Abstract
Accumulation of unfolded proteins and calcium dyshomeostasis induces endoplasmic reticulum (ER) stress, which can be resolved by the unfolded protein response (UPR). We have previously reported that activation of the PERK/ATF4 branch of the UPR, by overexpressing Presenilin in part of the vestigial domain of Drosophila wing imaginal discs, induces both a caspase-dependent apoptosis and a Slpr/JNK/Dilp8-dependent developmental delay that allows compensation of cell death in the tissue. Recently, dDad1 depletion in Drosophila in engrailed-expressing cells of wing imaginal discs was also reported to activate the PERK/ATF4 branch but induced Mekk1/JNK-dependent apoptosis. Here, we assessed whether the stressed cell location in the wing imaginal disc could explain these differences in response to chronic ER stress or whether the stress source could be responsible for the signaling discrepancy. To address this question, we overexpressed a Rhodopsin-1 mutant prone to aggregate either in vestigial- or engrailed-expressing cells. We observed similar responses to the Presenilin overexpression in the vestigial domain and to the dDad1 depletion in the engrailed domain. Therefore, the consequences of a PERK/ATF4 branch activation depend on the position of the cell in the Drosophila wing imaginal disc, suggesting interactions of PERK signaling with developmental pathways involved in the determination or maintenance of wing domains.
Electronic supplementary material
The online version of this article (10.1007/s12192-019-01009-8) contains supplementary material, which is available to authorized users.
Keywords: UPR, PERK, Apoptosis, Homeostasis, Wing imaginal disc
Introduction
The endoplasmic reticulum (ER) is the first site of the eukaryotic secretory pathway. After or during their synthesis, proteins that will transit this pathway are translocated into the ER where they are folded and glycosylated. Failure in these processes results in the accumulation of misfolded and aggregating proteins that tend to become toxic to the cell, leading to devastating pathologies including diabetes, atherosclerosis, neurodegenerative, and renal diseases (Wang and Kaufman 2012). An adaptive response, known as the unfolded protein response (UPR), normally prevents such accumulation by resolving the stress or eliminating the cell (Bravo et al. 2013). In mammals, three major signaling pathways, referred as “UPR arms,” activate the transcription of genes involved in the ER stress response. They are named according to their upstream ER transmembrane sensor, i.e., IRE1 (inositol-requiring enzyme 1), PERK (double-stranded RNA-activated protein kinase (PKR)-like ER kinase), or ATF6 (activating transcription factor 6). Drosophila orthologs of these sensors have been linked to the UPR (Kim et al. 2015; Ryoo 2015; Allen and Seo 2018).
The first in vivo characterization of the UPR to a strong chronic ER stress in Drosophila described the response to a stress induced by Rhodospin-1G69D (Rh-1G69D) mutant-encoded protein (Ryoo et al. 2007). This misfolded and aggregation-prone light-detecting protein leads to a retinal degeneration, which mimics autosomal dominant retinitis pigmentosa (ADRP). Overexpression of a transgene carrying this mutant allele during cell differentiation in larval eye imaginal discs induces an apoptosis, which is triggered by a Cdk5-Mekk1-JNK signaling and seems independent of the three main UPR sensors (Kang et al. 2012).
We have previously reported a different mode of apoptosis induction in a chronic ER stress provoked by overexpression of a Presenilin-encoding transgene in a subpopulation of vestigial-expressing cells of larval wing imaginal discs (Demay et al. 2014). Presenilins are eight to nine-pass transmembrane proteins described to function as the catalytic subunit of the γ-secretase complex. They are also known for their role in Ca2+ homeostasis in mammalian cells (Stutzmann et al. 2007; Rybalchenko et al. 2008; Cheung et al. 2008; Hayrapetyan et al. 2008) as well as in Drosophila ((Michno et al. 2009), our unpublished results). Psn overexpression in wing imaginal discs activates the PERK/ATF4 branch of the UPR, resulting in the transcriptional repression of diap1 (Drosophila inhibitor of apoptosis 1), and thus in a caspase-dependent apoptosis. Independently from cell death regulation, PERK/ATF4 also induced a Rac1/Slipper (Slpr)/JNK/Drosophila insulin-like peptide 8 (Dilp8) pathway in the dying cells (Demay et al. 2014). Dilp8 delayed the general development, increasing the period of cell proliferation in the wing disc, thereby favoring tissue homeostasis (Colombani et al. 2012; Garelli et al. 2012).
The discrepancy in the role of JNK signaling between these models suggests that response to ER stress could vary depending on the ER stress type, the cell status (proliferating vs. differentiated), or the tissue type (wing vs. eye imaginal disc). A recent report implies an additional hypothesis, which is the position of the stressed cells in a tissue. Indeed, impinging on N-glycosylation by depletion of a subunit of the oligosaccharyltransferase complex encoded by defender against apoptotic cell death 1 (dDad1) in engrailed-expressing cells of wing imaginal discs provokes a chronic ER stress. Here again, the PERK/ATF4 branch of the UPR is activated but it induces a Mekk1/JNK-dependent apoptosis (Zhang et al. 2016).
In this study, we tested whether the position of the stressed cells in a tissue—such as the wing imaginal disc—could explain the difference in UPR signaling. We used the UAS-GAL4 system to drive a UAS-Rh-1G69D transgene either by vg-GAL4 or en-GAL4, the two drivers used in the previous contrasted studies. As expected, we observed ER stress and activation of the PERK/ATF4 branch of the UPR. As predicted by our hypothesis, PERK/ATF4 triggered different responses according to the location of the cells. Therefore, responses to PERK/ATF4 activation do not seem to depend on the origin of ER stress but depends on the cell location in the Drosophila wing imaginal disc.
Methods
Drosophila crosses and strains
Flies were raised on standard corn-agar medium at 25 °C. Transgene expression was performed using the UAS-GAL4 system. Wing imaginal disc expression was driven in the dorso-ventral boundary by vg-Gal4 or in the posterior domain by en-GAL4e16-E. Drosophila strains could carry the dilp8MI00727 protein-trap-generating mutation (Bl#33079), UAS-xbp1::EGFP (Souid et al. 2007), tub-ATF4.5′UTR > dsRed (Kang et al. 2015), or TRE-red-2L (Chatterjee and Bohmann 2012) reporter genes.
A new UAS-Rh-1G69D insertion has been generated by a classical remobilization of the P element described in (Colley et al. 1995). It has been selected for its ability to provoke a fully penetrant notched-wing phenotype when driven by vg-GAL4 (Online Resource 1A).
Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study: UAS-atf4-RNAi (Bl#25985) from the Transgenic RNAi Project (TRIP). The UAS-slpr-RNAi (ID 106449), UAS-Cdk5-RNAi (ID 104491), UAS-Mekk1-RNAi (ID 110339), UAS-hep-RNAi (ID 109277), and UAS-bsk-RNAi (ID 104569) strains were obtained from the Vienna Drosophila RNAi Center (VDRC, Vienna, Austria). The efficiency of RNAi transgenes targeting atf4, Ire1, slpr, hep, and bsk mRNAs was previously verified (Demay et al. 2014). Genetic background control strains were selected according to the different transgenic lines we used. The y,w[1118];P{attP,y[+], w[3]} (ID 60100) line was used as a control for the KK library hosted by the VDRC, and P{CaryP}attP2 (Bl#25710) for the TRIP V20 library. Canton S flies were used as a reference for the other strains.
Immunostaining, TUNEL assay, and microscopy
Immunostaining, TUNEL assay, microscopy, and image processing were realized as described in Demay et al. (2014). The following primary antibodies were used: rabbit anti-Dcp-1 (Asp216, Cell Signaling, Danvers, MA, USA, 1:50) and rabbit anti-Phospho-eIf2-alpha (Ser51, Cell Signaling, Danvers, MA, USA, 1:25). Transverse sections were computationally generated after reslicing confocal stacks using ImageJ.
RNA extraction and RT-qPCR
RNA extraction and RT-qPCR were performed as previously described (Demay et al. 2014).
Quantifications
Stainings were quantified using ImageJ. The mean intensity of the imaginal disc area without staining was subtracted to the total imaginal disc mean intensity. The difference was then divided by the mean intensity of the imaginal disc area without staining to normalize data. ANOVA statistical analysis was done when data followed a normal distribution. Otherwise, a Mann-Whitney U test was performed.
Results
Rh-1G69D expression in the dorso-ventral boundary cells induces a similar response to Presenilin overexpression
We chose to overexpress Rh-1G69D as a source of ER stress for two reasons. Firstly, this stressor was previously validated when its expression in the Drosophila eye imaginal disc was shown to display a strong chronic ER stress (Ryoo et al. 2007). Secondly, potential Rh-1G69D-induced ER stress in the wing disc should only be due to the accumulation of misfolded Rh-1 since the Rh-1-encoding gene is not expressed in wing imaginal discs. Therefore, it is most unlikely that ectopic aggregate-prone Rh-1 has any function in this tissue.
As expected, Rh-1G69D expression driven by vg-GAL4 activated the IRE1 arm, as reported by the xbp1::EGFP reporter (Online Resource 1B). We also verified the activation of the PERK/ATF4 branch of the UPR when Rh-1G69D expression is driven by vg-GAL4. PERK directly phosphorylates eukaryotic initiation factor 2α (eIF2α) (Harding et al. 2000), and we could observe an increase of cells labeled by an anti-phosphorylated eIF2α in vg-GAL4 expression domain upon Rh-1G69D overexpression when compared with control (Online Resource 1C), thus showing PERK activation. Phosphorylation of eIF2α inhibits the general translation but activates ATF4 translation by modifying the usage of the multiple upstream open reading frames (uORFs) in its 5′UTR (Vattem and Wek 2004). This mode of regulation is conserved in Drosophila as shown by a tub-ATF4.5′UTR > dsRed reporter (Kang et al. 2015). We observed an increase of the activity of this reporter in the vg-GAL4 expression domain upon Rh-1G69D overexpression when compared with a control (Online Resource 1D), showing that ATF4 is translated in response to Rh-1G69D overexpression. Therefore, both IRE1 and the PERK/ATF4 arms of the UPR are activated by Rh-1G69D expression when driven by vg-GAL4.
Rh-1G69D expression driven by vg-GAL4 induced apoptosis, as assayed by immunostaining with an anti-cleaved Dcp-1 caspase antibody (Fig. 1), leading to notches at the margin of the adult wing (Online Resource 1A). Apoptosis is induced by the PERK/ATF4 branch of the UPR in both the Psn-induced ER stress model (Demay et al. 2014) and after dDad1 depletion (Zhang et al. 2016). We thus tested whether apoptosis induction is similar when UAS-Rh-1G69D is driven by vg-GAL4. Expression of perk-RNAi or atf4-RNAi significantly diminished anti-cleaved Dcp-1 immunostaining in this new ER wing imaginal disc cell stress model (Fig. 1), confirming the activation of the PERK/ATF4 pathway and its prevalence in the induction of apoptosis after ER stress in the wing imaginal disc.
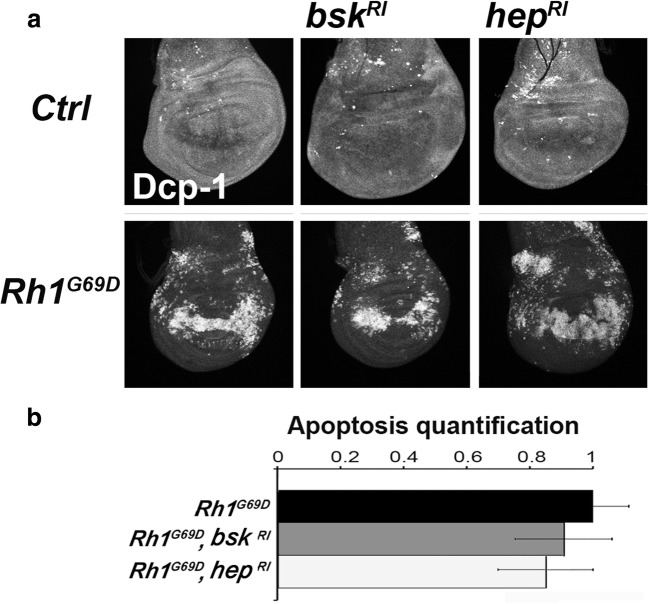
Fig. 1.
Rh-1G69D expression at the wing disc dorso-ventral boundary induces both cell death and JNK pathway activation in a PERK/ATF4-dependent manner. a Projections from confocal stacks of male third-instar larval wing imaginal discs immunostained with an anti-activated Dcp-1 antibody (top) and expressing the TRE reporter of JNK signaling (bottom). From left to right, genotypes are vg-Gal4, TRE-red-2L/+ (Ctrl), vg-Gal4, TRE-red-2L/+; UAS-Rh1G69D/+ (Rh1G69D), vg-Gal4, TRE-red-2L/+; UAS-Rh1G69D/UAS-Atf4-RNAi (Rh1G69D, Atf4RI) and vg-Gal4, TRE-red-2L/+; UAS-Rh1G69D/UAS-Perk-RNAi (Rh1G69D, PerkRI). b Quantification of apoptosis and JNK reporter stainings on wing discs of the previous genotypes. Error bars indicate S.E.M. Statistical difference with the control is indicated by * if p < 5% or *** if p < 10−3 (Mann-Whitney U test)
The PERK/ATF4 arm of the UPR activates the JNK signaling pathway in both chronic ER stress models in wing imaginal discs (i.e., Psn overexpression and dDad1 loss of function) (Demay et al. 2014; Zhang et al. 2016). Alternatively, JNK activation by Rh-1G69D expression seems independent of the three main UPR sensors (i.e., IRE1, ATF6, PERK) in the eye imaginal disc (Kang et al. 2012). We thus tested whether this observation could be recapitulated by vg-GAL4-driven Rh-1G69D expression. Activated AP1 (activator protein-1)—the downstream transcription factor of the JNK pathway—was observed in wing imaginal stressed cells, as reported by a TRE::RFP transgene (Chatterjee and Bohmann 2012) (Fig. 1). We assessed whether this activation depended on the PERK/ATF4 branch. RNAi-mediated depletion of PERK and ATF4 significantly reduced JNK activation in cells expressing Rh-1G69D (Fig. 1), showing that independently from the stress source, ER stress at the dorso-ventral boundary triggers a PERK/ATF4/JNK signaling.
Rh-1G69D-induced apoptosis has been reported to be mediated by a Cdk5/Mekk1/JNK pathway in eye imaginal discs (Kang et al. 2012). Similarly, ER stress-induced apoptosis due to dDad1 depletion in the posterior part of the wing imaginal disc depends on the JNK pathway (Zhang et al. 2016). By contrast, we have previously shown that ER stress-induced cell death is JNK independent in response to Psn overexpression in the dorso-ventral boundary of the wing disc (Demay et al. 2014). Therefore, we tested whether Rh-1G69D expression-induced apoptosis depended on the JNK pathway in this domain of wing imaginal discs. The JNK Bsk and JNKK Hep were depleted by specific RNAis in the presence of Rh-1G69D. Reduction of transcript levels of bsk or hep did not modify cleaved Dcp-1 immunodetection (Fig. 2). These results suggest that the apoptosis activation mode by the UPR does not depend on the source of stress but on the tissue and even more on the localization in the tissue, here the vg-GAL4 expression domain in the wing imaginal disc.
Fig. 2.
Rh-1G69D expression-induced apoptosis in the wing disc dorso-ventral boundary is JNK independent. a Projections from confocal stacks of male third-instar larval wing imaginal discs immunostained with an anti-activated Dcp-1 antibody. From left to right, genotypes of control discs on the top row are vg-GAL4/+, vg-GAL4/UAS-bsk-RNAi, and vg-GAL4/UAS-hep-RNAi. From left to right, wing imaginal discs expressing ectopically Rh1G69D (bottom row) display the following genotypes: vg-GAL4/+; UAS-Rh1G69D/+, vg-GAL4/UAS-bsk-RNAi; UAS-Rh1G69D/+ and vg-GAL4/UAS-hep-RNAi; UAS-Rh1G69D/+. b Quantification of apoptosis stainings on wing discs for the previously described genotypes allowing Rh-1G69D ectopic expression. Error bars indicate S.E.M. The Mann-Whitney U test revealed no statistic difference
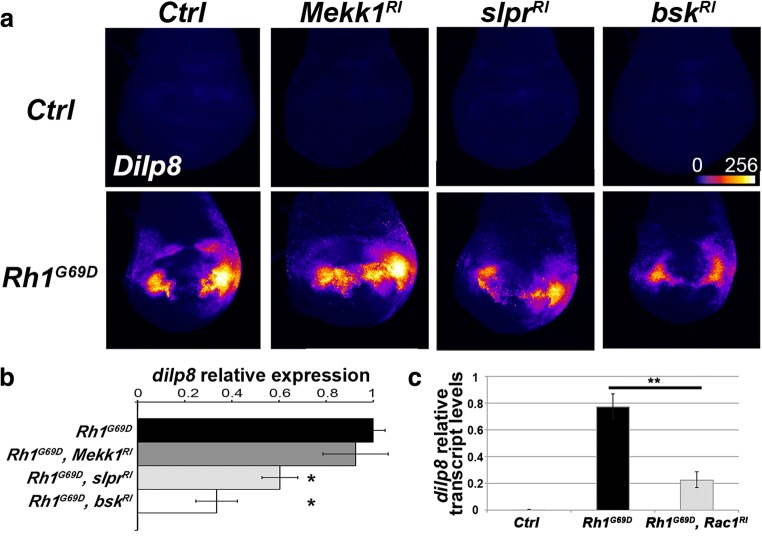
We have previously reported that Psn overexpression driven by vg-GAL4 activates a JNK pathway involving the Rac1 small GTPase and the MAP3K Slpr to upregulate dilp8 expression (Demay et al. 2014). We thus wondered whether this JNK pathway plays a similar role in wing imaginal disc cells expressing Rh-1G69D. Indeed, Rh-1G69D induced dilp8 expression and the depletion of Bsk or Slpr significantly reduced this induction, whereas Mekk1 depletion had no effect, as monitored with a dilp8::EGFP reporter gene (Garelli et al. 2012) (Fig. 3a, b). As all three mutant Rh-1G69Ddilp8::EGFP reporter and Rac1-targeting RNAi transgenes are located on the third chromosome, we checked Rac-1 implication by RT-qPCR. Depletion of Rac1 significantly reduced dilp8 transcripts levels (Fig. 3c). These results indicate that the previously described Psn overexpression-induced JNK pathway is also induced by a vg-GAL4-driven Rh-1G69D expression. Altogether, our data support the hypothesis that the role (i.e., inducing apoptosis or a homeostatic response) of the JNK signaling pathway in the ER stress UPR depends on the stressed cell location but not on the source of the stress.
Fig. 3.
Dilp8 expression in wing imaginal disc cells expressing Rh-1G69D depends on the Rac1/Slpr/JNK pathway. a Fluorescence intensity of GFP using a fire lookup table (ImageJ) reflecting the expression of a dilp8::EGFP reporter transgene in projections from confocal stacks of male third-instar larval wing imaginal discs. (Top row) From left to right, control genotypes are vg-GAL4/+; dilp8MI00727/+, vg-GAL4/UAS-Mekk1-RNAi; dilp8MI00727/+, vg-GAL4/UAS-slpr-RNAi; dilp8MI00727/+ and vg-GAL4/UAS-bsk-RNAi; dilp8MI00727/+. (Bottom row) From left to right, Rh-1G69D expressing wing disc genotypes are vg-GAL4/+; dilp8MI00727/UAS-Rh1G69D, vg-GAL4/UAS-Mekk1-RNAi; dilp8MI00727/UAS-Rh1G69D, vg-GAL4/UAS-slpr-RNAi; dilp8MI00727/UAS-Rh1G69D and vg-GAL4/UAS-bsk-RNAi; dilp8MI00727/UAS-Rh1G69D. b Quantification of Dilp8::EGFP was performed for the previously described genotypes allowing Rh-1G69D ectopic expression. Error bars represent the S.E.M. The asterisks indicate significant differences between the indicated genotype and its control (p < 10−3, Mann-Whitney U test). cdilp8 RNA levels measured by quantitative reverse PCR. Data represent mean ± S.E.M. of three independent experiments. RNA was extracted from control (vg-GAL4/+, open bar), from ER stressed (vg-GAL4/+; UAS-Rh1G69D/+, black bar), or from Rac1-depleted ER-stressed (vg-GAL4/UAS-rac1-RNAi; UAS-Rh1G69D/+, gray bar) wing imaginal discs. ** indicates a significant difference with the control (p < 1%, Student’s t test)
Rh-1G69D expression in posterior cells induces a similar response to dDad1 depletion
According to the stressed cell location hypothesis, Rh-1G69D expression driven by en-GAL4 in the posterior compartment of the wing imaginal disc should induce a similar response to dDad1 depletion in this domain, i.e., an activation of PERK/ATF4 pathway which in turn induces a Mekk1/JNK pathway-dependent apoptosis (Zhang et al. 2016).
As expected, Rh-1G69D expression driven by en-GAL4 activated the JNK signaling pathway, as reported by a TRE::RFP transgene, and induced apoptosis, as assayed by anti-cleaved Dcp-1 immunostaining (Fig. 4). Moreover, both responses depended on the PERK/ATF4 branch of the UPR. Indeed, RNAi-mediated depletion of PERK or ATF4 significantly reduced JNK activation and apoptosis induction in cells expressing Rh-1G69D, confirming that PERK/ATF4 branch activates JNK signaling in wing imaginal disc. JNK Bsk and JNKKK Mekk1 were depleted by using previously validated RNAis directed against the transcripts encoding these proteins (Demay et al. 2014; Zhang et al. 2016). We observed a significant decrease of cleaved Dcp-1 immunodetection in these conditions (Fig. 5), showing that apoptosis is induced by a Mekk1/JNK pathway.
Fig. 4.
Rh-1G69D expression in the wing posterior compartment activates both JNK signaling and apoptosis in a PERK/ATF4-dependent manner. a Projections from confocal stacks of male third-instar larval wing imaginal discs expressing the TRE reporter of JNK signaling (top) and immunostained with an anti-activated Dcp-1 antibody (bottom). From left to right, genotypes are en-GAL4, TRE-red-2L/+; UAS-Rh1G69D/+ (Rh1G69D), en-GAL4, TRE-red-2L/+; UAS-Rh1G69D/UAS-Atf4-RNAi (Rh1G69D, Atf4RI) and en-GAL4, TRE-red-2L/+; UAS-Rh1G69D/UAS-Perk-RNAi (Rh1G69D, PerkRI). b Quantification of apoptosis (top) and JNK reporter (bottom) stainings on wing discs of the previous genotypes. Error bars indicate S.E.M. Statistical difference with the control is indicated by * if p < 5% or *** if p < 10−3 (Mann-Whitney U test)
Fig. 5.
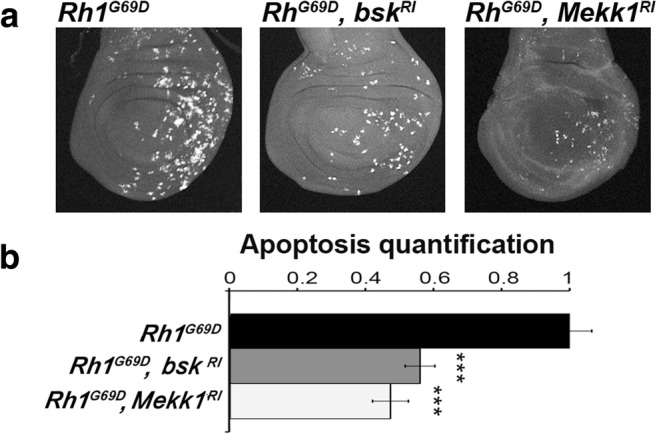
Rh-1G69D expression in the wing posterior compartment induces a Mekk1/JNK-dependent apoptosis. a Projections from confocal stacks of male third-instar larval wing imaginal discs immunostained with an anti-activated Dcp-1 antibody. From left to right, genotypes of wing imaginal discs expressing ectopically Rh1G69D are en-GAL4/+; UAS-Rh1G69D/+, en-GAL4/UAS-bsk-RNAi; UAS-Rh1G69D/+ and en-GAL4/UAS-Mekk1-RNAi; UAS-Rh1G69D/+. b Quantification of apoptosis stainings on wing discs of the previous genotypes. Error bars indicate S.E.M. Statistical difference with the control is indicated by *** (p < 10−3, Mann-Whitney U test)
Altogether, these results validate the hypothesis that the position of stressed cells in the wing imaginal disc determines the role and the nature of the JNK signaling pathway activated by the most prevalent arm of the UPR in this tissue, i.e., the PERK/ATF4 pathway.
Discussion
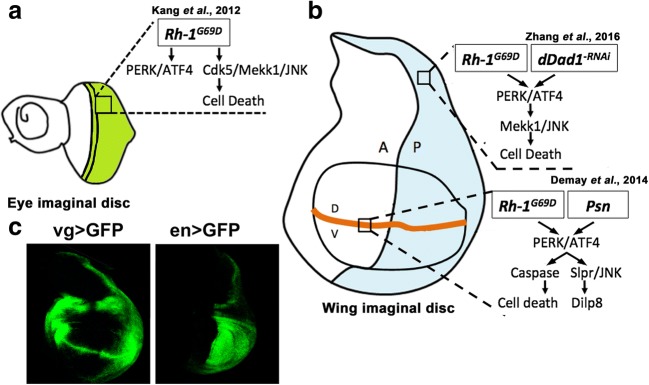
In the present study, by using the ectopic expression of Rh-1G69D as a unique stressor, we generalize the previous observations made in the wing imaginal disc on the responses to chronic ER stress induced by vg-GAL4-driven Psn overexpression (Demay et al. 2014) and en-GAL4-driven dDad1 depletion (Zhang et al. 2016). Contrarily to what was observed in eye imaginal discs (Kang et al. 2012), Rh-1G69D expression either in the dorso-ventral boundary or the posterior cells of wing imaginal discs induces a PERK/ATF4-dependent apoptosis, suggesting that this sensor is the prevalent arm of the UPR in Drosophila wing imaginal discs which face chronic ER stress. Moreover, these data show that the same stressor induces different pathways in different tissues (eye vs. wing imaginal disc), pointing out the tissue specificity of ER stress responses (Fig. 6).
Fig. 6.
Proposed model of ER stress pathways in Drosophila. Mutated aggregation-prone Rh1 induces different pathways depending on the tissue location. As in the compartment posterior to the morphogenetic furrow of the eye imaginal disc (a, green domain), ectopic Rh1G69D triggers a JNK-dependent cell death in the posterior compartment (blue domain) but not on the dorso-ventral frontier (orange line) of the wing imaginal disc (b). Both mutant Rh1G69D and depletion of dDad1, which is essential to N-glycosylation, induce the same unfolded protein response to ER stress in the posterior compartment of wing discs. On the dorso-ventral frontier, mutant Rh1G69D activates a different unfolded protein response to ER stress, which is identical to the signaling induced by overexpressing Psn. The exact pattern of expression of vg-GAL4 (left) and en-GAL4 (right), which drive the different transgenes used in this study, is revealed with a UAS-GFP reporter transgene (c). Our data together with the literature strongly suggest that the ER stress source is not crucial to specify the cell response, while cell location is
We have previously reported that the notched-wing phenotype induced by vg-GAL4-driven Psn overexpression is due to the imbalance between ER stress-induced cell death and an extended period of proliferation allowed by a Dilp8-dependent developmental delay (Demay et al. 2014). Ectopic expression driven by vg-GAL4 of Rh-1G69D confirmed these results, and in particular, that dilp8 expression is induced by a Rac1/Slpr/JNK pathway. Interestingly, this pathway differs from the signaling described for a neoplastic growth of the wing imaginal disc (Andersen et al. 2015). Indeed, depletion of Avalanche/Syntaxin 7 activates a Grindelwald/Traf2/Tak1/JNK pathway, which induces dilp8 expression. This discrepancy could result from either a difference in the type of stress (polarity loss/neoplastic growth vs. chronic ER stress) or the region of the disc (wing pouch vs. dorso-ventral boundary). Further studies should help to discriminate between these hypotheses.
A recent study pointed out that the transcriptional response to pharmacologically induced ER stress differed from the response to luminal load by unfolded proteins in mammalian cells (Bergmann et al. 2018). Surprisingly, the results presented in (i) this study on aggregate-prone Rh-1G69D, (ii) our previous work on the Ca2+ flux regulator Psn (Demay et al. 2014), (iii) our unpublished data on the depletion of the Ca2+ pump SERCA in the vg domain, and (iv) the literature on the inhibition of N-glycosylation (Zhang et al. 2016) in the wing disc indicate that different sources of ER stress trigger the same PERK-ATF4 pathway when affecting the same tissue domain. This apparent discrepancy may be explained by a lack of specificity of chemical drugs that trigger ER stress.
We report that the role of the JNK pathway that is activated by PERK/ATF4 differs according to the position of the cell in the wing imaginal disc. Indeed, expression of Rh-1G69D induces a JNK-dependent apoptosis in posterior cells, whereas cell death is JNK–independent at the dorso-ventral boundary. Interestingly, cells at this frontier support an increase in mechanical tension at the level of their adherens junctions (Aliee et al. 2012; Michel et al. 2016) and Rac1 seems necessary to recruit the actin at the adherens junctions (Eaton et al. 1995). One may wonder whether the activation of a Rac1/JNK pathway at the dorso-ventral boundary is related with the strength of cell bonds at this location of the wing imaginal disc and thus potentially contributing to the specific functional role of these cells throughout the development of the wing tissue.
The results of this study push further the concept of tissue specificity of ER stress responses to the regions of a same tissue, here the wing imaginal disc. Our study illustrates the importance to be careful when generalizing ER stress responses between cell types. This should be taken into account when targeting UPR branches to treat diseases.
Electronic supplementary material
(PDF 1493 kb)
(PDF 88 kb)
Acknowledgments
Confocal image acquisition and analysis were performed at the CYMAGES imaging facility. We acknowledge D. Bohmann, M.J. Kang, B. Mollereau, the Vienna Drosophila RNAi Center (VDRC, Vienna, Austria), and Drosophila Bloomington Stock Center (Bloomington, IN, USA) for providing fly stocks and the Developmental Studies Hybridoma Bank (University of Iowa, IA, USA) for providing monoclonal antibodies.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jessica Perochon, Email: jessica.perochon@glasgow.ac.uk.
Benjamin Grandon, Email: benjamin.grandon2@uvsq.fr.
Delphine Roche, Email: delphine.roche@uvsq.fr.
Christine Wintz, Email: christine.wintz@uvsq.fr.
Yohan Demay, Email: yohan.demay@uvsq.fr.
Bernard Mignotte, Email: bernard.mignotte@uvsq.fr.
Sébastien Szuplewski, Phone: +33 1 70 42 94 55, Email: sebastien.szuplewski@uvsq.fr.
Sébastien Gaumer, Phone: +33 1 70 42 94 55, Email: sebastien.gaumer@uvsq.fr.
References
- Aliee M, Röper J-C, Landsberg KP, Pentzold C, Widmann TJ, Jülicher F, Dahmann C. Physical mechanisms shaping the Drosophila dorsoventral compartment boundary. Curr Biol. 2012;22:967–976. doi: 10.1016/j.cub.2012.03.070. [DOI] [PubMed] [Google Scholar]
- Allen D, Seo J. ER stress activates the TOR pathway through Atf6. J Mol Signal. 2018;13:1. doi: 10.5334/1750-2187-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen DS, Colombani J, Palmerini V, Chakrabandhu K, Boone E, Röthlisberger M, Toggweiler J, Basler K, Mapelli M, Hueber AO, Léopold P. The Drosophila TNF receptor Grindelwald couples loss of cell polarity and neoplastic growth. Nature. 2015;522:482–486. doi: 10.1038/nature14298. [DOI] [PubMed] [Google Scholar]
- Bergmann TJ, Fregno I, Fumagalli F, Rinaldi A, Bertoni F, Boersema PJ, Picotti P, Molinari M. Chemical stresses fail to mimic the unfolded protein response resulting from luminal load with unfolded polypeptides. J Biol Chem. 2018;293:5600–5612. doi: 10.1074/jbc.RA117.001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R, Parra V, Gatica D, et al. Endoplasmic reticulum and the unfolded protein response: dynamics and metabolic integration. Int Rev Cell Mol Biol. 2013;301:215–290. doi: 10.1016/B978-0-12-407704-1.00005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee N, Bohmann D. A versatile PhiC31 based reporter system for measuring AP-1 and Nrf2 signaling in Drosophila and in tissue culture. PLoS One. 2012;7:e34063. doi: 10.1371/journal.pone.0034063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung K-H, Shineman D, Müller M, Cárdenas C, Mei L, Yang J, Tomita T, Iwatsubo T, Lee VMY, Foskett JK. Mechanism of Ca2+ disruption in Alzheimer’s disease by presenilin regulation of InsP3 receptor channel gating. Neuron. 2008;58:871–883. doi: 10.1016/j.neuron.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley NJ, Cassill JA, Baker EK, Zuker CS. Defective intracellular transport is the molecular basis of rhodopsin-dependent dominant retinal degeneration. Proc Natl Acad Sci U S A. 1995;92:3070–3074. doi: 10.1073/pnas.92.7.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombani J, Andersen DS, Léopold P. Secreted peptide Dilp8 coordinates Drosophila tissue growth with developmental timing. Science. 2012;336:582–585. doi: 10.1126/science.1216689. [DOI] [PubMed] [Google Scholar]
- Demay Y, Perochon J, Szuplewski S, Mignotte B, Gaumer S. The PERK pathway independently triggers apoptosis and a Rac1/Slpr/JNK/Dilp8 signaling favoring tissue homeostasis in a chronic ER stress Drosophila model. Cell Death Dis. 2014;5:e1452. doi: 10.1038/cddis.2014.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton S, Auvinen P, Luo L, Jan YN, Simons K. CDC42 and Rac1 control different actin-dependent processes in the Drosophila wing disc epithelium. J Cell Biol. 1995;131:151–164. doi: 10.1083/jcb.131.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garelli A, Gontijo AM, Miguela V, Caparros E, Dominguez M. Imaginal discs secrete insulin-like peptide 8 to mediate plasticity of growth and maturation. Science. 2012;336:579–582. doi: 10.1126/science.1216735. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Molecular Cell. 2000;5(5):897–904. doi: 10.1016/S1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- Hayrapetyan V, Rybalchenko V, Rybalchenko N, Koulen P. The N-terminus of presenilin-2 increases single channel activity of brain ryanodine receptors through direct protein-protein interaction. Cell Calcium. 2008;44:507–518. doi: 10.1016/j.ceca.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Kang M-J, Chung J, Ryoo HD. CDK5 and MEKK1 mediate pro-apoptotic signalling following endoplasmic reticulum stress in an autosomal dominant retinitis pigmentosa model. Nat Cell Biol. 2012;14:409–415. doi: 10.1038/ncb2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K, Ryoo HD, Park JE, Yoon JH, Kang MJ, Jan E. A drosophila reporter for the translational activation of ATF4 marks stressed cells during development. PLOS ONE. 2015;10(5):e0126795. doi: 10.1371/journal.pone.0126795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A-Y, Seo JB, Kim W-T, Choi HJ, Kim SY, Morrow G, Tanguay RM, Steller H, Koh YH. The pathogenic human Torsin A in Drosophila activates the unfolded protein response and increases susceptibility to oxidative stress. BMC Genomics. 2015;16:338. doi: 10.1186/s12864-015-1518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel M, Aliee M, Rudolf K, Bialas L, Jülicher F, Dahmann C. The selector gene apterous and notch are required to locally increase mechanical cell bond tension at the Drosophila dorsoventral compartment boundary. PLoS One. 2016;11:e0161668. doi: 10.1371/journal.pone.0161668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michno K, Knight D, Campusano JM, et al. Intracellular calcium deficits in Drosophila cholinergic neurons expressing wild type or FAD-mutant presenilin. PLoS One. 2009;4:e6904. doi: 10.1371/journal.pone.0006904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybalchenko V, Hwang S-Y, Rybalchenko N, Koulen P. The cytosolic N-terminus of presenilin-1 potentiates mouse ryanodine receptor single channel activity. Int J Biochem Cell Biol. 2008;40:84–97. doi: 10.1016/j.biocel.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Ryoo HD. Drosophila as a model for unfolded protein response research. BMB Rep. 2015;48:445–453. doi: 10.5483/BMBRep.2015.48.8.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryoo HD, Domingos PM, Kang M-J, Steller H. Unfolded protein response in a Drosophila model for retinal degeneration. EMBO J. 2007;26:242–252. doi: 10.1038/sj.emboj.7601477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souid S, Lepesant J-A, Yanicostas C. The xbp-1 gene is essential for development in Drosophila. Dev Genes Evol. 2007;217:159–167. doi: 10.1007/s00427-006-0124-1. [DOI] [PubMed] [Google Scholar]
- Stutzmann GE, Smith I, Caccamo A, et al. Enhanced ryanodine-mediated calcium release in mutant PS1-expressing Alzheimer’s mouse models. Ann N Y Acad Sci. 2007;1097:265–277. doi: 10.1196/annals.1379.025. [DOI] [PubMed] [Google Scholar]
- Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci. 2004;101(31):11269–11274. doi: 10.1073/pnas.0400541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Kaufman RJ. The impact of the unfolded protein response on human disease. J Cell Biol. 2012;197:857–867. doi: 10.1083/jcb.201110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Cui C, Lai Z-C. The defender against apoptotic cell death 1 gene is required for tissue growth and efficient N-glycosylation in Drosophila melanogaster. Dev Biol. 2016;420:186–195. doi: 10.1016/j.ydbio.2016.09.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1493 kb)
(PDF 88 kb)