Abstract
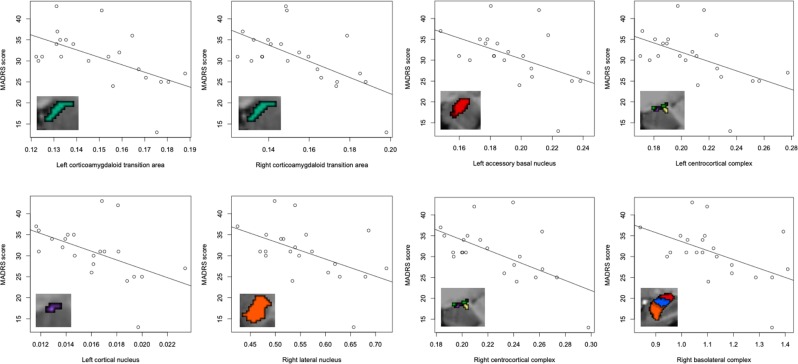
Subcortical volumetric changes in major depressive disorder (MDD) have been purported to underlie depressive symptomology, however, the evidence to date remains inconsistent. Here, we investigated limbic volumes in MDD, utilizing high-resolution structural images to allow segmentation of the hippocampus and amygdala into their constituent substructures. Twenty-four MDD patients and twenty matched controls underwent structural MRI at 7T field strength. All participants completed the Montgomery-Asberg Depression Rating Scale (MADRS) to quantify depressive symptomology. For the MDD group, volumes of the amygdala right lateral nucleus (p = 0.05, r2 = 0.24), left cortical nucleus (p = 0.032, r2 = 0.35), left accessory basal nucleus (p = 0.04, r2 = 0.28) and bilateral corticoamygdaloid transition area (right hemisphere p = 0.032, r2 = 0.38, left hemisphere p = 0.032, r2 = 0.35) each displayed significant negative associations with MDD severity. The bilateral centrocortical (right hemisphere p = 0.032, r2 = 0.31, left hemisphere p = 0.032, r2 = 0.32) and right basolateral complexes (p = 0.05, r2 = 0.24) also displayed significant negative relationships with depressive symptoms. Using high-field strength MRI, we report the novel finding that MDD severity is consistently negatively associated with amygdala nuclei, linking volumetric reductions with worsening depressive symptoms.
Subject terms: Cognitive neuroscience, Predictive markers, Amygdala
Introduction
Major depressive disorder (MDD) is a chronic and debilitating psychiatric illness characterized by numerous of symptoms, including, low mood, anhedonia, changes in appetite, hyper- or hyposomnia, concentration difficulties and suicidality1. At present, MDD is one of the leading causes of disability worldwide, with reports suggesting that one in six adults will suffer from MDD at some point during their lifetime2. MDD has a complex etiology, with a complex multifactorial interaction of genetic, neurobiological, and environmental components likely contributing to the individual differences seen in clinical liability and depressive symptomology1. To illustrate, molecular genetics investigations report narrow-sense heritability estimates of 37%3,4, suggesting a significant genetic component. Epidemiological research has found strong association between MDD and factors such as socioeconomic status, environmental stressors and neuroticism5,6. Further evidence from neurobiological studies suggests that the temporal limbic system is critical to the onset of MDD, due to its importance in emotion regulation, stress response, enhanced plasticity, and its sensitivity to MDD polygenic risk7–11.
Reported volumetric data focused on the hippocampal and amygdala structures in MDD have been notably inconsistent. Larger12,13, smaller14, and no significant differences15 in limbic volumes have all been reported in MDD cohorts compared to control subjects. Where amygdala findings appear to be more diverse, meta-analyses of large datasets point to reduced hippocampal grey matter volume being a commonly replicated finding in MDD16,17. Moreover, smaller hippocampi in major depression have frequently been demonstrated to be moderated by age of onset17–19. Heterogeneity within and between sample groups and significant confounding factors have been cited as reasons for disparate findings. One such confounding factor is the varying proportions of depressed patients currently taking antidepressant medication at the time of study20. Findings of hippocampal and amygdala volumetric deficits at the hippocampus and amygdala in MDD have often been attributed to excitotoxicity from elevated subcortical metabolic activity, which is consistent with increased activation at the amygdala and anterior cingulate cortex during negative stimuli processing on functional MRI21,22. In animal studies, depressive and stressed phenotypes are associated with reduced hippocampal neurogenesis, brain-derived neurotrophic factor (BDNF) levels and dendritic branching23–25 and antidepressant medication has been shown to inhibit these stress-induced processes while promoting neurogenesis26. Immunohistochemistry carried out on human hippocampi revealed a significantly increased number of neural progenitor cells and capillary area in dentate gyri of MDD patients on serotonin re-uptake inhibitor medication compared to both control subjects and unmedicated MDD patients, and furthermore that dentate gyrus volume was directly correlated with neural progenitor cell numbers27. Longitudinal assessment in humans has also shown that citalopram treatment over an 8 week period is associated with a regional increase in hippocampal grey matter28. Moreover, meta-analyses identified that significant enlargements of the amygdala are specifically associated with positive medication status in MDD patients compared to healthy controls, whereas unmedicated MDD patients exhibited decreased amygdala volumes29.
The amygdala and hippocampal formation are commonly treated as single entities in structural MRI in humans, however ex vivo high resolution human imaging and animal histological studies show that they are comprised of distinct substructures30–32. The amygdala is known to be comprised of multiple nuclei which exhibit differing connectivity and cellular profiles30,32–35, and the hippocampus is comprised of the cornu ammonis (CA), dentate gyrus and subiculum30. These regions have been shown in disease models to react differentially to pathological mechanisms, as well as having diverse functions in non-disease states21,36–38. It is therefore a prescient question in human in vivo MRI investigation which particular amygdala nuclei and hippocampal subfields are affected in major depression, and whether substructures are differentially affected. Much of the existing research on structural MRI in depression has utilized manual tracings of the amygdala and hippocampus8,20,21,39,40. Whilst manual tracing is regarded as the gold standard for anatomical precision and accuracy, individuals may vary in their tracing conventions, meaning comparisons between different investigator groups could be impaired in terms of replicability. Additionally, variability in spatial resolution and slice thickness have been implicated in differences between measurements, as these parameters may affect boundary demarcation of anatomical regions41. With the emergence of automated segmentation techniques, the feasibility and time commitment of investigating subcortical substructures in larger datasets is improved.
To the author’s knowledge, 7 Tesla MRI has not been leveraged to segment the amygdala in any clinical population. Hippocampal segmentation has become a recent prominent focus of ultra high-field MRI, the techniques and possible applications of which have been reviewed by Giuliano et al.42. The present study aimed to expand upon existing findings of whole subcortical volumes in major depression by utilizing the enhanced resolution of ultra high-field strength MRI and a computational segmentation atlas43. Recent findings are suggestive that hippocampal CA1 volume in particular is a potential biomarker of MDD status44. Here, we aimed to examine hippocampal subfield volumes at enhanced field strength, in addition to investigating whether, in a similar manner to subfield susceptibility, the amygdala nuclei displayed subregion-specific changes related to MDD. To minimize the aforementioned methodological issues of previous reports, we limited our population to an MDD patient cohort currently not taking antidepressant medication to investigate the association between (1) hippocampal subfield and amygdala nuclei volume (2) duration of illness and 3) MDD symptom severity. FreeSurfer (http://surfer.nmr.mgh.harvard.edu) automated segmentation of the volumes was used to eliminate intra- and inter-rater bias of manual tracing and maximize reproducibility43.
Methods
Subjects
Twenty-four participants (mean age = 39.6, SD = 10.4, 9 females) with a primary diagnosis of MDD were recruited through the Mood and Anxiety Disorders Program at the Icahn School of Medicine at Mount Sinai to take part in a pilot analysis. Twenty age-matched controls were also recruited (mean age = 39.5, SD = 12.5, 5 females). All participants were English-speaking and between 18 and 65 years of age. Age was not significantly different between groups (p = 1.0). Eligible patients had a primary diagnosis of major depressive disorder, without psychotic features, assessed by the Structured Clinical Interview for DSM-IV disorders (SCID-IV) or the Structured Clinical Interview for DSM-5 Research Version (SCID-5-RV)45,46. They were antidepressant free for at least 4 weeks prior to study participation and were currently experiencing a major depressive episode. No depressed participants had previously undergone electroconvulsive therapy and none were known to have a co-morbid anxiety disorder. Eleven of the MDD participants had undergone unsuccessful antidepressant treatment in their lifetime, 6 of these individuals receiving more than one unsuccessful drug. History of concussion or head injury was unknown for the sample. Healthy controls had no current or lifetime psychiatric disorder as determined by the SCID-IV or SCID-5-RV45,46. Participants with a current diagnosis of obsessive compulsive disorder (OCD), alcohol or substance abuse in the previous year, or lifetime history of a psychotic illness, bipolar disorder, or neurological disease were excluded. Participants with MRI contraindications, unstable medical conditions, or positive urine toxicology on day of scan were also excluded.
Depressive symptomology and severity was assessed using the Montgomery-Asberg Depression Rating Scale (MADRS; range 0–60; higher score indicates greater depression severity). The SCID and MADRS were all administered by trained clinical raters at screening, within 4 weeks of the MRI scan. The depressed sample had a mean duration of illness (current episode) of 80.7 months (SD = 85.3 months) and a mean age of onset of 17.6 years (SD = 10.4). All participants gave fully-informed written consent prior to investigation. This protocol was approved by the local Institutional Review Board, the Human Research Protection Program at the Icahn School of Medicine at Mount Sinai. All methods were performed in accordance with the relevant guidelines and regulations.
MRI acquisition
Structural MRI data was acquired for all participants on a 7 Tesla whole body scanner (Magnetom, Siemens Healthcare, Erlangen, Germany). A SC72CD gradient coil was used with a single coil transmit and a 32-channel head coil (Nova Medical, Wilmington, MA, USA). A T1-weighted MP2RAGE sequence was performed on each participant, with a 0.7 mm × 0.7 mm × 0.7 mm voxel resolution. Field of view (FOV) was 225 × 183, orientation of scan was coronal, repetition time (TR) was 6000 ms and echo time (TE) was 3.62 ms. A coronal-oblique T2-weighted turbo spin echo (T2-TSE) sequence was also obtained for all participants, with a 0.43 mm × 0.43 mm × 2.0 mm voxel resolution. FOV was 222 × 177, orientation of scan was coronal, TR was 9000 ms and TE was 69 ms.
Amygdala and hippocampal segmentations
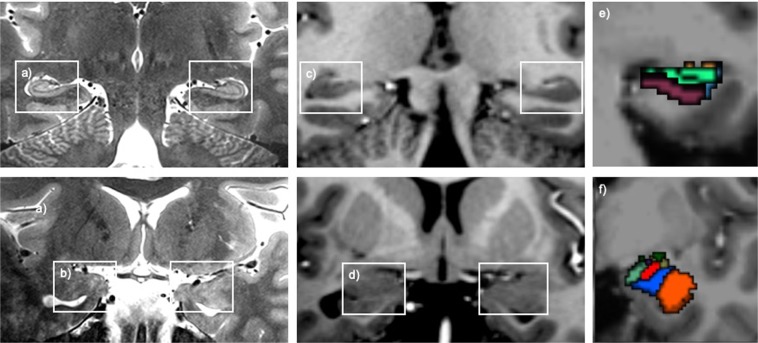
Image reconstruction and automated segmentation of the whole amygdala into subnuclei and the whole hippocampus into subfields was carried out in FreeSurfer (http://surfer.nmr.mgh.harvard.edu) version 6.0. The amygdala segmentation algorithm is based on Bayesian inference, and was developed using ten ex vivo human hemispheres, scanned at 7T field strength with a isotropic spatial resolution of 0.1 mm. Verification of substructures was carried out by a neuroanatomist and the segmentation was validated for performance using the publicly available ADNI and ABIDE neuroimaging datasets43. Similarly, the hippocampal subfield segmentation algorithm is built using Bayesian inference methodology, using data from fifteen 0.13 mm isotropic resolution autopsy scans and was validated using the ADNI dataset47. The segmentation processes use statistical inference to identify subregions of interest. Visual representation of the available level of anatomical detail in the present study is shown in Fig. 1.
Figure 1.
(a) T2-weighted images in the coronal orientation showing visibility of the hippocampus and (b) the amygdala at a resolution of 0.21 × 0.21 mm. (c) T1-weighted images in the coronal orientation showing visibility of the hippocampus and (d) the amygdala at an isotropic resolution of 0.7 mm. (e) Example coronal slice of the hippocampal subfield segmentation and (f) the amygdala nuclei segmentation.
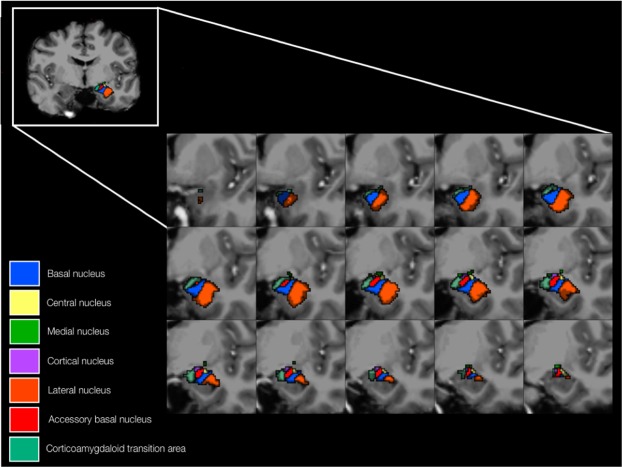
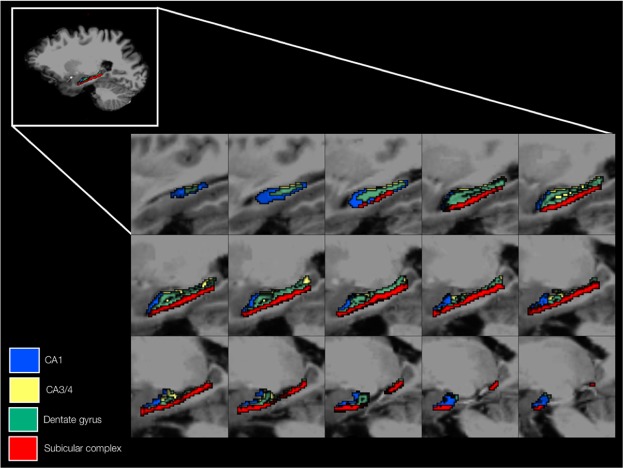
Both T1- and T2-weighted images were utilized to maximize accuracy of the segmentation process. All FreeSurfer outputs were manually inspected for quality, segmentation accuracy and correct co-registration during the analysis. The amygdala was segmented into the lateral, basal, accessory basal, cortical, medial and central nuclei and the corticoamygdaloid transition area (Fig. 2). The superficial structures and the deep structures were also investigated as the centrocortical complex (central, medial and cortical nuclei) and the basolateral complex (basal, lateral and accessory basal nuclei) respectively. The hippocampus was segmented into the subiculum, presubiculum, parasubiculum, CA1, CA3, CA4, the granule cell layer of the dentate gyrus, the molecular layer of the dentate gyrus, the hippocampal-amygdala transition area and the fimbria. Subfields were combined into CA1, CA3/4, the subicular complex (pre-, para- and subiculum) and the dentate gyrus (granule cell layer and molecular layer) to ensure subfield structures were large enough for accurate volume quantification (Fig. 3).
Figure 2.
An anatomical representation of the segmented amygdala nuclei, including the superficial structures (central, medial and cortical nuclei), the deep structures (basal, lateral and accessory basal nuclei) and the corticoamygdaloid transition area. The volumes of the lateral nucleus, cortical nucleus, accessory basal nucleus and corticoamygdaloid transition area displayed significant negative associations with MDD severity.
Figure 3.
An anatomical representation of the hippocampal subfields, with the subiculum, presubiculum, parasubiculum, CA1, CA3, CA4, granule layer of the dentate gyrus and molecular layer of the dentate gyrus grouped into CA1, CA3/4, dentate gyrus and subicular complex regions. Volume metrics of CA1 and CA3/4 exhibited negative associations with MDD severity.
Statistical analysis
Amygdala nuclei and hippocampal subfield volumes were normalized to intracranial volume (ICV). Normalization was favored over covariation in this case to be prudent, as ICV trended towards being lower in the MDD group (p = 0.2). Between group volumetric analyses were carried out using two-tailed independent t-testing, adjusting for age and gender. Linear regression analyses, also controlled for age and gender by the addition of the variables into the linear model as covariates, were performed on the MDD group only. Multiple comparisons were corrected for using false-discovery rate (FDR) on the p value outputs, accounting for separate testing of hippocampal subfields and amygdala nuclei. Orthogonalization was carried out on the data with a principle component analysis implemented using the generic ‘princomp’ function in R, computing eigenvalues and eigenvectors from the correlation matrix derived from the structural data and MADRS scores. All statistical analyses were carried out in R version 3.3.3. (https://www.r-project.org/). Significance level was assumed at p < 0.05 and determination coefficients are adjusted r2.
Results
Amygdala nuclei
Volumetric changes in the amygdala nuclei were t-tested, adjusting for age and gender. We did not find evidence to support the hypothesis that nuclei volume normalized to ICV are different between groups (Table 1).
Table 1.
Between group statistical analysis results for amygdala nuclei, whole amygdala, hippocampal subfields and whole hippocampus volumes.
| Subcortical region | Left hemisphere | Right hemisphere | ||
|---|---|---|---|---|
| p | Cohen’s d | p | Cohen’s d | |
| Corticoamygdaloid transition area | 0.97 | 0.29 | 0.92 | 0.41 |
| Lateral nucleus | 0.95 | 0.27 | 0.97 | 0.35 |
| Basal nucleus | 0.90 | 0.44 | 0.92 | 0.41 |
| Accessory basal nucleus | 0.95 | 0.39 | 0.93 | 0.49 |
| Central nucleus | 0.93 | 0.54 | 0.90 | 0.49 |
| Cortical nucleus | 0.95 | 0.32 | 0.93 | 0.34 |
| Medial nucleus | 0.95 | 0.05 | 0.92 | 0.29 |
| Basolateral complex | 0.84 | 0.36 | 0.79 | 0.40 |
| Centrocortical complex | 0.81 | 0.32 | 0.82 | 0.46 |
| Whole amygdala | 0.84 | 0.35 | 0.81 | 0.17 |
| CA1 | 0.92 | 0.05 | 0.92 | 0.52 |
| CA3/4 | 0.92 | 0.10 | 0.90 | 0.66 |
| Subicular complex | 0.92 | 0.20 | 0.88 | 0.09 |
| Dentate gyrus | 0.92 | 0.72 | 0.90 | 0.39 |
| Whole hippocampus | 0.64 | 0.09 | 0.96 | 0.07 |
Regression analyses in the MDD group, adjusted for age and gender, were used to investigate possible associations between amygdala nuclei volumetrics with MDD episode duration and severity, as quantified by MADRS score (Fig. 4). Significant negative associations of volumetric measurement with MADRS-rated depression symptomatology at both corticoamygdaloid transition areas (left hemisphere p = 0.032, r2 = 0.35, right hemisphere p = 0.032, r2 = 0.38), right lateral (hemisphere p = 0.050, r2 = 0.24), left accessory basal (p = 0.040, r2 = 0.28), left cortical (p = 0.032, r2 = 0.35) were found. When grouped, both centrocortical complexes (left hemisphere p = 0.032, r2 = 0.32, right hemisphere p = 0.032, r2 = 0.31) and the right basolateral complex (p = 0.050, r2 = 0.24) also showed a significant negative relationship with MADRS score. All reported p values are FDR corrected. The right accessory basal nucleus and right basal nucleus also showed associations with MDD severity, however the findings did not remain significant when the analysis was adjusted for age (p = 0.059, r2 = 0.18) and gender (p = 0.073, r2 = 0.16). Mean volumetrics upon grouping into mild, moderate and severe MADRS scores are presented in Supplementary Fig. S1. No significant associations were observed between amygdala nuclei volume and MDD duration. Additional analyses were carried out with ICV as a covariate in the regression model, in place of using normalization based on region of interest to ICV ratio. There were no changes to statistical significance between these two methods of controlling for ICV.
Figure 4.
Regression plots of the identified significant negative associations, surviving FDR correction, between amygdala nuclei and depressive symptoms rated by the MADRS.
Hippocampal subfields
Potential volumetric differences of the hippocampal subfields across study groups were investigated, adjusting for age and gender. There were no significant between group differences identified (Table 1).
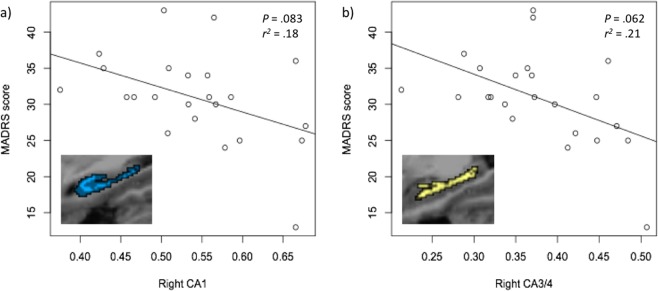
Regression analyses (adjusted for age and gender) revealed associations between MDD severity and volume of the right CA1 subfield and the right CA3/4 subfields, however these did not survive correction for false discovery (p = 0.083, r2 = 0.18 and p = 0.062, r2 = 0.21 respectively) (Fig. 5). Mean volumetrics upon grouping into mild, moderate and severe MADRS scores are presented in Supplementary Fig. S1. There were no significant associations between subfield volumetrics and duration of illness. Analyses adding ICV as a covariate into the regression model instead of using a ratio-based normalization did not influence statistical significance.
Figure 5.
Regression plots of the associations between hippocampal subfields CA1 and combined CA3/4 and depressive symptoms rated by the MADRS.
Principle component analysis
Interdependence of the volumetric nuclei and subfield measurements was assessed using principle component methodology. Variables above average contribution for the first component, where average contribution is defined as 1/length(variables), were left whole amygdala, left lateral nucleus, left basal nucleus, right lateral nucleus, left accessory basal nucleus, left corticoamygdaloid transition area, left subicular complex, right accessory basal nucleus, right CA1, right subicular complex, left CA1, left central nucleus and left dentate gyrus. For component 1, proportion of variance accounted for was 58.1%. Significantly contributing variables to the second component were MADRS score, group, right central nucleus, right CA3/4 and left medial nucleus, accounting for 9.1% of data variance. Component 3 comprised of the right medial nucleus, right central nucleus, right cortical nucleus, MADRS score, group, right accessory basal nucleus and left dentate gyrus, with a proportion of explained variance of 7.3%.
Discussion
To our knowledge, this is the first high-field MRI investigation that leverages enhanced signal from 7T scanning to examine amygdala nuclei and hippocampal subfield volumetrics in Major Depressive Disorder. MDD participants showed significant sensitivity of subcortical subregion volumes to depression severity. However, no significant volumetric changes in MDD compared to controls were identified when investigating hippocampal subfields, amygdala nuclei or whole structures with stringent control for the known effects of age, gender and antidepressant treatment.
Our present high-field imaging analysis of the amygdala shows that in the left hemisphere, the centrocortical complex (driven mainly by the cortical nucleus), the accessory basal nucleus and the corticoamygdaloid transition area are significantly associated with MDD severity. In the right hemisphere, all regions were significantly related to MDD symptomatology, although some nuclei only exhibited correlations when grouped into the basolateral and centrocortical complexes. Moreover, orthogonalization of the data showed that although many subregions of the amygdalo-hippocampal complex are cross-correlated, component loadings did not tally with volumes of significant association with MADRS score. This indicates that the present results do not reflect a general sensitivity of the overall complex to depression severity, but show changes of individual nuclei that are to some degree independent.
The amygdala sub-complexes and nuclei are considered anatomically, functionally, and connectively separate35,48,49, and previous studies of mood disorders and stress in animal models show differential GABAergic system modulatory responses of the amygdala nuclei to specific mood disorder-implicated behaviors, linking the basolateral complex to fear extinction, the medial nuclei to sociability and the central nuclei to anxiety and aggression50–54. Nevertheless, in rat models of stress, markers of neuronal inhibition in the amygdala were reduced uniformly across nuclei compared to controls. Evidence from animal models also shows that multiple amygdala nuclei exhibit associations between grey matter volume and depressive phenotypes55. The results reported here concur with a volumetric sensitivity of many amygdala substructures to depression symptom severity in humans, as opposed to vulnerability of a few specific nuclei. However, the literature of amygdala volume differences and association to depressive symptoms remains inconsistent. It is possible that during the development of the depressive phenotype amygdala volume may follow a trajectory of change which is shown at different relative time points by variation between study populations. This is somewhat supported by our data, as classifying MADRS symptom severities into mild, moderate and severe in our sample revealed that mild and moderate severities frequently exhibit a qualitative increase in regional volumes, which may be a causative factor in why a significant negative association was found between MADRS scores and amygdala nuclei volumes, but no significant between group difference was identified. Additionally, it is plausible that as the increased spatial resolution of this 7T study allows a greater degree of granular separation of amygdala nuclei, it therefore reflects a more detailed picture than amygdala volume as a whole. A previous study of in vivo parcellation of the human amygdala in healthy individuals showed that although the intra-amygdala regions appear mostly uniform at 3T field strength, notably increased signal-to-noise ratio at 7T allows for consistent segmentations of the amygdala based on structural image intensities52. Similarly, a 3T and 7T comparison study concluded that the amygdalo-hippocampal border, formed by cerebrospinal fluid in the temporal horns of the lateral ventricles and the thin sheet of white matter known as the alveus, was visualized with considerably higher detail at increased field strength53.
Regression analyses also suggested a relationship between hippocampal subfields CA1 and CA3/4 and depressive symptom severity, however these results did not survive correction for multiple comparisons. Nevertheless, in a relatively small sample size, this uncorrected association remains promising. Previous studies have found similar relationships at the whole hippocampal structure level, with number of depressive episodes negatively correlating with grey matter volume in the right hippocampus and amygdala using voxel-based morphometry (VBM) methodology56.
Our results suggest that MDD severity has a significant effect on multiple individual amygdala nuclei volumes, in addition to the superficial and deep grouped nuclei structures, and to a lesser degree, hippocampal subfields. A popular theory of the impact of stress and depression on reducing limbic volumes is neural excitotoxicity mediated by hypothalamic pituitary adrenal (HPA) axis hyperactivity and associated reduced levels of BDNF24,25,57,58. BDNF promotes survival, maturation and differentiation of neurons and synaptic connectivity, especially in the basal forebrain and cortex59, its signaling has been implicated in antidepressant efficacy60,61 and BDNF serum reductions have been associated with intense stress states, social defeat, despair behavior and reduced hippocampal neurogenesis and volume62,63. Along with excess corticosterone, it is possible that a combination of diminished neuronal survival promotion and stress-mediated overactivity may lead to a loss of grey matter integrity and volume in the temporal limbic system proportionate to symptomatology.
Phase and total duration of illness have also been shown to be associated with subcortical volumetrics in MDD, with limbic brain regions generally considered to be more structurally affected in persistent forms of MDD64. While early depression is associated with increased amygdala volume, with greater disease duration the amygdala volume declines65,66. The number of neurovascular cells in the accessory basal nucleus of the amygdala also appears to be associated with duration of depression, so that MDD patients with a disease duration of under 5 years exhibit significantly more cells than individuals with an MDD duration of 5 years or more67. Age plays an additional important role in hippocampal and amygdala volumetrics, neurochemistry and functionality, as well as serving as a source of variability in both in MDD and healthy controls68,69. Our results however do not suggest a relationship between any amygdala nuclei or hippocampal subfield volume and duration of depressive episode. This may be due to a lack of sufficient power in the present pilot sample to detect smaller effect sizes. Alternatively, variability within the depressed sample may have masked possible volumetric associations with duration.
Another likely possibility is that vulnerability to mood disorders may be conferred developmentally as a latent trait in hippocampal and amygdala volumetrics70. Animal models showing higher risk endophenotypes for MDD have been linked to hippocampal and amygdala remodeling62. One of the largest studies of amygdala volumes in MDD demonstrated increased volumes in depressed females without family history of MDD compared to depressed females with family history20 and population-based study utilizing polygenic risk has also suggested that hippocampal volume and MDD share a genetic basis7. The possibility of a genetic predisposition to reduced hippocampal and amygdala subvolumes in individuals susceptible to MDD likely mediates the known outcomes of HPA axis dysregulation and BDNF reduction, precipitating an association between decreasing subfield and nuclei size and increasing severity of depressive symptoms.
Despite the statistically stringent methodology used in the present study, some limitations of our results should be noted. Firstly, a small pilot sample was used, restricted in part by increased safety criteria at 7T field-strength. A meta-analysis of subcortical volume alterations in depression reports high sample size necessity for detection of between group differences in hippocampal volume, therefore lack of significant differences in the present study should be interpreted with caution17. Additionally, amygdala nuclei and hippocampal subfields exhibit collinearity which is inherent to brain anatomy (Supplementary Fig. 2), and this may have been a confounding factor in our statistical analysis of the dataset. Nevertheless, due to the survival of many of our significant correlative results after FDR correction, in addition to adjusting for interacting factors, our results remain promising. The clinical evaluation of depressive symptoms utilized a robust scale, the MADRS, which provides a measure of MDD severity71. However, the majority of participants did not fill out the MADRS questionnaire on the day of the scan, which may be considered to be a weakness when examining its associations with structural brain measures. Another limitation is that the duration of illness within our sample was varied, and existing research has suggested the existence of structural differences in the brain in long-term and short-term MDD39. However, when examined as a regressor the current sample showed no association between illness duration and any hippocampal or amygdala region volume, possibly due to the statistical control of age18. Also, though no participants were taking antidepressant medication at the time of scanning and clinical evaluation, it should be noted that a number of participants had taken antidepressant medication in the past, including individuals with a number of trials suggestive of possible treatment-resistant depression, which may or may not of influenced the present findings. Additionally, in vivo structural MRI alone cannot accurately predict biological or neuronal level causes of the identified sensitivity of the amygdala nuclei and hippocampal subfields volumes to depressive symptomatology, therefore potential mechanisms behind our findings should be treated cautiously. To the authors knowledge, the FreeSurfer hippocampal-amygdala segmentation technique has also not been independently validated. Whilst we acknowledge that FreeSurfer segmentations can fluctuate in their robustness and accuracy across field strengths72, the technique applied to the current data was developed at 7T using postmortem tissue and performance was analyzed at 3T on large datasets43. The analysis was therefore deemed suitable for the present 7T images, however further validation of this methodology will be a helpful future addition to the field.
We believe that this study’s strengths eclipse the relative limitations. Specifically, the novel use of ultra high-field 7T MRI to investigate the limbic region of MDD patients offers superior signal and resolution over conventional field strength imaging, enabling improved high resolution at the level of nuclei and subfields. Moreover, the utilization of automated segmentation methods is beneficial for possible future replication and offers some control over investigator bias. In all, we present novel findings that the amygdala nuclei and hippocampal subfields exhibit an almost uniform volumetric reactivity to the severity of depressive symptoms.
Supplementary information
Acknowledgements
NIH RO1 MH109544. NIH R01 CA202911. NARSAD Young Investigator Grant. Icahn School of Medicine Capital Campaign, Translational and Molecular Imaging. Institute and Department of Radiology, Icahn School of Medicine at Mount Sinai, Siemens Healthcare.
Author Contributions
The author’s S.B., P.B. and J.M. contributed substantially to the study conception and design, drafted and revised the article for important intellectual content and gave final approval of the version to be published. S.B. carried out data processing and all statistical analyses. J.R. contributed significantly to data pre-processing. G.V. and R.F. were substantial contributors to the MRI sequence development and data collection. J.A. carried out some aspects of data processing. M.S. made a substantial contribution to the collection of psychiatric and psychological data. B.D. was the expert neuroradiologist for the project, and examined all scans completed for the study.
Data Availability
The datasets generated and analyzed for the current study can be made available from the corresponding author upon reasonable request.
Competing Interests
Dr. Priti Balchandani (the Principal Investigator in this study) is a named inventor on patents relating to magnetic resonance imaging (MRI) and RF pulse design. The patents have been licensed to GE Healthcare, Siemens AG, and Philips international. Dr. Balchandani receives royalty payments relating to these patents. In the past 5 years, Dr. Murrough has provided consultation services to Boehreinger Ingelheim, Sage Therapeutics, FSV7, Novartis, Allergan, Fortress Biotech, Janssen Research and Development, Medavante-Prophase, and Global Medical Education (GME) and has received research support from Avanir Pharmaceuticals, Inc. No other authors report any conflicts of interest.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
J. M. Murrough and P. Balchandani contributed equally.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-46687-7.
References
- 1.Otte C, et al. Major depressive disorder. Nat Rev Dis Primers. 2016;2:16065. doi: 10.1038/nrdp.2016.65. [DOI] [PubMed] [Google Scholar]
- 2.Bromet E, et al. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011;9:90. doi: 10.1186/1741-7015-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geschwind DH, Flint J. Genetics and genomics of psychiatric disease. Science. 2015;349:1489–1494. doi: 10.1126/science.aaa8954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- 5.Navrady LB, et al. Genetic risk of major depressive disorder: the moderating and mediating effects of neuroticism and psychological resilience on clinical and self-reported depression. Psychol Med. 2018;48:1890–1899. doi: 10.1017/S0033291717003415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoebel J, Maske UE, Zeeb H, Lampert T. Social Inequalities and Depressive Symptoms in Adults: The Role of Objective and Subjective Socioeconomic Status. PLoS One. 2017;12:e0169764. doi: 10.1371/journal.pone.0169764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wigmore EM, et al. Do regional brain volumes and major depressive disorder share genetic architecture? A study of Generation Scotland (n = 19 762), UK Biobank (n = 24 048) and the English Longitudinal Study of Ageing (n = 5766) Transl Psychiatry. 2017;7:e1205. doi: 10.1038/tp.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munn MA, et al. Amygdala volume analysis in female twins with major depression. Biol Psychiatry. 2007;62:415–422. doi: 10.1016/j.biopsych.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siegle GJ, Konecky RO, Thase ME, Carter CS. Relationships between amygdala volume and activity during emotional information processing tasks in depressed and never-depressed individuals: an fMRI investigation. Ann N Y Acad Sci. 2003;985:481–484. doi: 10.1111/j.1749-6632.2003.tb07105.x. [DOI] [PubMed] [Google Scholar]
- 10.Anand A, et al. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry. 2005;57:1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 11.Sheline YI, et al. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/S0006-3223(01)01263-X. [DOI] [PubMed] [Google Scholar]
- 12.van Eijndhoven P, et al. Amygdala volume marks the acute state in the early course of depression. Biol Psychiatry. 2009;65:812–818. doi: 10.1016/j.biopsych.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 13.Frodl T, et al. Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biol Psychiatry. 2003;53:338–344. doi: 10.1016/S0006-3223(02)01474-9. [DOI] [PubMed] [Google Scholar]
- 14.Tang Y, et al. Reduced ventral anterior cingulate and amygdala volumes in medication-naive females with major depressive disorder: A voxel-based morphometric magnetic resonance imaging study. Psychiatry Res. 2007;156:83–86. doi: 10.1016/j.pscychresns.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- 16.Wise T, et al. Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: evidence from voxel-based meta-analysis. Mol Psychiatry. 2017;22:1455–1463. doi: 10.1038/mp.2016.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmaal L, et al. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry. 2016;21:806–812. doi: 10.1038/mp.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaworska N, et al. Influence of age of onset on limbic and paralimbic structures in depression. Psychiatry Clin Neurosci. 2014;68:812–820. doi: 10.1111/pcn.12197. [DOI] [PubMed] [Google Scholar]
- 19.Jaworska N, et al. Subgenual anterior cingulate cortex and hippocampal volumes in depressed youth: The role of comorbidity and age. J Affect Disord. 2016;190:726–732. doi: 10.1016/j.jad.2015.10.064. [DOI] [PubMed] [Google Scholar]
- 20.Saleh K, et al. Impact of family history and depression on amygdala volume. Psychiatry Res. 2012;203:24–30. doi: 10.1016/j.pscychresns.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998;9:2023–2028. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- 22.Groenewold NA, Opmeer EM, de Jonge P, Aleman A, Costafreda SG. Emotional valence modulates brain functional abnormalities in depression: evidence from a meta-analysis of fMRI studies. Neurosci Biobehav Rev. 2013;37:152–163. doi: 10.1016/j.neubiorev.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- 24.Covington HE, III., Vialou V, Nestler EJ. From synapse to nucleus: novel targets for treating depression. Neuropharmacology. 2010;58:683–693. doi: 10.1016/j.neuropharm.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yap JJ, et al. Repeated brief social defeat episodes in mice: effects on cell proliferation in the dentate gyrus. Behav Brain Res. 2006;172:344–350. doi: 10.1016/j.bbr.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 26.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 27.Boldrini M, et al. Hippocampal angiogenesis and progenitor cell proliferation are increased with antidepressant use in major depression. Biol Psychiatry. 2012;72:562–571. doi: 10.1016/j.biopsych.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arnone D, et al. State-dependent changes in hippocampal grey matter in depression. Mol Psychiatry. 2013;18:1265–1272. doi: 10.1038/mp.2012.150. [DOI] [PubMed] [Google Scholar]
- 29.Hamilton JP, Siemer M, Gotlib IH. Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Mol Psychiatry. 2008;13:993–1000. doi: 10.1038/mp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding SL, et al. Comprehensive cellular-resolution atlas of the adult human brain. J Comp Neurol. 2017;525:407. doi: 10.1002/cne.24130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amaral DG, Insausti R. Retrograde transport of D-[3H]-aspartate injected into the monkey amygdaloid complex. Exp Brain Res. 1992;88:375–388. doi: 10.1007/BF02259113. [DOI] [PubMed] [Google Scholar]
- 33.Chang SW, et al. Neural mechanisms of social decision-making in the primate amygdala. Proc Natl Acad Sci USA. 2015;112:16012–16017. doi: 10.1073/pnas.1514761112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho YT, Ernst M, Fudge JL. Cortico-amygdala-striatal circuits are organized as hierarchical subsystems through the primate amygdala. J Neurosci. 2013;33:14017–14030. doi: 10.1523/JNEUROSCI.0170-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abivardi A, Bach DR. Deconstructing white matter connectivity of human amygdala nuclei with thalamus and cortex subdivisions in vivo. Hum Brain Mapp. 2017;38:3927–3940. doi: 10.1002/hbm.23639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma DY, Xu MY, Yang HC, Yang LZ. Effect of inhibition of the central nucleus of the amygdala and drug experience on the regions underlying footshock-induced reinstatement of morphine seeking. J Int Med Res. 2008;36:992–1000. doi: 10.1177/147323000803600516. [DOI] [PubMed] [Google Scholar]
- 37.Oler JA, et al. Connectivity between the central nucleus of the amygdala and the bed nucleus of the stria terminalis in the non-human primate: neuronal tract tracing and developmental neuroimaging studies. Brain Struct Funct. 2017;222:21–39. doi: 10.1007/s00429-016-1198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu Y, Day TA, Buller KM. The central amygdala modulates hypothalamic-pituitary-adrenal axis responses to systemic interleukin-1beta administration. Neuroscience. 1999;94:175–183. doi: 10.1016/S0306-4522(99)00311-5. [DOI] [PubMed] [Google Scholar]
- 39.Zavorotnyy M, et al. Low left amygdala volume is associated with a longer duration of unipolar depression. J Neural Transm (Vienna) 2018;125:229–238. doi: 10.1007/s00702-017-1811-y. [DOI] [PubMed] [Google Scholar]
- 40.Vassilopoulou K, et al. A magnetic resonance imaging study of hippocampal, amygdala and subgenual prefrontal cortex volumes in major depression subtypes: melancholic versus psychotic depression. J Affect Disord. 2013;146:197–204. doi: 10.1016/j.jad.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 41.Arnone D, McIntosh AM, Ebmeier KP, Munafo MR, Anderson IM. Magnetic resonance imaging studies in unipolar depression: systematic review and meta-regression analyses. Eur Neuropsychopharmacol. 2012;22:1–16. doi: 10.1016/j.euroneuro.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Giuliano A, et al. Hippocampal subfields at ultra high field MRI: An overview of segmentation and measurement methods. Hippocampus. 2017;27:481–494. doi: 10.1002/hipo.22717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saygin ZM, et al. High-resolution magnetic resonance imaging reveals nuclei of the human amygdala: manual segmentation to automatic atlas. Neuroimage. 2017;155:370–382. doi: 10.1016/j.neuroimage.2017.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roddy Darren W., Farrell Chloe, Doolin Kelly, Roman Elena, Tozzi Leonardo, Frodl Thomas, O’Keane Veronica, O’Hanlon Erik. The Hippocampus in Depression: More Than the Sum of Its Parts? Advanced Hippocampal Substructure Segmentation in Depression. Biological Psychiatry. 2019;85(6):487–497. doi: 10.1016/j.biopsych.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 45.First, M. D., Spitzer, R. L., Williams, J. B. W. & Gibbon, M. (New York: New York Psychiatric Institute, 1995).
- 46.First, M. B., Williams, J. B. W., Karg, R. S., Spitzer, R. L. (American Psychiatric Association, Arlington, VA, 2015).
- 47.Iglesias, J. E. et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. Neuroimage115 (2015). [DOI] [PMC free article] [PubMed]
- 48.Solano-Castiella E, et al. Diffusion tensor imaging segments the human amygdala in vivo. Neuroimage. 2010;49:2958–2965. doi: 10.1016/j.neuroimage.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 49.Bach DR, Behrens TE, Garrido L, Weiskopf N, Dolan RJ. Deep and superficial amygdala nuclei projections revealed in vivo by probabilistic tractography. J Neurosci. 2011;31:618–623. doi: 10.1523/JNEUROSCI.2744-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanders SK, Shekhar A. Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacol Biochem Behav. 1995;52:701–706. doi: 10.1016/0091-3057(95)00153-N. [DOI] [PubMed] [Google Scholar]
- 51.Haller J, Mikics E, Halasz J, Toth M. Mechanisms differentiating normal from abnormal aggression: glucocorticoids and serotonin. Eur J Pharmacol. 2005;526:89–100. doi: 10.1016/j.ejphar.2005.09.064. [DOI] [PubMed] [Google Scholar]
- 52.Barad M, Gean PW, Lutz B. The role of the amygdala in the extinction of conditioned fear. Biol Psychiatry. 2006;60:322–328. doi: 10.1016/j.biopsych.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 53.Li G, Nair SS, Quirk GJ. A biologically realistic network model of acquisition and extinction of conditioned fear associations in lateral amygdala neurons. J Neurophysiol. 2009;101:1629–1646. doi: 10.1152/jn.90765.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trezza V, Campolongo P. Toward understanding the neurobiology of social attachment: role of estrogen receptors in the medial amygdala. J Neurosci. 2009;29:1–2. doi: 10.1523/JNEUROSCI.5193-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tzanoulinou S, et al. Long-term behavioral programming induced by peripuberty stress in rats is accompanied by GABAergic-related alterations in the Amygdala. PLoS One. 2014;9:e94666. doi: 10.1371/journal.pone.0094666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stratmann M, et al. Insular and hippocampal gray matter volume reductions in patients with major depressive disorder. PLoS One. 2014;9:e102692. doi: 10.1371/journal.pone.0102692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Magarinos AM, McEwen BS, Flugge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Becker C, et al. Repeated social defeat-induced depression-like behavioral and biological alterations in rats: involvement of cholecystokinin. Mol Psychiatry. 2008;13:1079–1092. doi: 10.1038/sj.mp.4002097. [DOI] [PubMed] [Google Scholar]
- 59.Yamada K, Nabeshima T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J Pharmacol Sci. 2003;91:267–270. doi: 10.1254/jphs.91.267. [DOI] [PubMed] [Google Scholar]
- 60.Castren E, Rantamaki T. Role of brain-derived neurotrophic factor in the aetiology of depression: implications for pharmacological treatment. CNS Drugs. 2010;24:1–7. doi: 10.2165/11530010-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 61.Castren E, Rantamaki T. The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Dev Neurobiol. 2010;70:289–297. doi: 10.1002/dneu.20758. [DOI] [PubMed] [Google Scholar]
- 62.Blugeot A, et al. Vulnerability to depression: from brain neuroplasticity to identification of biomarkers. J Neurosci. 2011;31:12889–12899. doi: 10.1523/JNEUROSCI.1309-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 64.Lorenzetti V, Allen NB, Fornito A, Yucel M. Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. J Affect Disord. 2009;117:1–17. doi: 10.1016/j.jad.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 65.Frodl TS, et al. Depression-related variation in brain morphology over 3 years: effects of stress? Arch Gen Psychiatry. 2008;65:1156–1165. doi: 10.1001/archpsyc.65.10.1156. [DOI] [PubMed] [Google Scholar]
- 66.Lange C, Irle E. Enlarged amygdala volume and reduced hippocampal volume in young women with major depression. Psychol Med. 2004;34:1059–1064. doi: 10.1017/S0033291703001806. [DOI] [PubMed] [Google Scholar]
- 67.Rubinow MJ, et al. Basolateral amygdala volume and cell numbers in major depressive disorder: a postmortem stereological study. Brain Struct Funct. 2016;221:171–184. doi: 10.1007/s00429-014-0900-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Driscoll I, et al. The aging hippocampus: cognitive, biochemical and structural findings. Cereb Cortex. 2003;13:1344–1351. doi: 10.1093/cercor/bhg081. [DOI] [PubMed] [Google Scholar]
- 69.Mouiha A, Duchesne S. Multi-decade hippocampal and amygdala volume analysis: equal variability and limited age effect. Neurosci Lett. 2011;499:93–98. doi: 10.1016/j.neulet.2011.05.041. [DOI] [PubMed] [Google Scholar]
- 70.Morey RA, et al. Amygdala volume changes in posttraumatic stress disorder in a large case-controlled veterans group. Arch Gen Psychiatry. 2012;69:1169–1178. doi: 10.1001/archgenpsychiatry.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fantino B, Moore N. The self-reported Montgomery-Asberg Depression Rating Scale is a useful evaluative tool in Major Depressive Disorder. BMC Psychiatry. 2009;9:26. doi: 10.1186/1471-244X-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heinen R, et al. Robustness of Automated Methods for Brain Volume Measurements across Different MRI Field Strengths. PLoS One. 2016;11:e0165719. doi: 10.1371/journal.pone.0165719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed for the current study can be made available from the corresponding author upon reasonable request.