Pregnant women’s engagement with the SmokeFree Baby app (v1) was low, and the app did not appear to help them stop smoking during pregnancy.
Keywords: Smartphone app, Intervention optimization, Factorial experiment, Engagement, Smoking cessation, Pregnant smokers
Abstract
Smartphone applications (apps) might be able to reach pregnant smokers who do not engage with face-to-face support. However, we do not know how far pregnant smokers will engage with smoking cessation apps or what components are likely to be effective. This study aimed to assess pregnant smokers’ engagement with the SmokeFree Baby app (v1) and to assess the short-term efficacy of selected components (“modules”) for smoking abstinence. Positive outcomes would provide a basis for further development and evaluation. SmokeFree Baby was developed drawing on behavior change theories and relevant evidence. Pregnant smokers (18+) who were interested in quitting and set a quit date were recruited. Following multiphase optimization development principles, participants (N = 565) were randomly allocated to one of 32 (2 × 2 × 2 × 2 × 2) experimental groups in a full factorial design to evaluate five modules (each in minimal and full version: identity, health information, stress management, face-to-face support, and behavioral substitution). Measures of engagement included duration and frequency of engagement with the app. Smoking abstinence was measured by self-reported number of smoke-free days up to 4 weeks from the quit date. Participants engaged with the app for a mean of 4.5 days (SD = 8.5) and logged in a mean of 2.9 times (SD = 3.1). Main effects of the modules on the number of smoke-free days were not statistically significant (identity: p = .782, health information: p = .905, stress management: p = .103, face-to-face support: p = .397, behavioral substitution: p = .945). Despite systematic development and usability testing, engagement with SmokeFree Baby (v1) was low and the app did not appear to increase smoking abstinence during pregnancy.
Implications
Practice: Engagement with the SmokeFree Baby app (v1) was low among pregnant smokers and individual modules did not have a statistically significant effect on smoking abstinence during pregnancy.
Policy: The optimization phase of digital behavior-change interventions might require several iterations before arriving at intervention components that are engaging and likely to be effective.
Research: Further research is needed on methods for predicting likely levels of engagement with digital behavior-change interventions before moving to the optimization phase.
INTRODUCTION
Digital behavior-change interventions (DBCIs), such as smartphone apps aimed at helping people to stop smoking, are being developed at a rapid rate [1,2] with hundreds of such apps available on app stores (e.g., Apple app store or Google play). Only a few apps have any evidence behind them [3] and high-quality randomized controlled trials (RCTs) have yet to provide clear evidence for their effectiveness to aid cessation [4–6]. Pregnant smokers might benefit from support from such apps but to date we do not have any evidence-based apps for this population. This paper reports on a study aimed at identifying potentially effective components to put into such an app, named SmokeFree Baby.
DBCIs could be attractive to pregnant smokers who do not engage with face-to-face support [7, 8] and who face numerous barriers, such as lack of access to specialized services [9]. There is some evidence that text messages can increase cessation rates during pregnancy [10] and when provided alongside routine care [11], but to date smoking cessation apps have not been evaluated among pregnant smokers [12].
This study followed the Multiphase Optimization Strategy (MOST) approach to intervention development [13]. MOST involves an optimization phase in which intervention components are evaluated in one or more factorial screening experiments to identify which components show promising effects prior to evaluating the intervention as a treatment package in an RCT. The primary aim of factorial screening experiments is to generate hypotheses for further evaluation [14]. Understanding the effectiveness of specific intervention components and using factorial designs to guide intervention development have been recognized as important but relatively neglected areas of smoking cessation research [15]. Only a few studies have reported using this approach to inform the development of DBCIs in other areas, e.g., to prevent substance use [16] and to reduce alcohol consumption [17].
Assessing participants’ engagement with DBCIs is key to understand intervention effectiveness [18]. However, the nature and level of engagement can vary extensively across DBCIs, behaviors, and populations [19, 20]. Moreover, an integrative definition of engagement with DBCIs proposes that it comprises more than one component: one’s subjective experience (e.g., interest in using the DBCI) and behavior (e.g., frequency of use) [21]. Therefore, although previous studies suggest that text messages can engage pregnant smokers with smoking cessation support [10, 11], the evidence is scarce regarding engagement with apps in this context, and this needs further research.
Prior to the optimization phase, intervention development should involve arriving at an intervention that is likely feasible and acceptable enough to generate sufficient engagement [22]. This phase of the pregnancy-specific smoking cessation app, SmokeFree Baby involved focus groups with health care providers who work with pregnant smokers [23], usability testing of the prototype app [24], and a think-aloud study with pregnant smokers to explore their views on the design, content, and usability of the app [25]. Results from this formative work suggested that there would be good engagement with the app.
The current study assessed the potential efficacy of five app components: (i) fostering a positive nonsmoker identity, (ii) providing health information about the consequences of smoking and benefits of cessation, (iii) promoting use of face-to-face support, (iv) improving stress management, and (v) promoting behavioral substitution. A detailed description of the modules and the rationale for their inclusion has been reported previously [24]. For example, findings from our formative work suggested that pregnant smokers wanted further cessation support (preferably face-to-face) in addition to an app. The “face-to-face” module was designed with these findings in mind to provide participants with easy access to local stop smoking services and stop smoking websites [26].
The following research questions were addressed as follows:
(1) What is the extent to which pregnant smokers engage with the app in terms of (i) duration and frequency of app use and (ii) use of app features (“active engagement”)?
(2) What are the main effects of, and two-way interactions between full and minimal version of five components (identity, health information, face-to-face support, stress management, and behavioral substitution) on self-recorded smoking abstinence during the 4 weeks after the quit date?
METHODS
Ethics
Ethical approval for this study was received from the UCL Psychology and Language Sciences Departmental Ethics Committee (Project ID: CEHP/2013/508).
Study design
Participants were randomly allocated to one of the 32 experimental groups in a 2 × 2 × 2 × 2 × 2 full factorial design. Intervention components that were experimentally varied were termed “modules.” Each of five modules (identity, health information, face-to-face support, stress management, and behavioral substitution) had a “minimal” version (brief quit advice) and a “full” version (interactive content). The CONSORT guideline for reporting RCTs was followed, and the completed checklist is reported in Supplementary Material.
Participants
Data were collected between October 2014 and October 2016. The SmokeFree Baby app (v1) was developed in England, but it was available to anyone through worldwide app stores, including the Apple app store (itunes.apple.com/us/app/smokefree-baby/id925671396) and Google play (play.google.com/store/apps/details?id=com.silverbackis.smokefreebaby). The app was in English.
Participants were recruited through a number of methods as follows. Stop smoking advisors who interact with pregnant smokers recommended the app to their patients. Printed information leaflets (https://osf.io/6usyp/ and https://osf.io/qj4gc/) were distributed in England. The leaflets could be ordered through Public Health England’s Start4Life campaign resources website (https://campaignresources.phe.gov.uk/resources/campaigns/2-start-4-life/resources/129). A dedicated website (www.smokefreebaby.co.uk) was developed and an online advertisement was placed on a pregnancy-related charity’s website (www.tommys.org/pregnancy-information/i’m-pregnant/smoking-and-pregnancy/get-help-stop-smoking). The app could also be found through independent worldwide searches on the app stores. Participants did not receive financial compensation for taking part in the study.
Participants were included if they opened the app with the study code (“9123”), provided consent to participate, were pregnant, aged 18+, smoked cigarettes at least once a week, were interested in stopping smoking, and set a quit date in the app.
The sample size was determined based on an a priori power calculation. To detect an assumed small to medium effect size of d = 0.3 for main effects on the number of smoke-free days with 80 per cent power and a two-tailed α = 0.05, a minimum of 352 participants (11 participants in each of 32 groups with an equal allocation ratio) had to be recruited.
Measures
At baseline, a unique device identifier (device ID) was automatically registered in the study database when the app was opened at the first time. If duplicated device IDs were registered, they were excluded and the first case of downloads was retained for further analysis. Uptake of the app (number of eligible participants who completed the registration) was automatically registered. Data on the operating system (iOS or Android) and participants’ country of registration were also automatically registered.
Participants completed a questionnaire that asked them about their age, highest completed educational qualification, employment status, week of pregnancy, and the number of children they had (Table 1). Nicotine dependence was assessed by the Heaviness of Smoking Index (a composite measure of number of cigarettes smoked per day and time to first cigarette) [27]. They were asked “When did your most recent quit attempt start?” (not yet attempting; in the last week; more than a week and up to a month; more than 1 month and up to 2 months; more than 2 months and up to 3 months; more than 3 months and up to 6 months; more than 6 months and up to a year) and “What types of support are you using in addition to the SmokeFree Baby app to help you quit smoking?” (see response options in Table 1). Motivation to stop smoking was assessed by asking participants how much they wanted to stop smoking during this pregnancy (not very; quite; very; extremely). Participants selected a behavior change goal and a date within 14 days from the date of registration to either stop smoking completely or cut down to fewer than three cigarettes per day. The cutting down option was offered to those who did not feel confident to quit abruptly, to assess whether or how far this would prove attractive and ultimately lead to cessation [28, 29]. Complete cessation was the primary target behavior and all participants were encouraged to stop smoking completely.
Table 1.
Baseline characteristics
| Reference group | Total sample | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | Identity | Health information | Face-to-face support | Stress management | Behavioural substitution | ||||||
| Level | Minimal | Full | Minimal | Full | Minimal | Full | Minimal | Full | Minimal | Full | |
| N | 565 | 277 | 288 | 288 | 277 | 292 | 273 | 266 | 299 | 283 | 282 |
| Operating system | |||||||||||
| iOS: % (N) | 63.0 (356) | 62.8 (174) | 63.2 (182) | 60.1 (173) | 66.1 (183) | 62.7 (183) | 63.4 (173) | 59.4 (158) | 66.2 (198) | 60.4 (171) | 65.6 (185) |
| Android: % (N) | 37.0 (209) | 37.2 (103) | 36.8 (106) | 39.9 (115) | 33.9 (94) | 37.3 (109) | 36.6 (100) | 40.6 (108) | 33.8 (101) | 39.6 (112) | 34.4 (97) |
| Country of registration | |||||||||||
| UK: % (N) | 51.0 (288) | 48.0 (133) | 53.8 (155) | 50.3 (145) | 51.6 (143) | 48.6 (142) | 53.5 (146) | 48.1 (128) | 53.3 (160) | 51.2 (145) | 50.7 (143) |
| USA: % (N) | 26.0 (147) | 28.9 (80) | 23.3 (67) | 25.0 (72) | 27.1 (75) | 27.4 (80) | 24.5 (67) | 28.2 (75) | 24.1 (72) | 28.3 (80) | 23.8 (67) |
| Other: % (N) | 23.0 (130) | 23.1 (64) | 22.9 (66) | 12.6 (71) | 10.4 (59) | 24.0 (70) | 22.0 (60) | 23.7 (63) | 22.4 (67) | 20.5 (58) | 25.5 (72) |
| Age: Mean (SD) | 27.3 (5.5) | 27.3 (5.5) | 27.3 (5.5) | 27.5 (5.5) | 27.0 (5.5) | 27.2 (5.3) | 27.4 (5.7) | 27.2 (5.7) | 27.4 (5.3) | 27.5 (5.7) | 27.1 (5.3) |
| Highest level of formal education achieved | |||||||||||
| No qualification: % (N) | 14.7 (83) | 14.4 (40) | 14.9 (43) | 12.5 (36) | 17.0 (47) | 14.7 (43) | 14.7 (40) | 13.2 (35) | 16.1 (48) | 15.9 (45) | 13.5 (38) |
| Secondary school: % (N) | 45.1 (255) | 41.5 (115) | 48.6 (140) | 43.8 (126) | 46.6 (129) | 47.3 (138) | 42.9 (117) | 45.9 (122) | 44.5 (133) | 45.6 (129) | 44.7 (126) |
| Undergraduate degree: % (N) | 26.9 (152) | 28.5 (79) | 25.3 (73) | 31.6 (91) | 22.0 (61) | 24.3 (71) | 29.7 (81) | 27.1 (72) | 26.8 (80) | 26.5 (75) | 27.3 (77) |
| Postgraduate degree: % (N) | 13.3 (75) | 15.5 (43) | 11.1 (32) | 12.2 (35) | 14.4 (40) | 13.7 (40) | 12.8 (35) | 13.9 (37) | 12.7 (38) | 12.0 (34) | 14.5 (41) |
| Employment status | |||||||||||
| Unemployed/on state benefit: % (N) | 27.8 (157) | 27.4 (76) | 28.1 (81) | 26.0 (75) | 29.6 (82) | 29.1 (85) | 26.4 (72) | 30.8 (82) | 25.1 (75) | 28.6 (81) | 27.0 (76) |
| Manual occupation: % (N) | 41.8 (236) | 42.2 (117) | 41.3 (119) | 41.7 (120) | 41.9 (116) | 42.5 (124) | 41.0 (112) | 38.0 (101) | 45.2 (135) | 41.0 (116) | 42.6 (120) |
| Non-manual occupation: % (N) | 30.4 (172) | 30.3 (84) | 30.6 (88) | 32.3 (93) | 28.5 (79) | 28.4 (83) | 32.6 (89) | 31.2 (83) | 29.8 (89) | 30.4 (86) | 30.5 (86) |
| Pregnancy gestation | |||||||||||
| 1–12 weeks: % (N) | 57.5 (325) | 55.2 (153) | 59.7 (172) | 58.0 (167) | 57.0 (158) | 58.9 (172) | 56.0 (153) | 59.4 (158) | 55.9 (167) | 57.6 (163) | 57.4 (162) |
| 13–28 weeks: % (N) | 36.5 (206) | 37.9 (105) | 35.1 (101) | 36.1 (104) | 36.8 (102) | 36.6 (107) | 36.3 (99) | 32.7 (87) | 39.8 (119) | 37.1 (105) | 35.8 (101) |
| 29+ weeks: % (N) | 6.0 (34) | 6.9 (19) | 5.2 (15) | 5.9 (17) | 6.1 (17) | 4.5 (13) | 7.7 (21) | 7.9 (21) | 4.3 (13) | 5.3 (15) | 6.7 (19) |
| Pregnancy parity | |||||||||||
| Pregnant with the first child: % (N) | 57.7 (326) | 58.8 (163) | 56.6 (163) | 55.9 (161) | 59.6 (165) | 57.5 (168) | 57.9 (158) | 57.9 (154) | 57.5 (172) | 56.2 (159) | 59.2 (167) |
| Heaviness of Smoking Index: Mean (SD) | 2.5 (1.4) | 2.6 (1.4) | 2.5 (1.4) | 2.5 (1.4) | 2.5 (1.4) | 2.5 (1.4) | 2.5 (1.4) | 2.5 (1.4) | 2.5 (1.4) | 2.5 (1.4) | 2.5 (1.4) |
| Past quit attempts | |||||||||||
| Quit attempt in past year: % (N) | 33.6 (190) | 33.9 (94) | 33.3 (96) | 35.8 (103) | 31.4 (87) | 33.2 (97) | 34.1 (93) | 35.7 (95) | 31.8 (95) | 34.6 (98) | 32.6 (92) |
| Use of cessation aidsa | |||||||||||
| Only SmokeFree Baby app b: % (N) | 38.8 (219) | 37.2 (103) | 40.3 (116) | 36.8 (106) | 40.8 (113) | 38.0 (111) | 39.6 (108) | 35.7 (95) | 41.5 (124) | 41.3 (117) | 36.2 (102) |
| Non-nicotine medication c: % (N) | 0.7 (4) | 0.7 (2) | 0.7 (2) | 1.0 (3) | 0.4 (1) | 1.0 (3) | 0.4 (1) | 0.4 (1) | 1.0 (3) | 1.1 (3) | 0.4 (1) |
| Nicotine-replacement therapy d: % (N) | 21.4 (121) | 22.4 (62) | 20.5 (59) | 21.2 (61) | 21.7 (60) | 21.6 (63) | 21.2 (58) | 20.7 (55) | 22.1 (66) | 21.2 (60) | 21.6 (61) |
| E-cigarettes: % (N) | 16.8 (95) | 18.4 (51) | 15.3 (44) | 14.9 (43) | 18.8 (52) | 16.4 (48) | 17.2 (47) | 15.8 (42) | 17.7 (53) | 16.3 (46) | 17.4 (49) |
| Books or leaflets: % (N) | 8.7 (49) | 9.0 (25) | 8.3 (24) | 9.0 (26) | 8.3 (23) | 8.9 (26) | 8.4 (23) | 11.3 (30) | 6.4 (19) | 8.8 (25) | 8.5 (24) |
| Websites: % (N) | 11.7 (66) | 11.2 (31) | 12.2 (35) | 12.2 (35) | 11.2 (31) | 12.3 (36) | 11.0 (30) | 13.5 (36) | 10.0 (30) | 11.3 (32) | 12.1 (34) |
| Other smoking cessation app: % (N) | 12.4 (70) | 11.6 (32) | 13.2 (38) | 14.9 (43) | 9.7 (27) | 13.7 (40) | 11.0 (30) | 11.3 (30) | 13.4 (40) | 12.4 (35) | 12.4 (35) |
| Support from a stop smoking advisor: % (N) | 10.8 (61) | 9.4 (26) | 12.2 (35) | 9.7 (28) | 11.9 (33) | 12.3 (36) | 9.2 (25) | 11.3 (30) | 10.4 (31) | 9.9 (28) | 11.7 (33) |
| Other: % (N) | 5.1 (29) | 5.4 (15) | 4.9 (14) | 6.6 (19) | 3.6 (10) | 6.2 (18) | 4.0 (11) | 6.0 (16) | 4.3 (13) | 5.7 (16) | 4.6 (13) |
| Motivation to stop smoking: Mean (SD) | 3.8 (0.6) | 3.7 (0.6) | 3.8 (0.5) | 3.8 (0.6) | 3.7 (0.5) | 3.8 (0.6) | 3.7 (0.6) | 3.7 (0.6) | 3.8 (0.5) | 3.8 (0.5) | 3.8 (0.6) |
| Goal setting | |||||||||||
| Stopping smoking completely: % (N) | 72.0 (407) | 72.9 (202) | 71.5 (205) | 71.2 (205) | 72.9 (202) | 69.9 (204) | 74.4 (203) | 70.3 (187) | 73.6 (220) | 73.9 (209) | 70.2 (198) |
| Cutting down: % (N) | 28.0 (75) | 27.1 (158) | 28.8 (83) | 28.8 (83) | 27.1 (75) | 30.1 (88) | 25.6 (70) | 29.7 (79) | 26.4 (79) | 26.1 (74) | 29.8 (84) |
| Quit date | |||||||||||
| Quit date set for the day of enrolment: % (N) | 41.8 (236) | 41.5 (115) | 42.0 (121) | 40.3 (116) | 43.3 (120) | 40.8 (119) | 42.9 (117) | 41.4 (110) | 42.1 (126) | 40.3 (114) | 43.3 (122) |
aMultiple cessation aids could be selected.
bParticipants selected SmokeFree Baby and did not select any other cessation aids.
cChampix (varenicline) or Zyban (bupropion).
dNicotine patch, gum, nasal spray, inhalator, or lozenge; Bonferroni-corrected statistically significant difference: p = .002.
Measures of engagement included duration of engagement (number of days between participants’ first and last log-in), frequency of engagement (number of log-ins), and active engagement (interacting with or rating the usefulness of an app component). Smoking abstinence was measured by the number of self-reported smoke-free days up to 4 weeks from participants’ target quit date. This measure was selected because it was an optimization study and we sought what we thought would be the most sensitive measure that would predict longer-term cessation. Smoking abstinence was assessed once a day, when participants first logged in to the app, by asking them “Did you smoke any cigarettes at all yesterday?”
Intervention
The full content specification of SmokeFree Baby is available through Open Science Framework (https://osf.io/nv8t2/). The intervention development process, including a detailed description of the theoretical underpinning and the selection of intervention components, is published elsewhere [24]. Forty-two distinct behavior change techniques (BCTs) were included in the app; these are defined as the smallest intervention components that on their own have the potential to change behavior [30,31], from the BCT Taxonomy v1 [32]. The BCT specification of the app has also been published [24]. Intervention components that were available to all participants were termed “general app features” (e.g., “Withdrawal symptom” features included tips to cope with withdrawal). The app was available for iOS (version 6.0 and later) and Android (version 4.1 and later) devices, and it was provided free of charge.
Procedure
Once SmokeFree Baby was downloaded to a digital device, a code had to be entered to open the app. The study code (“9123”) was offered as default on the main screen and a separate code (“5555”) was provided for those who wanted to opt out of the study; they could still access a minimal version of the app. To minimize contamination, a code (“1234”) was advertised for health professionals and researchers who were interested in the app.
Randomization was implemented using an algorithm embedded in the SmokeFree Baby program. The randomization matrix is reported in Supplementary Table 1. Participants had to be online at the time of randomization. To maximize recruitment, eligible participants were randomized immediately after opening the app for the first time. The background questionnaire could be completed and a quit date set at a later point, online or offline. A random number of 1–32 was generated when a new user entered the code for the experiment until one participant was added to each group. Then randomization started again with the next block of 32 groups. Participants were blinded to group allocation. The research team, who assessed the outcomes, was able to see the group allocation to check if the procedure was implemented correctly.
In the next step, information about the study was provided and consent was obtained from each participant. A brief questionnaire was included to collect background information and to assess participants’ eligibility to participate in the study. Because eligibility was checked after randomization, recruitment, and random allocation of participants commenced until each cell contained the minimum number of eligible participants. Follow-up was implemented automatically by using an in-app feature that prompted participants at the first login each day to record if they had smoked any cigarettes at all in the past 24 hr. The research team had no contact with study participants at any point during the RCT, apart from directing them to the app developers for technical support.
Analysis
Data analysis was conducted using SPSS 20.0. Descriptive statistics were used to report uptake of the app, device characteristics and participants’ baseline characteristics, and engagement with the app. Differences in baseline characteristics between minimal and full versions of each module were explored using Pearson’s chi-squared test (for categorical variables) and one-way ANOVA (for continuous variables). Between-subject factorial ANOVA was used to evaluate main effects of, and two-way interactions between (all interactions were included in the analysis, but not reported), the five modules on engagement and smoking abstinence. “Effect coding” was used so that main effects and interactions could be interpreted according to their classical definition, where the “minimal” level of each module was coded as −1 and the “full” level was coded as +1. Participants who were lost to follow-up were retained in the intention-to-treat (ITT) analysis and assumed to achieve zero smoke-free days. In case of a nonsignificant main effect, Bayes factors were calculated with half-normal distribution using mean difference parameter estimates to represent the alternative hypothesis. This was done using an online tool (http://www.lifesci.sussex.ac.uk/home/Zoltan_Dienes/inference/Bayes.htm). Bayes factors are indicators of the relative strength of evidence for one’s theory over the null-hypothesis given the data [33], and they allow determination of whether the results can be interpreted as evidence to support a null-hypothesis or the data are inconclusive as to whether the differences were present [34]. Conventional cut-offs [33, 35] were used to interpret Bayes factors (<1/3: evidence for null hypothesis; >3: evidence for alternative hypothesis; 1/3< and <3: the data were inconclusive). Sensitivity analyses were conducted among those who logged in at least once after their quit date, and Bayes factors were calculated for large effects.
RESULTS
Uptake and user characteristics
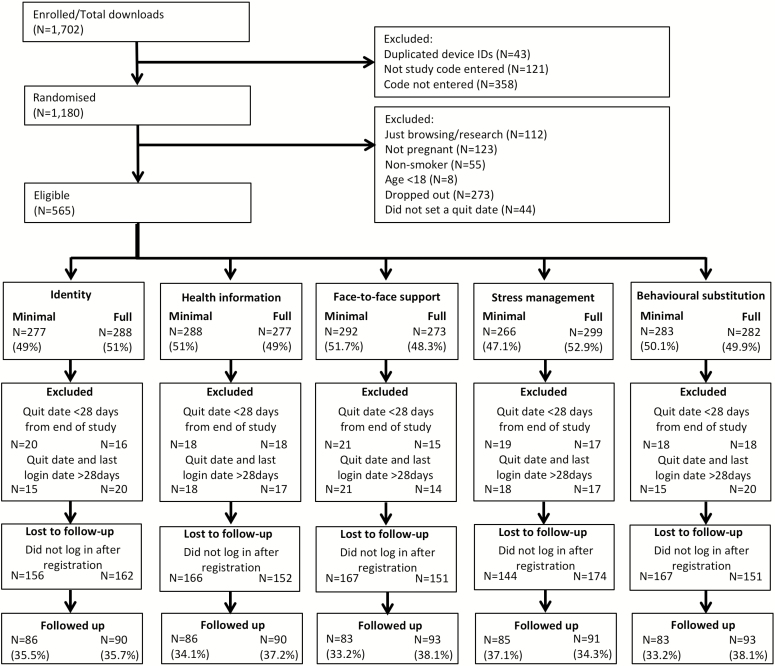
Participant flow is reported in Fig. 1. Of 1,702 downloads, 565 people (33.2%) met the eligibility criteria. The uptake of the app by eligible participants was a mean of 22.6 people per month (SD = 12.8). Of eligible participants, those who set a quit date more than a month prior to October 31, 2016 and engaged with the app up to 28 days from the quit date were included in the primary analysis (N = 494; 87.4%). The minimum requirement for engagement was the completion of registration. Of these, 318 (64.4%) did not respond to any follow-up questions regarding their smoking status (308 participants used the app only on the day of registration and 10 did not log in after the quit date).
Fig. 1.
Participant flow.
Baseline characteristics are reported in Table 1. Sixty-three per cent used an iOS device, 51 per cent were from the UK, 30.4 per cent had nonmanual occupation, 57.5 per cent were in the first trimester of pregnancy, 38.8 per cent used only SmokeFree Baby to aid cessation, and 72 per cent wanted to stop smoking completely. There were no statistically significant differences between the minimal and full versions of the modules in terms of participants’ baseline characteristics.
Participants’ engagement with the app
Participants engaged with the app for a mean period of 4.5 days (SD = 8.5; from registration until last login) and logged in a mean of 2.9 times (SD = 3.1) with 62.3 per cent (N = 308) logged in once or twice and 29.0 per cent (N = 143) logged in three to five times. Main effects of, and interactions between, modules on duration and frequency of engagement, respectively, are reported in Supplementary Tables 2 and 3. The full health information module had a statistically significant main effect on duration of engagement (F = 5.018; p = .026). The interaction between face-to-face support × behavioral substitution (F = 4.170; p = .042) was also statistically significant; the full version of both modules yielded longer engagement than other conditions. There were no statistically significant main effects on frequency of engagement, but the interaction between identity × behavioral substitution was statistically significant (F = 4.882; p = .028); those who received the full version of the modules logged in more frequently.
Participants’ active engagement with general app components is reported in Table 2. Five general app features (“How addicted are you?,” “Reasons to quit”—1 and 2, “Getting ready,” “Withdrawal symptoms”) were available after registration. Thirty-six per cent (N = 178) of all participants engaged with at least one of these (Mean = 0.6; SD = 1.1). A further four general app features (“Medicine,” “Phone support,” “Video memos,” “Social”) were available after the quit date, and 55.1 per cent (N = 272) engaged with at least one of these (Mean = 0.7; SD = 0.8).
Table 2.
Active engagement with general app features that were aimed at all participants
| General app feature | Active engagement type | N | Engaged with the feature at least once: % (N) |
|---|---|---|---|
| “How addicted are you?”a | Completing a two-item quiz as per the Heaviness of Smoking Index | 494 | 16.6 (82) |
| “Reasons to quit” – 1a | Listing why the person wants to quit smoking | 494 | 7.5 (37) |
| “Reasons to quit” – 2a | Indicating personal relevance of pre-defined reasons to quit | 494 | 4.9 (24) |
| “Getting ready”a | Indicating if pre-defined activities to prepare for the quit attempt have been completed | 494 | 10.1 (50) |
| “Withdrawal symptoms”a | Rating usefulness of tips to cope with withdrawal symptoms | 494 | 25.1 (124) |
| “Medicine”b | Indicating interest in trying out a nicotine replacement product c | 494 | 7.7 (38) |
| “Phone support”b | Adding contact details of people to get instant support | 494 | 1.2 (6) |
| “Video memos”b | Recording supportive video messages from friends and family and/or recording personal commitment to quitting smoking | 494 | 30.6 (151) |
| “Social”b | Rating usefulness of tips to cope with social situations and advice on using social support | 494 | 31.2 (154) |
aAvailable from registration.
bAvailable after quit date.
cIncluding nicotine gum, patch, lozenge, nasal spray, mouth spray, inhaler, and microtab.
Participants’ active engagement with the full version of the modules is reported in Table 3. Of those who received the full version, 25.8 per cent (N = 65) actively engaged with at least one of the four interactive content features in the identity module, no participant engaged with the “Tip of the day” in the health information module, 19.3 per cent (N = 47) engaged with at least one of the three interactive content features in the face-to-face module, 7.9 per cent (N = 21) engaged with at least one of the two interactive content features in the stress management module, and 2.9 per cent (N = 7) engaged with at least one of the two interactive content features in the behavioral substitution module.
Table 3.
Active engagement with interactive content in the full version of the modules
| Module | Interactive content | Active engagement type | N | Engaged with the feature at least once: % (N) |
|---|---|---|---|---|
| Identity | “I am…” | Endorsing statements about a new non-smoker identity | 252 | 16.7 (42) |
| Identity | “Video diary” | Recording progress with cessation and pregnancy | 252 | 2.0 (5) |
| Identity | “Ex-smokers” | Rating usefulness of videos of ex-smokers talking about their experiences with quitting | 252 | 4.0 (10) |
| Identity | “Tip of the day” | Rating usefulness of tips to establish a positive non-smoker identity | 252 | 15.1 (38) |
| Health information | “Tip of the day” | Rating usefulness of advice about the effects of smoking and cessation | 242 | 0 |
| Face-to-face support | “Tip of the day” | Rating usefulness of tips to engage with face- to-face support | 244 | 16.0 (39) |
| Face-to-face support | “Pro advice” | Rating usefulness of videos of stop smoking advisors talking about what face-to-face support involves | 244 | 3.3 (8) |
| Face-to-face support | “Local services” | Clicking on phone numbers/links to websites of local stop smoking services | 244 | 6.6 (16) |
| Stress | “Stress management tips” | Rating usefulness of tips to cope with stress | 265 | 6.0 (16) |
| Stress | “Stress plan” | Selecting strategies to plan how to cope with stress | 265 | 6.0 (16) |
| Behavioral substitution | “Behavioral substitution tips” | Rating usefulness of tips to distract oneself from smoking | 244 | 2.5 (6) |
| Behavioral substitution | “Distraction plan” | Selecting strategies to plan how to distract oneself from smoking | 244 | 2.9 (7) |
Effects of modules on smoking abstinence
Main effects of, and two-way interactions between, modules are reported in Table 4. Since main effects of the modules on smoking abstinence were not statistically significant, Bayes factors were calculated. Bayes factors suggested that the findings were either inconclusive (as to whether the differences were present) or supported the null hypothesis. The interaction between identity × behavioral substitution was statistically significant (F = 6.368; p = .012); those who received the full version of the modules attained more smoke-free days. In the sensitivity analysis, when only those were included who logged in at least once after the quit date (N = 176), the pattern of results remained the same (Supplementary Table 4).
Table 4.
Main effects and interactions between modules on the number of smoke-free days up to 4 weeks from quit date (N = 494)
| Factor | Level | Smoke-free days: Mean (SD) | F-ratio | df | p-value | Partial eta2 | Bayes factor | |
|---|---|---|---|---|---|---|---|---|
| 0.6 day | 1.6 days | |||||||
| Identity | Minimal | 0.40 (1.9) | 0.077 | 1 | .782 | <.000 | 0.36 | 0.14 |
| Full | 0.45 (1.9) | |||||||
| Health information | Minimal | 0.43 (2.0) | 0.014 | 1 | .905 | <.000 | 0.25 | 0.10 |
| Full | 0.41 (1.9) | |||||||
| Face-to-face support | Minimal | 0.50 (1.9) | 0.719 | 1 | .397 | .002 | 0.17 | 0.07 |
| Full | 0.35 (1.9) | |||||||
| Stress management | Minimal | 0.28 (1.9) | 2.668 | 1 | .103 | .006 | 1.81 | 0.77 |
| Full | 0.56 (1.9) | |||||||
| Behavioral substitution | Minimal | 0.43 (1.9) | 0.005 | 1 | .945 | <.000 | 0.22 | 0.08 |
| Full | 0.42 (2.0) | |||||||
| Identity × Health information | 1.019 | 1 | .313 | .002 | ||||
| Identity × Face-to-face support | 0.008 | 1 | .930 | <.000 | ||||
| Identity × Stress management | 1.089 | 1 | .297 | .002 | ||||
| Identity × Behavioral substitution | 6.368 | 1 | .012 | .014 | ||||
| Health information × Face-to- face support | 0.000 | 1 | .987 | <.000 | ||||
| Health information × Stress management | 1.310 | 1 | .253 | .003 | ||||
| Health information × Behavioral substitution | 0.627 | 1 | .429 | .001 | ||||
| Face-to-face support × Stress management | 1.349 | 1 | .246 | .003 | ||||
| Face-to-face support × Behavioral substitution | 0.012 | 1 | .911 | <.000 | ||||
| Stress management × Behavioral substitution | 0.045 | 1 | .832 | <.000 |
Bold values indicate p < .05.
DISCUSSION
Only a small proportion of pregnant smokers used the app following registration, responded to in-app follow-up questions, and engaged actively with the intervention content. The factorial screening experiment found that from the identity, health information, face-to-face support, stress management, and behavioral substitution modules none had a statistically significant main effect on smoking abstinence.
Low engagement is one of the main challenges of DBCIs [19] even when they are developed according to best practice [22, 30]. SmokeFree Baby was developed by systematically selecting BCTs using a rigorous methodology, including drawing on theory and evidence from the scientific literature [24]. Experts also rated the app as high quality based on its engagement, functionality, aesthetics, information, subjective qualities, and adherence to smoking cessation treatment guidelines [36]. Although participants were asked to complete a background questionnaire after downloading the app, only a minority (4.9%) of participants, who would have otherwise been eligible, disengaged prior to setting a quit date. Therefore, the process of registration prior to accessing the intervention itself does not appear to drive substantial disengagement.
Nevertheless, pregnant smokers, who met all inclusion criteria, engaged with SmokeFree Baby to a lesser extent than previously found in generic smoking cessation apps [37] and pregnancy-orientated smoking cessation text-messages [38]. Compared with a pre-/post-natal health-related app that did not specifically target smoking or pregnant smokers [39], engagement with SmokeFree Baby appears to be low. In high income countries, the vast majority (85%–94%) of adults (including women of childbearing age) own a smartphone with internet access [40, 41], and health-related apps are widely used among pregnant women [42, 43]. However, evidence regarding the use of smartphones and engagement with health-related apps specifically among pregnant smokers is scarce; it is therefore difficult to draw conclusions about the relative app usage and engagement in this sample. In terms of active engagement with SmokeFree Baby, it was also low overall, although some of the app features that provided practical tips to cope with social situations and withdrawal appeared to be more engaging than other features.
We did not have a priori hypotheses for interactions between modules and thus inferences from these effects should be regarded as tentative. The combined use of the full version of identity (aimed at prompting positive self-labels, self-images, and self-thoughts as a nonsmoker) and behavioral substitution modules (aimed at providing distraction from urges to smoke) yielded a small but statistically significant effect on smoking abstinence. This is in line with integrative behavior change theories proposing that reflective and automatic motivation interact in driving behavior [44–46]. For example, PRIME theory [44] suggests that identity (e.g., reflective motivation to become a nonsmoker mum) and impulses (e.g., automatic motivation to have a cigarette in response to smoking cues) are important sources of wants and needs, the strongest of which will drive behavior at any relevant moment. Acting in line with self-conscious intentions in the face of conflicting impulses requires self-regulation. Identity can strengthen self-regulation, but because resisting conflicting impulses is likely to be mentally effortful, distraction strategies such as behavioral substitution may help by saving the person’s mental resources. Although the interactive effect between identity and behavioral substitution warrants further research, another DBCI with better participant engagement should be used for testing this association further.
In terms of practical implications, our formative work involving qualitative studies (e.g., using think-aloud methodology) provided useful insights as to what potential users want and how they may interact with the app. However, these studies had small samples, were conducted in laboratory settings with potentially more motivated participants who also received incentives (e.g., vouchers) for taking part in the study. Therefore, engagement also needs to be tested in real-world settings from the early phases of intervention development. A potentially useful way of doing this is to draw on principles from agile methodology [47], where intervention modules are delivered and tested iteratively (e.g., with concurrent or sequential A-B testing) over short periods of time (usually within weeks). This approach might have been adopted in this study and the app revised until engagement was sufficient.
However, it may be that apps are not suitable for reaching and engaging pregnant smokers with smoking cessation, even if procedures for conducting formative work are improved. This may be because engaging with apps may be more effortful (e.g., they require users to log in and follow through a program) which requires higher motivation, as opposed to, for example, text-messages that are more difficult to ignore when received and thus easier to engage with. There may be issues around app literacy in that if apps are too complex or have too many components, it may be difficult for pregnant smokers to understand and engage with the content. It is also possible that pregnancy-specific smoking cessation apps might need to be integrated with face-to-face support in order to engage this population with digital support.
Engagement with the app plummeted within days of registration, despite various strategies in place to boost engagement. This included sending push notifications on three consecutive days after registration and then once a week for three consecutive weeks. Participants were also prompted (both by push notifications and in-app notifications) to view the new content that was released every day, and the content was presented in various modalities (e.g., videos, quizzes, and simple text). However, push notifications could have been turned off, and the in-app notifications could have remained unnoticed if participants did not log in. The low levels of active engagement with various app contents suggest that participants were not particularly interested in and did not particularly enjoy the content. Potential strategies to boost engagement with apps in the future may include multiple sources of health messaging and tailored provision of specific app components so that participants are only exposed to content that are most relevant and helpful for them.
One of the limitations of the study was that participants were screened against eligibility criteria after randomization, and there was a slight imbalance in sample size, reducing power to detect differences between modules. Bayes factors were calculated that supported the null hypothesis, except for the stress management module where it suggested that the data were inconclusive to detect an effect. Another limitation was high attrition and consequently low response rate to daily follow-ups on the primary outcome. Although there was no biochemical validation of smoking abstinence, data on self-reported smoking status were collected automatically with no contact from the researchers and no material rewards were given for participants for being abstinent; therefore, it is unlikely that smoking status would have been misreported. Although understanding the experiential and behavioral facets of engagement with DBCIs is important [21], we were not able to investigate this with the data collected in this study. Further research is needed to be able to disentangle factors related to engagement and effectiveness, and to test strategies to improve engagement with the app. The latter can lead to a better evaluation of the effects of intervention components.
Findings from this study do not support the effectiveness of individual modules in the SmokeFree Baby app to increase smoking abstinence during pregnancy. Pregnant smokers do not appear to engage with the intervention which is a key issue to be addressed in the future because, until satisfactory engagement with the intervention is achieved, it is not possible to test the effects of modules.
Supplementary Material
Acknowledgments
The authors are members of the UK Centre for Tobacco and Alcohol Control Studies. We are grateful for all our funders. We thank Hilary Wareing, and the Tommy’s and Best Beginning charities for their help with the recruitment. R. West’s, J. Brown’s, E. Beard’s, and I. Tombor’s salary is funded by a program grant from Cancer Research UK (CRUK; C1417/A22962). E. Beard also receives funding from the National Institute for Health Research School for Public Health Research. The Public Management Associates provided funding for the app promotion. The funders had no role in the study design, data collection, analysis, interpretation of data, writing of the manuscript and the decision to submit this manuscript for publication.
Compliance with Ethical Standards
Conflict of Interest: The authors Ildiko Tombor and Susan Michie have no competing interests. The authors Emma Beard, Jamie Brown, and Lion Shahab have received unrestricted research funding from Pfizer, a manufacturer of smoking cessation medications. Lion Shahab has received a research grant and honoraria for a talk and travel expenses from Pfizer. Robert West has received travel funds and hospitality from, and undertaken research and consultancy for, pharmaceutical companies that manufacture and/or research products aimed at helping smokers to stop.
Authors’ Contributions: The authors have full control of all primary data and allow the journal to review it if requested. This manuscript is not being simultaneously submitted elsewhere and the data/views have not been previously published.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethical approval for this study was received from the UCL Psychology and Language Sciences Departmental Ethics Committee (Project ID: CEHP/2013/508). This article does not contain any studies with animals performed by any of the authors.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
References
- 1. Abroms LC, Padmanabhan N, Thaweethai L, Phillips T. iPhone apps for smoking cessation: a content analysis. Am J Prev Med. 2011;40(3):279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abroms LC, Lee Westmaas J, Bontemps-Jones J, Ramani R, Mellerson J. A content analysis of popular smartphone apps for smoking cessation. Am J Prev Med. 2013;45(6):732–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haskins BL, Lesperance D, Gibbons P, Boudreaux ED. A systematic review of smartphone applications for smoking cessation. Transl Behav Med. 2017;7(2):292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bricker JB, Mull KE, Kientz JA, et al. Randomized, controlled pilot trial of a smartphone app for smoking cessation using acceptance and commitment therapy. Drug Alcohol Depend. 2014;143:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buller DB, Borland R, Bettinghaus EP, Shane JH, Zimmerman DE. Randomized trial of a smartphone mobile application compared to text messaging to support smoking cessation. Telemed J E Health. 2014;20(3):206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Regmi K Kassim N, Ahmad N, Tuah NAA. Effectiveness of mobile apps for smoking cessation: a review. Tob Prev Cessation. 2017;3(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Health and Social Care Information Centre. Statistics on Women’s Smoking Status at Time of Delivery, England – Quarter 4, 2014–15. London, UK:NHS Digital: 2015. [Google Scholar]
- 8. Health and Social Care Information Centre. Statistics on NHS Stop Smoking Services in England, April 2014 to March 2015. London, UK: Lifestyle Statistics Team, Health and Social Care Information Centre; 2015, http://www.hscic.gov.uk/catalogue/PUB18002/stat-stop-smok-serv-eng-2015-q4-rep.pdf. Accessibility verified October 12, 2017. [Google Scholar]
- 9. Butterworth SJ Sparkers E, Trout A, Brown K. Pregnant smokers’ perceptions of specialist smoking cessation services. J Smok Cessat. 2014;9(2):85–97. [Google Scholar]
- 10. Abroms LC, Johnson PR, Leavitt LE, et al. A randomized trial of text messaging for smoking cessation in pregnant women. Am J Prev Med. 2017;53(6):781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Naughton F, Cooper S, Foster K, et al. Large multi-centre pilot randomized controlled trial testing a low-cost, tailored, self-help smoking cessation text message intervention for pregnant smokers (MiQuit). Addiction. 2017;112(7):1238–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heminger CL, Schindler-Ruwisch JM, Abroms LC. Smoking cessation support for pregnant women: role of mobile technology. Subst Abuse Rehabil. 2016;7:15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Collins LM, Murphy SA, Strecher V. The multiphase optimization strategy (MOST) and the sequential multiple assignment randomized trial (SMART): new methods for more potent eHealth interventions. Am J Prev Med. 2007;32(5 Suppl):S112–S118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baker TB, Collins LM, Mermelstein R, et al. Enhancing the effectiveness of smoking treatment research: conceptual bases and progress. Addiction. 2016;111(1):107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baker TB. The 2016 ferno award address: three things. Nicotine Tob Res. 2017;19(8):891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wyrick DL, Rulison KL, Fearnow-Kenney M, Milroy JJ, Collins LM. Moving beyond the treatment package approach to developing behavioral interventions: addressing questions that arose during an application of the Multiphase Optimization Strategy (MOST). Transl Behav Med. 2014;4(3):252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garnett C, Crane D, Michie S, West R, Brown J. Evaluating the effectiveness of a smartphone app to reduce excessive alcohol consumption: protocol for a factorial randomised control trial. BMC Public Health. 2016;16:536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yardley L, Spring BJ, Riper H, et al. Understanding and promoting effective engagement with digital behavior change interventions. Am J Prev Med. 2016;51(5):833–842. [DOI] [PubMed] [Google Scholar]
- 19. Michie S, Yardley L, West R, Patrick K, Greaves F. Developing and evaluating digital interventions to promote behavior change in health and health care: recommendations resulting from an international workshop. J Med Internet Res. 2017;19(6):e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yardley L, Choudhury T, Patrick K, Michie S. Current issues and future directions for research into digital behavior change interventions. Am J Prev Med. 2016;51(5):814–815. [DOI] [PubMed] [Google Scholar]
- 21. Perski O Blandford A, West R, Michie, S. Conceptualising engagement with digital behaviour change interventions: a systematic review using principles from critical interpretive synthesis. Transl Behav Med. 2017;7(2):254–267. Published online: December 13, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Michie S, West R.. A Guide to Development and Evaluation of Digital Behaviour Change Interventions in Healthcare. London, UK: Silverback Publishing; 2016. [Google Scholar]
- 23. Tombor I., et al. , Healthcare providers’ views on digital smoking cessation interventions for pregnant women. J Smok Cessation, 2015;10(2):116–123. [Google Scholar]
- 24. Tombor I, Shahab L, Brown J, Crane D, Michie S, West R. Development of SmokeFree Baby: a smoking cessation smartphone app for pregnant smokers. Transl Behav Med. 2016;6(4):533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu J, Tombor I, Shahab L, West R. Usability testing of a smoking cessation smartphone application (‘SmokeFree Baby’): a think-aloud study with pregnant smokers. Digital Health, 2017;3:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tombor I. Development of a Smoking Cessation Smartphone Application for Pregnant Smokers Focusing on the Role of Identity (Doctoral Dissertation) in Department of Epidemiology and Public Health. London, UK: University College London; 2015. [Google Scholar]
- 27. Borland R, Yong HH, O’Connor RJ, Hyland A, Thompson ME. The reliability and predictive validity of the Heaviness of Smoking Index and its two components: findings from the International Tobacco Control Four Country study. Nicotine Tob Res. 2010;12 Suppl 1:S45–S50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yan J, Groothuis PA. Timing of prenatal smoking cessation or reduction and infant birth weight: evidence from the United Kingdom Millennium Cohort Study. Matern Child Health J. 2015;19(3):447–458. [DOI] [PubMed] [Google Scholar]
- 29. England LJ, Kendrick JS, Wilson HG, Merritt RK, Gargiullo PM, Zahniser SC. Effects of smoking reduction during pregnancy on the birth weight of term infants. Am J Epidemiol. 2001;154(8):694–701. [DOI] [PubMed] [Google Scholar]
- 30. Michie S, Atkins L, West R.. The Behaviour Change Wheel: A Guide to Designing Interventions. Great Britain: Silverback Publishing; 2014. [Google Scholar]
- 31. Michie S, Johnston M. Behavior change techniques. In: Turner JR, ed. Encyclopedia of Behavioral Medicine. New York, US: Springer; 2013:182–187. [Google Scholar]
- 32. Michie S, Richardson M, Johnston M, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46(1):81–95. [DOI] [PubMed] [Google Scholar]
- 33. Dienes Z. Using Bayes to get the most out of non-significant results. Front Psychol. 2014;5:781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Beard E, Dienes Z, Muirhead C, West R. Using Bayes factors for testing hypotheses about intervention effectiveness in addictions research. Addiction. 2016;111(12):2230–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jeffreys H. The Theory of Probability, 1st/3rd ed. Oxford, England: Oxford University Press; 1939/1961. [Google Scholar]
- 36. Thornton L, Quinn C, Birrell L, et al. Free smoking cessation mobile apps available in Australia: a quality review and content analysis. Aust N Z J Public Health. 2017;41(6):625–630. [DOI] [PubMed] [Google Scholar]
- 37. Ubhi HK, Michie S, Kotz D, Wong WC, West R. A mobile app to aid smoking cessation: preliminary evaluation of SmokeFree28. J Med Internet Res. 2015;17(1):e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Naughton F, Riaz M, Sutton S. Response parameters for SMS text message assessments among pregnant and general smokers participating in SMS cessation trials. Nicotine Tob Res. 2016;18(5):1210–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cooper S. App Pilot Evaluation Report: National in-App Data and in-App Data From Guys and St Thomas’ and Blackpool. London, UK: Best Beginnings; 2015. [Google Scholar]
- 40. Consultancy.uk. UK Smartphone Penetration Continues to Rise to 85% of Adult Population 2017. Available at https://www.consultancy.uk/news/14113/uk-smartphone-penetration-continues-to-rise-to-85-of-adult-population. Accessibility verified February 12, 2018.
- 41. Centraal Bureau voor de Statistiek. The Netherlands Internet Access, Use and Facilities. 2017. Available at https://www.cbs.nl/en-gb/news/2018/05/the-netherlands-leads-europe-in-internet-access. Accessibility verified May 10, 2018. [Google Scholar]
- 42. Lupton D, Pedersen S. An Australian survey of women’s use of pregnancy and parenting apps. Women Birth. 2016;29(4):368–375. [DOI] [PubMed] [Google Scholar]
- 43. Wallwiener S, Müller M, Doster A, et al. Pregnancy eHealth and mHealth: user proportions and characteristics of pregnant women using Web-based information sources-a cross-sectional study. Arch Gynecol Obstet. 2016;294(5):937–944. [DOI] [PubMed] [Google Scholar]
- 44. West R, Brown J.. Theory of Addiction. 2nd ed. West Sussex, UK: Wiley Blackwell; 2013. [Google Scholar]
- 45. Michie S West R, Gainforth H, Brown J, Campbell R.. ABC of Behaviour Change Theories Great Britain: Silverback publishing; 2014. [Google Scholar]
- 46. Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hekler EB, Klasnja P, Riley WT, et al. Agile science: creating useful products for behavior change in the real world. Transl Behav Med. 2016;6(2):317–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.