Abstract
A diagnosis of silicosis is made on the basis of exposure and typical radiological findings, according to the ILO's International Classification of Radiographs of Pneumoconiosis. Radiological patterns of silicosis can, however, resemble sarcoidosis. Sarcoidosis is a multi-systemic disorder of unknown etiology, although a role for initiating inorganic triggers such as metals or silica has been suggested. In this case report, we illustrate a patient previously diagnosed with silicosis based on exposure and radiological features, progressive under immunosuppressive treatment. In view of these findings, an open lung biopsy was performed and revealed sarcoidosis. The patient was effectively treated with infliximab. Further analysis showed the presence of silica in the granulomas. Sensitization to silica was also demonstrated, suggesting an association between silica exposure and sarcoidosis in this patient.
Keywords: Silicosis, Sarcoidosis, Sarcoidosis phenotypes, Silica, Infliximab
Abbreviations: 18 F-FDG PET/CT, 18F-fluorodeoxyglucose by positron emission tomography/computed tomography; CBD, Chronic beryllium disease; DLCO, diffusing capacity of the lung for carbon monoxide; EDXA, Energy-dispersive X-ray spectroscopy analysis; FVC, Forced Vital Capacity; HRCT, High-resolution computed tomography; LPT, Lymphocyte proliferation test
1. Introduction
Silicosis is caused by the inhalation of crystalline silicon dioxide, or silica, and is one of the most important occupational diseases worldwide [1]. A diagnosis of silicosis is made based on exposure and typical radiological findings, according to the ILO's International Classification of Radiographs of Pneumoconiosis [2]. In addition, competing diagnoses such as miliary tuberculosis or sarcoidosis should be excluded, as radiologic patterns of these diseases can be similar [1].
Sarcoidosis is a systemic disorder of unknown etiology, although initiating inorganic triggers such as metals or silica have been suggested in the literature to play a role [[3], [4], [5], [6], [7]]. Interestingly, some epidemiological studies have even demonstrated an elevated risk of sarcoidosis among occupationally silica-exposed individuals [8,9]. An underlying immunological mechanism to explain this has not yet been identified.
In this case report, we discuss a patient clinically diagnosed with silicosis based on exposure and radiological features, with progressive disease under immunosuppressive treatment. An open lung biopsy led to a diagnosis of sarcoidosis, though with silica present in the granulomas. By demonstrating sensitization to silica, we believe that this patient's exposure to silica could well have played a role in the pathogenesis of his sarcoidosis.
2. Case report
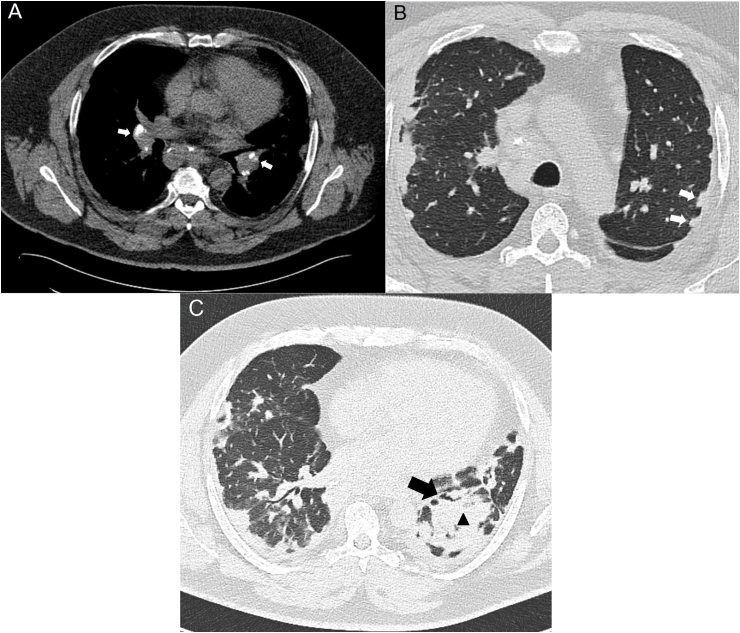
A 49-year-old male who had worked as a plasterer for 30 years presented with complaints of fatigue and progressive dyspnea, and was diagnosed as having silicosis. The patient was referred to our hospital because of deterioration, and to confirm the diagnosis. Occupational history was taken by an occupational hygienist and did indeed show exposure to construction dust including both crystalline and amorphous silica. High-resolution computed tomography (HRCT) showed mediastinal and bilateral hilar lymphadenopathy, subpleural nodules, consolidations, bronchiectasis and fibrotic lesions (Fig. 1).
Fig. 1.
HRCT scans showing mediastinal and bilateral hilar lymphadenopathy (arrows in A), subpleural nodules (arrows in B), consolidations (triangle in C) and bronchiectasis (arrow in C). Based on these images, no distinction could be made between silicosis and sarcoidosis.
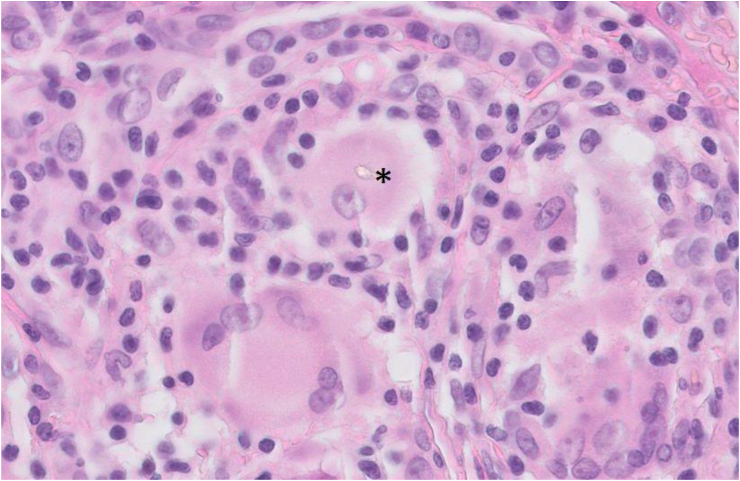
The radiology findings did not allow a diagnostic distinction to be made between silicosis and sarcoidosis. Hence, an open lung biopsy was performed, which showed inflammatory infiltration in the pleura, non-necrotizing granuloma including multinucleated giant cells with hyalinization, and birefringent material in the lung parenchyma (Fig. 2). Since yeast, mycobacterial and fungus cultures were negative and malignancy was excluded as well, the biopsy fitted with a diagnosis of sarcoidosis. In view of the presence of birefringent material in the tissue, most likely deposited as a result of the patient's masonry and plasterwork, an energy-dispersive X-ray spectroscopy analysis (EDXA) was performed. This revealed aluminum, silicon and titanium, elements that are all present in cement. A lymphocyte proliferation test (LPT) (MELISA®) was used to test whether the patient showed a hypersensitivity reaction to any of these compounds. Since beryllium is known to be capable of inducing a granulomatous reaction [10], the LPT was performed for beryllium as well. The stimulation indexes for the metals tested were all below 2.0, which is considered a negative test outcome [11]. Interestingly, a positive stimulation index of 3.2 was found for silica, indicating sensitization.
Fig. 2.
Open lung biopsy tissue demonstrating non-necrotizing granulomas including birefringent material, as indicated by the asterisk.
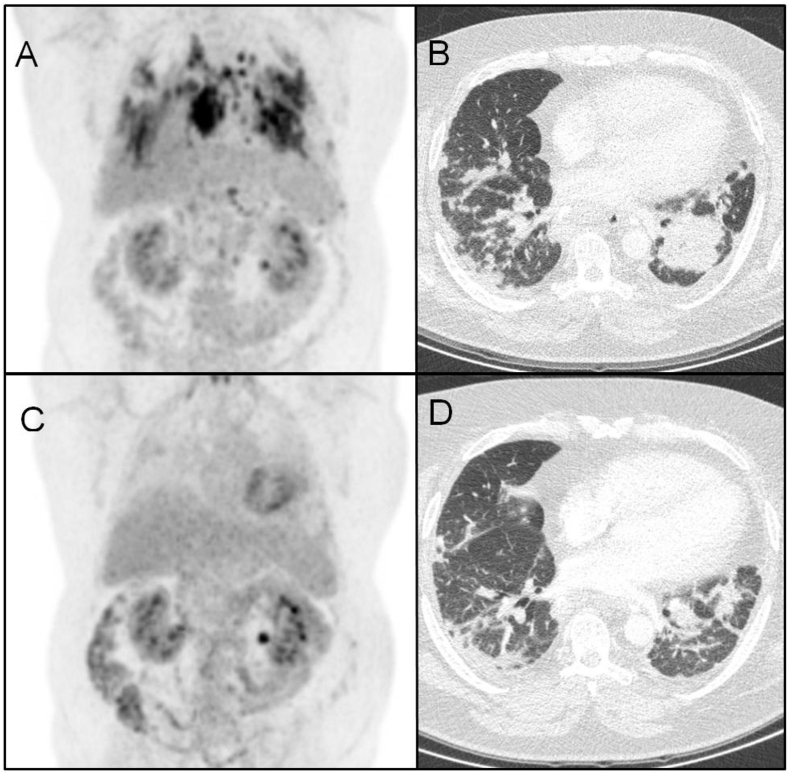
While being treated with a combination of prednisone and azathioprine, the patient deteriorated further, with increased dyspnea and pulmonary consolidations and worsening of the pulmonary function tests. In view of the final diagnosis of sarcoidosis instead of silicosis, treatment with infliximab was started. After 7 months of treatment with this agent, the patient's pulmonary function test had improved and pulmonary consolidations on HRCT as well as the inflammatory activity measured by 18F-fluorodeoxyglucose by positron emission tomography/computed tomography (18 F-FDG PET/CT) had decreased (Fig. 3). Forced Vital Capacity (FVC) rose from 1.97 L to 2.35 L (44 and 53% of predicted, respectively) and the diffusing capacity of the lung for carbon monoxide (DLCO) rose from 5.46 mmol/(min*kPa) (54% of predicted) to 6.26 mmol/(min*kPa) (62% of predicted).
Fig. 3.
PET and HRCT scans before and after 7 months of infliximab therapy. Upper part: PET (A) and HRCT scan (B) 2 months prior to the start of infliximab. At this time, the patient had been on azathioprine therapy for 8 months, and prednisone therapy had been stopped 14 months ago. Lower part: PET (C) and HRCT scans (D) after 7 months of infliximab therapy, showing decreased consolidations.
3. Discussion
This case highlights an interesting clinical problem in the differential diagnosis of pneumoconiosis versus sarcoidosis.
The radiological features, in combination with the history of silica exposure, led to a diagnosis of silicosis according to the International Classification of Radiographs of Pneumoconiosis. Histological features of silicosis include the presence of silicotic nodules [12]. The absence of silicotic nodules and the appearance of non-caseating granulomas in the biopsy of our patient, together with radiological features that were also compatible with sarcoidosis, made the initial diagnosis of silicosis for this patient unlikely, and pointed towards a diagnosis of sarcoidosis. The positive LPT for silica demonstrated sensitization to silica in this patient.
The link between silica and sarcoidosis is interesting, since previous retrospective studies have shown a higher risk of sarcoidosis in workers with occupational silica exposure [8,9].
In patients who are exposed to beryllium and have granulomatous lung disease, the finding of sensitization to beryllium makes a diagnosis of chronic beryllium disease (CBD) [10]. It is well known that the non-necrotizing granulomas found in CBD are identical to the granulomas found in sarcoidosis, so one could speculate that CBD can be seen as a form of beryllium-induced sarcoidosis [13,14]. The possibility that beryllium had been the cause of the granulomatous reaction in this patient was, however, ruled out by the fact that the occupational hygienist did not determine beryllium as one of the patient's occupational exposures, and by the negative beryllium LPT.
Keeping in mind the above-mentioned criteria to diagnose CBD, and applying them to silica in our case, a diagnosis of sarcoidosis associated with silica exposure seemed reasonable, which is why third-line sarcoidosis treatment options were considered for this patient. Treatment with infliximab has shown good responses in some patients with severe therapy-refractory sarcoidosis [[15], [16], [17]]. In CBD, infliximab treatment has shown effectiveness as well [18].
In conclusion, it can be difficult to distinguish between silicosis and sarcoidosis based only on exposure and radiological patterns. The fact that silica is related to both silicosis and sarcoidosis is, in our opinion, more than a mere coincidence. Sensitization to silica could be involved in the underlying immunological mechanism, explaining why workers with occupational silica exposure have an elevated risk of developing sarcoidosis, as demonstrated by our patient. Future studies in larger patients groups should be initiated to address the possible causality of silica exposure in the development of sarcoidosis, and clarify the underlying immunological mechanisms in more detail.
Conflicts of interest
None to declare.
Funding
This study is part of a research project (842002001) funded by ZonMw.
Notification of prior abstract publication/presentation
This case report has been presented as an oral presentation at the WASOG conference, Crete, Greece, June 7–9, 2018 and as a poster presentation at the Week of the Lungs, Ermelo, The Netherlands, April 11–12, 2018.
Contributor Information
Els Beijer, Email: e.beijer@antoniusziekenhuis.nl.
Marcel Veltkamp, Email: m.veltkamp@antoniusziekenhuis.nl.
References
- 1.Greenberg M.I., Waksman J., Curtis J. Silicosis: a review. Dis. Mon. 2007;53:394–416. doi: 10.1016/j.disamonth.2007.09.020. S0011-5029(07)00110-1 [pii] [DOI] [PubMed] [Google Scholar]
- 2.Muszynska-Graca M., Dabkowska B., Brewczynski P.Z. Guidelines for the use of the international classification of Radiographs of pneumoconioses of the international labour office (ILO): substantial changes in the currrent edition. Med. Pr. 2016;67:833–837. doi: 10.13075/mp.5893.00493. doi:63249 [pii] [DOI] [PubMed] [Google Scholar]
- 3.Werfel U., Schneider J., Rodelsperger K., Kotter J., Popp W., Woitowitz H.J., Zieger G. Sarcoid granulomatosis after zirconium exposure with multiple organ involvement. Eur. Respir. J. 1998;12:750. doi: 10.1183/09031936.98.12030750. [DOI] [PubMed] [Google Scholar]
- 4.Muller-Quernheim J., Gaede K.I., Fireman E., Zissel G. Diagnoses of chronic beryllium disease within cohorts of sarcoidosis patients. Eur. Respir. J. 2006;27:1190–1195. doi: 10.1183/09031936.06.00112205. 09031936.06.00112205 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Cao M., Cai H.R., Meng F.Q., Wei J.Y. Pulmonary sarcoidlike granulomatosis induced by aluminum dust: a case report and literature review. Zhonghua Jiehe He Huxi Zazhi. 2008;31:406–409. [PubMed] [Google Scholar]
- 6.Sola R., Boj M., Hernandez-Flix S., Camprubi M. Silica in oral drugs as a possible sarcoidosis-inducing antigen. Lancet (London, England) 2009;373:1943–1944. doi: 10.1016/S0140-6736(09)61057-6. ([doi]) [DOI] [PubMed] [Google Scholar]
- 7.Drent M., Wijnen P.A., Boots A.W., Bast A. Cat litter is a possible trigger for sarcoidosis. Eur. Respir. J. 2012;39:221–222. doi: 10.1183/09031936.00074411. ([doi]) [DOI] [PubMed] [Google Scholar]
- 8.Rafnsson V., Ingimarsson O., Hjalmarsson I., Gunnarsdottir H. Association between exposure to crystalline silica and risk of sarcoidosis. Occup. Environ. Med. 1998;55:657–660. doi: 10.1136/oem.55.10.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vihlborg P., Bryngelsson I.L., Andersson L., Graff P. Risk of sarcoidosis and seropositive rheumatoid arthritis from occupational silica exposure in Swedish iron foundries: a retrospective cohort study. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-016839. e016839-2017-016839. ([doi]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balmes J.R., Abraham J.L., Dweik R.A., Fireman E., Fontenot A.P., Maier L.A., Muller-Quernheim J., Ostiguy G., Pepper L.D., Saltini C., Schuler C.R., Takaro T.K., Wambach P.F., A.T.S.A.H.C. on B.S., Disease C.B. An official American Thoracic Society statement: diagnosis and management of beryllium sensitivity and chronic beryllium disease. Am. J. Respir. Crit. Care Med. 2014;190:e34–59. doi: 10.1164/rccm.201409-1722ST. ([doi]) [DOI] [PubMed] [Google Scholar]
- 11.Stejskal V.D., Cederbrant K., Lindvall A., Forsbeck M. MELISA-an in vitro tool for the study of metal allergy. Toxicol. In Vitro. 1994;8:991–1000. doi: 10.1016/0887-2333(94)90233-x. 0887-2333(94)90233-X [pii] [DOI] [PubMed] [Google Scholar]
- 12.Leung C.C., Yu I.T., Chen W. vol. 379. 2012. pp. 2008–2018. (Silicosis , Lancet). London, England. ([doi]) [DOI] [PubMed] [Google Scholar]
- 13.Rossman M.D., Kreider M.E. Is chronic beryllium disease sarcoidosis of known etiology? Sarcoidosis, Vasc. Diffus. Lung Dis. Off. J. WASOG. 2003;20:104–109. [PubMed] [Google Scholar]
- 14.Richeldi L. Chronic beryllium disease: a model for pulmonary sarcoidosis? Acta Biomed. 2005;76(Suppl 2):11–14. [PubMed] [Google Scholar]
- 15.Vorselaars A.D., Crommelin H.A., Deneer V.H., Meek B., Claessen A.M., Keijsers R.G., van Moorsel C.H., Grutters J.C. Effectiveness of infliximab in refractory FDG PET-positive sarcoidosis. Eur. Respir. J. 2015;46:175–185. doi: 10.1183/09031936.00227014. ([doi]) [DOI] [PubMed] [Google Scholar]
- 16.Schimmelpennink M.C., Vorselaars A.D.M., van Beek F.T., Crommelin H.A., Deneer V.H.M., Keijsers R.G.M., Veltkamp M. Efficacy and safety of infliximab biosimilar Inflectra((R)) in severe sarcoidosis. Respir. Med. 2018;138:S7–S13. doi: 10.1016/j.rmed.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Baughman R.P., Drent M., Kavuru M., Judson M.A., Costabel U., du Bois R., Albera C., Brutsche M., Davis G., Donohue J.F., Muller-Quernheim J., Schlenker-Herceg R., Flavin S., Lo K.H., Oemar B., Barnathan E.S., Investigators S. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am. J. Respir. Crit. Care Med. 2006;174:795–802. doi: 10.1164/rccm.200603-402OC. 200603-402OC [pii] [DOI] [PubMed] [Google Scholar]
- 18.Maier L.A., Barkes B.Q., Mroz M., Rossman M.D., Barnard J., Gillespie M., Martin A., Mack D.G., Silveira L., Sawyer R.T., Newman L.S., Fontenot A.P. Infliximab therapy modulates an antigen-specific immune response in chronic beryllium disease. Respir. Med. 2012;106:1810–1813. doi: 10.1016/j.rmed.2012.08.014. ([doi]) [DOI] [PMC free article] [PubMed] [Google Scholar]