Abstract
Glomus tumors are neoplasms arising from modified smooth muscle cells surrounding arteriovenous anastomosis in the dermis and subcutaneous tissues, which are contributing to blood flow regulation and temperature control on the skin surface. Glomus cells are sparse or absent in visceral organs, making extracutaneous presentation of glomus tumors an extremely rare finding. We briefly report histological considerations on glomus tumors of the trachea and sum the multidisciplinary aspects of their staged endoscopic and surgical management using the example of a rare case presentation.
Keywords: Glomus tumor, Trachea, Sleeve resection, Multidisciplinary treatment
1. Introduction
Glomus tumors, which contribute for less than 2% of all soft tissue tumors, are infrequent and generally benign neoplasms [1]. They originate from glomus cells, which surround arteriovenous anastomosis in the dermis and subcutaneous tissues, therefore contributing to blood flow regulation and temperature control. As these tumors typically occur in the skin corium, they are extremely uncommon within the tracheobronchial tree where glomus bodies are thought to be absent [2]. Up to the year 2019, no more than 82 glomus tumors of the tracheobronchial tree have been reported - the characteristics of these cases are summarized in Table 1. We hereby briefly report histological considerations on glomus tumors of the trachea and sum the multidisciplinary aspects of their staged endoscopic and surgical management using the example of a rare case presentation.
Table 1.
Summary characteristics of previously reported 82 patients with glomus tumor of the tracheobronchial tree (1950–2019).
| Characteristic | Value |
|---|---|
| Age in years, mean (range) |
50 (10–83) |
| Gender, n (%) | |
| Male | 55 (67%) |
| Female |
27 (33%) |
| Tumor size in cm, mean (range) |
1.9 × 1.5 (0.5 × 0.5–4.5 × 3.0) |
| Tumor location, n (%) | |
| Upper Trachea | 14 (17%) |
| Mid-trachea | 18 (22%) |
| Lower Trachea | 32 (39%) |
| Bronchus |
18 (22%) |
| Tumor typea | |
| Glomus tumor proper | 67 (86%) |
| Glomangioma | 5 (6%) |
| Glomangiomyoma | 2 (3%) |
| Glomangiosarcoma | 3 (4%) |
| Oncocytic glomus tumor |
1 (1%) |
| Treatment | |
| Tracheal resectionb | 46 (56%) |
| Endoscopic resectionc | 30 (37%) |
| Otherd |
6 (7%) |
| Presenting symptoms, n (%)e | |
| Cough | 47 (57%) |
| Dyspnea | 43 (53%) |
| Hemoptysis | 37 (46%) |
| Chest pain | 9 (11%) |
| Otherf | 4 (5%) |
| Asymptomatic | 6 (7%) |
| Follow-up in month, mean (range)g |
16.8 (1–72) |
|
Clinical findings of the presented case | |
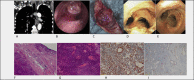 | |
(A) Sagittal computed tomographic scan revealing an obstructive tumor in the lower trachea with slightly irregular contour showing typically marked contrast enhancements in the tumors' periphery indicating transmural growth through the tracheal wall.
(B) Preoperative bronchoscopic view of the sessile multi-lobulated hypervascularized polypoid tumor, arising from the posterior wall of the trachea.
(C) Resected tracheal specimen.
(D) Bronchoscopic control two weeks postoperatively.
(E) Bronchoscopic control at 96-month-follow-up.
(F) Representative low-power magnification of a cut section of the glomus tumor showing a hypervascular tumor composed of branching, dilated, thick-walled, vascular channels and thin-walled, capillary-like vascular spaces (hematoxylin and eosin, 4×).
(G) Higher magnification of the tumor showing lobular arrangements of oval-to-spindle-shaped cells (hematoxylin and eosin, 20×).
(H) Cytoplasmic staining to show caldesmon in the spindle cells (immunohistochemistry; 20x).
(I) Immunonegativity for CD34 (immunohistochemistry; 10x).
Precise histological findings are less profound in most of the early cases, in four cases the exact histologic tumor type was not investigable, percentage values referring to 78 patients with accurately defined tumor types.
Including five bronchial sleeve resections.
Including neodymium:yttrium aluminum garnet (Nd-YAG) laser use and electroexcision.
Including three lobe resection, two carinal resections (one with ECMO), and one tangential resection with spiral tracheoplasty.
Certain patients presented multiple symptoms.
Including hoarseness, wheezing, asthma or stridor.
Follow-up documented only in 59 of the 82 reported cases.
2. Case presentation
A 66-year-old male was admitted to our hospital for a 3-month history of cough, hemoptysis and intermittent shortness of breath, without any past medical and smoking history. On physical examination, he had an audible expiratory stridor. There were no abnormalities in both lung auscultation and heart examination. Additional tests including blood exams (white cells, hemoglobin and platelets) and liver function, biochemistry, and coagulation panels were all within normal values. The chest X-ray was inconspicuous. A computed tomographic (CT) scan of the chest revealed a polypoid lesion ascending from the posterior wall of the lower trachea, with its size of approximately 1.1 cm × 2.2 cm (Table 1; A). A transmural expansion of the tumor had to be considered. There were no additional pathologies like lymphadenopathy, pulmonary nodules, pleural alterations, or any other indication of metastasis. Since the patient presented with severe dyspnea and risk of suffocation, urgent rigid bronchoscopy under general anesthesia was carried out at first step to restore patency of the airway and to define histology. Thereby, a sessile polypoid tumor was observed arising from the posterior wall of the trachea at 3 cm proximal to the main carina. The intraluminal part of the tumor occluded the trachea almost entirely, supplementary narrowed by a protuberance of the posterior wall caused by its transmural extension (Table 1; B). The endoluminal component of the tracheal tumor was sampled and partially resected using argon plasma coagulation/electrocautery snare that achieved at least 70% airway patency without any complications. Histology showed round epithelioid cells with eosinophilic cytoplasms, surrounding “nest-like” dilated vessels (Table 1; F, G). Immunohistochemistry (Table 1; H, I) was positive for both caldesmon and smooth muscle actin antibodies and negative for chromogranin, synaptophysin, CD-56, S-100, CD-31, CD-34 and anti-phospho-histon H3. The tumor was diagnosed as a glomangioma. To ensure durability of the airway, a covered tracheal stent (Boston Scientific, Galway, Ireland) was placed temporarily to overlap the residual tissue at the former tumor site. Tracheal surgery was performed following stent retrieval and patient preconditioning. Following video-assisted mediastinoscopic mobilization of the upper trachea, tracheal sleeve resection via right posterolateral thoracotomy with end-to-end anastomosis allowed complete en-bloc resection of the lesion with 0.5cm spare of both surgical margins (Table 1; C). Both recurrent laryngeal nerves were well protected. Postoperative bronchoscopy showed a successfully anastomosed trachea with no stricture or bleeding (Table 1; D). The patient left ICU within two days being discharged to rehabilitation in good condition. Long-time follow-up including annual bronchoscopy and chest CT was uneventful with no tumor recurrence over a 96-month period (Table 1; E).
3. Discussion
Although glomus tumors have been observed by some of the old writers dating back to the ancient Greek era (namely Hippocrates and Galen), special attention was paid to them not until the 18th century by such well-known authors as Morgagni, Bisset and Petit. William Wood (1812) gave the first accurate clinical description of the tumor of what he termed being a “painful subcutaneous tubercle”. Jan Kolaczek (1878) described a subungual tumor as a variant of an angiosarcoma. Besides these historical miscellanea, the deeper knowledge of “glomus tumors” starts only in the third decade of the last century when Barre (1920, 1922) described two cases which were later examined histologically by Masson (1924). The latter was the first to realize that these tumors arise from the muscular coat of arteriovenous anastomoses normally present in the vascular tree of the skin. Since these normal structures are also known as “neurovascular glomus” he gave the tumor the name: “neuromyoarterial tumor”. Following this description, glomus tumors attracted the attention of pathologists all over the world. Naming Masson's terminology “rather cumbersome”, several alternative terms were suggested through the years to come (i.e. “glomangioma”, “glomangiecton” and “angioneuromyoma”). Today, the tumor is generally referred to by the simple term “glomus tumor”. Apart being phrasing or inventing words, Hussarek (1950) reported about the first successful resection of a glomus tumor of the trachea [3].
From history to the facts of the 21st century: Glomus tumors are mesenchymal neoplasms that encompass up to 2% of soft tissue tumors [4]. They originate from altered smooth muscle cells surrounding the thermo-regulating arteriovenous anastomoses in the glomus bodies, which are typically existent within the profound dermis and adjacent subcutaneous tissue, particularly in the subungual regions of the digits, arms, and feet. They are regarded as cutaneous structures that support both regulation of body temperature and control of blood pressure [5]. Unusual extra-cutaneous localizations - where the “normal” glomus body is scarce or thought to be even absent - have been labeled comprising the gastrointestinal and respiratory tracts. These locations include (amongst others) stomach, colon, mediastinum, heart, lung, and trachea [5,6]. Mentioning the trachea as a last resort, it is an uncommon position for both benign and malignant neoplasms, with glomus tumors among the oddest of them all. Typically, glomus tumors arise from the posterior wall of the lower two-thirds of the trachea, where mucous glands and vessels are frequent [7]. The most common symptoms are cough, dyspnea, and hemoptysis (Table 1). Chest pain, stridor, and hoarseness are less frequent. The male to female ratio counts 2:1. The etiology of these tumors remains a puzzle with trauma, endocrine disorder or autosomal dominant inheritance being attributed [8]. Most glomus tumors are solitary, but about 10% exist as multiple variations with hereditary familial tendency. Assuming a connotation between germ line, somatic mutations, and glomus tumors, the gene for the inherited variant has been associated to chromosome 1p21–22.3 at which the mutation is conveyed in an autosomal dominant fashion [8].
Histologically, glomus tumors comprise medium-sized cells with round, regular nuclei and eosinophilic cytoplasm that are organized in a “nest-like” pattern around capillary-sized vessels [1,2]. Vessels surrounding glomus tumors have thick cellular walls and small lumen creating the Suquet-Hoyer canal [8]. Non-myelinated nerves lie along the margin of the cell wall in close vicinity to nearby Schwann cells and fibroblasts. The glomus tumors show differentiation toward smooth muscle tissue. They have characteristic immunohistochemical features: (1) they are consistently immunopositive for vimentin, smooth muscle actin and type IV collagen with variable expression of desmin, caldesmon, calponin and CD34; (2) they are immunonegative for neuroendocrine and epithelial markers, including S-100 protein, CD31, CKIT, chromogranin, cytokeratin, synaptophysin and factor VIII [7]. Based on the Pathology and Genetics of Tumors of Soft Tissue and Bone (World Health Organization Classification of Tumors), glomus tumors are divided into benign glomus tumors (>95%), glomus tumors of uncertain malignant potential, and malignant glomus tumors [8]. They are - besides the classification on the basis of their biologic activity - further categorized into three histologic groups, in view to the scopes of glomus cells, vascular structures, and smooth-muscle tissue: the Glomus tumor proper type, with the round glomus cells constituting the largest feature (ca. 75%); the glomangioma type, with vessels being primarily present (ca. 20%); and glomangiomyoma, where spindle cells represent the main cell type (<10%) [7]. An additional rather ultrastructural characterization describing abundant narrowly packed round or ovoid mitochondria, the so called “oncocytic type”, has also been pronounced (2%). Confusing the issue, tumors previously diagnosed as “atypical” or “malignant” by merit of nuclear atypia, infiltrative growth, or mitotic activity were further categorized into four main types: malignant glomus tumor (glomangiosarcoma), symplastic glomus tumor, glomus tumor of uncertain malignant potential, and glomangiomatosis [7]. The histologic touchstone of malignancy in glomus tumors are deep location, size >2 cm, presence of atypical mitotic figures, or a combination of moderate to high nuclear grade and ≥5 mitotic figures per 50 high-power fields [8]. There is a marked risk of metastasis and death from disease in patients whose tumor meets the above criteria.
The main histological characteristic of tracheal glomus tumor - the glomus cell cluster arrangement around dilated venous vessels - contributes to its main differential diagnosis with carcinoid tumor and myopericytoma (previous hemangiopericytoma) [7]. Carcinoid tumor has a reduced amount of the vascular pattern, stains positively for neuroendocrine markers (chromogranin, synaptophysin) and cytokeratin, does not develop smooth-muscle actin, and shows intracytoplasmic dense-core neurosecretory granules on electron microscopy. A myopericytoma contains spindle-shaped cells with elongated nuclei, the vessels show a “staghorn-like” arrangement, and the cells tint positive for vimentin (±CD34 and CD56). Smooth-muscle differentiation is infrequent [4]. Amending further differential diagnoses of endobronchial pathologies, these are (1) ulterior airway tumors (squamous cell carcinoma, adenoid cystic carcinoma, squamous cell papilloma, mucoepidermoid carcinoma, hamartoma, lipoma, epithelioid leiomyoma, chondromas, endobronchial plasmocytoma, paraganglioma, hemangioendotheliomas, and tracheal amyloidosis), (2) infections (mucus plugs, tuberculosis), and (3) inflammatory diseases (sarcoidosis, Wegener disease, rheumatoid granuloma) [9]. Narrowing down the differential diagnoses, morphological imaging such as CT or MRI provides excellent anatomical details, whereas functional imaging such as PET-CT only shows variable and thus discordant metabolic activity [10]. Preoperative suspicion, cautious morphologic observation and immunohistochemical staining with appropriate markers should allow making the diagnosis.
There is no “gold standard” in the treatment of glomus tumors of the trachea - there is rather a “first-choice” in the treatment process. As they frequently protrude polypoid-mass-like into the lumen of the trachea and thereby causing partial or complete obstruction, initial bronchoscopic intervention play a pivotal role in the multidisciplinary treatment approach. It allows prompt and actual airway recreation in a relentlessly symptomatic patient and provides diagnostic evidence before surgery. To avoid local recurrence, time shifted tracheal sleeve resection with primary reconstruction of the trachea should succeed in the multidisciplinary treatment scenario [4]. Particularly, when an involvement of the airway wall has been precisely assessed by computed tomography - as it shows typically marked contrast enhancements due to the tumors rich vasculature [10]. One has to keep in mind: thorough surgical resection is curative, does not necessitate adjuvant actions, and has an exceptional prognosis. Incomplete resection means decelerated local tumor recurrence; failure to perform surgical resection may result in failure to cure. Endoscopic intervention alone (i.e. bronchoscopic resection of the tumor using laser photocoagulation, electrocautery, or mechanical debulking) may be designated if (1) the alteration is rigorously restricted to the airway lumen without distension into the tracheal wall, (2) the tumor is histologically benign, (3) as a first-line action in critical conditions where instantaneous restoration of airway is compulsory allowing patient stabilization before surgery or (4) the patient is unfit or unwilling for surgery [2]. With the experience of at least 82 cases published until 2019 and an inconsistent yet increasing trend to resect these lesions endoscopically: exclusive bronchoscopic interventional techniques with or without adjuvant radiotherapy should be reserved for either high-risk patients or patients with inoperable tumors [11]. All other patients should not be withheld from the multidisciplinary aspects of the sequential treatment process. Taking our patient as an ideal example of a typical representative of the other 82 published cases, the prognosis of patients undergoing multidisciplinary approach including tracheal resection for glomus tumor is excellent with no evidence of tumor recurrence over an (in our case) eight-year follow-up period.
Conflicts of interest
None.
Footnotes
Presented at the 27th Annual Meeting of the German Society of Thoracic Surgery, Mannheim, Germany, 12th-14th September 2018.
References
- 1.Gombos Z., Zhang P.J. Glomus tumor. Arch. Pathol. Lab Med. 2008;132:1448–1452. doi: 10.5858/2008-132-1448-GT. [DOI] [PubMed] [Google Scholar]
- 2.Tan Y., Yang P., Deng X., Tang Y. Glomangioma of the trachea: a case report and literature review. Oncol. Lett. 2015;9:1273–1277. doi: 10.3892/ol.2015.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hussarek M., Rieder W. Glomus tumor of the trachea. Der Krebsarzt. 1950:208–212. [PubMed] [Google Scholar]
- 4.Wang C., Ma Y., Zhao X., Sun P.L., Zhang Y.M., Huang M., Zhu Y., Jin S.X. Glomus tumors of the trachea: 2 case reports and a review of the literature. J. Thorac. Dis. 2017;9:E815–E826. doi: 10.21037/jtd.2017.08.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schiefer T.K., Parker W.L., Anakwenze O.A., Amadio P.C., Inwards C.Y., Spinner R.J. Extradigital glomus tumors: a 20-year experience. Mayo Clin. Proc. 2006;81:1337–1344. doi: 10.4065/81.10.1337. [DOI] [PubMed] [Google Scholar]
- 6.Chabowski M., Paszkowski A., Skotarczak J., Dorobisz T., Leśniak M., Janczak D., Janczak D. Glomus tumor of the stomach - a case report and A literature review. Pol. Przegl. Chir. 2016;88:356–358. doi: 10.1515/pjs-2016-0076. [DOI] [PubMed] [Google Scholar]
- 7.Sakr L., Palaniappan R., Payan M.J., Doddoli C., Dutau H. Tracheal glomus tumor: a multidisciplinary approach to management. Respir. Care. 2011;56:342–346. doi: 10.4187/respcare.00761. [DOI] [PubMed] [Google Scholar]
- 8.Calonje E., Fletcher C.D.M. Vascular tumors. In: Fletcher C.D.M., editor. Diagnostic Histopathology of Tumors. third ed. Churchill Livingstone Elsevier; Philadelphia, PA: 2007. pp. 70–72. [Google Scholar]
- 9.Fernandez-Bussy S., Labarca G., Rodriguez M., Mehta H.J., Jantz M. Concomitant tracheal and subcutaneous glomus tumor: case report and review of the literature. Respir. Med. Case Rep. 2015;16:81–85. doi: 10.1016/j.rmcr.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham J.D., Plodkowski A.J., Giri D.D., Hwang S. Case report of malignant pulmonary parenchymal glomus tumor: imaging features and review of the literature. Clin. Imaging. 2016;40:144–147. doi: 10.1016/j.clinimag.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venegas O., Newton A., Vergara N., Singhal S., Predina J.D. Tracheal glomus tumor: a case report and review of the literature. Rare Tumors. 2017;9:6848. doi: 10.4081/rt.2017.6848. [DOI] [PMC free article] [PubMed] [Google Scholar]


