Abstract
AIM
To evaluate the light adjustable lens (LAL) vs a standard monofocal lens in achieving target astigmatic refraction and improving postoperative uncorrected distance visual acuity (UDVA).
METHODS
This randomized controlled clinical trial included 40 patients with pre-existing astigmatism and visually significant cataract. Twenty-eight patients received the LAL and 12 control patients received a monofocal intraocular lens (IOL) after cataract extraction at a single institution. The patients with the LAL underwent adjustment by ultraviolet (UV) light postoperatively plus subsequent lock-in procedures and all patients returned to clinic for follow up of study parameters at 6, 9, and 12mo. Manifest refraction, distance visual acuity, and adverse events were recorded at each visit.
RESULTS
The mean cylinder before adjustment in eyes with the LAL was -0.89±0.58 D (-2.00 to 0.00 D) and -0.34±0.34 D (-1.25 to 0.00 D) after lock-in (P=1.68x10−8). The mean cylinder in patients with the monofocal lens was -1.00±0.32 D (-1.50 to -0.50 D) at 17-21d postoperatively, which was statistically different from the LAL cylinder post lock-in (P=1.43x10−6). UDVA in the LAL group was 20/20 or better in 79% of patients post lock-in with good stability over 12mo compared with 33% of the control patients with UDVA of 20/20 or better.
CONCLUSION
These results demonstrate that the LAL is more effective in achieving target refractions and improving postoperative UDVA in patients with pre-existing corneal astigmatism than a standard monofocal lens.
Keywords: light adjustable lens, monofocal lens, astigmatism, cataract
INTRODUCTION
It is estimated that over 15 million cataract surgeries are performed worldwide each year[1]. In addition to the primary purpose of cataract removal, many patients undergo the procedure with high expectations of improved refractive outcomes. One reason for failing to reach target outcomes is the presence of astigmatism. As there is a high prevalence of astigmatism preoperatively in patients undergoing cataract surgery, achieving emmetropia after surgery may be hindered by either residual or surgically induced astigmatism. Xu and Zheng[2] found 33% of patients undergoing cataract surgery had at least 1.00 D of astigmatism, while other studies have shown 64.4% with 0.25-1.25 D[3]. The degree of surgically induced astigmatism varies by the size and location of the corneal incision but has been reported as 0.09-1.92 D, and while the temporal approach leads to the least amount of induced astigmatism, the effectiveness is limited by preoperative astigmatism[4].
Since the first in vivo study in rabbits in 2003 demonstrated successful power adjustment, the RxSight Inc. (Aliso Viejo, California, USA) light adjustable lens (LAL) has been explored as a potential advancement in improving postoperative visual outcomes after cataract surgery[5]. Multiple prospective human studies have since shown the ability of the LAL to achieve a target spherical and cylindrical refraction and the lens was approved by the food and drug administration (FDA) in November of 2017[6]–[13]. The LAL is a foldable, posterior chamber three-piece silicone lens with polymethylmethacrylate modified-C haptics and a 6.0 mm optic with a length of 13.0 mm. With application of ultraviolet (UV) light, it has the capacity to adjust spherical power from -2.00 to +2.00 D and cylindrical power from -0.75 to -2.00 D by 0.25 D increments. A light delivery device administers 365 nm UV light from a mercury arc light source on a standard slit lamp to adjust lens power and to lock-in the lens power once the desired refractive outcomes have been reached. This single site analysis of patients with pre-existing corneal astigmatism is the first report of a randomized controlled clinical study to evaluate the effectiveness of the LAL vs a monofocal intraocular lens (IOL) in achieving target refractive outcomes.
SUBJECTS AND METHODS
Ethical Approval
This is a primary site analysis of a prospective randomized controlled, multi-center phase III FDA clinical trial. Approval for this study was received from Salus IRB (Austin, Texas) and all participants gave informed consent according to the Declaration of Helsinki. This trial is registered with www.clinicaltrials.gov (NCT01496066).
Forty patients (40 total eyes) with visually significant cataract were selected to participate in this study. These subjects fulfilled all inclusion criteria including pupil diameter of ≥7.0 mm with full dilation and preoperative astigmatism between -0.75 and -2.50 D by manual keratometry with a steep axis between 70 and 110 degrees. Patients with irregular astigmatism, receiving photosensitizing systemic medication, or with significant anterior or posterior segment pathology with the potential to limit visual acuity were excluded from the study.
Each subject received a preoperative complete ophthalmic examination. This examination included ocular history, uncorrected distance visual acuity (UDVA) and best corrected distance visual acuity (CDVA), slit lamp examination, manifest refraction, manual keratometry, and fundus exam. Randomization was performed using Medrio EDC (San Francisco, California, USA) immediately before surgery by a 2:1 ratio to the LAL or control group, which received a single piece acrylic ZCBOO (Abbott, Santa Clara, California, USA) monofocal IOL. Patients were stratified based on preoperative cylinder power (0.75 to 1.25 D and 1.375 to 2.50 D). Selection of the implantable lens was based on ocular biometry and power calculation from IOL Master (V5.02, Carl Zeiss AG, Oberkochen, Germany) with the recommended manufacturer A-constant. Patients in the control group received an IOL targeted for postoperative emmetropia. Those in the LAL group were initially targeted to a postoperative manifest refraction spherical equivalent (MRSE) of +0.50 D to allow for a potential myopic shift from the lock-in treatment.
Both the LAL and control monofocal lenses were implanted using standard surgical technique including 2.4 mm clear temporal corneal incision, capsulorhexis (5.5 mm), and nuclear fragmentation using divide and conquer technique with phacoemulsification. All surgical instruments and methods were identical across the LAL and control groups. No limbal relaxing incision (LRI) was performed during surgery in either group. Postoperatively, all patients received moxifloxacin 0.5% (Alcon, Fort Worth, Texas, USA) and were instructed to use q.i.d. until the bottle was finished. Ketorolac 0.5% was also used q.i.d. until the bottle was finished and prednisolone acetate 1% was given with instructions on a tapering regimen over the course of four weeks. Patients in the LAL group were instructed to wear UV protective eyewear at all times while outdoors until after the final lock-in procedure was completed. Patients presented for an initial adjustment between 17-21d postoperatively. At this visit, those with a cylinder power ≤-0.75 D underwent spherocylindrical adjustment while patients with >-0.75 D of cylindrical power underwent spherical adjustment alone. Upon return 3-5d from the initial adjustment, a manifest refraction was again determined, and a second adjustment was performed if needed. After the desired refraction was obtained, patients in the LAL group received two lock-in procedures, also separated by 3-5d. Patients returned one week after the final lock-in procedure and post lock-in MRSE, UDVA, and CDVA were recorded. All patients returned to clinic at six months, nine months, and 12mo postoperatively to assess stability of these values over time. A masked observer measured refraction and visual acuity at each postoperative visit in both groups.
The data was collected and analyzed using Microsoft Excel (2016). Results are described by mean, standard deviation, and range. A student t-test was performed using a P value = 0.05 to determine significance. Statistical analysis of visual acuity was performed by conversion to logMAR as described by Holladay[14]. The London Clinic Standard Graphs for Refractive Surgery was used to create standard refractive graphs[15].
RESULTS
Patient demographics, axial length (AL), K1, K2, anterior chamber depth, mean spherical equivalent (SEQ), sphere, and cylinder were recorded for each group (Table 1). The mean SEQ of the patients in the LAL group before adjustment (17-21d postoperatively) was 0.54±0.39 D (-0.13 to 1.25 D) and after lock-in was 0.05±0.31 D (-0.50 to 0.88 D). The mean SEQ in patients who received a monofocal lens at 17-21d postoperatively was -0.29±0.49 D (-1.38 to 0.38 D) as shown in Table 2. Seventeen of the 28 patients in the LAL group required 2 adjustments before lock-in but no patients required more than 2 adjustments.
Table 1. Patient demographics.
| Value | Control | Light adjustable lens |
| Patients | 12 | 28 |
| Males | 6 | 14 |
| Females | 6 | 14 |
| Age (y) | 67 (42 to 79) | 66 (45 to 76) |
| Eyes (total) | 12 | 28 |
| Right eyes | 8 | 20 |
| Left eyes | 4 | 8 |
| Axial length (mm) | 24.54 (22.31 to 26.29) | 23.97 (22.07 to 27.06) |
| K1 (D) | 43.77 (41.56 to 48.56) | 43.68 (41.31 to 46.94) |
| K2 (D) | 44.94 (42.13 to 49.49) | 44.92 (42.03 to 47.67) |
| Anterior chamber depth (mm) | 3.23 (2.22 to 3.81) | 3.46 (2.21 TO 4.66) |
| Sphere (D) | -1.15 (-5.50 to 2.75) | -1.23 (-7.50 to 3.00) |
| Cylinder (D) | -1.06 (-1.75 to -0.25) | -1.04 (-3.25 to 0.00) |
Table 2. Spherical equivalent.
| Time period | Number of eyes | Spherical equivalent refraction | Sphere | Cylinder |
| Light adjustable lens (D) | ||||
| Preoperative | 28 | -1.75±3.04 (-8.13 to 2.50) | -1.23±3.00 (-7.50 to 3.00) | -1.04±0.81 (-3.25 to 0.00) |
| Preadjustment | 28 | 0.54±0.39 (-0.13 to 1.25) | 0.99±0.52 (0.00 to 2.00) | -0.89±0.58 (-2.00 to 0.00) |
| Post-lock-in | 28 | 0.05±0.31 (-0.50 to 0.88) | 0.22±0.31 (-0.25 to 1.00) | -0.34±0.34 (-1.25 to 0.00) |
| 6mo | 28 | -0.05±0.31 (-0.63 to 0.50) | 0.22±0.37 (-0.25 to 1.25) | -0.55±0.62 (-3.25 to 0.00) |
| 9mo | 28 | 0.10±0.35 (-0.50 to 1.00) | 0.32±0.45 (-0.25 to 1.50) | -0.45±0.48 (-2.00 to 0.00) |
| 12mo | 28 | 0.02±0.31 (-0.88 to 0.63) | 0.23±0.37 (-0.50 to 1.00) | -0.42±0.44 (-1.75 to 0.00) |
| Control (D) | ||||
| Preoperative | 12 | -1.68±3.00 (-6.25 to 2.50) | -1.15±2.96 (-5.50 to 2.75) | -1.06±0.50 (-1.75 to -0.25) |
| Postoperative | 12 | -0.29±0.49 (-1.38 to 0.38) | 0.21±0.50 (-0.75 to 0.75) | -1.00±0.32 (-1.50 to -0.50) |
| Post-lock-in | 0 | N/A | N/A | N/A |
| 6mo | 12 | -0.04±0.44 (-0.75 to 1.00) | 0.38±0.42 (-0.25 to 1.25) | -0.83±0.37 (-1.50 to -0.25) |
| 9mo | 12 | -0.16±0.44 (-0.75 to 0.50) | 0.23±0.45 (-0.50 to 0.75) | -0.77±0.29 (-1.25 to -0.25) |
| 12mo | 12 | 0.00±0.45 (-0.63 to 1.00) | 0.42±0.48 (-0.25 to 1.25) | -0.83±0.37 (-1.50 to -0.25) |
mean±SD (range)
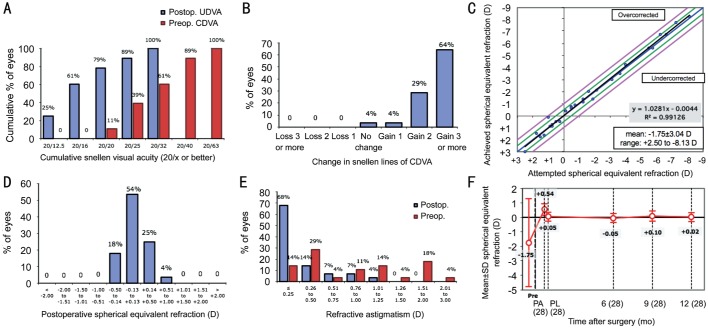
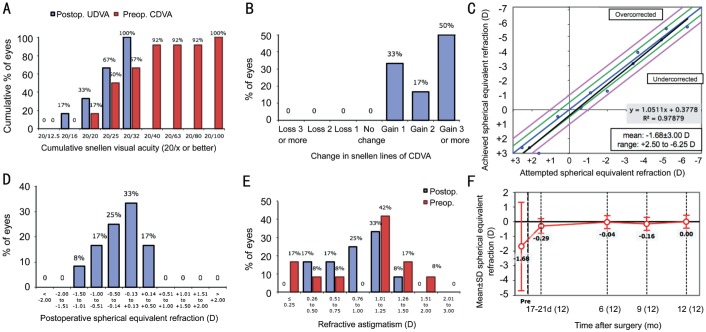
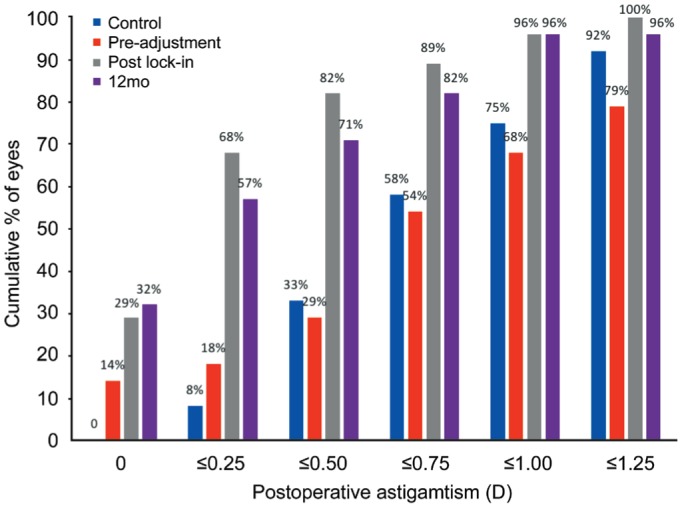
The mean cylinder before adjustment in the LAL group was -0.89±0.58 D (-2.00 to 0.00 D) and post lock-in was -0.34±0.34 D (-1.25 to 0.00 D) with a P value of 1.68×10−8. In the LAL group post lock-in, 68% of patients achieved cylindrical correction of 0.25 D or less and 82% achieved 0.50 D or less of astigmatism (Figure 1). Comparatively, no patients in the control group at 17-21d postoperatively achieved a cylindrical correction of 0.25 D or less and only 17% were found to have 0.50 D or less of astigmatism (Figure 2). The mean cylinder at 17-21d after surgery in the control eyes was -1.00±0.32 D (-1.50 to -0.50 D), which was statistically different from the mean cylinder at post lock-in LAL group (P=1.43×10−6).
Figure 1. Standard refractive surgery graphs for the patients who received the light adjustable lens.
Figure 2. Standard refractive surgery graphs for control patients.
The amount of cylinder in the LAL group post lock-in and at 12mo postoperatively was not statistically different (P=0.287), though the amount of cylinder was significantly different between the control and LAL groups at 12mo (P=0.007). Fifty-seven percent of eyes in the LAL group at 12mo were within a cylinder magnitude of 0.25 D compared with 8% in the control group while 96% in the LAL group and 75% of the controls were within 1.00 D (Figure 3). Sixty-eight percent of eyes at this final visit experienced a change in cylinder within 0.25 D of the post lock-in value and 89% were within 0.50 D of the post lock-in value.
Figure 3. Cumulative percentages of cylindrical power in patients who received the control lens (at 12mo postoperatively) vs LAL (light adjustable lens) pre-adjustment, after the final lock-in procedure, and at 12mo postoperatively.
Post lock-in results showed 79% of patients with the LAL to have UDVA of 20/20 or better, 89% 20/25 or better, and 100% of patients were 20/32 or better (Figure 4). Of the control patients at 17-21d postoperatively, 33% were found to have UDVA of 20/20 or better, 66% were 20/25 or better and 100% at 20/32 or better. UDVA in the LAL patients remained stable over 12mo postoperatively. Visual acuity at six, nine, and 12mo was significantly improved from pre-adjustment visual acuity in patients with the LAL when converted to logMAR. UDVA was also significantly improved at six and 12mo when compared with control groups while the nine-month data showed a P value=0.08. Additionally, 64% of eyes in the LAL group gained three lines of BCVA compared with 50% of eyes in the control group and no eye in either group lost lines of BCVA (Figures 1 and 2).
Figure 4. Uncorrected distance visual acuity in patients with the light adjustable lens vs monofocal lens pre-adjustment, post lock-in, and 12mo postoperatively.
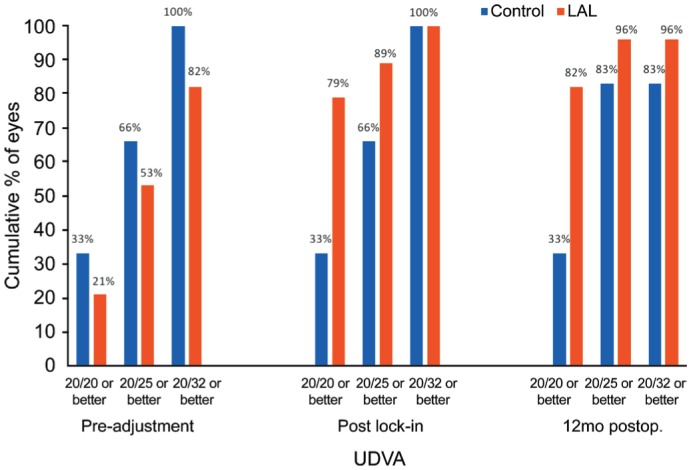
The control data for pre-adjustment and post lock-in was obtained at 17-21d postoperatively.
Adverse events included three patients with the LAL and one patient in the control group who developed increased intraocular pressure (IOP); one patient in each group required treatment for this increase in IOP which subsequently resolved within one week. The two patients in the LAL group not requiring pressure lowering therapy continued with mildly elevated IOP throughout the duration of the study. One patient in the LAL group developed trigeminal neuralgia and one patient had a stroke. One patient in both the LAL and control group described glare and halos postoperatively which resolved within one week. No patients developed macular edema or uveitis.
DISCUSSION
Many patients undergo cataract surgery with the goal of achieving spectacle independence postoperatively, yet various factors including keratometry, AL, anterior chamber depth, and correct IOL power and position contribute to the difficulty in consistently achieving optimal UDVA[16]. A number of studies have demonstrated the unpredictability of IOL power calculations, particularly in short eyes (AL<22 mm) and long eyes (AL>26 mm)[17]. Multiple formulas for calculating IOL power including Haigis, Hoffer Q, Holladay, Barrett Universal II, Olsen, SRK/T, and T2 have been created in an attempt to improve target refractions. While Kane found the Barrett Universal II formula to have the lowest mean absolute prediction error over the entire AL range, 27.7% of eyes after cataract surgery were more than 0.50 D from target refraction with no significant improvement when compared with newer formulas[18]–[19]. Even with careful preoperative calculations, the final position of the IOL cannot always be accurately predicted[16].
As the LAL allows for adjustment of the lens power postoperatively, this advancement allows the surgeon to overcome sources of residual refractive error in cataract surgery and significantly reduce the need for postoperative corrective lenses. Previous pilot studies and prospective clinical trials have demonstrated the success of the LAL in correcting myopia and hyperopia after cataract surgery with 88%-100% of patients achieving target spherical correction within 0.50 D[6]–[7],[10],[12],[20]. Comparatively, Liu et al[21] reported only 61.3% of patients are between -1.00 D and 1.00 D after cataract surgery. The fact that the accuracy of the LAL is within 0.25 D and the majority of patients in our study needed multiple adjustments further illustrates the need for technology capable of altering lens characteristics postoperatively. As noted above, postoperative MRSE was initially targeted for +0.5 D in the LAL group to compensate for a small myopic shift that has been described following lock-in treatment. The cause of this shift is not completely understood. Possible explanations have included an anterior axial shift of the IOL during lock-in treatment or variable UV penetration of the IOL secondary to corneal properties affecting the transmission of UV light through the cornea in a nonuniform manner. Further studies are needed to elucidate the etiology of these findings.
The LAL has also been demonstrated to effectively reduce cylindrical power with stability over time[8],[12]–[13],[22]. Although previous studies have examined the performance of RxSight's LAL, this is the first report of a randomized controlled study to evaluate visual and refractive outcomes in patients receiving the LAL vs monofocal lenses. While our study demonstrated slightly fewer patients achieving astigmatic correction of 0.50 D or less than previous studies (82% after lock-in and 71% at 12mo postoperatively), the mean cylinder in patients with an LAL was found to be significantly less when compared with the patients who received a monofocal lens[8],[22].
There are a number of alternatives to the LAL for astigmatic correction in cataract surgery that must be evaluated and compared with the efficacy and safety of the LAL. Insertion of a toric IOL or a peripheral corneal relaxing incision may be performed during surgery to correct pre-existing astigmatism. A recent meta-analysis found toric IOLs to be more effective than non-toric lenses combined with limbal relaxing insision (LRI), yet only 64.8% achieved an UDVA of 20/25 compared with 39.6% in the non-toric and LRI patients[23]. Our data found that 79% of patients who received the LAL reached 20/20 post lock-in and 89% reached 20/25 with relative stability over one year. Residual refractive cylindrical error has also been demonstrated in patients receiving toric lenses as 52% within a magnitude of 0.50 D and 40% in patients undergoing LRI at time of surgery[24]. Therefore, even though toric rotational stability has improved over recent years, it is susceptible to the same errors as monofocal lenses in achieving target refractive outcomes and our study demonstrates potential superiority of the LAL in reaching refractive goals.
Residual refractive error after cataract surgery may also be corrected by corneal refractive surgery[25]–[26]. However, there are additional risks associated with these procedures including dry eye and corneal ectasia[27]–[28]. The LAL and control groups in our study experienced similar rates of complications. The patient that had a stroke had pre-existing risk factors. While one patient developed trigeminal neuralgia, we do not believe this adverse event was due to implantation of the LAL. Of note, the literature is scarce regarding reports of this occurring after cataract surgery. While further studies with larger sample sizes are needed, the LAL does not appear to carry increased risks beyond what is associated with conventional implantable lenses in cataract surgery. Subgroup analysis of with-the-rule (WTR), against-the-rule (ATR), and oblique astigmatisms, including astigmatism vector analysis, would be beneficial to this study but were limited by a small sample size. Additional follow-up indices including corneal topography would be beneficial for further studies on the LAL.
Many other technologies are being developed as a means to correct residual refractive error after cataract surgery including the magnetically adjustable IOL, liquid crystal IOL, and adjustment by the femtosecond laser. Adjustment by the femtosecond laser is especially promising as laser application may cause shrinkage or a release of tension on the concentric IOL material, allowing for adjustment of the lens at any time postoperatively[29]. Currently, these have yet to undergo evaluation in a randomized clinical trial but may provide additional options for noninvasive adjustment in the future. While alternative treatments to limit astigmatism after cataract surgery are available, the LAL is at the forefront of technology that allows for postoperative refractive correction. Additionally, it has the potential to achieve more accurate target refractions than current intraoperative options without incurring the risks of additional procedures.
Acknowledgments
Foundation: Supported by Research to Prevent Blindness (New York, New York) and the clinical trial was sponsored by RxSight Inc. (formerly Calhoun Vision).
Conflicts of Interest: Moshirfar M, None; Wagner WD, None; Linn SH, None; Skanchy DF, None; Brown TW, None; Gomez AT, None; Goldberg JL, None; Ronquillo YC, None; Hoopes PC, None.
REFERENCES
- 1.Jadoon Z, Shah SP, Bourne R, Dineen B, Khan MA, Gilbert CE, Foster A, Khan MD, Pakistan National Eye Survey Study Group Cataract prevalence, cataract surgical coverage and barriers to uptake of cataract surgical services in Pakistan: the Pakistan National Blindness and Visual Impairment Survey. Br J Ophthalmol. 2007;91(10):1269–1273. doi: 10.1136/bjo.2006.106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu L, Zheng DY. Investigation of corneal astigmatism in phacoemulsification surgery candidates with cataract. Zhonghua Yan Ke Za Zhi. 2010;46(12):1090–1094. [PubMed] [Google Scholar]
- 3.Ferrer-Blasco T, Montés-Micó R, Peixoto-De-matos SC, González-Méijome JM, Cerviño A. Prevalence of corneal astigmatism before cataract surgery. J Cataract Refract Surg. 2009;35(1):70–75. doi: 10.1016/j.jcrs.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 4.Hashemi H, Khabazkhoob M, Soroush S, Shariati R, Miraftab M, Yekta A. The location of incision in cataract surgery and its impact on induced astigmatism. Curr Opin Ophthalmol. 2016;27(1):58–64. doi: 10.1097/ICU.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz DM. Light-adjustable lens. Trans Am Ophthalmol Soc. 2003;101:417–436. [PMC free article] [PubMed] [Google Scholar]
- 6.von Mohrenfels CW, Salgado J, Khoramnia R, Maier M, Lohmann CP. Clinical results with the light adjustable intraocular lens after cataract surgery. J Refract Surg. 2010;26(5):314–320. doi: 10.3928/1081597X-20090617-12. [DOI] [PubMed] [Google Scholar]
- 7.Hengerer FH, Conrad-Hengerer I, Buchner SE, Dick HB. Evaluation of the calhoun vision UV light adjustable lens implanted following cataract removal. J Refract Surg. 2010;26(10):716–721. doi: 10.3928/1081597X-20100408-02. [DOI] [PubMed] [Google Scholar]
- 8.Chayet A, Sandstedt C, Chang S, Rhee P, Tsuchiyama B, Grubbs R, Schwartz D. Use of the light-adjustable lens to correct astigmatism after cataract surgery. Br J Ophthalmol. 2010;94(6):690–692. doi: 10.1136/bjo.2009.164616. [DOI] [PubMed] [Google Scholar]
- 9.Brierley L. Refractive results after implantation of a light-adjustable intraocular lens in postrefractive surgery cataract patients. Ophthalmology. 2013;120(10):1968–1972. doi: 10.1016/j.ophtha.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Chayet A, Sandstedt C, Chang S, Rhee P, Tsuchiyama B, Grubbs R, Schwartz D. Correction of myopia after cataract surgery with a light-adjustable lens. Ophthalmology. 2009;116(8):1432–1435. doi: 10.1016/j.ophtha.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Griswold A. FDA approves implantable, adjustable lens for cataract patients-American Academy of Ophthalmology. [Accessed on July 16, 2018]. https://www.aao.org/headline/fda-approves-implantable-adjustable-lens-cataract-.
- 12.Hengerer FH, Dick HB, Conrad-Hengerer I. Clinical evaluation of an ultraviolet light adjustable intraocular lens implanted after cataract removal: eighteen months follow-up. Ophthalmology. 2011;118(12):2382–2388. doi: 10.1016/j.ophtha.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 13.Villegas EA, Alcon E, Rubio E, Marín JM, Artal P. Refractive accuracy with light-adjustable intraocular lenses. J Cataract Refract Surg. 2014;40(7):1075–1084.e2. doi: 10.1016/j.jcrs.2013.10.046. [DOI] [PubMed] [Google Scholar]
- 14.Holladay JT. Visual acuity measurements. J Cataract Refract Surg. 2004;30(2):287–290. doi: 10.1016/j.jcrs.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Standard Graphs for Reporting Refractive Surgery. London Vision Clinic. [Accessed on July 10, 2018]. https://www.londonvisionclinic.com/refractivesurgeryoutcomes/.
- 16.Norrby S. Sources of error in intraocular lens power calculation. J Cataract Refract Surg. 2008;34(3):368–376. doi: 10.1016/j.jcrs.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 17.Hoffer KJ, Savini G. IOL power calculation in short and long eyes. Asia Pac J Ophthalmol (Phila) 2017;6(4):330–331. doi: 10.22608/APO.2017338. [DOI] [PubMed] [Google Scholar]
- 18.Kane JX, Van Heerden A, Atik A, Petsoglou C. Intraocular Lens power formula accuracy: comparison of 7 formulas. J Cataract Refract Surg. 2016;42(10):1490–1500. doi: 10.1016/j.jcrs.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 19.Kane JX, Van Heerden A, Atik A, Petsoglou C. Accuracy of 3 new methods for intraocular lens power selection. J Cataract Refract Surg. 2017;43(3):333–339. doi: 10.1016/j.jcrs.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 20.Chayet A, Sandstedt CA, Chang SH, Rhee P, Tsuchiyama B, Schwartz D. Correction of residual hyperopia after cataract surgery using the light adjustable intraocular lens technology. Am J Ophthalmol. 2009;147(3):392–397.e1. doi: 10.1016/j.ajo.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 21.Liu B, Xu L, Wang YX, Jonas JB. Prevalence of cataract surgery and postoperative visual outcome in greater Beijing: the Beijing Eye Study. Ophthalmology. 2009;116(7):1322–1331. doi: 10.1016/j.ophtha.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 22.Lichtinger A, Sandstedt CA, Schwartz DM, Chayet AS. Correction of astigmatism after cataract surgery using the light adjustable lens: a 1-year follow-up pilot study. J Refract Surg. 2011;27(9):639–642. doi: 10.3928/1081597X-20110105-01. [DOI] [PubMed] [Google Scholar]
- 23.Kessel L, Andresen J, Tendal B, Erngaard D, Flesner P, Hjortdal J. Toric intraocular lenses in the correction of astigmatism during cataract surgery: a systematic review and meta-analysis. Ophthalmology. 2016;123(2):275–286. doi: 10.1016/j.ophtha.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Hirnschall N, Gangwani V, Crnej A, Koshy J, Maurino V, Findl O. Correction of moderate corneal astigmatism during cataract surgery: toric intraocular lens versus peripheral corneal relaxing incisions. J Cataract Refract Surg. 2014;40(3):354–361. doi: 10.1016/j.jcrs.2013.08.049. [DOI] [PubMed] [Google Scholar]
- 25.Kim P, Briganti EM, Sutton GL, Lawless MA, Rogers CM, Hodge C. Laser in situ keratomileusis for refractive error after cataract surgery. J Cataract Refract Surg. 2005;31(5):979–986. doi: 10.1016/j.jcrs.2004.08.054. [DOI] [PubMed] [Google Scholar]
- 26.Sánchez-Galeana CA, Smith RJ, Rodriguez X, Montes M, Chayet AS. Laser in situ keratomileusis and photorefractive keratectomy for residual refractive error after phakic intraocular lens implantation. J Refract Surg. 2001;17(3):299–304. doi: 10.3928/1081-597X-20010501-02. [DOI] [PubMed] [Google Scholar]
- 27.Hovanesian JA, Shah SS, Maloney RK. Symptoms of dry eye and recurrent erosion syndrome after refractive surgery. J Cataract Refract Surg. 2001;27(4):577–584. doi: 10.1016/s0886-3350(00)00835-x. [DOI] [PubMed] [Google Scholar]
- 28.Wolle MA, Randleman JB, Woodward MA. Complications of refractive surgery: ectasia after refractive surgery. Int Ophthalmol Clin. 2016;56(2):127–139. doi: 10.1097/IIO.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ford J, Werner L, Mamalis N. Adjustable intraocular lens power technology. J Cataract Refract Surg. 2014;40(7):1205–1223. doi: 10.1016/j.jcrs.2014.05.005. [DOI] [PubMed] [Google Scholar]