Abstract
Two cationic antimicrobial peptides (AMP) were designed based on the snake venom peptide, omwaprin, hypothesized to be shorter, cost effective and potent. Omw1 and omw2 demonstrated significant broad-spectrum antimicrobial activity against standard and clinical strains at a MIC ranging from 15.625 to 250 µg/ml for omw1 and from 31.3 to 500 µg/ml for omw2. Time–kill kinetics revealed that omw1 caused complete lysis of E. coli ATCC 25922 at 1× MIC and S. aureus ATCC 25923 at 2× MIC after 40 and 60 min of incubation, respectively. Membranolytic activity of the peptides was assessed by propidium iodide stain, where red fluorescence was observed in cells treated with the peptides compared to untreated cells. Notable morphological changes were observed in the microbes treated with peptides, as revealed by scanning electron micrographs. Omw1 and omw2 were also potent to inhibit the formation as well as dispersal of matured biofilms at 1/2× MIC against clinical strain, C. albicans. Further, minimal hemolytic activity demonstrated by both the peptides at microbicidal concentration against human erythrocytes proves that the designed peptides were less toxic and potent antimicrobial agents which could be considered for further studies with animal models to affirm its efficiency.
Electronic supplementary material
The online version of this article (10.1007/s13205-019-1801-x) contains supplementary material, which is available to authorized users.
Keywords: Antimicrobial peptides, Omwaprin, AMP derivative, Antibiofilm activity, Cationicity
Introduction
The rapid emergence of antibiotic resistance is a serious global threat manifesting the ineffectiveness of even the last resort antibiotics. It has been considered as the fourth major health issue by the United Nations (UN) after HIV, noncommunicable diseases and ebola, according to a commentary released by WHO in September 2016. If unchecked, the antimicrobial resistance (AMR) will cost the world economy $100 trillion dollars annually as per a survey undertaken by the UK. The status quo transparently demands to swap the current treatment strategy with an unconventional effective therapeutic option.
Antimicrobial peptides (AMPs) are small cationic peptides that fend off microbial intruders as part of innate immune mechanism in a living system. A plethora of studies conducted in the past decades authenticate that AMPs are effective and ideal solution to refrain multidrug resistance (Hancock and Diamond 2000). Their small size, rapid action, broad-spectrum activity, and minimal emergence of resistance are considerable to adopt this therapeutic molecule for treatment and disease prevention protocols. Despite many desirable properties as therapeutics, a few obstacles are holding back AMPs from wide applicability. The long size and complex design of the naturally occurring AMPs are one of the major limitations that need to be answered. Recent researches on AMP structure and function reveal that cationicity and hydrophobicity are the crucial factors required for AMP activity and stability, which is conceived nowhere in the whole AMP sequence but within a short region known as antimicrobial units (Pasupuleti et al. 2009a, b; Schmidtchen et al. 2009; Zapotoczna et al. 2017). In this scenario, developing de novo AMP derived from natural AMP sequence is significantly efficient and economical. Hence, various strategies were adopted by researchers working on AMP to develop highly potent α-helical AMPs (Kanthawong et al. 2012; Lin et al. 2013). Svendsen et al. (2019) has shown that lactoferricin-derived AMPs displayed inhibitory activities against broad range of microorganisms including resistant microbial and fungal strains. Given the significance and efficacy of short fragments reported by a few researchers (Bastos et al., 2011; Lin et al. 2013; Qu et al., 2016), it is conceived as an important line of research to modify the natural AMPs and identify the best analog which could curb the emergence of antibiotic resistance among infectious agents.
In this study, we have selected a snake venom peptide, omwaprin, as a template for creating AMPs with enhanced and broad spectrum antimicrobial and antibiofilm activity. Omwaprin, a 50-mer AMP isolated from the inland taipan (Oxyuranus microlepidotus) venom, has shown dose-dependent activity against certain pathogens and are salt and protease tolerant which are desirable factors to consider this molecule for drug development. According to Nair et al. (2007), six N-terminal amino acid residues are paramount for antibacterial activity of omwaprin; hence, the six N-terminal residues were taken for designing novel peptides. Two AMPs were created in this study, which consist of 11 residues from N-terminal and modified 10 residues from C-termini. This sequence was chosen based on the combination of simplest amino acid sequence with considerable antimicrobial potential (analyzed by AntiBP server) within the parent 50-mer omwaprin. Further, 11 N-terminal residues were retained and 10 C-terminal residues were replaced with one or other of the basic and hydrophobic residues as leucine, lysine, tryptophan, isoleucine, and alanine. Lysine was positioned randomly by replacing residues like phenylalanine, cysteine, and valine because literature indicates that activity depends on overall cationicity and not on positioning and accomplishing the hydrophobicity requirement of < 50% (Ebenhan et al. 2014). The designed AMPs were checked for antimicrobial and antibiofilm activities against reference and clinical bacterial and fungal strains.
Materials and methods
Microbial strains
In this study, Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 25923, Pseudomonas aeruginosa ATCC 27853 and clinical strains, E. coli, S. aureus, P. aeruginosa and Candida albicans were procured from Department of Microbiology, PSG Hospitals, Peelamedu, Coimbatore, Tamil Nadu, India. Bacterial strains were revived and maintained in nutrient medium and Mueller–Hinton broth (MHB) was used for microbiological assays. Candida isolates were cultured in Sabouraud dextrose broth (SDB).
Peptide designing, synthesis, and characterization
The two designed peptides were named as omw1 and omw2 with the sequence, NH2-KDRPKKPGLCPLIWWLIIKVG-CONH2 and NH2-KDRPKKPGLCPAAKKAAAAKA-CONH2, respectively. The potential of the designed peptides to serve as antimicrobial peptide (AMP) was determined with Antimicrobial Peptide Database 3 (APD3) and AntiBP server. The physiochemical properties of the peptides were checked using ProtParam, and HeliQuest was used to project the helical wheel diagram of omw1 and omw2.
The peptides were obtained in powder form from GENICBIO Limited (Shanghai, China), synthesized by solid-phase synthesis and standard 9-fluorenylmethoxy carbonyl chemistry. The peptides were purified by reverse-phase high-performance liquid chromatography (RP-HPLC) and the mass and purity of the peptides were verified by mass spectrometry and high-performance liquid chromatography (HPLC), respectively.
MIC and MBC determinations
The minimum inhibitory concentrations (MICs) of the two peptides were determined using the micro-broth dilution method in a 96-well ELISA plate against bacterial and fungal strains in MHB and SDB medium, respectively, following the guidelines from the Clinical and Laboratory Standards Institute (CLSI, http://www.clsi.org). Briefly, 50 µl of the bacterial inoculum containing 2–7 × 105 cfu/ml cells was added to the wells containing 50 µl of each of the serially diluted peptide and incubated at 37 °C for 20 h. Positive control without peptide and negative control with media alone were maintained for each set of experiments. The inhibition of growth was checked by measuring the absorbance at 595 nm using Epoch microplate spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA).
Minimum bactericidal concentration (MBC) was determined at the end of the experiment by taking 20 µl of the sample from the well with no visible growth followed by serial dilution and plating. Minimum bactericidal concentration was defined as the lowest peptide concentration, which caused 99% of cell lysis.
Time–kill kinetics
To determine the killing kinetics, E. coli 25922, S. aureus 25923 and C. albicans were grown overnight in MHB for bacteria and SDB for fungus. The inoculum was then transferred to fresh medium and grown to an absorbance of 0.2 containing 1.4 × 108 CFU/ml suspensions. Each microbial suspension was then incubated with either omw1 or omw2 at 1/2×, 1× and 2× MIC for 20, 40, 60, 80 and 100 min. Positive controls were maintained without any peptide. After the incubation, the bacterial suspension was serially diluted and spread on MH agar and incubated at 37 °C for 24 h. Colonies were counted and represented as log10 CFU/ml of each experiment that was repeated thrice. All the experiments were performed in triplicate and were averaged and standard deviation was calculated. The graphs were plotted using the Origin 8 software.
Fluorescence microscopic analysis
The effect of omw1 and omw2 on the membrane integrity of bacterial and fungal cells was determined using fluorescence microscopic analysis. Propidium iodide (PI) was used to assess the viability of cells, which intercalates with DNA of the cells whose cell membrane has been disrupted and emits red color while leaving the intact cells unstained. Bacterial and fungal cells treated with 2× and 4× MIC, respectively, with omw1 and omw2 were chosen for qualitative analysis of cell lysis. After incubation, the cells were centrifuged at 7000 rpm for 15 min, washed twice with PBS, and incubated with PI (5 µg/ml) for 15 min in dark. Positive control was treated similarly without peptide. All the samples were then observed under Inverted Fluorescence Microscope IM-3FLA (OPTIKA, Italy). Same microscopic field for all the samples was observed by phase contrast and fluorescence microscopy at 600X magnification and documented.
SEM analysis
Sample preparation for SEM analysis was done as described by Thankappan et al. 2013). Briefly, the overnight grown cultures of E. coli 25922, S. aureus 25923, and C. albicans were diluted to contain 2 × 108 cells/ml. E. coli 25922, S. aureus 25923 cells were treated with 2× MIC omw1 and omw2 and C. albicans with 4× MIC of the peptide concentration. After incubation for 1 h both the control and treated cells were collected by centrifugation at 4000 rpm for 10 min followed by PBS washing twice. The cells were fixed with 2.5% glutaraldehyde at − 20 °C for 8 h. The cells were then treated with gradation of ethyl alcohol ranging from 20 to 90% and centrifuged at 10,000 rpm for 10 min. The microbial cell pellet was then suspended in 100% ethyl alcohol and smeared on glass slide followed by gold sputtering and viewed under field emission scanning electron microscopy (FEI Quanta-250).
Biofilm formation assay
The ability of omw1 and omw2 to inhibit the formation of biofilms was assessed using crystal violet staining method (Durham-Colleran et al. 2010). Clinical strain, C. albicans was grown overnight in SDB medium supplemented with 1% dextrose and diluted to obtain a final concentration of 1 × 106 cfu/ml. 20 µl of the cell suspension was added to the wells of 96-well titre plate containing either omw1 or omw2 at a concentration of 1/16, 1/8, 1/4 and 1/2× MIC (50 µg/ml). Positive control carries media and cell alone without any peptides. The plate was then incubated for 24 h at static condition. Used media was aspirated and washed with 250 µl PBS three times and air-dried. The resulting biofilm was fixed by adding 200 µl of 99% methanol for 15 min, which was then aspirated and air-dried. Staining was done with 200 µl of 0.1% crystal violet (CV) for 5 min. Excess stain was aspirated and the wells were washed under running tap water and the plates were air-dried. A total of 200 µl of 95% ethanol was used to resolubilize the stain and absorbance was measured using Epoch Microplate Spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA) at 595 nm.
Biofilm dispersal assay
To determine the potential of the peptides to disperse established biofilms of C. albicans, 20 µl of 1 × 106 cfu/ml cells along with fresh SDB was allowed to form mature biofilms in the polystyrene 96-well titre plates for 48 h. Spent media was then aspirated and incubated with fresh media containing either omw1 and omw2 at a concentration of 1/16, 1/8, 1/2 and 1/2× MIC (50 µg/ml) for 3 h. The wells were then stained with CV following the procedure as described above and read at 595 nm. The antibiofilm activity was determined with triplicates’ data which were averaged and standard deviation was calculated. Origin 8 was used to plot the graph.
Fluorescence microscopic analysis
The 1/2× MIC concentration of omw1 and omw2 treated cells was used to analyse biofilm formation and dispersal of matured biofilms by staining with 0.1% Acridine orange (AO) and observed under fluorescence microscope. A small cover-slip piece was inserted into a 24-well plate for this assay. The experimental set-up was similar as described above; following incubation, the cover-slips were washed in PBS and stained with AO for 15 min and then observed under Inverted Fluorescence Microscope IM-3FLA (OPTIKA, Italy). Same microscopic field for all the samples was observed by phase contrast and fluorescence microscopy at 600× magnification and documented.
Hemolytic assay
Hemolytic assay was performed following the protocol of Aboudy et al. (1994). Briefly, 5 ml of human blood was taken in a sterile tube containing 5.4 mg of EDTA and centrifuged at 2000 rpm for 10 min. The erythrocyte pellets were then washed three times with 1× PBS (pH 7.4) for 5 min. About 100 µl of the erythrocytes was then suspended in 900 µl of PBS and added to wells containing serially diluted peptides at a concentration as used for MIC and incubated at 37 °C for 1 h. Erythrocytes treated with PBS and 0.2% Triton X-100 served as zero and 100% hemolysis, respectively. The sample was then centrifuged at 3000 rpm for 10 min and the supernatant was transferred to a fresh plate and read at 405 nm. The percentage of hemolysis was calculated using the following formula:
where As represents absorbance of the sample, A0 is absorbance of zero hemolysis (PBS) and A100 is absorbance of 0.2% triton X-100.
Results
Design and characterization of omw1 and omw2
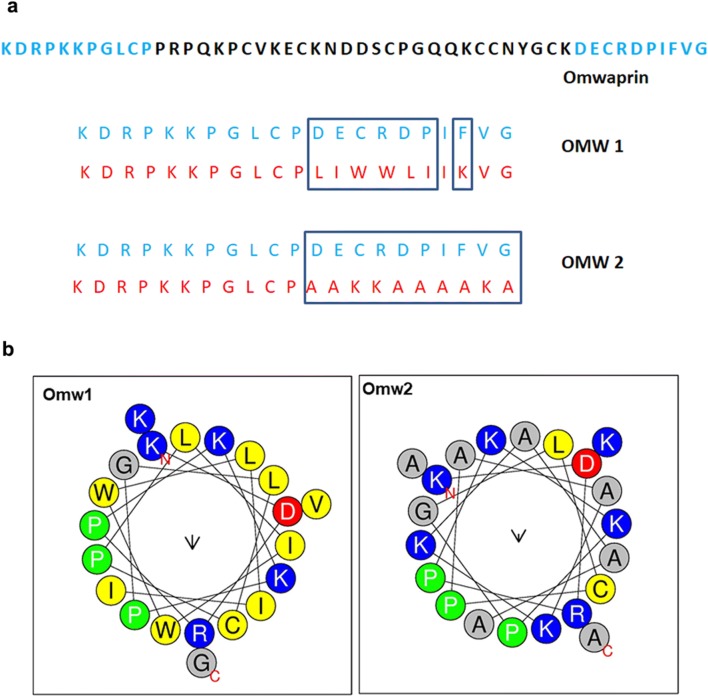
Two 21-aa designed AMPs were derived from omwaprin was designated to be omw1 and omw2. Based on the physiochemical properties analyzed using APD3 and AntiBP server, 11 residues from N-termini and 10 residues from C-termini were brought together as the template for creating short peptides (Fig. 1a). The factors considered for the selection of the sequence portion were charge and hydrophobicity, which are known to determine the activity of a AMP (Zelezetsky and Tossi 2006). 11 N-terminal residues were retained in the derivatives, six of which are indispensable for the peptide activity (Nair et al. 2007). The physicochemical properties of the omwaprin-derived peptides, omw1 and omw2, predicted using ProtParam is shown in Table 1. The isoelectric point of omw1 and omw2 was 10.03 and 10.31, while hydrophobicity was 47 and 42%, respectively. The net charge of the two AMPs was 4 and 6. The helical wheel projection of omw1 and omw2 is shown in Fig. 1b. The wheel interprets the accumulation of hydrophobic amino acids in the nonpolar phase.
Fig. 1.
Helical wheel projections of omw1 and omw2 (a). The distribution of hydrophilc and hydrophobic amino acids in omw1 and omw2 is given as helical wheel projection as determined by online tool, HeliQuest. Sequence map of parent peptide, omwaprin and the derived peptides, omw1 and omw2 (b)
Table 1.
Physicochemical properties of omwaprin-derived peptides, omw1 and omw2, as predicted by ProtParam and HeliQuest online tools
| Peptide | Mw | pI | Instability index | Net charge | Hydrophobic moment | Hydrophobic face | Hydrophobicity (%) |
|---|---|---|---|---|---|---|---|
| OMW1 | 2.4 | 10.03 | 14.43 | 4 | 0.140 | WPIPPW | 47 |
| OMW2 | 2.1 | 10.31 | 29.86 | 6 | 0.076 | PAPP | 42 |
| OMW | 5.6 | 8.69 | 51.69 | 4 | 0.174 | None | 26 |
The peptides synthesized by Fmoc were obtained at a purity of 95.81% and 96.27% for omw1 and omw2, respectively, as analysed by HPLC. Further, the predicted molecular weight of the two peptides confirmed by mass spectrometry was 2.4 and 2.1 kDa. The mass spectrum and HPLC profile of the peptides are shown in supplementary Figs. 1 and 2.
MIC and MBC of omw1 and omw2
Antimicrobial activity of omw1 and omw2 is shown in Table 2 against clinical and reference strains. Both the peptides showed activity against both Gram-positive and Gram-negative strains. Omw1 was effective against the reference ATCC strains of E. coli 25922, S. aureus 25923 and P. aeruginosa 27853 at a MIC ranging from 15.625 to 125 µg/ml and MBC ranging from 15.625 to 250 µg/ml, whereas a higher concentration was required to kill 90% of the clinical strains showing a MIC ranging from 31.25 µg for E. coli to 250 µg/ml for C. albicans.
Table 2.
MIC and MBC of omwaprin-derived peptides omw1 and omw2 against reference and clinical microbial strains
| Strains | Omw1 (µg/ml) | Omw2 (µg/ml) | ||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| E. coli ATCC 25922 | 15.625 | 15.625 | 31.25 | 125 |
| Staphylococcus aureus ATCC 25923 | 125 | 250 | 250 | 500 |
| Pseudomonas aeruginosa ATCC 27853 | 62.5 | 125 | 62.5 | 125 |
| E. coli | 31.25 | 125 | > 500 | > 500 |
| Candida albicans | 250 | > 500 | 500 | > 500 |
| Staphylococcus aureus | 125 | 500 | 250 | > 500 |
| Pseudomonas aeruginosa | 62.5 | 500 | 125 | 500 |
MIC for omw2 was 31.3, 250 and 62.5 µg/ml against E. coli 25922, S. aureus 25923 and P. aeruginosa 27853 and MBC was 125, 500 and 125 µg/ml, respectively. Greater concentration was required to render killing of clinical strains. MIC was 500, 250 and 125 µg/ml for C. albicans, S. aureus and P. aeruginosa and was > 500 µg/ml for E. coli. MBC of omw2 to inhibit the growth of clinical strains completely was either 500 µg/ml or above 500 µg/ml.
Time–kill kinetics
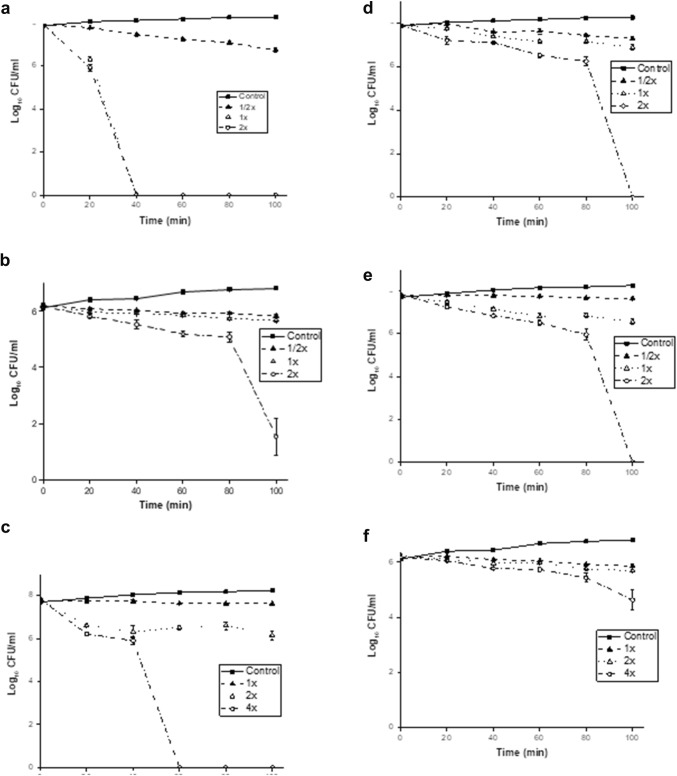
Time-killing results of omw1 and omw2 against the representative selected reference strains of E. coli 25922 and S. aureus 25923 and the clinical strain C. albicans are shown in Fig. 2. The results show that omw1 was more rapid in killing the target cells when compared to omw2. Complete inhibition of E. coli 25922 growth was observed at twofold MIC after 40 min of incubation with omw1. It took 60 min to lyse completely S. aureus 25923 and C. albicans at twofold and fourfold MIC, respectively.
Fig. 2.
Time-kill kinetics of omw1 and omw2. Time-kill kinetics of omw1 and omw2 against E. coli ATCC 25922 (a, d), S. aureus ATCC 25923 (b, e) and C. albicans (c, f), respectively at 1/2×, 1× and 2× MIC (15.625, 125 and 250 µg/ml for E. coli ATCC 25922, S. aureus ATCC 25923, respectively) and 1×, 2× and 4× MIC for C. albicans. Control is the individual bacterial culture without any peptide. Data represented are the mean ± SD of three independent experiments
In case of omw2, it took 100 min to kill E. coli 25922 and S. aureus 25923 at twofold MIC. However, complete lysis of cell death was not observed when omw2 was incubated with clinical strain, C. albicans, even after fourfold MIC.
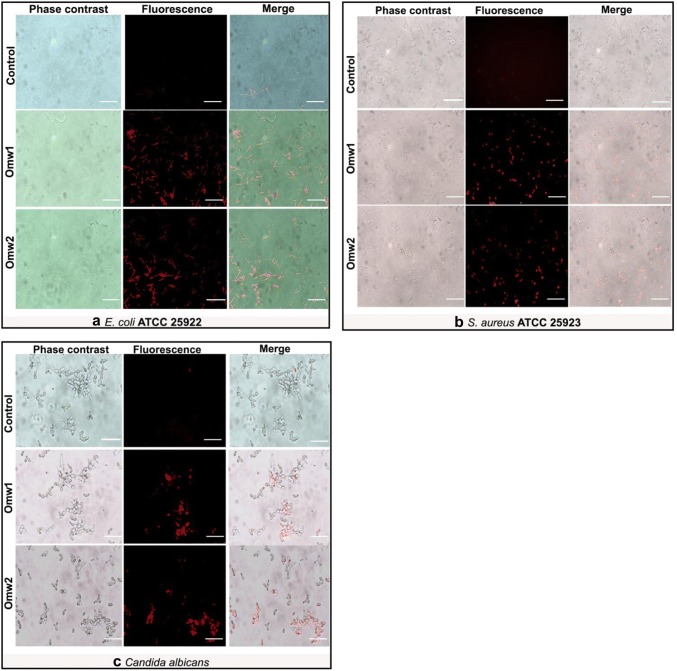
Results of time–kill assay obtained in plate method were further substantiated by qualitative analysis by staining with PI. The red color signal indicative of cell death was observed in all the treated microbial cells and thus preliminarily affirms the membranolytic effect of the two peptides (Fig. 3). The merged images show that most of the cells were emitting red signal, which represents dead cells of either E. coli, S. aureus or C. albicans.
Fig. 3.
Phase contrast and fluorescence microscopy images treated with Omw1 and Omw2 against a E. coli ATCC 25922, b S. aureus ATCC 25923 and c C. albicans. Propidium iodide (PI) stain is used for this experiment. PI stains the cells with damaged membrane while leaving the intact cells unstained or lightly stained. Red signal indicating membrane-lytic activity was observed in the cells treated with 2× MIC concentration of peptides for bacteria and 4× MIC for Candida cells. Columns 1 and 2 depict phase contrast and fluorescence images of the control and treated microbial cells. Column 3 is the merged image of 1 and 2
SEM analysis
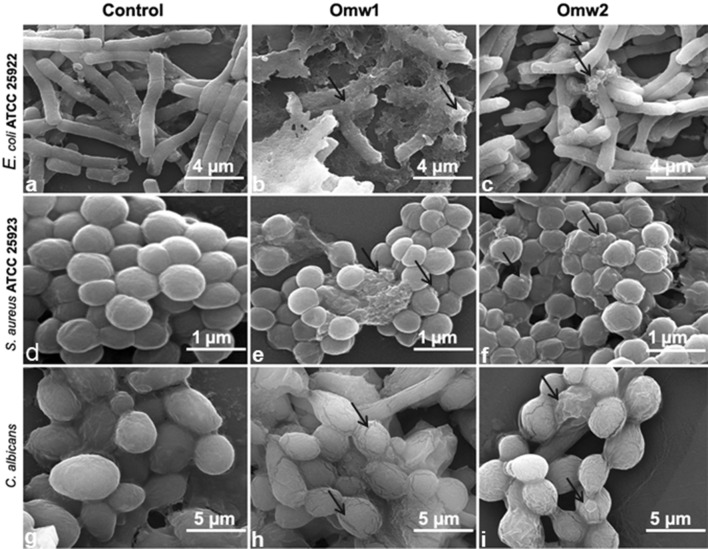
Greater understanding on the study of effect of omw1 and omw2 on microbial cell membrane was achieved from SEM data. E. coli 25922, S. aureus 25923 and C. albicans treated with the peptides exhibited structural abnormalities when compared to their untreated counterparts (Fig. 4). The appearance of the cell morphology changed drastically when treated with omw1 with occurrence of pore, displaying blebbing on the cell surface and cellular leakage in some of the cells. Morphologies of the E. coli 25922 and S. aureus 25923 cells treated with 2× MIC omw1 and omw2 were roughened and corrugated, as were the C. albicans cells treated with 4× of the peptide concentration.
Fig. 4.
SEM micrographs of E. coli ATCC 25922, S. aureus ATCC 25923 and C. albicans untreated and treated with omw1 and omw2. The untreated E. coli ATCC 25922 (a) displayed a smooth and undamaged cell membrane when compared to omw1-treated (b) which showed bleb formation and complete disruption of membrane. The E. coli treated with omw2 (c) also demonstrated a damaged cell membrane. Disrupted cell membrane was also observed in omw1 (e) and omw2 (f) treated S. aureus ATCC 25923 compared to untreated S. aureus cells (d). Spaces in the cell membrane of C. albicans were observed in omw1 (h) and omw2 (i) treated cells compared to unexposed cells (g)
Antibiofilm activity
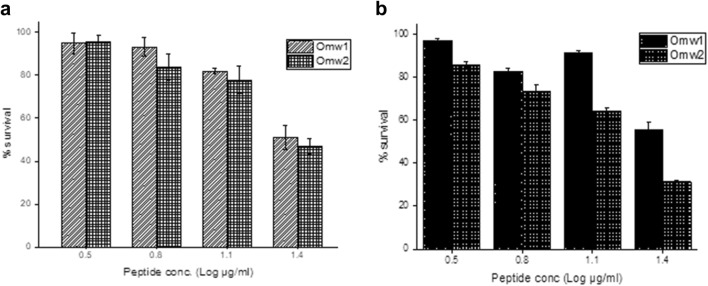
Quantitative and qualitative analysis of antibiofilm assay was carried out. 96-well titre plate was used to investigate the ability of omw1 and omw2 to inhibit the formation of biofilms as well as to dissolve the matured biofilms and the results expressed in percent survival are depicted in Fig. 5. At ½× MIC, omw1 and omw2 suppressed C. albicans to form biofilm by 51% and 46.9%, respectively. The peptides, omw1 and omw2, were also efficient to dissolve the established or mature biofilms by 55.6% and 31.4%, respectively.
Fig. 5.
Antibiofilm activity of omw1 and omw2. a Biofilm formation. Omw1 and omw2 exerted a significant effect on the formation of biofilm by clinical isolate, Candida albicans. Concentrations represent the log of 1/16×, 1/8×, 1/4× and 1/2× of MIC (50 µg/ml). b Dissolution of matured biofilms. The peptides were also equally potent to dissolute the established biofilm mass of Candida albicans. Values are mean ± SD of three independent experiments
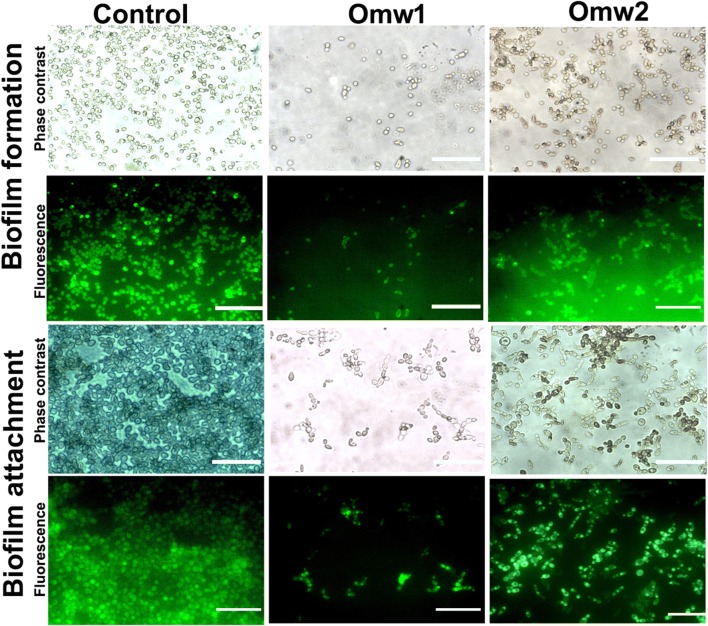
The quantitative results were further substantiated by qualitative analyses, where the treated cells were stained with acridine orange (Fig. 6). The untreated cells were found to appear as mat in the glass slide; however, a significant reduction in the number of cells of C. albicans was found in the slide treated with the peptides. It can be observed that the biofilm chain has been disturbed by the peptides resulting in the wash out of the cells in both biofilm formation and established biofilms.
Fig. 6.
Phase contrast and fluorescence micrographs of antibiofilm activity of omw1 and omw2 at 1/2× MIC. The treated and untreated cells were exposed to Acridine orange and observed under fluorescence microscope at 60×. The significant reduction in number of Candida cells in the treated plate reflects the antibiofilm activity of the two peptides both to inhibit the formation as well wash-out the established biofilms when compared to control. Row 1 depicts the phase contrast image of biofilm formation in the presence or absence of peptides, omw1 and omw2. Row 2 is the same microscopic field as Row 1 observed under blue filter. Rows 3 and 4 are the images showing mature biofilms disrupting properties of omw1 and omw2 under phase contrast and fluorescence lights, respectively
Hemolytic assay
The cytotoxicity of omw1 and omw2 was determined in human RBCs by measuring the extent of hemolysis at different concentrations of the two peptides. Omw2 rendered minimal hemolysis when compared to omw1. The hemolytic percentage at 31.25, 62.5, 125, 250 and 500 µg/ml for omw1 was 4.3, 4.82, 6.58, 10.01 and 14.46%, and for omw2, it was 1.26, 3.12, 2.32, 4.82 and 9.27%, respectively (Table 3).
Table 3.
Hemolytic activity of omw1 and omw2 against human red blood cells at MIC concentration
| Concentration (µg/ml) | 31.25 | 62.5 | 125 | 250 | 500 |
|---|---|---|---|---|---|
| Omw1 (%) | 4.30 ± 0.15 | 4.82 ± 0.33 | 6.58 ± 0.25 | 10.01 ± 0.52 | 14.46 ± 1.82 |
| Omw2 (%) | 1.26 ± 0.38 | 3.12 ± 0.38 | 2.32 ± 0.43 | 4.82 ± 0.74 | 9.27 ± 0.27 |
Values are mean ± SD of three independent experiments
Discussion
The non-toxicity of omwaprin toward mammalian cells, high salt tolerance, and minimal microbicidal dosage (Nair et al. 2007) renders omwaprin an interesting candidate for further optimization. The N-terminal amino sequence of omwaprin was chosen for designing short peptides with improved and broad-spectrum activity. Literature indicates that to curb the relentless global issue of antimicrobial resistance, several research groups are focusing on generating truncated variants of AMP, which exhibited strong microbicidal activity when compared to the parent peptide (Qu et al. 2016; Luo et al. 2017; Huertas et al. 2017). In order to design short and potent AMP, 11-N-terminal residues of omwaprin which are indispensable for activity (Nair et al. 2007) and 10 C-terminal were chosen; however, variations were made in the 10 C-terminal residues of the parent omwaprin completely deleting the central region of 29 residues. The chosen peptides were based on the best possible combination harnessing the possibility of forming an AMP as per APD3 database. The intention was to replace the 10 C-terminal residues with basic and hydrophobic residues so as to improve the efficiency the AMP with minimal toxicity as a result of optimizing the charge and hydrophobicity. Consequently, omw1 was designed by replacing DECRDP and F of the C-terminal to either leucine, isoleucine, tryptophan and lysine residues and omw2 was designed by replacing DECRDP and F of the C-terminal to lysine or alanine as shown in Fig. 1. The new sequence increased the hydrophobicity drastically from 26% of the parent to 47% in omw1 and to 42% in omw2 concomitantly with the exposure of a few residues to hydrophobic face substantiating the notion that optimal hydrophobicity reflects disruption of microbial membrane (Yin et al. 2012). Further, the presence of tryptophan in omw1 probably imparted membrane-disrupting properties to omw1 and hence might be more active than omw2 in clearing microbes (Pasupuleti et al. 2009a, b).
Omw1 and omw2 demonstrated significant antimicrobial activity with MIC ranging from 3.125 to 100 µg/ml against reference and clinical strains. This is comparable to the dosage of 2–10 µg of omwaprin against B. megaterium and Staph. Warneri; however, the parent peptide did not show any activity against other strains of Gram-positive or Gram-negative strains tested even at concentration of 5.6 mg/ml (Nair et al. 2007). As expected, omw1 and omw2 demonstrated broad-spectrum activity against reference and clinical strains although a higher concentration was required to inhibit the growth of clinical strains. The improved activity of AMP depends on certain important parameters such as cationicity, hydrophobicity and amphipathicity in combination (Yeaman and Yount 2003). The cationicity that determines the initial electrostatic interaction of AMP with the anionic target cell is 4 for the parent omwaprin as well as for omw1, but it is found to be higher for omw2, that is, 6. Cationicity could not be considered as the lone factor determining the antimicrobial activity but as described earlier the combinations of three parameters, charge, hydrophobicity and amphipathicity.
Hydrophobicity of both the derivatives is greater than their parent and is greatest for omw1, 47%. Optimal hydrophobicity is important for insertion of the peptide into the membrane bilayer and interacting with lipid moiety for disruption. Hence, the enhanced antimicrobial activity of omw1 could be attributed to the optimized hydrophobicity. Similar conclusion was drawn by Chen et al. (2007) who state that the antimicrobial activity for the peptide, A20L, was improved when hydrophobicity was increased in the peptide, V13KL. Amphipathicity, the segregation of hydrophobic and hydrophilic phases is another important efficacy determinant of AMPs. Hydrophobic moment is adopted as the quantitative measurement for amphipathicity. In the present study, the hydrophobic moment of parent omwaprin, omw1 and omw2, is given in Table 1. Hydrophobic moment is found to be highest for parent omwaprin followed by omw2 and omw1. However, antimicrobial activity is exhibited highest by omw1. This is in contrast to previous understanding that increasing the hydrophobic moment significantly increases the membrane permeabilizing potential as well as hemolysis (Yeaman and Yount 2003). The discrepancy can be explained with the results of Dathe et al. (1997), where it has been concluded that changes in hydrophobic moment is less pronounced in AMP with high hydrophobicity. Because all the three AMPs discussed in this paper has a higher hydrophobicity of > 40, the hydrophobic moment is modestly influential. Besides, the helical wheel projections of omwaprin, omw1 and omw2 show that the designed peptides have superior amphipathicity than the parent. In both derivatives, hydrophobic residues are confined to one face of the helix, whereas there were no residues in the hydrophobic face of parent omwaprin (Fig. 1b and Table 1). This clearly explains the enhanced efficiency of omwaprin-derived peptides.
The time–kill kinetics results of omw1 and omw2 show that both the truncated peptides showed a dose-dependent killing of strains, E. coli 25922, S. aureus 25923 and C. albicans. Omw1 was highly potent and fast to lyse all the inoculated microbial cells after 40 min of incubation at concentration of 1× and 2× MIC. Highly significant activity was observed against E. coli, where complete growth inhibition was observed at 1× MIC after 40 min of incubation. However, against S. aureus, approximately 6 log reductions were observed at 1× MIC even though complete growth inhibition was observed at 2× MIC concentration. It may be due to the higher hydrophobicity of 47% compared with 42% of omw2. Zero growth was observed with E. coli and S. aureus when incubated with omw2 at 2× MIC. Moreover, omw2 showed not > 4 log reduction of the Candida cells even at 4× MIC. Higher concentration requirement for killing fungal cells could be attributed difference in cell wall composition of bacteria and fungus. Cell wall of candida is different from bacteria comprising of glucan and chitin in higher proportion which makes it more resilient to any drug treatment. In this study, the requirement of high concentration by omw1 and inactivity of omw2 against yeast cells reflects the dependence of AMP on target membrane composition as indicated earlier (Ramírez-Carreto et al. 2015).
Literature indicates that AMP exerting amphipathic structures interact with membranes manifesting antimicrobial activity by forming pores, thereby disintegrating the membrane integrity (Hancock and Sahl 2006). In this study, to demonstrate the membrane damage, we employed DNA intercalating PI staining at 2× for E. coli and S. aureus and 4× for C. albicans. The untreated E. coli were unable to take the dye because of the intact cell membrane and so are the unexposed S. aureus cells. However, the E. coli cells treated with either omw1 or omw2 were found to rapidly acquire the dye as a result of membrane damage giving out the red signal as shown in Fig. 3. Similar result was observed at 2× MIC for S. aureus cells and at 4× MIC for C. albicans.
The membranolytic potential of omw1 and omw2 was further substantiated by Fe-SEM analysis. Irregular morphological appearance of cell membrane in treated cells in concurrence with blebbing confirms cell lysis as a result of membrane distortion. Similar changes were documented with other cationic α-helical peptide, such as porcine myeloid antimicrobial peptide-36 (PMAP) against E. coli and C. albicans (Lyu et al. 2016), NA-CATH and the truncated peptide, ATRA-1A, and its stereoisomer (Juba et al. 2015).
The difficult-to-treat candida biofilms are ever considered as a threat in treatment strategies and efforts are on way for its eradication. Hence, we were interested to study whether omw1 and omw2 were able to inhibit the formation as well as eradication of mature biofilms of C. albicans. The results obtained were very promising as both omw1 and omw2 were potent to inhibit the formation and disrupt matured biofilms by > 50% at 1/2× MIC as determined by standard crystal violet assay in a 96-well plate. The results were further substantiated by AO staining where the number of candida cells was significantly reduced in the treated plate when compared to microbial mat in the untreated plate. It can be deduced from Fig. 6 that both the peptides did not exert their membranolytic activity since the morphology of treated cells was intact but the network of cells as observed in untreated plate was disrupted. Omw1 by virtue of its rapid killing activity might have inhibited the formation of C. albicans biofilm by restricting the attachment to the substratum at sub-MIC concentration as observed with pleurocidin against S. aureus biofilms (Ko et al. 2019). Further, increased hydrophobicity and presence of tryptophan in the hydrophobic phase have contributed to the anti-biofilm activity of omw1 (Chan et al. 2006; Schmidtchen et al. 2014; Bi et al. 2014). However, it is noted that omw2, despite moderate bactericidal activity than omw1 showed equal anti-biofilm activity as omw1. The difference can be explained on the basis of the postulation that the mechanism of antibiofilm activity of an AMP is significantly distinct and unique from the antimicrobial activity (Dostert et al. 2018). A significant example for such peptide is the innate defense regulator-1018 (IDR-1018) which is reported to act on Gram-negative and Gram-positive bacterial biofilms with no effect on planktonic cells (de la Fuente-Núñez et al. 2014) by inhibiting the signaling molecule guanosine 5′-diphospahte 3′-diphosphate (ppGpp). Similarly, omw2 is hypothesized to bind and trigger the degradation of the signalling pathway Ras1-cAMP-Efg1 and MAP kinase involved in hyphae induction and biofilm formation in C. albicans (Leberer et al. 2001; Ramage et al. 2002; Biswas et al. 2007). As observed with other α-helical cationic AMPs, such as pleurocidin (Choi and Lee 2012), the antibiofilm activity of omw1 would be pore formation in the lipid component of EPS as observed with antimicrobial activity in planktonic cells.
The safety of omw1 and omw2 was tested by their ability to lyse human erythrocytes at microbicidal concentration ranging from 15.625 to 500 µg/ml. A very minimal of < 15% and < 10% hemolysis was observed at the maximal concentration used in this study.
In conclusion, the designed and synthesized two peptides derived from omwaprin demonstrated broad spectrum and increased antimicrobial and antibiofilm activity when compared to parent AMP. With the added advantage of simple amino acid residue and small size, the cost for synthesizing the peptides is significantly reduced. Because of the minimal hemolytic activity shown by these peptides, omw1 and omw2 could be considered for further studies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Purification of omw1 by HPLC (a) and determination of mass by MALDI-TOF (b). Omw1 was obtained at 95% purity and the observed molecular weight was 2460 Da.
Purification of omw2 by HPLC (a) and determination of mass by MALDI-TOF (b). Omw2 was obtained at 95% purity and the observed molecular weight was 2119 Da.
Acknowledgements
The first author greatly acknowledges the financial support from University Grants Commission (UGC), New Delhi, India in the form of fellowship and contingency. Both authors are thankful to the Bharathiar University administration for the instrumentation facilities in the department.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Aboudy Y, Mendelson E, Shalit I, et al. Activity of two synthetic amphiphilic peptides and magainin-2 against herpes simplex virus types 1 and 2. Int J Pept Protein Res. 1994;43:573–582. doi: 10.1111/j.1399-3011.1994.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Bastos M, Adão R, Nazmi K, Bolscher JM. C- and N-truncated antimicrobial peptides from LFampin 265–284: biophysical versus microbiology results. J Pharm Bioallied Sci. 2011;3:60. doi: 10.4103/0975-7406.76467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X, Wang C, Dong W, et al. Antimicrobial properties and interaction of two Trp-substituted cationic antimicrobial peptides with a lipid bilayer. J Antibiot. 2014;67:361–368. doi: 10.1038/ja.2014.4. [DOI] [PubMed] [Google Scholar]
- Biswas S, Van Dijck P, Datta A. Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiol Mol Biol Rev. 2007;71:348–376. doi: 10.1128/MMBR.00009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DI, Prenner EJ, Vogel HJ. Tryptophan- and arginine-rich antimicrobial peptides: structures and mechanisms of action. Biochim Biophys Acta Biomembr. 2006;1758:1184–1202. doi: 10.1016/j.bbamem.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Chen Y, Guarnieri MT, Vasil AI, et al. Role of peptide hydrophobicity in the mechanism of action of alpha-helical antimicrobial peptides. Antimicrob Agents Chemother. 2007;51:1398–1406. doi: 10.1128/AAC.00925-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Lee DG. Antimicrobial peptide pleurocidin synergizes with antibiotics through hydroxyl radical formation and membrane damage, and exerts antibiofilm activity. Biochim Biophys Acta. 2012;1820:1831–1838. doi: 10.1016/j.bbagen.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Dathe M, Wieprecht T, Nikolenko H, et al. Hydrophobicity, hydrophobic moment and angle subtended by charged residues modulate antibacterial and haemolytic activity of amphipathic helical peptides. FEBS Lett. 1997;403:208–212. doi: 10.1016/S0014-5793(97)00055-0. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Núñez C, Reffuveille F, Haney EF, et al. Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS Pathog. 2014;10:e1004152. doi: 10.1371/journal.ppat.1004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostert M, Belanger CR, Hancock RE. Design and assessment of anti-biofilm peptides: steps toward clinical application. J Innate Immun. 2018;11:193–204. doi: 10.1159/000491497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham-Colleran MW, Verhoeven AB, van Hoek ML. Francisella novicida forms in vitro biofilms mediated by an orphan response regulator. Microb Ecol. 2010;59:457–465. doi: 10.1007/s00248-009-9586-9. [DOI] [PubMed] [Google Scholar]
- Ebenhan T, Gheysens O, Kruger HG, et al. Antimicrobial peptides: their role as infection-selective tracers for molecular imaging. Biomed Res Int. 2014;2014:867381. doi: 10.1155/2014/867381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock RE, Diamond G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 2000;8:402–410. doi: 10.1016/S0966-842X(00)01823-0. [DOI] [PubMed] [Google Scholar]
- Hancock REW, Sahl H-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. 2006;24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- Huertas N, Monroy Z, Medina R, Castañeda J. Antimicrobial activity of truncated and polyvalent peptides derived from the FKCRRQWQWRMKKGLA sequence against Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 25923. Molecules. 2017;22:987. doi: 10.3390/molecules22060987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juba ML, Porter DK, Williams EH, et al. Helical cationic antimicrobial peptide length and its impact on membrane disruption. Biochim Biophys Acta Biomembr. 2015;1848:1081–1091. doi: 10.1016/j.bbamem.2015.01.007. [DOI] [PubMed] [Google Scholar]
- Kanthawong S, Bolscher JGM, Veerman ECI, et al. Antimicrobial and antibiofilm activity of LL-37 and its truncated variants against Burkholderia pseudomallei. Int J Antimicrob Agents. 2012;39:39–44. doi: 10.1016/j.ijantimicag.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Ko SJ, Kang NH, Kim MK, et al. Antibacterial and anti-biofilm activity, and mechanism of action of pleurocidin against drug resistant Staphylococcus aureus. Microb Pathog. 2019;127:70–78. doi: 10.1016/j.micpath.2018.11.052. [DOI] [PubMed] [Google Scholar]
- Leberer E, Harcus D, Dignard D, et al. Ras links cellular morphogenesis to virulence by regulation of the MAP kinase and cAMP signalling pathways in the pathogenic fungus Candida albicans. Mol Microbiol. 2001;42:673–687. doi: 10.1046/j.1365-2958.2001.02672.x. [DOI] [PubMed] [Google Scholar]
- Lin M-C, Hui C-F, Chen J-Y, Wu J-L. Truncated antimicrobial peptides from marine organisms retain anticancer activity and antibacterial activity against multidrug-resistant Staphylococcus aureus. Peptides. 2013;44:139–148. doi: 10.1016/j.peptides.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Luo Y, McLean DTF, Linden GJ, et al. The Naturally occurring host defense peptide, LL-37, and its truncated mimetics KE-18 and KR-12 have selected biocidal and antibiofilm activities against Candida albicans, Staphylococcus aureus, and Escherichia coli in vitro. Front Microbiol. 2017;8:544. doi: 10.3389/fmicb.2017.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu Y, Yang Y, Lyu X, et al. Antimicrobial activity, improved cell selectivity and mode of action of short PMAP-36-derived peptides against bacteria and Candida. Sci Rep. 2016;6:27258. doi: 10.1038/srep27258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair DG, Fry BG, Alewood P, et al. Antimicrobial activity of omwaprin, a new member of the waprin family of snake venom proteins. Biochem J. 2007;402:93–104. doi: 10.1042/BJ20060318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasupuleti M, Chalupka A, Mörgelin M, et al. Tryptophan end-tagging of antimicrobial peptides for increased potency against Pseudomonas aeruginosa. Biochim Biophys Acta. 2009;1790:800–808. doi: 10.1016/j.bbagen.2009.03.029. [DOI] [PubMed] [Google Scholar]
- Pasupuleti M, Schmidtchen A, Chalupka A, et al. End-tagging of ultra-short antimicrobial peptides by W/F stretches to facilitate bacterial killing. PLoS ONE. 2009;4:e5285. doi: 10.1371/journal.pone.0005285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu P, Gao W, Chen H, et al. The central hinge link truncation of the antimicrobial peptide fowlicidin-3 enhances its cell selectivity without antibacterial activity loss. Antimicrob Agents Chemother. 2016;60:2798–2806. doi: 10.1128/AAC.02351-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage G, VandeWalle K, López-Ribot JL, Wickes BL. The filamentation pathway controlled by the Efg1 regulator protein is required for normal biofilm formation and development in Candida albicans. FEMS Microbiol Lett. 2002;214:95–100. doi: 10.1111/j.1574-6968.2002.tb11330.x. [DOI] [PubMed] [Google Scholar]
- Ramírez-Carreto S, Jiménez-Vargas JM, Rivas-Santiago B, et al. Peptides from the scorpion Vaejovis punctatus with broad antimicrobial activity. Peptides. 2015;73:51–59. doi: 10.1016/j.peptides.2015.08.014. [DOI] [PubMed] [Google Scholar]
- Schmidtchen A, Pasupuleti M, Mörgelin M, et al. Boosting antimicrobial peptides by hydrophobic oligopeptide end tags. J Biol Chem. 2009;284:17584–17594. doi: 10.1074/jbc.M109.011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtchen A, Pasupuleti M, Malmsten M. Effect of hydrophobic modifications in antimicrobial peptides. Adv Colloid Interface Sci. 2014;205:265–274. doi: 10.1016/j.cis.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Svendsen JSM, Grant TM, Rennison D, et al. Very short and stable lactoferricin-derived antimicrobial peptides: design principles and potential uses. Acc Chem Res. 2019;52:749–759. doi: 10.1021/acs.accounts.8b00624. [DOI] [PubMed] [Google Scholar]
- Thankappan B, Jeyarajan S, Hiroaki S, et al. Antimicrobial and antibiofilm activity of designed and synthesized antimicrobial peptide, KABT-AMP. Appl Biochem Biotechnol. 2013;170:1184–1193. doi: 10.1007/s12010-013-0258-3. [DOI] [PubMed] [Google Scholar]
- Yeaman MR, Yount NY. Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev. 2003;55:27–55. doi: 10.1124/pr.55.1.2. [DOI] [PubMed] [Google Scholar]
- Yin LM, Edwards MA, Li J, et al. Roles of hydrophobicity and charge distribution of cationic antimicrobial peptides in peptide–membrane interactions. J Biol Chem. 2012;287:7738–7745. doi: 10.1074/jbc.M111.303602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapotoczna M, Forde É, Hogan S, et al. Eradication of Staphylococcus aureus biofilm infections using synthetic antimicrobial peptides. J Infect Dis. 2017;215:975–983. doi: 10.1093/infdis/jix062. [DOI] [PubMed] [Google Scholar]
- Zelezetsky I, Tossi A. Alpha-helical antimicrobial peptides—using a sequence template to guide structure-activity relationship studies. Biochim Biophys Acta. 2006;1758:1436–1449. doi: 10.1016/j.bbamem.2006.03.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Purification of omw1 by HPLC (a) and determination of mass by MALDI-TOF (b). Omw1 was obtained at 95% purity and the observed molecular weight was 2460 Da.
Purification of omw2 by HPLC (a) and determination of mass by MALDI-TOF (b). Omw2 was obtained at 95% purity and the observed molecular weight was 2119 Da.