Abstract
Behavioral modification (BM) is a strategy designed to sustain or restore well-being through effects such as enhanced relaxation, reduced stress, and improved sleep. Few studies have explored the role of BM delivered in the context of fitness programs for healthy adults. Thus, the purpose of this investigation was to examine whether BM combined with aerobic and resistance training programs would improve health and fitness measures more than the exercise training alone. Thirty-two healthy fitness club members (19 men) were randomized to receive a BM program (n=15) or an equal-attention (EA) control (n=17). BM consisted of twelve, 10-min education sessions between a trained fitness professional and the participant, coupled with weekly, individualized relaxation, stress reduction, and sleep improvement assignments. All participants engaged in 1 h of coached resistance training and remotely guided aerobic exercise thrice weekly for 12 weeks. Fitness measures (aerobic performance, body composition, muscle strength and endurance, lower-body power), sleep characteristics, and heart rate variability (HRV) were obtained at baseline and after the 12-week program. BM resulted in greater improvements in aerobic performance (increased maximum oxygen uptake, metabolic (lactate) threshold, and percent of maximum oxygen uptake at which metabolic threshold occurred), peak and average lower-body power, and body composition (decreased body fat percentage and fat mass) compared to EA. BM also positively influenced parasympathetic tone through increased High-frequency HRV. BM resulted in greater improvements in fitness measures, body composition, and heart rate variability compared with EA. These findings have intriguing implications regarding the role of BM in augmenting health and physical performance.
Key words: behavioral modification, physical fitness, exercise, maximum oxygen uptake, heart rate variability, sleep intervention
Introduction
Behavioral modification (BM) is a strategy designed to sustain or restore well-being through effects such as enhanced relaxation, reduced stress, and improved sleep. It is often implemented as a primary or adjunct therapy in managing a multitude of disorders ranging from cardiovascular disease 18 to psychiatric illness 41 . Specifically, one of the most common applications of BM is to enhance participation in physical activity and exercise interventions. Over the past decade alone, accumulating evidence suggests that BM improves exercise adherence in patients with obesity 7 , cancer 34 , and heart failure 11 . Although these results show promise for individuals impacted by disease, few studies exist that examine the potential role of BM in the healthy, active individual. While promoting exercise adherence in athletes is advantageous, its potential impact is limited due to the high baseline adherence rate in this population. Instead, BM may offer improved physiological benefits of training secondary to stress reduction and enhanced sleep.
Sleep deprivation and sleep disorders affect 50–70 million Americans and result in billions of dollars in medical costs annually 25 , yet sleep health is frequently overlooked as part of maintaining wellness. Poor sleep adversely affects physiological functioning, causing decreased autonomic tone, increased blood pressure, and deleterious effects on inflammation and hormone balance, even in healthy adults without medical comorbidities 37 45 . Despite clear evidence that poor or insufficient sleep adversely impacts health, access to high quality behavioral interventions outside of medical settings remains rare and has not been formally studied.
Physical activity itself is known to improve sleep quality, total sleep time, and decreased sleep onset latency 27 . In contrast, psychological stress has been shown to impede individuals’ capability to exercise at optimal levels 44 . Thus, implementation of BM into an exercise training program could be an important component of both health and performance optimization. Studies of athletes suggest that working to improve sleep habits can increase total sleep time 16 31 46 and may improve performance, however, studies have been small and to our knowledge, controlled trials of these behavioral approaches have not been conducted.
This study investigated, using a randomized controlled study design, the potential benefits to fitness club members of incorporating BM in the form of a novel, multicomponent intervention, delivered by fitness professionals with specialized training in behavior change theory as well as in aerobic and resistance training. We hypothesized that participants who received BM in addition to an exercise training program would demonstrate greater improvements in fitness and favorable changes in heart rate variability (HRV) than those who engaged in the exercise training program alone.
Methods
Participants
We recruited club members meeting the following inclusion criteria: (i) apparently healthy men and women, (ii) 22–44 years of age; (iii) history of exercising 4–8 days per month at the fitness club over the previous three months. In total, 43 members were enrolled into the study. All volunteers completed a pre-participation physical activity readiness questionnaire (PAR-Q) 1 and an exercise history questionnaire. Exclusion criteria were unstable cardiovascular, pulmonary, musculoskeletal or metabolic disorders that would preclude high intensity exercise training as well as anabolic drug use in the past 6 months. All participants were determined to be low risk for sleep apnea as measured by the Berlin Sleep Apnea Questionnaire 39 . Five participants failed to complete baseline assessments, resulting in a total of 38 randomized participants (22 men; age: 33.0±5.4 years; height: 1.74±0.8 m). Randomization was 1:1 (19 per group) following CONSORT guidelines ( Fig. 1 ). The UCLA Institutional Review Board reviewed and approved the study and all participants gave their written informed consent. This study was conducted according to international standards for sport and exercise science research 22 .
Fig. 1.
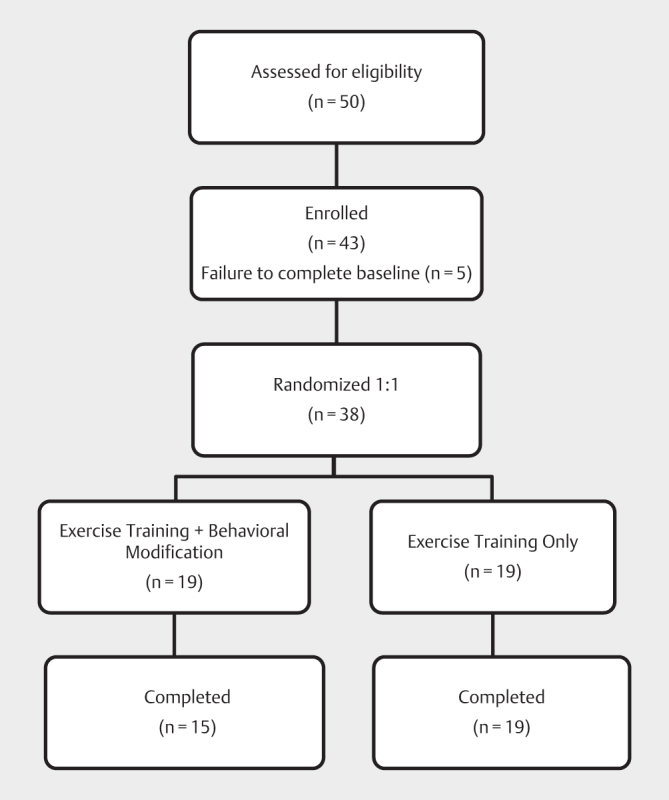
CONSORT diagram showing participant flow through the study.
Study design
All participants received a 12-week evidence-based, remotely guided aerobic exercise program 15 and a recently validated, periodized functional resistance training regimen 43 in combination with either the behavioral modification intervention (BM group) or a non-directive, equal-attention wellness education program (EA group). Training sessions were 3 times weekly (for a total of 36 sessions) and participants were asked to refrain from additional vigorous activity. Both groups received basic nutritional information via weekly emails (Precision Nutrition, Inc., Toronto, Canada); however, dietary intake and macronutrient portions were not assessed or controlled in either group.
Interventions
Certified personal trainers conducted the BM and EA interventions and coached all participants through the resistance training portions of the exercise program.
Intervention group: Behavioral Modification (BM)
The weekly BM intervention comprised twelve, 10 min interactive slide presentations (PowerPoint; Microsoft Corporation, Redmond, WA), each covering a different topic related to enhancing relaxation, reducing stress, or improving sleep, coupled with individualized recommendations for implementation ( Table 1 ). Between sessions, participants tracked progress in a paper-and-pencil sleep diary, which was reviewed weekly by the trainer. The BM intervention content was centered on education commonly provided to individuals with behaviorally induced insufficient sleep syndrome, including sleep education 6 , 35 plus components of motivational interviewing 33 , to encourage participants to improve healthy sleep habits (e. g., avoiding sleep deprivation during the work week), problem solving around challenges to adherence, and stress management techniques such as relaxation. Coaches had a weekly consultation call with an experienced clinical sleep psychologist (JLM), who reviewed recommendations and provided guidance in facilitating client motivation to implement strategies for enhanced relaxation, reduced stress, and improved sleep.
Table 1 Topics and information provided at each session of the behavioral modification (BM) intervention.
| Week | Material Covered | Activities | Homework |
|---|---|---|---|
| 1 | Topic: Welcome to the SCORE study | ||
|
|
|
|
| 2 | Topic: Introduction to relaxation and healthy sleep habits | ||
|
|
|
|
| 3 | Topic: Biological Sleep Need: Part 1 | ||
|
|
|
|
| 4 | Topic: Biological Sleep Need: Part II | ||
|
|
|
|
| 5 | Topic: The Circadian Clock | ||
|
|
|
|
| 6 | Topic: Resetting the circadian clock | ||
|
|
|
|
| 7 | Topic: Travel and Jet Lag | ||
|
|
|
|
| 8 | Topic: Summary and review of progress | ||
|
|
|
|
| 9 | Topic: Stress impairs sleep and well-being | ||
|
|
|
|
| 10 | Topic: Stress Reduction - Putting the mind to rest | ||
|
|
Action plan: sleep hygiene+sleep schedule+bedtime routine+mindfulness Daily sleep diary | |
| 11 | Topic: Relaxation - Separating from thoughts | ||
| Techniques for relaxationObstacles to long-term prioritization of sleep | Thoughts as leaves on a stream |
|
|
| 12 | Topic: Surfing the sleep wave | ||
|
|
|
|
Control group: Equal Attention (EA)
Participants randomized to the EA group received equal time periods of attention with their assigned trainer viewing 10 min slide presentations covering generalized healthy lifestyle advice absent of any specific guidance related to relaxation, stress reduction or sleep. The EA intervention included the following topics: healthy relationships, brain power, movement therapy (ergonomics), general health screening, environmental health, cancer screening, tobacco/nicotine, time management, basic hygiene practices, and preventable diseases/immunizations.
Exercise training
Supervised, periodized, functional resistance training
Resistance training was provided to all participants using a previously validated, evidence-based functional resistance training program 43 . The training regimen consisted of a 3-cycle, nonlinear program in which acute program variables including exercise selection, volume, and intensity were varied over both the 4-week mesocycles and within the weekly microcycles. The volume and/or intensities of each training session were categorized as high (H), moderate (M), or low (L) and applied to both upper- and lower-body exercises on a given day during the course of each week of training. Individual sessions lasted approximately 20–25 min.
Remotely-guided aerobic exercise training
Aerobic training was performed on treadmill ergometers and each session lasted 30 min. Heart rate was recorded using a physiological status monitor affixed to a chest strap (BioHarness-3™; Zephyr Technologies, Annapolis, MD, USA) paired via Bluetooth to a smartphone. Individualized target heart rate zones (THRZ) were displayed and at the end of each training session, the data was uploaded remotely to a storage server. Prior to the next session, study personnel reviewed the data via the UCLA Exercise Physiology Research Laboratory Digital Health Network (DHN) – a secured, encrypted web portal used to gather and analyze participant data 5 – and adjusted exercise parameters for future sessions accordingly. The remote-guidance process, outlined in more detail in a previous publication 15 , was used to define individualized program progression without requiring a dedicated strength coach to supervise each session. The automated system enabled aerobic training zones to be defined for each participant based on the physiological events underlying the incremental exercise response. These zones were presented in terms of lower- and upper-limit heart rates at the ventilatory and metabolic thresholds, Respectively. Individualized exercise prescription for all subsequent treadmill training sessions. The target heart rate zones were adjusted during the study to account for progression of training effects.
Outcome measures
All outcomes were measured at baseline and at completion of the 12-week intervention.
Fitness measures
Aerobic performance
Aerobic capacity (VO 2 max), the metabolic threshold (lactate threshold determined by gas exchange, VO 2 θ) and the ventilatory threshold (the onset of hyperventilation in response to metabolic acidosis, VCO 2 θ) were measured from an incremental, symptom-limited maximal treadmill exercise test using a validated portable metabolic measurement system (Oxycon™ Mobile; CareFusion, Yorba Linda, CA, USA) and standardized approach 14 .
Muscle strength and endurance
Upper- and lower-body isotonic muscle strength was measured by determining 1-repetition maximum (1-RM) on a seated chest press (Technogym, Cesena, Italy) and a seated leg press (Eagle NX; Cybex International, Medway, MA, USA) using the procedure described by the National Strength and Conditioning Association 3 . After 10 min of rest, muscle endurance was measured as the number of repetitions to failure using 85% of 1-RM values.
Lower-body power
Participants stood on a previously validated electronic jump mat (Just Jump; Probotics, Inc., Huntsville, AL, USA) 28 with feet hip-width apart and then performed a countermovement jump for maximal height. Three trials were given with 20–30 s rest between each attempt. The best trial was used to calculate peak and average lower-body power using published equations that require vertical jump height and body mass 21 .
Body composition
Percentage body fat (BF%) was measured using a recently validated 13 octipolar, multi-frequency, multi-segmental bioelectrical impedance device (R20; InBody Co., Seoul, South Korea). Because hydration state has a marked influence on bioelectrical impedance analysis (BIA) results, participants were instructed to remain hydrated and not exercise during the 2 h period before testing. Data were collected after at least 3 h of fasting and voiding.
Sleep measures
All participants completed a battery of sleep questionnaires using the DHN. Questionnaires included the Pittsburgh Sleep Quality Index (PSQI) 9 to measure general sleep quality, the Sleep Hygiene Index (SHI) 32 to assess sleep hygiene behaviors, and the Morningness-Eveningness Questionnaire (MEQ) 23 to determine chronotype tendency and circadian phase preference. The information was used both to measure outcomes and to inform individualization of the sleep coaching intervention recommendations. Participants were also asked to complete a daily sleep diary, which was used to compute average time in bed (TIB), total sleep time (TST), and sleep efficiency (SE) calculated as TST/TIB. The sleep diary, which was completed electronically on a smartphone-type device and uploaded to the DHN, was based on that described by Carney et al. 10 and included items to assess bedtime at night, time to fall asleep, time awake at night, final morning wake time and morning rise time.
Heart rate variability
Autonomic balance, which is thought to reflect stress, was evaluated using heart rate variability (HRV) derived from a 5 min, resting single-channel electrocardiogram chest strap biosensor (Zephyr Bioharness) obtained with participants seated in a darkened, quiet room. Time domain indices (ms) included standard deviation of normal-to-normal intervals (SDNN), and root mean square differences of the standard deviation (RMSSD). Frequency domain analysis included low-frequency (LF) component (frequency range 0.04–0.15 Hz) and high-frequency (HF) component (frequency range 0.15–0.4 Hz), in absolute units (ms 2 ), and the low-frequency to high-frequency ratio (LF/HF). Elaborated methodology is presented in Supplemental Digital Content.
Statistical analysis
We determined from pilot testing, and allowing for 15% missing data, that a sample size of 44 participants would be sufficient to assess changes in fitness outcomes based on α=0.05 and β=0.20. All data were exported to IBM SPSS Statistics for Windows, version 24 (IBM Corp., Armonk, N.Y., USA) for analysis. Descriptive statistics are presented as mean (SD). Grubbs’ test was employed to detect potential outliers and none were found. Prior to comparisons, all variables were assessed for normality via Shapiro-Wilk tests. Within group comparisons at baseline and after 12 weeks were made by paired t -tests and Wilcoxon signed-rank tests for normally and non-normally distributed variables, respectively. Changes between groups were analyzed by Welch’s independent t -tests (normal) or Mann-Whitney U tests (non-normal). Statistical significance was determined by α=0.05 and all tests were two-tailed. Given that this is one of the first randomized trials testing a behavioral modification model, we did not employ strict type 1 error control; however, we limited the number of main outcome measures and based our interpretation on the pattern of results seen for each domain rather than on individual statistical tests.
Results
Thirty-eight participants completed the 12-week training program (36 aerobic exercise and resistance sessions) without injuries or serious adverse events, although three participants required an additional week to complete the program due to minor illness or vacation. Due to scheduling conflicts, six participants were not able to return for 12-week follow-up testing, therefore 32 participants (15 receiving BM and 17 receiving EA) were included in the final analysis of fitness measures ( Table 2 ). HRV data for one additional participant was corrupted during recording and was, therefore, not included in the analysis ( Table 3 ). Eleven participants did not perform 12-week follow-up sleep measurements ( Table 3 ). Sleep diary data were available for 19 participants. The losses to follow-up did not result in uneven group size for final analyses.
Table 2 Fitness measures at baseline and after 12 weeks for the equal-attention control and behavioral modification groups.
| Fitness Measures | Equal-Attention (n=17; 10 males) | Behavioral Modification (n=15; 9 males) | P-between | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 Weeks† | Change | P-within | Baseline | 12 Weeks‡ | Change | P-within | ||
| Age | 33.3 (6.3) | - | - | - | 32.7 (4.7) | - | - | - | 0.748 |
| Body weight (kg) | 72.7 (12.6) | 72.5 (12.0) | −0.3 (1.8) | 0.530 | 74.5 (11.6) | 74.1 (10.5) | −0.4 (2.7) | 0.538 | 0.859 |
| BMI (kg/m 2 ) | 23.8 (2.6) | 23.7 (2.3) | −0.1 (0.6) | 0.577 | 25.0 (3.1) | 24.9 (2.9) | −0.1 (0.9) | 0.630 | 0.922 |
| Body fat (%) | 20.1 (7.1) | 18.6 (6.8) | −1.4 (1.9) | 0.006 | 22.1 (6.5) | 18.5 (6.5) | −3.6 (2.6) | <0.001 | 0.011 |
| Fat mass (kg) | 14.6 (5.7) | 13.3 (5.1) | −1.3 (1.7) | 0.006 | 16.5 (5.7) | 13.8 (5.6) | −2.7 (1.7) | <0.001 | 0.021 |
| Fat-free mass (kg) | 58.3 (12.1) | 59.2 (12.1) | 0.9 (1.9) | 0.083 | 58.1 (10.5) | 60.4 (9.4) | 2.3 (3.4) | 0.021 | 0.165 |
| CP 1-RM (kg) | 62.1 (26.5) | 73.8 (28.9) | 11.6 (9.7) | <0.001 | 58.9 (25.4) | 75.0 (31.0) | 16.1 (16.3) | 0.002 | 0.551 |
| LP 1-RM (kg) | 92.4 (33.4) | 120.7 (36.2) | 28.3 (14.5) | <0.001 | 104.8 (39.3) | 134.2 (54.4) | 29.5 (20.4) | 0.001 | 0.766 |
| CP 85% 1-RM (kg) | 52.8 (22.5) | 62.7 (24.6) | 9.9 (8.3) | <0.001 | 50.0 (21.6) | 63.7 (26.4) | 13.7 (13.8) | 0.002 | 0.551 |
| CP 85% 1-RM (reps) | 5.2 (1.9) | 7.1 (2.0) | 1.9 (2.6) | 0.008 | 5.0 (1.4) | 7.9 (1.9) | 2.9 (1.7) | 0.001 | 0.207 |
| LP 85% 1-RM (kg) | 78.5 (28.4) | 102.6 (30.8) | 24.0 (12.3) | <0.001 | 89.1 (33.4) | 114.1 (46.2) | 25.0 (17.3) | 0.001 | 0.766 |
| LP 85% 1-RM (reps) | 7.2 (2.6) | 9.2 (4.1) | 1.9 (3.7) | 0.067 | 7.5 (3.9) | 8.6 (2.3) | 1.1 (4.0) | 0.076 | 0.823 |
| Leg power peak (W) | 7861 (349) | 8161 (471) | 300 (208) | <0.001 | 7760 (339) | 8283 (374) | 523 (190) | <0.001 | 0.006 |
| Leg power avg (W) | 1597 (145) | 1733 (126) | 137 (58) | <0.001 | 1534 (138) | 1746 (167) | 212 (79) | <0.001 | 0.005 |
| VO 2 max (L/min) | 3.06 (0.64) | 3.29 (0.65) | 0.23 (0.08) | <0.001 | 3.13 (0.53) | 3.57 (0.51) | 0.44 (0.12) | <0.001 | <0.001 |
| rVO 2 max (mL/min/kg) | 42.1 (5.1) | 45.4 (4.9) | 3.3 (1.0) | <0.001 | 42.6 (7.9) | 48.7 (7.1) | 6.1 (2.1) | <0.001 | <0.001 |
| VO 2 θ (L/min) | 2.03 (0.39) | 2.38 (0.42) | 0.34 (0.13) | <0.001 | 2.12 (0.32) | 2.76 (0.37) | 0.64 (0.13) | <0.001 | <0.001 |
| VO 2 θ/VO 2 max (%) | 67 (5) | 73 (6) | 6 (3) | <0.001 | 68 (6) | 77 (5) | 9 (3) | <0.001 | 0.009 |
| VCO 2 θ (L/min) | 2.79 (0.54) | 3.01 (0.59) | 0.22 (0.10) | <0.001 | 2.90 (0.53) | 3.26 (0.50) | 0.36 (0.13) | <0.001 | 0.098 |
Values are mean (SD). No significant differences were observed between groups at baseline. VO 2 max=maximum oxygen uptake; rVO 2 max=maximum oxygen uptake normalized by body mass; VO 2 θ=oxygen uptake at metabolic threshold; VO 2 θ/VO 2 max=metabolic threshold as a percent of maximum oxygen uptake; VCO 2 θ=oxygen uptake at ventilatory threshold; BMI=body mass index; CP=chest press; 1-RM=1-repetition maximum; LP=leg press
Table 3 Sleep and heart rate variability measures at baseline and after 12 weeks for the equal-attention control and behavioral modification groups.
| Sleep Measures | Equal-Attention (n=14) | Behavioral Modification (n=13) | P-between | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 Weeks† | Change | P-within | Baseline | 12 Weeks‡ | Change | P-within | ||
| PSQI | 4.9 (3.0) | 3.1 (1.5) | −1.7 (3.1) | 0.049 | 4.5 (2.7) | 3.0 (1.3) | −1.5 (2.9) | 0.063 | 0.830 |
| MEQ | 39.0 (8.1) | 40.4 (8.1) | 1.4 (2.7) | 0.070 | 37.2 (6.8) | 38.9 (5.3)† | 1.8 (2.7) | 0.044 | 0.322 |
| SHI | 17.1 (5.5) | 16.5 (4.2) | −0.6 (4.2) | 0.577 | 17.2 (4.5) | 15.2 (2.6) | −2.0 (4.3) | 0.121 | 0.416 |
| TST (hrs) | 6.5 (0.7)‡ | 6.9 (0.6)‡ | 0.4 (0.7) | 0.119 | 6.7 (0.6)# | 6.9 (1.0)# | 0.2 (1.1) | 0.237 | 0.898 |
| TIB (hrs) | 7.3 (0.8)‡ | 7.4 (0.8)‡ | 0.1 (0.7) | 0.619 | 7.6 (0.4)# | 7.7 (1.0)# | −0.1 (1.1) | 0.604 | 0.816 |
| SE (%) | 88.7 (5.9)‡ | 92.6 (4.7)‡ | 3.9 (4.3) | 0.015 | 88.6 (7.1)# | 89.8 (7.2)# | 1.2 (8.0) | 0.866 | 0.368 |
| HRV Measures | Equal-Attention (n=16) | Behavioral Modification (n=15) | P-between | ||||||
| Baseline | 12 Weeks† | Change | P-within | Baseline | 12 Weeks‡ | Change | P-within | ||
| fC (beats/min)§ | 68.1 (1.9) | 67.2 (1.8) | −0.9 (1.6) | 0.031 | 69.3 (1.3) | 67.2 (2.1) | −2.1 (2.2) | 0.002 | 0.104 |
| SDNN (ms) | 60.7 (4.8) | 63.5 (3.9) | 2.8 (3.0) | 0.002 | 59.5 (4.1) | 63.0 (4.3) | 3.5 (3.5) | 0.002 | 0.446 |
| RMSSD (ms) | 53.8 (3.9) | 56.5 (3.4) | 2.7 (3.5) | 0.008 | 52.7 (4.8) | 57.5 (3.3) | 4.8 (4.3) | 0.001 | 0.086 |
| HF (ms 2 )§ | 43.0 (2.9) | 43.2 (2.0) | 0.3 (2.3) | 0.616 | 40.1 (3.0) | 42.7 (1.5) | 2.6 (3.3) | 0.010 | 0.036 |
| LF (ms 2 ) | 53.6 (2.3) | 52.3 (2.5) | −1.3 (2.7) | 0.075 | 53.6 (2.1) | 51.0 (3.0) | −2.6 (2.8) | 0.003 | 0.197 |
| LF/HF | 2.6 (0.4) | 2.4 (0.4) | −0.2 (0.2) | 0.002 | 2.6 (0.5) | 2.1 (0.4) | −0.5 (0.8) | 0.007 | 0.740 |
Values are mean (SD). PSQI=Pittsburgh Sleep Quality Index; MEQ=Morningness-Eveningness Questionnaire; SHI=Sleep Hygiene Index; TST=total sleep time derived from sleep diary; TIB=time in bed; SE=sleep efficiency (TST/TIB); HRV=heart rate variability; fC=heart rate; SDNN=standard deviation of normal-to-normal intervals; RMSSD=root mean square differences of the standard deviation; HF=high-frequency component; LF=low-frequency component; LF/HF=ratio of low- to high-frequency components; †n=12; ‡n=10; #n=9; §significantly different at baseline ( P <0.05).
Fitness measures ( Table 2 )
Aerobic performance: improved significantly in both groups, with an almost 2-fold greater improvement in nearly all measures in participants who received BM. VO 2 max increased in the BM group compared to the EA group. Additionally, metabolic threshold (VO 2 θ), and the percentage of maximum oxygen uptake at which metabolic threshold occurred (VO 2 θ/VO 2 max) increased in the BM compared to the EA group.
Both upper- and lower-body muscle strength and endurance improved significantly in all participants, although there was no difference in the magnitude of improvement between groups. The number of repetitions performed during lower-body endurance testing did not decrease despite performing the test with roughly 30% more weight. Peak and average lower-body power also increased significantly in both groups; however, there was significantly greater improvement in the BM group versus the EA group for both variables.
Furthermore, body composition improved in both groups, with two-fold greater improvement in the BM group. Body fat percentage decreased in the BM group compared to the EA group. Similarly, absolute fat mass decreased nearly 2-fold more in the BM group.
Sleep measures ( Table 3 )
For all symptom questionnaires and sleep diary variables, mean baseline values fell within normal ranges and significant differences between groups for changes in sleep measures were not observed. However, within the EA group sleep quality was improved (reduced PSQI score, increased diary SE), whereas the BM group demonstrated a shift toward morningness in circadian tendency (MEQ).
Heart rate variability ( Table 3 )
High-frequency signal (HF) improved in the BM group compared to no change in the EA group. Although this improvement between groups was statistically significant, it is notable that this measure was also significantly different between groups at baseline.
Discussion
Research in behavioral modification evaluates the efficacy of various interventions designed to enhance relaxation, reduce stress, and improve sleep. Sleep research mainly addresses issues specific to patients with clinically diagnosed sleep disorders in an effort to restore sleep quality to healthy levels. Considering that a high proportion of the general population receives inadequate sleep on a nightly basis 30 47 , even in the absence of diagnosable sleep disorders, the potential exists for improvements in health and fitness in broader subclinical populations as well. We believe this to be the first randomized, double-blind controlled trial integrating a novel, multicomponent BM intervention into an exercise training program in healthy volunteers, delivered by fitness professionals under the supervision of a clinical sleep psychologist. This delivery method is novel and contributes to existing literature by expanding the models through which BM interventions related to sleep can be delivered.
Physical performance
Prior research has shown decrements in aerobic performance resulting from sleep loss. Mougin et al. 36 observed reduced VO 2 max and earlier onset of VO 2 θ following partial sleep loss. Several other researchers have concluded chronic sleep deprivation leads to decreased athletic performance in various athletes 19 20 . The current study demonstrates that this decrement in aerobic performance can be reversed when behavioral strategies designed to improve sleep are introduced. Interestingly, these aerobic improvements were realized without compromising development of muscular strength or endurance.
Similarly, a few other studies have examined the effect sleep has on lower-body power. Using a jump mat similar to the one in the present investigation, Prentice et al. 40 reported a significant decrease in lower-body power immediately following a night of curtailed sleep. As with aerobic performance, we believe our study is the first to demonstrate an increase in this measure after an intervention targeting sleep-related habits and behaviors. Therefore, our data strengthens the evidence for a relationship between sleep and physical performance; however, the mechanism by which such a relationship exists remains to be established.
Body composition
Recent evidence suggests that sleep deprivation is linked to increased fat mass and obesity 26 49 ; however, the literature is equivocal on the effect of exercise as an additional factor. Farnsworth et al. 17 found that body composition, independent of physical activity levels, predicted the risk for developing a sleep disorder. Conversely, a meta-analysis of patients with obstructive sleep apnea concluded that exercise—and not solely a decrease in body mass—was responsible for the reduction in symptom severity 2 . Although the results in the present study suggest improved sleep habits augment the effect of exercise on body composition, the lack of significant changes in traditional sleep metrics makes it difficult to draw conclusions about the pathway through which these improvements may occur. It is possible that improved regularity of sleep habits, which is not easily captured in questionnaires or short-term sleep diaries, leads to improved health habits in general.
Sleep quality
Interestingly, although our BM program had a substantial component focused on improving sleep, the only significant change in sleep outcomes was a slight shift toward morningness on the MEQ in the BM group. It is possible that regular exercise in combination with sleep coaching served to strengthen the sense of alertness during the daytime hours via changing circadian rhythmicity and perhaps improve overall daytime energy levels, subjectively. Despite these improvements, we did not see major differences between groups in other sleep outcome measures. At baseline, participants did not demonstrate clinically significant sleep disorders, and commonly used sleep questionnaires may not have captured the degree of improvements in nighttime sleep quality one would see in clinical populations. Since our population consisted of healthy volunteers, any sleep deficiencies would be considered sub-clinical and, therefore, improvement in sleep resulting from our intervention could constitute a shift from adequate to optimal, rather than pathologic to healthy. In addition, because the intervention was individualized for each participant, some participants improved the regularity of their sleep schedule across weekdays and weekends, whereas others increased the total amount of time they spent in bed on a nightly basis, and others reduced sleep-disruptive environmental factors to decrease nighttime awakenings. This variation cannot be captured with aggregate measures. One limitation of our ability to monitor changes in sleep habits and routines was low adherence to completion of the daily sleep diaries during the assessment phases. The reason for this is somewhat unclear but may have been related to a preference for the paper-and-pencil sleep diaries used during the intervention (rather than the electronic diaries used during the assessment phases).
Heart rate variability
Another important goal of our BM program was to reduce stress, and we attempted to capture such an effect by measuring HRV – a reliable, noninvasive marker that reflects the balance of the autonomic nervous system (i. e., sympathetic and vagal neural influences) on heart rate. Previous studies have shown that increased sympathetic activity and decreased parasympathetic activity results in reduced HRV, which has been strongly associated with developing cardiovascular pathology 12 24 . In the present investigation, SDNN and RMSSD increased in both groups while resting fC and LF/HF ratio decreased. Because a lower LF/HF ratio suggests dominance of parasympathetic activity over sympathetic activity, these results suggest that exercise may exhibit a cardioprotective effect by simultaneously reducing sympathetic outflow and augmenting vagal tone. Furthermore, a significantly greater change in HF in the BM group compared to the EA group suggests that the BM intervention may have had an additive effect of increasing parasympathetic modulation. Many investigators have previously shown a strong positive association between HF and sleep quality in a variety of populations 8 48 . This observed difference in HF HRV improvement parallels the benefit of our BM intervention.
Strengths and limitations
One intriguing physiologic process that should be explored in future studies is circadian rhythms. A recent investigation found that, independent of sleep deprivation, chronobiological misalignment led to decreased insulin sensitivity and increased systemic inflammation 29 . In addition to being linked to caloric intake and glucose metabolism 4 , misaligned circadian rhythms have been shown to interfere with sleep and hinder athletic performance 38 . As described above, we did see a change in MEQ scores coupled with reduced fatigue during the intervention. Prescribed sleep scheduling—a component of the BM intervention—is regarded as a potential treatment for circadian rhythm disruption 42 ; therefore, it is plausible that sleep coaching stimulated circadian realignment which, in turn, increased the productivity of exercise training sessions even if it did not improve sleep quality as measured by the questionnaires employed in this trial. The use of targeted physiological measures (e. g., melatonin levels, dim light melatonin onset time) is needed to definitively test this hypothesis.
Although statistical power was calculated in advance, one limitation is the relatively small sample included in our study, which may have increased the risk for both type 1 and type 2 errors. This is partially ameliorated by careful selection of outcome measures and interpretation of results based on the pattern of findings in each domain; however, future larger studies are recommended to confirm our findings. Another limitation was the inability to directly detect meaningful changes in sleep quality using traditional clinical questionnaires in addition to the limited adherence to daily sleep diaries. Future studies should incorporate 24 h objective monitoring with actigraphy to evaluate whether objective changes in sleep can be achieved with this sleep coaching model. Additionally, although all participants received generalized dietary guidance, we did not assess or control dietary intake. It is possible that nutritional differences may have emerged between the groups and impacted our results. Similarly, we did not control for other behavioral and lifestyle factors, such as stress, emotional and/or spiritual health, or family and community support.
A unique aspect of our study was the use of trained fitness professionals to deliver the BM intervention program. The intervention program was developed by an experienced sleep psychologist (JLM) and was structured so it could be delivered within the health club setting (e. g., use of visual materials that can be shown on a computer screen or printed) in a relatively short period of time (10 min). This has the likelihood of increasing access to accurate information about sleep for fitness clients, and for delivering messages about the importance of sleep health by linking sleep-related behavioral changes (e. g., getting out of bed at a consistent time, avoiding stimulating activities near bed time) to fitness goals. Notably, the trainers in this study did consult weekly with the sleep psychologist (JLM), which facilitated their ability to master the sleep coaching program and work effectively with clients in changing their sleep-related behaviors. We cannot conclude that use of the intervention materials in the absence of this ongoing learning would have lead to the same positive outcomes.
The improved aerobic fitness profiles in both the BM and EA groups over baseline measures serve to further validate the use of a remotely-guided digital health system in directing an effective aerobic training program. In this study, as in previous studies that have incorporated the DHN, participants were able to capture and transmit biometric data from a fitness club or their home, facilitating rapid translation of this information into personalized exercise progression and performance feedback, which was then available to inform the very next exercise session. Previously validated in a population of emergency responders 15 , semi-automated systems, such as our DHN-enabled platform, could serve to enhance the reach and productivity of medical practitioners, personal trainers, and health coaches working with a wide variety of clientele without sacrificing fitness results.
Conclusions
This study demonstrates that a novel, multicomponent behavioral modification intervention delivered by fitness professionals with the goal of optimizing fitness outcomes was more efficacious at improving various measures of aerobic performance, body composition, lower-body power, and HRV compared with identical exercise training with an equal-attention control. Our findings indicate not only that sleep interventions have the potential to directly enhance physical performance and autonomic cardiac regulation, but that these interventions can be successfully administered by trained fitness professionals in a non-medical setting. Future investigations should explore the replicability and utility of these results in special populations such as elite athletes and those afflicted with lifestyle diseases.
Acknowledgments
This study was supported by a research grant from Equinox Fitness. The authors gratefully acknowledge the support of David Harris and the contributions of Jon Adams, Joe Geraghty, Mattew Berenc, Dr. Justin Mager, and the Equinox personal trainers in recruiting participants and implementing the protocol. The authors also thank Dr. John Berardi and Precision Nutrition for consultation in designing the biochemical analysis and providing nutrition education content.
Footnotes
Conflict of Interest BAD, JLM, and CBC served on the Equinox Health Advisory Board without compensation during the conduct of this study. The results of this study do not constitute endorsement of Equinox Fitness or its programs by the authors. The authors declare that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
References
- 1.Adams R.Revised physical activity readiness questionnaire Can Fam Physician 199945992, 9951004–1005. [PMC free article] [PubMed] [Google Scholar]
- 2.Aiello K D, Caughey W G, Nelluri B, Sharma A, Mookadam F, Mookadam M. Effect of exercise training on sleep apnea: A systematic review and meta-analysis. Respir Med. 2016;116:85–92. doi: 10.1016/j.rmed.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 3.National Strength & Conditioning Association Baechle T R, Earle R W.edsEssentials of Strength Training and Conditioning 3 rd ed Champaign, IL: Human Kinetics; 2008 [Google Scholar]
- 4.Baron K G, Reid K J. Circadian misalignment and health. Int Rev Psychiatry. 2014;26:139–154. doi: 10.3109/09540261.2014.911149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batalin M, Yuen E, Dolezal B, Smith D, Cooper C, Mapar J. IEEE International Conference on Body Sensor Networks; Cambridge, Massachusetts: 2013. PHASER: Physiological Health Assessment System for Emergency Responders. [Google Scholar]
- 6.Borbely A A. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 7.Burgess E, Hassmen P, Welvaert M, Pumpa K L. Behavioural treatment strategies improve adherence to lifestyle intervention programmes in adults with obesity: A systematic review and meta-analysis. Clin Obes. 2017;7:105–114. doi: 10.1111/cob.12180. [DOI] [PubMed] [Google Scholar]
- 8.Burton A R, Rahman K, Kadota Y, Lloyd A, Vollmer-Conna U. Reduced heart rate variability predicts poor sleep quality in a case-control study of chronic fatigue syndrome. Exp Brain Res. 2010;204:71–78. doi: 10.1007/s00221-010-2296-1. [DOI] [PubMed] [Google Scholar]
- 9.Buysse D J, Reynolds C F, 3rd, Monk T H, Berman S R, Kupfer D J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 10.Carney C E, Buysse D J, Ancoli-Israel S, Edinger J D, Krystal A D, Lichstein K L, Morin C M. The consensus sleep diary: Standardizing prospective sleep self-monitoring. Sleep. 2012;35:287–302. doi: 10.5665/sleep.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deka P, Pozehl B, Williams M A, Yates B. Adherence to recommended exercise guidelines in patients with heart failure. Heart Fail Rev. 2017;22:41–53. doi: 10.1007/s10741-016-9584-1. [DOI] [PubMed] [Google Scholar]
- 12.Dekker J M, Crow R S, Folsom A R, Hannan P J, Liao D, Swenne C A, Schouten E G. Low heart rate variability in a 2 min rhythm strip predicts risk of coronary heart disease and mortality from several causes: The ARIC Study. Atherosclerosis Risk In Communities. Circulation. 2000;102:1239–1244. doi: 10.1161/01.cir.102.11.1239. [DOI] [PubMed] [Google Scholar]
- 13.Dolezal B A, Lau M J, Abrazado M, Storer T W, Cooper C B. Validity of two commercial-grade bioelectrical impedance analyzers for measurement of body fat percentage. J Exerc Physiol Online. 2013;16:74–83. [Google Scholar]
- 14.Dolezal B A, Storer T W, Neufeld E V, Smooke S, Tseng C H, Cooper C B. A systematic method to detect the metabolic threshold from gas exchange during incremental exercise. J Sports Sci Med. 2017;16:396–406. [PMC free article] [PubMed] [Google Scholar]
- 15.Dolezal B A, Waite J G, Neufeld E V, Boland D M, Cooper C B. Remotely guided feedback enhances exercise training adherence and physical performance in firefighters. Int J Sports Sci. 2015;5:221–227. [Google Scholar]
- 16.Duffield R, Murphy A, Kellett A, Reid M. Recovery from repeated on-court tennis sessions: Combining cold-water immersion, compression, and sleep recovery interventions. Int J Sports Physiol Perform. 2014;9:273–282. doi: 10.1123/ijspp.2012-0359. [DOI] [PubMed] [Google Scholar]
- 17.Farnsworth J L, Kim Y, Kang M. Sleep disorders, physical activity, and sedentary behavior among U.S. adults: National Health and Nutrition Examination Survey. J Phys Act Health. 2015;12:1567–1575. doi: 10.1123/jpah.2014-0251. [DOI] [PubMed] [Google Scholar]
- 18.Farrell T C, Keeping-Burke L. The primary prevention of cardiovascular disease: nurse practitioners using behaviour modification strategies. Can J Cardiovasc Nurs. 2014;24:8–15. [PubMed] [Google Scholar]
- 19.Fowler P, Duffield R, Vaile J. Effects of simulated domestic and international air travel on sleep, performance, and recovery for team sports. Scand J Med Sci Sports. 2015;25:441–451. doi: 10.1111/sms.12227. [DOI] [PubMed] [Google Scholar]
- 20.Fullagar H H, Skorski S, Duffield R, Hammes D, Coutts A J, Meyer T. Sleep and athletic performance: the effects of sleep loss on exercise performance, and physiological and cognitive responses to exercise. Sports Med. 2015;45:161–186. doi: 10.1007/s40279-014-0260-0. [DOI] [PubMed] [Google Scholar]
- 21.Harman E A, Rosenstein M T, Frykman P N, Rosenstein R M, Kraemer W J. Estimation of human power output from vertical jump. J Strength Cond Res. 1991;5:116–120. [Google Scholar]
- 22.Harriss D J, Macsween A, Atkinson G. Standards for ethics in sport and exercise science research: 2018 update. Int J Sports Med. 2017;38:1126–1131. doi: 10.1055/s-0043-124001. [DOI] [PubMed] [Google Scholar]
- 23.Horne J A, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 24.Huikuri H V, Jokinen V, Syvanne M, Nieminen M S, Airaksinen K E, Ikaheimo M J, Koistinen J M, Kauma H, Kesaniemi A Y, Majahalme S, Niemela K O, Frick M H. Heart rate variability and progression of coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 1999;19:1979–1985. doi: 10.1161/01.atv.19.8.1979. [DOI] [PubMed] [Google Scholar]
- 25.Institute of Medicine (US) Committee on Sleep Medicine and Research Colten H R, Altevogt B M.edsSleep Disorders and Sleep Deprivation: An Unmet Public Health Problem Washington, DC: National Academies Press (US)2006 [PubMed] [Google Scholar]
- 26.Kim K, Shin D, Jung G U, Lee D, Park S M. Association between sleep duration, fat mass, lean mass and obesity in Korean adults: The fourth and fifth Korea National Health and Nutrition Examination Surveys. J Sleep Res. 2017;26:453–460. doi: 10.1111/jsr.12504. [DOI] [PubMed] [Google Scholar]
- 27.Kredlow M A, Capozzoli M C, Hearon B A, Calkins A W, Otto M W. The effects of physical activity on sleep: A meta-analytic review. J Behav Med. 2015;38:427–449. doi: 10.1007/s10865-015-9617-6. [DOI] [PubMed] [Google Scholar]
- 28.Leard J S, Cirillo M A, Katsnelson E, Kimiatek D A, Miller T W, Trebincevic K, Garbalosa J C. Validity of two alternative systems for measuring vertical jump height. J Strength Cond Res. 2007;21:1296–1299. doi: 10.1519/R-21536.1. [DOI] [PubMed] [Google Scholar]
- 29.Leproult R, Holmback U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63:1860–1869. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Wheaton A G, Chapman D P, Cunningham T J, Lu H, Croft J B. Prevalence of healthy sleep duration among adults–United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:137–141. doi: 10.15585/mmwr.mm6506a1. [DOI] [PubMed] [Google Scholar]
- 31.Mah C D, Mah K E, Kezirian E J, Dement W C. The effects of sleep extension on the athletic performance of collegiate basketball players. Sleep. 2011;34:943–950. doi: 10.5665/SLEEP.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mastin D F, Bryson J, Corwyn R. Assessment of sleep hygiene using the Sleep Hygiene Index. J Behav Med. 2006;29:223–227. doi: 10.1007/s10865-006-9047-6. [DOI] [PubMed] [Google Scholar]
- 33.Miller W R, Rollnick S.Motivational Interviewing: Preparing People for Change3rd edNew York: Guilford Press; 2012 [Google Scholar]
- 34.Minton O, Jo F, Jane M. The role of behavioural modification and exercise in the management of cancer-related fatigue to reduce its impact during and after cancer treatment. Acta Oncol. 2015;54:581–586. doi: 10.3109/0284186X.2014.996660. [DOI] [PubMed] [Google Scholar]
- 35.Morgenthaler T, Kramer M, Alessi C, Friedman L, Boehlecke B, Brown T, Coleman J, Kapur V, Lee-Chiong T, Owens J, Pancer J, Swick T. Practice parameters for the psychological and behavioral treatment of insomnia: An update. An American Academy of Sleep Medicine report. Sleep. 2006;29:1415–1419. [PubMed] [Google Scholar]
- 36.Mougin F, Simon-Rigaud M L, Davenne D, Renaud A, Garnier A, Kantelip J P, Magnin P. Effects of sleep disturbances on subsequent physical performance. Eur J Appl Physiol Occup Physiol. 1991;63:77–82. doi: 10.1007/BF00235173. [DOI] [PubMed] [Google Scholar]
- 37.Mullington J M, Haack M, Toth M, Serrador J M, Meier-Ewert H K. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nedelec M, Halson S, Delecroix B, Abaidia A E, Ahmaidi S, Dupont G. Sleep hygiene and recovery strategies in elite soccer players. Sports Med. 2015;45:1547–1559. doi: 10.1007/s40279-015-0377-9. [DOI] [PubMed] [Google Scholar]
- 39.Netzer N C, Stoohs R A, Netzer C M, Clark K, Strohl K P. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 40.Prentice C, Stannard S R, Barnes M J. Effects of heavy episodic drinking on physical performance in club level rugby union players. J Sci Med Sport. 2015;18:268–271. doi: 10.1016/j.jsams.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 41.Roemer L, Orsillo S M, Salters-Pedneault K. Efficacy of an acceptance-based behavior therapy for generalized anxiety disorder: Evaluation in a randomized controlled trial. J Consult Clin Psychol. 2008;76:1083–1089. doi: 10.1037/a0012720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sack R L, Auckley D, Auger R R, Carskadon M A, Wright K P, Jr., Vitiello M V, Zhdanova I V. Circadian rhythm sleep disorders: Part I, basic principles, shift work and jet lag disorders. An American Academy of Sleep Medicine review. Sleep. 2007;30:1460–1483. doi: 10.1093/sleep/30.11.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Storer T W, Dolezal B A, Berenc M N, Timmins J E, Cooper C B. Effect of supervised, periodized exercise training vs. self-directed training on lean body mass and other fitness variables in health club members. J Strength Cond Res. 2014;28:1995–2006. doi: 10.1519/JSC.0000000000000331. [DOI] [PubMed] [Google Scholar]
- 44.Stults-Kolehmainen M A, Sinha R. The effects of stress on physical activity and exercise. Sports Med. 2014;44:81–121. doi: 10.1007/s40279-013-0090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tobaldini E, Costantino G, Solbiati M, Cogliati C, Kara T, Nobili L, Montano N. Sleep, sleep deprivation, autonomic nervous system and cardiovascular diseases. Neurosci Biobehav Rev. 2017;74:321–329. doi: 10.1016/j.neubiorev.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Van Ryswyk E, Weeks R, Bandick L, O’Keefe M, Vakulin A, Catcheside P, Barger L, Potter A, Poulos N, Wallace J, Antic N A. A novel sleep optimisation programme to improve athletes’ well-being and performance. Eur J Sport Sci. 2017;17:144–151. doi: 10.1080/17461391.2016.1221470. [DOI] [PubMed] [Google Scholar]
- 47.Watson N F, Badr M S, Belenky G, Bliwise D L, Buxton O M, Buysse D, Dinges D F, Gangwisch J, Grandner M A, Kushida C, Malhotra R K, Martin J L, Patel S R, Quan S F, Tasali E. Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society on the Recommended Amount of Sleep for a Healthy Adult: Methodology and Discussion. Sleep. 2015;38:1161–1183. doi: 10.5665/sleep.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Werner G G, Ford B Q, Mauss I B, Schabus M, Blechert J, Wilhelm F H. High cardiac vagal control is related to better subjective and objective sleep quality. Biol Psychol. 2015;106:79–85. doi: 10.1016/j.biopsycho.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu Y, Zhai L, Zhang D. Sleep duration and obesity among adults: A meta-analysis of prospective studies. Sleep Med. 2014;15:1456–1462. doi: 10.1016/j.sleep.2014.07.018. [DOI] [PubMed] [Google Scholar]


