank Helge Küster (Leibniz University Hannover, Germany) for providing seeds of dmi3 and Ramona Schubert (IPB Halle) for help with statistics. This work was funded by a grant in the SAW-PAKT “Chemical Communication in the Rhizosphere” framework provided by the Leibniz Association.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Wink M. Evolution of secondary metabolites in legumes (Fabaceae) South African J Bot. 2013;89:164–175. [Google Scholar]
- 2.van Dam N.M., Bouwmeester H.J. Metabolomics in the rhizosphere: Tapping into belowground chemical communication. Trends Plant Sci. 2016;21:256–265. doi: 10.1016/j.tplants.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Dudareva N., Klempien A., Muhlemann J.K., Kaplan I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013;198:16–32. doi: 10.1111/nph.12145. [DOI] [PubMed] [Google Scholar]
- 4.Loreto F., Schnitzler J.-P. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010;15:154–166. doi: 10.1016/j.tplants.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Dudareva N., Pichersky E., Gershenzon J. Biochemistry of plant volatiles. Plant Physiol. 2004;135:1893–1902. doi: 10.1104/pp.104.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erb M., Lenk C., Degenhardt J., Turlings T.C.J. The underestimated role of roots in defense against leaf attackers. Trends Plant Sci. 2009;14:653–659. doi: 10.1016/j.tplants.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Doyle J.J., Luckow M.A. The rest of the iceberg. Legume diversity and evolution in a phylogenetic context. Plant Physiol. 2003;131:900–910. doi: 10.1104/pp.102.018150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonfante P., Genre A. Plants and arbuscular mycorrhizal fungi: an evolutionary-developmental perspective. Trends Plant Sci. 2008;13:492–498. doi: 10.1016/j.tplants.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Rich M.K., Nouri E., Courty P.-E., Reinhardt D. Diet of arbuscular mycorrhizal fungi: Bread and butter? Trends Plant Sci. 2017;22:652–660. doi: 10.1016/j.tplants.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Smith S.E., Read D.J. 3rd ed. Academic Press; London: 2008. Mycorrhizal symbiosis. [Google Scholar]
- 11.Parniske M. Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nature Rev Microbiol. 2008;6:763–775. doi: 10.1038/nrmicro1987. [DOI] [PubMed] [Google Scholar]
- 12.Gaulin E., Jacquet C., Bottin A., Dumas B. Root rot disease of legumes caused by Aphanomyces euteiches. Mol Plant Pathol. 2007;8:539–548. doi: 10.1111/j.1364-3703.2007.00413.x. [DOI] [PubMed] [Google Scholar]
- 13.Hughes T, Teresa J, Grau C. Aphanomyces root rot (common root rot) of legumes. https://www.apsnet.org/edcenter/intropp/lessons/fungi/Oomycetes/Pages/Aphanomyces.aspx; 2014.
- 14.Nurnberger T., Kemmerling B. Receptor protein kinases - pattern recognition receptors in plant immunity. Trends Plant Sci. 2006;11:519–522. doi: 10.1016/j.tplants.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Mitra R.M., Gleason C.A., Edwards A., Hadfield J., Downie J.A., Oldroyd G.E.D. A Ca2+/calmodulin-dependent protein kinase required for symbiotic nodule development: Gene identification by transcript-based cloning. Proc Natl Acad Sci USA. 2004;101:4701–4705. doi: 10.1073/pnas.0400595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Declerck S, Strullu D, Fortin A, editors. In vitro culture of mycorrhizas. In: Soil biology series, vol. 4. Berlin Heidelberg New York: Springer; 2005.
- 17.Javot H., Pumplin N., Harrison M.J. Phosphate in the arbuscular mycorrhizal symbiosis: transport properties and regulatory roles. Plant, Cell Environ. 2007;30:310–322. doi: 10.1111/j.1365-3040.2006.01617.x. [DOI] [PubMed] [Google Scholar]
- 18.Hilou A., Zhang H., Franken P., Hause B. Do jasmonates play a role in arbuscular mycorrhiza-induced local bioprotection of Medicago truncatula against root rot disease caused by Aphanomyces euteiches? Mycorrhiza. 2014;24:45–54. doi: 10.1007/s00572-013-0513-z. [DOI] [PubMed] [Google Scholar]
- 19.St-Arnaud M., Hamel C., Vimard B., Caron M., Fortin J.A. Enhanced hyphal growth and spore production of the arbuscular mycorrhizal fungus Glomus intraradices in an in vitro system in the absence of host roots. Mycol Res. 1996;100:328–332. [Google Scholar]
- 20.Schaarschmidt S., González M.-C., Roitsch T., Strack D., Sonnewald U., Hause B. Regulation of arbuscular mycorrhization by carbon. The symbiotic interaction cannot be improved by increased carbon availability accomplished by root-specifically enhanced invertase activity. Plant Physiol. 2007;143:1827–1840. doi: 10.1104/pp.106.096446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Errard A., Ulrichs C., Kühne S., Mewis I., Drungowski M., Schreiner M. Single- versus multiple-pest infestation affects differently the biochemistry of tomato (Solanum lycopersicum ‘Ailsa Craig’) J Agric Food Chem. 2015;63:10103–10111. doi: 10.1021/acs.jafc.5b03884. [DOI] [PubMed] [Google Scholar]
- 22.Broeckling C.D., Broz A.K., Bergelson J., Manter D.K., Vivanco J.M. Root exudates regulate soil fungal community composition and diversity. App Environl Microbiol. 2008;74:738–744. doi: 10.1128/AEM.02188-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soković M., Glamočlija J., Marin P.D., Brkić D., Griensven L.J.L.D.V. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules. 2010;15:7532. doi: 10.3390/molecules15117532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heil M., Silva Bueno J.C. Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc Natl Acad Sci USA. 2007;104:5467–5472. doi: 10.1073/pnas.0610266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Degenhardt J., Gershenzon J. Demonstration and characterization of (E)-nerolidol synthase from maize: a herbivore-inducible terpene synthase participating in (3E)-4,8-dimethyl-1,3,7-nonatriene biosynthesis. Planta. 2000;210:815–822. doi: 10.1007/s004250050684. [DOI] [PubMed] [Google Scholar]
- 26.Abisado R.G., Benomar S., Klaus J.R., Dandekar A.A., Chandler J.R. Bacterial quorum sensing and microbial community interactions. mBio. 2018;9 doi: 10.1128/mBio.02331-17. e02331-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albuquerque P., Casadevall A. Quorum sensing in fungi – a review. Med Mycol. 2012;50:337–345. doi: 10.3109/13693786.2011.652201. [DOI] [PMC free article] [PubMed] [Google Scholar]