Abstract
AIM
To explore the process of retinal vascularization and risk factors for retinopathy of prematurity (ROP) treated with intravitreal ranibizumab (IVR) as monotherapy.
METHODS
Infants with type 1 ROP who received IVR as primary treatment from August 2014 to October 2016 at Peking University People's Hospital's Ophthalmology Department were included in the study. All eyes received 0.25 mg ranibizumab at initial treatment. Retinal vascularization was evaluated clinically. Potential risk factors were also recorded and examined.
RESULTS
Retinal vascularization was completed in 126 eyes (62.7%), and retinal vascularization terminated in zone II and zone III with 16 eyes (7.9%) and 44 eyes (21.9%), respectively, after more than 1-year follow-up. In multivariate regression analysis, lower birth weight (BW), severity of ROP and repeated injections were found to be risk factors for peripheral avascular area (P<0.05).
CONCLUSION
In our retrospective study, 29.8% of the ROP eyes treated with ranibizumab have peripheral avascular area at the last follow-up. Lighter BW and the severity of ROP are risk factors. Furthermore, repeated injections also increase the risk of retinal peripheral avascular area remaining in ROP patients.
Keywords: retinal vascularization, retinopathy of prematurity, ranibizumab, avascular retinal area
INTRODUCTION
Retinopathy of prematurity (ROP) is a vascular proliferative disease caused by the abnormal development of retinal blood vessels in premature infants[1]. ROP is the leading cause of visual morbidity in children worldwide, and responsible for up to 60% in middle-income countries[2]. Ablation of the peripheral avascular retina is the standard treatment for ROP, thus, since the 1980s cryotherapy and laser photocoagulation have been the gold standards to treat ROP[3].
Vascular endothelial growth factor (VEGF) was discovered to be related to retinal neovascularization in the 1990s. In the last several years, a number of studies have demonstrated that the use of anti-VEGF agents could prevent the progression of ROP. A prospective, multicenter trial about intravitreal bevacizumab for ROP—Bevacizumab Eliminates the Angiogenic Threat of Retinopathy of Prematurity (BEAT-ROP) showed that bevacizumab was effective in treating ROP and was more effective than laser treatment in zone I ROP infants[4]. Ranibizumab is a monoclonal antibody fragment (Fab) derived from antibody as bevacizumab but with greater affinity for VEGF. Recently, a large retrospective study showed that intravitreal ranibizumab (IVR) could result in a positive response in 94% of ROP eyes[5]. VEGF also plays an important role in retinal vascular development. Some studies have discovered peripheral avascular areas and abnormal vascular patterns in ROP eyes after anti-VEGF treatment[6]–[7].
The purpose of the present study was to explore the effect of ranibizumab as monotherapy for ROP on the process of retinal vascularization and to examine the influential risk factors.
SUBJECTS AND METHODS
Ethical Approval
From August 2014 to October 2016, a retrospective study was performed at the Department of Ophthalmology, Peking University People's Hospital. Consecutive patients who were diagnosed with ROP and accepted IVR as primary treatment were included. This study was conducted with the approval of the Institutional Review Board of Peking University People's Hospital and adhered to the tenets of the Declaration of Helsinki. All subjects provided informed consent.
Patient Screening and Treatment
Infants who were born at gestational age (GA) <32wk and birth weight (BW) <2000 g were screened for ROP according to Chinese guidelines[8]. The International Classification of Retinopathy of Prematurity 2005 was also referenced [9]. Digital retina images for all infants were obtained by a RetCam 3 Imaging System (Clarity Medical System, Pleasanton, CA, USA). Fluorescein angiograms (FFA) were examined for infants who had peripheral avascular area.
Infants who were diagnosed with type 1 ROP, as defined by the Early Treatment for Retinopathy of Prematurity (ETROP) criteria, were treated with IVR as initial treatment. A 0.25 mg/0.025 mL dose of ranibizumab (Lucentis, Novartis, Switzerland) was injected into the eye by experienced surgeons. The intravitreal injection was performed under sterile conditions in an operating theater. After administering topical anesthesia to the cornea and conjunctiva by repeated application of oxybuprocaine hydrochloride eye drops and after inducing a slight systemic sedation of each child, the external eye and the surrounding skin were disinfected with a povidone-iodine 5% ophthalmic solution (Betadine; Alcon Inc, Fort Worth, Texas, USA) and the eyes were draped. A lid speculum was inserted. The surgeon injected ranibizumab with a sterile 30-gauge 0.5-inch needle at 1.5 mm posterior to the limbus, then removed the needle with simultaneous compression using a sterile cotton tip. Instillation of topical tobramycin and removal of the speculum were performed. If the other eye was to be treated, new equipment was used. After the injection, we applied an antibiotic eye drop combination 4 times per day for 1wk. Infants who had cataracts, glaucoma, cornea opacity, or 4b ROP or worse or who were not able to undergo the examinations regularly were excluded.
Follow-up Procedure
The patients were monitored on the first day, first week, and depending on the retinal findings, until full retinal vascularization. Disease regression, recurrence and peripheral vascularization were evaluated on each follow-up. In case of ROP recurrence, additional treatment including repeated injections, laser photocoagulation or surgery was applied. Additional treatment was not considered if peripheral avascular areas remained stable during the follow-up.
Data were collected from patient charts including gender, GA at birth, BW, stage of ROP at presentation, retinal vascularization, additional treatments, and any adverse side effects. Potential risk factor including red blood cell (RBC) count, platelet count and hemoglobin levels were also recorded.
Statistical Analysis
Descriptive continuous variables are presented using the mean and standard deviation. Categorical variables were compared between the groups using the Chi-square test and Fisher's exact test. The potential risk factors were included in univariate analysis. A logistic multivariate regression was constructed to identify independent risk factors for ROP retinal vascularization. Statistical analyses were performed using SPSS software for Windows version 22.0 (SPSS, Inc., Chicago, USA). P<0.05 was considered significant.
RESULTS
Patients Characteristics
This study consisted of 201 eyes of 104 patients, all of whom received IVR at primary treatment. Of these patients, 58.6% were male. The mean GA and BW of the patients were 29±3.2wk (range, 25 to 33.8wk) and 1236.87±343.99 g (range, 670 to 2500 g). The mean follow-up was 69.8±13.7wk by adjusted age (GA and postnatal age). The patient demographics are listed in Table 1. While most of the eyes (80.6%) had zone II ROP, 26 eyes (12.9%) had aggressive posterior retinopathy of prematurity (APROP), and 13 eyes (6.5%) had zone I ROP. Repeated intravitreal anti-VEGF treatment was administered to 73 eyes (36.3%) due to recurrence and plus disease. Furthermore, 12 eyes (5.9%) and 3 eyes (1.5%) received additional laser treatment and surgical treatment, respectively. There were no significant differences in GA between the zone of ROP group. In this study, we collected RBC count, platelet count and hemoglobin level as potential risk factors, and the mean RBC count, platelet count and hemoglobin level were 3.49×109/L, 318.31×109/L and 105.4 g/L, respectively (Table 1). Hemoglobin level and platelet count were significantly associated with severity of ROP (P=0.011 and 0.026, respectively).
Table 1. Demographic features of the patients.
| Variable | Severity of ROP |
P | |||
| Zone I (n=13) | APROP (n=26) | Zone II (n=162) | Total (n=201) | ||
| BW (g) | 1293.07±402.81 | 1409.81±333.94 | 1204.61±333.85 | 1236.87±343.99 | 0.015 |
| GA (wk) | 29.1±2.1 | 30.3±1.95 | 28.9±2.7 | 29±3.2 | 0.830 |
| RBC (×109/L) | 3.62±0.89 | 3.67±0.69 | 3.46±0.62 | 3.49±0.65 | 0.244 |
| PLT (×109/L) | 386.69±158.842 | 287.77±122.86 | 317.31±108.69 | 318.31±108.69 | 0.026 |
| HGB (g/L) | 110.92±26.22 | 114.81±17.67 | 103.44±18.38 | 105.40±19.206 | 0.011 |
| Repeated injections (n) | 9 | 23 | 41 | 73 | 0.043 |
| Additional laser treatment | 3 | 5 | 4 | 12 | |
| Additional surgery | 0 | 3 | 2 | 5 | |
BW: Birth weight; GA: Gestational age; RBC: Red blood cell; PLT: Platelet; HGB: Hemoglobin; APROP: Aggressive posterior retinopathy of prematurity.
mean±SD
No serious complications, such as endophthalmitis, cataract, retinal detachment or hemorrhage were observed after injection. Furthermore, no adverse systemic side effects were observed.
Retinal Vascularization
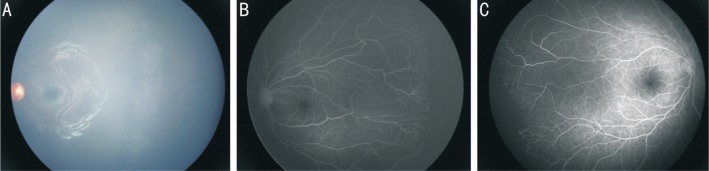
Retinal vascularization was completed in 126 eyes (62.7%), and retinal vascularization terminated in zone II and zone III in 16 eyes (7.9%) and 44 eyes (21.9%), respectively. The results are shown in Table 2. In eyes in which retinal vascularization terminated in zone II, zone I ROP identified in was 1 eye (7.69%), APROP was identified in 5 eyes (20%) and zone II ROP was identified in 10 eyes (6.17%). After more than 1y of follow-up, 23.1% (3 eyes) with zone I ROP, 23.1% (6 eyes) with APROP and 21.5% (35 eyes) with zone II ROP terminated retinal vascularization in zone III. Zone II ROP comprised 88.09% of eyes with completed retinal vascularization eyes. The degree of retinal vascularization was significantly associated with severity of ROP. The fundus photography and FFA of degree of retinal vascularization are shown in Figure 1.
Table 2. Characteristics of retinal vascularization of ROP eyes with IVR at initial treatment.
| Variable | Peripheral avascular zone |
Additional laser treatment (n=12) | Additional surgery treatment (n=3) | Total (n=201) | ||
| Zone II (n=16) | Zone III (n=44) | Completed vascularization (n=126) | ||||
| BW (g) | 1100±347.1 | 1185.45±348.5 | 1274.38±348.1 | 1216.67±249.4 | 1226.67±316.5 | 1236.87±343.99 |
| GA (wk) | 28.9±2.2 | 28.5±1.7 | 29.2±2.9 | 29.6±2.4 | 29.7±2.8 | 29±3.2 |
| Severity of ROP | ||||||
| Zone I (n) | 1 | 3 | 6 | 3 | 0 | 13 |
| APROP (n) | 5 | 6 | 9 | 5 | 1 | 26 |
| Zone II (n) | 10 | 35 | 111 | 4 | 2 | 162 |
| Repeated injections (n) | 9 | 18 | 38 | 7 | 1 | 73 |
| RBC (×109/L) | 3.53±0.44 | 3.59±0.74 | 3.46±0.64 | 3.51±0.72 | 3.19±0.43 | 3.50±0.65 |
| PLT (×109/L) | 295.63±127.38 | 309.64±106.29 | 323.52±110.30 | 310.75±80.31 | 377.67±88.59 | 318.31±108.69 |
| HGB (g/L) | 109.25±15.25 | 107.14±19.89 | 103.95±19.77 | 110.83±16.47 | 98.33±11.93 | 105.40±19.21 |
BW: Birth weight; GA: Gestational age; APROP: Aggressive posterior retinopathy of prematurity; RBC: Red blood cell; PLT: Platelet; HGB: Hemoglobin.
mean±SD
Figure 1. Ocular fundus and fundus fluorescein angiography of ROP eyes.
A: ROP eyes with completed retinal vascularization; B, C: ROP eyes with retinal vascularization terminated in zone II (B) and zone III (C).
In multivariate regression analysis, three factors were identified as being significantly associated with ROP retinal vascularization: infants BW (P=0.001), severity of ROP (P=0.005) and repeated injections (P=0.045). The levels of significance, odds ratio (OR) and 95% confidence interval (CI) are presented in detail in Table 3.
Table 3. Multivariable analysis of risk factor associated with retinal vascularization of ROP eyes with IVR at initial treatment.
| Variable | Peripheral avascular eyes | Completed vascularization eyes | P | OR (95%CI) |
| BW (g) | 1163.66±347.31 | 1274.38±348.17 | 0.001 | 0.997 (0.996, 0.999) |
| GA (wk) | 200.88±13.1 | 204.97±20.62 | 0.627 | 0.994 (0.969,1.019) |
| Severity of ROP | ||||
| Zone I (n) | 4 | 6 | 0.005 | 0.388 (0.2, 0.775) |
| APROP (n) | 11 | 9 | ||
| Zone II (n) | 45 | 111 | ||
| Repeated injections (n) | 0.5 | 0.33 | 0.045 | 1.754 (1.013, 3.038) |
| RBC (×109/L) | 3.58±0.67 | 3.47±0.64 | 0.111 | 0.263 (0.051, 1.357) |
| PLT (×109/L) | 305.9±111.34 | 323.5±110.3 | 0.525 | 0.999 (0.996, 1.002) |
| HGB (g/L) | 107.7±18.66 | 103.95±19.77 | 0.057 | 1.056 (0.998, 1.118) |
BW: Birth weight; GA: Gestational age; APROP: Aggressive posterior retinopathy of prematurity; RBC: Red blood cell; PLT: Platelet; HGB: Hemoglobin. OR: Odds ratio; CI: Confidence interval; SD: Standard deviation.
mean±SD
DISCUSSION
Retinal blood vessels begin at the optic nerve at 16wk gestation, branch outward, and then reach the edges of the retina at 40wk gestation[10]. According to previous reports, complete vascularization is clinically defined as the accession of retinal vessels to the temporal ora serrata, and ROP eyes should be monitored until complete retinal vascularization[11]–[12]. However, in a recent study, children with congenital glaucoma or cataract had retinal vascular abnormalities and peripheral nonperfusion up to 7.5 years old, indicating that complete vascularization to the ora may not be the rule[13]. Blair et al[14] performed FFA in normal children and found that the avascular retina extends ≤1.0 disk diameters (DD) nasally and ≤1.5 DD temporally from the ora serrata in children up to 13 years of age. This suggests that only a distance of more than 2 DD from the ora serrata to the vascularized retinal margin should be considered abnormal. The advantage of intravitreal anti-VEGF therapy for ROP are the short duration of the procedure, the absence of anesthesia-related complications and ongoing retinal vascularization[4]. However, persistent avascular retina has been reported after anti-VEGF treatment. Karkhaneh et al[15] reported that avascular areas in the peripheral retina existed remained in 45% of the infants at 90wk postmenstrual age after bevacizumab treatment. Alyamaç Sukgen et al[16] found that 18% of the infants in the IVB group and 26% of the infants in the IVR group had remaining avascular areas at 1 year old. Tahija et al[17] reported that 50% of the eyes with ROP showed remaining peripheral avascular areas of more than 2 DD up to 4y after bevacizumab treatment, even though the outcome was considered satisfactory.
In our study, we found that 29.8% of the eyes had peripheral avascular area at the last follow-up, approximately 1/4 of their retinal vascularization reached zone II, and these patients did not develop pathological neovascularization within the follow-up period. In multivariate regression analysis, we found that patients who retained avascular area had significantly lower BW than patients who completed retinal vascularization (P=0.001). Similarly, the severity of ROP was found to be another risk factor for retinal avascular area (P=0.005).
Previous studies showed that ranibizumab treatment may be associated with a higher incidence of ROP reactivation. Hu et al[18] found that 26.2% of eyes had a recurrence of ROP after the IVR initial treatment. Huang et al[5] reported a reactivation rate of 44.1% in Chinese ROP patients. In our study, 26.9% of the eyes needed retreatment, including repeated injections, laser treatment or surgery.
Moreover, in our study, infants who received repeated ranibizumab injections were more likely to retain peripheral avascular area in follow-up. VEGF is an important angiogenic factor in development and essential in the homeostasis of developed vasculature[19]–[20]. Our results indicate that intravitreal anti-VEGF treatment also had a significant impact on normal retinal vascularization.
Our study was limited as a retrospective and nonrandomized controlled trial. The timing at which the fluorescein angiography was performed was not uniform among all patients. In line with this, no control group of imaging data from healthy infants was included.
In conclusion, in our retrospective study, 29.8% of the eyes treated with ranibizumab had peripheral avascular area at the last follow-up. Lighter BW and the severity of ROP were risk factors. Furthermore, repeated injections also increase the risk of retinal peripheral avascular area remaining in ROP patients.
Acknowledgments
We would like to thank all the patients for kindly participating in the study.
Authors' contributions: Meng QY, Zhao MW and Liang JH were involved in the study design, data and interpretation, and manuscript writing. Meng QY, Cheng Y and Liang JH worked on the data collection, analysis and manuscript writing. All authors read and approved the final manuscript.
Foundation: Supported by Peking University People's Hospital Research and Development Funds (No.RDY2017-17). Funding institutions had no role in the study design, data collection, analysis, the decision to publish, or the preparation of the manuscript.
Conflicts of Interest: Meng QY, None; Cheng Y, None; Zhao MW, None; Liang JH, None.
REFERENCES
- 1.Section on Ophthalmology American Academy of Pediatrics; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2006;117(2):572–576. doi: 10.1542/peds.2005-2749. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert C, Fielder A, Gordillo L, Quinn G, Semiglia R, Visintin P, Zin A, International NO-ROP Group Characteristics of infants with severe retinopathy of prematurity in countries with low, moderate, and high levels of development: implications for screening programs. Pediatrics. 2005;115(5):e518–e525. doi: 10.1542/peds.2004-1180. [DOI] [PubMed] [Google Scholar]
- 3.Early Treatment For Retinopathy Of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121(12):1684–1694. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- 4.Mintz-Hittner HA, Kennedy KA, Chuang AZ, BEAT-ROP Cooperative Group Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364(7):603–615. doi: 10.1056/NEJMoa1007374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang QJ, Zhang Q, Fei P, Xu Y, Lyu J, Ji XD, Peng J, Li YA, Zhao PQ. Ranibizumab injection as primary treatment in patients with retinopathy of prematurity: anatomic outcomes and influencing factors. Ophthalmology. 2017;124(8):1156–1164. doi: 10.1016/j.ophtha.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Mehta S, Hubbard GB., 3rd Delayed recurrent neovascularization and persistent avascular retina following intravitreal bevacizumab for retinopathy of prematurity. Retin Cases Brief Rep. 2013;7(3):206–209. doi: 10.1097/ICB.0b013e318285238e. [DOI] [PubMed] [Google Scholar]
- 7.Lepore D, Quinn GE, Molle F, Orazi L, Baldascino A, Ji MH, Sammartino M, Sbaraglia F, Ricci D, Mercuri E. Follow-up to age 4y of treatment of type 1 retinopathy of prematurity intravitreal bevacizumab injection versus laser: fluorescein angiographic findings. Ophthalmology. 2018;125(2):218–226. doi: 10.1016/j.ophtha.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Feng J, Gilbert C, Yin H, Liang JH, Li XX. Time at treatment of severe retinopathy of prematurity in China: recommendations for guidelines in more mature infants. PLoS One. 2015;10(2):e0116669. doi: 10.1371/journal.pone.0116669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123(7):991–999. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 10.Ashton N. Retinal angiogenesis in the human embryo. Br Med Bull. 1970;26(2):103–106. doi: 10.1093/oxfordjournals.bmb.a070758. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Stahl A, Hellstrom A, Smith LE. Current update on retinopathy of prematurity: screening and treatment. Curr Opin Pediatr. 2011;23(2):173–178. doi: 10.1097/MOP.0b013e3283423f35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah PK, Prabhu V, Karandikar SS, Ranjan R, Narendran V, Kalpana N. Retinopathy of prematurity: past, present and future. World J Clin Pediatr. 2016;5(1):35–46. doi: 10.5409/wjcp.v5.i1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HY, Hodapp E, Grajewski AL, Sarraf D, John VJ, Hess DJ, Berrocal AM. Peripheral retinal vasculopathy in childhood glaucoma. Retina. 2015;35(5):1028–1035. doi: 10.1097/IAE.0000000000000415. [DOI] [PubMed] [Google Scholar]
- 14.Blair MP, Shapiro MJ, Hartnett ME. Fluorescein angiography to estimate normal peripheral retinal nonperfusion in children. J AAPOS. 2012;16(3):234–237. doi: 10.1016/j.jaapos.2011.12.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karkhaneh R, Khodabande A, Riazi-Eafahani M, Roohipoor R, Ghassemi F, Imani M, Dastjani Farahani A, Ebrahimi Adib N, Torabi H. Efficacy of intravitreal bevacizumab for zone-II retinopathy of prematurity. Acta Ophthalmol. 2016;94(6):e417–e420. doi: 10.1111/aos.13008. [DOI] [PubMed] [Google Scholar]
- 16.Alyamaç Sukgen E, Çömez A, Koçluk Y, Cevher S. The process of retinal vascularization after anti-VEGF treatment in retinopathy of prematurity: a comparison study between ranibizumab and bevacizumab. Ophthalmologica. 2016;236(3):139–147. doi: 10.1159/000449530. [DOI] [PubMed] [Google Scholar]
- 17.Tahija SG, Hersetyati R, Lam GC, Kusaka S, McMenamin PG. Fluorescein angiographic observations of peripheral retinal vessel growth in infants after intravitreal injection of bevacizumab as sole therapy for zone I and posterior zone II retinopathy of prematurity. Br J Ophthalmol. 2014;98(4):507–512. doi: 10.1136/bjophthalmol-2013-304109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu QR, Bai YJ, Chen XL, Huang L, Chen Y, Li XX. Recurrence of retinopathy of prematurity in zone II stage 3+ after ranibizumab treatment: A retrospective study. J Ophthalmol. 2017;2017:5078565. doi: 10.1155/2017/5078565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saint-Geniez M, Kurihara T, Sekiyama E, Maldonado AE, D'Amore PA. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc Natl Acad Sci U S A. 2009;106(44):18751–18756. doi: 10.1073/pnas.0905010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saint-Geniez M, Maharaj AS, Walshe TE, Tucker BA, Sekiyama E, Kurihara T, Darland DC, Young MJ, D'Amore PA. Endogenous VEGF is required for visual function: evidence for a survival role on Müller cells and photoreceptors. PLoS One. 2008;3(11):e3554. doi: 10.1371/journal.pone.0003554. [DOI] [PMC free article] [PubMed] [Google Scholar]