Abstract
Aim: The cardio-ankle vascular index (CAVI) represents the blood pressure-independent arterial stiffness from the origin of the aorta to the ankle. CAVI0 has been proposed as a variant index. We aimed to clarify the difference between CAVI and CAVI0 among large populations, and to explore reasons of the difference.
Methods: The subjects were 5,293 Japanese healthy and 3,338 hypertensive people. Simple and multiple regression analyses were performed using age, sex, body mass index, systolic, and diastolic blood pressure (Pd) as variables. Sub-group analysis was performed by sex and age. The CAVI values with and without adjustment by reference pressure were also compared.
Results: CAVI had a positive correlation with Pd, while CAVI0 had a negative correlation with Pd in the healthy population. The CAVI values of the hypertensive group were higher than those of healthy group in both men and women, but the CAVI0 values in women of the hypertensive group in the 30–39 age group was significantly lower than that of the corresponding healthy group. Differences of CAVI values with or without modification using the reference pressure were 1.09% ± 1.38% for the healthy group and 3.68% ± 1.66% for the hypertensive group.
Conclusion: CAVI showed the expected values, but CAVI0 showed inexplicable results in the healthy and hypertensive populations. The differences were due to the strong dependency of CAVI0 on Pd. Differences of CAVI values with or without reference pressure were negligible. These results indicate that CAVI obtained by the VaSera system is appropriate, but CAVI0 is not.
Keywords: CAVI, CAVI0, haPWV, Bramwell-Hill's equation, Stiffness Parameter β
See editorial vol. 26: 601–602
Introduction
It is known that arterial stiffness reflects arteriosclerosis and it is related to cardiovascular events. Measurements of arterial stiffness have been attempted from the late 19th century1). It is established that the velocity of the pulse that arises from the heart is correlated with the stiffness of the artery2). Consequently, pulse wave velocity (PWV) has been used as an index of arterial stiffness 3). However, PWV essentially changes according to changes in blood pressure at the measuring time. This has been shown theoretically and also experimentally4). Therefore, PWV is an inappropriate index for research studying the effect of blood pressure on arterial stiffness.
An attempt to establish an arterial stiffness index independent of blood pressure at measuring time was made by Hayashi et al.5). They found that the relationship between a change in vascular diameter and internal blood pressure showed an exponential curve, using isolated arteries. Whereas, when blood pressure change was expressed as a natural logarithm, the relationship between diameter change and blood pressure change showed nearly a straight line. The inclination of this line, regarded as an arterial stiffness parameter which does not change according to changes in blood pressure at the measuring time, was defined as stiffness parameter β (Supplement Seq. 1)5). Later, Kawasaki et al. showed a method to measure β using an ultrasonic diagnostic imaging apparatus by setting the diastolic pressure (Pd) in place of the reference pressure (Supplement Seq. 7)6).
In 2004, the cardio–ankle vascular index (CAVI) was developed by applying the Bramwell–Hill equation to Kawasaki's β formula using PWV, as an index reflecting arterial stiffness of the arterial tree from the origin of the aorta to the ankle. The VaSera system was invented to measure CAVI. The precise methods were already published7, 8) and the index was defined by eq. 18).
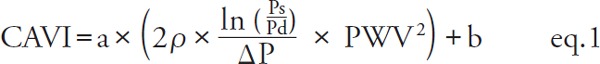 |
[PWV: pulse wave velocity of the arterial tree from the origin of the aorta to the ankle, Ps: systolic blood pressure, Pd: diastolic blood pressure, ρ: blood density, Δ P: Ps–Pd, a, b: coefficients8)].
Using this index, many studies have been published over the last 15 years; these studies include various valuable findings confirmed by researchers throughout the world9). Though both CAVI and PWV values increase with aging, the increase is more marked with CAVI10). Further, CAVI is more closely correlated to aortic distensibility than PWV11). Patients with arteriosclerotic diseases, such as coronary arterial disease, cerebrovascular diseases, and chronic kidney disease show high CAVI values12–14). Furthermore, CAVI is known to be high among those with coronary risk factors, such as hypertension15, 16), diabetes mellitus17, 18), dyslipidemia19), and sleep apnea syndrome20, 21). Many studies have shown that CAVI is a predictor of cardiovascular events22–25). In addition, several studies on the acute changes of CAVI showed reasonable changes as a result of the administration of metoprolol, doxazosin, and nitroglycerin8, 26).
However, recently, Spronck et al. proposed a similar index, termed CAVI0, which is based on the assumption that CAVI is dependent on blood pressure, whereas, CAVI0 is not. The calculation formula for CAVI0 is defined in eq. 2 27–29).
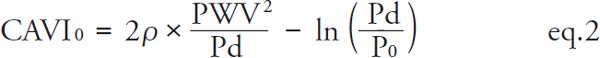 |
[P0: reference pressure (100 mmHg)]
According to Spronck et al., there are two reasons for the blood pressure dependence of CAVI. The first reason is that the slight difference between the β of Hayashi and that of Kawasaki et al. is expressed by the second term of the right side of eq. 2, which is related to the reference pressure, and this is the first element of blood pressure dependency. The second reason is the 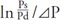 of the CAVI expression, which is different from 1/Pd. Spronck et al. compared CAVI and CAVI0 values based on mathematical simulations using blood pressure values from a randomized list, and showed the difference between CAVI and CAVI0, as it related to the dependency on the blood pressure at measuring time27).
of the CAVI expression, which is different from 1/Pd. Spronck et al. compared CAVI and CAVI0 values based on mathematical simulations using blood pressure values from a randomized list, and showed the difference between CAVI and CAVI0, as it related to the dependency on the blood pressure at measuring time27).
Based on the assumption that the artery is completely monotonous and blood pressure is the same at both sides of the artery, Spronck's equation (eq. 2) could be mathematically reasonable. However, Spronck's equation (eq. 2) includes only Pd. This raises one concern that CAVI0 might be influenced too strongly by Pd30). The pulse transition time measured in CAVI is not just a foot-to-foot period of the pulse waves at the pressure level of Pd, and it is not suitable to apply the CAVI0 theory simply to this condition.
To date, there has been no detailed report comparing CAVI and CAVI0 with a large population; carotid-femoral PWV has been compared to CAVI and resulted in a relatively small correlation [r2 = 0.18 10) or r2 = 0.31 31)].
Aim
The aim of this study was to compare and clarify the actual differences between CAVI and CAVI0 in the large population. Both values were compared in a healthy group and a hypertensive group by simple and multiple regression analyses using age, sex, body mass index (BMI), Ps, and Pd as variables. Also, the comparison was performed in subgroups by sex and age. As a result, there were differences between CAVI and CAVI0. The reason for the differences is discussed from the viewpoint of the properties of heart-ankle PWV (haPWV) used in the VaSera system.
Furthermore, Spronck et al. pointed out that the arterial stiffness value should be corrected with a reference pressure27), and 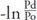 (see eq. 2) was added in the CAVI0 equation. In this article, the actual differences between the CAVI values with or without reference pressure were calculated and compared among the healthy group and the hypertensive group, respectively.
(see eq. 2) was added in the CAVI0 equation. In this article, the actual differences between the CAVI values with or without reference pressure were calculated and compared among the healthy group and the hypertensive group, respectively.
Methods
Subjects
The population-based sample used in the present analysis was comprised of n = 8,631 (n = 5,293, healthy and n =3,338, hypertensive) Japanese subjects aged ≥ 20 years residing in major cities nationwide who underwent a health check at the Japan Health Promotion Foundation with the complete data set n = 28,400 among n =32,627 of the published data32, 33). A flow diagram of the population is shown in Fig. 1.
Fig. 1.
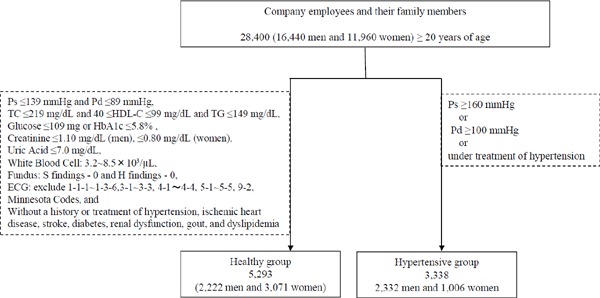
Flow diagram of the population
Abbreviations: Ps, systolic blood pressure; Pd, diastolic blood pressure; Pm, mid blood pressure; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride.
Analysis Method
1. Comparison of CAVI and CAVI0 by Simple and Multiple Regression Analysis
Firstly, in order to overview the basic feature of CAVI and CAVI0 as a whole, the coefficients of correlation between CAVI and CAVI0 were obtained in the healthy and hypertensive groups. Then, simple and multiple regression analyses were performed in the healthy and hypertensive groups using age, sex, BMI, Ps, and Pd as variables. Dummy variables were set for sex (men: 1, women: 2). To avoid confounding by blood pressure, regression analysis was performed in separate models with Ps and with Pd. The criteria of the healthy group and the hypertensive group are shown in Fig. 1.
2. Comparison of the Differences between CAVI and CAVI0 among the Healthy and Hypertensive Groups in Sex and Age subgroups
To investigate the differences between CAVI and CAVI0 in detail, subjects were divided into 12 groups by sex and age strata. The age groups were 20–29, 30–39, 40–49, 50–59, 60–69, and ≥ 70 years. The mean values of CAVI and CAVI0 and the statistical significance between the healthy group and the hypertensive group were calculated, respectively, in men and women.
Also, in order to investigate the relationship between CAVI and CAVI0 with blood pressure, Ps and Pd of the healthy group and the hypertensive group were obtained in the same manner in each age stratum in men and women.
3. Correlation between haPWV and Ps, Pd, and Mid Pressure (Pm) in the Healthy Group
Shirai et al. reported that CAVI without coefficient “a” and “b” can be described as indicated in eq. 3 34):
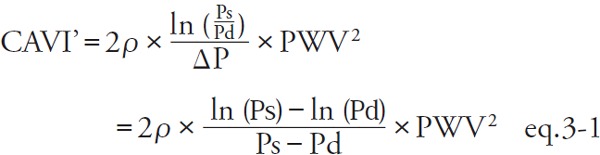 |
 |
[CAVI': CAVI without coefficients “a” and “b,” Pm: mid blood pressure 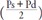 ]
]
The reason why CAVI is based on Pm is as follows: CAVI is calculated from haPWV, which is the PWV of the arterial tree from the origin of the aorta to the ankle. As is well known, PWV has blood pressure dependency16). Therefore, also in the cardiac cycle, PWV takes different values depending on the point of measurement between at Pd and at Ps; Shirai et al. showed that haPWV in CAVI corresponded mostly to Pm rather than Ps and Pd34). It means that the formula of CAVI is substantially based on Pm, whereas, Spronck et al. assumed that Pd should be used in CAVI0 because PWV measured at the foot-to-foot of pulse wave corresponds to at Pd. This is the essential difference. The influence of the second term of CAVI0, which is related to the reference pressure, will be described later.
In order to reconfirm this, the relationship of haPWV with Ps, Pd, and Pm were studied in a healthy group, which is considered to have less influence of vascular remodeling with hypertension, by age strata with simple regression analysis.
4. Analysis of the Over-Estimation of CAVI0: Comparison of Pm/Pd in Men and Women
Since in CAVI0, PWV squared is divided by incompatible Pd instead of compatible Pm, there is a concern that the CAVI0 value is overestimated by Pm/Pd, which corresponds to CAVI0/CAVI as a whole. In order to confirm the influence of this over-estimation, the values of Pm/Pd were calculated in the healthy group and the hypertensive group in each age stratum in men and women
5. Comparison of CAVI Values with and without Reference Pressure
A combination of Bramwell–Hill's equation and Stiffness Parameter β gives eq. 4 (Supplement Seq. 15).
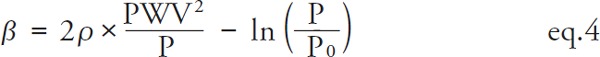 |
When PWV corresponds to Pd, by substituting P=Pd, the CAVI0 equation of eq. 2 is derived. For CAVI, PWV corresponds to Pm, and by substituting P= Pm, eq. 5 is derived.
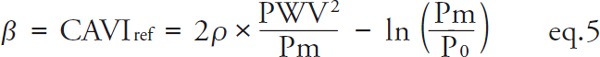 |
[CAVIref: the value with reference pressure P0.]
CAVI' described in eq. 3-1 is the actual measured value without the coefficients “a” and “b,” and is comparable to CAVIref, the mathematical value with reference pressure described in eq. 5. It has been reported that there is no discrepancy in the clinical significance between CAVI with and without the coefficients “a” and “b”8).
In order to evaluate the differences of values, CAVIref, CAVI' and CAVI0 were compared and the difference ratio to CAVIref were calculated in the healthy group and hypertensive group.
Statistical Analysis
Unpaired Welch's t-test was used to evaluate comparisons of CAVI and CAVI0 among the groups. Results were expressed as the mean ± standard deviation, and p < 0.05 was considered significant.
In the regression analysis, the dependent variables were CAVI and CAVI0, and the independent variables used for regression analysis were sex, age, body mass index (BMI), Ps, and Pd. Co-linearity between independent variables was confirmed by the Durbin-Watson test and Variance Inflator Factor.
In the simple regression analysis of haPWV with Ps, Pd, and Pm, the coefficient of correlation was obtained in each age stratum of the healthy group. In the comparison of βm, βm', and CAVI0, the results were expressed as the mean ± standard deviation. All statistical analyses were performed using the SPSS software package (SPSS Inc., Chicago, IL, USA).
Results
1. Comparison of CAVI and CAVI0 with Simple and Multiple Regression Analysis
The coefficients of correlation between CAVI and CAVI0 in the healthy group and the hypertensive group were 0.923 and 0.955, respectively, with p < 0.001 for both. Subsequently, regression analyses with age, sex, BMI, Ps, and Pd in CAVI and CAVI0 were studied. The results are shown in Table 1. In the relationship with Ps (Table 1a, b), no major discrepancy was seen between CAVI and CAVI0, except for that in CAVI; Ps was not significant in the multiple regression analysis in the healthy group.
Table 1. Comparison of the relationship of CAVI and CAVI0 with blood pressure by simple and multiple regression analysis with Ps (a, b) and with Pd (c, d).
|
(a) Simple and multiple regression analysis of CAVI with Ps | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Healthy (N = 5,293) |
Hypertension (N = 3,338) |
|||||||||||||
| Simple Regression |
Multiple Regression |
Simple Regression |
Multiple Regression |
|||||||||||
| r | p value | β | B | 95%CI | p value | r | p value | β | B | 95%CI | p value | |||
| Age | 0.607 | p < 0.001 | 0.644 | 0.050 | 0.048 | 0.051 | p < 0.001 | 0.525 | p < 0.001 | 0.515 | 0.064 | 0.060 | 0.067 | p < 0.001 |
| Sex | −0.117 | p < 0.001 | −0.182 | −0.317 | −0.353 | −0.280 | p < 0.001 | −0.081 | p < 0.001 | −0.115 | −0.292 | −0.363 | −0.221 | p < 0.001 |
| BMI | −0.049 | p < 0.001 | −0.197 | −0.058 | −0.064 | −0.051 | p < 0.001 | −0.224 | p < 0.001 | −0.146 | −0.050 | −0.059 | −0.040 | p < 0.001 |
| Ps | 0.119 | p < 0.001 | NS | 0.127 | p < 0.001 | 0.166 | 0.010 | 0.009 | 0.012 | p < 0.001 | ||||
|
(b) Simple and multiple regression analysis of CAVI0 with Ps | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Healthy (N = 5,293) |
Hypertension (N = 3,338) |
|||||||||||||
| Simple Regression |
Multiple Regression |
Simple Regression |
Multiple Regression |
|||||||||||
| r | p value | β | B | 95%CI | p value | r | p value | β | B | 95%CI | p value | |||
| Age | 0.531 | p < 0.001 | 0.564 | 0.084 | 0.081 | 0.088 | p < 0.001 | 0.553 | p < 0.001 | 0.548 | 0.182 | 0.173 | 0.191 | p < 0.001 |
| Sex | −0.080 | p < 0.001 | −0.143 | −0.480 | −0.558 | −0.403 | p < 0.001 | −0.027 | p = 0.122 | −0.057 | −0.388 | −0.574 | −0.202 | p < 0.001 |
| BMI | −0.088 | p < 0.001 | −0.221 | −0.125 | −0.139 | −0.112 | p < 0.001 | −0.211 | p < 0.001 | −0.123 | −0.112 | −0.137 | −0.087 | p < 0.001 |
| Ps | 0.114 | p < 0.001 | 0.027 | 0.004 | 0.000 | 0.008 | p < 0.05 | 0.180 | p < 0.001 | 0.224 | 0.038 | 0.033 | 0.042 | p < 0.001 |
|
(c) Simple and multiple regression analysis of CAVI with Pd | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Healthy (N = 5,293) |
Hypertension (N = 3,338) |
|||||||||||||
| Simple Regression |
Multiple Regression |
Simple Regression |
Multiple Regression |
|||||||||||
| r | p value | β | B | 95%CI | p value | r | p value | β | B | 95%CI | p value | |||
| Age | 0.607 | p < 0.001 | 0.633 | 0.049 | 0.047 | 0.051 | p < 0.001 | 0.525 | p < 0.001 | 0.548 | 0.068 | 0.064 | 0.071 | p < 0.001 |
| Sex | −0.117 | p < 0.001 | −0.175 | −0.304 | −0.341 | −0.266 | p < 0.001 | −0.081 | p < 0.001 | −0.097 | −0.246 | −0.319 | −0.173 | p < 0.001 |
| BMI | −0.049 | p < 0.001 | −0.205 | −0.060 | −0.066 | −0.054 | p < 0.001 | −0.224 | p < 0.001 | −0.146 | −0.049 | −0.059 | −0.040 | p < 0.001 |
| Pd | 0.214 | p < 0.001 | 0.040 | 0.004 | 0.002 | 0.006 | p = 0.001 | −0.031 | p = 0.076 | 0.145 | 0.014 | 0.011 | 0.017 | p < 0.001 |
| (d) Simple and multiple regression analysis of CAVI0 with Pd | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Healthy (N = 5,293) | Hypertension (N = 3,338) | |||||||||||||
| Simple Regression | Multiple Regression | Simple Regression | Multiple Regression | |||||||||||
| r | p value | β | B | 95%CI | p value | r | p value | β | B | 95%CI | p value | |||
| Age | 0.531 | p < 0.001 | 0.641 | 0.096 | 0.092 | 0.099 | p < 0.001 | 0.553 | p < 0.001 | 0.534 | 0.178 | 0.168 | 0.187 | p < 0.001 |
| Sex | −0.080 | p < 0.001 | −0.195 | −0.655 | −0.729 | −0.580 | p < 0.001 | −0.027 | p = 0.122 | −0.069 | −0.468 | −0.661 | −0.276 | p < 0.001 |
| BMI | −0.088 | p < 0.001 | −0.162 | −0.092 | −0.105 | −0.079 | p < 0.001 | −0.211 | p < 0.001 | −0.114 | −0.104 | −0.130 | −0.078 | p < 0.001 |
| Pd | −0.064 | p < 0.001 | −0.257 | −0.050 | −0.055 | −0.045 | p < 0.001 | −0.166 | p < 0.001 | NS | ||||
Abbreviations: BMI, Body Mass Index; Ps, systolic blood pressure; Pd, diastolic blood pressure; r, regression coefficient; β, standardized partial regression coefficient; B, unstandardized partial regression coefficient; CI, confidential interval.
However, in the relationship with Pd (Table 1c, d), an inexplicable result was observed for CAVI0. For CAVI, the correlation coefficient (r) in simple regression with Pd in the healthy group was 0.214 (p < 0.001). In multiple regression analysis, the standardized partial regression coefficient (β) was 0.040 (p = 0.001). In the hypertensive group, for a simple regression with Pd, the result was −0.031 (p = 0.076). In the multiple regression analysis, the standardized partial regression coefficient (β) was 0.145 (p < 0.001). From these results, a positive correlation with Pd was observed for CAVI.
For CAVI0, the correlation coefficient (r) in the simple regression analysis with Pd in the healthy group was −0.064 (p < 0.001). In the multiple regression analysis, the standardized partial regression coefficient (β) was −0.257 (p < 0.001). In the hypertensive group, in the simple regression analysis with Pd, the result was −0.166 (p < 0.001). In multiple regression analysis, the standardized partial regression coefficient (β) was not significant. As shown above, a negative correlation with Pd was observed for CAVI0.
2. Comparison of the Significant Differences of CAVI and CAVI0 between the Healthy and the Hypertensive Groups in Sex and Age Subgroups
As shown in Fig. 2a–d, in general, a similarity was seen between CAVI and CAVI0, but inexplicable results were found for CAVI0. In the analysis of CAVI, the value of the hypertensive group exceeded the value of the healthy group in both men and women, and the differences were significant, except for women at the age of 30–39 years.
Fig. 2.
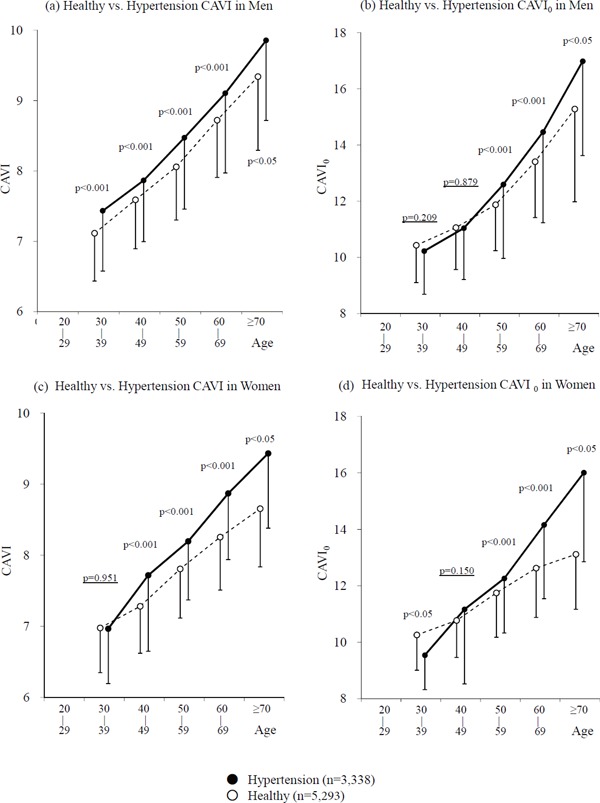
Comparison of the significant differences of CAVI and CAVI0 between healthy group and the hypertensive group in each age stratum in men (a, b) and women (c, d)
However, in the analysis of CAVI0, in the group with the age of 30–39 years, the values of the hypertensive group were less than the values of the healthy group in both men and women. (10.23 ± 1.54 vs. 10.43 ± 1.33, p = 0.21 in men, and 9.54 ± 1.22 vs. 10.26 ± 1.25, p = 0.024 in women, respectively). In the group with women at the age of 30–39 years, the values of the hypertensive group were significantly less than the values of the healthy group. Thus, a contradiction was observed between CAVI and CAVICAVI0 among large healthy and hypertensive populations.
Additionally, the differences between the healthy and hypertensive groups were not significant in the age groups; 30–39 years and 40–49 years in men and 40–49 years in women (p = 0.209, p = 0.879, and p = 0.150, respectively), whereas in CAVI, the differences were significant with p < 0.001 or p < 0.05. The results of the blood pressure in each age stratum in men and women are shown in Fig. 3. In the healthy group, both Ps and Pd increased with age in both men and women. In the hypertensive group, both Ps and Pd decreased with age in both men and women, in conjunction with the antihypertensive treatments.
Fig. 3.
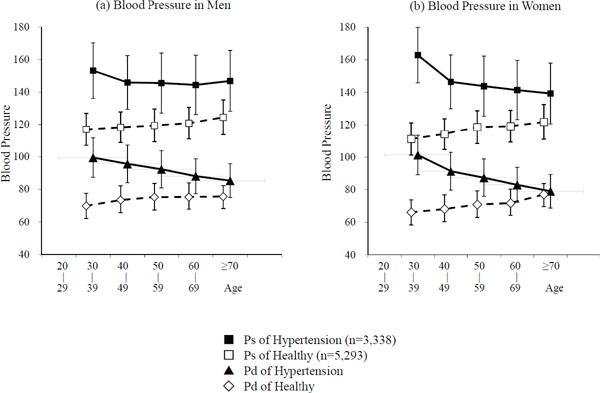
Blood pressure in each age stratum in men (a) and women (b)
Abbreviations: Ps, systolic blood pressure; Pd, diastolic blood pressure.
3. Correlation between haPWV and Ps, Pd, and Pm in the Healthy Group
Coefficients of correlation between haPWV and Ps, Pd, and Pm in the healthy group were obtained, and the results are shown in Table 2. In all age strata, Pm showed a stronger correlation with haPWV than Ps and Pd, indicating that Pm was a stronger factor in the determination of haPWV than Ps and Pd. This result was consistent with the previous report by Shirai et al.34).
Table 2. Correlation of coefficients between haPWV and Ps, Pd, and Pm in the healthy group (n = 5,293).
| Age n |
20–29 980 |
30–39 1,948 |
40–49 1,260 |
50–59 818 |
60–69 255 |
≥ 70 32 |
Total 5,593 |
|---|---|---|---|---|---|---|---|
| Ps | .565 (***) | .529 (***) | .556 (***) | .520 (***) | .464 (***) | .332 (NS) | .544 (***) |
| Pd | .599 (***) | .587 (***) | .632 (***) | .567 (***) | .536 (***) | .082 (NS) | .624 (***) |
| Pm | .637 (***) | .604 (***) | .641 (***) | .599 (***) | .546 (***) | .290 (NS) | .631 (***) |
***means p < 0.001 and NS means p ≥ 0.05
Abbreviations: Ps, systolic blood pressure; Pd, diastolic blood pressure; Pm, mid blood pressure.
4. Analysis of the Over-Estimation in CAVI0; Comparison of Pm/Pd in Men and Women
The changes of Pm/Pd at each age group were studied to clarify how the contradictory results (i.e., higher CAVI0 in healthy women than in hypertensive women at the age of 30–39 years), were obtained. The results are shown in Fig. 4. Pm/Pd values in the hypertensive group were less than those aged 30–39 and 40–49 in the healthy group in both men and women.
Fig. 4.
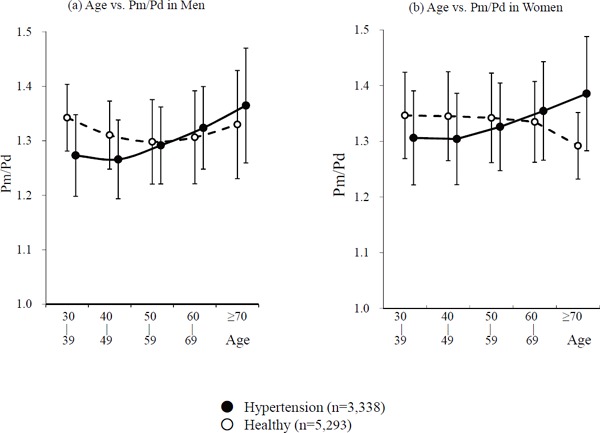
Comparison of the Pm/Pd between healthy group and the hypertensive group in each age stratum in men (a) and women (b)
Abbreviations: Pm, mid blood pressure; Pd, diastolic blood pressure.
5. Comparison between CAVI Values with and without Reference Pressure
The difference between CAVIref (with reference pressure P0), CAVI' (actual measured value without the coefficients “a” and “b”), and CAVI0 are shown in Fig. 5. The difference ratio of CAVI' and CAVIref in the healthy group and in the hypertensive group were 1.09% ± 1.39% and 3.68 ± 1.66%, respectively, while the difference ratio of CAVI0 to CAVIref in the healthy group and in the hypertensive group were 37.58 ± 8.66% and 34.75 ± 9.48%, respectively.
Fig. 5.
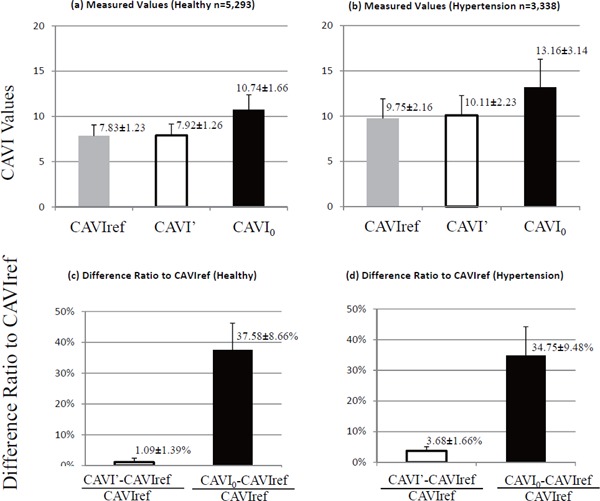
Comparison of measured values with and without the reference pressure
CAVI ref is with reference pressure and CAVI' is without reference pressure. (a) values in the healthy group, (b) values in the hypertensive group, (c) difference ratio of CAVI' and CAVI0 with CAVIref in the healthy group, and (d) difference ratio in the hypertensive group.
Discussion
In general, some similarity was seen between CAVI and CAVI0 with high values more than 0.9 in coefficients of correlation both in the healthy and hypertensive groups.
However, as shown in Table 1 (d), the Pd was a significantly negative contributing factor for CAVI0 in both the healthy group and hypertensive group by simple regression analysis, and it was also a negatively contributing factor for CAVI0 in the healthy group by multiple regression analysis. Generally, those results were unreasonable. Whereas the Pd was a significantly positive contributing factor for CAVI in the healthy group by simple regression analysis and multiple regression analysis, and there was no significance between Pd and CAVI in the hypertensive group by both simple and multiple regression analysis. These results were acceptable.
Next, CAVI and CAVI0 values were compared in subgroups of sex and age, as shown in Fig. 2. a–d. The CAVI0 values of the hypertensive young women group were significantly lower than the value of the healthy young women group, and the significantly higher CAVI0 was observed age > 50 years in both men and women. Whereas, CAVI values of the hypertensive young women group were almost the same as those of the healthy young women group, and the significantly higher CAVI in the hypertensive group was observed in all age strata of men and women, except for young women in their 30s. It is unreasonable that arterial stiffness in young hypertensive women is lower than that of the young healthy women in their 30s. Therefore, we tried to clarify the reason.
At first, the equations of CAVI and CAVI0 (see eq. 1 and eq. 2 as described in the introduction), were compared. The main component of CAVI0 is composed of pwv2/pd, so it is supposed that CAVI0 is overly negatively influenced by Pd. This is considered the reason for lower CAVI0 with higher Pd and higher CAVI0 with lower Pd, seen in Fig. 2–3.
Furthermore, in order to clarify another reason for these contradictory results between CAVI0 and CAVI, the properties of PWV, which is an important component for CAVI and CAVI0, were analyzed. As previously reported, PWV changes during the cardiac cycle according to the blood pressure35, 36).
Spronck et al. assumed in the analysis of CAVI0 that haPWV should be the PWV at Pd based on the assumption that PWV was measured at the foot-to-foot of the pulse wave and used Pd in the CAVI0 equation27–29). To confirm this, the correlation of Ps, Pd, and Pm with haPWV used in the VaSera system were studied. Among Ps, Pd, and Pm, Pm was correlated with haPWV most strongly in the healthy group in all age strata as shown in Table 2. This data was consistent with the previously published data in all populations (n = 3,591)34). The reason is not clear yet; however, one of the reasons may be due to the detection system of PWV in the VaSera system. In order to identify the period from the starting time of the wave at the origin of the aorta, the time from the 2nd heart sound to the corresponding notch of the upper brachial artery, and the time from the upper brachial artery to the ankle are measured. The former time is measured in the end-systolic period, and the pressure level is far higher than Pd. This detection system may render the strong dependency of haPWV on Pm rather than Pd. We have already reported this item34). Spronck et al. gave a further comment on previous data34) in an attempt to explain that the dependency of CAVI on Pm might be due to arterial remodeling in hypertensive patients because Pm is considered to be related to the remodeling. However, the data of Table 2 in this paper was obtained from the healthy group, and the results were consistent in all age strata, irrespective of the influence of age-related remodeling. This data was essentially consistent with the data in our previous report.
Then, it can be concluded that CAVI was obtained at Pm during the pulse cycle between Ps and Pd in the VaSera system.
Furthermore, to confirm the reason why CAVICAVI0 in healthy women was higher than in hypertensive women at the age of 30–39 years, the contribution of Pd was studied. Fig. 4 shows Pm/Pd in men and women of all ages. Pm/Pd in the hypertensive group was significantly less than those of the healthy group aged 30–39 years and 40–49 years in both men and women. This corresponds to the results of the comparison of the significant contradiction between CAVI and CAVI0 among women at the age of 30–39 years in the healthy group and the hypertensive group, and is considered to be the reason why the CAVI0 values were reversed between the young hypertensive group and the healthy group, and that there were no significant differences between them in men.
Also, as Pd increases, Pm/Pd becomes relatively small, so the CAVI0 value decreases. As a result, CAVI0 correlated negatively with Pd. This is considered to be the main reason why CAVI0 showed unreasonable results.
These results suggest that CAVI0 was strongly dependent on Pd changes and the CAVI0 value was underestimated in the case of the elevated Pd group.
Another important difference between CAVI and CAVI0 is that CAVI0 included 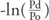 to be corrected with P0. The influence of the reference pressure on CAVI was studied using clinical data without coefficients “a” and “b.” As shown in Fig. 5, the difference ratio between CAVI' and CAVIref was small and practically negligible in clinical usage, while the difference ratio between CAVI0 and CAVIref was large.
to be corrected with P0. The influence of the reference pressure on CAVI was studied using clinical data without coefficients “a” and “b.” As shown in Fig. 5, the difference ratio between CAVI' and CAVIref was small and practically negligible in clinical usage, while the difference ratio between CAVI0 and CAVIref was large.
From the above results, the difference between CAVI and CAVI0 is due to the application of Pm in place of Pd, rather than the difference with or without the reference pressure.
Recently, two studies comparing CAVI and CAVI0 in pediatric subjects of Slovakia reported a slight blood pressure dependency of CAVI. One study compared between normotensive boys and those with white-coat hypertension (WCH) with higher CAVI37), and the other study compared between normotensive girls and boys with spurious systolic hypertension (SSH) with lower CAVI38). No significant differences were seen in CAVI0 in both cases.
However, this article did not show any other standard of arterial stiffness, and it is actually difficult to decide which index is more proper. Only the values of CAVI and CAVI0 were presented on the assumption that there was no difference in either group regarding arterial stiffness. Although it is known that WCH is associated with sympathetic hyperactivity39), which can modulate arterial stiffness40), and it is also known that SSH is seen in young boys with highly elastic arteries41), some possibilities remain, in that arterial stiffness of the persons with WCH is actually high, and that of boys with SSH is actually low.
Conclusion
CAVI showed reasonable values, but CAVI0 showed unreasonable values between healthy and hypertensive populations, especially in young women. This difference was due to the strong dependency of CAVI0 on Pd. The differences in CAVI values with or without the reference pressure were negligible. These results indicate that CAVI obtained by the VaSera system is appropriate, whereas CAVI0 is not. We concluded that the CAVI value obtained by using the VaSera system is reasonable and appropriate as a clinical index of blood pressure-independent arterial stiffness of the arterial tree from the origin of the aorta to the ankle, and the accumulated data published for the last 15 years is reliable.
Disclosures
Kohji Shirai, Kenji Suzuki, Kazuhiro Shimizu, and Masanobu Takata had no conflict of interest concerning this article.
Shinichi Tsuda, Tomoyuki Yamamoto, Mitsuya Maruyama, and Koji Takahashi belong to Fukuda Denshi Co., Ltd. and are involved in the development of CAVI.
Supplement
β Described with PWV and Blood Pressure
Hayashi defied the β as blood pressure-independent parameter of arterial stiffness in Seq. 1 5).
 |
(β: specific stiffness of the blood vessel, P: blood pressure, P0: reference pressure, D: blood vessel diameter, D0: blood vessel diameter at P0)
By dividing both side of Seq. 1 by P0 and taking natural logarithm,
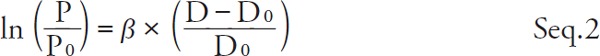 |
Therefore,
 |
By substituting P = Ps and P = Pd for Seq. 2 and transforming,
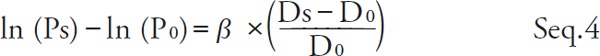 |
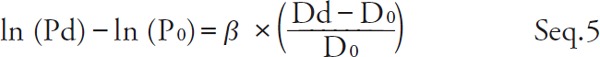 |
By taking the difference between both sides of Seq. 4 and Seq. 5 and transforming,
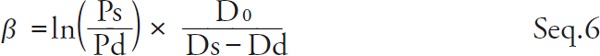 |
Kawasaki et al. defined the β' as practically measurable stiffness parameter in Seq. 7 6).
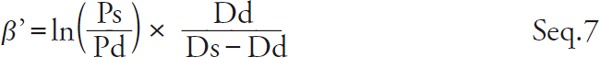 |
 |
From Seq. 2,
 |
By substituting Seq. 9 for Seq. 8 with D = Dd
 |
Since in the physiological range, the second term of right side of Seq. 10 is generally small compared to the first term, β is approximated by β'.
On the other hand, the Bramwell-Hill equation is represented by Seq. 11 2, 3).
 |
(ρ: blood density)
Here, 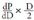 is the volume elastic modulus, and Seq. 11 is a general equation expressing the relationship between the wave velocity and the volume elastic modulus. If D changes in proportion to P, the volume elastic modulus is constant and the wave velocity PWV is also constant. In blood vessels, however, D changes exponentially with respect to P, which is indicated by the Stiffness Parameter β equation of Seq. 1
is the volume elastic modulus, and Seq. 11 is a general equation expressing the relationship between the wave velocity and the volume elastic modulus. If D changes in proportion to P, the volume elastic modulus is constant and the wave velocity PWV is also constant. In blood vessels, however, D changes exponentially with respect to P, which is indicated by the Stiffness Parameter β equation of Seq. 1
The blood pressure dependency of PWV arises from the exponential nature of this blood vessel as follows:
When both sides of Seq. 1 are differentiated with D, the exponent 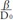 becomes a coefficient from the differential formula of exponential,
becomes a coefficient from the differential formula of exponential,
 |
Substituting Seq. 12 for the right side of Seq. 11
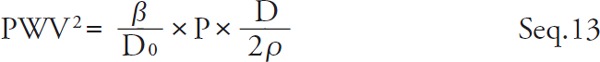 |
β is represented by Seq. 14 by transforming Seq. 13.
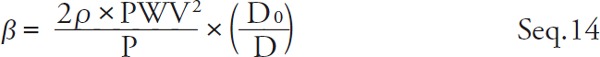 |
Substitute Seq. 9 for Seq. 14 and organize it with β,
 |
Since in the physiological range, the second term of right side of Seq. 15 is generally small compared to the first term, PWV2 is approximately proportional to β and P.
References
- 1). Young T. The Croonian Lecture: on the functions of the heart and arteries. Phil Trans R Soc Lond, 1809; 99: 1-31 [Google Scholar]
- 2). Moens AI. Die Pulskurve [The Pulse Curve]. Leiden, The Netherlands: Brill E. J.; 1898 [Google Scholar]
- 3). Bramwell JC, Hill AV. The velocity of the pulse wave in man. Proc R Soc Lond B Biol Sci, 1922; 93: 298-306 [Google Scholar]
- 4). Bramwell JC, McDowali RJS, McSwiney BA. The variation of arterial elasticity with blood pressure in man. (Part I). Proc R Soc Lond B, 1923; 94: 450-454 [Google Scholar]
- 5). Hayashi K, Handa H, Nagasawa S, Okumura A, Moritake K. Stiffness and elastic behavior of human intracranial and extracranial arteries. J Biomech, 1980; 13: 175-184 [DOI] [PubMed] [Google Scholar]
- 6). Kawasaki T, Sasayama S, Yagi S, Asakawa T, Hirai T. Noninvasive assessment of the age related changes in stiffness of major branches of the human arteries. Cardiovasc Res, 1987; 21: 678-687 [DOI] [PubMed] [Google Scholar]
- 7). Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure-independent arterial wall stiffness parameter; cardio- ankle vascular index (CAVI). J Atheroscler Thromb, 2006; 13: 101-107 [DOI] [PubMed] [Google Scholar]
- 8). Takahashi K, Yamamoto T, Tsuda S, Okabe F, Shimose T, Tsuji Y, Suzuki K, Otsuka K, Takata M, Shimizu K, Uchino J, Shirai K. Coefficients in the CAVI Equation and the Comparison Between CAVI With and Without the Coefficients Using Clinical Data. J Atheroscler Thromb, 2019; 26: 465-475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Hayashi K, Yamamoto T, Takahara A, Shirai K. Clinical assessment of arterial stiffness with cardio-ankle vascular index: theory and applications. J Hypertens, 2015; 33: 1742-1757 [DOI] [PubMed] [Google Scholar]
- 10). Topouchian J, Labat C2, Gautier S, Bäck M, Achimastos A, Blacher J, Cwynar M, de la Sierra A, Pall D, Fantin F, Farkas K, Garcia-Ortiz L, Hakobyan Z, Jankowski P, Jelakovic A, Kobalava Z, Konradi A, Kotovskaya Y, Kotsani M, Lazareva I, Litvin A, Milyagin V, Mintale I, Persson O, Ramos R, Rogoza A, Ryliskyte L, Scuteri A, Sirenko Y, Soulis G, Tasic N, Udovychenko M, Urazalina S, Wohlfahrt P, Zelveian P, Benetos A, Asmar R. Effects of metabolic syndrome on arterial function in different age groups: the Advanced Approach to Arterial Stiffness study. J Hypertens, 2018; 36: 824-833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Boardman H, Lewandowski AJ, Lazdam M, Kenworthy Y, Whitworth P, Zwager CL, Francis JM, Aye CY, Williamson W, Neubauer S, Leeson P. Aortic stiffness and blood pressure variability in young people: a multimodality investigation of central and peripheral vasculature. J Hypertens, 2017; 35: 513-522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Horinaka S, Yabe A, Yagi H, Ishimura K, Hara H, Iemura T, Matsuoka H. Comparison of atherosclerotic indicators between cardio ankle vascular index and brachial ankle pulse wave velocity. Angiology, 2009; 60: 468-476 [DOI] [PubMed] [Google Scholar]
- 13). Suzuki J, Sakakibara R, Tomaru T, Tateno F, Kishi M, Ogawa E, Kurosu T, Shirai K. Stroke and cardio-ankle vascular stiffness index. J Stroke Cerebrovasc Dis, 2013; 22: 171-175 [DOI] [PubMed] [Google Scholar]
- 14). Kubozono T, Miyata M, Ueyama K, Nagaki A, Hamasaki S, Kusano K, Kubozono O, Tei C. Association between arterial stiffness and estimated glomerular filtration rate in the Japanese general population. J Atheroscler Thromb, 2009; 16: 840-845 [DOI] [PubMed] [Google Scholar]
- 15). Okura T, Watanabe S, Kurata M, Manabe S, Koresawa M, Irita J, Enomoto D, Miyoshi K, Fukuoka T, Higaki J. Relationship between cardio-ankle vascular index (CAVI) and carotid atherosclerosis in patients with essential hypertension. Hypertens Res, 2007; 30: 335-340 [DOI] [PubMed] [Google Scholar]
- 16). Takaki A, Ogawa H, Wakeyama T, Iwami T, Kimura M, Hadano Y, Matsuda S, Miyazaki Y, Hiratsuka A, Matsuzaki M. Cardio-ankle vascular index is superior to brachial-ankle pulse wave velocity as an index of arterial stiffness. Hypertens Res, 2008; 31: 1347-1355 [DOI] [PubMed] [Google Scholar]
- 17). Ibata J, Sasaki H, Kakimoto T, Matsuno S, Nakatani M, Kobayashi M, Tatsumi K, Nakano Y, Wakasaki H, Furuta H, Nishi M, Nanjo K. Cardio-ankle vascular index measures arterial wall stiffness independent of blood pressure. Diabetes Res Clin Pract, 2008; 80: 265-270 [DOI] [PubMed] [Google Scholar]
- 18). Izuhara M, Shioji K, Kadota Y, Baba O, Takeuchi Y, Uegaito T, Mutsuo S, Matsuda M. Relationship of cardiovascular index (CAVI) to carotid and coronary arteriosclerosis. Circ J, 2008; 72: 1762-1767 [DOI] [PubMed] [Google Scholar]
- 19). Takaki A, Ogawa H, Wakeyama T, Iwami T, Kimura M, Hadano Y, Matsuda S, Miyazaki Y, Matsuda T, Hiratsuka A, Matsuzaki M. Cardio-ankle vascular index is a new noninvasive parameter of arterial stiffness. Circ J, 2007; 71: 1710-1714 [DOI] [PubMed] [Google Scholar]
- 20). Kumagai T, Kasai T, Kato M, Naito R, Maeno K, Kasagi S, Kawana F, Ishiwata S, Narui K. Establishment of the cardio-ankle vascular index in patients with obstructive sleep apnea. Chest, 2009; 36: 779-786 [DOI] [PubMed] [Google Scholar]
- 21). Kasai T, Inoue K, Kumagai T, Kato M, Kawana F, Sagara M, Ishiwata S, Ohno M, Yamaguchi T, Momomura S, Narui K. Plasma pentraxin3 and arterial stiffness in men with obstructive sleep apnea. Am J Hypertens, 2011; 24: 401-407 [DOI] [PubMed] [Google Scholar]
- 22). Otsuka K, Fukuda S, Shimada K, Suzuki K, Nakanishi K, Yoshiyama M, Yoshikawa J. Serial assessment of arterial stiffness by cardio-ankle vascular index for prediction of future cardiovascular events in patients with coronary artery disease. Hypertens Res, 2014; 37: 1014-1020 [DOI] [PubMed] [Google Scholar]
- 23). Gohbara M, Iwahashi N, Sano Y, Akiyama E, Maejima N, Tsukahara K, Hibi K, Kosuge M, Ebina T, Umemura S, Kimura K. Clinical Impact of the Cardio-Ankle Vascular Index for Predicting Cardiovascular Events After Acute Coronary Syndrome. Circ J, 2016; 80: 1420-1426 [DOI] [PubMed] [Google Scholar]
- 24). Sato Y, Nagayama D, Saiki A, Watanabe R, Watanabe Y, Imamura H, Yamaguchi T, Ban N, Kawana H, Nagumo A, Ohira M, Endo K, Kurosu T, Tomaru T, Shirai K, Tatsuno I. Cardio-Ankle Vascular Index is Independently Associated with Future Cardiovascular Events in Outpatients with Metabolic Disorders. J Atheroscler Thromb, 2016; 23: 596-605 [DOI] [PubMed] [Google Scholar]
- 25). Hitsumoto T. Clinical usefulness of the cardio-ankle vascular index as a predictor of primary cardiovascular events in patients with chronic kidney disease. J Clin Med Res, 2018; 10: 883-890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Shirai K, Song M, Suzuki J, Kurosu T, Oyama T, Nagayama D, Miyashita Y, Yamamura S, Takahashi M. Contradictory effects of β1- and α1-aderenergic receptor blockers on cardio-ankle vascular stiffness index (CAVI) - CAVI is independent of blood pressure-. J Atheroscler Thromb, 2011; 18: 49-55 [DOI] [PubMed] [Google Scholar]
- 27). Spronck B, Avolio AP, Tan I, Butlin M, Reesink KD, Delhaas T. Arterial stiffness index beta and cardio-ankle vascular index inherently dependent on blood pressure but can be readily corrected. J Hypertens, 2017; 35: 98-104 [DOI] [PubMed] [Google Scholar]
- 28). Spronck B, Avolio AP, Tan I, Butlin M, Reesink KD, Delhaas T. Reply: physics cannot be disputed. J Hypertens, 2017; 35: 1523-1525 [DOI] [PubMed] [Google Scholar]
- 29). Spronck B, Avolio AP, Tan I, Butlin M, Reesink KD, Delhaas T. Medical science is based on facts and evidence. J Hypertens, 2018; 36: 960-962 [DOI] [PubMed] [Google Scholar]
- 30). Shirai K, Shimizu K, Takata M, Suzuki K. Independency of the cardio-ankle vascular index from blood pressure at the time of measurement. J Hypertens, 2017; 35: 1521-1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Gomez-Sanchez L, et al. The Association Between the Cardio-ankle Vascular Index and Other Parameters of Vascular Structure and Function in Caucasian Adults: MARK Study. J Atheroscler Thromb, 2015; 22: 901-911 [DOI] [PubMed] [Google Scholar]
- 32). Suzuki K, Ishizuka N, Miyashita Y, Shirai K. Epidemiological examination about the standard value and the validity for the standardization as the examination of CAVI (cardio-ankle vascular index) noninvasive blood pressure-independent arteriosclerosis test. Niigata J Med Technol, 2008; 48-1: 2-10; (in Japanese) [Google Scholar]
- 33). Namekata T, Suzuki K, Ishizuka N, Shirai K. Establishing baseline criteria of cardio-ankle vascular index as a new indicator of arteriosclerosis: a cross-sectional study. BMC Cardiovasc Disord, 2011; 11: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34). Shirai K, Shimizu K, Takata M, Suzuki K. Medical science is based on evidence (answer to Spronck et al.' refutation: physics cannot be disputed). J Hypertens, 2018; 36: 958-960 [DOI] [PubMed] [Google Scholar]
- 35). Mirault T, Pernot M, Frank M, Couade M, Niarra R, Azizi M, Emmerich J, Jeunemaître X, Fink M, Tanter M, Messas E. Carotid stiffness change over the cardiac cycle by ultrafast ultrasound imaging in healthy volunteers and vascular Ehlers-Danlos syndrome. J Hypertens, 2015; 33: 1890-1896 [DOI] [PubMed] [Google Scholar]
- 36). Kenyhercz WE, Rterman B, Sita V, Illapani P, Dowell J, Mo X, White RD, Kolipaka A. Quantification of aortic stiffness using magnetic resonance elastography: measurement reproducibility, pulse wave velocity comparison, changes over cardiac cycle, and relationship with age. Magn Reson Med, 2016; 75: 1920-1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). Mestanik M, Jurko A, Spronck B, Avolio AP, Butlin M, Jurko T, Visnovcova Z., Mestanikova A, Langer P, Tonhajzerova I. Improved assessment of arterial stiffness using corrected cardio-ankle vascular index (CAVI0) in overweight adolescents with white-coat and essential hyper tension. Scand J Clin Lab Invest, 2017; 77: 665-672 [DOI] [PubMed] [Google Scholar]
- 38). Jurko T, Mestanik M, Jurko A, Jr, Spronck B, Avolio AP, Mestanikova A, Sekaninova N, Tonhajzerova I. Pediatric reference values for arterial parameters cardio-ankle vascular index (Cavi) and Cavi0. J Am Soc Hypertens, 2018; 12: e35-e43 [DOI] [PubMed] [Google Scholar]
- 39). Smith PA, Graham LN, Mackintosh AF, MA, Stoker JB, Mary DA. Sympathetic neural mechanisms in white-coat hypertension. J Am Coll Cardiol, 2002; 40: 126-132 [DOI] [PubMed] [Google Scholar]
- 40). Nardone M, Incognito AV, Millar PJ. Evidence for pressure-independent sympathetic modulation of central pulse wave velocity. J Am Heart Assoc, 2018; 7: e007971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Mahmud A, Feely J. Spurious systolic hypertension of youth: fit young men with elastic arteries. Am J Hypertens, 2003; 16: 229-232 [DOI] [PubMed] [Google Scholar]


