Abstract
The dipeptidyl peptidase-4 inhibitor saxagliptin is a widely used antihyperglycemic agent in patients with type 2 diabetes. The purpose of this study was to evaluate the effects of saxagliptin on endothelial function in patients with type 2 diabetes. This was a prospective, multicenter, interventional study. A total of 34 patients with type 2 diabetes were enrolled at four university hospitals in Japan. Treatment of patients was initially started with saxagliptin at a dose of 5 mg daily. Assessment of endothelial function assessed by flow-mediated vasodilation (FMD) and measurement of stromal cell-derived factor-1α (SDF-1α) were conducted at baseline and at 3 months after treatment with saxagliptin. A total of 31 patients with type 2 diabetes were included in the analysis. Saxagliptin significantly increased FMD from 3.1 ± 3.1% to 4.2 ± 2.4% (P = 0.032) and significantly decreased total cholesterol from 190 ± 24 mg/dL to 181 ± 25 mg/dL (P = 0.002), glucose from 160 ± 53 mg/dL to 133 ± 25 mg/dL (P < 0.001), HbA1c from 7.5 ± 0.6% to 7.0 ± 0.6% (P < 0.001), urine albumin-to-creatinine ratio from 63.8 ± 134.2 mg/g to 40.9 ± 83.0 mg/g (P = 0.043), and total SDF-1α from 2108 ± 243 pg/mL to 1284 ± 345 pg/mL (P < 0.001). These findings suggest that saxagliptin is effective for improving endothelial function.
Subject terms: Interventional cardiology, Outcomes research
Introduction
Endothelial dysfunction occurs in the early stage of atherosclerosis and plays a key role in the progression of atherosclerosis1,2. Measurements of flow-mediated vasodilation (FMD), which is an index of endothelium-dependent vasodilation, have frequently been utilized to evaluate endothelial function3–6. Endothelial dysfunction is an independent predictor of vascular events7–10. Type 2 diabetes is associated with endothelial dysfunction and is a risk factor for systemic atherosclerosis and cardiovascular events11–14. Hyperglycemia in diabetes induces oxidative stress, which is a trigger of endothelial dysfunction by reducing nitric oxide (NO) bioavailability13,14. Therefore, it is necessary to identify interventions that can prevent endothelial dysfunction in patients with type 2 diabetes.
Dipeptidyl peptidase-4 (DPP-4) inhibitors are widely used antihyperglycemic agents in patients with type 2 diabetes15–17. It has been demonstrated that DPP-4 inhibition has vascular protective benefits via the regulation of several substrate factor activities18. Stromal cell-derived factor-1α (SDF-1α), one of the DPP-4 substrates, participates in the repair of vascular injury by mobilization of endothelial progenitor cells19,20. Several experimental studies have shown that a DPP-4 inhibitor has a beneficial effect on the endothelial function through increasing SDF-1α levels21,22. However, there is no information on the effects of saxagliptin on SDF-1α in humans.
The relationship between treatment with saxagliptin and endothelial function in patients with type 2 diabetes has been reported23,24. However, previous studies were single center studies with a limited number of patients. Therefore, we conducted a prospective, multicenter study to evaluate the effects of saxagliptin on endothelial function and circulating SDF-1α levels in patients with type 2 diabetes.
Results
Clinical characteristics
We enrolled 34 patients with type 2 diabetes. Three patients including 1 patient who discontinued the intervention and 2 patients who had a protocol deviation were excluded from the analysis. The baseline clinical characteristics of the 31 patients before and after treatment with saxagliptin are summarized in Table 1. The 31 patients included 22 men (71.0%) and 9 women (29.0%), and 29 (93.5%) of the patients had hypertension, 23 (74.2%) had dyslipidemia, 18 (58.1%) had a history of smoking, 10 (32.3%) had history of coronary artery disease, and 2 (6.5%) had a history of stroke.
Table 1.
Patient characteristics and changes in parameters before and after treatment.
| Variables | Baseline n = 31 | 12 weeks n = 31 | P value |
|---|---|---|---|
| Age, yr | 64 ± 13 | ||
| Gender, men/women | 22/9 | ||
| Body mass index, kg/m2 | 27.8 ± 5.6 | 27.7 ± 5.9 | 0.354 |
| Body weight, kg | 75.8 ± 19.2 | 75.4 ± 20.7 | 0.341 |
| Systolic blood pressure, mmHg | 126 ± 17 | 126 ± 17 | 0.877 |
| Diastolic blood pressure, mmHg | 78 ± 8 | 76 ± 9 | 0.473 |
| eGFR, mL/min/1.73 m2 | 71.2 ± 16.5 | 70.2 ± 14.9 | 0.162 |
| Total cholesterol, mg/dL | 190 ± 24 | 181 ± 25 | 0.002 |
| Triglycerides, mg/dL | 175 ± 167 | 144 ± 74 | 0.247 |
| HDL cholesterol, mg/dL | 55 ± 18 | 53 ± 17 | 0.300 |
| LDL cholesterol, mg/dL | 105 ± 26 | 99 ± 24 | 0.208 |
| Glucose, mg/dL | 160 ± 53 | 133 ± 25 | < 0.001 |
| HbA1c, (%) | 7.5 ± 0.6 | 7.0 ± 0.6 | < 0.001 |
| ACR, (mg/g) | 63.8 ± 134.2 | 40.9 ± 83.0 | 0.043 |
| Medical history, n (%) | |||
| Diabetes duration, years | 7.9 ± 10.3 | ||
| Hypertension, n (%) | 29 (93.5) | ||
| Dyslipidemia, n (%) | 23 (74.2) | ||
| Previous coronary heart disease, n (%) | 10 (32.3) | ||
| Previous stroke, n (%) | 2 (6.5) | ||
| Current smoker, n (%) | 5 (16.1) | ||
| Former smoker, n (%) | 18 (58.1) | ||
| Medications, n (%) | |||
| Calcium-channel blockers, n (%) | 18 (58.1) | 18 (58.1) | NA |
| Renin angiotensin system inhibitors, n (%) | 22 (71.0) | 22 (71.0) | NA |
| Statins, n (%) | 17 (54.8) | 17 (54.8) | NA |
| Biguanides, n (%) | 7 (22.6) | 7 (22.6) | NA |
| Sulfonylurea, n (%) | 3 (9.7) | 3 (9.7) | NA |
| Thiazolidinedione, n (%) | 0 (0.0) | 0 (0.0) | NA |
| Alpha-glucosidase inhibitors, n (%) | 3 (9.7) | 3 (9.7) | NA |
| SGLT-2 inhibitors, n (%) | 7 (22.6) | 7 (22.6) | NA |
| Insulin, n (%) | 0 (0.0) | 0 (0.0) | NA |
Results are presented as mean ± SD for continuous variables and percentages for categorical variables.
eGFR indicates estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; ACR, albumin-to-creatinine ratio; SGLT-2, sodium glucose cotransporter-2; NA, not applicable.
Changes in parameters after treatment were evaluated using paired t test.
Effects of saxagliptin on endothelial function and parameters
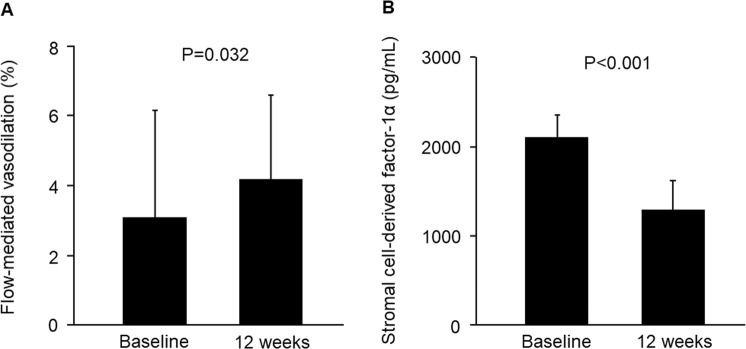
Saxagliptin significantly increased FMD from 3.1 ± 3.1% to 4.2 ± 2.4% (P = 0.032, Fig. 1A). Saxagliptin significantly decreased total cholesterol, glucose, HbA1c, urine albumin-to-creatinine ratio (ACR) (Table 1), and SDF-1α (from 2108 ± 243 pg/mL to 1284 ± 345 pg/mL, P < 0.001, Fig. 1B). There were no significant differences in body mass index, body weight, systolic blood pressure, diastolic blood pressure, eGFR, triglycerides, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol before and after 12 weeks of saxagliptin treatment. Changes in FMD did not correlate with changes in systolic blood pressure (r = 0.22, P = 0.36), changes in diastolic blood pressure (r = −0.15, P = 0.58), changes in glucose (r = 0.19, P = 0.32), changes in HbA1c (r = −0.08, P = 0.68), changes in ACR (r = 0.29, P = 0.11), or changes in SDF-1α (r = −0.03, P = 0.89).
Figure 1.
Bar graphs show flow-mediated vasodilation (A) and stromal cell-derived factor-1α (B) before the beginning of treatment and after 12 weeks of treatment.
Adverse effects
None of the patients withdrew from the study because of adverse effects associated with the treatment. One patient reported mild constipation. Two patients had mild liver enzyme elevation. One patient reported bone fracture after an incidental fall. There were no hypoglycemic events during the study period.
Discussion
This study was a prospective, multicenter, interventional study to evaluate the effects of saxagliptin on endothelial function in patients with type 2 diabetes. Treatment with saxagliptin significantly increased FMD and significantly decreased SDF-1α and ACR.
We showed that saxagliptin significantly improved endothelial function. Several potential mechanisms by which saxagliptin improves endothelial function has been proposed. It is well known that DPP-4 inhibitors enhance systemic and tissue glucagon-like peptide-1 (GLP-1) levels18,25. Previous studies showed that GLP-1 per se directly enhances phosphorylation of adenosine monophosphate-activated protein kinase (AMPK) and Akt in endothelial cells26,27. DPP-4 inhibitor-mediated AMPK activation has been shown to improve endothelial function by counteracting oxidative stress in endothelial cells25,26. However, there is controversy about the effects of treatment with DPP-4 inhibitors on FMD23,24,28. Kitao et al. showed that FMD does not alter after administration of vildagliptin28. They enrolled type 2 diabetic patients treated with metformin and the mean value of baseline FMD was 5.48%. Nafisa et al. showed that metformin improves endothelial function in patients with diabetes mellitus29. It is thought that endothelial function was already improved by pretreatment with metformin.
SDF-1α increased by a DPP-4 inhibitor has been shown to enhance homing of endothelial progenitor cells and thereby exert vascular protection19–22,25,30. In the present study, a DPP-4 inhibitor significantly decreased total SDF-1α levels. Several clinical studies and the present study have shown that treatment with DPP-4 inhibitors significantly decreases the total amount of SDF-1α31,32. Lovshin et al. reported that administration of sitagliptin significantly increased intact SDF-1α and decreased truncated SDF-1α, resulting in an decrease in the total amount of SDF-1α33. The reason for this discrepancy between clinical observations and experimental studies is due to the methodological differences in SDF-1α assays. In addition, experimental studies have shown that a DPP-4 inhibitor significantly increased SDF-1α levels in a murine model of type 1 diabetes34,35. Further studies in which the relationship between effects of DPP-4 inhibitors on SDF-1α levels is evaluated in a murine model of type 2 diabetes may reveal the reason for this discrepancy.
Chronic kidney disease is one of the complications of type 2 diabetes mellitus. Urine albumin excretion (random urine ACR) is a marker for kidney damage, and increased ACR is a risk factor for end-stage renal disease (ESRD) and cardiovascular events36,37. Although angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers are recommended to reduce the prevalence of ESRD in patients with diabetes, it is well known that patients with diabetes have a high residual risk of ESRD38–40. Several experimental studies have suggested that saxagliptin improves renal function41,42. Recently, a large clinical trial has shown that treatment with saxagliptin improved ACR compared with that in the placebo group after a median follow-up period of 2.1 years43. In the present study, we confirmed that 3-month treatment with saxagliptin significantly decreased ACR. However, the effects of saxagliptin on the risk of renal outcomes remains inconclusive30. Further studies with a longer duration are needed to evaluate the effects of saxagliptin on renal outcomes.
Several factors are known to affect vascular tone through NO metabolism in endothelial cells. The β2 adrenergic receptors and glucose metabolism are involved in the release of NO, leading to alteration in vasoconstriction and vasodilation of blood vessels11,12,44. In the present study, changes in FMD were not associated with changes in systolic blood pressure, changes in diastolic blood pressure, changes in glucose, changes in HbA1c, or changes in ACR, suggesting that saxagliptin improves endothelial function independently of its effects on glucose metabolism and renal function. In addition, there was no significant relationship between changes in FMD and changes in SDF-1α. However, there was not enough power to draw a negative conclusion. We cannot deny the possibility that saxagliptin improves endothelial function by improving glucose metabolism and renal function and by inducing an increase in SDF-1α-related endothelial progenitor cells. A large clinical trial is needed to confirm the factors that improve endothelial function in patients treated with saxagliptin.
The present study has some limitations. First, this was not a randomized and placebo-control study design and was a single-arm. In addition, the number of subjects was relatively small. However, it was clearly shown that saxagliptin improves endothelial function assessed by FMD in this prospective, multicenter study. In addition, the integrity of the data and the accuracy of the data analysis are ensured by regulatory authorities (independent data center, data monitoring committee, and audit team). Second, we evaluated only the 3-month effects of saxagliptin on endothelial function. Long-term interventions are needed to determine whether the 3-month effects of saxagliptin are sustained over time. Third, although measurements of reactive hyperemia index and endothelial progenitor cells as an index of endothelial function would enable more specific conclusions concerning the role of saxagliptin in endothelial function to be drawn, we cannot perform additional experiments to evaluate endothelial function. In the present study, measurement of FMD was performed by sonographers specialized in FMD measurement. To decrease the measurement variability of FMD, all of the sonographers received training for a standard protocol of FMD measurement at the core laboratory located in Tokyo Medical University. Previously, we confirmed that the FMD values measured at each hospital had a good correlation with the FMD values measured at a core laboratory (r = 0.838, P < 0.001)45. Finally, some antidiabetic agents such as metformin have been shown to improve endothelial function29. Of the 31 patients, 14 patients (45.2%) took antidiabetic agents. Although none of patients changed medications at any time throughout the study, we cannot deny the possibility that medications affected the results of this study.
In conclusion, treatment with saxagliptin is effective for improving endothelial function. Further studies are needed to assess the long-term effects of saxagliptin on vascular function, onset of cardiovascular disease, and cardiovascular events.
Methods
Study participants
Between June 2016 and June 2017, we enrolled 34 patients with type 2 diabetes at four university hospitals in Japan. Diabetes mellitus was defined according to the American Diabetes Association46. Estimated glomerular filtration rate (eGFR) was calculated by the following equation: 194 × serum creatinine−1.094 × age−0.287 (×0.739 if women)47. The inclusion criteria were as follows: (1) patients with type 2 diabetes, (2) age ≥20 years, and (3) HbA1c level ≥7.0% and <9.0%. The exclusion criteria were as follows: (1) treatment with DPP-4 inhibitors, GLP-1, or insulin, (2) a history of myocardial infarction or cerebrovascular disease within three months prior to the study, (3) a history of diabetic ketoacidosis or diabetic coma within three months prior to the study, (4) serious hepatic dysfunction, (5) eGFR < 50 mL/min per 1.73 m2, (6) pregnancy or possible pregnancy, and (7) a history of malignant disease within five years prior to the study. This study was approved by the ethical committee of Hiroshima University Graduate School of Medicine. The study was executed in accordance with the Good Clinical Practice guidelines. All patients gave written informed consent for participation in the study.
Study protocol
This was a prospective, multicenter, interventional study. Treatment of patients was initially started with saxagliptin at a dose of 5 mg daily. Active treatment was then carried out for 12 weeks, and the time course of the effects of saxagliptin was evaluated.
The subjects were instructed not to eat, smoke, take caffeine and drink alcohol for about 12 hours before investigations. Data of investigations were obtained as each subject were put in the supine position in a quiet, dark, air-conditioned room (constant temperature of 22–25 °C). Venous blood samples were drawn from the left antecubital vein. FMD was measured after 30 minutes of resting in the supine position.
Study management
Details of the organization of this study is as provided in the online-only Data Supplement (Supplementary Text). The independent data monitoring committee independently reviewed accrual, safety, and maturity of the data. The funding source had no role in study design or conduct, data collection, data management, analysis and interpretation of the data, and manuscript preparation. We abide with the relevant guidelines and regulations in performing the methods of this study.
Measurement of FMD
FMD evaluation was performed using the high-resolution ultrasonography system (UNEXEF18G, UNEX Co, Nagoya, Japan). The protocol for measurement of FMD was as previously described48. In brief, the longitudinal images of the brachial artery were assessed at before and after a vascular response were generated by reactive hyperemia after a 5-min period of forearm occlusion. FMD was defined as the maximal percentage change in vessel diameter from the baseline value.
Measurement of total SDF-1α level and urinary albumin and creatinine levels
SDF-1α was measured by using an enzyme-linked immunosorbent assay kit (Human CXCL12/SDF-1α immunoassay, R&D Systems, Minneapolis, USA). Urinary albumin and creatinine were measured in single voided urine samples, and the albumin-to-creatinine ratio (ACR) was calculated.
Statistical analysis
For the present study, we estimated that 28 patients were needed with α = 0.05 and a power of 0.8 and with the expectation of at least 1.0% difference between the pre- and post-intervention values of FMD49. Finally, we enrolled 34 patients with consideration for 20% dropouts. Results are shown as the means ± SD for continuous variables and numbers (%) for categvorical variables. P < 0.05 was considered statistical significant. Changes in FMD and parameters before and after treatment with saxagliptin were evaluated using the paired t-test. Correlations between variables were performed by Pearson’s correlation analysis. The data were processed using the software package Stata version 9 (Stata Co., College Station, Texas, USA).
Supplementary information
Acknowledgements
We thank Megumi Wakisaka, Ki-ichiro Kawano, and Satoko Michiyama of Hiroshima University, Research Institute for Radiation Biology and Medicine, for their excellent secretarial assistance. This study was supported financially by Kyowa Hakko Kirin Co. Ltd.
Author Contributions
M.K. and Y.H., drafting the article and conception of this study; M.K., Y.N., S.K., T.M., T.H., S.K., S.M., H.H., Y.T., F.M.Y., C.G., K.N., A.N., K.S., S.Y., R.A. and H.Y., acquisition of data; Y.K., K.C., A.H., H.T., T.H. and M.S., revising the article critically for important intellectual content. Y.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Competing Interests
A.N. received grants from TWOCELLS Co. Ltd., MSD K.K., Astellas Pharma Incorporated, and Teijin Pharma Limited, and honoraria from Kyowa Hakko Kirin Co. Ltd. and CHUGAI Pharmaceutical Co. Ltd. K.N. received honoraria and grants from Daiichi Sankyo Co. Ltd. and MSD K.K., and honoraria from Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Co. Ltd., Mochida Pharmaceutical Co. Ltd., Otsuka Pharmaceutical Co. Ltd., Amgen Astellas BioPharma K.K., Bayer Holding Limited, Boehringer Ingelheim GmbH, Eli Lilly K.K., Astellas Pharma Incorporated, Toa Eiyo K.K., and Abbott Japan Co. Ltd. H.T. had grants from Teijin Pharma Limited, Asahi Calpis Wellness Company, and Omron Health Care Company. A.H. received consulting fees from Toa-Eiyo Co. Ltd. as well as honoraria from Boehringer Ingelheim GmbH, Merck Sharp & Dohme Corporation, Sanofi K.K., AstraZeneca K.K., Astellas Pharma Incorporated, Daiichi Sankyo Co. Ltd., Amgen Astellas BioPharma K.K., Bayer Pharmaceutical Co., Bristol-Mayer Squibb Pharmaceutical Co. Y.H. received consulting fees from Kyowa Hakko Kirin Corporation related to this study, as well as honoraria and grants from Mitsubishi Tanabe Pharma Corporation, Teijin Pharma Limited, Boehringer Ingelheim GmbH, Merck Sharp & Dohme Corporation, Sanofi K.K., AstraZeneca K.K., Kyowa Hakko Kirin Co. Ltd., Takeda Pharmaceutical Co. Ltd., Astellas Pharma Incorporated, Daiichi Sankyo Co. Ltd., Mochida Pharmaceutical Co. Ltd., Nihon Kohden Corporation, Shionogi Co. Ltd., Nippon Sigmax Co. Ltd., Sanwa Kagaku Kenkyusho Co. Ltd., Unex Corporation, and Kao Corporation, and honoraria from Radiometer Limited, Omron Corporation, Sumitomo Dainippon Pharma Co. Ltd., Otsuka Pharmaceutical Co. Ltd., Torii Pharmaceutical Co. Ltd., Kowa Co. Ltd., Fujiyakuhin Co. Ltd., Amgen Astellas BioPharma K.K., Nippon Shinyaku Co. Ltd., Itamar Medical Limited, Bayer Holding Limited, Eli Lilly K.K., and Ono Pharmaceutical Co. Ltd. T.H. received honoraria or grants from Kyowa Hakko Kirin Co. Ltd., Mitsubishi Tanabe Pharma Corporation, Boehringer Ingelheim GmbH, Sanofi K.K., AstraZeneca K.K., Takeda Pharmaceutical Co. Ltd., Astellas Pharma Incorporated, Daiichi Sankyo Co. Ltd., Mochida Pharmaceutical Co. Ltd., Shionogi Co. Ltd., Sumitomo Dainippon Pharma Co. Ltd., Otsuka Pharmaceutical Co. Ltd., Kowa Co. Ltd., Amgen Astellas BioPharma K.K., and Ono Pharmaceutical Co. Ltd., MSD K.K., Eisai Co. Ltd., Pfizer Japan Incorporated and Bristol-Myers Squibb Company. Y.K received honoraria from Mitsubishi Tanabe Pharma Corporation, Teijin Pharma Limited, Boehringer Ingelheim GmbH, Merck Sharp & Dohme Corporation, Sanofi K.K., Astra Zeneca K.K., Takeda Pharmaceutical Co. Ltd., Daiichi Sankyo Co. Ltd., Otsuka Pharmaceutical Co. Ltd., Kowa Co. Ltd., Nippon Shinyaku Co. Ltd., Bayer Holding Limited, and Ono Pharmaceutical Co. Ltd.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-46726-3.
References
- 1.Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Higashi Y, Noma K, Yoshizumi M, Kihara Y. Oxidative stress and endothelial function in cardiovascular diseases. Circ J. 2009;73:411–418. doi: 10.1253/circj.CJ-08-1102. [DOI] [PubMed] [Google Scholar]
- 3.Celermajer DS, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-F. [DOI] [PubMed] [Google Scholar]
- 4.Corretti MC, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/S0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin EJ, et al. Clinical correlates and heritability of flow-mediated dilation in the community: the Framingham Heart Study. Circulation. 2004;109:613–619. doi: 10.1161/01.CIR.0000112565.60887.1E. [DOI] [PubMed] [Google Scholar]
- 6.Kajikawa M, et al. Ratio of serum levels of AGEs to soluble form of RAGE is a predictor of endothelial function. Diabetes Care. 2015;38:119–125. doi: 10.2337/dc14-1435. [DOI] [PubMed] [Google Scholar]
- 7.Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol. 2002;40:505–510. doi: 10.1016/S0735-1097(02)01976-9. [DOI] [PubMed] [Google Scholar]
- 8.Gokce N, et al. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 2002;105:1567–1572. doi: 10.1161/01.CIR.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- 9.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 10.Morimoto H, et al. Endothelial function assessed by automatic measurement of enclosed zone flow-mediated vasodilation using an oscillometric method is an independent predictor of cardiovascular events. J Am Heart Assoc. 2016;5:e004385. doi: 10.1161/JAHA.116.004385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Izzo R, et al. Enhanced GRK2 expression and desensitization of betaAR vasodilatation in hypertensive patients. Clin Transl Sci. 2008;1:215–220. doi: 10.1111/j.1752-8062.2008.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shu J, et al. Diabetes, body fat, skeletal muscle, and hypertension: The ominous chiasmus? J Clin Hypertens (Greenwich). 2019;21:239–242. doi: 10.1111/jch.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ceriello A, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57:1349–1354. doi: 10.2337/db08-0063. [DOI] [PubMed] [Google Scholar]
- 14.Mäkimattila S, et al. Chronic hyperglycemia impairs endothelial function and insulin sensitivity via different mechanisms in insulin-dependent diabetes mellitus. Circulation. 1996;94:1276–1282. doi: 10.1161/01.CIR.94.6.1276. [DOI] [PubMed] [Google Scholar]
- 15.Scirica BM, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 16.Green JB, et al. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2015;373:232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 17.White WB, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 18.Lei Y, et al. Dipeptidyl Peptidase-IV Inhibition for the Treatment of Cardiovascular Disease - Recent Insights Focusing on Angiogenesis and Neovascularization. Circ J. 2017;81:770–776. doi: 10.1253/circj.CJ-16-1326. [DOI] [PubMed] [Google Scholar]
- 19.Cheng X, et al. The Role of SDF-1/CXCR4/CXCR7 in Neuronal Regeneration after Cerebral Ischemia. Front Neurosci. 2017;11:590. doi: 10.3389/fnins.2017.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Marco GS, et al. Cardioprotective effect of calcineurin inhibition in an animal model of renal disease. Eur Heart J. 2011;32:1935–1945. doi: 10.1093/eurheartj/ehq436. [DOI] [PubMed] [Google Scholar]
- 21.Huang CY, et al. Dipeptidyl peptidase-4 inhibitor improves neovascularization by increasing circulating endothelial progenitor cells. Br J Fharmacol. 2012;167:1506–1519. doi: 10.1111/j.1476-5381.2012.02102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shih CM, et al. MK-0626, a dipeptidyl peptidase-4 inhibitor, improves neovascularization by increasing both the number of circulating endothelial progenitor cells and endothelial nitric oxide synthetase expression. Curr Med Chem. 2014;21:2012–2022. doi: 10.2174/09298673113206660273. [DOI] [PubMed] [Google Scholar]
- 23.Li F, Chen J, Leng F, Lu Z, Ling Y. Effect of Saxagliptin on Circulating Endothelial Progenitor Cells and Endothelial Function in Newly Diagnosed Type 2 Diabetic Patients. Exp Clin Endocrinol Diabetes. 2017;125:400–407. doi: 10.1055/s-0042-124421. [DOI] [PubMed] [Google Scholar]
- 24.Dell’Oro R, et al. Long-term Saxagliptin Treatment Improves Endothelial Function but not Pulse Wave Velocity and Intima-Media Thickness in Type 2 Diabetic Patients. High Blood Press Cardiovasc Prev. 2017;24:393–400. doi: 10.1007/s40292-017-0215-2. [DOI] [PubMed] [Google Scholar]
- 25.Higashi Y. Incretin-related drugs and cardiovascular events: A comparison of GLP-1 analogue and DPP-4 inhibitor. J Cardiol. 2017;69:508–510. doi: 10.1016/j.jjcc.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Tang ST, et al. Sitagliptin inhibits endothelin-1 expression in the aortic endothelium of rats with streptozotocin-induced diabetes by suppressing the nuclear factor-κB/IκBα system through the activation of AMP-activated protein kinase. Int J Mol Med. 2016;37:1558–1566. doi: 10.3892/ijmm.2016.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu L, et al. Dipeptidyl peptidase 4 inhibitor sitagliptin protects endothelial function in hypertension through a glucagon-like peptide 1-dependent mechanism. Hypertension. 2012;60:833–841. doi: 10.1161/HYPERTENSIONAHA.112.195115. [DOI] [PubMed] [Google Scholar]
- 28.Kitao N, et al. The effects of vildagliptin compared with metformin on vascular endothelial function and metabolic parameters: a randomized, controlled trial (Sapporo Athero-Incretin Study 3) Cardiovasc Diabetol. 2017;16:125. doi: 10.1186/s12933-017-0607-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nafisa A, et al. Endothelial function and dysfunction: Impact of metformin. Pharmacol Ther. 2018;192:150–162. doi: 10.1016/j.pharmthera.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Scheen AJ. Cardiovascular Effects of New Oral Glucose-Lowering Agents: DPP-4 and SGLT-2 Inhibitors. Circ Res. 2018;122:1439–1459. doi: 10.1161/CIRCRESAHA.117.311588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aso Y, et al. Sitagliptin, a dipeptidyl peptidase-4 inhibitor, increases the number of circulating CD34+CXCR4+ cells in patients with type 2 diabetes. Endocrine. 2015;50:659–664. doi: 10.1007/s12020-015-0688-5. [DOI] [PubMed] [Google Scholar]
- 32.Park KS, et al. Vildagliptin reduces plasma stromal cell-derived factor-1α in patients with type 2 diabetes compared with glimepiride. J Diabetes Investig. 2017;8:218–226. doi: 10.1111/jdi.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lovshin JA, et al. Dipeptidyl Peptidase 4 Inhibition Stimulates Distal Tubular Natriuresis and Increases in Circulating SDF-1α1-67 in Patients with Type 2 Diabetes. Diabetes Care. 2017;40:1073–1081. doi: 10.2337/dc17-0061. [DOI] [PubMed] [Google Scholar]
- 34.Li CJ, et al. Saxagliptin Induces β-Cell Proliferation through Increasing Stromal Cell-Derived Factor-1α In Vivo and In Vitro. Front Endocrinol. 2017;8:326. doi: 10.3389/fendo.2017.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang YP, et al. Saxagliptin Attenuates Albuminuria by Inhibiting Podocyte Epithelial- to-Mesenchymal Transition via SDF-1α in Diabetic Nephropathy. Front Pharmacol. 2017;8:780. doi: 10.3389/fphar.2017.00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerstein HC, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 37.Matsushita K, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brenner BM, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 39.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. doi: 10.1038/kisup.2012.73. [DOI] [Google Scholar]
- 40.de Boer IH, et al. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang YP, et al. Saxagliptin attenuates albuminuria by inhibiting podocyte epithelial- to-mesenchymal transition via SDF-1α in diabetic nephropathy. Front Pharmacol. 2017;8:780. doi: 10.3389/fphar.2017.00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uchii M, Kimoto N, Sakai M, Kitayama T, Kunori S. Glucose-independent renoprotective mechanisms of the tissue dipeptidyl peptidase-4 inhibitor, saxagliptin, in Dahl salt-sensitive hypertensive rats. Eur J Pharmacol. 2016;783:56–63. doi: 10.1016/j.ejphar.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Mosenzon O, et al. Effect of Saxagliptin on Renal Outcomes in the SAVOR-TIMI 53 Trial. Diabetes Care. 2017;40:69–76. doi: 10.2337/dc16-0621. [DOI] [PubMed] [Google Scholar]
- 44.Molinari C, et al. The role of nitric oxide in the peripheral vasoconstriction caused by human placental lactogen in anaesthetized pigs. Exp Physiol. 2006;91:603–610. doi: 10.1113/expphysiol.2005.032755. [DOI] [PubMed] [Google Scholar]
- 45.Tomiyama H, et al. Reliability of measurement of endothelial function across multiple institutions and establishment of reference values in Japanese. Atherosclerosis. 2015;242:433–442. doi: 10.1016/j.atherosclerosis.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 46.American Diabetes Association Classification and diagnosis of diabetes. Diabetes Care. 2017;40(Suppl 1):S11–24. doi: 10.2337/dc17-S005. [DOI] [PubMed] [Google Scholar]
- 47.Matsuo S, et al. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 48.Maruhashi T, et al. Nitroglycerine-induced vasodilation for assessment of vascular function: a comparison with flow-mediated vasodilation. Arterioscler Thromb Vasc Biol. 2013;33:1401–1408. doi: 10.1161/ATVBAHA.112.300934. [DOI] [PubMed] [Google Scholar]
- 49.Inaba Y, et al. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging. 2010;33:631–640. doi: 10.1007/s10554-010-9616-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.