Graphical abstract
Keywords: Salinity, Endophytes, nifH, Halophytes, Diazotrophs, Soil
Highlights
-
•
Revealing of the community composition of diazotrophic endophytes of S. europaea.
-
•
The abundance of bacterial diazotrophs in plant organs of S. europaea.
-
•
Domination of endophytic diazotrophs from Actinobacteria in higher salinity.
-
•
Indication of new diazotrophic species associated with halophytes.
-
•
Selection of diazotrophic endophytes useful in agriculture.
Abstract
Despite the great interest in using halophyte Salicornia europaea L. as a crop in extreme saline habitats, little is known about the role played by associated endophytic bacteria in increasing tolerance of the host-plant to nutrient deficiency. Main objectives of this study were to investigate the community composition of diazotrophic endophytes of S. europaea grown under natural conditions, and determine the proportion of plant-growth promoting bacterial strains able to fix N2. To quantify the abundance of diazotrophic bacterial endophytes in stems and roots of S. europaea, nifH gene and 16S rDNA copy numbers were assessed by quantitative real-time PCR, and characterized the taxonomic structure of cultivable bacteria based on selective medium for diazotrophs. The highest copy numbers of nifH and 16S rDNA were observed in the stems of plants growing at the test site characterized by lower salinity, and correlated with high N concentrations in plant tissues. The abundance of bacterial diazotrophs isolated from plant tissues ranged from 3.6 to 6.3 (log10 of cfu per gram dry plant tissue) and varied in a site- and plant-organ manner. Proteobacteria dominated in plants growing in lower salinity while Actinobacteria prevailed in plants originating from higher salinity, what suggest better adaptation of this group of bacteria to extreme salinity. The results provide insights into new species of diazotrophs associated with halophytes that can be used to optimize strategies for selecting biostimulants useful in saline soils.
Introduction
Soil salinization is increasing day by day and over 7% of the total world landmass is currently affected by salinity [1]. A significant proportion of this area belongs to agricultural land, resulting in nearly 45 million ha being excluded from cultivation [2]. Salinization can be an effect of natural processes (e.g. geological, hydrological, or weather events) as well as anthropogenic activity (e.g. improper methods of irrigation and fertilization, deforestation, chemical contamination, and poor water management) [3], [4], [5]. Increased salinity has a negative influence not only on physico-chemical soil parameters (e.g. pH, availability of nutrients) but also on plants (e.g. photosynthesis, water management) and soil microorganisms (e.g. diversity, activity).
The plant kingdom contains only a small group of plant species (∼1%) belonging to halophytes, which possess highly distinctive capacities for salt tolerance as a result of evolutionary adaptation to their environments. These plants are able to survive and reproduce at concentrations of sodium and chloride ions that would be toxic to most plant species [6]. Halophytes have received particular attention in the last few years not just as model species in salt tolerance research, but also as potential forage, fiber, and biomass crops, as well as platforms for developing crop systems that use saline water and/or ameliorate salinized soils [7].
This study focuses on Salicornia europaea L., a salt marsh halophyte belonging to the Amaranthaceae, which is one of the most salt-tolerant plant species worldwide [8]. Significant advances have been made in understanding how halophytes have adapted to high salinity conditions [6], [9]. It is well known that halophytes can cope with high soil salinity due to morphological and biochemical adaptations [1]. However, recently special attention has also been paid to the effect of specific microbial associations, e.g. endophytes, which can increase the tolerance of host-plants to high salinity and other unfavorable environmental conditions [10], [11], [12]. Salicornia as a wild plant, not modified by plant breeding, has probably retained over millennia the natively established plant-microbiome functional interaction at specific salt-affected areas. Such a naturally developed association between a plant and its microbiota may help the plant to grow and successfully compete with other plants.
Some plant-associated bacteria can biologically fix atmospheric N2 to plant-available ammonia (NH3). This process is ecologically important as an input of fixed nitrogen (N) into many habitats [13], and represents a promising substitute for chemical N fertilizers. Microorganisms catalyze nitrogen fixation via the enzyme nitrogenase, which has been highly conserved throughout evolution. All N2 fixers carry the nif (nitrogen fixation) genes, which encode the nitrogenase complex [14]. The nif operon includes the nitrogenase structural gene nifH, which has been sequenced to provide a large database from diverse environments [14], [15]. One of the best-reported outcomes of this plant-microbe association is the promotion of plant growth by direct and indirect mechanisms. Besides fixing atmospheric N2, these bacteria can also produce plant growth hormones, and some species are reported to improve nutrient uptake and increase plant tolerance against biotic and abiotic stresses [16], [17].
Since crop production is highly dependent on chemical nitrogen fertilizers and their extensive use may have negative effects on human and environment health, as well as on generating greenhouse gasses and reducing the ozone layer [18], the existence and application of bacterial diazotrophs adapted to saline conditions in non-host plants could greatly increase the production of glycophytic crops in saline soils.
The hypothesis is that associative diazotrophic bacteria, which are known to fix atmospheric nitrogen and provide nitrogen to their host plants, may be established in the microbiota of S. europaea and probably live there endophytically. If diazotrophs exist in the endophyllosphere of S. europaea the questions are: (1) which bacterial species do they belong to; (2) are these species also known to be related to crop plants, and finally (3) how does the level of salinization affect the distribution and community structure of diazotrophic endophytes of S. europaea? More extensive knowledge about diazotrophic endophytes associated with halophytes will facilitate the understanding of possible mechanisms involved in the interaction and possible role of microbiota in adaptation to saline stress conditions.
In this study halophyte S. europaea plant samples were collected and analyzed from two sites representing moderate and high soil salinity. The microbiota of S. europaea was assessed by quantitative real-time PCR of both the nifH genes and 16S rDNA. Although the overall abundance of diazotrophs isolated from stems and roots was higher in plants growing at lower salinity, the proportion of potential diazotrophic bacteria in the total bacterial community revealed an enrichment of diazotrophs in the plants grown in highly saline soil. A total of 141 endophytic diazotrophs were cultured and identified based on their phylogenetic affiliations by comparing their 16S rRNA gene sequences.
Material and methods
Site description and sampling
Plant samples were collected at two test sites located in central Poland and characterized by extreme anthropogenic (S1) and natural (S2) origin of salinity. Site S1 (N 52°48, E 18°15) comprised meadows near a soda factory (Soda Poland CIECH SA) in Inowrocław-Mątwy, where a long-term process of inappropriate waste storage from soda production has caused strong alkalization and salinization of soils. The salt meadows are dominated by halophytic species, e.g. Salicornia europaea, Aster tripolium, Spergularia salina, Glaux maritima, Triglochin maritimum, Puccinellia distans and Atriplex prostrata spp. prostrata var. salina. Site S2 (N 52°53, E 18°47) was located in Ciechocinek (close to a brine graduation tower), at the largest lowland health resort in Poland involved in the treatment of respiratory illnesses. In this area a Halophytes Nature Reserve Park and a landscape park “Natura 2000” were established to protect rare plant species [19].
At each test site, three sub-plots (2 m × 2 m) were designated at a distance of 10 m from each other. From each sub-plot five plants of S. europaea with adjacent soil (20 cm × 20 cm × 20 cm soil cubes) were collected in autumn 2013 (15 plants per test site and 30 plants in total). Each sample was taken using sterile tools, placed in a separate plastic bag to avoid contamination and immediately transported to the laboratory for analysis.
Soil samples (three replicates per site) were analyzed according to methods described by van Reeuvijk [20]. The soil at S1 was classified [21] as mineral-organic (organic matter content 10–20%) and at S2 as mineral (organic matter content < 10%). Salinity at both sites was related to the presence of chloride and other anions: Cl− ≫ SO42− > HCO3−. Salinity, measured as the electrical conductivity of a saturated extract, were 55 dS∙m−1 at S1 and 112 dS∙m−1 at S2, and resulted from the domination of calcium ions (Ca2+ > Na+ ≫ Mg2+ > K+) at S1 and sodium ions (Na+ ≫ Ca2+ > Mg2+ > K+) at S2 (Table 1).
Table 1.
Physico-chemical soil parameters of the two test sites: S1 (Inowroclaw) and S2 (Ciechocinek). Listed are the content of cations and anions in saturated extract.
| Test site | ECe, dS/m | NaCl, % | Org. matter, % | N total, % | Moisture, % | pH | HCO3−, mg/L | Cl−, mg/L | SO42−, mg/L | K+, mg/L | Ca2+, mg/L | Mg2+, mg/L | Na+, mg/L | Ca/Na |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 55 | 3.52 | 13.4 | 0.539 | 150 | 7.3 | 742 | 23,770 | 573 | 70 | 9079 | 102 | 8283 | 1.096 |
| S2 | 112 | 7.168 | 6.88 | 0.203 | 73 | 7.6 | 714 | 22,473 | 2216 | 256 | 1117 | 275 | 14,579 | 0.077 |
Preparation of plant samples for analysis
Endophytic bacteria were analyzed in two different organs of S. europaea: stems and roots. One averaged sample of analyzed plant organs (stem and root) was prepared (10 g) from five plants sampled at each sub-plot. Finally, from each test site (S1 and S2) three representative samples of stems and roots were analyzed. Plant samples were surface sterilized in 100 ml of 15% hydrogen peroxide by shaking (5 min), then washed three times with 100 mL of sterile 2% NaCl solution. The liquid obtained after third washing was used to assess the sterilization process. Only successfully surface-sterilized plant organs were used for further analysis.
Analysis of nifH gene and 16S rDNA copy numbers in plant organs of S. europaea (real-time PCR assays)
DNA was extracted from the surface-sterilized and lyophilized plant samples (stems and roots) using the DNeasy Plant Mini Kit (Qiagen, Hilden GmbH, Germany), according to the manufacturer’s instructions. DNA concentrations were measured photometrically at λ = 260 nm (Nano-drop, ThermoFisher). Quantitative real-time PCR was conducted using a Bio-Rad detection system (Germany). Amplification was performed with primer pairs for: nifH (19F: 5′-GCIWTYTAYGGIAARGGIGG-3′ and 388R: 5′-AAICCRCCRCAIACIACRTC-3′) [22] and 16S rDNA (519f 5′-CAGCMGCCGCGGTAANWC-3′ and 907r 5′-CCGTCAATTCMTTTRAGTT3′) [23]. To ensure appropriate template plant DNA concentrations enabling quantification of the 16S rDNA and the nifH gene, PCR was performed using undiluted, 1:10 diluted, and 1:100 diluted plant DNA. PCR was performed using the program described by Jurayeva et al. [24]. Abundance of nifH (involved in N2–fixation) and 16S rDNA (corresponding to the total number of bacteria) was expressed as copy number of target gene μg−1 of DNA used for amplification. The proportion of nifH-gene copy number per number of 16S rDNA was calculated after both qPCR equilibration curves were equalized in their efficiency.
Abundance of endophytic diazotrophs associated with S. europaea
Surface-sterilized plant samples were homogenized in mortars under sterile conditions. The material obtained was used as a starting sample for preparing serial dilutions. Bacterial strains were isolated using medium selective for the growth of diazotrophs [25]. From each variant of isolation (18 in total) three replicates of each dilution (10−1–10−5) were investigated (15 plates per variant, 180 plates in total). Bacteria were grown at 26 °C for 7 d and counted (one representative dilution with an average number of colonies per plate between 30 and 300; in most cases 10−2–10−4 was chosen for each variant of the experiment). Results are presented as cfu (colony-forming units per gram of fresh plant tissue). From each plant organ (stem and root) and each test site (S1 and S2) 22–47 bacterial colonies were chosen based on their different morphological features in order to detect a broader spectrum of bacterial taxa. In total, 141 strains were selected for further investigation (Supplementary materials, Table A). Selected bacterial strains were purified and maintained on agar slants with Rennie medium [25]. All strains were proved for their potential biological nitrogen fixing ability by identifying the presence of the diazotrophic marker gene nifH in the bacterial genomes.
Molecular identification of bacterial strains by PCR and sequencing
Single colonies cultivated on Rennie medium were suspended in 0.85% NaCl and centrifuged. DNA was isolated using the DNeasy Plant Mini Kit (Qiagen, Hilden GmbH, Germany) according to the manufacturer’s instructions. The 16S rRNA gene was amplified by PCR using the primers 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492R (3′-CTACGG CTACCTTGTTACGA-5′) according to procedure described by Szymańska et al. [10] and sequenced using the same primers. Sequencher 5.1 (Gene Codes 20) software was used to assemble the forward and reverse sequences obtained. The sequences acquired were used for identification based on comparing these sequences with reference sequences deposited in the GenBank nucleotide database by BLASTn [26]. A minimum of 99% similarity was required for appropriate identification. All DNA sequences obtained were submitted to GenBank and accession numbers were assigned: MK398004-MK398122 (Supplementary materials, Table A).
Phylogenetic analysis
The phylogenetic affiliation of the cultivated strains is based on aligning the sequenced 16S rRNA gene fragments, and their closely related sequences, derived from the GenBank comparison (BLASTn) [26], using MUSCLE v3.8.31 (https://www.drive5.com/muscle/) [27]. The multiple sequence alignment was trimmed with trimAl v1.2 (http://trimal.cgenomics.org) [28] filtering out positions with 20% or more gaps across the sequences, unless less than 60% of the positions remain as conserved sites. Subsequently, the best-scoring maximum likelihood tree was inferred with RAxML v8.2.12 (https://github.com/stamatak/ standard-RAxML) [29] using the GTRCAT approximation model and rapid bootstrap analysis based on 1000 replicates. Visualization and annotation of the phylogenetic tree was conducted with iTOL v4 (https://itol.embl.de) [30], taking the binary information of test sites (S1/S2), plant type (non-/halophytic) and plant organ (stem/root) into account.
Nitrogen (Nt) and carbon (Ct) concentrations in plant tissues
The freeze-dried samples of stems and roots sampled from both test sites (S1 and S2) were ground to <0.5 mm by a mill (Retsch GmbH, Haan, Germany) to determine Nt and Ct concentrations. Analysis was performed using the CHN-O Rapid Elemental Analyser (Elementar Analysensysteme GmbH, Henau, Germany).
Statistical analyses
The data obtained for nutrient concentration in plant tissues, copy number of nifH and 16S rDNA and total number of cultivable bacterial diazotrophs were analyzed by one- and two-factorial analyses of variance (ANOVA). The statistics were computed using STATISTICA (StatSoft, 2010).
Results
Nutrient concentrations in the plant organs of S. europaea
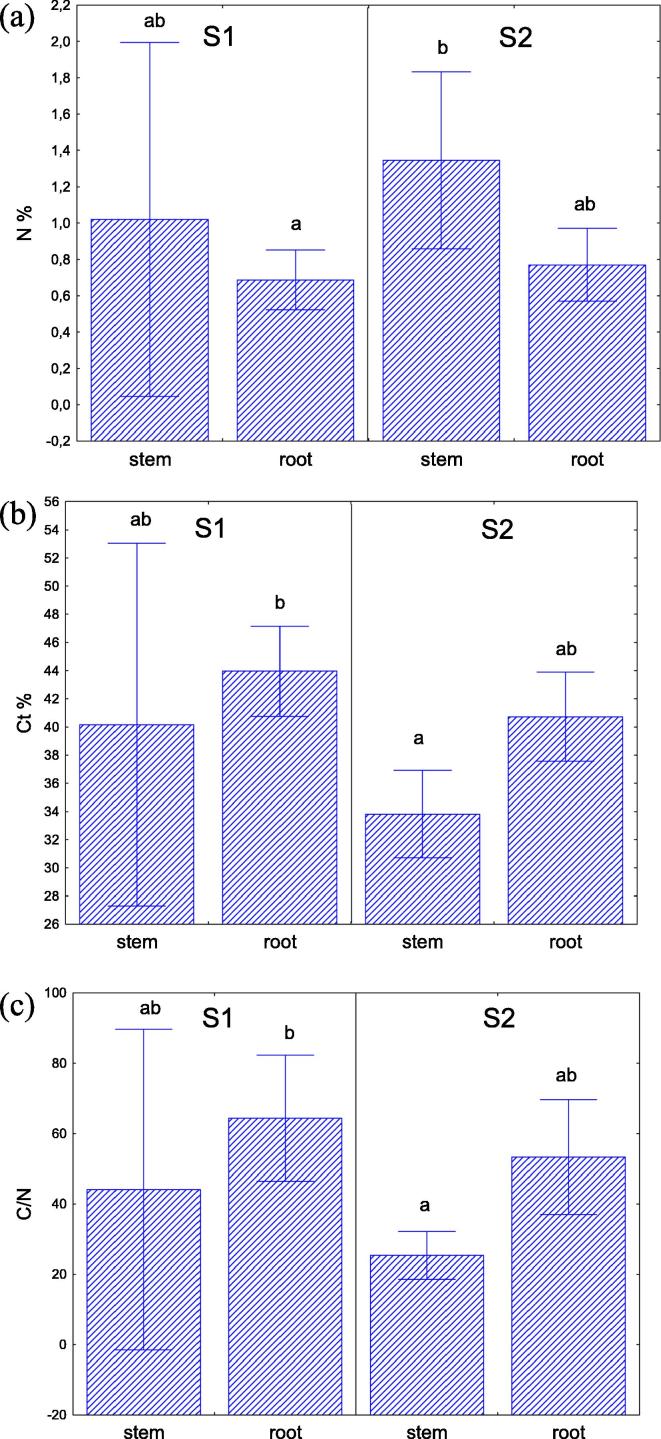
The halophyte Salicornia europaea L was sampled from two saline environments with contrasting salinity (ECe 55 dS m−1 at test site S1, and 112 dS m−1 at test site S2). Although the soil total nitrogen content was higher in the lower saline soil S1, the nitrogen concentration in stems and roots of S. europaea L. was not significantly different between plants grown on S1 and S2. Generally the N concentration in stems was higher than in roots (Fig. 1; Supplementary materials: Table A). In contrast to N, significantly higher levels of carbon were found in the roots, and at the test site S1 (lower soil salinity) the concentration of this element in plant tissues was generally higher than at S2 (higher salinity). Analysis of the carbon-nitrogen ratio was correlated with the results obtained for carbon.
Fig. 1.
Nutrient concentrations in stems and roots of S. europaea. (a) Nutrient concentrations of total nitrogen (N in %), (b) total carbon (C in %) and (c) the C/N ratio in stems and roots of S. europaea (mean ± SD, n = 9) growing at the two test sites, S1 and S2. Bars indicate standard deviation p < 0.05.
Proportion of nifH gene at 16S rDNA copy numbers in stems and roots of S. europaea depend on the soil salinity level
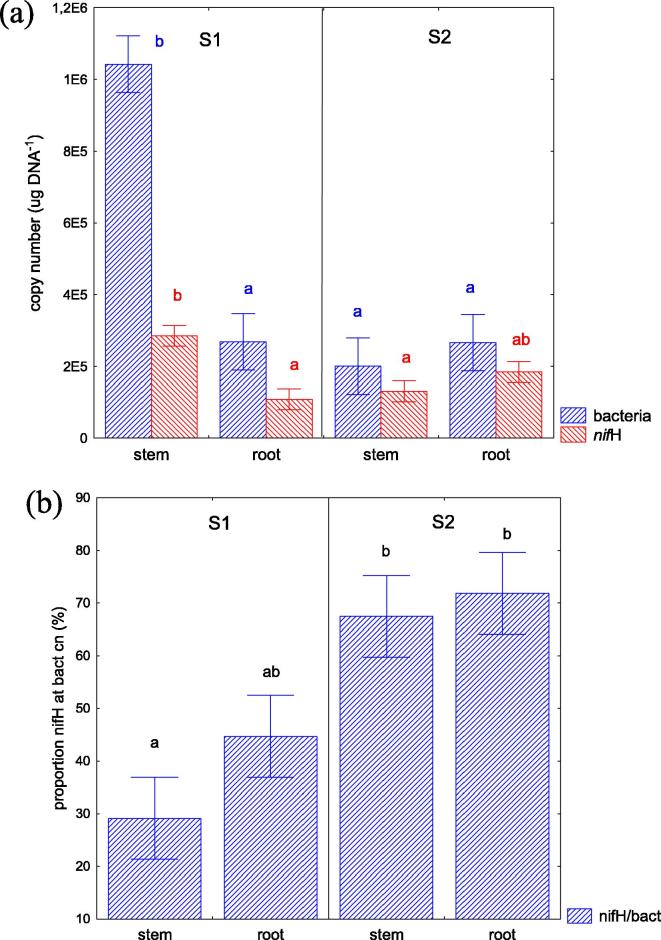
To assess the number of potential diazotrophic organisms in the population, the nifH genes (encoding potential diazotrophic bacteria) and the total bacterial community was determined in plant tissues of S. europaea by analyzing both nifH gene and 16S rDNA copy numbers in the same DNA sample using quantitative real-time PCR (qPCR). Knowing, that both, the 16S rDNA and the nifH gene copy numbers are variable between various bacterial species and the absolute numbers can be affected by this variation, the present calculation was conducted to proximately estimate a proportional shift within the endophytic bacterial community. The highest abundance of 16S rDNA and nifH genes (1.04 × 106 and 2.85 × 105, respectively) was observed in the stems of S. europaea growing at S1, characterized by lower salinity levels in the soil. In the other variants of the experiment, the copy number of the genes did not differ significantly from each other, and ranged between 2.01 and 2.69 × 105 for 16S rDNA copy number, and 1.08–1.84 × 105 for nifH copy number (Fig. 2A). The proportion of potential diazotrophic bacterial numbers at total bacterial counts was calculated using the molecular information obtained from DNA samples extracted from roots and stems. After both qPCR equilibration curves were equalized in their efficiency, the data revealed an enrichment of potential diazotrophic bacteria within the total bacterial community in the halophyte S. europaea grown in highly saline conditions (S2) (Fig. 2B).
Fig. 2.
Soil salinity levels determine the proportion of nifH at 16S rDNA copy numbers in stems and roots of S. europaea. (a) The gene copy number of nifH and 16S rDNA (per µg of DNA) in different plant organs of S. europaea (stems and roots) growing at two saline test sites (S1 and S2) was analyzed using quantitative real-time PCR (qPCR), and (b) the data used to calculate the proportion of potential diazotrophic bacteria (nifH gene copy number) at total bacterial counts (16S rDNA copy number). Bars indicate standard error at P ≤ 0.05.
Abundance and taxonomic structure of cultivable endophytic diazotrophs
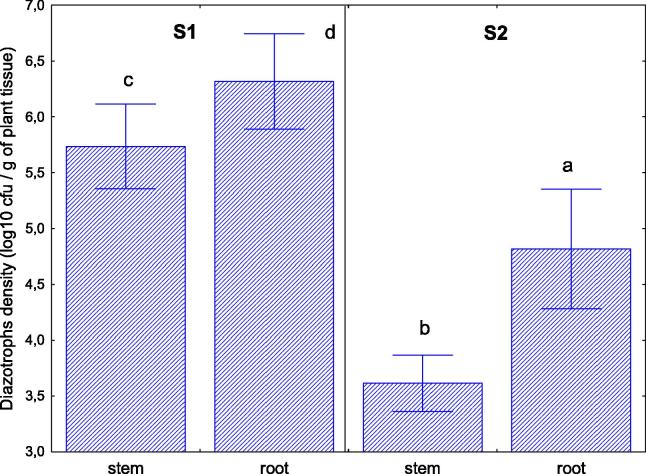
Analysis of bacterial diazotrophs which were isolated and cultivable from stems and roots of S. europaea L. growing at both test sites S1 and S2 showed a significantly higher abundance of this group of microorganisms in roots that had direct contact with highly diverse microbial communities in the rhizosphere soil (Fig. 3). The abundance of diazotrophs isolated from stems and roots at S1 was 1.5- and 1.3-fold higher (respectively) than in plants growing at higher salinity (S2), ranging from 5.7 to 6.3 log10 cfu g−1 fresh plant tissue at S1 and from 3.6 to 4.8 log10 cfu g−1 fresh plant tissue at S2.
Fig. 3.
Abundance of cultivable endophytic diazotrophs. Abundance (log10 cfu per 1 g of fresh plant tissue) of endophytic bacterial diazotrophs isolated from stems and roots of S. europaea growing at the two saline test sites (S1 and S2).
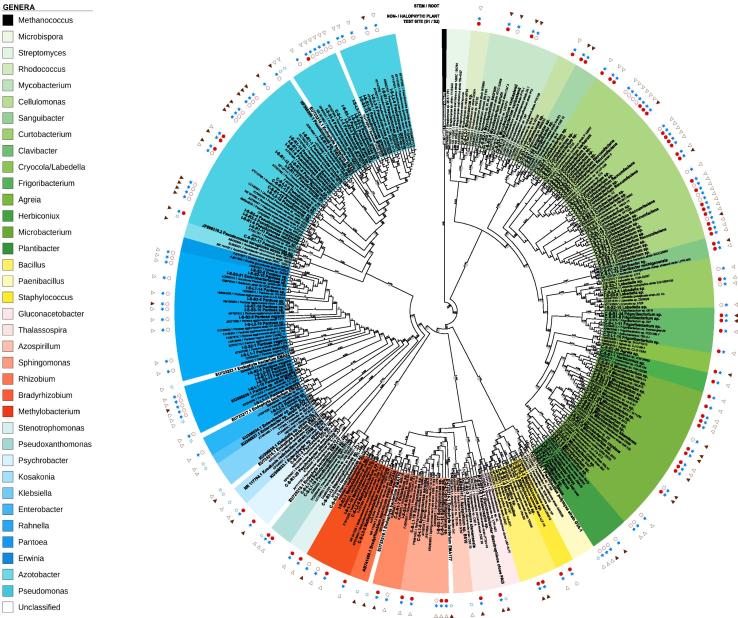
In total, 141 endophytic diazotrophs were isolated from stems and roots of S. europaea L. sampled at the two test sites: 70/71 strains from plants growing at test site S1/S2 (47 isolates/site from stems and 23–24 from roots) (Fig. 4). Fig. 4 shows the phylogenetic affiliations (based on 16S rRNA genes) of diazotrophic strains isolated from the endophyllosphere (stems, roots) of the halophyte S. europaea L. compared to endospheric diazotrophs originating from non-halophytic crop plants. Most of the endophytic diazotrophs from S. europaea L belonged to the phyla Actinobacteria (50%) and Proteobacteria (47%), and only a few to Firmicutes (3%). At S2 (higher salinity, marked with a red filled point) diazotrophs were strongly dominated by Actinobacteria and at S1 (lower salinity, marked by red empty point) by Proteobacteria. The genus Curtobacterium and Microbacterium (phylum Actinobacteria) were isolated from both test sites, but were more abundant at S2, and the genus Rhodococcus, Cellulomonas, Sanguibacter, Clavibacter, Frigoribacterium, Agreia and Herbiconiux were found exclusively at S2. The genus Pseudomonas (phylum Proteobacteria) was isolated from both test sites, but dominated at S1, and Pantoea was characteristic only for S1, with higher abundance in stems. In general, diazotrophs isolated from plant organs of S. europaea growing at S2 were affiliated to 13 different genera that were not observed at all at S1, while at S1 strains from only two genera were isolated which were not detected at S2. These observations confirm a higher diversity within the diazotrophic endophytic microbiota of the halophytic plant S. europaea when growing in higher saline soils (S2). Surprisingly, for the first time, from the endophyllosphere and endorhizosphere of the halophytic plant S. europaea a wide range of genera from Actinobacteria phylum were isolated, while these diazotrophs were not yet detected in the plant endorhizosphere or endophyllosphere of glyophytes (Fig. 4).
Fig. 4.
Taxonomic structure of cultivable endophytic diazotrophs. Dendrogram displaying phylogenetic affiliations (based on 16S rRNA genes) of diazotrophic strains isolated from the endophyllosphere (stems, roots) of the halophyte Salicornia europaea L. grown at the two different saline test sites (S1/S2) compared to endospheric diazotrophs originating from non-halophytic crop plants. Abbreviations: Branch lengths are assigned as numbers and the bootstrap support values, ranging from 70% to 100%, as gray circles to the edges. All strains are colored according to their genus. Methanococcus voltae PS served as outgroup. Diazotrophic strains are written in bold font style, whereas phylogenetic closely related species are written in normal style. The surrounding circles describe: strains of halophytic ( ) and non-halophytic plants (
) and non-halophytic plants ( ), the plant isolation site, stem (
), the plant isolation site, stem ( ) and root (
) and root ( ), and the two distinct isolation test sites, S1 (
), and the two distinct isolation test sites, S1 ( ) and S2 (
) and S2 ( ).
).
Discussion
Crop production in saline areas is a huge challenge since all plant species currently used in agriculture belong to glycophytes, and these are relatively sensitive to medium and high salt concentrations in soils. S. europaea belongs to a family of underutilized plants with naturally developed tolerance to unfavorable environmental conditions. In previous studies with the use of two endophytic bacterial strains isolated from S. europaea (Pseudomonas sp. ISE-12 and Xanthomonadales sp. CSE-34 [12] we have revealed more evident effect of strain containing nifH gene on the growth of Beta vulgaris under different concentrations of salinity. This is why the presented work, for the first time, focused on diazotrophic endophytes of this halophyte and characterized this important group of bacteria. Diazotrophic endophytes are known to protect the host-plant against harsh environmental conditions by increasing nutrient availability or producing phytohormones [17].
Bacterial endophytes of S. europaea: The total bacterial community vs. diazotrophs
Since the abundance of total bacterial counts from S. europaea growing at the test site characterized by higher salinity (S2: 112 dS/m) was significantly lower than in moderate saline soil (S1), we suggest that bacteria are relatively sensitive to extreme concentrations of salt, but tolerate medium soil salinity (S1: 55 dS/m). Similarly, an overall lower number of microorganisms (bacteria and fungi) in root and rhizosphere samples of S. europaea from the same S2 test site were identified earlier by Szymańska et al. [10]. Therefore it seems correct to conclude that extreme salinity generally inhibits microbial abundance. However, proportionally the number of potential diazotrophic bacteria was enriched in both stem and root samples of S. europaea when grown in extremely salty conditions. Moreover, observed at both S1 and S2 sites, the significantly higher number of diazotrophs isolated from roots than stems confirms that the main source of diazotrophs of S. europaea is rhizosphere soil, an environment with the highest microbial diversity and activity.
A culture-independent approach achieved by analyzing nifH transcripts provided results on active diazotrophs in the rhizosphere, and nif gene amplification and sequencing has been used to identify nitrogen-fixing bacteria associated with rice, sorghum, wheat and maize [18]. Present results using real-time qPCR show significantly higher nifH and 16S rDNA copy numbers in the stems than in the roots of S. europaea from S1 (lower salinity), which is not consistent with the results obtained for cultivable diazotrophs. This contradiction could originate from the lack of suitable media to cultivate as yet non-cultured diazotrophic organisms, especially when they grow within the stem endophyllosphere, where specific nutritional and environmental niches exist that have only recently started to discover [31].
Nitrogen is one of the most abundant elements in plants (after carbon: about 40% of plant dry matter and oxygen: about 45%); however N concentrations in plant tissues is plant species specific and changes during developmental stages (higher in young tissues and lower in mature or senesced parts) [32], [33]. In the present experiments at both analyzed test sites, nitrogen concentrations in plant tissues of S. europaea were in the range of 0.68–1.34% of dry plant weight, with significantly higher N values observed for shoots than roots. However this is a relatively low level of N compared to other crops, e.g. wheat (Triticum aestivum) with N contents in shoots between 1.5 and 3% of plant dry weight [32]. There are three possible reasons for such low nitrogen concentrations in S. europaea tissues: (i) the generally low nitrogen demand of this crop; (ii) the late growing season when samples were collected for testing (autumn), or (iii) the extremely low N content in the soil at both analyzed test sites (0.2–0.5 Nt). Since plant-associated microorganisms (rhizosphere, symbiotic or endophytic) are key factors in increasing plant nutrient efficiency [e.g. [34], [35]], it is assumed that S. europaea plants growing in poor soils are stimulated by unique microorganisms that allow them to overcome deficiencies in the environment.
Endophytic diazotrophs of S. europaea
Previous studies revealed that the microbiome of S. europaea is dominated by the phyla Proteobacteria, Bacteroidetes, Actinobacteria and Firmicutes [11], [36], [37], and many of their representatives belong to typical halotolerant bacteria with a potential role in mitigating salt stress, e.g. Salinicola sp., Kushneria sp. [10]. In general, diazotrophs can be found among alphaproteobacteria, gammaproteobacteria, Firmicutes, betaproteobacteria and cyanobacteria; however they generally correspond to minor components of the ecosystem since nitrogen fixation is an energetically expensive process for bacteria [18]. Endophytic diazotrophs isolated from S. europaea represent a broad range of N2-fixing bacteria with Actinobacteria dominating at the test site characterized by higher salinity (S2), and Proteobacteria at lower salinity (S1). Most of the isolates from Actinobacteria (70 isolates, in total) belonged to the genus Curtobacterium (30 isolates) (Curtobacterium sp., C. flaccumfaciens, C. herbarum) and Microbacterium (17 isolates) (Microbacterium sp., M. kitamiense, M. oxydans), and some of them to Rhodococcus, Mycobacterium, Cellulomonas, Sanguibacter, Clavibacter, Cryocola/Labedella, Frigoribacterium, Agreia, Herbiconiux, and Plantibacter. But all of them have been identified as endophytic diazotrophs for the first time. These organisms mostly originated from the endophyllosphere of the halophyte S. europaea grown on the high saline test site S2. It is worth to mention that salt-tolerant diazotrophic Actinobacteria: Brachybacterium saurashtrense sp. nov. [38] and Zhihengliuella somnathii sp. nov. [39] were isolated earlier from other Salicornia spp. (e.g. S. brachiata). Further diazotrophic endophytes in the present study belong to Proteobacteria (66 isolates in total), dominated by two genera: Pseudomonas (29 isolates) (Pseudomonas sp., P. marincola, P. fluorescens, P. koreensis) and Pantoea (17 isolates) (Pantoea sp., P. vagans, P. ananatis). Other examples of diazotrophic bacteria belonging to Pseudomonas and are also associated with crops are: P. aeruginosa [JF899310] isolated from Pennisetum glaucum (L.) [40], and Pseudomonas sp. SB2 [HF566309] isolated from a grape variety [41].
Diazotrophs from the genera Glucanacetobacter, Azospirillum, Kosakonia and Enterobacter, which are often detected and described as endophytes in glyophytic crops [e.g. [42] were not isolated from the halophyte S. europaea. The phylum Firmicutes was represented by only 4 strains isolated from S2 (higher salinity) (belonging to Staphylococcus sp., Bacillus sp., B. pumilus). This phylum in general is known as a source of diazotrophs; however only one example of a crop endophyte was noted so far (Paenibacillus polymyxa [KU306835]) (Fig. 4).
Halotolerant diazotrophic bacteria can positively promote growth of many crops under salt stress conditions, e.g. Hartmannibacter diazotrophicus E19T isolated from the rhizosphere of Plantago winteri stimulated growth of barley (Hordeum vulgare L.) by reducing ethylene emission [43], [44], five diazotrophic bacteria (Klebsiella, Pseudomonas, Agrobacterium, and Ochrobactrum) isolated from the roots of a halophyte Arthrocnemum indicum successfully colonized the peanut roots and were capable of promoting the plant growth by maintaining ion homeostasis and ROS levels under salt stress condition [45], Pseudomonas stutzeri ISE12 isolated from S. europaea alleviated the salt stress of rape (Brassica napus L.) by activating their antioxidant defence system and triggering the rearrangement of cell walls [46].
In summary, the present results reveal enrichment of diazotrophic endophytes in S. europaea represented by a highly diverse community, and many isolates were identified for the first time. Such isolates have not yet been detected within the endorhizo- or endophyllosphere of other crop plants, which confirms the high specificity of this group of microorganisms to the saline environment. Notably, N2-fixing bacteria represented by Actinobacteria seem to have higher tolerance to salinity than Proteobacteria, which were more often observed at lower salinity. Whether and which of these organisms alleviate salt stress conditions or help the plant by supplying nitrogen or specific hormones to adapt to such harsh conditions remains to be confirmed in future studies with the use of microscopic methods, e.g. fluorescent in situ hybridization (FISH) or confocal laser scanning microscopy (CLSM). Interestingly, the number of cultivable diazotrophic strains was higher in roots of S. europaea suggesting that this plant organ possess unique properties for selecting N2-fixing bacteria.
Conclusions
S. europaea represents a convenient host-plant system for analyzing a range of diazotrophs. Although extreme soil salinity slightly decreases their abundance it profoundly affects their taxonomic structure. Selection of diazotrophic endophytes compatible with specific plant genotypes could become a common practice for improving plant tolerance to unfavorable saline soils to complement advanced technologies applied in green plant biotechnology. The herewith established collection of halotolerant diazotrophic bacteria could provide an excellent source of such biostimulants to be tested in agriculture.
Conflict of Interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Acknowledgements
This study was financially supported by Leibniz Institute of Vegetable- and Ornamental Crops (Grossbeeren) and the National Science Centre (Poland) (DEC-2012/07/B/NZ9/01801).
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2019.05.002.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Xu C., Xiaoli T., Hongbo S., Hongyan W. Salinity tolerance mechanisms of economic halophytes from physiological to molecular hierarchy for improving food quality. Curr Genom. 2016;17(3):2017–2214. doi: 10.2174/1389202917666160202215548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.FAO. Terrastat Database, http://www.fao.org/about/en/.
- 3.Evelin H., Kapoor R., Giri B. Arbuscular mycorrhizal fungi in alleviation of salt stress: a review. Ann Bot. 2009;104:1263–1280. doi: 10.1093/aob/mcp251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rueda-Puente E.O., Farmohammadi S., Moghaddam A., Zakeri O. Plant growth promoting bacteria associated to Salicornia rhyzosphere in Abbas, Iran. Agric Sci Res J. 2011;1:155–165. [Google Scholar]
- 5.Yadav S., Irfan M., Ahmad A., Hayat S. Causes of salinity and plant manifestations to salt stress: a review. J Environ Biol. 2011;32:667–685. [PubMed] [Google Scholar]
- 6.Flowers T.J., Galal H.K., Bromham L. Evolution of halophytes: multiple origins of salt tolerance in land plants. Funct Plant Biol. 2010;37:604–612. [Google Scholar]
- 7.Van Oosten M.J., Maggio A. Functional biology of halophytes in the phytoremediation of heavy metal contaminated soils. Environ Exp Bot. 2015;111:135–146. [Google Scholar]
- 8.Salicornia Patel S. evaluating the halophytic extremophile as a food and a pharmaceutical candidate. 3 Biotech. 2016;6:104. doi: 10.1007/s13205-016-0418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bressan R.A., Park H.C., Orsini F., Oh D., Dassanayake M., Inan G. Biotechnology for mechanisms that counteract salt stress in extremophile species: a genome-based view. Plant Biotechnol Rep. 2013;7:27–37. [Google Scholar]
- 10.Szymańska S., Płociniczak T., Piotrowska-Seget Z., Hrynkiewicz K. Endophytic and rhizosphere bacteria associated with the roots of the halophyte Salicornia europaea L. – community structure and metabolic potential. Microbiol Res. 2016;192:37–51. doi: 10.1016/j.micres.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Szymańska S., Borruso L., Brusetti L., Hulisz P., Furtado B., Hrynkiewicz K. Bacterial microbiome of root-associated endophytes of Salicornia europaea in correspondence to different levels of salinity. Environ Sci Pollut Res. 2018;25:25420–25431. doi: 10.1007/s11356-018-2530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piernik A., Hrynkiewicz K., Wojciechowska A., Szymańska S., Lis M., Muscolo A. Effect of halotolerant endophytic bacteria isolated from Salicornia europaea L. on the growth of fodder beet (Beta vulgaris L.) under salt stress. Arch Agron Soil Sci. 2017;63(10):1404–1418. [Google Scholar]
- 13.Arp D.J. The nitrogen cycle. In: Triplett E.W., editor. Prokaryotic nitrogen fixation. Horizon Scientific Press; Wymondham: 2000. pp. 1–14. [Google Scholar]
- 14.Argandona M., Fernandez-Carazo R., Llamas I., Martinez-Checa F., Caba J.M., Quesada E. The moderately halophilic bacterium Halomonas maura is a free-living diazotroph. FEMS Microbiol Lett. 2005;244:69–74. doi: 10.1016/j.femsle.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 15.Zehr J.P., Jenkins B.D., Short S.M., Steward G.F. Nitrogenase gene diversity and microbial community structure: a cross-system comparison. Environ Microbiol. 2003;5:539–554. doi: 10.1046/j.1462-2920.2003.00451.x. [DOI] [PubMed] [Google Scholar]
- 16.Carvalho T.L.G., Ferreira P.C.G., Hemerly A.S. Sugarcane genetic controls involved in the association with beneficial endophytic nitrogen fixing bacteria. Trop Plant Biol. 2011;4:31–41. [Google Scholar]
- 17.Becker M., Patz S., Becker Y., Berger B., Drungowski M., Bunk B. Comparative genomics reveal a flagellar system, a type VI secretion system and Plant Growth-Promoting gene clusters unique to the endophytic bacterium Kosakonia radicincitans. Front Microbiol. 2018;9:1997. doi: 10.3389/fmicb.2018.01997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenblueth M., Ormeño-Orrillo E., López-López A., Rogel M.A., Reyes-Hernández B.J., Martínez-Romero J.C. Nitrogen fixation in cereals. Front Microbiol. 2018;9:1794. doi: 10.3389/fmicb.2018.01794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piernik A. Growth of three meadow species along a salinity gradient in an inland saline habitat: transplant experiment. Pol J Ecol. 2006;54:117–126. [Google Scholar]
- 20.Van Reeuwijk LP, editor. Procedures for soil analysis. Wageningen: ISRIC; 2002.
- 21.Polish Soil Classification, Systematyka gleb Polski. Soil Sci Annu (Roczniki Gleboznawcze) 2011;62(3):5–142.
- 22.Ueda T., Suga Y., Yahiro N., Matsuguchi T. Remarkable N2-fixing bacterialdiversity detected in rice roots by molecular evolutionary analysis of nifH genesequences. J Bacteriol. 1995;177:1414–1417. doi: 10.1128/jb.177.5.1414-1417.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lane D.J. 16S/23S rRNA sequencing. In: Stackebrandt E., Goodfellow M., editors. Nucleic acid techniques in bacterial systematics. Wiley; Chchester, England: 1991. pp. 205–248. [Google Scholar]
- 24.Juraeva D., George E., Davranov K., Ruppel S. Detection and quantification of the nifH gene in shoot and root of cucumber plants. Can J Microbiol. 2006;52:731–739. doi: 10.1139/w06-025. [DOI] [PubMed] [Google Scholar]
- 25.Rennie R.J. A single medium for the isolation of acetylene-reducing (dinitrogen fixing) bacteria from soils. Can J Microbiol. 1981;27:8–14. doi: 10.1139/m81-002. [DOI] [PubMed] [Google Scholar]
- 26.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J Mol Biol Mol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 27.Chojnacki S., Cowley A., Lee J., Foix A., Lopez R. Programmatic access to bioinformatics tools from EMBL-EBI update. Nucl Acids Res. 2017;3:W550–W553. doi: 10.1093/nar/gkx273. 45(Web Server issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Capella-Gutiérrez S., Silla-Martínez J.M., Gabaldón T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;1(15):1972–1973. doi: 10.1093/bioinformatics/btp348. 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;1(21):2688–2690. doi: 10.1093/bioinformatics/btl446. 22. [DOI] [PubMed] [Google Scholar]
- 30.Letunic I., Bork P. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucl Acids Res. 2016;8(W1):W242–W245. doi: 10.1093/nar/gkw290. 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarhan M., Patz S., Hamza M., Youssef H., Mourad E., Fayez M. G3 PhyloChip analysis confirms the promise of plant-based culture media for unlocking the composition and diversity of the maize root microbiome and for recovering unculturable candidate dicisions/phyla. Microbes Environ. 2018;33:317–325. doi: 10.1264/jsme2.ME18023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duncan E.G., O'Sullivan C.A., Roper M.M., Biggs J.S., Peoples M.B. Influence of co-application of nitrogen with phosphorus, potassium and sulphur on the apparent efficiency of nitrogen fertiliser use, grain yield and protein content of wheat: review. Field Crop Res. 2018;226:56–65. [Google Scholar]
- 33.Parveen S., Ranjan R.K., Anand A., Singh B. Combined deficiency of nitrogen and iron increases senescence induced remobilization of plant immobile iron in wheat. Acta Physiol Plant. 2018;40:211. [Google Scholar]
- 34.Ruppel S., Ruehlmann J., Merbach W. Quantification and localization of bacteria in plant tissues using quantitative real-time PCR and online emission fingerprinting. Plant Soil. 2006;286:21–35. [Google Scholar]
- 35.Hrynkiewicz K., Baum C. The potential of rhizosphere microorganisms to promote the plant growth in disturbed soils. In: Malik A., Grohmann E., editors. Environmental protection strategies for sustainable development. Springer-Verlag; Berlin: 2012. pp. 35–64. [Google Scholar]
- 36.Shi Y., Lou K., Li C., Wang L., Zhao Z., Zhao S. Illumina-based analysis of bacterial diversity related to halophytes Salicornia europaea and Sueada aralocaspica. J Microbiol. 2015;53(10):678–685. doi: 10.1007/s12275-015-5080-x. [DOI] [PubMed] [Google Scholar]
- 37.Zhao S., Zhou N., Zhao Z.Y., Zhang K., Tian C.Y. High-throughput sequencing analysis of the endophytic bacterial diversity and dynamics in roots of the halophyte Salicornia europaea. Curr Microbiol. 2016;72:557–562. doi: 10.1007/s00284-016-0990-3. [DOI] [PubMed] [Google Scholar]
- 38.Gontia I., Kavita K., Schmid M., Hartmann A., Jha B. Brachybacterium saurashtrense sp. nov., a halotolerant root-associated bacterium with plant growth-promoting potential. Int J Syst Evol Microbiol. 2011;61:2799–2804. doi: 10.1099/ijs.0.023176-0. [DOI] [PubMed] [Google Scholar]
- 39.Jha B., Singh V.K., Weiss A., Hartmann A., Schmid M. Zhihengliuella somnathii sp. nov., a halotolerant actinobacterium from the rhizosphere of a halophyte Salicornia brachiate. Int J Syst Evol Microbiol. 2015;65:3137–3142. doi: 10.1099/ijsem.0.000391. [DOI] [PubMed] [Google Scholar]
- 40.Gupta G., Panwar J., Jha P.N. Natural occurrence of Pseudomonas aeruginosa, a dominant cultivable diazotrophic endophytic bacterium colonizing Pennisetum glaucum (L.) R. Br. Appl Soil Ecol. 2013;64:252–261. [Google Scholar]
- 41.Martins G., Lauga B., Miot-Sertier C., Mercier A., Lonvaud A., Soulas M.L. Characterization of epiphytic bacterial communities from grapes, leaves, bark and soil of grapevine plants grown, and their relations. PLoS ONE. 2013;8(8):E73013. doi: 10.1371/journal.pone.0073013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elnemr R, Ruppel S, Hegazi N, Youssef H. Direct Submission. NCBI. Cairo University-Faculty of Agriculture, Egypt; 2017;5:539–554.
- 43.Suarez C., Ratering S., Geissler-Plaum R., Schnell S. Hartmannibacter diazotrophicus gen. nov., sp. nov., a phosphate-solubilizing and nitrogen-fixing alphaproteobacterium isolated from the rhizosphere of a natural salt-meadow plant. Int J Syst Evol Microbiol. 2014;64:3160–3167. doi: 10.1099/ijs.0.064154-0. [DOI] [PubMed] [Google Scholar]
- 44.Suarez C., Cardinale M., Ratering S., Steffens D., Jung S., Zapata Montoya A.M. growth-promoting effects of Hartmannibacter diazotrophicus on summer barley (Hordeum vulgare L.) under salt stress. Appl Soil Ecol. 2015;95:23–30. [Google Scholar]
- 45.Sharma S., Kulkarni J., Jha B. Halotolerant rhizobacteria promote growth and enhance salinity tolerance in peanut. Front Microbiol. 2016;7:1600. doi: 10.3389/fmicb.2016.01600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szymańska S., Dąbrowska G.B., Tyburski J., Niedojadło K., Piernik A. Hrynkiewicz K. Boosting the Brassica napus L. tolerance to salinity by the halotolerant strain Pseudomonas stutzeri ISE12. Environ Exp Bot. 2019;163:55–68. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.