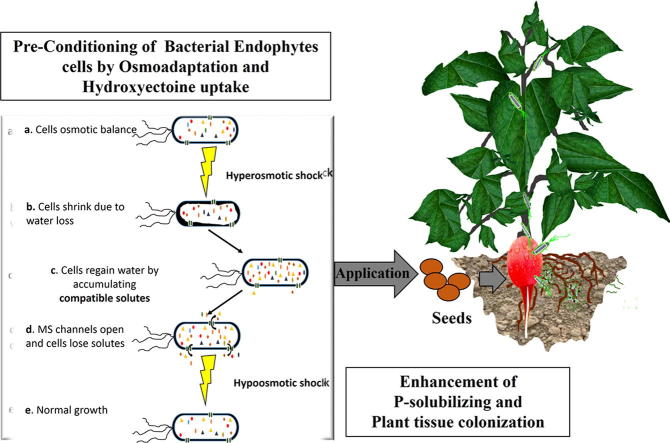
Graphical abstract
Keywords: Bacterial endophytes, Osmoadaptation, Hydroxyectoine, Compatible solutes, Phosphate solubilisation, Plant tissue colonisation
Highlights
-
•
K. radicincitans enhance P solubilization responding to osmotic stress and hydroxyectoine.
-
•
Hydroxyectoine uptake by K. radicincitans cells can increase the production of acid phosphatases.
-
•
Pre-conditioning of K. radicincitans by osmoadaptation improved radish plant yields.
-
•
Osmoadaptation and the enrichment with hydroxyectoine increase plant colonisation.
-
•
Successful microfermentation of K. radicincitans cells via the BioLector® approach.
Abstract
Gram-negative bacterial endophytes have attracted research interest caused by their advantageous over epiphytic bacteria in plant nutrition and protection. However, research on these typically Gram-negative endophytes has deficiencies concerning the role of cultivation and pre-formulation strategies on further plant colonisation capabilities. Besides, the influence of cultivation conditions and osmotic stress within bacterial endophytes on their phosphate solubilising ability has not yet been addressed. By pre-conditioning cells with an osmoadaptation and a hydroxyectoine accumulation approach, this research aimed at enhancing the capability of the plant growth promoting bacterium Kosakonia radicincitans strain DSM 16656T to both solubilise phosphate and colonise plant seedlings. The results showed that halotolerant bacterial phenotypes increased the root-colonising capability by approximately 3-fold and presented growth-promoting effects in radish plants. Interestingly, findings also demonstrated that salt stress in the culture media along with the accumulation of hydroxyectoine led to an increase in the in vitro phosphate-solubilising ability by affecting the production of acid phosphatases, from 1.24 to 3.34 U mg-1 for non-salt stressed cells and hydroxyectoine-added cells respectively. Thus, this approach provides a useful knowledge upon which the salt stress and compatible solutes in bacteria endophytes can confer phenotypic adaptations to support the eco-physiological performance concerning phosphate-solubilising abilities and endosphere establishment.
Introduction
Bacterial endophytes have attracted considerable attention because of their capability to promote plant growth through direct or indirect mechanisms [1], [2]. Many bacterial endophytes can support host plants by counteracting negative impacts in the environment and are classified as plant growth-promoting endophytic bacteria (PGPEB) [3], [4]. Colonisation by PGPEB is essential for providing benefits to host plants. Endophytic colonisation involves the entry, growth and proliferation of bacterial populations within the plant [2], [5]. The colonisation patterns of PGPEB in plant tissues are strongly dependent on several biotic and abiotic factors [6], [7]. Moreover, rhizosphere microbial communities and metabolic pathway profiles may be entirely different from those of endophytes [8]. Hence, variations in abiotic factors such as temperature [9], soil type [10], pH [11], and nutrients availability have been described to influence bacterial plant colonisation behaviour. Especially, soil salinity alters bacterial community composition and their functional activities [5], [12], [13]. Thus, bacterial endophytes may help the plant to withstand such sudden osmolarity peak conditions in their ecological niche.
Organic osmolytes enable organisms to adapt to environmental conditions by protecting cells or molecules against salt stress. These organic osmolytes are generally ‘‘compatible” with the metabolism of the cell without adversely affecting macromolecules or physiological processes and are referred to as compatible solutes [14]. Solutes accumulate either by synthesis or by transport from the extracellular medium through osmotically regulated transporters and mechanosensitive channels [15]. Moreover, compatible solutes provide beneficial enzyme functions, protecting against high temperature, desiccation, salinity, freeze-thaw procedures and even drying [16], [17], [18]. Among these, intracellular solutes such hydroxyectoine is well known in halophytic bacteria [19], [20] and is considered a protein protectant [21]. Despite these benefits, the effect of hydroxyectoine enrichment in bacterial cells on bacterial plant colonisation behaviour is so far missing.
To date, four genomes of endophytes belonging to Kosakonia radicincitans have been completed and published [22], [23], [24]. Isolation procedures of these strains revealed their endophytic capacity in different crops such as wheat, maize, banana and yerba mate. Within these isolates, the endophytic capability of the Gram-negative bacterium Kosakonia radicincitans DSM 16656T (syn. Enterobacter radicincitans) [25], formerly Pantoea agglomerans has been previously demonstrated [26]. K. radicincitans was isolated from the winter wheat leaves of the temperate regions [27]. Additionally, this facultative endophyte is known to stimulate the growth and yield of a range of plant hosts such as wheat (Triticum aestivum), maize (Zea mays) and radish (Raphanus sativus), among others [26], [28], [29], [30]. The strain is able to fix atmospheric nitrogen biologically, solubilise calcium phosphate and to produce phytohormones such as auxins and cytokinins [31], [32], [33]. Finally, there already exists an efficient commercial product “AbiVital”, which increases crop yield and even fruit quality parameters of radish and tomato plants [28], [29], [34].
The course of nutrients use including the phosphorus uptake emphasises the necessity to stimulate its availability during early plant growth stages and the endophytic establishment [33], [35]. Thus, beyond the well-documented plant growth promoting benefits of this bacterial strain, and its inorganic phosphate solubilisation capability, the response of this endophyte to both the osmotic stress and the accumulation of compatible solutes, and how these conditions may influence its phosphate solubilisation ability and subsequent plant colonisation activity are still unknown. Here, we hypothesised that providing exogenous hydroxyectoine during the adaptation at high salinities in culture media may synergistically influence the phosphatase enzymes of K. radicincitans, enhancing its physiological machinery for phosphate solubilisation and subsequent plant colonisation activity.
Members of the Brassicaceae family are economically important crops, which the benefits of associated bacterial endophytes have tested successfully [36]. Previously studies demonstrated the ability of K. radicincitans to colonise internal tissue of Brassicaceae species such as radish (Raphanus sativus L. var. sativus) [29], independently of the site of application. Radish has features that favour the assessing of effects caused by environmental variables such as the short growth cycle (24–30 days), small size enabling a large number of treatments, easy determination of growth parameters and nutrient uptake from soil [37]. In particular, radish easily responds to the application of PGP and especially P-solubilizing bacteria with leave and tuber growth improvement and P-uptake responses [38]. Therefore, this plant was selected as a model for testing plant growth-promoting and endophytic activity of pre-conditioned cells. Thus, this research investigates the effects of osmotic stress and hydroxyectoine accumulation in K. radicincitans DSM 16656T cells as endophytic bacteria model on the radish colonisation capability and P-solubilising activity.
Material and methods
Compatible solute standard hydroxyectoine (H-ectoine) was acquired from Sigma Aldrich (Cat: 70709, Sigma Aldrich Corporation, Darmstadt, Germany). All other materials used were of analytical reagent grade and used as received.
Bacteria and growth conditions
The bacterial strain K. radicincitans DSM 16656T was provided by the Leibnitz Institute of Vegetable and Ornamental Crops in Grossbeeren, Germany. Chemically defined growth medium (DM) was routinely used (g L-1): glycerol (15), yeast extract (8), K2HPO4 (2.74), KH2PO4 (1.31), MgSO4 7H2O (0.5), FeSO4H2O (60 ppm), MnSO4 (10 ppm) at pH 7.4. Pre-conditioning of bacteria before plant colonisation assays was carried out by amending the DM with NaCl [1, 4%] and providing hydroxyectoine [1 mM] to DM 4% NaCl.
Bacterial suspensions for plant colonisation experiments were prepared as follows: DM (100 mL) was poured into 250 mL baffled Erlenmeyer flasks that were autoclaved at 121 °C under 1.5 atm, for 30 min. The initial inoculum concentration in the media was adjusted at 106 cells mL−1. The cultures were maintained at 190 rpm in a rotary incubator at 30 ± 1 °C (IKA KS 4000 IC Control, Staufen, Germany). Hydroxyectoine were sterilised separately by filtration through a 0.2 μm membrane filter (Durapore® 0.2 µm polyvinylidene fluoride (PVDF), Millipore, Darmstadt, Germany). Actively growing cells were harvested at the exponential phase after 20 h (OD600 of 0.7–0.9) by centrifugation at 6875g for 15 min (Centrifuge 5810R, Eppendorf, Wesseling, Germany), and the obtained pellet of bacteria was washed and centrifuged twice with corresponding NaCl solution [1, 4%] to maintain the osmotic pressure. The three bacteria type treatments [NaCl 1%, 4% and 4% + H-ectoine] were stored in the same NaCl solution adjusted to OD600 ∼ 1.0 until use in plant colonisation assays. The intracellular hydroxyectoine concentration before endophytic establishments experiments was determined via HPLC [39].
Mineral phosphate solubilisation in liquid media
The efficiency of K. radicincitans of both pre-conditioned osmoadapted cells and osmoadapted hydroxyectoine-added cells for phosphate solubilisation were measured via Pikovkaya’s (PVK) liquid media (pH 7.2 ± 0.2) [40]. PVK media were amended with 4% NaCl and 4% NaCl plus hydroxyectoine [1 mM] to reveal the effects of osmotic stress and the osmolyte on bacterial phosphate solubilisation ability. Each flask containing 100 mL of PVK was inoculated with 100 µL of bacterial suspension at 108 cells mL−1. The flasks were incubated for approximately nine days at 30 ± 1 °C under shaking at 190 rpm on a rotary shaker (IKA KS 4000 IC Control, Staufen, Germany). Four independent replicates per treatment were tested. Quantitative spectrophotometric analysis of the soluble phosphate was performed at 24 h, 48 h, 120 h and 200 h according to a standard protocol [41]. In parallel, the influence of osmotic stress and the addition of hydroxyectoine on pH evolution in PVK medium was monitored online, using microtiter plate cultivations (MPCs) (RoboLector-BioLector system, m2p-labs, Baesweiler, Germany). The following adjustments were used: Scattered light (620 nm filter, gain 3), pH-optode (Filter pH[HP8] Ex (nm) = 470 nm; Em(nm) = 525, gain 7), and 1000 μL of PVK media incubated in 48-well MTP-48-BH flower- plates, Lot No: 1808 at 30 °C and 1200 rpm.
Phosphatase enzyme activity
Phosphatase activity was determined using p- nitrophenyl phosphate disodium (PNPP, 0.025 M) as a colourimetric substrate. For the assay, 2 mL of 0.5 M modified universal buffer (MUB) buffer adjusted to pH 6.5 (acid phosphatases) and 11 (alkaline phosphatases), and 0.5 mL of the substrate were added to 0.5 mL of PVK supernatant medium. Cell-free supernatant samples were obtained by centrifuging 2 mL of culture at 21382g for 10 min (Mikro HT 200R, Hettich GmbH & Co. KG, Tuttlingen, Germany). Reactions were carried out at 37 ± 1 °C for 60 min and stopped by the addition of 0.5 mL of 0.5 M CaCl2 and 2 mL of 0.5 M NaOH. Samples were filtered by using filter paper (grade 401) retention time 12–15 µm pore size. The p-nitrophenol (PNP) formed was measured spectrophotometrically at 400 nm (Genesys 10S UV–Vis, Thermo Fisher Scientific, Waltham, MA, USA) [42]. Four independent replicates per treatment were tested. Controls including the chemical hydroxyectoine were analysed under the same experimental conditions. The unit of enzyme activity (U) were expressed as micrograms of PNPP released per millilitre per hour (µg mL-1h−1) and normalised to dry biomass (mg) produced after 24 h.
Promotion of radish plant growth by osmoadapted K. radicincitans cells
The effects of both osmoadaptation and the inclusion of hydroxyectoine into K. radicincitans on plant growth promotion were tested under glasshouse conditions. Radish (Raphanus sativus L. var. sativus) seeds of cultivar Rondar (an F1 hybrid; S & G GmbH, Kleve, Germany) were used in all experiments. Radish inoculation with osmoadapted bacterial cells and hydroxyectoine-added cells was conducted by immersing radish seeds into a bacterial suspension (108 cells mL−1) for 5 min. Afterwards, 10 inoculated seeds were placed in pots (10 pots per treatment), filled with 1.5 L of a 1:1 (v/v) quartz-sand soil mixture (Fruhstorfer Erde type T25: P2O5: 200–300 mg L-1, Hawita Gruppe GmbH Vechta, Germany). The pots were then placed randomly on trivets to avoid transfer of bacteria between individual pots [29]. Seeds treated with NaCl solution [1, 4%] were used as controls. Seedlings were irrigated manually with 50 mL of tap water per day (conductivity 0.005–0.05 S/m). Plants were maintained under natural light conditions in the glasshouse at an average temperature of 18 ± 4 °C and an air humidity >45%. At one and four weeks post-planting, the plants were harvested.
One-week-old seedlings samples from three different locations per pot were taken, rinsed thoroughly with sterile water for removing soil with loosely adhering bacteria and flash frozen for nucleic acid extraction. Afterwards, the plants were equally thinned to five plants per pot. Total fresh mass of tuber and leaves material, as well as the tuber diameter of each plant were measured at four weeks old plants. The leaves were separated from the roots, and oven dried at 60 °C until constant weight, after which the dry weight of tubers and leaves were measured. The complete experiment was repeated twice.
Nucleic acid extraction and quantification of K. radicincitans in planta using qPCR
DNA was extracted from approximately 50 mg of lyophilised plant root material (one-week-old seedlings) using DNeasy Plant Mini Kit (Qiagen, Hilden GmbH, Germany) according to the manufacturer’s instructions. The lysis of bacterial cells was ensured by the addition of 5 mm sterile metal beads and by mechanical cell disruptor (Retsch MM200, Haan, Germany) at 30 rpm for 5 min. DNA quality and purity were assessed photometrically (NanoDrop, Thermo Fischer Scientific, Darmstadt, Germany). Quantitative real-time PCR (qPCR) was conducted using Advanced TM Universal SYBR® Green I Dye Supermix (Bio-Rad Laboratories, Hercules, CA, USA). K. radicincitans DSM 16656T species-specific primers and the plant TEF reference gene were used for in planta bacterial quantification [43]. The fold colonisation of K. radicincitans treated plants with respect to the reference gene and the control plants was calculated and represented with the 2−ΔΔcq method [44].
Statistical analysis
The data were analysed using SPSS Statistics v.2 software (SPSS, Chicago, IL). Data are presented as mean values ± standard deviations (SD) or standard error (SE). The means were tested for significant differences by one-way analysis of variance (ANOVA) followed by the Tukey post hoc test. Repeated measures ANOVA was carried out for the phosphate solubilisation data. The level of significance was set at P < 0.05.
Results
Effects of osmotic stress on phosphate solubilisation capability
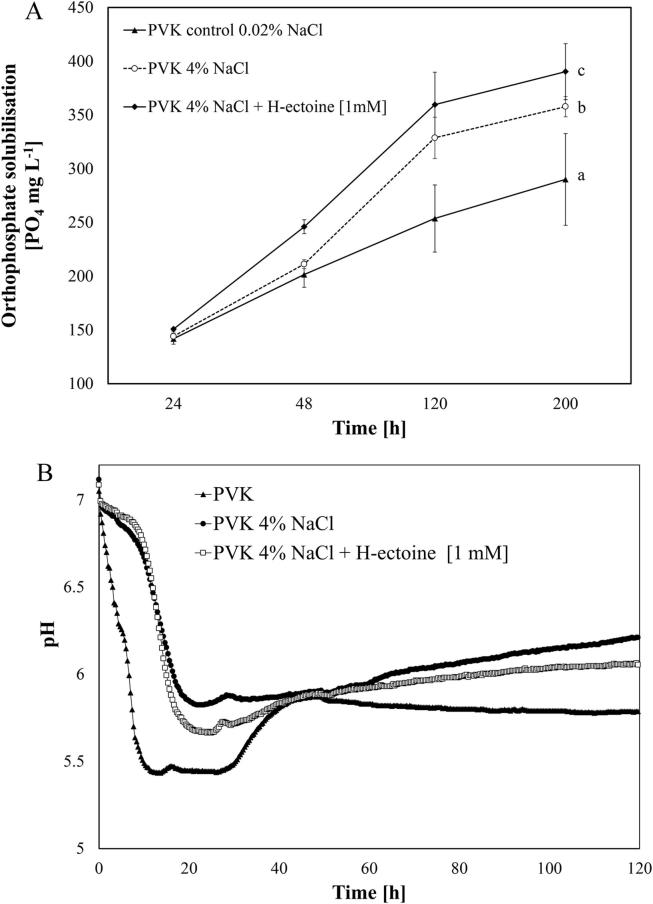
To look into the influence of osmotic unbalance on phosphate solubilisation capability PVK-amended liquid media with NaCl was used. Fig. 1A shows the positive effects on K. radicincitans ability to solubilise calcium phosphate in the PVK media under osmotic stress caused by 4% NaCl, compared with that in the PVK control media (NaCl 0.02%), solubilisation in the salt-amended media increased by up to 23.3%. The bacterial uptake of hydroxyectoine was detected by HPLC analysis after 15 h in response to high salinity, reaching more than 500 µmol g−1 of dry biomass at 24 h. No hydroxyectoine was detected in cells grown in PVK in the absence of salt. Interestingly, repeated measures ANOVA indicated significant differences (F2, 9 = 28.229; P < 0.001) in response to the accumulation of hydroxyectoine on phosphate solubilisation capability during cultivation in PVK medium at 4% NaCl, and additional phosphate release increased by up to 9.39% after five days (Fig. 1A). Thus, K. radicincitans can solubilise phosphate in a range of 150–400 mg L-1 after four days of cultivation. Online pH monitoring by the BioLector during the cultivation in PVK media, demonstrated a decrease in pH, with minimal pH values of 5.43, 5.66 and 5.82 recorded after 12.3 h, 21.6 h and 22.3 h for PVK control, PVK 4% NaCl with hydroxyectoine and PVK 4% NaCl media respectively. (Fig. 1B).
Fig. 1.
(A) Effects of the pre-conditioning of K. radicincitans in culture media by osmoadaptation and by the addition of hydroxyectoine [1 mM] on the solubilisation of orthophosphate, when grown in salt and hydroxyectoine amended PVK media. (B) BioLector pH online monitoring during the cultivation of K. radicincitans in PVK. The Statistics were determined using repeated measures ANOVA and the Tukey-HSD post hoc test at P < 0.05. The sphericity of the matrix was assessed with the Mauchly sphericity test.
Phosphatase enzyme activity
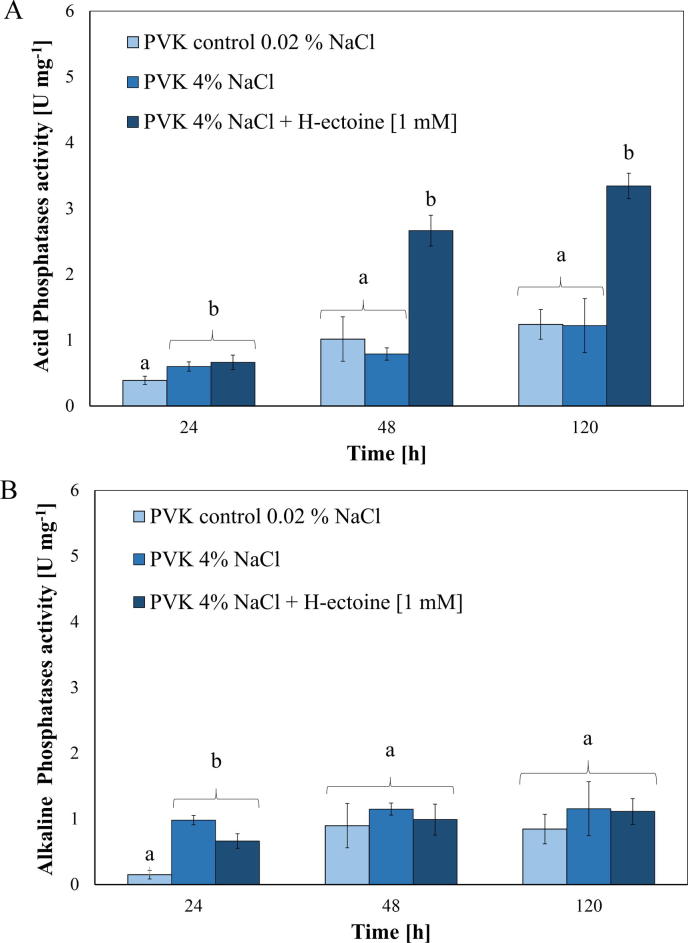
Compared to the PVK control and the PVK 4% NaCl media, the addition of hydroxyectoine induced a positive effect on phosphatase enzyme activity. Acid phosphatase activity was highly induced by the presence of hydroxyectoine in the PVK media (4% NaCl) after 24 h (F2, 11 = 12.00; P = 0.0029), 48 h (F2, 11 = 70.67; P < 0.0001) and 120 h (F2, 11 = 35.22; P = 0.0001) (Fig. 2A). Conversely, alkaline phosphatase activity was affected only after 24 h (F2, 11 = 35.52; P = 0.0001), since at 48 h (F2, 11 = 1.17; P = 0.3542) and at 120 h (F2, 11 = 1.32; P = 0.3140), all treatments presented similar activities (Fig. 2B). Chemical control hydroxyectoine had not a detectable reaction for inducing P-solubilising.
Fig. 2.
Phosphatase enzyme activity of K. radicincitans by osmoadaptation and by the addition of Hydroxyectoine [1 mM] in PVK media. (A) Acid phosphatases; (B) Alkaline phosphatases. Different letters above bars indicate significant differences according to the Tukey post hoc test at P < 0.05 (means ± SD, n = 4).
Plant growth promotion in radish
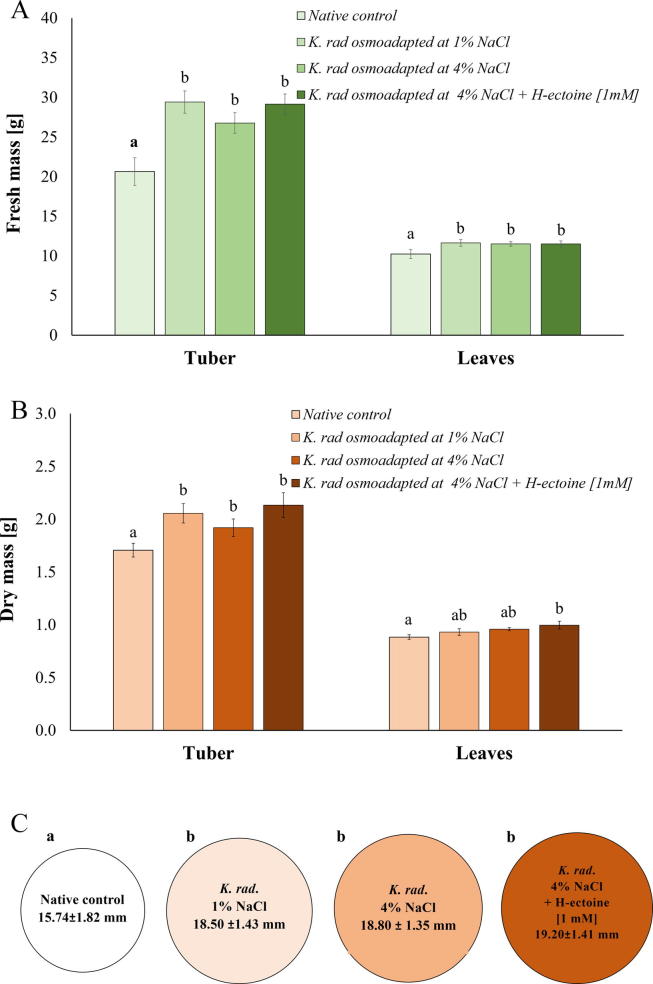
Generally, plant growth promotion by non-osmoadapted and osmoadapted K. radicincitans cells was observed in all inoculated radish plants. Interestingly, when hydroxyectoine at 1 mM was added during the cultivation of bacterial cells before plant seed inoculation, the fresh matter of tuber and leaves increased significantly by 41.1% (F3, 39 = 9.80, P = 0.0001) and 5.4% (F3, 39 = 3.86, P = 0.0172) in comparison to the non-inoculated control (Fig. 3A). Notably, compared with the osmoadapted cells in DM 4% NaCl, the hydroxyectoine amended cells increased the dry matter of either tubers or leaves by 16.20% (F3, 39 = 3.01, P = 0.0426) and 3.96% (F3, 39 = 3.60, P = 0.0672) respectively (Fig. 3B). In line with the plant weight increase, the tuber diameter also significantly increased in all cases of K. radicincitans cells compared to the non-inoculated control (F3, 34 = 6.70, P = 0.0013). The tuber diameter varied from 15.74 ± 1.82 mm in the native control up to 19.18 ± 1.41 mm for the hydroxyectoine pre-conditioned treatment (Fig. 3C).
Fig. 3.
Radish growth promotion in a glasshouse inoculated with pre-conditioned K. radicincitans cells by osmoadaptation at 4% NaCl and by the addition of hydroxyectoine at 1 mM. (A) Fresh mass of tubers and leaves. Fresh tuber mass (F3, 39 = 9.80, P = 0.0001); leaves fresh mass (F3, 39 = 3.86, P = 0.0172). (B) Dry mass of tubers and leaves. Dried tuber mass (F3, 39 = 3.01, P = 0.0426) and dry leaves mass (F3, 39 = 3.60, P = 0.0672) (means ± SE, n = 10). (C). Tuber diameter after 4 weeks of planting (F3, 34 = 6.70, P = 0.0013) (means ± SD, n > 5 of 5 plant measurements each). Different letters represent significant differences according to the Tukey post hoc test at P < 0.05.
K. radicincitans plant colonisation
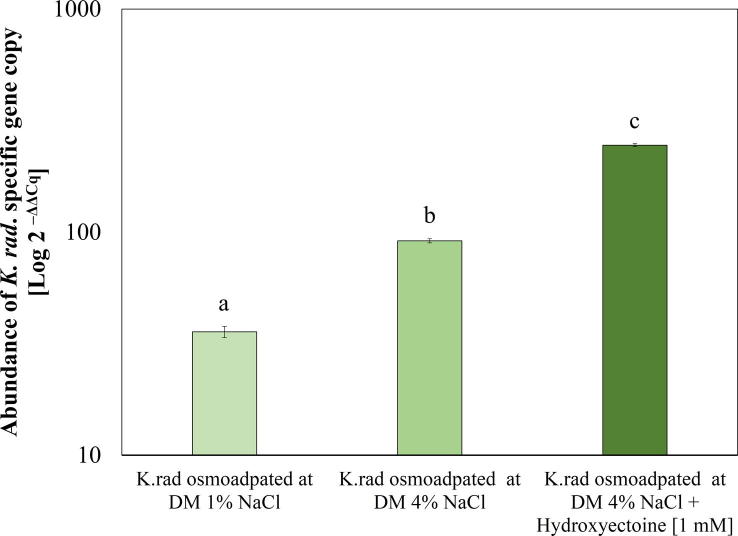
Regarding the relative gene expression response, compared with non-pre-conditioned bacteria cells at 1% NaCl, bacteria cells pre-conditioned with 4% NaCl colonised significantly tissue of eight-day-old seedlings (F2, 14 = 10.803; P = 0.033), in comparison to non-pre-conditioned cells at 1% NaCl (Fig. 4). Consistent with the biomass production of radish plants, the endophytic colonisation was relatively strong in the presence of intracellular hydroxyectoine in K. radicincitans cells that were osmoadapted at 4% NaCl; the colonisation was approximately 3-fold greater under the evaluated treatment conditions (F2, 14 = 10.803; P = 0.002) (Fig. 4).
Fig. 4.
Abundance of K. radicincitans specific gene copy numbers in inoculated radish plants with osmoadapted and hydroxyectoine pre-conditioned cells. Relative gene abundance measured using qPCR and calculated according to the methods of Livak [44]. Different letters above the bars indicate significant differences between treatments according to Dunnett’s post hoc test at P < 0.05, (means ± SE, n > 4).
Discussion
Beyond the undeniable plant growth-promoting capability of K. radicincitans, results of this study suggest that it is feasible to significantly improve plant colonisation ability of bacterial endophytes, by physiologically growing them under conditions that promote the uptake or synthesis of advantageous compatible solutes such as hydroxyectoine. The bacterial ability to solubilise rock phosphate has been extensively reported as a vital mechanism that promotes plant growth [45], [46]. In this study, was demonstrated that K. radicincitans cells under osmotic stress could increase its phosphate solubilisation capability up to 357.7 ± 9.38 mg L-1 in comparison to non-stressed cells with 290.3 ± 42.6 mg L-1 after four days of cultivation. Comparable yields are congruent with other pure culture experiments with Gram-negative bacterial endophytes [47]. Therefore, bacteria may respond to high salinity no only by accumulating exogenous osmolytes such as hydroxyectoine but also by up-regulating central metabolic pathways involved in the organic acid synthesis, which is well known for its role as crucial factors in phosphate solubilisation [48]. Since the pH values were lower in the treatment with hydroxyectoine compared to the treatment in which the bacterial cells were grown solely in PVK 4% NaCl media, a higher concentration of organic acids or the consumption of alkaline components within the media is feasible in conjunction with the osmoadaptation process [33].
Widely distributed in microorganisms, plants and animals, phosphatases are hydrolases that catalyse the hydrolysis of orthophosphate monoesters thereby releasing phosphate [49]. The general classification of acid and alkaline phosphatases relies only on the optimum pH for enzymatic activity. A major production of enzymes may be correlated with the intracellular content of amino acids, since phosphatases are built on the basis of amino acid sequences and the phosphoryl group acceptors. It is expected that the physiological changes caused by either osmoadaptation or the addition of hydroxyectoine trigger metabolic pathways for induction of amino acids, either aromatic or branched-chain ones, which can favour enzyme biosynthesis and secretion. At high salinity, as a homeostasis response, trehalose biosynthesis in K. radicincitans cells may also lead to high concentrations of trehalose-6-phosphate phosphatase (T6PP), which catalyses the hydrolysis of trehalose 6-phosphate (T6P) to not only trehalose but also inorganic phosphate [50], [51]. A greater production of enzymes, including phosphatases, may also be attributed to the construction of osmo-remedial mutations-epimutations in K. radicincitans cells, since some proteins are nonfunctional when the cells are grown in media of low osmotic strength but regain activity at elevated osmolarities, suggesting that cells may be undergoing phenotypic modulation [52], [53]. Here the increase in acid phosphatase activity of the endophyte K. radicincitans exposed to osmotic shock in the presence of hydroxyectoine was evident. However, further research is needed to elucidate the types of phosphatases (nonspecific vs specific for certain substrates) that have been up-regulated in the production process.
Since osmoadaptation in this bacterium leads to increased acid phosphatase enzyme production, it can be expected that this sub-lethal pre-conditioning procedure also supports the metabolite-rich arsenal to face the extremely competitive rhizosphere conditions before entering the plant. It has been demonstrated with bacteria such as Pantoea agglomerans, that osmotic stress can alter quorum sensing or the quenching of produced metabolites such as 1,3-propanediol, which are suggested to act as signals in the plant microbiome, resulting in tolerance to abiotic stress [54], [55]. Therefore, K. radicincitans has a fraction of genes involved in propanediol degradation (PDD-pduABDEFLMPQ) and dha genes (dhaBDKLMT) involved in glycerol transformation (GT) [56].
K. radicincitans cells were able to promote growth in radish plants. These findings are in line with previous studies [29], in which the weight of radish tubers and leaves increased from 20 to roughly 50%, in response to either seed-inoculated or two-leaf sprayed plants with fresh cultivated cells. However, since in these pot experiments the majority of plant growth yields were not significantly different between K. radicincitans cells grown in DM 1% and DM 4% NaCl, the physiological advantages conferred to the osmoadapted cells may affect mainly during early colonisation stages, as shown here for one-week old plants. Thus, the relative high endophytic lifestyle preference for salt-stressed bacteria cells and hydroxyectoine-added cells may be due to the alteration of signalling types of metabolites that K. radicincitans can secrete into the microenvironments surrounding plant roots, modifying plant-defence and plant-competition mechanisms along with plant metabolite synthesis [57]. Besides, external stimuli such as the presence of root exudates may up-regulate the expression of the chemotaxis and motility-related genes of these bacteria [56].
Consistent with the biomass production of radish plants, plant colonisation was stronger in the presence of intracellular hydroxyectoine within K. radicincitans cells, in which the approximately 3-fold increase in colonisation was significant under the evaluated conditions. Generally, these results may indicate that symbiotic performance with plants increased by synergistic effects of pre-conditioned cells by osmoadaptation and the physiological changes caused by hydroxyectoine. However, despite the endophytic colonisation ability of the strain, it also growth and colonises the root surfaces [58], [59]. These cells may contribute to phosphorous solubilization and improved plant P-uptake which has been previously demonstrated [33]. Nevertheless, additional studies are required to determine the performance of halotolerant bacterial endophytic cells on plant phosphorous uptake under saline conditions.
The reinforcement of in vitro phosphate solubilisation capability, including the production of acid phosphatase enzymes and the accumulation of hydroxyectoine, also suggests an intracellular metabolic re-ordering within bacterial cells in response to high salinity. It is proposed that these substantial and significant alterations in metabolites levels represent the activation of a phenotypic shift as an osmoadaptation mechanism for conferring both survivals under such stress conditions and advantages during rhizosphere competition establishment, orchestrating nutrient exchange and mediating associations within the plant. To the best of our knowledge, the current study is the first to address the pre-conditioning of bacterial endophytic cells as an alternative to increasing plant colonisation abilities.
Conclusions
The results of this study showed that physiological modifications of K. radicincitans by osmotic stress treatments and by the accumulation of compatible solutes during cultivation constitute a feasible strategy to improve the ability of the bacterium to solubilise phosphate and to promote the root colonisation. Nevertheless, additional studies are needed for understanding the related mechanisms that are triggered by osmoadaptation and compatible solutes inclusions that favour endophyte-plant signalling. Moreover, pre-conditioning could be a promising alternative prior to formulating bacterial endophytes.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics requirements
This article does not contain any studies with human or animal subjects.
Acknowledgements
This study was supported by the University of Applied Sciences Bielefeld, in cooperation with the Leibnitz Institute of Vegetable and Ornamental Crops, Grossbeeren, and ABiTEP GmbH in Germany; by Universidad Nacional, Agrosavia; and Colciencias in Colombia (Grant 647, 2015). The authors are grateful to Dr. rer. nat. Beatrice Berger and Birgit Wernitz for their technical assistance during glasshouse and qPCR experiments at Leibnitz Institute of Vegetable and Ornamental Crops in Grossbeeren.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Santoyo G., Moreno-Hagelsieb G., Del Carmen Orozco-Mosqueda M, Glick B.R. Plant growth-promoting bacterial endophytes. Microbiol Res. 2016;183:92–99. doi: 10.1016/j.micres.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Reinhold-Hurek B., Hurek T. Living inside plants: bacterial endophytes. Curr Opin Plant Biol. 2011;14(4):435–443. doi: 10.1016/j.pbi.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Rosenblueth M., Martinez-Romero E. Bacterial endophytes and their interactions with hosts. Mol Plant Microbe Interact. 2006;19(8):827–837. doi: 10.1094/MPMI-19-0827. [DOI] [PubMed] [Google Scholar]
- 4.Ryan R.P., Germaine K., Franks A., Ryan D.J., Dowling D.N. Bacterial endophytes: recent developments and applications. FEMS Microbiol Lett. 2008;278(1):1–9. doi: 10.1111/j.1574-6968.2007.00918.x. [DOI] [PubMed] [Google Scholar]
- 5.Kandel S.L., Joubert P.M., Doty S.L. Bacterial endophyte colonization and distribution within plants. Microorganisms. 2017;5(4):2–26. doi: 10.3390/microorganisms5040077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hallmann J., QuadtHallmann A., Mahaffee W.F., Kloepper J.W. Bacterial endophytes in agricultural crops. Can J Microbiol. 1997;43(10):895–914. [Google Scholar]
- 7.Gaiero J.R., McCall C.A., Thompson K.A., Day N.J., Best A.S., Dunfield K.E. Inside the root microbiome: bacterial root endophytes and plant growth promotion. Am J Bot. 2013;100(9):1738–1750. doi: 10.3732/ajb.1200572. [DOI] [PubMed] [Google Scholar]
- 8.Timm C.M., Campbell A.G., Utturkar S.M., Jun S.R., Parales R.E., Tan W.A. Metabolic functions of Pseudomonas fluorescens strains from Populus deltoides depend on rhizosphere or endosphere isolation compartment. Front Microbiol. 2015;6:1118. doi: 10.3389/fmicb.2015.01118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mocali S., Bertelli E., Di Cello F., Mengoni A., Sfalanga A., Viliani F. Fluctuation of bacteria isolated from elm tissues during different seasons and from different plant organs. Res Microbiol. 2003;154(2):105–114. doi: 10.1016/S0923-2508(03)00031-7. [DOI] [PubMed] [Google Scholar]
- 10.Gottel N.R., Castro H.F., Kerley M., Yang Z.M., Pelletier D.A., Podar M. Distinct microbial communities within the endosphere and rhizosphere of populus deltoides roots across contrasting soil types. Appl Environ Microbiol. 2011;77(17):5934–5944. doi: 10.1128/AEM.05255-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardoim P.R., Hardoim C.C.P., van Overbeek L.S., van Elsas J.D. Dynamics of seed-borne rice endophytes on early plant growth stages. PLoS ONE. 2012;7(2) doi: 10.1371/journal.pone.0030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szymanska S., Borruso L., Brusetti L., Hulisz P., Furtado B., Hrynkiewicz K. Bacterial microbiome of root-associated endophytes of Salicornia europaea in correspondence to different levels of salinity. Environ Sci Pollut Res. 2018;25(25):25420–25431. doi: 10.1007/s11356-018-2530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yaish M.W., Al-Lawati A., Jana G.A., Patankar H.V., Glick B.R. Impact of soil salinity on the structure of the bacterial endophytic community identified from the roots of caliph medic (Medicago truncatula) PLoS ONE. 2016;11(7) doi: 10.1371/journal.pone.0159007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown A.D. Compatible solutes and extreme water stress in eukaryotic micro-organisms. Adv Microb Physiol. 1978;17:181–242. doi: 10.1016/s0065-2911(08)60058-2. [DOI] [PubMed] [Google Scholar]
- 15.Wood J.M., Bremer E., Csonka L.N., Kraemer R., Poolman B., van der Heide T. Osmosensing and osmoregulatory compatible solute accumulation by bacteria. Comp Biochem Physiol Part A: Mol & Integr Physiol. 2001;130(3):437–460. doi: 10.1016/s1095-6433(01)00442-1. [DOI] [PubMed] [Google Scholar]
- 16.Lippert K., Galinski E.A. Enzyme stabilization be ectoine-type compatible solutes: protection against heating, freezing and drying. Appl Microbiol Biotechnol. 1992;37(1):61–65. [Google Scholar]
- 17.Sleator R.D., Hill C. Bacterial osmoadaptation: the role of osmolytes in bacterial stress and virulence. FEMS Microbiol Rev. 2002;26(1):49–71. doi: 10.1111/j.1574-6976.2002.tb00598.x. [DOI] [PubMed] [Google Scholar]
- 18.Manzanera M., Vilchez S., Tunnacliffe A. High survival and stability rates of Escherichia coli dried in hydroxyectoine. FEMS Microbiol Lett. 2004;233(2):347–352. doi: 10.1016/j.femsle.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 19.del Moral A., Severin J., Ramos-Cormenzana A., Trüper H.G., Galinski E.A. Compatible solutes in new moderately halophilic isolates. FEMS Microbiol Lett. 1994;122(1–2):165–172. [Google Scholar]
- 20.Ono H., Okuda M., Tongpim S., Imai K., Shinmyo A., Sakuda S. Accumulation of compatible solutes, ectoine and hydroxyectoine, in a moderate halophile, Halomonas elongata KS3 isolated from dry salty land in Thailand. J Ferment Bioeng. 1998;85(4):362–368. [Google Scholar]
- 21.Wang C., Zhu D., Nagata S. Supplementation effects of hydroxyectoine on proline uptake of downshocked Brevibacterium sp. JCM 6894. J Biosci Bioeng. 2006;101(2):178–184. doi: 10.1263/jbb.101.178. [DOI] [PubMed] [Google Scholar]
- 22.Witzel K., Gwinn-Giglio M., Nadendla S., Shefchek K., Ruppel S. Genome sequence of enterobacter radicincitans DSM16656(T), a plant growth-promoting endophyte. J Bacteriol. 2012;194(19):5469. doi: 10.1128/JB.01193-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suhaimi N.S., Yap K.P., Ajam N., Thong K.L. Genome sequence of Kosakonia radicincitans UMEnt01/12, a bacterium associated with bacterial wilt diseased banana plant. FEMS Microbiol Lett. 2014;358(1):11–13. doi: 10.1111/1574-6968.12537. [DOI] [PubMed] [Google Scholar]
- 24.Bergottini V.M., Filippidou S., Junier T., Johnson S., Chain P.S., Otegui M.B. Genome sequence of Kosakonia radicincitans strain YD4, a plant growth-promoting Rhizobacterium isolated from Yerba Mate (Ilex paraguariensis St. Hill.) Genome Announc. 2015;3(2) doi: 10.1128/genomeA.00239-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brady C., Cleenwerck I., Venter S., Coutinho T., De Vos P. Taxonomic evaluation of the genus Enterobacter based on multilocus sequence analysis (MLSA): Proposal to reclassify E. nimipressuralis and E. amnigenus into Lelliottia gen. nov. as Lelliottia nimipressuralis comb. nov. and Lelliottia amnigena comb. nov. Syst Appl Microbiol. 2013;36:309–319. doi: 10.1016/j.syapm.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Remus R., Ruppel S., Jacob H.-J., Hecht-Buchholz C., Merbach W. Colonization behaviour of two enterobacterial strains on cereals. Biol Fert Soils. 2000;30(5):550–557. [Google Scholar]
- 27.Kampfer P., Ruppel S., Remus R. Enterobacter radicincitans sp nov., a plant growth promoting species of the family Enterobacteriaceae. Syst Appl Microbiol. 2005;28(3):213–221. doi: 10.1016/j.syapm.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Berger B., Patz S., Ruppel S., Dietel K., Faetke S., Junge H. Successful formulation and application of plant growth-promoting Kosakonia radicincitans in maize cultivation. Biomed Res Int. 2018 doi: 10.1155/2018/6439481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berger B., Wiesner M., Brock A.K., Schreiner M., Ruppel S. K-radicincitans, a beneficial bacteria that promotes radish growth under field conditions. Agron Sustain Dev. 2015;35(4):1521–1528. [Google Scholar]
- 30.Krey T., Caus M., Baum C., Ruppel S., Eichler-Lobermann B. Interactive effects of plant growth-promoting rhizobacteria and organic fertilization on P nutrition of Zea mays L. and Brassica napus L. J Plant Nutr Soil Sci. 2011;174(4):602–613. [Google Scholar]
- 31.Ruppel S., Merbach W. Effects of different nitrogen sources on nitrogen fixation and bacterial growth of Pantoea agglomerans and Azospirillum sp. in bacterial pure culture: An investigation using 15N2 incorporation and acetylene reduction measures. Microbiol Res. 1995;150(4):409–418. [Google Scholar]
- 32.Scholz-Seidel C., Ruppel S. Nitrogenase- and phytohormone activities of Pantoea agglomerans in culture and their reflection in combination with wheat plants. Zentralbl Mikrobiol. 1992;147(5):319–328. [Google Scholar]
- 33.Schilling G., Gransee A., Deuhel A., Ležoviž G., Ruppel S. Phosphorus availability, root exudates, and microbial activity in the rhizosphere. J Plant Nutr Soil Sci. 1998;161(4):465–478. [Google Scholar]
- 34.Berger B., Baldermann S., Ruppel S. The plant growth-promoting bacterium Kosakonia radicincitans improves fruit yield and quality of Solanum lycopersicum. J Sci Food Agric. 2017;14:4865–4871. doi: 10.1002/jsfa.8357. [DOI] [PubMed] [Google Scholar]
- 35.Singh R.K., Malik N., Singh S. Improved nutrient use efficiency increases plant growth of rice with the use of IAA-overproducing strains of endophytic Burkholderia cepacia strain RRE25. Microb Ecol. 2013;66(2):375–384. doi: 10.1007/s00248-013-0231-2. [DOI] [PubMed] [Google Scholar]
- 36.Card S.D., Hume D.E., Roodi D., McGill C.R., Millner J.P., Johnson R.D. Beneficial endophytic microorganisms of Brassica - A review. Biol Control. 2015;90:102–112. [Google Scholar]
- 37.Kostka-Rick R., Manning W.J. Radish (Raphanus sativus L.): a model for studying plant responses to air pollutants and other environmental stresses. Environ Pollut. 1993;82(2):107–138. doi: 10.1016/0269-7491(93)90109-2. [DOI] [PubMed] [Google Scholar]
- 38.Lara C., Sanes S.C., Oviedo L.E. Impact of native phosphate solubilizing bacteria on the growth and development of radish (Raphanus sativus L.) plants. Biotecnol Apl. 2013;30:276–279. [Google Scholar]
- 39.Teixido N., Canamas T.P., Usall J., Torres R., Magan N., Vinas I. Accumulation of the compatible solutes, glycine-betaine and ectoine, in osmotic stress adaptation and heat shock cross-protection in the biocontrol agent Pantoea agglomerans CPA-2. Lett Appl Microbiol. 2005;41(3):248–252. doi: 10.1111/j.1472-765X.2005.01757.x. [DOI] [PubMed] [Google Scholar]
- 40.Schoebitz M., Ceballos C., Ciamp L. Effect of immobilized phosphate solubilizing bacteria on wheat growth and phosphate uptake. J Soil Sci Plant Nutr. 2013;13:1–10. [Google Scholar]
- 41.Murphy J., Riley J.P. A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta. 1962;27:31–36. [Google Scholar]
- 42.Tabatabai M.A., Bremner J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem. 1969;1(4):301–307. [Google Scholar]
- 43.Witzel K., Strehmel N., Baldermann S., Neugart S., Becker Y., Becker M. Arabidopsis thaliana root and root exudate metabolism is altered by the growth-promoting bacterium Kosakonia radicincitans DSM 16656(T) Plant Soil. 2017;419(1–2):557–573. [Google Scholar]
- 44.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 45.Hayat R., Ali S., Amara U., Khalid R., Ahmed I. Soil beneficial bacteria and their role in plant growth promotion: a review. Ann Microbiol. 2010;60(4):579–598. [Google Scholar]
- 46.Gyaneshwar P., Kumar G.N., Parekh L.J., Poole P.S. Role of soil microorganisms in improving P nutrition of plants. Plant Soil. 2002;245(1):83–93. [Google Scholar]
- 47.Oteino N., Lally R.D., Kiwanuka S., Lloyd A., Ryan D., Germaine K.J. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front Microbiol. 2015;6:745. doi: 10.3389/fmicb.2015.00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He G.Q., Wu C.D., Huang J., Zhou R.Q. Effect of Exogenous Proline on Metabolic Response of Tetragenococcus halophilus under Salt Stress. J Microbiol Biotechn. 2017;27(9):1681–1691. doi: 10.4014/jmb.1702.02060. [DOI] [PubMed] [Google Scholar]
- 49.Bull H., Murray P.G., Thomas D., Fraser A.M., Nelson P.N. Acid phosphatases. J Clin Pathol-Mol Pathol. 2002;55(2):65–72. doi: 10.1136/mp.55.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kong X., Liu Y., Gou X., Zhang H., Wang X., Zhang J. Directed evolution of operon of trehalose-6-phosphate synthase/phosphatase from Escherichia coli. Biochem Biophys Res Commun. 2001;280(1):396–400. doi: 10.1006/bbrc.2000.3819. [DOI] [PubMed] [Google Scholar]
- 51.Liu C., Dunaway-Mariano D., Mariano P.S. Rational design of reversible inhibitors for trehalose 6-phosphate phosphatases. Eur J Med Chem. 2017;128:274–286. doi: 10.1016/j.ejmech.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 52.Kunin C.M., Tong H.H., Maher W.E. Naturally-occurring, osmo-remedial variants of Escherichia coli. J Med Microbiol. 1993;38(3):216–221. doi: 10.1099/00222615-38-3-216. [DOI] [PubMed] [Google Scholar]
- 53.Csonka L.N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989;53(1):121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bacon C.W., White J.F. Functions, mechanisms and regulation of endophytic and epiphytic microbial communities of plants. Symbiosis. 2016;68(1–3):87–98. [Google Scholar]
- 55.van Kessel J.C., Rutherford S.T., Cong J.P., Quinodoz S., Healy J., Bassler B.L. Quorum sensing regulates the osmotic stress response in vibrio harveyi. J Bacteriol. 2015;197(1):73–80. doi: 10.1128/JB.02246-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Becker M., Patz S., Becker Y., Berger B., Drungowski M., Bunk B. Comparative genomics reveal a flagellar system, a type VI secretion system and plant growth-promoting gene clusters unique to the endophytic bacterium Kosakonia radicincitans. Front Microbiol. 2018;9(1997) doi: 10.3389/fmicb.2018.01997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brader G., Compant S., Mitter B., Trognitz F., Sessitsch A. Metabolic potential of endophytic bacteria. Curr Opin Biotechnol. 2014;27:30–37. doi: 10.1016/j.copbio.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ruppel S., Hecht-Buchholz C., Remus R., Ortmann U., Schmelzer R. Settlement of the diazotrophic, phytoeffective bacterial strain Pantoea agglomerans on and within winter wheat: An investigation using ELISA and transmission electron microscopy. Plant Soil. 1992;145(2):261–273. [Google Scholar]
- 59.Ruppel S., Ruehlmann J., Merbach W. Quantification and localization of bacteria in plant tissues using quantitative real-time PCR and online emission fingerprinting. Plant Soil. 2006;286(1–2):21–35. [Google Scholar]