Abstract
The heat-inducible expression system has been widely used to produce recombinant proteins in Escherichia coli. However, the rise in temperature affects cell growth, activates the bacterial Heat-Shock Response (HSR), and promotes the formation of insoluble protein aggregates known as inclusion bodies (IBs). In this work, we evaluate the effect of the culture scale (shake flasks and bioreactors) and induction temperature (39 and 42 °C) on the kinetic behavior of thermoinducible recombinant E. coli ATCC 53606 producing rESAT-6 (6-kDa early-secretory antigenic target from Mycobacterium tuberculosis), compared with cultures grown at 30 °C (without induction). Also, the expression of the major E. coli chaperones (DnaK and GroEL) was analyzed. We found that almost twice maximum biomass and rESAT-6 production were obtained in bioreactors (~ 3.29 g/L of biomass and ~ 0.27 g/L of rESAT-6) than in shake flasks (~ 1.41 g/L of biomass and ~ 0.14 g/L of rESAT-6) when induction was carried out at 42 °C, but similar amounts of rESAT-6 were obtained from cultures induced at 39 °C (~ 0.14 g/L). In all thermo-induced conditions, rESAT-6 was trapped in IBs. Furthermore, DnaK was preferably expressed in the soluble fraction, while GroEL was present in IBs. Importantly, IBs formed at 39 °C, in both shake flasks and bioreactors, were more susceptible to degradation by proteinase-K, indicating a lower amyloid content compared to IBs formed at 42 °C. Our work presents evidence that the culture scale and the induction temperature modify the E. coli metabolic response, expression of chaperones, and structure of the IBs during rESAT-6 protein production in a thermoinducible system.
Electronic supplementary material
The online version of this article (10.1007/s12192-019-01006-x) contains supplementary material, which is available to authorized users.
Keywords: Shake flasks, Bioreactors, Thermoinduction, Recombinant protein, Chaperones, Inclusion bodies
Background
A wide variety of recombinant proteins (RP) with pharmaceutical applications are produced using the Escherichia coli bacteria owing to its fast growth on inexpensive substrates, well-studied genetics, availability of several mutant strains and vectors, and its high productivity compared to another cells (Baneyx 1999; Baneyx and Mujacic 2004; Baeshen et al. 2015; Sánchez-García et al. 2016). However, this bacterial factory tends to promote protein aggregation under heterologous protein overexpression, stress conditions, and shortage of chaperones (Gatti-Lafranconi et al. 2011; Mitraki and King 1989; Valdez-Cruz et al. 2010; Valdez-Cruz et al. 2011; de Marco et al. 2018). The aggregates of RP are called inclusion bodies (IBs) (Williams et al. 1982), which are electron-dense and dynamic particles with spherical/cylindrical shapes of size ranging from 50 to ~ 800 nm (Rinas and Bailey 1992; Bowden et al. 1991; Carrió and Villaverde 2002; Carrió and Villaverde 2003; Margreiter et al. 2008; Baig et al. 2014; Calcines-Cruz et al. 2018; de Marco et al. 2018). For many years, formation of IBs was considered an undesired event during production of soluble proteins; however, nowadays biologically active heterologous proteins can be obtained from IBs (García-Fruitós et al. 2007; García-Fruitós 2010; Peternel et al. 2008; Rinas et al. 2017; de Marco et al. 2018). IBs have different arquitecture and composition depending on the RP produced, host strain background, culture medium, and environmental conditions, such as temperature, culture time, pH, and inductor concentration (Strandberg and Enfors 1991; Jürgen et al. 2010; Baig et al. 2014; Castellanos-Mendoza et al. 2014; Przybycien et al. 1994; García-Fruitós et al. 2011; Calcines-Cruz et al. 2018; de Marco et al. 2018).
Temperature-induced systems are widely used to produce high amounts of RP and reduce production costs, even at large scale (Valdez-Cruz et al. 2010; Valdez-Cruz et al. 2011). The thermoinducible system used in E. coli is λpR/pL-cI857, which is based on the insertion of a gene of interest into vectors containing the strong pR and/or pL promoters and the thermolabile mutant repressor cI857 from λ bacteriophage that blocks the transcription of RNA polymerase at temperatures below to 37 °C (Villaverde et al. 1993; Dodd et al. 2004; Lieb 1966; Villaverde et al. 1993; Menart et al. 2003). The thermoinduction culture strategy consists of a growth phase between 28 and 32 °C and a subsequent production period between 38 and 42 °C (Valdez-Cruz et al. 2010; Valdez-Cruz et al. 2011; Caspeta et al. 2013). This system avoids the use of special media, antibiotics, or chemical inducers, such as IPTG (isopropyl-β-D-1-thiogalactopyranoside), which may be expensive and toxic to cells and humans (Miroux and Walker 1996; Kosinski et al. 1992). Thus, the risks of contamination by manipulation are minimized since temperature is controlled externally (Makrides 1996; Remaut et al. 1981; Caspeta et al. 2009).
The overproduction of RP stimulates the formation of IBs, and a rise in temperature can negatively affect cell growth and trigger the Heat-Shock Response (HSR) (Gill et al. 2000; Rosen and Ron 2002; Zhao et al. 2005; Valdez-Cruz et al. 2011). In E. coli, the HSR is regulated by an alternative RNA polymerase sigma factor (σ32) that controls the expression of genes encoding the Heat-Shock Proteins (HSPs) (Grossman et al. 1987). HSPs assist the assembly of proteins by sensing and responding to the folding state through interactions with nascent polypeptide chains and aggregates (Gill et al. 2000; Jürgen et al. 2000; Hoffmann and Rinas 2000; Carrió and Villaverde 2005; Smith 2007). Depending on their activity, the HSPs have been classified into the following three groups: (1) folding HSPs that include the trigger factor (TF) and the DnaK/DnaJ and GroEL/GroES chaperones; (2) stabilizing HSPs that include the small chaperones IbpA and IbpB; and (3) disaggregating HSPs of the ClpB family (Baneyx and Mujacic 2004). Particularly, DnaK and GroEL bind to the hydrophobic residues of polypeptide chains and work cooperatively to catalyze their de novo folding through ATP-driven conformational changes (Gragerov et al. 1992; Hayer-Hartl et al. 2015). A transcriptomic analysis showed that mRNA levels of DnaK and GroEL increased several times when cultures were thermoinduced at 38 and 42 °C, compared to the levels at 30 °C (Caspeta et al. 2009; Valdez-Cruz et al. 2011). Moreover, DnaK and GroEL have been identified in association with IBs of E. coli by in situ immunodetection (Carrió and Villaverde 2005) or 2D gels (Hoffmann and Rinas 2000).
The 6-kDa early-secretory antigenic target (ESAT-6) produced by Mycobacterium tuberculosis (Mtb) is a small protein composed of 95 amino acids. This protein is encoded by the esxA gene that is in the RD1 region of the virulent Mtb genome (Berthet et al. 1998) but was deleted from all subgroups of virulent Mycobacterium bovis and attenuated M. bovis (BCG) (Mahairas et al. 1996). ESAT-6 stimulates the production of interferon gamma (IFN-γ) from T lymphocytes (Van Pinxteren et al. 2000; Brock et al. 2001; Araujo et al. 2008; Pimienta-Rodríguez et al. 2012) and participates in the increase of IL-4 and IL-10 levels, contributing to the development of anti-tuberculosis immunity (Torabi et al. 2013). ESAT-6 has been proposed to be used as a diagnostic tool and for innovative vaccines against tuberculosis (Van Pinxteren et al. 2000; Brock et al. 2001; Brodin et al. 2004; Nemes et al. 2019). The production of rESAT-6 in E. coli by IPTG induction has been achieved in IBs with low productivities (Meher et al. 2006; Vutla et al. 2011; Peng et al. 2016). Also, rESAT-6 has been detected in the soluble fraction of E. coli using low IPTG concentration (0.1 mM/L) and low temperature (28 °C) (Pimienta-Rodríguez et al. 2012). The tendency of ESAT-6 to aggregate has been related to its structure because this protein has a hydrophobic nature with high content (~ 56%) of α-helical structures (Renshaw et al. 2002). This feature allows ESAT-6 to form a thermodynamically stable heterodimer with CFP10, another Mtb antigen, important for the secretion of both native proteins (Meher et al. 2006).
In the present study, we evaluated the soluble and insoluble production of rESAT-6 in E. coli (Migula) Castellani and Chalmers ATCC® 53606™ and the expression of the chaperones DnaK and GroEL using shake flasks and bioreactors at two different induction temperatures (39 and 42 °C). We also quantified the biomass growth, glucose consumption, and acetate production, monitoring pH and dissolved oxygen tension. Additionally, we determined the extractability of proteins from the IBs through evaluation of their susceptibility to proteolytic degradation.
Methods
Bacterial strain, culture media, and culture conditions
E. coli (Migula) Castellani and Chalmers ATCC® 53606™ that constitutively expresses the temperature-sensitive cI857 repressor was used and was transformed with pdeltablue (ATCC® 77334™) plasmid encoding the native ESAT-6 (GenBank accession number FJ014499), with an optimized gene sequence under the control of the lambda pL promoter. About 200 μL aliquots of cryopreserved recombinant E. coli (− 70 °C) with optical density (OD600 nm) of 1.6 AU (absorbance units) were used as pre-inoculum in shake flasks, incubated overnight at 30 °C and 200 rpm (New Brunswick Scientific Classic C25, Orbital shaker, USA).
Both shake flasks and bioreactor cultures were grown on defined medium described previously by Caspeta et al. (2013) with some modifications. Composition in distilled water (g/L) is as follows: (NH4)2HPO4, 4.0; KH2PO4, 13.3; citric acid, 1.7; MgSO4·7H2O, 1.2; thiamine, 0.045; ampicillin, 0.1; glucose, 17.5; casamino acids, 3.0; and trace element solution, 2.0 mL/L. The composition of the concentrated stock solution of trace elements in g/L is as follows: Fe-(III)-citrate, 100.8; ZnSO4·7H2O, 32.0; MnCl2·4H2O, 15.0; Na2-EDTA·2H2O, 14.1; H3BO3, 3.0; CoCl2·6H2O, 2.5; Na2MoO4, 2.1; and CuCl2, 0.1. Culture medium was adjusted to pH 7.0 ± 0.1 with 1 M NaOH and sterilized at 121 °C and 22 psig for 30 min. Solutions of glucose, MgSO4, and trace elements were separately sterilized and added to the medium once it was cold. Thiamine, casamino acids, and ampicillin solutions were sterilized by filtration through 0.22 μm filters (mixed cellulose ester membrane filter, Merck-Millipore, Billerica, MA, USA) and added to the medium just before the inoculum.
Experimental cultures in shake flasks were grown in 250 mL conventional Erlenmeyer flasks with 20% of filling volume (50 mL of culture medium, Duran® Erlenmeyer flask, narrow neck, Borosilicate Glass, USA) using initial OD600 nm of 0.1 AU at constant agitation rate (200 rpm). The inoculum volume required was calculated and sterile-pipetted in each shake flask. Dissolved oxygen tension (DOT) measurement in shake flasks during culture was recorded online using the oxygen optical meter Fibox 3 coupled to PSt3 sensor (PreSens Precision Sensing GmbH, Germany) (Reynoso-Cereceda et al. 2016). Measurement of pH was done offline using a Corning 430 pH meter (Corning, NY, USA).
Bioreactor experiments were inoculated with overnight shake flask cultures at an initial OD600 nm of 0.1 AU, and the inoculum volume was calculated and sterile-pipetted into the bioreactor. Bioreactor cultures were instrumented with pH, DOT, and temperature sensors (Applisens, Applikon Biotechnology, Netherlands) controlled by ADI-1010 Biocontroller, and data acquisition was followed online (BioXpert® Software, Applikon Biotechnology, Netherlands). DOT was controlled at 35% of air saturation by cascade changing the agitation speed (between 100 and 1000 rpm) and maintaining the airflow at 1.0 L/min (1 vvm) by using a proportional-integral-derivative (PID) control strategy (Trujillo-Roldán et al. 2001). The pH was maintained at 7.0 ± 0.4 by using an automatic addition of NaOH or HCl (3 M) through an on–off control strategy. Temperature was controlled by PID with a heating jacket.
Thermal-induction of recombinant protein
Shake flasks and bioreactor cultures were grown at 30 °C until pre-stationary growth phase. The induction of the expression of rESAT-6 was started by a shift from 30 to 39 °C or 42 °C when culture reaches OD600 nm of 1.4–2.0 AU (5 h) in shake flask cultures and 3.0–4.0 AU (6 h) in bioreactor cultures. The heating rate was 0.5 °C/min in bioreactors and 0.2 °C/min in shake flasks.
Analytical methods
Bacterial growth of E. coli ATCC® 53606 (rESAT-6) was determined by measuring the OD600 nm (Spectronic Genesys 20, Thermo Fisher Sci., Waltham, MA, USA). The OD was converted to dry cell mass through a linear correlation standard curve. For E. coli ATCC® 53606, one absorbance unit was equivalent to 0.31 ± 0.05 g/L of cell dry weight. Biomass was recovered and determined by gravimetry by centrifugation at 7000 ×g for 10 min and filtered (0.22 μm mixed cellulose ester pre-weighed membrane filter, Merck-Millipore, Billerica, MA, USA). The supernatant (1.0 mL) was used to measure glucose concentration using the Biochemistry Analyzer YSI 2900 (YSI Life Sciences, Yellow Springs, OH, USA). Acetate was quantified by high-performance liquid chromatography (HPLC; Shimadzu, Kyoto, Japan) using an Aminex HPX-87H column (300 × 7.8 mm, 9-μm internal diameter; Bio-Rad, Hercules, CA, USA), at 0.6 mL/min (mobile phase: H2SO4 0.008 N) and 30 °C, and UV detection at 210 nm. Data analysis was done using the LCsolution software (Shimadzu, Kyoto, Japan). The commercial organic acid analysis standard was used as recommended by the supplier (Catalog No. 125-0586, Bio-Rad, USA).
Separation of the soluble and insoluble protein fractions and inclusion body purification
Biomass was recovered by centrifugation of the bacterial culture at 10,000 ×g for 10 min, and the wet weight of the pellet was determined. The cell pellet was resuspended in BugBuster™ (Merck-Millipore, Billerica MA, USA) by gently pipetting (5.0 mL reagent per gram of wet cells), and 1.0 μL (25 units) Benzonase per 1.0 mL of BugBuster™ and 1.0 mM PMSF were added. Cell suspension was agitated constantly for 20 min at room temperature and centrifuged at 16,000 ×g for 20 min. The supernatant corresponded to cytoplasmic soluble proteins (S in Figs. 3 and 4) and the pellet to insoluble proteins (I in Figs. 3 and 4). This pellet was resuspended in the same previous volume of BugBuster™ and a solution of Lysozyme (Sigma, St. Louis, MO, USA) to a final concentration of 200 μg/mL and incubated for 5 min at room temperature. Six volumes of diluted BugBuster™ in deionized water (1:10) were then added and mixed by vortexing for 1 min. This solution was centrifuged at 16,000 ×g for 15 min, and the supernatant was removed. The pellet was resuspended in half the original volume of 1:10 diluted BugBuster™, mixed, and centrifuged at 16,000 ×g for 15 min twice. Finally, the pellet was washed twice with deionized water by centrifuging at 16,000 ×g for 15 min, and the purified IBs were stored at − 20 °C.
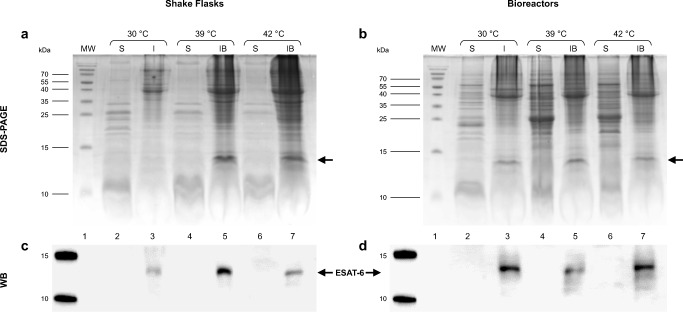
Fig. 3.
Expression of rESAT-6 in E. coli ATCC® 53606™ by SDS-PAGE (18%) with a Coomassie staining (a,b) and Western blot (c,d) with anti-ESAT-6 antibody. Soluble (S), insoluble (I) protein fractions, and purified inclusion bodies (IBs) obtained at the end of the thermo-induced cultures (27 h) in shake flasks (a,c) and bioreactors (b,d) are presented. Lane 1, molecular weight standards (MW). Lanes 2 and 3, S and I fractions from non-induced culture at 30 °C, respectively. Lanes 4 and 5, S and IBs from thermo-induced culture at 39 °C, respectively. Lanes 6 and 7, S and IBs from thermo-induced culture at 42 °C, respectively. Arrowheads indicate the position of rESAT-6 between 10 and 15 kDa. The SDS-PAGE and Western blots were made for each culture independently, and the duplicates are shown in the supplementary material (Figs. 1S and 2S)
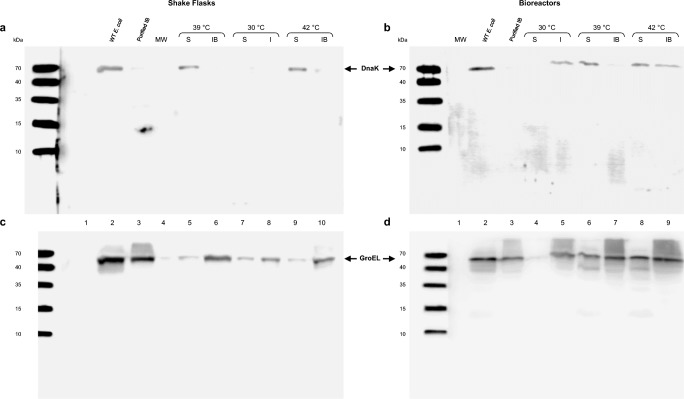
Fig. 4.
Immunoblotting of chaperones DnaK (a,b) and GroEL (c,d) in soluble (S), insoluble (I) protein fractions, and purified inclusion bodies (IBs) obtained in E. coli ATCC® 53606™ (rESAT-6) cultures. In shake flasks (a,c), lane 1, empty; lane 2, cell extract of wild-type E. coli 53606 grown at 42 °C as a control; lane 3, purified IBs harvested from shake flask culture induced at 39 °C with complex medium (LB) as a thermoinduction control; lane 4, molecular weight standards (MW); lanes 5 and 6, S and IBs from induced culture at 39 °C, respectively; lanes 7 and 8, S and I fractions from non-induced culture at 30 °C, respectively; and lanes 9 and 10, S and IBs from induced culture at 42 °C, respectively. In bioreactors, (b,d) lane 1, molecular weight standards (MW); lane 2, cell extract of wild-type E. coli 53606 grown at 42 °C as a control; lane 3, purified IBs harvested from culture induced at 39 °C with complex medium (LB); lanes 4 and 5, S and I fractions from non-induced culture at 30 °C, respectively; lanes 6 and 7, S and IBs from induced culture at 39 °C, respectively; and lanes 8 and 9, S and IBs from induced culture at 42 °C, respectively. Arrowheads indicate the position of chaperones DnaK (~ 70 kDa) and GroEL (~ 60 kDa)
Analysis of recombinant protein expression by SDS-PAGE and Western blot
The rESAT-6 production was analyzed in soluble fraction (S) and IBs using 18% SDS-PAGE (Sambrook et al. 1989). The protein concentration was determined by Bradford (Bradford 1976; Bio-Rad, Hercules, CA, USA). Calibration curves were obtained using bovine serum albumin (BSA), and the samples were suspended in isoelectric focusing (IEF) buffer (1:5). Absorbance was measured at 600 nm in a 96-well plate reader (Stat Fax® 4200, Awareness Technology, Inc., Palm City, FL, USA). Samples were analyzed in biological duplicates and by technical duplicates. SDS-PAGE were stained with Coomassie Brilliant Blue R-250 (Bio-Rad, Hercules, CA, USA), and the excess dye was removed using a solution containing 10% acetic acid, 50% methanol, and water. Quantification was done by densitometry in the SDS-PAGE using the Image-Lab™ software and Gel Doc™ EZ Imager (Bio-Rad, Hercules, CA, USA).
Proteins in the gel were transferred to a polyvinylidene difluoride (PVDF) membrane (Merck-Millipore, Billerica, MO, USA) using semi-wet system (Bio-Rad, Hercules, CA, USA). Membrane was blocked with 5% dry milk in 1× Tris-buffered saline (TBS) buffer for 40 min, washed twice with washing solution (TBS + 0.06% Tween 20), and incubated with anti-ESAT-6 rabbit polyclonal antibody (Cat. No. PA1-19446, Thermo Fisher Sci., Waltham, MA, USA) for 50 min. Three washes (TBS + 0.06% Tween 20) were made followed by incubation with HRP-conjugated anti-rabbit IgG as the secondary antibody (Cat. No. A0545, Merck-Sigma-Aldrich, St. Louis, MI, USA) for 50 min. The bands were detected by chemiluminescence using SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Sci., Waltham, MA, USA) and visualized using the C-DIGIT blot scanner (LI-COR, Lincoln, NE, USA). A home-made luminescent strip put on top of PVDF membrane for immunoreactive protein detection was used and arranged in the same place as the SDS-PAGE molecular weight marker. The SDS-PAGE and Western blots were made for each culture independently, and the repeats are shown in Supplementary Figs. 1S and 2S.
Identification of chaperones DnaK and GroEL by immunoblotting
The PVDF membranes were incubated with the respective anti-chaperone primary antibody. First, each membrane was blocked (5% dry milk in TBS 1× for 40 min) at room temperature, washed twice with TBS + 0.06% Tween 20, and incubated with anti-DnaK mouse polyclonal antibody (Cat. No. ADI-SPA-880, Enzo Life Sciences, Farmingdale, NY, USA). After 1 h, HRP-conjugated anti-mouse IgG was used as the secondary antibody (Cat. No. A9044, Merck-Sigma-Aldrich, St. Louis, MI, USA). Similarly, we used the anti-GroEL rabbit primary antibody (Cat. No. ADI-SPA-875, Enzo Life Sciences, Farmingdale, NY, USA) and HRP-conjugated anti-rabbit IgG as the corresponding secondary antibody (Cat. No. A0545, Merck-Sigma-Aldrich, St. Louis, MI, USA). The immunoreactive proteins were revealed by chemiluminescence using SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Sci., Waltham, MA, USA) and visualized using the C-DIGIT blot scanner (LI-COR, Lincoln, NE, USA). A home-made luminescent strip put on top of PVDF membrane for immunoreactive protein detection was used and arranged in the same place as the SDS-PAGE molecular weight marker.
Proteolytic digestion of inclusion bodies
IBs obtained were subject to proteolytic digestion as previously reported by Castellanos-Mendoza et al. (2014) using proteinase-K (Merck-Sigma-Aldrich, St. Louis, MI, USA) at 12 μg/mL (final concentration). Digestion was performed with 50 μg/mL of purified IBs diluted in buffer (50 mM Tris–HCl and 150 mM NaCl at pH 8.0) to a final volume of 1.0 mL. Absorbance was monitored at 350 nm for 100 min (DU®730 UV/Vis Spectrophotometer, Beckman Coulter, Brea, CA, USA). All measurements were made in duplicate, and absorbance obtained was normalized (De Groot and Ventura 2006; Upadhyay et al. 2012; Castellanos-Mendoza et al. 2014).
Statistical measures
All cultures were grown at least in duplicate. Independent samples and multiple-comparison tests were used to estimate statistical significance of differences in the culture parameters (two-way analysis of variance [ANOVA] and Tukey’s post-hoc test were used). A threshold significance level of 0.1 was applied.
Results
Study of kinetic parameters in recombinant E. coli cultures producing rESAT-6 using a thermoinducible system
The bacterial growth, glucose consumption, and acetate accumulation were evaluated in shake flasks and bioreactor cultures thermoinduced at 39 and 42 °C (Fig. 1). All cultures were started with an inoculum of 0.1 AU and were compared with non-induced cultures maintained at 30 °C as a control (closed dots in Fig. 1). The increase in temperature for induction was performed when cells were in pre-stationary phase at 1.4–2.0 AU (~ 5 h) in shake flasks and 3.0–4.0 AU (~ 6 h) in bioreactors.
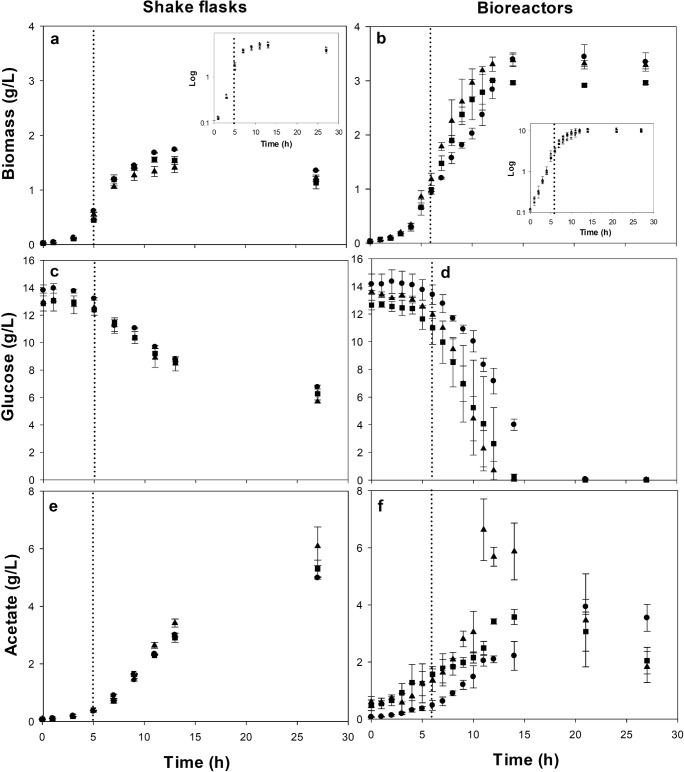
Fig. 1.
Bacterial growth kinetics (a,b), glucose uptake (c,d) and acetate accumulation (e,f) of recombinant E. coli ATCC® 53606™ (rESAT-6) in 250 mL shake flasks (a,c,e) and 1.2 L bioreactors (b,d,f) at different induction temperatures. Non-induced culture as a control was kept at 30 °C (closed dots), and thermo-induced cultures were heated up to 39 °C (closed squares) and 42 °C (closed triangles). Thermoinduction (dotted line) of rESAT-6 production was started at OD600 nm of 1.4–2.0 AU (5 h) in shake flasks and 3.0–4.0 AU (6 h) in bioreactors. Average and standard deviation of at least two independent experiments are shown
In non-induced cultures (30 °C) carried out in shake flasks, the maximal cell biomass concentration (Xmax) was 1.74 ± 0.01 g/L, whereas a statistically significant lower Xmax of 1.54 ± 0.07 and 1.41 ± 0.09 g/L were obtained when cultures were induced at 39 and 42 °C, respectively (Fig. 1a, Table 1). In bioreactors, almost twice-higher Xmax was obtained compared to shake flasks; the Xmax was 3.35 ± 0.17 g/L in non-induced cultures (30 °C), while 2.96 ± 0.03 g/L was reached when cultures were induced at 39 °C and 3.29 ± 0.07 g/L when induced at 42 °C, without significant differences (P > 0.1) (Fig. 1b, Table 1). In shake flasks and bioreactors, the specific growth rates before induction (μs) were similar (0.59 ± 0.01 h−1) as was expected (Table 1), since μs was calculated before the temperature increase. Moreover, no significant differences (P > 0.1) were found in the pre-stationary specific growth rate after induction (μi) in shake flask cultures (0.16 ± 0.01 h−1 at 30 °C, 0.19 ± 0.03 h−1 at 39 °C, and 0.14 ± 0.01 h−1 at 42 °C) and bioreactors (0.18 ± 0.01 h−1 at 30 °C, 0.18 ± 0.00 h−1 at 39 °C, 0.16 ± 0.02 h−1 at 42 °C). This suggests that thermoinduction does not affect the specific growth rate in pre-stationary phase, even when the heat stress is present.
Table 1.
Growth and production parameters of recombinant E. coli ATCC®-53606 (rESAT-6) using two different culture scales (shake flasks and bioreactors) and two induction temperatures (39 and 42 °C). Non-induced culture (kept at 30 °C) was used as a control. Data show the mean and standard deviation for at least two independent experiments
| Parameter | Shake flasks | Bioreactors | ||||
|---|---|---|---|---|---|---|
| 30 °C | 39 °C | 42 °C | 30 °C | 39 °C | 42 °C | |
| μs (h−1) | 0.59 ± 0.02 | 0.59 ± 0.01 | ||||
| AμI (h−1) | 0.16 ± 0.01 a | 0.19 ± 0.03 b | 0.14 ± 0.01 a | 0.18 ± 0.01 a | 0.18 ± 0.00 a | 0.16 ± 0.02 a |
| BXmax (g/L) | 1.74 ± 0.01 b | 1.54 ± 0.07 a | 1.41 ± 0.09 a | 3.35 ± 0.17 a | 2.96 ± 0.03 a | 3.29 ± 0.07 a |
| C Glucose consumed (g/L) | 7.05 ± 0.25 a | 6.59 ± 0.22 a | 7.24 ± 0.54 a | Completely consumed | ||
| DYX/S (g/g) | 0.35 ± 0.02 a | 0.37 ± 0.02 a | 0.31 ± 0.01 b | 0.24 ± 0.00 a | 0.23 ± 0.01 a | 0.24 ± 0.01 a |
| qS (g/g.h) | 0.47 ± 0.02 a | 0.53 ± 0.03 b | 0.45 ± 0.01 a | 0.75 ± 0.05 a | 0.75 ± 0.02 a | 0.67 ± 0.06 a |
| E Acetate (g/L) | 4.99 ± 0.04 a | 5.30 ± 0.30 a | 6.08 ± 0.67 a | 3.93 ± 0.27 a | 3.57 ± 0.27 a | 7.37 ± 0.99 b |
| Final pH | 5.46 ± 0.02 a | 5.13 ± 0.00 a | 4.96 ± 0.01 a | Controlled around 6.45 and 7.35 | ||
| F Total insoluble protein (g/L) | – | 0.95 ± 0.14 a | 0.87 ± 0.23 a | – | 1.35 ± 0.22 a | 1.53 ± 0.33 a |
| G rESAT-6 in inclusion bodies (%) | – | 15 ± 4 a | 16 ± 5 a | – | 11 ± 5 a | 18 ± 6 a |
| rESAT-6 (g/L) | – | 0.15 ± 0.4 a | 0.14 ± 0.4 a | – | 0.14 ± 0.5 a | 0.27 ± 0.7 b |
| YrESAT-6/X (g/g) | – | 0.09 ± 0.03 a | 0.09 ± 0.02 a | – | 0.04 ± 0.02 a | 0.08 ± 0.02 a |
The statistical differences between two or more temperatures are represented with a (the result is not significant at P > 0.1) and b (the result is significant at P < 0.1) indices
A, The pre-stationary specific growth rate after induction (μi) was calculated as the slope of remaining growth after induction (points correspond to 5–11 h in shake flasks and 6–12 h in bioreactors), even in those cultures kept at 30 °C
B, The maximal concentration biomass (Xmax) was reached at 13 h in shake flasks and 25 h in bioreactors (Fig. 1a,b)
C, The glucose consumed in shake flasks corresponds to the initial concentration minus the final glucose in culture medium. In bioreactors, the glucose was completely consumed (Fig. 1c,d)
D, The biomass per glucose yield (YX/S) was calculated using the glucose consumed until the point of the maximal concentration biomass in both systems
E, The maximal acetate accumulated in shake flasks was at the end of culture (25 h) and after of 10 h of culture in bioreactors (Fig. 1e,f)
F, Total insoluble protein was quantified by the Bradford’s method
G, The percentage corresponding to the recombinant protein based on the densitometric data generated from the bands identified as rESAT-6 in the SDS-PAGE gels (arrowheads between 10 and 15 kDa, Fig. 3a,b)
In shake flasks, about 40% of the glucose was consumed in all cultures (Fig. 1c), while in bioreactors, the carbon source was almost completely consumed (Fig. 1d, Table 1). No significant differences were observed in the biomass per glucose yields (YX/S) between induction temperatures using bioreactors (~ 0.24 g/g, Table 1). However, in shake flask cultures induced at 42 °C, YX/S was 12% lower (0.31 ± 0.01 g/g) than in control non-induced (30 °C) cultures (0.35 ± 0.02 g/g) (Table 1). Additionally, the specific glucose uptake rates in shake flasks (qs, calculated by using the pre-stationary specific growth rate after induction) do not present significant differences between non-induced (0.47 ± 0.05 g/g h at 30 °C) and thermo-induced cultures (0.53 ± 0.03 g/g h at 39 °C and 0.45 ± 0.01 g/g h at 42 °C; Table 1). In bioreactor cultures, qs values were higher than those in shake flask cultures, but no significant differences were observed between non-induced and thermo-induced cultures (0.75 ± 0.05 g/g h at 30 °C, 0.75 ± 0.02 g/g h at 39 °C, and 0.67 ± 0.06 g/g h at 42 °C; Table 1).
On the other hand, acetate accumulation in thermo-induced cultures at 42 °C (6.08 ± 0.67 g/L in shake flasks and 7.37 ± 0.99 g/L in bioreactors) was greater compared to that in non-induced cultures at 30 °C (4.99 ± 0.04 g/L in shake flasks and 3.93 ± 0.27 in bioreactors) and those thermo-induced at 39 °C (5.30 ± 0.30 in shake flasks and 3.57 ± 0.27 in bioreactors) (Table 1, Fig. 1e,f). In thermo-induced bioreactors, the acetate accumulated was consumed during stationary phase. After 14 h of culture at 39 °C, acetate decreased to 2.04 ± 0.46 g/L, and, at 42 °C, acetate diminished to 1.82 ± 0.54 g/L (Fig. 1f).
In summary, although there was no glucose limitation in shake flasks, it reached lower biomass compared to bioreactors, possibly due to acetate accumulation, decrease in pH, and/or oxygen limitation. To verify this assumption, we recorded dissolved oxygen tension (DOT) online and pH offline during shake flask cultures. The profiles obtained were compared with controlled cultures grown in bioreactors (Fig. 2).
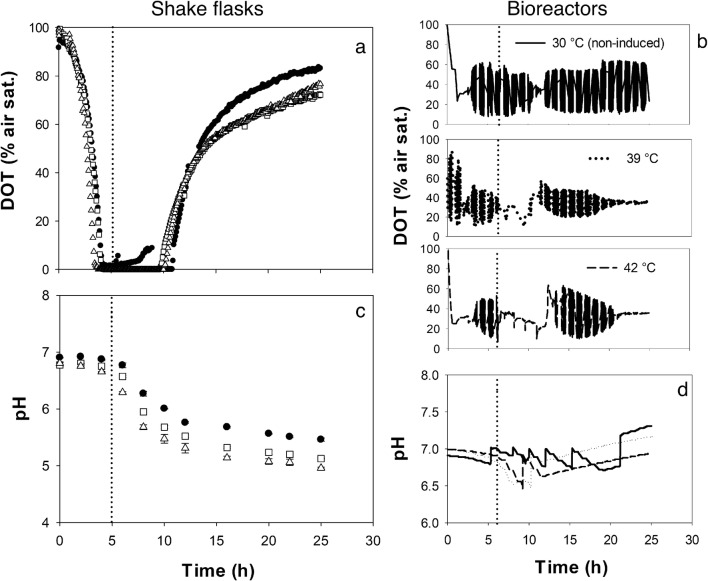
Fig. 2.
Profiles of dissolved oxygen tension (a,b) and pH (c,d) of recombinant E. coli ATCC® 53606™ (rESAT-6). In shake flasks (a,c), non-induced cultures were kept at 30 °C (closed dots) and thermo-induced cultures were heated up to 39 °C (closed squares) and 42 °C (closed triangles). An average and standard deviation of at least two independent experiments are shown. In bioreactors (b,d), a typical trend is shown in solid line (─) of a non-induced culture kept at 30 °C, in dotted line (∙∙∙) of a thermo-induced culture at 39 °C, and in discontinuous line (---) of a thermo-induced culture at 42 °C. Thermoinduction of recombinant protein (vertical dotted line) was started at OD600 nm of 1.4–2.0 AU (5 h) in shake flasks and 3.0–4.0 AU (6 h) in bioreactors
Dissolved oxygen tension and pH profiles of recombinant E. coli using a thermoinducible system
In shake flasks, an oxygen depletion (DOT = 0% air saturation) was observed from ~ 4.0 to ~ 10.0 h in all cultures (Fig. 2a). This period coincides with the decline in the specific growth rate and the start of the stationary phase. In the case of bioreactors, DOT oscillated between 20 and 60% around the set point (35%), controlled by agitation in cascade (Fig. 2b). On the other hand, a decrease in the pH was observed in shake flasks (Fig. 2c), with a final pH of 5.46 ± 0.02 in non-induced cultures, but, in thermo-induced cultures at 39 and 42 °C, 5.13 ± 0.00 and 4.96 ± 0.01 were reached, respectively (Fig. 2c, Table 1). This result agrees with the higher acetate accumulation in cultures induced at 42 °C. In bioreactors, the pH was automatically controlled close to 7.00 with an interval between 6.45 and 7.35, adjusting with NaOH or HCl 3N (Fig. 2d).
Analysis of rESAT-6 accumulation in soluble and insoluble fractions
The effect of induction temperature and culture scale on the production of rESAT-6 in the soluble (S), insoluble (I) protein fractions and purified IBs was analyzed (Fig. 3). We refer to insoluble protein fraction (I) when it was obtained at 30 °C (without induction). The IBs are obtained from thermo-induced cultures at 39 and 42 °C. We did not find rESAT-6 in the soluble fractions of all cultures. For IBs obtained from shake flasks and bioreactors, a band corresponding to rESAT-6 was seen around 13–14 kDa using SDS-PAGE (lanes 5 and 7 in Fig. 3a,b, respectively) and was confirmed by Western blot using anti-ESAT-6 rabbit polyclonal antibody (Fig. 3c,d, respectively). This result established that rESAT-6 was accumulated entirely in IBs after thermoinduction. In the insoluble fraction (I) of shake flasks and bioreactors non-induced (30 °C), a mild band was observed close to 13–14 kDa in SDS-PAGE (lane 3 in Fig. 3a,b, respectively) and was recognized by the anti-ESAT-6 antibody (Fig. 3c,d), indicating an incomplete repression of the pL promoter upstream of the recombinant gene of ESAT-6. The SDS-PAGE gels and Western blots were made for each culture independently, and the duplicates are shown in the Supplementary material (Figs. 1S and 2S).
When cultures were induced, the total protein from IBs was 0.95 ± 0.14 and 0.87 ± 0.23 g/L in shake flasks and 1.35 ± 0.22 and 1.53 ± 0.33 g/L in bioreactors, at 39 and 42 °C, respectively (Table 1). The rESAT6 accumulation in shake flasks was lower, probably due to the lower biomass concentration and/or cells under this condition undergo more stress (oxygen limitation, pH change, and acetate accumulation) compared to those in the bioreactors. Analysis by densitometry in SDS-PAGE gels indicated greater accumulation of rESAT-6 in thermo-induced cultures at 42 °C (~ 16% and ~ 18% in shake flasks and bioreactors, respectively) compared to thermo-induced cultures at 39 °C (Fig. 3a,b, Table 1).
Expression of chaperones DnaK and GroEL
The expression of two major chaperones of E. coli, DnaK and GroEL, related with protein folding was evaluated in shake flasks and bioreactor cultures under two induction temperatures (39 and 42 °C) by Western blot using antibodies against DnaK and GroEL (Fig. 4). The comparison was made using the same volumes for protein extraction recovered at the end of the cultures. DnaK (~ 70 kDa) was mostly detected in soluble fractions at 39 and 42 °C in shake flasks (lanes 5 and 9, Fig. 4a), as also in cultures of the wild-type E. coli ATCC 53606 grown at 42 °C, used as control (lane 2, Fig. 4a). DnaK was not observed in IBs (lanes 6 and 10, Fig. 4a) and in non-induced shake flask cultures at 30 °C (lanes 7 and 8, Fig. 4a). In bioreactor cultures, DnaK was detected in the insoluble fraction (I) in cultures grown at 30 °C (lane 5, Fig. 4b), and more was observed in the soluble fraction at 39 and 42 °C (lanes 6 and 8, Fig. 4b) compared to DnaK detected in the IBs (lanes 7 and 9, Fig. 4b). DnaK localizes preferentially on the surface of the IBs produced under chemically induced system and has also been observed in the cytoplasm (Carrió and Villaverde 2005). In our case, the absence or low detection of DnaK observed in IBs in shake flask cultures was probably due to the effect of culture scale, the low DnaK concentration around the IBs after their isolation from cell, or the exposure time of antibody.
On the other hand, GroEL (~ 60 kDa) was identified in all evaluated conditions, regardless of induction temperature or culture scale (Fig. 4c,d). This emphasizes their importance during production of a recombinant protein in E. coli using a thermoinducible system. GroEL was detected preferentially in the IBs of shake flask cultures (lanes 6 and 10, Fig. 4c). A similar behavior was observed for bioreactor cultures with an enrichment of GroEL in the IBs formed at 39 and 42 °C (lanes 7 and 9, Fig. 4d) compared to soluble fractions (lanes 6 and 8, Fig. 4d). As in control, in wild-type E. coli ATCC 53606 supernatants, from cultures carried out at 42 °C, DnaK (lane 2, Fig. 4a,b) and GroEL (lane 2, Fig. 4c,d) were detected.
Resistance of rESAT-6 IBs to proteinase-K degradation
To understand the effect of scale and induction temperature on the molecular architecture of IBs, the kinetic degradation by proteinase-K on purified IBs recovered from shake flasks and bioreactors was evaluated (Fig. 5). This assay consists of incubating the IBs obtained at the different conditions with the proteinase-K and measuring the changes in the absorbance for 100 min as an indicator that the protein within the aggregate is degrading due to the enzymatic action. All IBs obtained showed resistance to proteolytic degradation; however, IBs formed in shake flasks and bioreactors induced at 39 °C were degraded ~ 24% more after 100 min of reaction (0.756 ± 0.002 and 0.873 ± 0.004 AU, respectively) compared with IBs formed under thermoinduction at 42 °C (0.905 ± 0.017 and 0.942 ± 0.035 AU, respectively). Moreover, a faster degradation by proteinase-K was observed in IBs harvested from shake flasks than those from bioreactors, since the end of the proteinase-K reaction was reached on first 20 min of incubation (Fig. 5a), whereas, only ~ 13% and ~ 6% of disintegration was achieved in IBs obtained from thermo-induced bioreactor cultures at 39 and 42 °C, respectively (Fig. 5b). The average rate of IB degradation by proteinase-K was 0.0106 and 0.0048 AU/min in shake flasks induced at 39 and 42 °C, respectively, whereas in bioreactors, it was 0.0041 AU/min at 39 °C and 0.0015 AU/min at 42 °C.
Fig. 5.
Kinetic comparison of proteolytic degradation of rESAT-6 IBs (50 μg/mL) harvested at the end of culture in shake flasks (a) and bioreactors (b). Proteinase-K was used for degradation at final concentration of 12 μg/mL. The progressive degradation was followed by absorbance at 350 nm for 100 min, and data were normalized. Average and standard deviation of at least two IBs obtained from independent cultures are shown. IBs were purified from thermo-induced cultures at 39 °C (closed dots) and 42 °C (open triangles)
Discussion
In shake flask cultures, thermoinduction (39 and 42 °C) had a slightly negative effect on maximal biomass concentration compared with the non-induced cultures at 30 °C (Fig. 1a and Table 1). This could be due to the host redirecting a significant amount of energy resources from growth and maintenance toward expression of a heterologous protein, thereby developing a metabolic burden and decreasing biomass yield (Glick 1995; Hoffmann and Rinas 2004; Bentley et al. 1990; Jensen and Carlsen 1990). Also, in a thermoinducible system, an increase in energy demand is not only associated with cell growth and the production of recombinant proteins but also with the synthesis of heat-shock proteins (Wittmann et al. 2007; Hoffmann and Rinas 2004). After thermoinduction (30 to 42 °C) of recombinant hFGF-2 in E. coli, a significant change in metabolic fluxes occurs (Hoffmann et al. 2002). The synthesis of tricarboxylic acid cycle (TCA) enzymes that respond to increased energy demand, such as succinyl dehydrogenase (SdhA) and dihydrolipoamide dehydrogenase (LpdA), was stimulated. With thermoinduction, the cells seem to readjust their metabolic activities according to their energy requirements and, if necessary, at the cost of their biosynthetic capabilities (Hoffmann et al. 2002; Weber et al. 2002). On the other hand, the transcriptomic profiles of recombinant E. coli were subjected to 50 °C and IPTG induction (Harcum and Haddadin 2006). These cultures exhibited lower growth rates under double stress, and more than 25% of genes associated with metabolism were affected (Harcum and Haddadin 2006). During thermoinduction, a higher fraction of the substrate may be required for energy-generating purposes, thereby directing glucose utilization from cell growth toward recombinant protein synthesis, cell maintenance, and response to heat stress (Schmidt et al. 1999).
Furthermore, in shake flask cultures, the major deficiency is the insufficient supply of oxygen through the gas–liquid interface (oxygen-transfer limitation) that affects cell growth (Büchs 2001; Reynoso-Cereceda et al. 2016) stressing the culture even more. However, in bioreactors, high values of mass transfer rates and avoidance of oxygen limitation owing to DOT control can be attained. This is reflected in the maximal biomass obtained in bioreactors that is almost two-folds greater than in shake flask cultures (Fig. 1a,b; Table 1). In the same sense, in shake flask cultures, the residual glucose was around 6.0 g/L (Fig. 1c) and the maximal biomass was almost half of that in bioreactor cultures (Fig. 1a,b). Glucose may not be the growth-limiting factor in shake flasks, but it may be the accumulation of acetate (Luli and Strohl 1990; Roe et al. 2002; Eiteman and Altman 2006), or oxygen limitation (Li et al. 1992; Reynoso-Cereceda et al. 2016), or the decrease of the pH in the culture media (Salmond et al. 1984; Maurer et al. 2005), or the synergistic effect of these factors (Figs. 1e, 2a,c). On the other hand, the carbon source in the bioreactor cultures was completely consumed (Fig. 1d) owing to oxygen availability and pH control (Fig. 2b,d, respectively), and, in this case, the entry into stationary phase is associated with glucose depletion (Neubauer et al. 1995; Hewitt et al. 1999) and acetate production (Luli and Strohl 1990; Eiteman and Altman 2006). In thermo-induced cultures, acetate production has been widely reported (Hoffmann et al. 2002; Wittmann et al. 2007; Caspeta et al. 2009; Caspeta et al. 2013) and is related to two phenomena—metabolic overflow triggered by glucose excess and oxygen-limitation conditions (Rinas et al. 1989; Lara et al. 2006; Wolfe 2005; Bernal et al. 2016). In the first case, the rate of accumulation of acetyl-CoA exceeds the rate at which it is fed into the tricarboxylic acid (TCA) cycle, owing to low availability of TCA enzymes and low NADH oxidation rate in the respiratory chain (Xu et al. 1999; Lara 2011; Bettenbrock et al. 2014). Thus, the metabolic overflow causes the branching of glycolysis toward acetate production (Eiteman and Altman 2006). In the second case, the oxygen limitation in shake flasks favors the oxidation of accumulated NADH to NAD+ through organic acid production; if NADH is not regenerated, the cell growth is reduced (Losen et al. 2004; Phue and Shiloach 2005). In the bioreactor cultures, acetate reached its maximum concentration at the beginning of the stationary phase and was then re-assimilated (Fig. 1f). E. coli can utilize the accumulated acetate as a secondary carbon source in order to maintain cellular homeostasis and survive under stress conditions (Phue and Shiloach 2004; Shiloach et al. 2010). This phenomenon, known as “acetate switch,” is accompanied by a molecular change in cells which activates the enzymatic machinery (acetyl-CoA synthase or phosphotransacetylase-kinase) responsible for converting acetate into acetyl-CoA (Wolfe 2005). In shake flasks, the period of oxygen limitation (DOT near the 0% of air saturation) occurred after 4 h of culture (Fig. 2a) and coincided with the increase in acetate accumulation (Fig. 1e) and consequently with the decrease in the pH of the culture media (Fig. 2c). The absence of oxygen in E. coli inhibits the expression of many TCA cycle enzymes, branching metabolic pathways to the production of secondary metabolites like acetate (Wolfe 2005). Oxygen-limited conditions when growing E. coli in shake flasks have been widely documented and are related to the capacity of the agitated container to compensate the oxygen demand of the microorganisms (Büchs 2001; Klöckner and Büchs 2012). However, the effect of thermoinduction on respiratory activity of recombinant E. coli cultures has not been extensively studied.
Some reports suggest that recombinant protein production by a thermoinducible system can be associated with an increase in respiratory activity of the cells. Prior to thermoinduction, the oxygen uptake rate is lower compared to the carbon dioxide production rate (respiratory quotient < 1); however, after a temperature upshift, both rates are similar (respiratory quotient ≈ 1), indicating a more efficient glucose oxidation (Schmidt et al. 1999). Furthermore, cultures induced at a constant temperature of 42 °C required a higher oxygen-sparging flow rate to maintain the DOT at the set point (Caspeta et al. 2013), suggesting cells under this condition have a higher respiration rate. The enhanced respiration can be directly correlated to the increase in the synthesis rates of plasmid-encoded and stress proteins (Hoffmann and Rinas 2001). In our experimental conditions, since there was an oxygen limitation in shake flasks and DOT control in bioreactors, it was not possible to observe differences in DOT profiles after thermoinduction (Fig. 2a,b). Thus, further studies are necessary to understand the phenomenon of oxygen transfer upon temperature induction.
The rESAT-6 was exclusively found in IBs in both induction temperatures (39 and 42 °C) in shake flasks and bioreactors (Fig. 3). The formation of insoluble aggregates of recombinant protein in E. coli has been widely reported (Kane and Hartley 1988; Fink 1998; Rosen et al. 2002; Hartl and Hayer-Hartl 2002; de Marco et al. 2018). Upon thermoinduction, the overexpression of a recombinant protein and the temperature increase alters protein folding, which favors their aggregation and accumulation into IBs (Babu et al. 2000; Caspeta et al. 2009; Valdez-Cruz et al. 2010; Caspeta et al. 2013). Although the ESAT-6 antigen has not been previously expressed in a thermoinducible system, rESAT-6 was found in IBs of E. coli upon chemical induction (Wang et al. 2005; Meher et al. 2006; Vutla et al. 2011; Peng et al. 2016). Likewise, rESAT-6 has been observed in the soluble fraction of E. coli cytoplasm upon using low IPTG concentration (0.1 mmol/L) and low temperature (28 °C) (Pimienta-Rodríguez et al. 2012).
An important contribution of this paper to the current knowledge about thermoinducible systems is the differential expression of DnaK and GroEL chaperones in relation to induction by temperature and culture scale. DnaK was preferentially detected in the soluble fractions of thermo-induced shake flasks and bioreactor cultures (Fig. 4a,b). This could be owing to the fact that DnaK is part of the first complex of chaperones (in conjunction with TF chaperone—trigger factor—and DnaJ-GrpE co-chaperones) that directly receive the nascent polypeptide chains from the ribosome, where large and compact aggregates have not formed (Szabo et al. 1994). In contrast, GroEL was expressed in all conditions but in larger amounts in IBs (Fig. 4c,d). This result is supported by the previous study of Carrió and Villaverde (2003) where the importance of GroEL during the aggregation process was demonstrated. Deficient GroEL mutants reduce the extent of protein aggregation and prevent IBs from being assembled from small protein aggregation cores (Carrió and Villaverde 2003). Although GroEL is overexpressed under thermal shock, this protein is also required for cell growth over a wide range of temperatures (from 17 to 42 °C), but DnaK is preferably required only at high temperatures (Fayet et al. 1989; Kusukawa and Yura 1988). In thermo-induced cultures, an increase between 3.2- and 9.9-fold of GroEL protein has been reported, representing 21% of all synthesized proteins (Yamamori et al. 1978; Caspeta et al. 2009; Valdez-Cruz et al. 2011). Moreover, DnaK has been detected on the surface of recombinant VP1LAC IBs and some amounts in the E. coli cytoplasm by in situ immunodetection, but not inside the aggregates. In contrast, GroEL was preferentially distributed inside IBs (Carrió and Villaverde 2005).
For an E. coli thermo-induced system (from 30 to 42 °C), only DnaK was observed in the purified recombinant protein fractions from bioreactor cultures and not in the fractions from shake flask cultures (Yang and Enfors 1996). The oxygen limitation and/or the pH change (associated with the production of organic acids) in shake flasks might be the determinants in the limited expression of heat-shock proteins (as observed with DnaK and GroEL in Fig. 4). In order to quantify this phenomenon, it will be necessary to use more powerful tools, such as two-dimensional difference gel electrophoresis (2D DIGE) or mass spectrometry.
The differences in the expression of DnaK and GroEL in IBs (in shake flasks and bioreactors at two temperatures) allow us to propose that a possible biotechnological strategy might be the manipulation of the post-induction conditions, in order to modify the expression of the heat-shock proteins. For example, the presence/absence of GroEL and DnaK indicates the partial unfolded/misfolded ratio of the recombinant protein produced (de Marco et al. 2000). Moreover, IBs are normally bigger and with more similar folding to the native protein without the presence of DnaK, when compared to the IBs produced in the wild-type E. coli strain (Gonzalez-Montalban et al. 2007; Garcia-Fruitos et al. 2007).
On the other hand, proteinase-K exhibits its activity against globular domains and disordered regions of proteins but not against amyloid fibrils (Sabaté et al. 2010; Macedo et al. 2015). Since partial amyloid structures have a protein core, they will be less resistant to proteinase-K (Chiti and Dobson 2006). In this sense, IBs from cultures, in both shake flasks and bioreactors, induced at 39 °C, were less resistant to proteinase-K than IBs formed in cultures induced at 42 °C. Also, IBs obtained from shake flasks were more susceptible (Fig. 5a) compared with IBs obtained from bioreactors (Fig. 5b). These results indicate that IBs from cultures thermo-induced at 42 °C were highly compacted and structured (major β-sheet conformation) compared to those from cultures at 39 °C. To the best of our knowledge, there is no mechanism that can explain the formation and structuring of IBs in thermo-induced systems, since many factors are involved in the degree of compaction. However, the stability of the hydrophobic interactions and structural compaction are likely reinforced with the increase of temperature. Therefore, future studies are necessary to better understand the thermodynamics of IBs obtained during the production of a recombinant protein using a thermoinducible system.
Electronic supplementary material
(DOCX 315 kb)
Acknowledgements
SRP and CGBC thank the scholarships (308750 and 366135, respectively) from CONACYT-México. SRP is a doctoral student from Programa de Doctorado en Ciencias Biológicas, Universidad Nacional Autónoma de México (UNAM). CGBC is a doctoral student from Programa de Doctorado en Ciencias Bioquímicas, Universidad Nacional Autónoma de México (UNAM). We thank Abel Blancas-Cabrera Eng. and Lorena López-Griego Biol. for technical assistance. We thank Editage (www.editage.com) for the English language editing. This project was developed under the Institutional Program of the Instituto de Investigaciones Biomédicas-UNAM: “La producción de biomoléculas de interés biomédico en bacterias y hongos.”
Notation
- AU
Absorbance units
- BSA
Bovine serum albumin
- DOT
Dissolved oxygen tension, % air saturation
- HSPs
Heat-shock proteins
- HSR
Heat-shock response
- IBs
Inclusion bodies
- Mtb
Mycobacterium tuberculosis
- OD600 nm
Optical density at 600 nm, AU
- PID
Proportional-integral-derivative control strategy
- qacetate
Specific acetate production rate, g/g h
- qs
Specific glucose consumption rate, g/g h
- rESAT6
Recombinant 6-kDa early-secretory antigen from Mycobacterium tuberculosis
- SDS-PAGE
Sodium dodecyl-sulfate polyacrylamide gel electrophoresis
- TBS
Tris-buffered saline
- YX/S
Yield of biomass on glucose, g/g
- μs
Specific growth rate before induction, h−1
- μi
Specific growth rate after induction, h−1
- Xmax
Maximal biomass concentration, g/L
Funding
This work was partially financed by the “Consejo Nacional de Ciencia y Tecnología” (CONACYT 247473, 220795) and “Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica, Universidad Nacional Autónoma de México” (PAPIIT-UNAM IT-200719, IN-208415).
Compliance with ethical standards
Conflict of interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sara Restrepo-Pineda, Email: sarestrepo90@gmail.com.
Carlos G. Bando-Campos, Email: drgiroshi@gmail.com
Norma A. Valdez-Cruz, Email: adrivaldez1@gmail.com
Mauricio A. Trujillo-Roldán, Phone: +525556229192, Email: maurotru@gmail.com, Email: maurotru@biomedicas.unam.mx
References
- Araujo Z, Acosta M, Escobar H, Baños R, Fernández de Larrea C, Rivas-Santiago B. Respuesta inmunitaria en tuberculosis y el papel de los antígenos de secreción de Mycobacterium tuberculosis en la protección, patología y diagnóstico: Revisión. Investig Clin. 2008;49(3):411–441. [PubMed] [Google Scholar]
- Babu KR, Swaminathan S, Marten S, Khanna N, Rinas U. Production of interferon-alpha in high cell density cultures of recombinant Escherichia coli and its single step purification form refolded inclusion body proteins. Appl Microbiol Biot. 2000;53:655–660. doi: 10.1007/s002530000318. [DOI] [PubMed] [Google Scholar]
- Baeshen MN, Al-Hejin AM, Bora RS, Ahmed MM, Ramadan HA, Saini KS, Redwan EM. Production of biopharmaceuticals in E. coli: current scenario and future perspectives. J Microbiol Biotechnol. 2015;25(7):953–962. doi: 10.4014/jmb.1412.12079. [DOI] [PubMed] [Google Scholar]
- Baig F, Fernando L, Salazar MA, Powell R, Bruce T, Harcum S. Dynamic transcriptional response of Escherichia coli to inclusion body formation. Biotechnol Bioeng. 2014;9999:1–20. doi: 10.1002/bit.25169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baneyx F. Recombinant protein expression in Escherichia coli. Curr Opin Biotechnol. 1999;10:411–421. doi: 10.1016/s0958-1669(99)00003-8. [DOI] [PubMed] [Google Scholar]
- Baneyx F, Mujacic M. Recombinant protein folding and misfolding in Escherichia coli. Nat Biotechnol. 2004;22:1399–1408. doi: 10.1038/nbt1029. [DOI] [PubMed] [Google Scholar]
- Bentley WE, Mirjalili N, Andersen DC, Davis RH, Kompala DS. Plasmid-encoded protein: the principal factor in the “metabolic burden” associated with recombinant bacteria. Biotechnol Bioeng. 1990;35(7):668–681. doi: 10.1002/bit.260350704. [DOI] [PubMed] [Google Scholar]
- Bernal V, Castaño-Cerezo S, Cánovas M. Acetate metabolism regulation in Escherichia coli: carbon overflow, pathogenicity, and beyond. Appl Microbiol Biot. 2016;100(21):8985–9001. doi: 10.1007/s00253-016-7832-x. [DOI] [PubMed] [Google Scholar]
- Berthet F, Rasmussen P, Rosenkrands I, Andersen P, Gicquel B. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular mass culture filtrate protein (CFP-10) Microbiol. 1998;144:3195–3203. doi: 10.1099/00221287-144-11-3195. [DOI] [PubMed] [Google Scholar]
- Bettenbrock K, Bai H, Ederer M, Green J, Hellingwerf KJ, Holcombe M, Sharma P. Towards a systems level understanding of the oxygen response of Escherichia coli. Adv Microb Physiol. 2014;64:65–114. doi: 10.1016/B978-0-12-800143-1.00002-6. [DOI] [PubMed] [Google Scholar]
- Bowden GA, Paredes AM, Georgiou G. Structure and morphology of protein inclusion bodies in Escherichia coli. Biotechnol. 1991;9:725–730. doi: 10.1038/nbt0891-725. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brock I, Munk ME, Kok-Jensen A, Andersen P. Performance of whole blood IFN test for tuberculosis diagnosis based on PPD or the specific antigens ESAT-6 and CFP-10. Int J Tuberc Lung D. 2001;5:462–467. [PubMed] [Google Scholar]
- Brodin P, Rosenkrands I, Andersen P, Cole S, Brosch R. ESAT-6 proteins: protective antigens and virulence factors? Trends Microbiol. 2004;12:500–508. doi: 10.1016/j.tim.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Büchs J. Introduction to advantages and problems of shaken cultures. Biochem Eng J. 2001;7(2):91–98. doi: 10.1016/s1369-703x(00)00106-6. [DOI] [PubMed] [Google Scholar]
- Calcines-Cruz C, Olvera A, Castro-Acosta RM, Zavala G, Alagón A, Trujillo-Roldán MA, Valdez-Cruz NA. Recombinant-phospholipase A2 production and architecture of inclusion bodies are affected by pH in Escherichia coli. Int J Biol Macromol. 2018;108:826–836. doi: 10.1016/j.ijbiomac.2017.10.178. [DOI] [PubMed] [Google Scholar]
- Carrió MM, Villaverde A. Construction and deconstruction of bacterial inclusion bodies. J Biotechnol. 2002;96:3–12. doi: 10.1016/s0168-1656(02)00032-9. [DOI] [PubMed] [Google Scholar]
- Carrió MM, Villaverde A. Role of molecular chaperones in inclusion body formation. FEBS Lett. 2003;537:215–221. doi: 10.1016/s0014-5793(03)00126-1. [DOI] [PubMed] [Google Scholar]
- Carrió MM, Villaverde A. Localization of chaperones DnaK and GroEL in bacterial inclusion bodies. J Bacteriol. 2005;187(10):3599–3601. doi: 10.1128/JB.187.10.3599-3601.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspeta L, Flores N, Pérez NO, Bolívar F, Ramírez OT. The effect of heating rate on Escherichia coli metabolism, physiological stress, transcriptional response, and production of temperature-induced recombinant protein: a scale-down study. Biotechnol Bioeng. 2009;102(2):468–482. doi: 10.1002/bit.22084. [DOI] [PubMed] [Google Scholar]
- Caspeta L, Lara AR, Pérez NO, Flores N, Bolívar F, Ramírez OT. Enhancing thermo-induced recombinant protein production in Escherichia coli by temperature oscillations and post-induction nutrient feeding strategies. J Biotechnol. 2013;167(1):47–55. doi: 10.1016/j.jbiotec.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Castellanos-Mendoza A, Castro-Acosta RM, Olvera A, Zavala G, Mendoza-Vera M, García-Hernández E, Alagón A, Trujillo-Roldán MA, Valdez-Cruz NA. Influence of pH control in the formation of inclusion bodies during production of recombinant sphingomyelinase-D in E. coli. Microb Cell Factories. 2014;13:137. doi: 10.1186/s12934-014-0137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiti F, Dobson CM. Protein misfolding, functional amyloid and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- De Groot NS, Ventura S. Effect of temperature on protein quality in bacterial inclusion bodies. FEBS Lett. 2006;580(27):6471–6476. doi: 10.1016/j.febslet.2006.10.071. [DOI] [PubMed] [Google Scholar]
- de Marco A, Ferrer-Miralles N, Garcia-Fruitós E, Mitraki A, Peternel S, Rinas U, Trujillo-Roldán MA, Valdez-Cruz NA, Villaverde A. Bacterial inclusion bodies are industrially exploitable amyloids. FEMS Microbiol Rev. 2018;43(1):53–72. doi: 10.1093/femsre/fuy038. [DOI] [PubMed] [Google Scholar]
- de Marco A, Volrath S, Bruyere T, Law M, Fonné-Pfister R. Recombinant maize protoporphyrinogen IX oxidase expressed in Escherichia coli forms complexes with GroEL and DnaK chaperones. Protein Expr Purif. 2000;20(1):81–86. doi: 10.1006/prep.2000.1274. [DOI] [PubMed] [Google Scholar]
- Dodd IB, Shearwin KE, Perkins AJ, Burr T, Hochschild A, Egan JB. Cooperativity in long-range gene regulation by the λ CI repressor. Genes Dev. 2004;18(3):344–354. doi: 10.1101/gad.1167904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiteman MA, Altman E. Overcoming acetate in Escherichia coli recombinant protein fermentations. Trends Biotechnol. 2006;24(11):530–536. doi: 10.1016/j.tibtech.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Fayet O, Ziegelhoffer T, Georgopoulos C. The groES and groEL heat shock gene products of Escherichia coli are essential for bacterial growth at all temperatures. J Bacteriol. 1989;171(3):1379–1385. doi: 10.1128/jb.171.3.1379-1385.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink AL. Protein aggregation: folding aggregates, inclusion bodies and amyloid. Fold Des. 1998;3(1):R9–R23. doi: 10.1016/S1359-0278(98)00002-9. [DOI] [PubMed] [Google Scholar]
- García-Fruitós E, Arís A, Villaverde A. Localization of functional polypeptides in bacterial inclusion bodies. Appl Environ Microbiol. 2007;73:289–294. doi: 10.1128/AEM.01952-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Fruitos E, Martínez-Alonso M, González-Montalbán N, Valli M, Mattanovich D, Villaverde A. Divergent genetic control of protein solubility and conformational quality in Escherichia coli. J Mol Biol. 2007;374(1):195–205. doi: 10.1016/j.jmb.2007.09.004. [DOI] [PubMed] [Google Scholar]
- García-Fruitós E (2010) Inclusion bodies: a new concept. Microb Cell Factories 9:80. 10.1186/1475-2859-9-80 [DOI] [PMC free article] [PubMed]
- García-Fruitós E, Sabate R, de Groot NS, Villaverde A, Ventura S. Biological role of bacterial inclusion bodies: a model for amyloid aggregation. FEBS J. 2011;278(14):2419–2427. doi: 10.1111/j.1742-4658.2011.08165.x. [DOI] [PubMed] [Google Scholar]
- Gatti-Lafranconi P, Natalello A, Ami D, Doglia SM, Lotti M. Concepts and tools to exploit the potential of bacterial inclusion bodies in protein science and biotechnology. FEBS J. 2011;278(14):2408–2418. doi: 10.1111/j.1742-4658.2011.08163.x. [DOI] [PubMed] [Google Scholar]
- Gill RT, Valdes JJ, Bentley WE. A comparative study of global stress gene regulation in response to overexpression of recombinant proteins in Escherichia coli. Metab Eng. 2000;2(3):178–189. doi: 10.1006/mben.2000.0148. [DOI] [PubMed] [Google Scholar]
- Glick BR. Metabolic load and heterologous gene expression. Biotechnol Adv. 1995;13(2):247–261. doi: 10.1016/0734-9750(95)00004-a. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Montalban N, Garcia-Fruitos E, Villaverde A. Recombinant protein solubility—does more mean better? Nat Biotechnol. 2007;25(7):718–720. doi: 10.1038/nbt0707-718. [DOI] [PubMed] [Google Scholar]
- Gragerov A, Nudler E, Komissarova N, Gaitanaris GA, Gottesman ME, Nikiforov V. Cooperation of GroEL/GroES and DnaK/DnaJ heat shock proteins in preventing protein misfolding in Escherichia coli. PNAS. 1992;89(21):10341–10344. doi: 10.1073/pnas.89.21.10341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman AD, Straus DB, Walter WA, Gross CA. Sigma 32 synthesis can regulate the synthesis of heat shock proteins in Escherichia coli. Genes Dev. 1987;1:179–184. doi: 10.1101/gad.1.2.179. [DOI] [PubMed] [Google Scholar]
- Harcum S, Haddadin F. Global transcriptome response of recombinant Escherichia coli to heat-shock and dual heat-shock recombinant protein induction. J Ind Microbiol Biotechnol. 2006;33(10):801–814. doi: 10.1007/s10295-006-0122-3. [DOI] [PubMed] [Google Scholar]
- Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295(5561):1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- Hayer-Hartl M, Bracher A, Hartl FU. The GroEL–GroES chaperonin machine: a nano-cage for protein folding. Trends Biochem Sci. 2015;41(1):62–76. doi: 10.1016/j.tibs.2015.07.009. [DOI] [PubMed] [Google Scholar]
- Hewitt CJ, Nebe-Von Caron G, Nienow AW, McFarlane CM. The use of multi-parameter flow cytometry to compare the physiological response of Escherichia coli W3110 to glucose limitation during batch, fed-batch and continuous culture cultivations. J Biotechnol. 1999;75(2):251–264. doi: 10.1016/s0168-1656(99)00168-6. [DOI] [PubMed] [Google Scholar]
- Hoffmann F, Rinas U. Kinetics of heat-shock response and inclusion body formation during temperature-induced production of basic fibroblast growth factor in high-cell-density cultures of recombinant Escherichia coli. Biotechnol Prog. 2000;16(6):1000–1007. doi: 10.1021/bp0000959. [DOI] [PubMed] [Google Scholar]
- Hoffmann F, Rinas U. On-line estimation of the metabolic burden resulting from the synthesis of plasmid-encoded and heat-shock proteins by monitoring respiratory energy generation. Biotechnol Bioeng. 2001;76(4):333–340. doi: 10.1002/bit.10098. [DOI] [PubMed] [Google Scholar]
- Hoffmann F, Weber J, Rinas U. Metabolic adaptation of Escherichia coli during temperature-induced recombinant protein production: 1. Readjustment of metabolic enzyme synthesis. Biotechnol Bioeng. 2002;80(3):313–319. doi: 10.1002/bit.10379. [DOI] [PubMed] [Google Scholar]
- Hoffmann F, Rinas U. Stress induced by recombinant protein production in Escherichia coli. Adv Biochem Eng Biot. 2004;89:73–92. doi: 10.1007/b93994. [DOI] [PubMed] [Google Scholar]
- Jensen EB, Carlsen S. Production of recombinant human growth hormone in Escherichia coli: expression of different precursors and physiological effects of glucose, acetate, and salts. Biotechnol Bioeng. 1990;36(1):1–11. doi: 10.1002/bit.260360102. [DOI] [PubMed] [Google Scholar]
- Jürgen B, Lin HY, Riemschneider S, Scharf C, Neubauer P, Schmid R, Hecker M, Schweder T. Monitoring of genes that respond to overproduction of an insoluble recombinant protein in Escherichia coli glucose-limited fed-batch fermentations. Biotechnol Bioeng. 2000;70(2):217–224. doi: 10.1002/1097-0290(20001020)70:2<217::aid-bit11>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Jürgen B, Breitenstein A, Urlacher V, Büttner K, Lin H, Hecker M, Schweder T, Neubauer P. Quality control of inclusion bodies in Escherichia coli. Microb Cell Factories. 2010;9:41. doi: 10.1186/1475-2859-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane JF, Hartley DL (1988) Formation of recombinant protein inclusion bodies in Escherichia coli. Trends Biotechnol 6:95–101. 10.1016/0167-7799(88)90065-0
- Klöckner W, Büchs J (2012) Advances in shaking technologies. Trends Biotechnol 30(6):307–314. 10.1016/j.tibtech.2012.03.001 [DOI] [PubMed]
- Kosinski MJ, Rinas U, Bailey JE (1992) Isopropyl-β-D-thiogalactopyranoside influences the metabolism of Escherichia coli. Appl Microbiol Biot 36(6):782–784. 10.1007/BF00172194
- Kusukawa N, Yura T. Heat shock protein GroE of Escherichia coli: key protective roles against thermal stress. Genes Dev. 1988;2(7):874–882. doi: 10.1101/gad.2.7.874. [DOI] [PubMed] [Google Scholar]
- Lara AR, Leal L, Flores N, Gosset G, Bolivar F, Ramírez OT. Transcriptional and metabolic response of recombinant Escherichia coli to spatial dissolved oxygen tension gradients simulated in a scaledown system. Biotechnol Bioeng. 2006;93:372–385. doi: 10.1002/bit.20704. [DOI] [PubMed] [Google Scholar]
- Lara AR. Recombinant protein production in Escherichia coli. Rev Mex Ing Quim. 2011;2011:209–223. [Google Scholar]
- Li X, Robbins JW, Taylor KB. Effect of the levels of dissolved oxygen on the expression of recombinant proteins in four recombinant Escherichia coli strains. J Ind Microbiol. 1992;9:10–11. doi: 10.1007/BF01576362. [DOI] [PubMed] [Google Scholar]
- Lieb M (1966) Studies of heat-inducible lambda bacteriophage, I. order of genetics sites and properties of mutant prophages. J Mol Biol 16:149–163. 10.1016/S0022-2836(66) [DOI] [PubMed]
- Losen M, Frölich B, Pohl M, Büchs J. Effect of oxygen limitation and Medium composition on Escherichia coli fermentation in shake-flask cultures. Biotechnol Progr. 2004;20(4):1062–1068. doi: 10.1021/bp034282t. [DOI] [PubMed] [Google Scholar]
- Luli GW, Strohl WR. Comparison of growth, acetate production, and acetate inhibition of Escherichia coli strains in batch and fed-batch fermentations. Appl Environ Microb. 1990;56(4):1004–1011. doi: 10.1128/aem.56.4.1004-1011.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo B, Sant’Anna R, Navarro S, Cordeiro Y, Ventura S. Mammalian prion protein (PrP) forms conformationally different amyloid intracellular aggregates in bacteria. Microb Cell Factories. 2015;14(1):1. doi: 10.1186/s12934-015-0361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahairas GG, Sabo PJ, Hickey MJ, Singh DC, Stover CK. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J Bacteriol. 1996;178(5):1274–1282. doi: 10.1128/jb.178.5.1274-1282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makrides SC. Strategies for achieving high-level expression of genes in Escherichia coli. Microbiol Rev. 1996;60(3):512–538. doi: 10.1128/mr.60.3.512-538.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margreiter G, Messner P, Caldwell K, Bayer K. Size characterization of inclusion bodies by sedimentation field-flow fractionation. J Biotechnol. 2008;138:67–73. doi: 10.1016/j.jbiotec.2008.07.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer LM, Yohannes E, Bondurant SS, Radmacher M, Slonczewski JL. pH regulates genes for flagellar motility, catabolism, and oxidative stress in Escherichia coli K-12. J Bacteriol. 2005;187(1):304–319. doi: 10.1128/JB.187.1.304-319.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meher AK, Bal NC, Chary KV, Arora A. Mycobacterium tuberculosis H37Rv ESAT-6–CFP-10 complex formation confers thermodynamic and biochemical stability. FEBS J. 2006;273(7):1445–1462. doi: 10.1111/j.1742-4658.2006.05166.x. [DOI] [PubMed] [Google Scholar]
- Menart V, Jevševar S, Vilar M, Trobiš A, Pavko A. Constitutive versus thermoinducible expression of heterologous proteins in Escherichia coli based on strong pR/pL promoters from phage lambda. Biotechnol Bioeng. 2003;83(2):181–190. doi: 10.1002/bit.10660. [DOI] [PubMed] [Google Scholar]
- Miroux B, Walker JE. Over-production of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J Mol Biol. 1996;260(3):289–298. doi: 10.1006/jmbi.1996.0399. [DOI] [PubMed] [Google Scholar]
- Mitraki A, King J. Protein folding intermediates and inclusion body formation. Nat Biotechnol. 1989;7(7):690–697. [Google Scholar]
- Nemes E, Abrahams D, Scriba TJ, Ratangee F, Keyser A, Makhethe L, Erasmus M, Mabwe S, Bilek N, Rozot V, Geldenhuys H, Hatherill M, Lempicki MD, Holm LL, Bogardus L, Ginsberg AM, Blauenfeldt T, Smith B, Ellis RD, Loxton AG, Walzl G, Andersen P, Ruhwald M (2019) Diagnostic accuracy of ESAT-6 free IGRA compared to QuantiFERON-TB gold in-tube. Clin Infect Dis. 10.1093/cid/ciz034 [DOI] [PMC free article] [PubMed]
- Neubauer P, Häggström L, Enfors SO. Influence of substrate oscillations on acetate formation and growth yield in Escherichia coli glucose limited fed-batch cultivations. Biotechnol Bioeng. 1995;47(2):139–146. doi: 10.1002/bit.260470204. [DOI] [PubMed] [Google Scholar]
- Peng X, Jiang G, Liu W, Zhang Q, Qian W, Sun J. Characterization of differential pore-forming activities of ESAT-6 proteins from Mycobacterium tuberculosis and Mycobacterium smegmatis. FEBS Lett. 2016;590(4):509–519. doi: 10.1002/1873-3468.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peternel Š, Grdadolnik J, Gaberc-Porekar V, Komel R. Engineering inclusion bodies for non-denaturing extraction of functional proteins. Microb Cell Fact. 2008;7(1):34. doi: 10.1186/1475-2859-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phue JN, Shiloach J. Transcription levels of key metabolic genes are the cause for different glucose utilization pathways in E. coli B (BL21) and E. coli K (JM109) J Biotechnol. 2004;109:21–30. doi: 10.1016/j.jbiotec.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Phue JN, Shiloach J. Impact of dissolved oxygen concentration on acetate accumulation and physiology of E. coli BL21, evaluating transcription levels of key genes at different dissolved oxygen conditions. Metab Eng. 2005;7(5):353–363. doi: 10.1016/j.ymben.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Pimienta-Rodríguez ET, Rodríguez-Valdés C, Sarzo-Gómez M, Vallín-Plou C (2012) Producción de los antígenos ESAT-6 y CFP-10 de Mycobacterium tuberculosis y evaluación de sus potencialidades en el serodiagnóstico de la tuberculosis activa en una población cubana. Revista CENIC. Ciencias Biológicas (2):43
- Przybycien TM, Dunn JP, Valax P, Georglou G. Secondary structure characterization of ß-lactamase inclusion bodies. Protein Eng. 1994;7(1):131–136. doi: 10.1093/protein/7.1.131. [DOI] [PubMed] [Google Scholar]
- Remaut E, Stanssens P, Fiers W. Plasmid vectors for high-efficiency expression controlled by the pL promoter of coliphage lambda. Gene. 1981;15(1):81–93. doi: 10.1016/0378-1119(81)90106-2. [DOI] [PubMed] [Google Scholar]
- Renshaw PS, Panagiotidou P, Whelan A, Gordon SV, Hewinson RG, Williamson RA, Carr MD. Conclusive evidence that the major T-cell antigens of the Mycobacterium tuberculosis complex ESAT-6 and CFP-10 form a tight, 1:1 complex and characterization of the structural properties of ESAT-6, CFP-10, and the ESAT-6· CFP-10 complex implications for pathogenesis and virulence. J Biol Chem. 2002;277(24):21598–21603. doi: 10.1074/jbc.M201625200. [DOI] [PubMed] [Google Scholar]
- Reynoso-Cereceda GI, García-Cabrera RI, Valdez-Cruz NA, Trujillo-Roldán MA. Shaken flasks by resonant acoustic mixing versus orbital mixing: mass transfer coefficient kLa characterization and Escherichia coli cultures comparison. Biochem Eng J. 2016;105:379–390. [Google Scholar]
- Rinas U, Bailey JE. Protein compositional analysis of inclusion bodies produced in recombinant Escherichia coli. Appl Microbiol Biotechnol. 1992;37:609–614. doi: 10.1007/BF00240735. [DOI] [PubMed] [Google Scholar]
- Rinas U, Kracke-Helm HA, Schügerl K. Glucose as a substrate in recombinant strain fermentation technology. Appl Microbiol Biotechnol. 1989;31(2):163–167. [Google Scholar]
- Rinas U, Garcia-Fruitós E, Corchero JL, Vázquez E, Seras-Franzoso J, Villaverde A. Bacterial inclusion bodies: discovering their better half. Trends Biochem Sci. 2017;42(9):726–737. doi: 10.1016/j.tibs.2017.01.005. [DOI] [PubMed] [Google Scholar]
- Roe AJ, O’Byrne C, McLaggan D, Booth IR. Inhibition of Escherichia coli growth by acetic acid: a problem with methionine biosynthesis and homocysteine toxicity. Microbiol. 2002;148(7):2215–2222. doi: 10.1099/00221287-148-7-2215. [DOI] [PubMed] [Google Scholar]
- Rosen R, Ron EZ. Proteome analysis in the study of the bacterial heat shock response. Mass Spectrom Rev. 2002;21(4):244–265. doi: 10.1002/mas.10031. [DOI] [PubMed] [Google Scholar]
- Rosen R, Biran D, Gur E, Becher D, Hecker M, Ron EZ. Protein aggregation in Escherichia coli: role of proteases. FEMS Microbiol Lett. 2002;207:9–12. doi: 10.1111/j.1574-6968.2002.tb11020.x. [DOI] [PubMed] [Google Scholar]
- Sabaté R, Espargaró A, de Groot NS, Valle-Delgado JJ, Fernàndez-Busquets X, Ventura S. The role of protein sequence and amino acid composition in amyloid formation: scrambling and backward reading of IAPP amyloid fibrils. J Mol Biol. 2010;404(2):337–352. doi: 10.1016/j.jmb.2010.09.052. [DOI] [PubMed] [Google Scholar]
- Salmond CV, Kroll RG, Booth IR. The effect of food preservatives on pH homeostasis in Escherichia coli. Microbiol. 1984;130(11):2845–2850. doi: 10.1099/00221287-130-11-2845. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritschh EF, Maniatis T (1989) Molecular cloning, vol 2, 2da edn. Cold Spring Harbor Lab Press, New York, pp 9–14
- Sánchez-García L, Martín L, Mangues R, Ferrer-Miralles N, Vázquez E, Villaverde A. Recombinant pharmaceuticals from microbial cells: a 2015 update. Microb Cell Factories. 2016;15(1):33. doi: 10.1186/s12934-016-0437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Babu KR, Khanna N, Marten S, Rinas U. Temperature-induced production of recombinant human insulin in high-cell density cultures of recombinant Escherichia coli. J Biotechnol. 1999;68(1):71–83. doi: 10.1016/s0168-1656(98)00189-8. [DOI] [PubMed] [Google Scholar]
- Shiloach J, Reshamwala S, Noronha SB, Negrete A. Analyzing metabolic variations in different bacterial strains, historical perspectives and current trends—example E. coli. Curr Opin Biotech. 2010;21(1):21–26. doi: 10.1016/j.copbio.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Smith HE. The transcriptional response of Escherichia coli to recombinant protein insolubility. J Struct Funct Genom. 2007;8(1):27–35. doi: 10.1007/s10969-007-9030-7. [DOI] [PubMed] [Google Scholar]
- Strandberg L, Enfors SO. Factors influencing inclusion body formation in the production of a fused protein in Escherichia coli. Appl Environ Microbiol. 1991;57(6):1669–1674. doi: 10.1128/aem.57.6.1669-1674.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo A, Langer T, Schröder H, Flanagan J, Bukau B, Hartl FU. The ATP hydrolysis-dependent reaction cycle of the Escherichia coli Hsp70 system DnaK, DnaJ, and GrpE. PNAS. 1994;91(22):10345–10349. doi: 10.1073/pnas.91.22.10345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torabi A, Tahmoorespur M, Vahedi F, Mosavari N, Nassiri M. Quantitation of IL-4, IL-10 and IFN-ɣ genes expression after immunization of mice with CFP-10 and ESAT-6 containing vectors. Iran J Immunol. 2013;10(4):205–215. [PubMed] [Google Scholar]
- Trujillo-Roldán MA, Peña C, Ramírez OT, Galindo E. Effect of oscillating dissolved oxygen tension on the production of alginate by Azotobacter vinelandii. Biotechnol Prog. 2001;17(6):1042–1048. doi: 10.1021/bp010106d. [DOI] [PubMed] [Google Scholar]
- Upadhyay AK, Murmu A, Singh A, Panda AK. Kinetics of inclusion body formation and its correlation with the characteristics of protein aggregates in Escherichia coli. PLoS One. 2012;7:e33951. doi: 10.1371/journal.pone.0033951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez-Cruz NA, Caspeta L, Pérez NO, Ramírez OT, Trujillo-Roldán MA. Production of recombinant proteins in E. coli by the heat inducible expression system based on the phage lambda pL and/or pR promoters. Microb Cell Factories. 2010;9:18. doi: 10.1186/1475-2859-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez-Cruz NA, Ramírez OT, Trujillo-Roldán MA (2011) Heat shock molecular responses caused by the production of recombinant proteins in the heat inducible expression system: E. coli with lambda phage pL and/or pR promoters. Bioeng Bugs 2(2). 10.4161/bbug.2.2.14316
- Van Pinxteren LAH, Ravn P, Agger EM, Pollock J, Andersen P. Diagnosis of tuberculosis based on the two specific antigens ESAT-6 and CFP10. Clin Diagn Lab Immun. 2000;7(2):155–160. doi: 10.1128/cdli.7.2.155-160.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villaverde A, Benito A, Viaplana E, Cubarsi R. Fine regulation of cI857-controlled gene expression in continuous culture of recombinant Escherichia coli by temperature. Appl Environ Microbiol. 1993;59(10):3485–3487. doi: 10.1128/aem.59.10.3485-3487.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vutla J, Chandran D, Veerasami M, Sugumar P, Shahana PV, Das D, Narasu ML, Srinivasan VA. Cloning, expression and purification of ESAT-6 and CFP-10 and their use in the detection of IFN-γ responses in tuberculosis infected cattle. Biotechnol Bioinf Bioeng. 2011;1(2):255–264. [Google Scholar]
- Wang BL, Xu Y, Wu CQ, Xu YM, Wang HH. Cloning, expression, and refolding of a secretory protein ESAT-6 of Mycobacterium tuberculosis. Protein Expres Purif. 2005;39(2):184–188. doi: 10.1016/j.pep.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Weber J, Hoffmann F, Rinas U. Metabolic adaptation of Escherichia coli during temperature-induced recombinant protein production: 2. Redirection of metabolic fluxes. Biotechnol Bioeng. 2002;80(3):320–330. doi: 10.1002/bit.10380. [DOI] [PubMed] [Google Scholar]
- Williams DC, Van Frank RM, Muth WL, Burnett JP. Cytoplasmic inclusion bodies in Escherichia coli producing biosynthetic human insulin proteins. Science. 1982;215(4533):687–689. doi: 10.1126/science.7036343. [DOI] [PubMed] [Google Scholar]
- Wittmann C, Weber J, Betiku E, Krömer J, Böhm D, Rinas U. Response of fluxome and metabolome to temperature-induced recombinant protein synthesis in Escherichia coli. J Biotechnol. 2007;132:375–384. doi: 10.1016/j.jbiotec.2007.07.495. [DOI] [PubMed] [Google Scholar]
- Wolfe AJ. The acetate switch. Microbiol Mol Biol R. 2005;69(1):12–50. doi: 10.1128/MMBR.69.1.12-50.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Jahic M, Blomsten G, Enfors SO. Glucose overflow metabolism and mixed-acid fermentation in aerobic large-scale fed-batch processes with Escherichia coli. Appl Microbiol Biotech. 1999;51(5):564–571. doi: 10.1007/s002530051433. [DOI] [PubMed] [Google Scholar]
- Yamamori T, Ito K, Nakamura Y, Yura T. Transient regulation of protein synthesis in Escherichia coli upon shift-up of growth temperature. J Bacteriol. 1978;134(3):1133–1140. doi: 10.1128/jb.134.3.1133-1140.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Enfors SO. Process conditions affecting proteolysis of recombinant proteins in Escherichia coli. Biotechnol Lett. 1996;18(1):7–12. [Google Scholar]
- Zhao K, Liu M, Burgess RR. The global transcriptional response of Escherichia coli to induced sigma 32 protein involves sigma 32 regulon activation followed by inactivation and degradation of sigma 32 in vivo. J Biol Chem. 2005;280(18):17758–17768. doi: 10.1074/jbc.M500393200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 315 kb)