Abstract
GBM cells can easily gain resistance to conventional therapy, and therefore treatment of glioblastoma multiforme (GBM) is difficult. One of the hallmark proteins known to be responsible for this resistance is heat shock protein 27 (Hsp27) which has a key role in the cell survival. Resveratrol, a natural compound, exhibits antitumor effects against GBM, but there are no reports regarding its effect on Hsp27 expression in gliomas. The aim of the present study was to asses the effect of resveratrol on Hsp27 expression and apoptosis in non-transfected and transfected U-87 MG human glioblastoma cells. In order to block the Hsp27 expression, siRNA transfection was performed. Non-transfected and transfected cells were treated with either 10 or 15 μM resveratrol. The effects of resveratrol were compared with quercetin, a well-known Hsp27 inhibitor. Resveratrol was found to induce apoptosis more effectively than quercetin. Our data showed that resveratrol induces dose- and time-dependent cell death. We also determined that silencing of Hsp27 with siRNA makes the cells more vulnerable to apoptosis upon resveratrol treatment. The highest effect was observed in the 15 μM resveratrol and 25 nM siRNA combination group (suppressed Hsp27 expression by 93.4% and induced apoptosis by 101.2%). This study is the first report showing that resveratrol reduces Hsp27 levels, and siRNA-mediated Hsp27 silencing enhances the therapeutic effects of resveratrol in glioma cells. Our results suggest that resveratrol administration in combination with Hsp27 silencing has a potential to be used as a candidate for GBM treatment.
Keywords: Resveratrol, siRNA, Combined therapy, Hsp27, Glioma, Apoptosis
Introduction
Glioblastoma multiforme (GBM-grade IV astrocytoma) is the most common type of the primary malignant brain tumors. The median survival times are < 15 months, and the mortality rate is over 50%. Despite surgical, radiological, and chemotherapeutic interventions, effective therapy for such tumors is still very limited, and the prognosis is poor (Westphal and Lamszus 2011; Rajesh et al. 2017) largely due to the resistance of glioma cells against various therapy strategies. Numerous studies have emphasized that overexpression of stress proteins (also known as Heat shock protein, Hsps) is responsible for therapy resistance, thus reducing Hsp levels that can be an important strategy for improving treatment efficacy of gliomas (Chatterjee and Burns 2017; Calderwood 2018).
Hsps, which are shown to be overexpressed in many cancer types, constitute a large family of proteins with conserved structures. While some Hsps are responsible for maintaining protein homeostasis by acting as molecular chaperones in normal cells, some of them can also help tumor progression in cancer cells. Elevated Hsp levels are particularly effective in promoting cell growth, invasion and metastasis, and inhibition of apoptosis (Chatterjee and Burns 2017; Calderwood 2018). Hsp27, one of the best-studied Hsps, is highly expressed in different brain tumors such as astrocytomas, gliomas, oligodendrogliomas (Zhang et al. 2003; Khalil 2007). Hsp27 functions as an anti-apoptotic molecule, and there is a great deal of evidence which suggest that it inhibits apoptotic signaling pathways, in particular by inhibiting caspase activation (Concannon et al. 2003). Increased Hsp27 levels can stimulate carcinogenesis and are associated with drug resistance, tumor cell proliferation and survival (Garrido et al. 2006; Khalil et al. 2011). Today, different strategies are being tested to downregulate Hsp27 and other Hsps that are overexpressed in cancer cells. One of these strategies is the targeting of Hsps with natural compounds (Önay-Uçar 2015; Önay Uçar et al. 2017). Antioxidant agents have long been studied in cancer research as potential therapeutic agents due to their free radical-scavenging capacity, ability to interact with cell-signaling pathways and to modulate of gene expression. One of the best known Hsp inhibitors as an antioxidant agent is quercetin (3,3′,4′,5-7-pentahydroxyflavone), a natural flavonoid. It has been detected in many fruits and vegetables (Khan et al. 2016; Önay Uçar et al. 2018), and shown to inhibit Hsp27 protein and to facilitate apoptosis of tumor cells by activation of caspases in glioma cells (Jakubowicz-Gil et al. 2013a; Jakubowicz-Gil et al. 2013b; Li et al. 2016; Şengelen and Önay-Uçar 2018).
Resveratrol (trans-3,4′,5-trihydroxystilbene) is a natural polyphenol. It is found in many plant species such as various berries, grapes, peanuts, pines, and herbs (Catalgol et al. 2012; Elshaer et al. 2018). It is known to have antioxidant (Rubiolo and Vega 2008), antiviral (Docherty et al. 1999), antibacterial (Chan 2002), and neuroprotective effects (Rocha-González et al. 2008). Also, current findings indicate that resveratrol has high potential as an anticancer agent (Ko et al. 2017; Elshaer et al. 2018). Many studies have been focused on defining the anticancer effects of resveratrol in different cancers such as brain (Jiang et al. 2009; Lin et al. 2012; Wang et al. 2015), breast (Díaz-Chávez et al. 2013), colorectal (Juan et al. 2008; Sengottuvelan et al. 2009; Buhrmann et al. 2015), kidney (Zhao et al. 2018), prostate (Benitez et al. 2009), myeloma (Shimizu et al. 2006), and leukemia (Chakraborty et al. 2008; Banerjee Mustafi et al. 2010). Studies investigating the effect of resveratrol on Hsp expression in cancer cells are limited. Only a limited number of studies examined the effect of resveratrol on Hsp27 and Hsp70 expressions in breast cancer (Díaz-Chávez et al. 2013), colorectal cancer (Sengottuvelan et al. 2009), and leukemia (Chakraborty et al. 2008; Banerjee Mustafi et al. 2010). However, changes in the expression levels of Hsp in glioma cells upon resveratrol treatment has not been studied so far. Also, resveratrol-induced specific molecular mechanisms which can affect Hsp expression are not very clear in tumor cells and need to be further investigated.
Today, advances in gene silencing technology offer promising approaches for cancer therapy. Small interfering (si)RNA-mediated silencing, one of the RNA interference (RNAi) technologies, is known as a powerful tool to modulate gene expression against cancer (Elbashir et al. 2001; Wang et al. 2011; Shim et al. 2018). For glioma cells, specificity and potency of siRNAs to inhibit Hsp gene expression have been reported in many studies (Önay-Uçar et al. in press). Herein, we used siRNA-mediated Hsp27 silencing to enhance the therapeutic effect of resveratrol.
Thus far, there is no data available regarding the effect of resveratrol on Hsp27 expression in gliomas. The aim of the present study was to investigate the effect of resveratrol treatment on the Hsp27 expression and caspase-3 activity in U-87 MG human glioblastoma cell line. Moreover, we also explored the resveratrol treatment in combination with Hsp27-specific siRNA knockdown. Our findings demonstrated that siRNA-mediated silencing of Hsp27 expression can make the cells more vulnerable to apoptosis when combined with resveratrol treatment. Furthermore, our results revealed that resveratrol is more effective than quercetin as a Hsp inhibitor. These findings highlight the potential use of resveratrol and siRNA in combination as an effective therapeutic in Hsp27 inhibition for the treatment of patients with GBM.
Materials and methods
Experimental reagents and siRNAs
Resveratrol and quercetin (Santa Cruz Biotechnology, Dallas, Texas, USA) were dissolved in dimethyl sulfoxide (DMSO). Standard tissue culture reagents were from Gibco (Carlsbad, CA, USA). siRNA oligonucleotides targeting Hsp27 and a negative control were purchased from Ambion (Carlsbad, CA, USA) and dissolved in ultrapure DNase/RNase-free distilled water to a final concentration of 10 μM. In this study, two different Hsp27 siRNA molecules were used. The sense strand of Hsp27 siRNA 1 (Hsp27 si1) was 5′-GCGUGUCCCUGGAUGUCAAtt-3′, and the antisense strand was 5′-UUGACAUCCAGGGACACGCgc-3′; the sense strand of Hsp27 siRNA 2 (Hsp27 si2) was 5′-GCCGCCAAGUAAAGCCUUAtt-3′, and antisense strand was 5′-UAAGGCUUUACUUGGCGGCag-3′. Scrambled siRNA was used as a negative control. DharmaFECT 4 transfection reagent was obtained from Dharmacon (Cambridge, UK), and Opti-MEM transfection medium was from Invitrogen (Carlsbad, CA, USA). EDTA-free protease inhibitor cocktail was from Roche (Darmstadt, Germany). SMART™ BCA Protein Assay Kit was purchased from iNtRON Biotechnology (Seongnam, Gyeonggi, Korea). Polyvinylidene fluoride (PVDF) membrane for Western blotting and Caspase-3 Colorimetric Activity Assay Kit were purchased from Millipore (Darmstadt, Germany). Mouse anti-Hsp27 monoclonal antibody and horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG were purchased from Enzo Life Sciences (Farmingdale, NY, USA). HRP conjugated GAPDH Loading Control Monoclonal Antibody and Pierce™ ECL Western Blotting Substrate were from ThermoFisher Scientific (Kwartsweg, Bleiswijk, Holland). All other chemicals were obtained from Sigma (St. Louis, MO, USA).
Cells and culture conditions
U-87 MG human glioblastoma cells were maintained at exponential growth in Dulbecco’s modified Eagle’s medium/high glucose (DMEM/High) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS), 1% (v/v) antibiotic-antimycotics solution (100 U/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin B) and 1% (v/v) non-essential amino acids in a humidified atmosphere with 5% CO2 at 37 °C. The cells were passaged every 3 days, always before they reached full confluence. Experiments were performed using cells from passages 3 to 10.
Cell survival assay
MTT (3-(4,5-dimethylthiazol-2-yl)2, 5-diphenyl-tetrazolium bromide) assay was carried out for detecting cytotoxic activities of resveratrol alone or in combination with siRNAs. Briefly, U-87 MG cells at the density 1 × 104 per well were seeded in a 96-well tissue culture plate 24 h prior to treatment. For resveratrol treatments, 1, 10, and 100 μM resveratrol added into the cells, and cell death was analyzed both at 24, 48, and 72 h following treatment. To determine half-maximal inhibitory concentration (IC50) of resveratrol, the agent was added to the culture medium with final concentrations ranging from 0 to 200 μM. The final concentration of DMSO in the culture medium did not exceed 0.2%. To determine whether DMSO influences cell viability, the cells were incubated with 0.2% of DMSO. For detecting cytotoxic activity of cell transfection and combined therapy, the applications were performed as described in the title “Cell transfection and combined therapy” and two different doses of siRNA (25 and 50 nM) were used as final concentrations. After 48 h post-incubation at 37 °C, the cells incubated with 30 μL of MTT (5 mg/mL in sterile D-PBS) for 4 h, and then 150 μL of DMSO was added to dissolve the colored formazan crystals produced from MTT. OD values of the solutions were measured at 540 nm in a microplate reader (EON, BioTek Instruments Inc.). All experiments were performed in quadruplicate in three independent experiments.
Resveratrol and quercetin treatments
For cell treatment with resveratrol or quercetin, U-87 MG human glioblastoma cells were plated in a 6-well plate at a density of 2.8 × 105 and the cells kept overnight. The cells were treated with quercetin (10 and 30 μM) or resveratrol (10 and 15 μM) at different final concentrations. The doses were selected keeping cell viability ≥ 80% at the highest dose applied. The final concentration of DMSO in the culture medium was less than 0.05% (v/v) in treatments. We treated the cells with resveratrol or quercetin for 48 h. Each experiment was performed at least three times.
Cell transfection and combined therapy
Optimization of the cell transfection method and detailed protocols have been described previously (Şengelen and Önay-Uçar 2018). In order to block the expression of Hsp27 gene (HSPB1) in U-87 MG cells, a transfection method with specific siRNAs was used according to the manufacturer’s protocol. Briefly, siRNA transfections were performed in 6-well and 96-well tissue culture plates. Cells were cultured in 6-well plate at seeding density of 2.8 × 105 cells/well or in 96-well plate at a density of 1 × 104 cells/well, and the cells incubated at 37 °C in a CO2 incubator for 24 h until cell confluence reached 60–80%. Prior to transfection, transfection reagent/siRNA complexes were prepared in Opti-MEM media and incubated for 20 min. For each transfection, the final concentration of the transfection reagent was 2.5 μL/mL, siRNA concentrations were 25 and 50 nM. The cells were washed with the D-PBS, fresh growth medium without antibiotics, the complexes were added directly to each well, and the cells were incubated for 5 h at 37 °C. After this time, the medium was replaced with fresh medium alone or with fresh medium containing quercetin or resveratrol for combined therapy groups. For mock transfection, cells were transfected using only transfection reagent without any siRNA. Non-transfected cells were used as a control. Assays were done at 48 h after transfection. The effectiveness of Hsp27 gene silencing or combined therapy was assessed at the protein level by immunoblotting. Three independent experiments were performed.
Immunoblotting assays
Samples were analyzed by Western blotting, as previously described (Şengelen and Önay-Uçar 2018). Briefly, the cells were harvested by trypsinization and centrifugation at 700×g for 10 min. The pellets were resuspended in lysis buffer [20 mM Tris-HCl (pH 6.8), 0.04% (w/v) EDTA, 1% (v/v) Triton X-100, and EDTA-free PIC (one tablet per 50 mL of buffer), 1 mM PMSF], and then the extracts were centrifuged at 20,000×g for 20 min at 4 °C. BCA protein assay kit was used to determine total protein concentration. Equal quantities of protein (30 μg/well) were separated under reducing conditions by SDS-PAGE gel and subsequently transferred to PVDF membranes. All membranes were blocked using 5% non-fat milk in Tris-buffered saline/Tween 20 (TBST) for 1 h before an overnight anti-Hsp27 primer antibody (working dilution 1:1000) incubation at 4 °C. Membranes were washed five times with TBST × 5 min, incubated with IgG-HRP secondary antibody (working dilution 1:5000) for 2 h at RT, and washed again. Protein bands were visualized using an ECL kit. The data were normalized relative to GAPDH (antibody diluted 1:2000). The levels of protein expression were determined using the ImageLab 5.2.1 software (Bio-Rad). At least three independent experiments were performed.
Determination of caspase activity
Caspase-3 activity was assayed by using the Caspase-3 Colorimetric Activity Assay Kit according to the manufacturer’s protocol. Briefly, cells were treated with Cell Lysis Buffer, incubated on ice for 10 min, and centrifuged at 10,000×g for 5 min at 4 °C. The supernatants were incubated with Caspase-3 substrate (1 mM Ac-DEVD-pNA) at 37 °C for 2 h. The caspase-3 activity was measured by a microplate reader at 405 nm. Three independent assays were performed.
Statistical analysis
The quantitative data were presented as mean ± standard deviation (SD) based on at least three independent experiments (n denoting the number of experiments). Nonlinear regression analysis of sigmoidal dose-response curve to calculate IC50 value was performed. All statistical analysis and graph generation were performed using the GraphPad Prism (version 7.00; GraphPad Software, San Diego, CA, USA). The statistical evaluation was performed with one-way analysis of variance (ANOVA) followed by Dunnett’s or Tukey post hoc tests to multiple comparisons. The criterion for statistical significance was P < 0.05.
Results
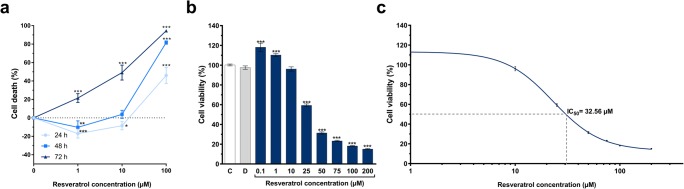
Resveratrol blocks proliferation of human glioblastoma cells in a dose- and time-dependent manner
In order to evaluate the cytotoxic effect of resveratrol in U-87 MG cells and to determine the IC50 value, a modified MTT cell viability assay was performed. Our results showed that resveratrol treatments (1, 10, and 100 μM) in U-87 MG cells induced cell death in a dose- and time-dependent manner (Fig. 1a). The MTT results indicated that 24 h of resveratrol treatment showed proliferative effects at low doses (1 and 10 μM) and inhibited cell viability at high dose (100 μM) by less than 50%. Treatment with each dose (1, 10, and 100 μM) of 72 h resulted in inhibition of cell viability at high levels. Forty-eight hours of treatment did not show a proliferative or cytotoxic effect for 10 μM. According to the results, we examined the cytotoxic activity of resveratrol in wider dose range (0–200 μM) for 48 h and determined the IC50 value. The final concentration of DMSO in the culture medium did not exceed 0.2% (the concentration which did not affect cell viability). We observed that resveratrol blocked U-87 MG cell proliferation in a dose-dependent manner (Fig. 1b). The IC50 value of resveratrol was determined as 32.56 μM (Fig. 1c). The value of IC50 was calculated by fitting the data to a sigmoidal dose-response curve using GraphPad Prism 7.00 software. In our previous study, IC50 value of quercetin was found to be 109.29 μM (Şengelen and Önay-Uçar 2018). We chose two doses of resveratrol and quercetin to be used in experiments according to cell viability = 100% and ≥ 80%. Therefore, resveratrol at 10 and 15 μM, and quercetin at 10 and 30 μM were used to investigate the effects on Hsp27 expression and apoptosis in U-87 MG cells.
Fig. 1.
Effect of resveratrol on the viability of the U-87 MG cell line. The cells (1 × 104 per well) were seeded into 96-well plates and cultured for 24 h. After resveratrol added into the cells, cell viability was determined by MTT assay at the indicated time. a Resveratrol treatments (1, 10, and 100 μM) were associated with an increase in cell death in a dose- and time-dependent manner. b Dose response for 48 h on cell viability of resveratrol treatment versus untreated group. The cells either left untreated (C, control), or treated with 0.2% DMSO (D) or resveratrol (between 0.1 and 200 μM). Resveratrol was found to affect cell viability in a dose-dependent manner. DMSO (the final concentration in the culture medium did not exceed 0.2%) which used to dissolve resveratrol had no effect on cell viability. c Dose-response curve (R2 = 0.96) and IC50 value (32.56 μM) of resveratrol for 48 h. The value of IC50 was calculated by fitting the data to a sigmoidal dose-response curve using nonlinear regression analysis. The data points were presented as mean ± SD of three independent experiments performed in quadruplicate (n = 12). ***P < 0.001 versus untreated group (C) determined by one-way ANOVA using Dunnett’s multiple comparison test
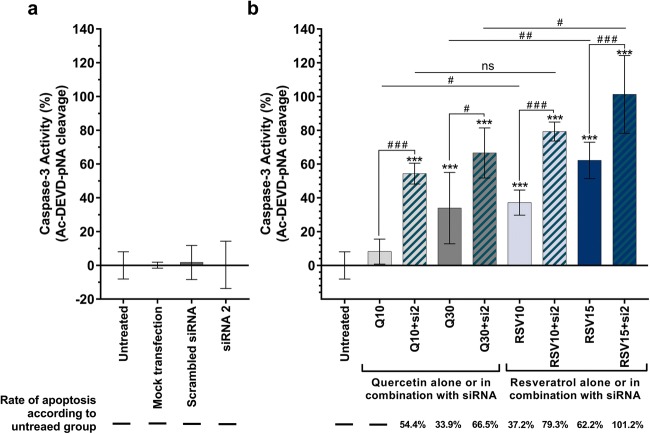
siRNA knockdown efficiency
In order to determine Hsp27 gene (HSPB1) knockdown efficiency, the U-87 MG cells were transfected with different concentrations of siRNAs as described in the “Materials and Methods.” Two different commercial siRNAs (si1 and si2) targeting different regions of Hsp27 mRNA were tested in the cells. Optimization of the amount of transfection reagent to be used in blocking HSPB1 has been previously examined (Şengelen and Önay-Uçar 2018). Our experiments showed that transfection of U-87 MG cells was successful and the level of Hsp27 expression was markedly reduced (Fig. 2a). Although both si1 and si2 treatments reduced Hsp27 levels, si2 was found to be more effective (P < 0.001), and it was used in further experiments. After transfection with 25 nM si1 or si2, expression of Hsp27 decreased by 18.5% and 39.8%, respectively. Results of transfection with 50 nM si1 or si2 resulted in knockdown efficiency of 27.5% and 48.2%, respectively. Also, there was no statistically significant effect of scrambled siRNA or transfection agent (mock transfection) versus untreated group on Hsp27 expression (P > 0.05) levels. Similarly, we did not observe significant cytotoxic effects (Fig. 2b) in the experimental groups except for 50 nM si2-treated group when compared to the untreated cells. However, the cytotoxic effect in this group did not exceed 20%.
Fig. 2.
Knockdown efficiency of Hsp27 by siRNA in U-87 MG cells. Five different groups were used for the evaluation: untreated cells, cells transfected with the transfection reagent only (mock transfection), the cells transfected with scrambled siRNA or two different commercial siRNAs. Below each set of western blots are the values of the knockdown efficiency of siRNAs on Hsp27 protein levels, which were normalized to those of GAPDH. a On western blot data, bars represent the mean ± SD from at least three individual experiments. ***P < 0.001 versus untreated group. ###P < 0.001, siRNA 1 versus siRNA 2. P values were determined by one-way ANOVA followed by Tukey’s post hoc test to multiple comparisons. b On cell viability data determined by MTT assay, bars represent the mean ± SD from at least three individual experiments. *P < 0.05 versus untreated group determined by one-way ANOVA followed by Dunnett’s multiple comparison
The effects of resveratrol alone or in combination with siRNA on human glioblastoma cell viability
We examined the effect of resveratrol treatment on cellular proliferation in U-87 MG cells either alone or in combination with Hsp27 knockdown (resveratrol and siRNA). For this purpose, we used a modified MTT assay and presented our results (Fig. 3) in comparison with the previously reported cytotoxicity data for quercetin (Şengelen and Önay-Uçar 2018). Quercetin and resveratrol at 10 μM doses showed no statistically significant difference on cell viability compared to the untreated group, and the effects of these non-cytotoxic doses in experiments were analyzed. The cell viability was found to be less than 80% in the combined treatment groups transfected with 50 nM siRNA, except for the combination of 50 nM si2 and 10 μM quercetin. Therefore, 50 nM siRNA concentration was not studied for further analysis, and combined therapy groups that were more than 20% cytotoxic on the cells were not used in experiments.
Fig. 3.
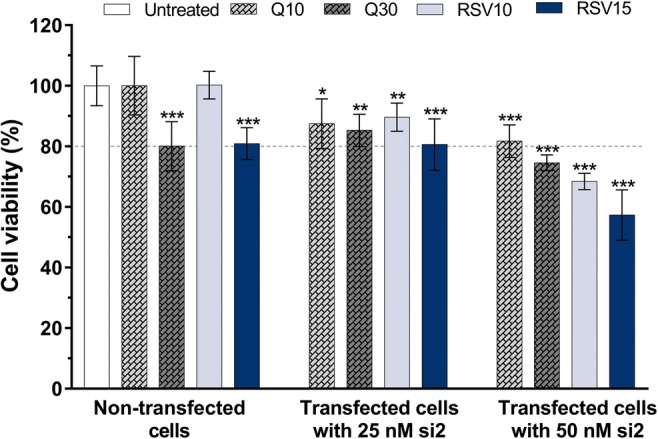
Effects of quercetin and resveratrol treatments, alone or in combination with Hsp27 siRNA on U-87 MG human glioblastoma cells. The cells (1 × 104 per well) were seeded into 96-well plates 24 h prior to treatment. Then, the cells either left untreated, or treated with quercetin (10 and 30 μM) or resveratrol (10 and 15 μM) alone or in combination with Hsp27 si2 for 48 h. Cell viability was determined by MTT assay. The cell viability was less than 80% in the combined treatment groups transfected with only 50 nM si2 (except for the combination of 50 nM si2 and 10 μM Q). Graph represents the mean ± SD (n = 6). **P < 0.01 and ***P < 0.001 versus untreated group. P values were determined by one-way ANOVA using Dunnett’s multiple comparison test. si siRNA, Q quercetin, RSV resveratrol
Combined therapy inhibits Hsp27 expression more powerfully in human glioblastoma cells
Western blot analysis (Fig. 4) was performed to evaluate the effect of resveratrol treatment on Hsp27 expression in U-87 MG cells. We observed that resveratrol-alone treatment inhibited the expression of Hsp27 as much as treatment with quercetin, which is known to be a good Hsp27 inhibitor. There was no statistically significant difference between the resveratrol or quercetin administered groups (P > 0.05). Analysis of data showed that resveratrol (10 and 15 μM) reduced the expression of Hsp27 by 21.1% and 47.7%, respectively. Similarly, 10 μM and 30 μM quercetin decreased the level of Hsp27 by 25.8% and 45.8%, respectively. For combined therapy, cells were exposed to resveratrol or quercetin treatment after cell transfection with 25 nM si2. The effect of Hsp27 knockdown was assessed by decrease immunoblotting. Western blot data showed that Hsp27 was effectively silenced in the combined therapy groups. Quantification of western blot results revealed that 10 μM and 30 μM quercetin reduced the level of Hsp27 in the cells transfected with siRNA by 27.6% and 81.5%, respectively. The use of 10 and 15 μM resveratrol and siRNA combinations resulted in a decrease in the level of Hsp27 by 49.4% and 93.4%, respectively. These findings suggest that the combined treatments were much more effective in comparison to the individual compound (excepting 10 μM quercetin alone) (P < 0.001). Among all treatments, the combination of 15 μM resveratrol and 25 nM si2 resulted in the highest level (93.4%) of Hsp27 silencing.
Fig. 4.

Expression of Hsp27 in response to quercetin (10 and 30 μM) and resveratrol (10 and 15 μM) treatments, alone or in combination with Hsp27 si2 (25 nM) for 48 h. Western blot analysis showed that the combined treatments were much more effective in comparison to the individual compound (excepting 10 μM quercetin alone). All data were normalized to GAPDH. Graph represents the mean ± SD (n = 7). ***P < 0.001 versus untreated group. &&&P < 0.001 versus si2. ns indicates P > 0.05 and ###P < 0.001, which show multiple comparisons between different groups. P values were determined by one-way ANOVA using Tukey’s multiple comparison test. si siRNA, Q quercetin, RSV resveratrol, ns not significant
siRNA-mediated silencing makes U-87 MG cells more vulnerable to apoptosis upon resveratrol treatment
Caspase-3 activity analysis was used to estimate the level of apoptosis induction in U-87 MG cells. According to the results (Fig. 5), resveratrol was found to induce apoptosis more effectively than quercetin. Resveratrol and quercetin administrations (with only 10 μM quercetin as an exemption) showed statistically significant apoptosis induction when compared with the untreated group (P < 0.001). Quercetin at 30 μM final concentrations increased caspase-3 activity by 33.9%, whereas 10 μM and 15 μM resveratrol increased the apoptotic rate by 37.2% and 62.2%, respectively. For combined therapy, 25 nM si2 was used for cell transfection, in accordance with western blot data and MTT cell viability assays. After cell transfection, resveratrol or quercetin was administered to the cells. Caspase-3 activity analysis results revealed that apoptosis was induced at a higher level in combined therapy groups when compared to resveratrol- or quercetin-treated cells alone. When the cells were treated with 10 μM and 15 μM resveratrol in combination with Hsp27 siRNA, caspase-3 activity was increased by 79.3% and 101.2%, respectively. Moreover, combination of 10 μM and 30 μM quercetin and siRNA increased caspase-3 activity by 54.4% and 66.5%, respectively. The 15 μM resveratrol and 25 nM si2 combined group showed apoptotic activity at the highest rate (101.2%). These results demonstrate that transfection of cells with siRNA prior to antioxidant agent treatment can make the cells more vulnerable to apoptosis.
Fig. 5.
Effect of a siRNA transfection, b quercetin (10 and 30 μM), and resveratrol (10 and 15 μM) treatments alone or in combination with Hsp27 siRNA on caspase-3 activity in U-87 MG cells. Caspase-3 activity analysis showed that siRNA-mediated silencing makes U-87 MG cells more vulnerable to apoptosis upon quercetin or resveratrol treatment, while siRNA transfection alone did not induce apoptosis. The most effective apoptotic activity was observed in the RSV15 + si2 group. Graph represents the mean ± SD (n = 6). ***P < 0.001 versus untreated group. ns indicates P > 0.05 and ###P < 0.001, which show multiple comparisons between different groups. P values were determined by one-way ANOVA using Dunnett’s or Tukey’s multiple comparison tests. si siRNA, Q quercetin, RSV resveratrol, ns not significant
Discussion
Hsp27 acts as an anti-apoptotic molecule in tumor cells and is highly expressed in brain tumors (Concannon et al. 2003; Zhang et al. 2003). In a study conducted by Khalil (2007), it has been shown that Hsp27 can be used as a biomarker for GBM. Many reports indicate that a wide range of natural products, especially antioxidant compounds, have the high potential to inhibit Hsp27 in many cancers, including gliomas (Önay-Uçar 2015; Önay Uçar et al. 2017). Our earlier experiments conducted to inhibit Hsp27 protein in gliomas revealed that the Viscum album extract (Uçar et al. 2012), quercetin, and rosmarinic acid (Şengelen and Önay-Uçar 2018) are effective agents in reducing Hsp27 levels and inducing apoptosis. However, there is no report on the effect of resveratrol on Hsp levels in glioma cells.
Resveratrol, a natural polyphenolic compound, is efficiently absorbed after oral administration, but rapidly metabolized and well tolerated in humans. This leads to poor bioavailability of resveratrol and thus may require use of high doses. Numerous studies have reported that it has many beneficial biological and pharmacological activities and that low daily doses of resveratrol have potent chemopreventive effects in vivo. More importantly, evidence suggests anticancer properties of resveratrol (Ko et al. 2017; Elshaer et al. 2018). Herein, our results indicated that resveratrol significantly inhibited U-87 MG human glioblastoma cell growth in a dose- and time-dependent manner. The highest rate of cell death was observed after 72 h incubation with 100 μM resveratrol, but even at low concentration (1 μM), it was significantly effective (21.4%). Conversely, a significantly lower cell death was observed after 24 h of resveratrol treatment (only 46% cell death at 100 μM). According to MTT assay results, the study was continued with 48 h of treatment, and IC50 value of resveratrol was determined as 32.56 μM. The value was lower when compared with IC50 value of quercetin which was found to be 109.29 μM in our previous study (Şengelen and Önay-Uçar 2018). While the 10 μM concentration of both quercetin and resveratrol did not show proliferative or cytotoxic effects on the cells, it was observed that the difference between the IC50 values was high, which suggests that higher doses (≥ 25 μM) of resveratrol are more toxic to U-87 MG cells compared with those of quercetin.
We previously showed that quercetin suppressed the expression of Hsp27 in U-87 MG human glioblastoma cells (Şengelen and Önay-Uçar 2018). Similar results were obtained again when quercetin was applied to the cells for the present study. This finding is in agreement with other studies, which examined the effect of quercetin on Hsp27 expression in glioblastoma cells (Jakubowicz-Gil et al. 2013a; Jakubowicz-Gil et al. 2013b; Li et al. 2016). The results of western blot analysis (Fig. 4) revealed that resveratrol treatment inhibited Hsp27 expression at similar rates to quercetin, and there was no statistically significant difference between quercetin (10 or 30 μM, respectively) and resveratrol (10 or 15 μM, respectively) treatment groups. The best result for resveratrol alone treatment (15 μM) resulted in a reduction by 47.7% in Hsp27 expression compared the untreated cells (P < 0.001). This shows that resveratrol has a high potential to be a used as an effective Hsp27 inhibitor such as quercetin.
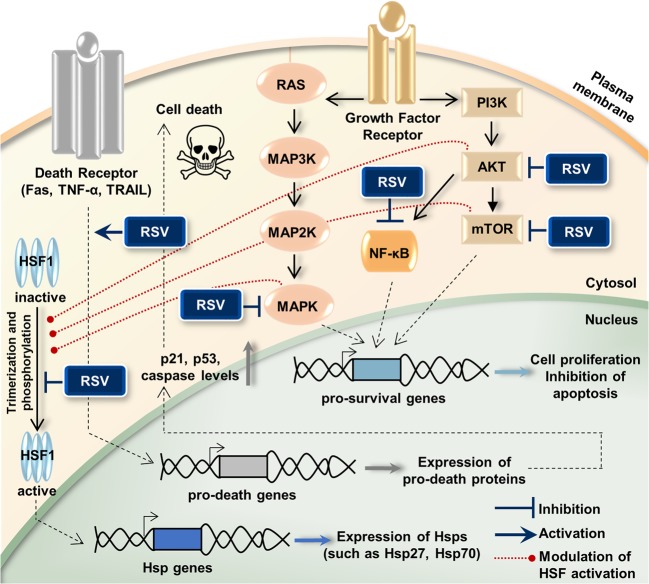
Being a lipophilic compound, resveratrol can trigger many intracellular pathways by crossing the plasma membrane (Pervaiz and Holme 2009). Investigations into the anticancer effects of resveratrol have revealed that it interacts with many molecular targets which are the members of cell survival or cell death signaling pathways (Fig. 6). Among these, the well-defined ones are the PI3K, AKT, mTOR (Jiang et al. 2009; Banerjee Mustafi et al. 2010; He et al. 2011), NF-κB (Benitez et al. 2009; Ren et al. 2013), MAPKs (Banerjee Mustafi et al. 2010; Parekh et al. 2011), Fas, TNF-α, TRAIL (Delmas et al. 2003; Bickenbach et al. 2008; Ganapathy et al. 2010), p21, p53, Bax, and caspases (Kim et al. 2003; Li et al. 2018). Additionally, one of the most important targets is the stress proteins (e.g., Hsp27, Hsp70), but there are few studies showing the effect of resveratrol on Hsp levels in cancer cells. In a study conducted on K562 leukemia cells, it has been shown that resveratrol inhibits proliferation by downregulating phosphorylation and activity of AKT, modulating the function of heat shock factor 1 (HSF1), and inhibiting expression of Hsp70 (Banerjee Mustafi et al. 2010). Another study in the same cell type emphasized that resveratrol induces apoptosis and inhibits expression of Hsp70 by preventing HSF1 transcriptional activity (Chakraborty et al. 2008). Resveratrol has also been shown to inhibit Hsp27 expression, induce caspase-3 and caspase-9 activation, and sensitize breast cancer cells to doxorubicin treatment (Díaz-Chávez et al. 2013). It has been shown that resveratrol inhibits Cox-2, a cellular signaling protein, and suppresses the overexpression of Hsp70 and Hsp27 in colon cancer (Sengottuvelan et al. 2009). For brain tumors, there are no reports on the effect of resveratrol on Hsp levels. In the present study, we have evaluated for the first time the effect of resveratrol on Hsp27 inhibition in human glioblastoma cells. The current evidence can help to better understand the signaling pathways involved in resveratrol-mediated downregulation of Hsp27. The reduction of Hsp27 expression levels in the cells treated with resveratrol may be associated with downregulation of HSF1, the common transcription factor of Hsp. The modulation of HSF1 activation (trimerization and phosphorylation) by resveratrol is also likely to occur as a result of suppression of proteins which are involved in HSF activation such as MAPKs, AKT, or mTOR (Wang et al. 2006; Banerjee Mustafi et al. 2010; Dayalan Naidu et al. 2016; Holmes et al. 2018).
Fig. 6.
Resveratrol (RSV) interacts with various intracellular molecules, regulates Hsp expressions, and induces cell death. RSV inhibits cell proliferation by blocking the PI3K/AKT/mTOR, NF-κB, and MAPK pathways, which are effective in cell survival. It induces apoptosis by interacting with the cell death pathways. Also, an important role of RSV is its effect in reducing elevated levels of Hsp which are effective in promoting the cell growth and inhibiting apoptosis. RSV modulates activation of HSF1, the common transcription factor of Hsp, by blocking the protein kinases (MAPKs, AKT, or mTOR), and so inhibits Hsp expression. AKT (or PKB) protein kinase B, HSF heat shock factor, Hsp heat shock protein, MAPK mitogen-activated protein kinase, MAP2K mitogen-activated protein kinase kinase, MAP3K mitogen-activated protein kinase kinase kinase, mTOR mammalian target of rapamycin, NF-κB nuclear factor kappa B, PI3K phosphatidylinositol 3-kinase, TNF-α tumor necrosis factor alpha, TRAIL TNF-related apoptosis-inducing ligand (TRAIL)
Our study and some other studies mentioned above emphasize that resveratrol can be used as an Hsp inhibitor for cancer therapy. On the contrary, there are also studies in which resveratrol has been reported to be the activator of Sirtuin 1 (SIRT1), which prolongs HSF1 binding to heat shock promoter and thereby enhances Hsp expression (Borra et al. 2005; Westerheide et al. 2009). In contrast with this phenomenon, the literature also shows that resveratrol is not a direct activator of SIRT1 (Beher et al. 2009). Moreover, evidence indicates that SIRT1 is required for resveratrol-mediated antitumoral effects in cancer cells (Frazzi et al. 2013; Yang et al. 2013). It is well-known that SIRT1 and PI3K/AKT/NF-κB pathways are closely related, and resveratrol shows the anticancer effect by SIRT1-dependent inhibition of NF-κB (Buhrmann et al. 2016; Elshaer et al. 2018). In addition, resveratrol induces SIRT1-dependent apoptosis by suppressing PI3K/AKT signaling activity (Chen et al. 2012; Chai et al. 2017). Resveratrol is also found to activate ERK through induction of SIRT1 (Huang et al. 2008). There is also information in the literature that the increase in ERK1/2 activity and decrease in AKT activity by resveratrol treatment negatively affect HSF1 activation in cancer cells, and therefore, Hsp expression is downregulated (Banerjee Mustafi et al. 2010). In the light of this information, although SIRT1 is known to increase the activity of HSF1, it is understood that increased levels of SIRT1 under resveratrol treatment may result in a reduction of HSF1 and Hsp levels via ERK and AKT pathways in the cancer cells. This supports our result of Hsp27 inhibition as a result of resveratrol treatment in glioma cells, and more research is needed to clarify this matter.
There are examples of various combined therapy applications to enhance the anticancer effect of antioxidant compounds in the literature: antioxidant-antioxidant (Narayanan et al. 2009; Del Follo-Martinez et al. 2013), antioxidant-drug (Jakubowicz-Gil et al. 2010; Staedler et al. 2011; Lin et al. 2012; Jakubowicz-Gil et al. 2013a), antioxidant-oligonucleotide (Jakubowicz-Gil et al. 2013b; Chen et al. 2016; Şengelen and Önay-Uçar 2018), etc. Del Follo-Martinez et al. (2013) found that resveratrol and quercetin combination is an effective mixture to induce apoptosis in colon cancer cells. In a study by Lin et al. (2012), resveratrol (an antioxidant agent) has been shown to enhance the therapeutic effect of temozolomide (a drug) by inhibiting autophagy in gliomas. In studies conducted by Jakubowicz-Gil and colleagues, co-administration of quercetin (an antioxidant agent) and temozolomide has been shown to be a useful, potent, and promising combination for cell death in the treatment of brain tumors (Jakubowicz-Gil et al. 2013a; Jakubowicz-Gil et al. 2013b). Chen et al. (2016) reported that suppression of Hsp27 by short hairpin RNA increases the anti-tumor effects of quercetin in human leukemia cells. In our previous study, we combined rosmarinic acid, an antioxidant agent, with Hsp27 siRNAs to enhance its effects on Hsp27 expression and caspase-3 induction in human glioblastoma cells. We demonstrated that the combined therapy suppressed Hsp27 expression by 90.5% and increased caspase-3 activity by 58%. We have shown that an antioxidant agent and siRNA in combination can be a powerful combination for glioblastoma treatment (Şengelen and Önay-Uçar 2018). The aim of the present study was to demonstrate the effect of resveratrol alone or in combination with siRNA on the Hsp27 expression and apoptosis in human glioblastoma cells.
Today, siRNA-mediated silencing, one of the targeted gene silencing techniques, is presented as a powerful tool against cancer (Elbashir et al. 2001; Wang et al. 2011; Shim et al. 2018). Several studies have demonstrated the efficacy of using siRNA to silence Hsp genes in gliomas (Önay-Uçar et al. in press). In our previous study, we reported that siRNA-mediated silencing is an effective tool for the inhibition of Hsp27 expression (Şengelen and Önay-Uçar 2018). In the present study, after cell transfection, resveratrol or quercetin treatment was administered to the cells, and the effect of combined therapy on Hsp27 expression was examined. Combined treatment was observed as a much more effective treatment in comparison to the individual compound (except for the only 10 μM quercetin). Among all treatments, a 15 μM resveratrol and 25 nM si2 combination provided the highest level of Hsp27 silencing (by 93.4%). The combination of 30 μM quercetin with 25 nM si2 provided 81.5% silencing (Fig. 4). The synergistic effect between resveratrol or quercetin and siRNA compared with the single application of either demonstrated that the combined therapy has strong potential in the inhibition of Hsp27 for GBM treatment.
Caspases are a family of protease enzymes which play a central role in mediating various apoptotic responses. During the activation of the caspases, a cascade of signaling is generated, and caspase-3 activation is involved in the final step. To examine the apoptotic induction in resveratrol- or quercetin-treated U-87 MG cells, we evaluated the caspase-3 activation after incubation with resveratrol (10 and 15 μM) or quercetin (10 and 30 μM) for 48 h. Herein, we showed that apoptosis is induced in the presence of resveratrol or quercetin in a dose-dependent manner in human glioblastoma cells, and resveratrol shows good potential as an inducer of apoptosis (Fig. 5). Induction of caspase-3 activity was observed after resveratrol treatment with 10 μM and 15 μM final concentrations at ratio of 37.2% and 62.2%, respectively. In contrast to resveratrol, quercetin at 10 μM final concentration did not induce caspase-3 activation, while in the presence of 30 μM quercetin, induction of apoptosis was observed in 33.9%. In addition, we found that the reduction of Hsp27 expression as a result of siRNA transfection alone was ineffective in activating of caspase-3. The fact that the siRNA-mediated silencing of Hsp27 did not reveal a statistically significant result on caspase-3 induction suggests that the death program may be induced in a different way. Studies supporting this are available in the literature. The decreased level of molecular chaperones is likely to lead to cell death by causing the accumulation of many protein aggregates and the promotion of endoplasmic reticulum (ER) stress (Jakubowicz-Gil et al. 2013a; Jakubowicz-Gil et al. 2013b), or caspase-independent death pathways (Nylandsted et al. 2000). Even so, further studies are required to clarify this matter.
Besides, the reduction of Hsp27 levels as a result of siRNA-mediated silencing was also shown to make the glioma cells more vulnerable to apoptotic induction after resveratrol or quercetin treatment. Interestingly, in healthy cells having less Hsp27 levels than in cancer cells, resveratrol has been shown to be antiapoptotic and to protect cells against stress via Hsp27 upregulation (Zhang et al. 2015; Zhou et al. 2018). The antiapoptotic effect in healthy cells and apoptotic activity in cancer cells make resveratrol a potential agent for cancer treatment. In our study, both doses of quercetin (10 and 30 μM) induced caspase-3 induction in human glioblastoma cells transfected with siRNA (54.4% and 66.5%, respectively). Moreover, caspase-3 activities were increased at higher levels (79.3% and 101.2%) after siRNA transfection and subsequent resveratrol incubations (10 and 15 μM, respectively). These findings indicate that resveratrol and siRNA in combination seems to be a potent and promising anticarcinogen agent, and it may be useful for glioma therapy by inducing caspase-3 activity.
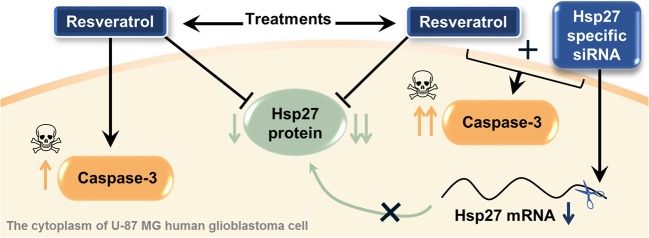
In conclusion, the present study is the first to demonstrate that resveratrol suppresses Hsp27 expression, and silencing Hsp27 with siRNA enhances the therapeutic effects of resveratrol in U-87 MG human glioblastoma cells. Briefly, herein, it was determined that the combined therapy is a more effective treatment alternative for both Hsp27 suppression and caspase-3-dependent apoptosis induction, when compared to resveratrol-treated cells alone (Fig. 7). This indicates that siRNA-mediated knockdown of Hsp27 makes U-87 MG cells vulnerable to apoptosis induction upon resveratrol treatment. This confirms that Hsp27, a molecular preservative, is responsible for resistance to apoptosis in glioma cells. This study has also showed that resveratrol represses Hsp27 expresion and triggers caspase-3-induced apoptosis at a higher level than the Hsp27 inhibitor quercetin (used in this as a positive control). This suggests that resveratrol may be an effective adjuvant in the treatment of brain tumors by targeting inhibition of Hsp27 and induction of apoptosis. Furthermore, we have also shown that combined therapy with siRNA and resveratrol is a promising combination and can be an ideal candidate for therapy strategies against glioma cells. However, it would be necessary to explain the exact mechanism of this combined therapy approach and to determined long-term outcomes in vivo to make it a universally accepted treatment strategy. Further research will be needed to determine the mechanisms by which resveratrol is effective in modulating Hsp expression in brain tumors. Our further studies will focus on dissecting the exact mechanism of action of resveratrol in glioma cells.
Fig. 7.
Overview of the study outputs. The results showed that combined therapy (resveratrol + Hsp27-specific siRNA) is more effective than the resveratrol treatment alone for both Hsp27 suppression and caspase-3-dependent apoptosis induction in U-87 MG human glioblastoma cells
Abbreviations
- AKT
Protein kinase B (PKB)
- DMSO
Dimethyl sulfoxide
- ER
Endoplasmic reticulum
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- GBM
Glioblastoma multiforme
- HSF
Heat shock factor
- Hsp
Heat shock protein
- IC50
The half-maximal inhibitory concentration
- MAPK
Mitogen-activated protein kinase
- mTOR
Mammalian target of rapamycin
- MTT
3-(4,5-Dimethylthiazol-2-yl) 2, 5-diphenyl-tetrazolium bromide
- NF-κB
Nuclear factor kappa B
- PI3K
Phosphatidylinositol 3-kinase
- RNAi
RNA interference
- siRNA
Small interfering RNA
- Sirtuin 1
SIRT1
- TNF-α
Tumor necrosis factor alpha
- TRAIL
TNF-related apoptosis-inducing ligand
Funding information
This work was supported by the Istanbul University Research Foundation, Turkey (Project numbers 57959 and BEK-24987).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Banerjee Mustafi S, Chakraborty PK, Raha S. Modulation of Akt and ERK1/2 pathways by resveratrol in chronic myelogenous leukemia (CML) cells results in the downregulation of Hsp70. PLoS One. 2010;5(1):e8719. doi: 10.1371/journal.pone.0008719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beher D, Wu J, Cumine S, Kim KW, Lu SC, Atangan L, Wang M. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem Biol Drug Des. 2009;74(6):619–624. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- Benitez DA, Hermoso MA, Pozo-Guisado E, Fernández-Salguero PM, Castellón EA. Regulation of cell survival by resveratrol involves inhibition of NFκB-regulated gene expression in prostate cancer cells. Prostate. 2009;69(10):1045–1054. doi: 10.1002/pros.20953. [DOI] [PubMed] [Google Scholar]
- Bickenbach KA, Veerapong J, Shao MY, Mauceri HJ, Posner MC, Kron SJ, Weichselbaum RR. Resveratrol is an effective inducer of CArG-driven TNF-alpha gene therapy. Cancer Gene Ther. 2008;15(3):133–139. doi: 10.1038/sj.cgt.7701103. [DOI] [PubMed] [Google Scholar]
- Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280(17):17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- Buhrmann C, Shayan P, Kraehe P, Popper B, Goel A, Shakibaei M. Resveratrol induces chemosensitization to 5-fluorouracil through up-regulation of intercellular junctions, epithelial-to-mesenchymal transition and apoptosis in colorectal cancer. Biochem Pharmacol. 2015;98(1):51–68. doi: 10.1016/j.bcp.2015.08.105. [DOI] [PubMed] [Google Scholar]
- Buhrmann C, Shayan P, Popper B, Goel A, Shakibaei M. Sirt1 is required for resveratrol-mediated Chemopreventive effects in colorectal Cancer cells. Nutrients. 2016;8(3):145. doi: 10.3390/nu8030145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood SK. Heat shock proteins and cancer: intracellular chaperones or extracellular signalling ligands? Philos Trans R Soc Lond Ser B Biol Sci. 2018;373(1738):20160524. doi: 10.1098/rstb.2016.0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalgol B, Batirel S, Taga Y, Ozer NK. Resveratrol: French paradox revisited. Front Pharmacol. 2012;3:141. doi: 10.3389/fphar.2012.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai R, Fu H, Zheng Z, Liu T, Ji S, Li G. Resveratrol inhibits proliferation and migration through SIRT1 mediated post-translational modification of PI3K/AKT signaling in hepatocellular carcinoma cells. Mol Med Rep. 2017;16(6):8037–8044. doi: 10.3892/mmr.2017.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty PK, Mustafi SB, Ganguly S, Chatterjee M, Raha S. Resveratrol induces apoptosis in K562 (chronic myelogenous leukemia) cells by targeting a key survival protein, heat shock protein 70. Cancer Sci. 2008;99(6):1109–1116. doi: 10.1111/j.1349-7006.2008.00809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MM. Antimicrobial effect of resveratrol on dermatophytes and bacterial pathogens of the skin. Biochem Pharmacol. 2002;63(2):99–104. doi: 10.1016/s0006-2952(01)00886-3. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Burns TF. Targeting heat shock proteins in cancer: a promising therapeutic approach. Int J Mol Sci. 2017;18(9):E1978. doi: 10.3390/ijms18091978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Xiao X, Feng X, Li W, Zhou N, Zheng L, Sun Y, Zhang Z, Zhu W. Resveratrol induces Sirt1-dependent apoptosis in 3T3-L1 preadipocytes by activating AMPK and suppressing AKT activity and survivin expression. J Nutr Biochem. 2012;23(9):1100–1112. doi: 10.1016/j.jnutbio.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Chen X, Dong XS, Gao HY, Jiang YF, Jin YL, Chang YY, Chen LY, Wang JH. Suppression of HSP27 increases the anti-tumor effects of quercetin in human leukemia U937 cells. Mol Med Rep. 2016;13(1):689–696. doi: 10.3892/mmr.2015.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concannon CG, Gorman AM, Samali A. On the role of Hsp27 in regulating apoptosis. Apoptosis. 2003;8(1):61–70. doi: 10.1023/a:1021601103096. [DOI] [PubMed] [Google Scholar]
- Dayalan Naidu S, Sutherland C, Zhang Y, Risco A, de la Vega L, Caunt CJ, Hastie CJ, Lamont DJ, Torrente L, Chowdhry S, Benjamin IJ, Keyse SM, Cuenda A, Dinkova-Kostova AT. Heat shock factor 1 is a substrate for p38 mitogen-activated protein kinases. Mol Cell Biol. 2016;36(18):2403–2417. doi: 10.1128/MCB.00292-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Follo-Martinez A, Banerjee N, Li X, Safe S, Mertens-Talcott S. Resveratrol and quercetin in combination have anticancer activity in colon cancer cells and repress oncogenic microRNA-27a. Nutr Cancer. 2013;65(3):494–504. doi: 10.1080/01635581.2012.725194. [DOI] [PubMed] [Google Scholar]
- Delmas D, Rebe C, Lacour S, Filomenko R, Athias A, Gambert P, Cherkaoui-Malki M, Jannin B, Dubrez-Daloz L, Latruffe N, Solary E. Resveratrol-induced apoptosis is associated with Fas redistribution in the rafts and the formation of a death-inducing signaling complex in colon cancer cells. J Boil Chem. 2003;278(42):41482–41490. doi: 10.1074/jbc.M304896200. [DOI] [PubMed] [Google Scholar]
- Díaz-Chávez J, Fonseca-Sánchez MA, Arechaga-Ocampo E, Flores-Pérez A, Palacios-Rodríguez Y, Domínguez-Gómez G, Marchat LA, Fuentes-Mera L, Mendoza-Hernández G, Gariglio P, López-Camarillo C. Proteomic profiling reveals that resveratrol inhibits HSP27 expression and sensitizes breast cancer cells to doxorubicin therapy. PLoS One. 2013;8(5):e64378. doi: 10.1371/journal.pone.0064378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty JJ, Fu MM, Stiffler BS, Limperos RJ, Pokabla CM, DeLucia AL. Resveratrol inhibition of herpes simplex virus replication. Antivir Res. 1999;43(3):145–155. doi: 10.1016/s0166-3542(99)00042-x. [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411(6836):494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Elshaer M, Chen Y, Wang XJ, Tang X. Resveratrol: an overview of its anti-cancer mechanisms. Life Sci. 2018;207:340–349. doi: 10.1016/j.lfs.2018.06.028. [DOI] [PubMed] [Google Scholar]
- Frazzi R, Valli R, Tamagnini I, Casali B, Latruffe N, Merli F. Resveratrol-mediated apoptosis of hodgkin lymphoma cells involves SIRT1 inhibition and FOXO3a hyperacetylation. Int J Cancer. 2013;132(5):1013–1021. doi: 10.1002/ijc.27748. [DOI] [PubMed] [Google Scholar]
- Ganapathy S, Chen Q, Singh KP, Shankar S, Srivastava RK. Resveratrol enhances antitumor activity of TRAIL in prostate cancer xenografts through activation of FOXO transcription factor. PLoS One. 2010;5(12):e15627. doi: 10.1371/journal.pone.0015627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumourigenic properties. Cell Cycle. 2006;5(22):2592–2601. doi: 10.4161/cc.5.22.3448. [DOI] [PubMed] [Google Scholar]
- He X, Wang Y, Zhu J, Orloff M, Eng C. Resveratrol enhances the anti-tumor activity of the mTOR inhibitor rapamycin in multiple breast cancer cell lines mainly by suppressing rapamycin-induced AKT signaling. Cancer Lett. 2011;301(2):168–176. doi: 10.1016/j.canlet.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Holmes B, Benavides-Serrato A, Freeman RS, Landon KA, Bashir T, Nishimura RN, Gera J. mTORC2/AKT/HSF1/HuR constitute a feed-forward loop regulating Rictor expression and tumor growth in glioblastoma. Oncogene. 2018;37(6):732–743. doi: 10.1038/onc.2017.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Gan Q, Han L, Li J, Zhang H, Sun Y, Zhang Z, Tong T. SIRT1 overexpression antagonizes cellular senescence with activated ERK/S6K1 signaling in human diploid fibroblasts. PLoS One. 2008;3(3):e1710. doi: 10.1371/journal.pone.0001710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowicz-Gil J, Langner E, Bądziul D, Wertel I, Rzeski W. Apoptosis induction in human glioblastoma multiforme T98G cells upon temozolomide and quercetin treatment. Tumour Biol. 2013;34(4):2367–2378. doi: 10.1007/s13277-013-0785-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowicz-Gil J, Langner E, Bądziul D, Wertel I, Rzeski W. Silencing of Hsp27 and Hsp72 in glioma cells as a tool for programmed cell death induction upon temozolomide and quercetin treatment. Toxicol Appl Pharmacol. 2013;273(3):580–589. doi: 10.1016/j.taap.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Jakubowicz-Gil J, Langner E, Wertel I, Piersiak T, Rzeski W. Temozolomide, quercetin and cell death in the MOGGCCM astrocytoma cell line. Chem Biol Interact. 2010;188(1):190–203. doi: 10.1016/j.cbi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Jiang H, Shang X, Wu H, Gautam SC, Al-Holou S, Li C, Kuo J, Zhang L, Chopp M. Resveratrol downregulates PI3K/Akt/mTOR signaling pathways in human U251 glioma cells. J Exp Ther Oncol. 2009;8(1):25–33. [PMC free article] [PubMed] [Google Scholar]
- Juan ME, Wenzel U, Daniel H, Planas JM. Resveratrol induces apoptosis through ROS-dependent mitochondria pathway in HT-29 human colorectal carcinoma cells. J Agric Food Chem. 2008;56(12):4813–4818. doi: 10.1021/jf800175a. [DOI] [PubMed] [Google Scholar]
- Khalil AA. Biomarker discovery: a proteomic approach for brain cancer profiling. Cancer Sci. 2007;98(2):201–213. doi: 10.1111/j.1349-7006.2007.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil AA, Kabapy NF, Deraz SF, Smith C. Heat shock proteins in oncology: diagnostic biomarkers or therapeutic targets? Biochim Biophys Acta. 2011;1816(2):89–104. doi: 10.1016/j.bbcan.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Khan F, Niaz K, Maqbool F, Ismail Hassan F, Abdollahi M, Nagulapalli Venkata KC, Nabavi SM, Bishayee A. Molecular targets underlying the anticancer effects of quercetin: an update. Nutrients. 2016;8(9):529–548. doi: 10.3390/nu8090529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YA, Lee WH, Choi TH, Rhee SH, Park KY, Choi YH. Involvement of p21WAF1/CIP1, pRB, Bax and NF-κB in induction of growth arrest and apoptosis by resveratrol in human lung carcinoma A549 cells. Int J Oncol. 2003;23(4):1143–1149. [PubMed] [Google Scholar]
- Ko JH, Sethi G, Um JY, Shanmugam MK, Arfuso F, Kumar AP, Bishayee A, Ahn KS. The role of resveratrol in cancer therapy. Int J Mol Sci. 2017;18(12):E2589. doi: 10.3390/ijms18122589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Tang C, Li L, Li R, Fan Y. Quercetin blocks t-AUCB-induced autophagy by Hsp27 and Atg7 inhibition in glioblastoma cells in vitro. J Neuro-Oncol. 2016;129(1):39–45. doi: 10.1007/s11060-016-2149-2. [DOI] [PubMed] [Google Scholar]
- Li L, Qiu RL, Lin Y, Cai Y, Bian Y, Fan Y, Gao XJ. Resveratrol suppresses human cervical carcinoma cell proliferation and elevates apoptosis via the mitochondrial and p53 signaling pathways. Oncol Lett. 2018;15(6):9845–9851. doi: 10.3892/ol.2018.8571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CJ, Lee CC, Shih YL, Lin TY, Wang SH, Lin YF, Shih CM. Resveratrol enhances the therapeutic effect of temozolomide against malignant glioma in vitro and in vivo by inhibiting autophagy. Free Radic Biol Med. 2012;52(2):377–391. doi: 10.1016/j.freeradbiomed.2011.10.487. [DOI] [PubMed] [Google Scholar]
- Narayanan NK, Nargi D, Randolph C, Narayanan BA. Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int J Cancer. 2009;125(1):1–8. doi: 10.1002/ijc.24336. [DOI] [PubMed] [Google Scholar]
- Nylandsted J, Rohde M, Brand K, Bastholm L, Elling F, Jäättelä M. Selective depletion of heat shock protein 70 (Hsp70) activates a tumor-specific death program that is independent of caspases and bypasses Bcl-2. Proc Natl Acad Sci U S A. 2000;97(14):7871–7876. doi: 10.1073/pnas.97.14.7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Önay Uçar E, Pekmez M, Arda N. Targeting of heat shock proteins by natural products in cancer. In: Farooqi A, Ismail M, editors. Molecular oncology: underlying mechanisms and translational advancements. Cham: Springer; 2017. pp. 173–192. [Google Scholar]
- Önay Uçar E, Şengelen A, Mertoğlu E, Pekmez M, Arda N (2018) Suppression of HSP70 expression by quercetin and its therapeutic potential against cancer. In: Asea A, Kaur P (eds) HSP70 in Human diseases and disorders. heat shock proteins, 14th edn. Springer, Cham, pp 361–379 ISBN: 978–3–319-89550-5. doi: 10.1007/978-3-319-89551-2_19
- Önay-Uçar E. Heat shock proteins and cancer: plant based therapy. In: Asea AAA, Calderwoo SK, editors. Heat shock protein-based therapies. 9. Cham: Springer; 2015. pp. 27–48. [Google Scholar]
- Önay-Uçar E, Şengelen A, Güngör E, Mertoğlu E, Pekmez M, Arda N (in press) Can hsp targeted gene therapy be a new hope for gliomas? In: Asea A, Kaur P (eds) Heat shock proteins in neuroscience, 20th edn. Springer
- Parekh P, Motiwale L, Naik N, Rao KV. Downregulation of cyclin D1 is associated with decreased levels of p38 MAP kinases, Akt/PKB and Pak1 during chemopreventive effects of resveratrol in liver cancer cell. Exp Toxicol Pathol. 2011;63(1–2):167–173. doi: 10.1016/j.etp.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Pervaiz S, Holme AL. Resveratrol: its biologic targets and functional activity. Antioxid Redox Signal. 2009;11(11):2851–2897. doi: 10.1089/ars.2008.2412. [DOI] [PubMed] [Google Scholar]
- Rajesh Y, Pal I, Banik P, Chakraborty S, Borkar SA, Dey G, Mukherjee A, Mandal M. Insights into molecular therapy of glioma: current challenges and next generation blueprint. Acta Pharmacol Sin. 2017;38(5):591–613. doi: 10.1038/aps.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z, Wang L, Cui J, Huoc Z, Xue J, Cui H, Mao Q, Yang R. Resveratrol inhibits NF-kB signaling through suppression of p65 and IkappaB kinase activities. Pharmazie. 2013;68(8):689–694. [PubMed] [Google Scholar]
- Rocha-González HI, Ambriz-Tututi M, Granados-Soto V. Resveratrol: a natural compound with pharmacological potential in neurodegenerative diseases. CNS Neurosci Ther. 2008;14(3):234–247. doi: 10.1111/j.1755-5949.2008.00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubiolo JA, Vega FV. Resveratrol protects primary rat hepatocytes against necrosis induced by reactive oxygen species. Biomed Pharmacother. 2008;62(9):606–612. doi: 10.1016/j.biopha.2008.06.034. [DOI] [PubMed] [Google Scholar]
- Şengelen A, Önay-Uçar E. Rosmarinic acid and siRNA combined therapy represses Hsp27 (HSPB1) expression and induces apoptosis in human glioma cells. Cell Stress Chaperones. 2018;23(5):885–896. doi: 10.1007/s12192-018-0896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengottuvelan M, Deeptha K, Nalini N. Influence of dietary resveratrol on early and late molecular markers of 1,2-dimethylhydrazine-induced colon carcinogenesis. Nutrition. 2009;25(11–12):1169–1176. doi: 10.1016/j.nut.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Shim G, Kim D, Le QV, Park GT, Kwon T, Oh YK. Nonviral delivery systems for cancer gene therapy: strategies and challenges. Curr Gene Ther. 2018;18(1):3–20. doi: 10.2174/1566523218666180119121949. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Nakazato T, Xian MJ, Sagawa M, Ikeda Y, Kizaki M. Resveratrol induces apoptosis of human malignant B cells by activation of caspase-3 and p38 MAP kinase pathways. Biochem Pharmacol. 2006;71(6):742–750. doi: 10.1016/j.bcp.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Staedler D, Idrizi E, Kenzaoui BH, Juillerat-Jeanneret L. Drug combinations with quercetin: doxorubicin plus quercetin in human breast cancer cells. Cancer Chemother Pharmacol. 2011;68(5):1161–1172. doi: 10.1007/s00280-011-1596-x. [DOI] [PubMed] [Google Scholar]
- Uçar EÖ, Arda N, Aitken A. Extract from mistletoe, Viscum album L., reduces Hsp27 and 14-3-3 protein expression and induces apoptosis in C6 rat glioma cells. Genet Mol Res. 2012;11(3):2801–2813. doi: 10.4238/2012.August.24.5. [DOI] [PubMed] [Google Scholar]
- Wang G, Dai F, Yu K, Jia Z, Zhang A, Huang Q, Kang C, Jiang H, Pu P. Resveratrol inhibits glioma cell growth via targeting oncogenic microRNAs and multiple signaling pathways. Int J Oncol. 2015;46(4):1739–1747. doi: 10.3892/ijo.2015.2863. [DOI] [PubMed] [Google Scholar]
- Wang X, Khaleque MA, Zhao MJ, Zhong R, Gaestel M, Calderwood SK. Phosphorylation of HSF1 by MAPK-activated protein kinase 2 on serine 121, inhibits transcriptional activity and promotes HSP90 binding. J Biol Chem. 2006;281(12):782–791. doi: 10.1074/jbc.M505822200. [DOI] [PubMed] [Google Scholar]
- Wang Z, Raoi DD, Senzer N, Nemunaitis J. RNA interference and cancer therapy. Pharm Res. 2011;28(12):2983–2995. doi: 10.1007/s11095-011-0604-5. [DOI] [PubMed] [Google Scholar]
- Westerheide SD, Anckar J, Stevens SM, Jr, Sistonen L, Morimoto RI. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science. 2009;323(5917):1063–1066. doi: 10.1126/science.1165946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal M, Lamszus K. The neurobiology of gliomas: from cell biology to the development of therapeutic approaches. Nat Rev Neurosci. 2011;12(9):495–508. doi: 10.1038/nrn3060. [DOI] [PubMed] [Google Scholar]
- Yang Q, Wang B, Zang W, Wang X, Liu Z, Li W, Jia J. Resveratrol inhibits the growth of gastric cancer by inducing G1 phase arrest and senescence in a Sirt1-dependent manner. PLoS One. 2013;8(11):e70627. doi: 10.1371/journal.pone.0070627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Guo X, Xie W, Li Y, Ma M, Yuan T, Luo B. Resveratrol exerts an anti-apoptotic effect on human bronchial epithelial cells undergoing cigarette smoke exposure. Mol Med Rep. 2015;11(3):1752–1758. doi: 10.3892/mmr.2014.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Tremblay TL, McDermid A, Thibault P, Stanimirovic D. Identification of differentially expressed proteins in human glioblastoma cell lines and tumors. Glia. 2003;42(2):194–208. doi: 10.1002/glia.10222. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Tang H, Zeng X, Ye D, Liu J. Resveratrol inhibits proliferation, migration and invasion via Akt and ERK1/2 signaling pathways in renal cell carcinoma cells. Biomed Pharmacother. 2018;98:36–44. doi: 10.1016/j.biopha.2017.12.029. [DOI] [PubMed] [Google Scholar]
- Zhou F, Huang X, Pan Y, Cao D, Liu C, Liu Y, Chen A. Resveratrol protects HaCaT cells from ultraviolet B-induced photoaging via upregulation of HSP27 and modulation of mitochondrial caspase-dependent apoptotic pathway. Biochem Biophys Res Commun. 2018;499(3):662–668. doi: 10.1016/j.bbrc.2018.03.207. [DOI] [PubMed] [Google Scholar]