Abstract
AIM
To review imaging characteristics and surgical outcomes of orbital neurilemmoma.
METHODS
Retrospective review of 21 patients with orbital neurilemmoma managed at the Zhongshan Ophthalmic Center of Sun Yat-sen University from June 2005 to December 2016. All patients underwent surgical excision following preoperative imaging including ultrasonography, computed tomography (CT) and magnetic resonance imaging (MRI).
RESULTS
Among these patients, 11 were male and 10 were female, with age ranging from 12 to 75y (average, 40.3y). Ultrasound of the orbit showed a roundish well-demarcated orbital mass with low or middle internal reflectivity in each case. Dark inner liquid fields were detected in 28.6% of these cases. Doppler ultrasound demonstrated blood flow signals in these masses. CT showed that the tumors were either homogeneous or heterogeneous. MRI of T1WI revealed isointense or hypointense tumors, while the T2WI indicated heterogeneous hyperintense lesions. Gd contrast MRI demonstrated heterogenous or homogeneous enhancement initiating from the wide area of the lesion. Six patients underwent lateral orbitotomy and 15 anterior orbitotomy. All tumors were completely removed. After a mean follow-up of 1.8y, 3 patients experienced reduced vision while the remaining 10 patients showed improved vision after surgery. One patient experienced a mild limitation of upward motility. No recurrence occurred.
CONCLUSION
Orbital neurilemmoma is a relatively rare, benign orbital tumor. Effective diagnosis requires a combination of ultrasonography, CT and/or MRI. These imaging techniques are also vital to differentiate neurilemmomas from other orbital masses like that of cavernous hemangiomas and meningiomas. Successful treatment requires complete resection of the neurilemmomas as performed either by lateral or anterior orbitotomy. Recurrence is rare after complete removal.
Keywords: neurilemmoma, orbital tumor, imaging, orbitotomy, orbital surgery
INTRODUCTION
Neurilemmoma (also known as schwannoma) is a benign, slow growing, encapsulated tumor originating from Schwann cells of the peripheral nerve sheath. It occurs mostly in adults between 30 to 70 years old[1]. Although uncommon in the orbit, peripheral nerve tumors comprise 2% of all orbital neoplasms. Of these, neurilemmomas are the most common, accounting for 50% of these tumors[2]. Orbital neurilemmomas tend to arise not only from branches of the oculomotor, trochlear and abducens nerves, but also from sympathetic and parasympathetic fibers as well as the frontal branch of the trigeminal nerve. This origin of orbital neurilemmomas accounts for their location to be primarily present in the supraorbital region[3]. Although there are some reports on the clinical characteristics and imaging findings of orbital neurilemmoma, the information available on this condition is quite limited and accurate preoperative diagnoses in clinical practice remain difficult. Notably, differentiating among neurilemmoma, cavernous hemangioma and meningioma remains challenging. Clinically, it is critical to distinguish these three orbital tumors as they require different treatments and have different final visual prognoses. The purpose of this study is to describe the relatively unique imaging findings of orbital neurilemmoma. Such information will greatly aid in the presurgical diagnosis, and thus proper surgical approaches for patients with orbital neurilemmomas.
SUBJECTS AND METHODS
Ethical Approval
The Ethics Committee of the Zhongshan Ophthalmic Center approved this retrospective study, which was conducted according to the principles expressed in the Declaration of Helsinki. The committee specifically waived the need for consent.
A retrospective review of the records of 21 patients with orbital neurilemmoma over the period from June 2005 to December 2016 was conducted. All records were from a single tertiary eye hospital, the Zhongshan Ophthalmic Center, of Sun Yat-sen University, Guangzhou, China. The inclusion criteria were that all patients had been treated by a single surgeon (Yan JH) with a minimum follow-up period of 5mo. All identified cases were histologically diagnosed as neurilemmoma. Other histological types of peripheral orbital nerve tumors were excluded from our series.
The data collected in this study included general information on the patient's age, sex and duration of orbital lesion at presentation. Ocular information included the affected orbit, laterality, symptoms (visual problems, redness or swelling, proptosis, diplopia, palpable mass), signs (best corrected vision, proptosis, ocular motility deficit, strabismus). Tumor information included orbital location (superior, inferior, anterior, posterior), configuration (round, ovoid, diffuse), size, margin (ill-defined, well-defined), quality (rigid, soft, medium), tenderness (present, absent), tissues or areas involved, imaging findings and histopathologic examination.
All patients underwent ultrasonography and computed tomography (CT) or magnetic resonance imaging (MRI) prior to surgery. Either a lateral or anterior orbitotomy was performed according to each patient's image features. Histopathologic features, including the presence of Antoni A or Antoni B, were recorded. All patients were followed up over a period from 5mo to 3y, with a mean of 1.8y.
RESULTS
Symptoms and Signs
A total of 21 cases (11 males and 10 females; 11 right and 10 left orbits) with a histopathological diagnosis of orbital neurilemmoma were identified. This number represented 1.9% of all orbital mass lesions treated by the corresponding author over this same period. Ages ranged from 12 to 75y, with a mean of 40.3y. Two cases of recurrence who had previously received orbital surgery at local hospitals were included. The main symptoms and signs of orbital neurilemmoma at presentation are listed in Table 1. The most common symptom was a painless, gradually progressive proptosis (57.1%), while the second most common symptom was visual deterioration (33.3%). The remaining less common symptoms included palpable orbital masses, pain, conjunctival hyperemia and ptosis. Two cases were identified serendipitously by imaging. The main signs included proptosis (61.9%), visual deterioration (33.3%) and globe dystopia (33.3%). However, other signs such as retinal folds, tangible lumps, strabismus, and papilloedema were also relatively common. The mean reading of proptosis was 6.1 mm. Preoperative corrected visual acuity was <20/200 in 2 cases, 20/200-20/40 in 5 cases, 20/30-20/25 in 7 cases and >20/20 in 7 cases.
Table 1. Clinical symptoms and signs of orbital neurilemmoma (n=21).
| Symptom | n (%) | Sign | n (%) |
| Proptosis | 12 (57.1) | Proptosis | 13 (61.9) |
| Visual deterioration | 7 (33.3) | Visual deterioration | 7 (33.3) |
| Visible lump | 4 (19.0) | Globe dystopia | 7 (33.3) |
| Pain | 2 (9.5) | Retinal folds | 6 (28.6) |
| Conjunctival hyperemia | 1 (4.8) | Tangible lump | 5 (23.8) |
| Lid swelling | 1 (4.8) | Strabismus | 5 (23.8) |
| Ptosis | 1 (4.8) | Papilloedema | 4 (19.0) |
| Asymptomatic | 2 (9.5) |
Imaging Examination
All patients received both ultrasonography (n=21) and CT (n=15) or MRI (n=11) scans. Among whom 8 had a post-gadolinium (Gd) contrast MRI examination (Figures 1–3). The mean length and width of the lesions was 26.3 mm×19.1 mm, respectively. Ultrasonography findings, listed in Table 2, revealed that a roundish well-demarcated orbital mass possessing low or middle internal reflectivity was detected in each case, with 90.5% of these masses showing well-demarcated borders. Dark inner liquid fields were commonly observed in 28.6% of these cases (Figure 1). The mass was completely cystic in 1 case. In 3 cases subjected to Doppler ultrasound, blood flow signals were found inside these masses (Figure 3). On precontrast CT scan, tumors were classified as well-defined heterogeneous or homogeneous soft-tissue occupying lesions in all 15 cases, with CT values between 19.6 and 40 HU (Table 3). The mean CT value was 33.15 HU (Figure 2). Post-contrast CT showed centripetal enhancement of tumor parenchyma. Expansion of the bony orbit was also observed including orbital wall deformation (n=4) and orbital apex widening (n=2). T1-weighted MRI images revealed the neurilemmomas as isointense or hypointense relative to muscle. On T2-weighted images, tumors were heterogeneous hyperintense relative to muscle. On post-gadolinium (Gd) contrast MRI, tumors demonstrated heterogenous or homogeneous enhancement initiating from the wide area of the lesion (Table 4, Figures 1 and 3). The shape of the tumor was ovoid in 17 cases, dumbbell in 2 cases, irregularly shape in 1 case and a multilobulated chain in 1 case. Twenty cases had only one mass in the orbit, while the remaining case had 2 masses within the same orbit (Figure 2). Tumor positions based on imaging findings are listed in Table 5.
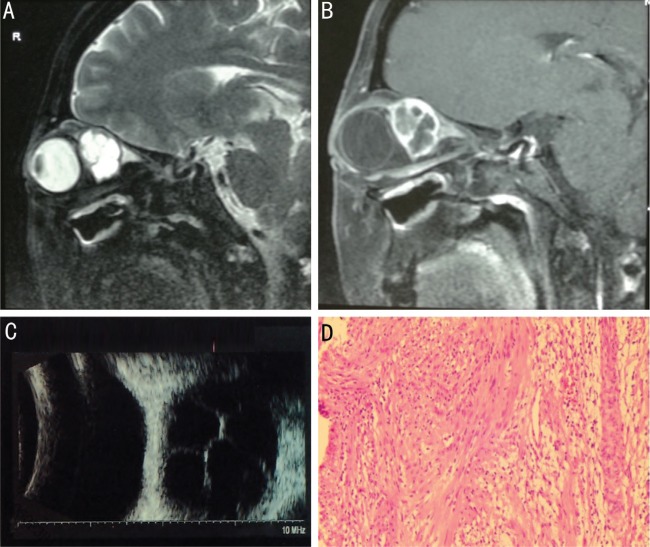
Figure 1. A 47-year-old woman experienced a gradually progressive proptosis with visual deterioration of the right eye and diplopia for one year.
A, B: T2-weighted sagittal MRI and enhanced T1-weighted MRI showed a well-defined multicystic-like lesion mass; C: B-mode ultrasound examination revealed liquid dark areas with central septa; D: The tumor was histologically confirmed as neurilemmoma with both Antoni A and Antoni B portions.
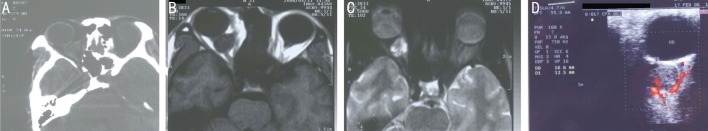
Figure 3. A 31-year-old male experienced right eye discomfort for two months.
The patient had a history of a right orbital mass, with orbital surgery being performed in a local hospital 6y previously. Histological diagnosis confirmed the tumor as neurilemmoma. A: Axial CT-scan revealed a well-defined heterogeneous oval mass with a CT value of 35 HU; B: Axial T-1 signal MRI indicated a retrobulbar mass with relatively homogenous hypointensity; C: Axial T-2 signal MRI showed a mass with heterogeneous hyperintensity; D: Doppler ultrasound revealed the presence of blood flow signals within the tumor.
Table 2. Ocular ultrasonography features in cases with orbital neurilemmoma (n=21).
| Feature | n (%) |
| Tumor border | |
| Clear | 19 (90.5) |
| Unclear | 2 (9.5) |
| Internal reflectivity | |
| Low | 12 (57.1) |
| Low to medium | 5 (23.8) |
| Medium | 4 (19.1) |
| Parenchyma | |
| Homogeneous | 9 (42.9) |
| Heterogeneous | 12 (57.1) |
| Inner liquid dark field | 6 (28.6) |
Table 3. CT features in cases with neurilemmoma (n=15).
| Feature | n (%) |
| Density | |
| Isodensity | 5 (33.3) |
| Iso to hypodensity | 10 (66.7) |
| Parenchyma | |
| Homogeneous | 5 (33.3) |
| Heterogeneous | 10 (66.7) |
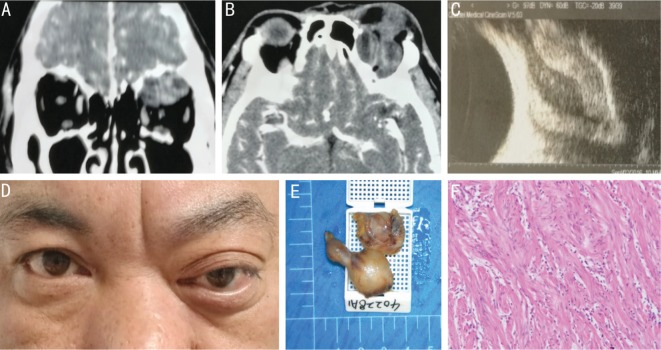
Figure 2. A 44-year-old male experienced a progressive proptosis of the left eye for one year.
A, B: Axial and coronal CT scans revealed two well-defined heterogeneous low CT value masses; C: B-mode ultrasound indicated a double wall sign; D: Ptosis and downward globe dystopia of the left eye were observed; E: The appearance of the tumors after complete surgical removal; F: The tumor was confirmed as neurilemmoma based on histological analysis with both Antoni A and Antoni B components.
Table 4. MRI features in cases with neurilemmoma (n=11).
| Feature | n (%) |
| T-1 signal | |
| Hypointense | 7 (63.6) |
| Isointense | 4 (36.4) |
| Hyperintense | 0 |
| T-2 signal | |
| Hyper and hypointense | 2 (18.2) |
| Hyper and Isointense | 4 (36.4) |
| Hyperintense | 5 (45.4) |
| Gd contrast MRI (n=8) | |
| Enhanced | 8 (100) |
| Unenhanced | 0 |
| Parenchyma | |
| Homogeneous | 1 (9.1) |
| Heterogeneous | 10 (90.9) |
Table 5. Neurilemmoma location in cases with orbital neurilemmoma (n=21).
| Position | n (%) |
| Relative position | |
| Out the muscle cone | 4 (19.0) |
| In the muscle cone | 17 (81.0) |
| Site of tumor in the orbit | |
| Medial | 4 (19.0) |
| Superior | 6 (28.6) |
| Lateral | 5 (23.8) |
| Inferior | 2 (9.5) |
| Center | 4 (19.0) |
Surgical Techniques
All cases underwent surgical excision either by lateral orbitotomy (28.6%) or anterior orbitotomy (71.4%). Among those with anterior orbitotomy, 14 cases were performed by a transcutaneous approach and 1 case by a transconjunctival approach. The tumors had a smooth and well-encapsulated oval or rounded shape with a gray/white to yellow color. No significant adhesions were present in 4 cases, while 17 cases had varying degrees of adhesion. Adhesive tissues included the supraorbital nerve, optic nerve, extraocular muscles, surrounding connective tissues and lacrimal gland (Table 6). The tumors were removed completely in all 21 cases. However, in 5 cases, the encapsulated lesions ruptured during surgical excision due to their huge size or vulnerable nature.
Table 6. Adhesive tissue sites in cases with orbital neurilemmoma (n=17).
| Tissue | n (%) |
| Supraorbital nerve | 4 (19.0) |
| Optic nerve | 6 (28.6) |
| Superior oblique muscle | 2 (9.5) |
| Surrounding connective tissues | 2 (9.5) |
| Inferior rectus muscle | 1 (4.8) |
| Lateral rectus muscle | 1 (4.8) |
| Lacrimal gland | 1 (4.8) |
Follow-up Outcomes
The duration of follow-up ranged from 5mo to 3y, with a mean of 1.8y. Results on final postoperative visual acuities are listed in Table 7. Central retinal artery occlusion was present in 2 cases and an occlusion within a branch of the retinal artery was present in 1 case. These three patients experienced severe postoperative visual deterioration. Postoperative strabismus (diplopia) occurred in 8 (38.1%) patients, while ptosis was observed in 4 (19.0%) patients. With the exception of 1 patient who showed a mild limitation of upward motility at 2y after surgery, postoperative diplopia and ptosis resolved spontaneously within 6 to 12mo in all remaining patients. There was no tumor recurrence in any of the cases.
Table 7. Pre and post-operative visual acuities in cases with orbital neurilemmoma (n=21).
| Cases | Preoperative visual acuity | Postoperative visual acuity |
| 1a | 20/25 | NLP |
| 2 | 20/22 | 20/20 |
| 3 | 20/25 | 20/25 |
| 4 | 20/12.5 | 20/12.5 |
| 5 | 20/20 | 20/16 |
| 6 | 20/16 | 20/16 |
| 7b | 20/32 | 20/200 |
| 8 | 20/25 | 20/16 |
| 9 | 20/20 | 20/16 |
| 10 | 20/600 | 20/400 |
| 11 | 20/16 | 20/16 |
| 12 | 20/50 | 20/50 |
| 13 | 20/22 | 20/20 |
| 14 | 20/32 | 20/20 |
| 15 | 20/16 | 20/16 |
| 16 | 20/28 | 20/22 |
| 17 | 20/63 | 20/25 |
| 18 | 20/200 | 20/22 |
| 19 | 20/40 | 20/22 |
| 20a | 20/50 | NLP |
| 21 | HM/10 cm | HM/10 cm |
aCentral retinal artery occlusion was present. bBranch retinal artery occlusion was present.
Histopathologic Findings
All cases were diagnosed as neurilemmoma by histopathological examination. Antoni A pattern (tightly packed Schwann cells with nuclear palisading) was observed in 6 cases. Both Antoni A and B patterns were observed in 15 cases. Immunohistochemical examination showed positive staining for S-100 and vimentin, and negative staining for cytokeratin and desmin.
DISCUSSION
Although relatively rare, neurilemmoma is the most common orbital neural neoplasm. In our series, there were no sex differences (11 men versus 10 women), which is similar to that of the previously reported findings of Konrad and Thiel[3] and Pushker et al[4]. In contrast, a greater prevalence within males was reported by Pointdujour-Lim et al[5] who found that males accounted for 73.3% and by Young et al[6] who reported that 61.5% of the orbital neurilemmomas were in males, whereas Wang and Xiao[7] found that only 42.0% of their cases were male. The age distribution of our patients ranged from 12 to 75y (mean 40.3y) and there was no differences between right and left eyes (11 right versus 10 left), which is similar to results reported by Pointdujour-Lim et al[5].
As a slow-growing benign tumor, orbital neurilemmoma is usually asymptomatic during its initial stages. As a result, these lesions are often only found by serendipitous imaging examinations. This was the case for 2 of the patients in our series. One orbital neurilemmoma was identified by brain MRI examination that was performed due to a sudden fainting episode, while the other was found by orbital MRI due to bilateral papilledema during uveitis treatment. In the report of Pointdujour-Lim et al[5], 26.7% of their cases were found incidentally. Eventually, the progressive growth of the tumor often results in compression of surrounding orbital tissues, leading to proptosis, globe displacement, vision disturbance and strabismus[1]–[2]. Therefore, similar to other benign slowly-growing tumors such as cavernous hemangioma and meningioma, orbital neurilemmoma has no specific clinical symptoms or signs. The first two most common signs, as observed in our study, included a painless insidious proptosis and visual deterioration. In the report of Kim et al, the most common symptom was a painless palpable mass (100%)[8], while in the series of Young et al[6] proptosis was present in all cases. As reported by Pushker et al[4], the most common presenting condition was a painless progressive proptosis (92.9%), followed by deterioration of vision (73.8%), diplopia (14.3%), pain (14.3%) and eyelid swelling (4.8%). These differences in signs observed among patients are largely attributable to the location and size of the tumor. Findings of multiple neurilemmomas in one orbit are rare. However, Kron et al[9] reported a 59-year-old male who showed recurrence at 6 years after complete excision of the initial tumor, resulting from two independent orbital schwannomas being present in this case. Moreover, Koktekir et al[10] described 3 cases of primary orbital schwannomatosis who showed multiple orbital schwannomas. In our series, we found only one patient (a 44-year-old male) with both tumors located within the superior region of the orbit. No patients with intracranial involvement were observed in this study. This result, likely reflects a referral bias, as patients with intracranial involvement are usually referred to other hospitals for evaluation.
Imaging plays a vital role in preoperative diagnosis of orbital neurilemmoma. Typically, with use of ultrasonography, orbital neurilemmoma appears as an oval or lobulated mass with a heterogeneous middle-to-low internal reflection. This imaging feature was present in 57.1% of our cases, while cystic degeneration occurred in about 41% of all cases. Regions of degeneration corresponded to the site of hemorrhagic, necrotic portion of the tumor or Antoni B areas, which had mucus or microencapsulated areas[4]. Doppler ultrasound showed blood flow signals inside the tumor. Kashyap et al[11] found that purely cystic schwannoma showed a double wall sign. On CT, most neurilemmomas appear as isodense to hypodense oval or rounded soft-tissue masses. In our cases, 81.0% displayed such features, while Young et al[6] reported this description in only 61.6% of their cases. Compared to other types of soft-tissue tumors, neurilemmomas have a lower CT value, generally under 50 HU. This was also true in our series, as CT values ranged from 19.6 to 40 HU with a mean of 33.15. A significant advantage of CT is its capacity for observing secondary orbital bone remodeling. For example, extension of the orbital apex may indicate a neurilemmoma projecting from the intracranial to the orbital region[12]. MRI has a greater value than CT in the diagnosis of neurilemmoma. On T1WI, the tumors were hypointense (63.6%) or isointence (36.4%) while on T2WI, the tumors were hyperintense. Both the T1 and T2 signals were heterogeneous in 90.1% of our cases. On post GD-enhanced T1WI, all neurilemmomas were homo- or heterogeneously enhanced. Such findings are similar to that of other reports[4]–[9],[11]. The heterogeneous feature of neurilemmomas on MRI may relate to the pathological types of the lesions. For example, the Antoni B part contains more mucus than Antoni A, which will result in a higher MRI signal. As most neurilemmomas contain both pathological types (71.4%), the MRI imaging of neurilemmoma will more likely appear heterogeneous[5]–[7],[12]. With the benefit of GD-enhanced, high-resolution thin-section MRI, it is possible to locate lesions with sizes of less than 10 mm in diameter[13].
In general, clinical and imaging features of orbital neurilemmoma, cavernous hemangioma and meningioma all appear quite similar. All typically present with a gradually progressive proptosis and visual deterioration[14]–[15] and all are located deep within the orbit, usually near or within the muscle cone. Differentiation among orbital neurilemmoma, cavernous hemangioma and meningioma is critical as they require different surgical approaches and have different visual prognoses. Patients with retrobulbar cavernous hemangioma often can be surgically treated by anterior orbitotomy and show a favorable postoperative visual prognosis. When the lesion involves a retrobulbar neurilemmoma or menigioma, lateral orbitotomy is usually required and their postoperative visual outcomes are inauspicious as compared with that of cavernous hemangioma. Visual prognosis is worst in patients with orbital meningioma. Orbital neurilemmoma can be easily distinguished from orbital cavernous hemangioma with use of ultrasonography. As revealed with Doppler, orbital neurilemmoma presents as a middle or low reflectivity mass with dark liquid areas and relatively rich blood signals, while cavernous hemangioma shows a homogeneous high reflectivity mass with no blood signal[16]. Orbital meningiomas often originate from the optic nerve sheath and surround the nerve, which is an important imaging feature that discriminates it from cavernous hemangioma and neurilemmoma. Visual deterioration is usually observed in early stages of orbital meningiomas. In a review by Dutton[17], 97% of their orbital meningioma cases showed visual deterioration at the time of presentation. On CT, an enlarged optic nerve with increased peripheral intensity and a decreased central intensity, described as a “tram-track” sign is typically observed, an image that has been observed in 64% of orbital meningiomas cases[18]. The CT value of meningioma is often higher than that of neurilemmoma. On MRI, orbital meningiomas often show homogeneous isointense on T1WI and hyperintense on T2WI.
The surgical removal of orbital neurilemmomas is required for patients with visual deterioration and remarkable proptosis while the asymptomatic patient can be closely followed. Currently, complete removal of the neurilemmoma is the only treatment without recurrence. Orbital neurilemmomas often adhere to surrounding tissues, as was observed in 17/21 cases in this study. Even with careful surgical separation, injury to the optic nerve may result. Due to its relatively thin wall, the tumor can easily rupture during surgery, especially in cases with cystic degeneration. Moreover, the origins of these tumors are oftentimes difficult to locate, with the origin of some 50% of orbital neurilemmoma failing to be identified during surgery[19]. In our cases, the origin of the tumor was located in only 4 cases, including 3 cases from the supra orbital nerve and 1 case from the trochlear nerve. Both lateral and anterior orbitotomy are widely used surgical approaches for orbital neurilemmoma[20]. Tumor location is the most important factor in selecting the surgical approach. Anterior orbitotomy is used for lesions located at the anterior portion of the orbit, while lateral orbitotomy is required for retrobulbar lesions[21]–[22]. Recently, endoscopic approaches have been applied for tumors located in the inferior and medial regions of the orbit[23]. If the tumor has extended into the cranial cavity, a cranio-orbitotomy can be performed[24]. Subramanian et al[25] even reported one patient who underwent an eye enucleation due to loss of vision, gross proptosis and severe corneal exposure. The surgical approaches used in previous reports for orbital neurilemmoma and their complications are summarized in Table 8. Surgical complications include visual deterioration, globe dystopia, strabismus, ptosis and facial numbness resulting from the damage of nerves and extraocular muscles during separation of the tumor from surrounding tissues. Though surgical complications are usually common, most patients show a spontaneous recovery within 1 to 6mo. Sun et al[26] reported that cranial nerve VI recovery is more likely after surgical resection of neurilemmoma, if the tumor did not extend into the cavernous sinus. A piece-meal or a subtotal removal, sometimes by evacuation of the tumor within the capsule can be performed to avoid surgical complications, especially in cases where serious visual loss may result owing to the close proximity to the optic nerve in the apex of the orbit. Recurrence is rare if the lesion has been removed completely. Rootman et al[27] found no recurrence in patients who had complete tumor excision. In contrast, in four patients who underwent subtotal tumor excision recurrence occurred within 3y after their initial surgery. We found no recurrence in our series. However, patients should be warned of recurrence in cases when an incomplete tumor excision was performed[9].
Table 8. Summary of reported surgically managed orbital neurilemmomas including current series.
| Author, year | Cases | Surgical approach | Extent of resection | Complication | Visual acuity |
| Rootman et al, 1982[27] | 5 | Lateral orbitotomy (n=1); combined frontal craniotomy and lateral orbitotomy (n=1); anteriororbitomy (n=2); ethmoidectomy (n=1) | Total (n=5) | Temporarily ptosis (n=1) | 6/7-5 or better (n=4); 6/12 (n=1) |
| Butt and McNab, 1998[24] | 7+1a | Anterior orbitotomy (n=4); lateral orbitotomy (n=3); cranio-orbitotomy (n=1) | Subtotal (n=2); total (n=5) | Recurrence (n=1); diplopia (n=2); partial lateral rectus palsy supraorbital anaesthesia, and temporary ptosis (n=1); underaction of the superior rectus (n=1) | Improved (n=3); normal (n=2); not mentioned (n=2) |
| Schick et al,2003[20] | 5 | Extradural pterional approach (n=2); Supraorbital approach (n=2); Transconjunctival approach (n=1) | Total (n=5) | Transient palsy of the abducens nerve (n=1); amaurotic with dilated pupil (n=1); temporarily proptosis (n=1) | Improved (n=3); equal (n=1); decreased (n=1) |
| Subramanian et al, 2005[25] | 4+1a | Combined medial and lateral orbitotomy (n=1); lateral orbitotomy (n=2); second procedure lateral orbitotomy (n=1); eye enucleation (n=1) | Total (n=3); subtotal (n=2) | Recurrence (n=1) | Improved (n=1); equal (n=1); decreased (n=2); anopia (n=1) |
| Irace et al, 2008[21] | 1 | Lateral orbitomy (n=1) | Total | VI nerve partial palsy (n=1) | Not mentioned |
| Rato et al, 2012[22] | 1 | Lateral orbitomy (n=1) | Total | Temporarily proptosis and VI nerve palsy | Not mentioned |
| Pushker et al, 2015[4] | 42 | Lateral orbitotomy (28.6%); anterior orbitomy (71.4%) | Total (76.2%); Subtotal (23.8%) | Not mentioned | Not mentioned |
| Champagne et al, 2014[23] | 1 | Endoscopic resection | Total | No complication | Not mentioned |
| Kim et al, 2015[8] | 5 | Supraorbital transcutaneous incision (n=1); subciliary incision (n=1); not recorded (n=3) | Total (n=5) | Temporary numbness of forehead and scalp (n=1); temporary numbness of forehead (n=1) | Normal |
| Sun et al, 2017[26] | 33 | Suboccipital (n=11); anterior transpetrosal (n=3); subtemporal (n=6); transcondylar (n=1); orbitotomy-lateral (n=3); orbitotomy-superior (n=1); frontotemporal (n=4); orbitozygomatic (n=2); not recorded (n=2) | Gross total (n=18); subtotal (n=14) | Postsurgical CN VI recovery (n=14; 42.4%) | Not mentioned |
| Pointdujour-Lim et al, 2018[5] | 15 | Orbitotomy via superior eyelid crease incision (n=9); lower eyelid crease incision (n=3); transconjunctival approach (n=2); craniotomy (n=1) | Total (n=14; 93.3%); piecemeal excision (n=1; 6.7%) | Not mentioned | Not mentioned |
| Young et al, 2018[6] | 13 | Lateral orbitotomy (51.5%); anterior orbitomy (30.8%); craniotomy (7.7%) | Total (92.3%); subtotal (7.7%) | Temporary hypesthesia (92.3%); temporary ptosis (23.1%); temporary diplopia (7.7%) | Not mentioned |
| Current series | 21 | Lateral orbitotomy (71.4%); anterior orbitotomy (28.6%) | Total (100%) | Temporary ptosis (19.0%); temporary globe dystopia (28.6%); central retinal artery occlusion (9.5%); branch retinal artery occlusion (4.8%) | Improved (n=10); equal (n=8); decreased (n=3) |
aSecond surgery procedure.
As with all retrospective reviews, there exist a number of limitations in this study. The most notable limitations include: 1) retrospective observations; 2) small sample size (n=21); 3) not all patients received both CT and MRI examinations and 4) an absence of identical studies, due to the variations present in the patients' conditions and resultant differences in surgical techniques. In conclusion, orbital neurilemmoma is a slow growing benign tumor. It occurs mostly in adults ranging in age from 12 to 75y (average 40.0y). The most common symptoms include proptosis and visual deterioration. Ultrasonography revealed a low or middle internal reflectivity of a rounded well-demarcated orbital mass. Dark inner liquid fields were detected in 28.6% of these cases. Blood flow signals were found inside these masses using Doppler ultrasound. CT scans indicated a homogeneous or heterogeneous lesion with a mean CT value of 33.15 HU, a value which is quite lower than that obtained in orbital cavernous hemangioma and meningioma. T1-weighted MRI images revealed the neurilemmomas as isointense or hypointense relative to the muscle, while on T2-weighted images, tumors were heterogeneous hyperintense relative to the muscle. Currently, complete removal of the lesion by either anterior or lateral orbitotomy is the most widely used treatment for orbital neurilemmomas. Recurrence is rare after complete removal.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No.81670885); the Science and Technology Program of Guangdong Province, China (No.2013B020400003); Science and Technology Program of Guangzhou, China (No.15570001).
Conflicts of Interest: Chen MH, None; Yan JH, None.
REFERENCES
- 1.Shields JA, Shields CL. Orbital schwannoma. In: Shields JA, Shields CL, editors. Eyelid, Conjunctival and Orbital Tumors. An Atlas and Textbook. 3rd ed. Philadelphia, PA: Wolters Kluwer; 2016. pp. 552–555. [Google Scholar]
- 2.Shields JA, Shields CL, Scartozzi R. Survey of 1264 patients with orbital tumors and simulating lesions: The 2002 Montgomery Lecture, part 1. Ophthalmology. 2004;111(5):997–1008. doi: 10.1016/j.ophtha.2003.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Konrad EA, Thiel HJ. Schwannoma of the orbit. Ophthalmologica. 1984;188(2):118–127. doi: 10.1159/000309352. [DOI] [PubMed] [Google Scholar]
- 4.Pushker N, Khurana S, Kashyap S, Sen S, Shrey D, Meel R, Chawla B, Bajaj MS. Orbital schwannoma: a clinicopathologic study. Int Ophthalmol. 2015;35(4):481–486. doi: 10.1007/s10792-014-9973-1. [DOI] [PubMed] [Google Scholar]
- 5.Pointdujour-Lim R, Lally SE, Shields JA, Eagle RC, Jr, Shields CL. Orbital schwannoma: radiographic and histopathologic correlation in 15 cases. Ophthalmic Plast Reconstr Surg. 2018;34(2):162–167. doi: 10.1097/IOP.0000000000000900. [DOI] [PubMed] [Google Scholar]
- 6.Young SM, Kim YD, Jeon GS, Woo KI. Orbital frontal nerve schwannoma-distinctive radiological features. Am J Ophthalmol. 2018;186:41–46. doi: 10.1016/j.ajo.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Xiao LH. Orbital schwannomas: findings from magnetic resonance imaging in 62 cases. Eye (Lond) 2008;22(8):1034–1039. doi: 10.1038/sj.eye.6702832. [DOI] [PubMed] [Google Scholar]
- 8.Kim KS, Jung JW, Yoon KC, Kwon YJ, Hwang JH, Lee SY. Schwannoma of the orbit. Arch Craniofac Surg. 2015;16(2):67–72. doi: 10.7181/acfs.2015.16.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kron M, Bohnsack BL, Archer SM, McHugh JB, Kahana A. Recurrent orbital schwannomas: clinical course and histopathologic correlation. BMC Ophthalmol. 2012;12:44. doi: 10.1186/1471-2415-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koktekir BE, Kim HJ, Geske M, Bloomer M, Vagefi R, Kersten RC. Orbital schwannomatosis in the absence of neurofibromatosis. J Craniofac Surg. 2014;25(6):2109–2111. doi: 10.1097/SCS.0000000000001064. [DOI] [PubMed] [Google Scholar]
- 11.Kashyap S, Pushker N, Meel R, Sen S, Bajaj MS, Khuriajam N, Mehta M, Chawla B. Orbital schwannoma with cystic degeneration. Clin Exp Ophthalmol. 2009;37(3):293–298. doi: 10.1111/j.1442-9071.2009.02029.x. [DOI] [PubMed] [Google Scholar]
- 12.Skolnik AD, Loevner LA, Sampathu DM, Newman JG, Lee JY, Bagley LJ, Learned KO. Cranial nerve schwannomas: diagnostic imaging approach. Radiographics. 2016;36(5):1463–1477. doi: 10.1148/rg.2016150199. [DOI] [PubMed] [Google Scholar]
- 13.Yulek F, Demer JL. Isolated schwannoma involving extraocular muscles. J AAPOS. 2016;20(4):343–347. doi: 10.1016/j.jaapos.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan J, Wu Z. Cavernous hemangioma of the orbit: analysis of 214 cases. Orbit. 2004;23(1):33–40. doi: 10.1076/orbi.23.1.33.28992. [DOI] [PubMed] [Google Scholar]
- 15.Paulsen F, Doerr S, Wilhelm H, Becker G, Bamberg M, Classen J. Fractionated stereotactic radiotherapy in patients with optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. 2012;82(2):773–778. doi: 10.1016/j.ijrobp.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Yang M, Yan J. Long term surgical outcomes of orbital cavernous haemangiomas (low-flow venous malformations) as performed in a tertiary eye hospital in China. J Craniomaxillofac Surg. 2014;42(7):1491–1496. doi: 10.1016/j.jcms.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Dutton JJ. Optic nerve sheath meningiomas. Surv Ophthalmol. 1992;37(3):167–183. doi: 10.1016/0039-6257(92)90135-g. [DOI] [PubMed] [Google Scholar]
- 18.Ghassibi MP, Ulloa-Padilla JP, Dubovy SR. Neural tumors of the orbit: what is new? Asia Pac J Ophthalmol (Phila) 2017;6(3):273–282. doi: 10.22608/APO.2017157. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Lin J, Liu R, Li J, Yan J. Orbital schwannoma originating from the superior oblique muscle. J Craniofac Surg. 2015;26(2):559–560. doi: 10.1097/SCS.0000000000001230. [DOI] [PubMed] [Google Scholar]
- 20.Schick U, Bleyen J, Hassler W. Treatment of orbital schwannomas and neurofibromas. Br J Neurosurg. 2003;17(6):541–545. doi: 10.1080/02688690310001627786. [DOI] [PubMed] [Google Scholar]
- 21.Irace C, Davì G, Corona C, Candino M, Usai S, Gambacorta M. Isolated intraorbital schwannoma arising from the abducens nerve. Acta Neurochir (Wien) 2008;150(11):1209–1210. doi: 10.1007/s00701-008-0134-z. [DOI] [PubMed] [Google Scholar]
- 22.Rato RM, Correia M, Cunha JP, Roque PS. Intraorbital abducens nerve schwannoma. World Neurosurg. 2012;78(3-4):375.e1–375.e4. doi: 10.1016/j.wneu.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Champagne PO, Desrosiers M, Moumdjian R. Endoscopic resection of an infraorbital nerve schwannoma. Clin Neurol Neurosurg. 2014;119:106–109. doi: 10.1016/j.clineuro.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 24.Butt ZA, McNab AA. Orbital neurilemmoma: report of seven cases. J Clin Neurosci. 1998;5(4):390–393. doi: 10.1016/s0967-5868(98)90268-5. [DOI] [PubMed] [Google Scholar]
- 25.Subramanian N, Rambhatia S, Mahesh L, Menon SV, Krishnakumar S, Biswas J, Noronha OV. Cystic schwannoma of the orbit-a case series. Orbit. 2005;24(2):125–129. doi: 10.1080/01676830590922020. [DOI] [PubMed] [Google Scholar]
- 26.Sun H, Sharma K, Kalakoti P, Thakur JD, Patra DP, Konar S, Maiti T, Akbarian-Tefaghi H, Bollam P, Notarianni C, Nanda A. Factors associated with abducens nerve recovery in patients undergoing surgical resection of sixth nerve schwannoma: a systematic review and case illustration. World Neurosurg. 2017;104:883–899. doi: 10.1016/j.wneu.2017.04.146. [DOI] [PubMed] [Google Scholar]
- 27.Rootman J, Goldberg C, Robertson W. Primary orbital schwannomas. Br J Ophthalmol. 1982;66(3):194–204. doi: 10.1136/bjo.66.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]