Abstract
Tapeworms of the family Diphyllobothriidae, commonly known as broad tapeworms, are predominantly large-bodied parasites of wildlife capable of infecting humans as their natural or accidental host. Diphyllobothriosis caused by adults of the genera Dibothriocephalus, Adenocephalus and Diphyllobothrium is usually not a life-threatening disease. Sparganosis, in contrast, is caused by larvae (plerocercoids) of species of Spirometra and can have serious health consequences, exceptionally leading to host's death in the case of generalised sparganosis caused by ‘Sparganum proliferum’. While most of the definitive wildlife hosts of broad tapeworms are recruited from marine and terrestrial mammal taxa (mainly carnivores and cetaceans), only a few diphyllobothriideans mature in fish-eating birds. In this review, we provide an overview the recent progress in our understanding of the diversity, phylogenetic relationships and distribution of broad tapeworms achieved over the last decade and outline the prospects of future research. The multigene family-wide phylogeny of the order published in 2017 allowed to propose an updated classification of the group, including new generic assignment of the most important causative agents of human diphyllobothriosis, i.e., Dibothriocephalus latus and D. nihonkaiensis. Genomic data of selected representatives have also begun to accumulate, promising future developments in understanding the biology of this particular group of parasites. The list of nominal species of taxonomically most complicated genus Spirometra as well as host-parasite list of 37 species of broad tapeworms parasitising marine mammals (pinnipeds and cetaceans) are also provided.
Graphical abstract
Highlights
-
•
Recent progress in our understanding of the diversity, interrelations and distribution of broad tapeworms is overviewed.
-
•
A list of nominal taxa of Spirometra, causative agents of human sparganosis, is provided.
-
•
Revised host-parasite list of broad tapeworms parasitising marine mammals is presented.
-
•
Prospects of future research are outlined.
1. Introduction
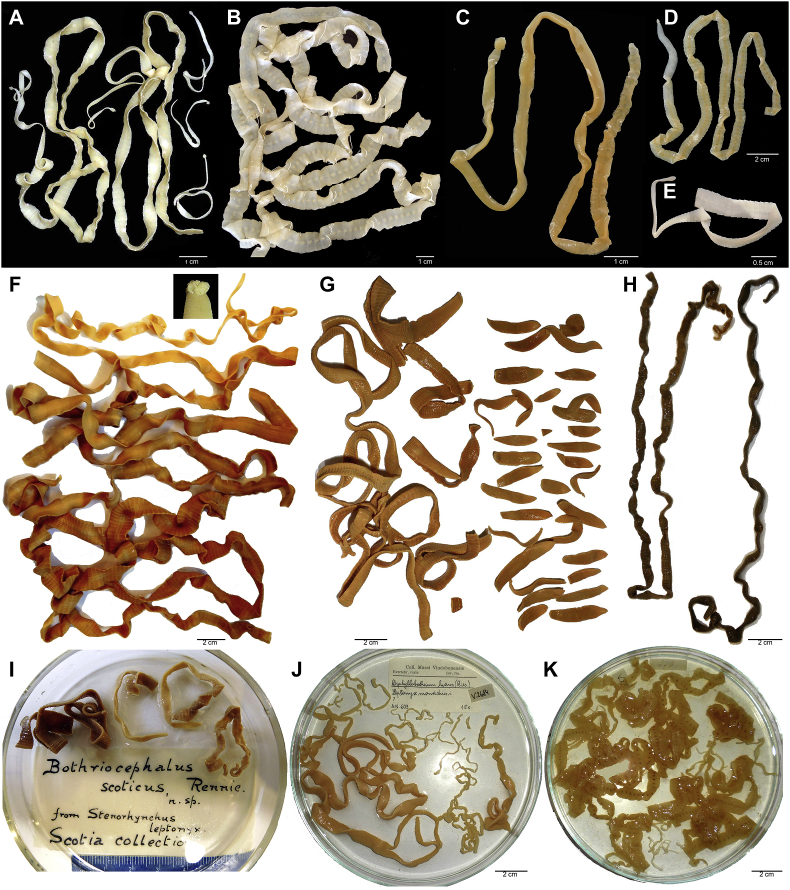
Broad tapeworms (members of the family Diphyllobothriidae of the order Diphyllobothriidea) are notorious for their impact on public health, especially species formerly placed in genus Diphyllobothrium (now Dibothriocephalus; see Waeschenbach et al., 2017); to prevent any confusion, the genus name of Diphyllobothrium will be abbreviated to Diph. and Dibothriocephalus to Dibo. throughout the text), and species of Spirometra, whose larvae called sparganum can cause human sparganosis (Daly, 1981; Kuchta et al., 2015; Kuchta and Scholz, 2017). However, vast majority of broad tapeworm species parasitise as adults in wildlife, especially in marine and terrestrial mammals. They are among the largest tapeworms on the Earth (Fig. 1), with some specimens from sperm whale reaching up to 30 m in length (Yurakhno, 1992; Yurakhno and Maltsev, 1997).
Fig. 1.
Microphotographs of diphyllobothriid tapeworms. A – Adenocephalus pacificus from Callorhinus ursinus, St. Paul Island, Alaska; fixed with hot water by T. Kuzmina. B – Dibothriocephalus latus spontaneously shed from experimentally infected man; fixed by R. Kuchta. C – Pyramicocephalus phocarum from Erignathus barbatus, Saint Lawrence Island, Alaska; fixed after relaxation by R. Rausch (MSBP 16648). D – Dibothriocephalus alasensis from Canis familiaris, Chevak, Alaska, 28 June 1958; fixed after relaxation by R. Rausch; type series (MSBP 17496). E – Dibothriocephalus dalliae from C. familiaris experimentally infected with plerocercoids from Dallia pectoralis, Gene Lake, Alaska, 12 March 1958; fixed after relaxation by R. Rausch; type series (MSBP 17092). F – Plicobothrium globicephalae from Orcinus orca, Newfoundland, Canada; inset: detail of the scolex; ‘cold’ (= in room temperature) fixation, museum sample (CMNPA 1999-0009). G – Diphyllobothrium cordatum (larger), Diphyllobothrium lanceolatum and Diphyllobothrium schistochilos (smaller) from E. barbatus; Greenland, 5 May 1916, "Crocker Land Expedition"; ‘cold’ fixation (SNM). H – Diphyllobothrium cordatum from E. barbatus, Greenland; 24 July 1890; decomposed material; museum sample (SNM). I – Diphyllobothrium scotium from Hydrurga leptonyx, Antarctica; type material (NMS Z.1921.1.43.1501). J – Diphyllobothrium hians from Monachus monachus; captured in Austria; collected by K.M. Diesing; type material (NMW 2684). K – Spirometra decipiens from Puma concolor, Brazil; collected by J. Natterer; type material (NMW 2682). Acronyms of museum collections: CMNPA – Canadian Museum of Nature, Parasitology Collection, Ottawa, Ontario, Canada; MSBP – Museum of Southwestern Biology, Division of Parasitology, University of New Mexico, Albuquerque, New Mexico, U.S.A.; NMS – National Museum of Scotland, Edinburgh, Scotland, U.K.; NMW – Das Naturhistorische Museum Wien, Vienna, Austria; SNM – Swedish Museum of Natural History, Stockholm, Sweden.
The family Diphyllobothriidae contains the well-known and most species-rich genus Diphyllobothrium (and Dibothriocephalus) housing more than 60 nominal species appearing in the literature since 1758, when the first species, Taenia lata, was described by Linnaeus (1758). After decades of being neglected, broad tapeworms now attract growing attention thanks to the increasing number of reports of human cases but also thanks to considerable advancement achieved by application of molecular methods in diagnosis and epidemiological studies. Several review articles of book chapters on broad tapeworms have been published recently, but most of them focused mainly on human-infecting taxa (Dick, 2008; Scholz et al., 2009; Kuchta et al., 2015; Scholz and Kuchta, 2016). In the present review, historical data are briefly reviewed, some of recent achievements pinpointed and key questions for future research highlighted, with the focus on broad tapeworms occurring in wildlife.
2. A brief excursion to history
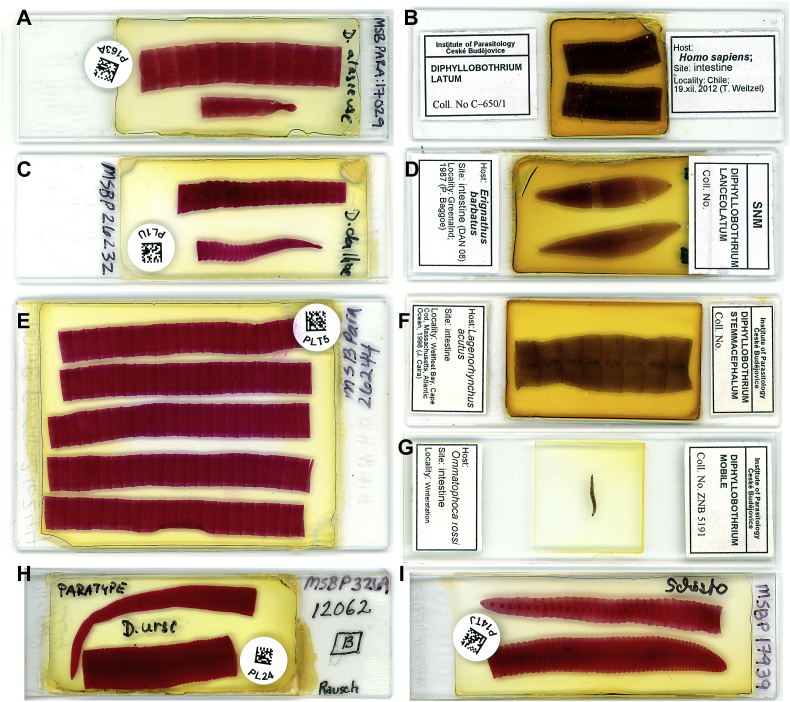
Broad tapeworms were among the first helminths to be recognised as human parasites because of their large size, with the body reaching up to several metres (Fig. 1, Fig. 2). Long co-existence of broad tapeworms with humans is evidenced by archeoparasitological data from mummies and coprolites, which showed the presence of eggs of diphyllobothriid tapeworms at least since the early Neolithic period in Europe and South America (Mitchell, 2013). In 1592, two Swiss physicians, Thaddeus Dunus in Lucarno and somewhat later Gaspard Wolphius in Zurich described in a recognisable form tapeworms now known as the broad fish tapeworm (Dibothriocephalus latus; see Grove, 1990). However, these researchers did not recognise the differences between this tapeworm and human-infecting Taenia saginata and T. solium. As a result, all large-sized human tapeworms were collectively called as ‘Lumbricus latus’. The first person who undoubtedly recognised these two groups and called them as species prima (= Dibotriocephalus) and species secunda (= Taenia) was another Swiss, Felix Platter (Platerus) in Basel in 1602 (see Grove, 1990).
Fig. 2.
Microphotographs of permanent slides of diphyllobothriid tapeworms. A – Dibothriocephalus alasensis from Canis familiaris, Hooper Bay, Alaska, March 18, 1958; fixed after relaxation by R. Rausch (MSBP 17029). B – Dibothriocephalus latus from Homo sapiens, Chile, 19 November 2012; contracted clinical sample fixed with ‘cold’ fixative by T. Weitzel. C – Dibothriocephalus dalliae from C. familiaris, Alaska, 5 November 1970; fixed after relaxation by R. Rausch (MSBP 26232). D – Diphyllobothrium lanceolatum from Erignathus barbatus, Greenland, 7 October 1987; collected by P. Baagoe (SNM). E – Dibothriocephalus cf. nihonkaiensis from Homo sapiens, Newtok, Alaska, 26 March 1967; fixed after relaxation by R. Rausch (MSBP 26244). F – Diphyllobothrium stemmacephalum from Lagenorhynchus acutus, Wellfleet Bay, Cape Cod, Massachusetts, 1998; collected by J.N. Caira. G – Diphyllobothrium mobile from Ommatophoca rossii, Antarctica, 11 August 1901, Deutsche Südpolar-Expedition; collected by E. Dagobert von Drygalski (ZNB 5188). H – Dibothriocephalus ursi from Ursus arctos middendorfi, Karluk Lake, Kodiak Island, 9 October 1952; fixed after relaxation by R. Rausch; paratype (MSBP 3269). I – Schistocephalus sp. from C. familiaris, Newtok, Alaska, 4 April 1958; fixed after relaxation by R. Rausch (MSBP 17939). Acronyms of museum collections: MSBP – Museum of Southwestern Biology, Division of Parasitology, University of New Mexico, Albuquerque, New Mexico, U.S.A.; SNM – Swedish Museum of Natural History, Stockholm, Sweden; ZNB – Zoologische Museum Berlin, Berlin, Germany.
Confusion in differentiation of human tapeworms continued throughout the following two centuries. The first illustration of broad fish tapeworm (lacking a scolex) was provided by Nicholas Andry in 1718, but the scolex was first illustrated by Bonnet in 1750 (Grove, 1990). Finally, Linnaeus (1758) named the broad fish tapeworm as Taenia lata. In the same book, he also described another diphyllobothrid cestodes, Fasciola intestinorum (now Ligula intestinalis), which was found as a larva (plerocercoid) in fish. A few years later, Müller (1776) described adults of Taenia solida (now Schistocephalus solidus) as the first diphyllobothriid species found in birds.
Numerous species of broad tapeworms were described in the 19th century from mammals and, much less frequently, birds, such as the human-infecting Dibothriocephalus dendriticus (Nitzsch, 1824), Dibothriocephalus ditremus (Creplin, 1825) from birds, Diphyllobothrium stemmacephalum Cobbold, 1858 (type species of the genus) from dolphins, and ‘Diphyllobothrium’ tetrapterum (von Siebold, 1848) from seals. The first documented case of human diphyllobothriosis outside Europe was likely caused by the Japanese broad tapeworm, now Dibothriocephalus nihonkaiensis (Yamane, Kamo, Bylund and Wikgren, 1986), and was described in the traditional Japanese medical book ‘Shinsen-Yamainosoushi’ published in 1850 (see Yamane et al., 1998).
In 1854, Diesing (1854) established the genus Sparganum as a collective group to house larvae (plerocercoids) of broad tapeworms with similar morphological features. Subsequently, immature forms of diphyllobothriids were placed provisionally in this genus until the corresponding adult form was found. The first human case of sparganosis was reported as early as in 1596 (see Qiu and Qiu, 2009), but the first scientifically documented human case was that by Patrick Manson in 1881 (Cobbold, 1883). When studying lymphatic filariosis caused by the nematode Wuchereria bancrofti in Xiamen, China, Manson carried out a secret (in the night) autopsy of a 34-year-old man with elephantiasis. Manson found adult filariae, but also dozen ribbon-like worms in the retroperitoneal adipose tissue and pleural cavity. He sent these specimens to Cobbold in London who named them Ligula mansoni Cobbold (1883), but they were later renamed as Bothriocephalus liguloides Leuckart, 1886 and finally Spirometra mansoni by Faust et al. (1929).
In the 20th century, research on broad tapeworms was quite intensive, especially in North America (pivotal papers by Justus F. Mueller on Spirometra and Robert L. Rausch's accounts on species of ‘Diphyllobothrium’ mainly from North America) and former Soviet Union (Siberia and Far East of Russia by several authors) (Delyamure et al., 1985). From the latter country, as many as 1,725 studies on diphyllobothriids were published in Russia between 1950 and 1972 (Rosenberg, 1977). Noteworthy are also numerous studies from Scandinavia, e.g., K. Andersen, B. von Bonsdorff, G. Bylund, O. Halvorsen, R. Vik and others (see von Bonsdorff, 1977), North America (e.g. T. Dick, T.V.M. Cameron, H.E. Essex, T.B. Magath, L. Margolis, T. Vergeer, R.A. Wardle) and Japanese researchers (e.g., E. Eguchi, H. Kamo and Y. Yamane). However, attention paid to broad tapeworms sharply declined in the last decades of the 20th century.
Life cycles of only a few broad tapeworms are known currently. The source of human infection with broad fish tapeworm was first identified by the Finnish researcher H. D. Spöring, in 1747. He noticed that people who lived on the banks of rivers, rapids and lakes with abundant fish suffered from the tapeworm infections more than human populations living in other localities. In 1881, M. Braun experimentally infected dogs and three medical students with plerocercoids from pike; later, he found a large number of eggs of D. latus in students’ stool. However, the full three-host life cycle that includes also copepods as the first intermediate host was completely elucidated by Janicki and Rosen (1917) in Switzerland for Dibo. latus and just 2 years later by Okumura (1919) in Japan for S. mansoni (= S. erinaceieuropaei). Another breakpoint in our knowledge of the biology of broad tapeworms was made by Gnezdilov (1957) in former Soviet Union who discovered that golden hamsters could be easily infected with plerocercoids of the genus Dibothriocephalus to produce adult worms in the laboratory. In contrast, attempts to culture broad tapeworms in vitro have not been successful with exception of almost whole life-cycle of Schistocephalus solidus and Spirometra mansonoides (Berntzen and Mueller, 1972; Mueller, 1974; Jakobsen et al., 2012).
3. Diversity, phylogenetic relationships and updated classification
Considerable contribution to the present knowledge of the diversity and morphology of broad tapeworms has been made, among others, by Delyamure et al. (1985), Yurakhno (1992), Kamo (1999), to mention just a few (see Kuchta and Scholz, 2017 for more data). Remarkable advancement has been made also recently, mainly thanks to application of methods of integrative taxonomy including generation of molecular data, which proved critical for differentiation of morphologically similar taxa. Kuchta and Scholz (2017) presented a list of 58 species in 13 genera of broad tapeworms recognised as valid (see Table 1).
Table 1.
List of genera of diphyllobothriid tapeworms with numbers of their species, definitive hosts and habitats.
| genus | spp. | definitive host | habitat | reference |
|---|---|---|---|---|
| Adenocephalus | 1 | Ma: Otariidae, (Canidae, Hominidae) | marine | Hernández-Orts et al. (2015) |
| Baylisia | 2 | M: Phocidae | marine | Kuchta and Scholz (2017) |
| Baylisiella | 1 | M: Phocidae | marine | Kuchta and Scholz (2017) |
| Dibothriocephalus | 7 | M: Canidae, Felidae, Hominidae, Ursidae, (Muridae, Mustelidae); Ab Accipitridae, Alcidae, Anatidae, Ardeidae, Corvidae, Gaviidae, Laridae, Pandionidae, Pelecanidae, Phalacrocoracidae, Podicipedidae, Sternidae | terrestrial | Waeschenbach et al. (2017) |
| Diphyllobothrium | 7 | M: Balaenidae, Balaenopteridae, Delphinidae, Eschrichtidae, Monodontidae, Phocoenidae, Ziphiidae | marine | Waeschenbach et al. (2017) |
| ‘Diphyllobothrium’ | 20 | M: Otariidae, Phocidae, (Mustelidae); A: Spheniscidae | marine | Waeschenbach et al. (2017) |
| Flexobothrium | 1 | M: Phocidae | marine | Kuchta and Scholz (2017) |
| Glandicephalus | 2 | M: Phocidae | marine | Kuchta and Scholz (2017) |
| Ligula | 5 | A: Anatidae, Laridae, Podicipedidae and many othersc | terrestrial | Dubinina (1980) |
| Plicobothrium | 1 | M: Delphinidae | marine | Kuchta and Scholz (2017) |
| Pyramicocephalus | 1 | M: Phocidae, (Mustelidae) | marine | Kuchta and Scholz (2017) |
| Schistocephalus | 5 | A: Anatidae, Laridae and many othersc | terrestrial | Dubinina (1980) |
| Spirometra | 4 | M: Canidae, Felidae, Herpestidae, Hyaenidae, Mephitidae, Mustelidae, Viverridae, Procyonidae, (Didelphidae) | terrestrial | Kuchta and Scholz (2017) |
| Tetragonoporus | 1 | M: Physeteridae | marine | Kuchta and Scholz (2017) |
| Total | 58 |
Waeschenbach et al. (2017) provided a robust phylogenetic hypothesis of interrelationships of diphyllobothriidean cestodes, using 30 representative species of the whole order and on two ribosomal plus two mitochondrial genes. They confirmed that the genus Diphyllobothrium is polyphyletic and includes closely unrelated taxa of independent evolutionary origin. As a result, the most important human-infecting species were placed in the resurrected genus Dibothriocephalus Lühe, 1899 (in total 7 species – see Table 1). ‘True’ Diphyllobothrium including the type species D. stemmacephalum comprises only 7 species from pinnipeds, including Diplogonoporus balaenopterae, type species of Diplogonoporus, which is invalidated (for diplogonadic forms, separate genera should not be proposed because the number of genital complexes may vary even within the strobila of a single worm (Hernández-Orts et al., 2017). Non-monopyletic ‘Diphyllobothrium’ provisionally comprises 20 species (8 characterised molecularly) that do not form a monophyletic assemblage; these species are considered as incertae sedis (Waeschenbach et al., 2017).
4. Host associations and life cycles
Tapeworms of the order Diphyllobothriidea are peculiar among all other tapeworm orders but one (Cyclophyllidea) cestode orders in successful colonising all major tetrapod groups. Molecular data revealed that the main lineages corresponding to three families recognised by Kuchta et al. (2008) reflect the evolutionary history of their tetrapod definitive hosts and the complexity of parasites' life-cycles (Waeschenbach et al., 2017). Species of the most speciose and relatively more derived lineage of the order, the Diphyllobothriidae, parasitise mammals including humans and, to a lesser extent, birds as definitive hosts. Earliest diverging groups of diphyllobothriids (Spirometra and Schistocephalus) colonised freshwater-terrestrial ecosystems followed by radiation in marine mammals (pinnipeds and cetaceans – ‘Diphyllobothrium’ Adenocephalus, etc.). In contrast, species of the most recently diverging groups (Ligula and Dibothriocephalus) use terrestrial mammals and fish-eating birds as their definitive hosts and larvae developed in freshwater environment (Waeschenbach et al., 2017).
The life cycles of the Diphyllobothriidae are always connected with aquatic environment (freshwater or marine), because the first larva (coracidium) is aquatic and needs to pass to the first aquatic intermediate host – a copepod. Two intermediate hosts are involved: copepods as first intermediate hosts and vertebrates as second intermediate hosts (Janicki and Rosen, 1917; Okumura, 1919; Kamo et al., 1973; Dubinina, 1980; Hatsushika et al., 1981). The second intermediate hosts (with the exception of the genus Spirometra) are freshwater or brackishwater fish (Dubinina, 1980; Kuchta et al., 2015; Kuchta and Scholz, 2017). In contrast, the life cycle of species of Spirometra includes a wide spectrum of amphibians, reptiles, birds or mammals as second intermediate hosts, but never fish (see chapter 7 below).
Adults of Dibo. dendriticus and Dibo. ditremus have been reported from fish-eating birds (Markowski, 1949; Delyamure et al., 1985; Kuchta et al., 2013). The spectrum of these hosts is rather wide, but they are primarily core water birds (clade Aequornithes), less frequently members of the orders Anseriformes, Charadriiformes and Podicipediformes, whereas records from the Accipitriformes, Gruiformes and Passeriformes are considered accidental (Delyamure et al., 1985). Only one species, Diph. scoticum (Rennie et Reid, 1912) was reported to parasitise penguins, along with its typical definitive hosts, seals (Kuchta and Scholz, 2017).
Most species of Ligula and Schistocephalus are euryxenous at the level of definitive hosts, with adults of these genera having been reported from almost 80 species of fish-eating birds (Dubinina, 1980). For example, S. solidus has been reported from as many as 42 species in eight bird orders (Vik, 1954). In contrast, three-spined sticklebacks (Gasterosteus aculeatus) serve as the only second intermediate host species of this tapeworm: plerocercoids continue to grow for an unusually long time (several months), and nearly reach sexual maturity in the fish host. In the definitive host, adults of S. solidus survive only few days, producing high numbers of eggs meanwhile (Dubinina, 1980). However, gravid adults of Ligula and Schistocephalus were occasionally (accidently?) as well as after experimental infection found also from mammals as dogs and cats (Rausch et al., 1967; Dubinina, 1980; Fig. 2I).
5. Broad tapeworms and human-infections (diphyllobothriosis and sparganosis)
Humans get infected mainly by terrestrial species of the genera Dibothriocephalus and Spirometra (Fig. 1, Fig. 2), but also by a few species that normally mature in marine mammals such as Adenocephalus pacificus and Diph. balaenopterae. Scholz and Kuchta (2016) critically reviewed records of 19 species of broad tapeworms (except for species of Spirometra) previously reported from humans. They found many of the records of human cases doubtful, accidental or apparently erroneous, and concluded that only the following six species can be considered genuine human parasites (all confirmed with molecular techniques): A. pacificus, Dibo. dendriticus, Dibo. latus, Dibo. nihonkaiensis, Diph. balaenopterae and Diph. stemmacephalum. The remaining 13 species should not be considered typical human parasites because they have been reported from humans only rarely and their identification based on morphological characters only (Scholz and Kuchta, 2016).
Waeschenbach et al. (2017) provided evidence that human parasitism evolved on minimum of four independent occasions within the broad tapeworm clade. Two of those lineages include tapeworms living in freshwater/terrestrial habitats (species of Spirometra and Dibothriocephalus) and two in marine habitats (A. pacificus and Diph. balaenopterae with Diph. stemmacephalum). The origin of most of the human infections with broad tapeworms is likely zoonotic, i.e. the parasites of wildlife infecting humans accidently (Waeschenbach et al., 2017). Humans likely represent the principal definitive hosts only for Dibo. latus and, probably, Dibo. nihonkaiensis; the former species has the ability to grow faster in humans compared to other experimental hosts (dogs, cats, wolves, foxes, etc. Essex and Magath, 1931; Andersen, 1975; von Bonsdorff, 1977).
Humans are usually infected with euryxenous species, e.g., Dibo. dendriticus, Dibo. nihonkaiensis, Dibo. latus, A. pacificus, Diph. balaenopterae, Diph. stemmacephalum, whereas reports of human cases caused by presumably stenoxenous or oioxenous species (‘Diph.’ scoticum, ‘Diph.’ cf. cameroni and ‘Diph.’ cf. hians) are rare and validity of Dibo. alascencesis, Dibo. daliae and Dibo. ursi reported exclusively from Alaska by R. Rausch needs verification (Scholz and Kuchta, 2016; Waeschenbach et al., 2017) (Fig. 1, Fig. 2). Numerous unverified or questionable records further bias reliable assessment of the actual host spectrum of broad tapeworms including those parasitising humans. In addition, definitive hosts of broad tapeworms are often predators and may thus represent postcyclic or incidental hosts.
Broad tapeworms of terrestrial and marine mammals are seemingly capable of better survival in the environment of human intestines compared to species occurring in birds such as Dibo. ditremus that is likely incapable of infecting humans (Waeschenbach et al., 2017). Therefore, it was probable that ecological rather than evolutionary factors played a key role in the origin of broad tapeworms infections in humans, i.e., consumption of raw or poorly cooked fish in the case of species of Adenocephalus, Dibothriocephalus and Diphyllobothrium, and amphibians, snakes or mammals in the case of Spirometra (Waeschenbach et al., 2017).
Based on habitat, diphyllobothriids can be divided into two ecological groups: (i) parasites of marine mammals (confirmed from 32 species of phocids and 28 species of cetaceans) that are relatively narrowly host specific (37 species – see Supplementary Table 1), and (ii) parasites of terrestrial mammals (mainly canids and felids), less frequently fish-eating birds, both displaying lower levels of host specificity (euryxeny; 21 species). The majority of the members of the terrestrial group (i.e., species of the genera Dibothriocephalus, Ligula and Schistocephalus) use freshwater and anadromous teleosts as second intermediate hosts, whereas species of Spirometra use tetrapods, mainly amphibians and reptiles, but never fish as second intermediate hosts (Mueller, 1974; Daly, 1981; Kuchta and Scholz, 2017).
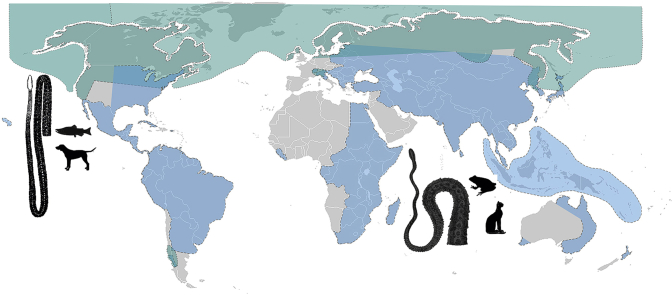
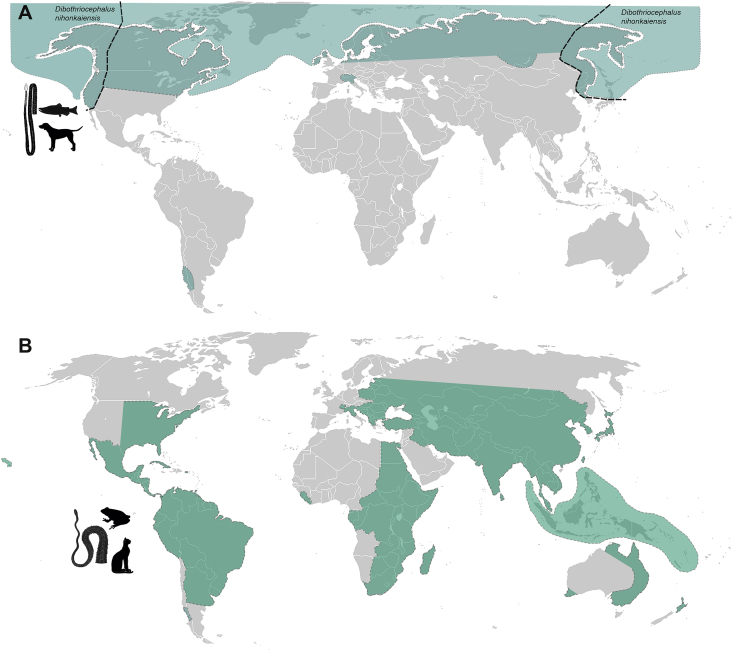
A better knowledge of the host associations and geographical distribution of causative agents of human diphyllobothriosis (Fig. 3) is impeded by erroneous diagnosis (numerous misidentifications with Spirometra) and the existence of doubtful or erroneous records, especially from the subtropical and tropical regions, including Ethiopia (Abere et al., 2013), India (Ramana et al., 2011; Sasikala et al., 2018), Nigeria (Umar, 2009; Alayande et al., 2013), or Pakistan (Kuntz, 1960).
Fig. 3.
Distribution of two genera of diphyllobothriid tapeworms from wildlife and man. A – Dibothriocephalus spp., B – Spirometra spp. Based on available literature data by countries except for some large countries where occurrence was estimated based on data available (Russia, China, etc.).
6. Broad tapeworms in wildlife
Marine mammals are hosts of almost 2/3 (64%) of diphyllobothriids, i.e., 37 of a total number of 58 known species of broad tapeworms (Kuchta and Scholz, 2017; Supplementary Table 1). They infect mainly pinnipeds (reported from 17 of 19 species of the Phocidae and 15 of 16 species of the Otariidae), but also cetaceans (reported from 7 of 13 species of the Mysticeti and 21 of 55 species of the Odonoceti – Supplementary Table 1). Pinnipeds or seals (Phocidae and Otariidae) serve as definitive hosts for 30 diphyllobothriid species (52% of broad tapeworm diversity). Out of these, phocids host a much richer (28 species) fauna compared to otariids with only four species, A. pacificus being the only species specific to eared seals. Cetaceans of the families Balaenidae, Balaenopteridae, Delphinidae, Eschrichtiidae, Monodontidae, Phocoenidae, Physeteridae and Ziphiidae host nine broad tapeworm species (Supplementary Table 1).
Infection rate in seals can be extraordinarily high, with prevalence reaching up to 100% (Kuzmina et al., 2015, 2018) and with extremely high intensity of infections, especially in Antarctic seals. For example, the Weddell seal (Leptonychotes weddellii) is a host of 7 diphyllobothriid species and the mean intensity reported by Yurakhno and Maltsev (1997) reaching an estimated 1,300,000 tapeworms per host (based on examination of 28 individuals). The most heavily infected seal harboured as many as 3,600,000 specimens of D. mobile, but majority of specimens were juvenile (Yurakhno and Maltsev, 1997). The leopard seal (Hydrurga leptonyx) was also found heavily infected with four diphyllobothriid species, with Diph. quadratum and Diph. scoticum being the most abundant (mean intensity of 180,000 specimens per seal; 60 of 67 seals examined were infected). The highest intensity of infection was recorded in a one-year-old H. leptonyx with 1,600,000 individuals of Diph. quadratum (see Yurakhno and Maltsev, 1997).
Intensity of infection of seals (Phocidae) in the Northern hemisphere, compared to the seals in the Southern hemisphere, is generally much lower, but still remains relatively high. Nearly every single bearded seal (Erignathus barbatus) is usually infected with diphyllobothriids and the maximum intensity of 50,000 individuals of Diph. lanceolatum and Diph. schistochilos has been reported (Delyamure and Popov, 1975; Delyamure et al., 1976; Fiscus et al., 1976; Shulman and Popov, 1982; Schaeffner et al., 2018) (Fig. 1G). Even the endemic Hawaiian monk seal, Neomonachus schauinslandi, is heavily infected with as many as three species of broad tapeworms with prevalence of 78% (282 seals examined – Reif et al., 2006). However, great majority of broad tapeworms found in these heavily infected pinnipeds are juvenile or immature. For example, Kuzmina et al. (2015) found that 70% of 14,660 tapeworms of three species in 756 northern fur seals (Callorhinus ursinus) were immature.
Cetaceans are also common hosts of diphyllobothriids with nine species reported including the type species Diph. stemmacephalum (Supplementary Table 1). The infection rates, however, reach much lower levels compared to pinnipeds, with common prevalence being about 10% and intensity of infection much lower than that in seals (Brattey and Stenson, 1995; Herreras et al., 1997; Gibson et al., 1998; Krivokhizhyn, 2000; Kleinertz et al., 2014). High prevalence (52%; 25 hosts examined) of diphyllobothriid eggs, most likely a species of Tetragonoporus, was found in the sperm whale (Physeter macrocephalus) in the Mediterranean Sea (Hermosilla et al., 2018).
Terrestrial species of broad tapeworm genera such as Spirometra or Dibothriocephalus are not able to mature in pinnipeds or cetaceans and only plerocercoids of Dib. dendriticus and Schistocephalus solidus have been rarely found in seals from Lake Baikal and Baltic Sea, most likely representing accidental infections (Pronin and Zhaltsanova, 1999; Sinisalo et al., 2003). However, several previous checklists erroneously listed as many as eight pinnipeds and cetaceans as definitive hosts of the human broad fish tapeworm Dibo. latus (see Felix, 2013). Broad tapeworms of terrestrial vertebrates are represented by 21 species of four genera, Dibothriocephalus, Ligula, Schistocephalus and Spirometra (36% of all known diphyllobothriids). They infect significantly wider spectrum of hosts and generally exhibit far lower host specificity compared to marine species (see above). Members of the genera Dibothriocephalus and Spirometra are typical parasites of carnivores (several unrelated families), occasionally of fish-eating birds (some species of Dibothriocephalus).
The prevalence and intensity of infection with diphyllobothriids in terrestrial mammals are very variable and differ across localities/regions studied; majority of records comes from colder latitudes (Fig. 3). Dogs and cats are infected with species of Dibothriocephalus with the prevalence ranging from 2 to 50% (Rausch and Hilliard, 1970; Salb et al., 2008; Schurer et al., 2013). Muratov (1993) provided comprehensive data on the prevalence of Dib. nihonkaiensis (as Diphyllobothrium klebanovskii) in the Russian Far East based on a survey of 2,198 terrestrial mammals of 18 species. He found that the brown bear (Ursus arctos) and black bear (Selenarctos tibetanus) were most heavily infected (prevalence up to 50%) with the intensity of infection up to 538 tapeworms per brown bear individual. The prevalence and intensity of infection were much lower in other mammals such as wolf, domestic dog, American mink or domestic pig, prevalence not reaching more than 2% with intensity of infection 1 or 2 tapeworms per host.
Species of Spirometra occur in warmer latitudes (Fig. 3) than species of Dibothriocephalus. Dogs are also infected with Spirometra but with lower infection rates compared to cats (Mueller, 1974; Daly, 1981). Prevalence of infection in dogs varied from 0.6% (500 examined hosts) in Thailand to 44% (34) in Laos (Rojekittikhun et al., 2013; Otake Sato et al., 2017) and in cats from 1% in New Jersey, U.S.A. and 13% (55) in Laos to 40% in China (116) up to 61% in New South Wales, Australia (146) (Mueller, 1974; Ryan, 1976; Scholz et al., 2003; Hong et al., 2016). Some mammals such as raccoons or man (see below) may serve as second intermediate as well as definitive hosts of species of Spirometra (Mueller, 1974; Daly, 1981).
7. Spirometra & sparganosis: current problems and prospects
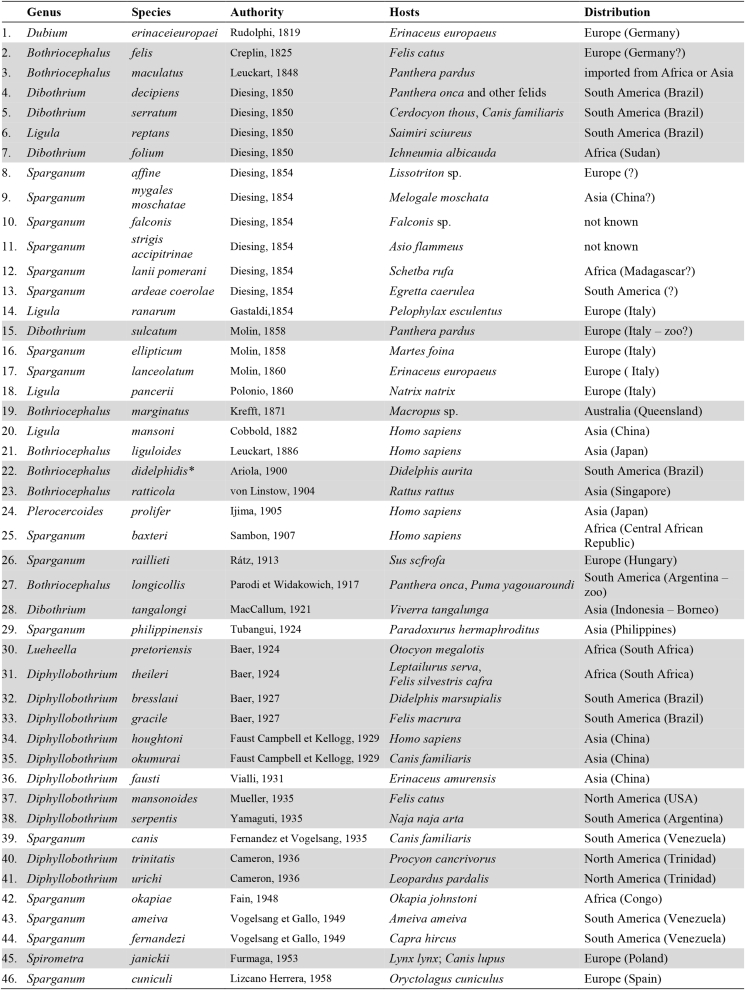
Species of the genus Spirometra have been recognised as intestinal parasites of carnivores for a long time, but still remain unsatisfactorily known (Daly, 1981; Kuchta and Scholz, 2017). Despite numerous attempts to clarify their taxonomy, host specificity and geographical distribution (Faust et al., 1929; Wardle et al., 1974), the genus remains one of the most complicated groups of tapeworms. Iwata (1934, 1972), Mueller (1974) and Odening (1985) concluded that it is almost impossible to distinguish some of nearly 50 nominal species of Spirometra based on morphological characteristics only (Table 2). In the last revision of the family, Kamo (1999) recognised only four species as valid; Kuchta and Scholz (2017) accepted this taxonomic view. Our preliminary molecular data (LSU and cox1) comparisons of isolates of Spirometra from several biogeographical regions indicate that the actual species diversity of the genus is higher and different than assumed by previous authors including Kamo (1999) and Kuchta and Scholz (2017). Taxonomy of this genus should thus be regarded as in its infancy thanks to the numerous problems with species circumscription and distinguishing remain to be resolved using methods of integrative taxonomy applied to newly collected and properly processed material (Kuchta and Scholz, 2017).
Table 2.
Nominal species of Spirometra (adults in grey frame, plerocercoids not highlighted).
Unfortunately, general uniformity of most species, their high intraspecific variability and lack of agreement among investigators as to species circumscription has led to confusion about the classification of Spirometra (Mueller, 1974; Daly, 1981; Kuchta and Scholz, 2017). Moreover, most of the available material was obtained from host examined long time post mortem or even from decomposed carcasses, which may have caused significant morphological changes (Hernández-Orts et al., 2015). Live tapeworms obtained from experimentally infected hosts were usually fixed under pressure or following their relaxation in saline or tap water, which may have also led to unnatural changes in worms’ morphology and anatomy. As a result, morphological and biometrical data in some species descriptions may be misleading or even erroneous, which seriously impedes reliable species identification of individual taxa.
Similarly, most clinical samples of larval stages (plerocercoids called ‘sparganum’; plural ‘spargana’) were not characterised molecularly and were described under four different names (Table 2). In many cases, no voucher material has been preserved for many records to confirm identification. Poorly resolved taxonomy and classification of the genus have also led to apparently erroneous reports, such as repeated identification of isolates from southeastern Asia as the South American species S. decipiens and the European S. ranarum in Myanmar (Jeon et al., 2015, 2018). Cosmopolitan distribution of the type species, S. erinaceieuropaei, is also questionable and should be confirmed by a critical study of isolates from throughout the world, mainly from Europe. This species was described from Europe and its occurrence may be limited.
Another serious limitation that has considerably contributed to the existing deplorable situation in the taxonomy of Spirometra is very poor morphological description of the type species, S. erinaceieuropaei, originally based on larvae (plerocercoids) from a European hedgehog (Erinaceus europaeus) from an unknown locality in Europe. It is desirable to obtain presumably conspecific adults and larvae from Europe for a detailed morphological and molecular characterisation of the type species and its differentiation from other congeneric species (Odening and Bockhardt, 1982; Qiu and Qiu, 2009; Kuchta and Scholz, 2017). However, adults of Spirometra are reported from Europe very rarely and prevalence of infection of carnivorans with adults is apparently very low and restricted to a few localities such as Białowieża National Park in Poland, Belarus, Lithuania, Ukraine or Bulgaria (Odening, 1985; Kornyushin et al., 2011; Kołodziej-Sobocińska and Miniuk, 2018).
Molecular data on Spirometra have been collected intensively, but nearly exclusively for isolates from China and Korea, including complete characterisation of a total number of eight mitochondrial genomes (Zhang et al., 2017). While the available mitochondrial sequence-based comparisons document only very low levels of genetic differentiation within the Asian isolates (Eom et al., 2015; Jeon et al., 2015; Zhang et al., 2015, 2016, 2017), the relatively sporadic (and mostly highly fragmentary) sequence data on Spirometra from remaining regions suggest there is far greater diversification among geographically distant (and presumably individual species-level) taxa (Almeida et al., 2016; Eberhard et al., 2015; Petrigh et al., 2015; Waeschenbach et al., 2017). Based on this simple observation, one could expect that any sequence data of isolates from under-sampled geographical localities (Europe, Africa, the Americas) will allow to obtain far greater insights into the taxonomical richness of the genus.
The life cycle is known only partially for a few species of Spirometra (Daly, 1981; Kuchta and Scholz, 2017). Planktonic crustaceans (copepods) serve as the first and a wide range of tetrapods as the second intermediate or paratenic hosts. Identification of spargana from these hosts to the species level using morphological tools is impossible. The larvae are most commonly found in frogs and reptiles that serve as source of infections of mammals (Magnino et al., 2009; Oda et al., 2016). They may also occur in a spectrum of wild mammals such as badgers, baboons, feral swines, macaques, monotremes, raccoons, but also in cats and dogs and other domestic animals (Keeling et al., 1993; Nobrega-Lee et al., 2007; Stief and Enge, 2011; Woldemeskel, 2014). As many as 128 spargana have been found in a single badger (Meles meles) in the Białowieża Primeval Forest in Poland (Kołodziej-Sobocińska et al., 2014). It is of special interest that some of the above-listed mammals may serve as both intermediate/paratenic and definitive hosts – raccoons, foxes, hyenas, etc. (Daly, 1981; Buergelt et al., 1984; Bengtson and Rogers, 2001; Bauchet et al., 2013).
Species of Spirometra are distributed around the globe, throughout much of the tropics and subtropics, but also in part of Europe and Americas (Daly, 1981; Kuchta et al., 2015); Fig. 3). The prevalence of infection in the definitive as well as intermediate hosts seems to be usually very low (around 1.5% in South America – Oda et al., 2016). Exceptions include some endemic areas in Asia (e.g., in China prevalence up to 40% – Hong et al., 2016) or few studies from Serbia reporting prevalence in pigs up to 57% and from Russia in grass snake (Natrix natrix) up o 100% (Dubinina, 1951; Rukavina et al., 1957; Ryzhenko, 1969).
Whereas adults of Spirometra hardly cause any pathology, the penetration and migration of plerocercoids through tissues of intermediate and incidental hosts typically result in clinical manifestations (Daly, 1981). Adults of Spirometra are capable of maturing in the human intestine, causing rather rare disease spirometrosis, usually lacking any clinical symptoms (Lee et al., 1984; Wang et al., 2012; Le et al., 2017). In contrast, spargana often cause serious disease in humans and other vertebrates (mammals) called sparganosis (Daly, 1981; Bauchet et al., 2013). More than 1,600 human cases have been reported globally so far, with the number of patients recently increasing in endemic areas, especially in China and South East Asia (Liu et al., 2015). Larvae are usually located in the subcutaneous tissue and muscles of the host or alternatively invade other internal organs (Liu et al., 2015; Kuchta et al., 2015). In rare cases, sparganosis may result in fatalities, manifesting mostly as a so-called proliferative sparganosis caused by closely uncharacterised taxonomic entity called Sparganum proliferum (Moulinier et al., 1982; Kuchta et al., 2015). Fatal proliferative sparganosis was also reported from domestic cats in North America (Buergelt et al., 1984; Woldemeskel, 2014) and dog in Europe (Stief and Enge, 2011).
Spargana may cause rapid growth in hypophysectomised or thyroidectomised rats, and exert an ameliorative effect in the diabetic rat (Mueller, 1974; Odening, 1985). The growth response is due to a protein that is synthesised and released by plerocercoids in the host called ‘sparganum growth factor’ (SGF) or ‘plerocercoid growth factor’ (PGF). It is transported by the blood and interacts with growth hormone receptors (Phares, 1996). This activity is not yet well understood, but it illustrates the clear impact of larvae of Spirometra on the second intermediate, paratenic or accidental host.
8. Genomics of broad tapeworms
Broad tapeworms have found a few of their representatives in a noteworthy position of being involved in high-throughput sequencing efforts during the recently established era of genomics. Presumably thanks to the fact that the family incorporates human parasites (as well as a long-time appreciated laboratory model), three representatives of the broad tapeworms were included within the genome sequencing project run by the 50 Helminth Genomes Initiative (Coghlan et al., 2018). While not reaching comparable levels of attention to the flagship human-infecting tapeworm species (Echinococcus and Taenia), the sequencing effort resulted in gathering notable amounts of high-throughput sequence data and their assemblies into the following draft genomes in various state of completeness: Dibothriocephaus latus and Schistocephalus solidus (Coghlan et al., 2018) and Spirometra erinaceieuropaei (Bennett et al., 2014). The laboratory model S. solidus represents the most advanced genome assembly of the three at the moment, according to various genome completeness assessment measures, e.g. BUSCO (Simão et al., 2015). All of the data are publicly available from the WormBase ParaSite portal (http://parasite.wormbase.org; (Howe et al., 2017).
Schistocephalus solidus genome sequencing further benefited from independent de novo transcriptome sequencing undertaken by Hébert et al. (2016). In their effort, Hébert et al. (2016) generated high-throughput sequence data (more than 290,000 transcript assemblies, 10,285 high-confidence unique genes) from three developmental stages of the parasite allowing them to validate the gene prediction models previously generated based solely on the genomic sequence by the 50 Helminth Genomes Initiative. Hébert et al. (2016) then followed with careful comparisons of the relative gene expression levels of the three developmental stages, finding striking differences between gene expression patterns illustrative of the biological processes characteristic to the individual life cycle stages.
The adult worm transcriptome was found significantly enriched for reproduction-related genes, including energy metabolism genes exclusively upregulated in specific tissues, e.g., the elevated production of glyceraldehyde-3-phosphate dehydrogenase in testes as opposed to the remaining body tissues. Endocytosis-related genes were also found upregulated in the adult worm tissues. Even more interestingly, two broadly specified groups of genes lacking homology to any known genes (i.e., without functional annotation or resemblance to other parasitic helminths deposited in public sequence repositories) were found both specific and among the most differentially upregulated in either the adult or the infective plerocercoid stages. These two facts make those pools of genes notably peculiar to the parasite, potentially representing evolutionary novelties linked to the unique life histories of the parasite, and thus, the most attractive points of attention of future research (Hébert et al., 2016).
Aside of the above mentioned sequencing efforts, there has been only a single additional transcriptomic resource devoted to a broad tapeworm: an expressed sequence tags (EST) database derived from plerocercoids of S. erinaceieuropaei naturally infecting snakes. While the dedicated SpiroESTdb (Kim et al., 2012) database can be no longer accessed online, the EST data consisting of a total of 5,634 ESTs corresponding to 1,787 unique ESTs are available in GenBank (Kim et al., 2014). Similar to the situation in S. solidus, 443 ETSs of Spirometra did not match any of the sequences deposited in public databases based on similarity searches (at the time of the publication), potentially rendering those genes Spirometra lineage-specific. The EST data of Kim et al. (2014) were also directly compared to the gene models of S. erinaceieuropaei derived from an independent parasite tissue source (Bennett et al., 2014). Reminiscent of S. solidus, all of the ESTs by Kim et al. (2014) had a significant match within the draft genome and conversely, the gene models of Bennett et al. (2014) predicted 73% of the ESTs, demonstrating the utility of the gene prediction tools employed.
Altogether, the high-throughput sequence data generated recently from an essential, broadly accessible pool of data to benefit from in future explorations into various aspects of parasitic flatworms' biology. Appealing examples from research on taeniid tapeworm models already exist, e.g., the survey of tapeworm nervous system components by Koziol et al. (2016) and Preza et al. (2018) demonstrating the conservation and/or loss of individual neurotransmitters within this evolutionary lineage. Given the relative state of the genome assembly as well as the availability (and manageability) of its life cycle in the laboratory, S. solidus might be the first among the broad tapeworms to join the established taeniid models and allow for further validating various biological phenomena studied in cestodes. While the life cycle of S. erinaceieuropaei has also been successfully established as a laboratory model (Okino et al., 2017), we suspect its sustainable maintenance would be too complicated by the requirement to keep the parasite's definitive host in the animal husbandry and thus, unfortunately, hindering developments in functional research on this human pathogen.
9. Conclusions and prospects
Broad tapeworms represent a peculiar group of cestodes because of the large size of most of their species and ability to colonise all major groups of tetrapods including humans. After a long period of being neglected, this group of predominantly parasites of wildlife began to regain some attention by the scientific community driven, in part, by the increasing number of human case reports from developed countries caused by the popularity of consuming raw meat. While benefiting from the application of recent molecular methods, most of the attention remains devoted to broad tapeworm taxa of medical importance, leaving the wildlife parasite majority neglected. Reliable estimates of human cases are still missing to allow assessment of the actual effect of broad tapeworms on human and wildlife health. Genomic data could be utilised for design of future tools for diagnosis and epidemiological surveys, including environmental DNA screenings for presence of life cycle stages of broad tapeworms in the environment. Several basic questions remain to be adequately addressed within this group: species composition of the genus Spirometra; ability of individual species to cause human sparganosis or spirometrosis; current occurrence and geographical distribution of causative agents of human disease; or explanation of the recent outbreak of diphyllobothriosis in Alpine lakes. Previous data on broad tapeworms of wildlife are only of limited use thanks to the general unavailability of samples suitable for DNA sequencing and the poor quality of material for morphological descriptions. Future surveys should focus on the diverse tapeworm fauna of marine, often endangered host species, as well as adult life cycle stages of Spirometra in terrestrial mammals and their larvae in second intermediate, paratenic or accidental hosts.
Conflicts of interest
There is no conflict of interest in submitted manuscript titled " Broad tapeworms (Diphyllobothriidae), parasites of wildlife and humans: recent progress and future challenges".
Acknowledgements
The senior author (T.S.) thanks Andrew Thompson for invitation to write this review article. Financial support of the Institute of Parasitology, Biology Centre of the Czech Academy of Sciences (RVO: 60077344) is also much appreciated. The authors are indebted to Ian Beveridge (Australia), Sonja Kleinertz (Germany) who kindly provided valuable material. We would also like to acknowledge Sara Brant (MSBP), Yemisi Dare (CMNPA), Eileen Harris (BMNH), Reinhardt Møbjerg Kristensen (SNM), Birger Neuhaus (ZMB), Anna Phillips (USNM), Sankurie Pye (NMS) and Helmut Sattmann (NMW) for providing access to the museum collections and the permission to assess material for the present study. Visits to museum institutions were supported financially by the SYNTHESYS programme of the European Communities (project Nos. DE-TAF-703, DK-TAF-4500, GB-TAF-735 and 926).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2019.02.001.
Appendix A. Supplementary data
The following are the supplementary data to this article:
Host-parasite list of diphyllobothriid tapeworms in marine mammals (only most pertinent references are provided, whereas doubtful records or records needing verification are usually not included; type hosts of a given tapeworm are marked with an asterisk).
References
- Abere T., Bogale B., Melaku A. Gastrointestinal helminth parasites of pet and stray dogs as a potential risk for human health in Bahir Dar town, north-western Ethiopia. Vet. World. 2013;6:388–392. [Google Scholar]
- Alayande M.O., Faleke O.O., Salihu M.D., Mahmuda A., Muhammad M.B. Short communication: prevalence of intestinal helminths of dog (Canis familaris) in some new layouts of Sokoto metropolis. Niger. Vet. J. 2013;34:801–804. [Google Scholar]
- Andersen K.I. Studies of the helminth fauna of Norway XXXIV: the morphological stability of Diphyllobothrium Cobbold. A comparison of adult D. dendriticum (Nitzsch), D. latum (L.) and D. ditremum (Creplin) developed in different hosts. Norw. J. Zool. 1975;23:45–53. [Google Scholar]
- Bauchet A.L., Joubert C., Helies J.M., Lacour S.A., Bosquet N., Le Grand R., Guillot J., Lachapelle F. Disseminated Sparganosis in a cynomolgus macaque (Macaca fascicularis) J. Comp. Pathol. 2013;148:294–297. doi: 10.1016/j.jcpa.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Bengtson S.D., Rogers F. Prevalence of sparganosis by county of origin in Florida feral swine. Vet. Parasitol. 2001;97:241–244. doi: 10.1016/s0304-4017(01)00409-5. [DOI] [PubMed] [Google Scholar]
- Bennett H., Mok H., Gkrania-Klotsas E., Tsai I., Stanley E., Antoun N., Coghlan A., Harsha B., Traini A., Ribeiro D., Steinbiss S., Lucas S., Allinson K., Price S., Santarius T., Carmichael A., Chiodini P., Holroyd N., Dean A., Berriman M. The genome of the sparganosis tapeworm Spirometra erinaceieuropaei isolated from the biopsy of a migrating brain lesion. Genome Biol. 2014;15:510. doi: 10.1186/s13059-014-0510-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bonsdorff B.v. Academic Press; London: 1977. Diphyllobothriasis in Man. [Google Scholar]
- Brattey J., Stenson G.B. Helminth parasites of the alimentary tract of the harbor porpoise Phoacena phoacena (L.), from Newfoundland and Labrador. J. Helminthol. Soc. Wash. 1995;62:209–216. [Google Scholar]
- Buergelt C.D., Greiner E.C., Senior D.F. Proliferative sparganosis in a cat. J. Parasitol. 1984;70:121–125. [PubMed] [Google Scholar]
- Cobbold T.S. Description of Ligula mansoni, a new human cestode. J. Linn. Soc. Lond. Zool. 1883;17:78–83. [Google Scholar]
- Coghlan A., Tyagi R., Cotton J.A., Holroyd N., Rosa B.A., Tsai I.J., Laetsch D.R., Beech R.N., Day T.A., Hallsworth-Pepin K., Ke H.-M., Kuo T.-H., Lee T.J., Martin J., Maizels R.M., Mutowo P., Ozersky P., Parkinson J., Reid A.J., Rawlings N.D., Ribeiro D.M., Swapna L.S., Stanley E., Taylor D.W., Wheeler N.J., Zamanian M., Zhang X., Allan F., Allen J.E., Asano K., Babayan S.A., Bah G., Beasley H., Bennett H.M., Bisset S.A., Castillo E., Cook J., Cooper P.J., Cruz-Bustos T., Cuéllar C., Devaney E., Doyle S.R., Eberhard M.L., Emery A., Eom K.S., Gilleard J.S., Gordon D., Harcus Y., Harsha B., Hawdon J.M., Hill D.E., Hodgkinson J., Horák P., Howe K.L., Huckvale T., Kalbe M., Kaur G., Kikuchi T., Koutsovoulos G., Kumar S., Leach A.R., Lomax J., Makepeace B., Matthews J.B., Muro A., O'Boyle N.M., Olson P.D., Osuna A., Partono F., Pfarr K., Rinaldi G., Foronda P., Rollinson D., Samblas M.G., Sato H., Schnyder M., Scholz T., Shafie M., Tanya V.N., Toledo R., Tracey A., Urban J.F., Wang L.-C., Zarlenga D., Blaxter M.L., Mitreva M., Berriman M., International Helminth Genomes C. Comparative genomics of the major parasitic worms. Nat. Genet. 2018;51:163–174. doi: 10.1038/s41588-018-0262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J.J. Sparganosis. In: Steele J.H., Beran G.W., editors. CRC Handbook Series in Zoonoses. Section C: Parasitic Zoonoses. CRC Press; Boca Raton: 1981. pp. 293–312. [Google Scholar]
- Delyamure S.L., Popov V.N. Contribution to the study of the helminth fauna of the bearded seal inhabiting Sakhalin Bay. Nauch. Dokl. Vys. Skol. Biol. Nauk. 1975;10:7–10. [Google Scholar]
- Delyamure S.L., Skryabin A.S., Serdiukov A.M. Nauka; Moscow, Russia: 1985. Diphyllobothriata – Flatworm Parasites of Man, Mammals and Birds. [Google Scholar]
- Delyamure S.L., Yurakhno M.V., Popov V.N. On the helminth fauna of pennipeds form the Karaginsk gulf (The Bering Sea) Parazitologiya. 1976;10:325–332. [PubMed] [Google Scholar]
- Dick T.A. Diphyllobothriasis: the Diphyllobothrium latum human infection conundrum and reconciliation with a worldwide zoonosis. In: Darwin Murrell K., Fried B., editors. Food-borne Parasitic Zoonoses: Fish and Plant-Borne Parasites (World Class Parasites) Springer; London: 2008. pp. 151–184. [Google Scholar]
- Diesing K.M. Über eine naturgemässe Vertheilung der Cephalocotyleen. Sitzungsber. K. Akad. Wissenschaft., Wien, Mat.-Naturwissenschaft. Classe. 1854;13:556–616. [Google Scholar]
- Dubinina M.N. On the biology and distribution of Diphyllobothrium erinacei-europaei (Rud., 1819) Iwata, 1933. Zool. Zh. 1951;30:421–429. [Google Scholar]
- Dubinina M.N. Amerind Publishing Company; Leningrad and Washington D.C: 1980. Tapeworms (Cestoda, Ligulidae) of the Fauna of the USSR. [Google Scholar]
- Eom K.S., Park H., Lee D., Choe S., Kim K.-H., Jeon H.-K. Mitochondrial genome sequences of Spirometra erinaceieuropaei and S. decipiens (Cestoidea: Diphyllobothriidae) Kor. J. Parasitol. 2015;53:455–463. doi: 10.3347/kjp.2015.53.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex H.E., Magath T.B. Comparison of the variability of ova of the broad fish tapeworm, Diphyllobothrium latum, from man and dogs: its bearing on the spread of infestation with this parasite. Am. J. Hyg. 1931;14:698–704. [Google Scholar]
- Faust E.C., Campbell H.E., Kellogg C.R. Morphological and biological studies on the species of Diphyllobothrium in China. Am. J. Hyg. 1929;9:560–583. [Google Scholar]
- Felix J.R. Zea Books; Lincoln: 2013. Reported Incidences of Parasitic Infections in Marine Mammals from 1892 to 1978. [Google Scholar]
- Fiscus C., Braham H., Krogman B. Environmental Assessment of the Alaskan Continental Shelf: Receptors (Biota) Marine Mammals. 1976. Distribution and abundance of Bowhead and Beluga whales in the Bering, Chukchi and Beaufort Seas; pp. 273–324. [Google Scholar]
- Gibson D.I., Harris E.A., Bray R.A., Jepson P.D., Kuiken T., Baker J.R., Simpson V.R. A survey of the helminth parasites of cetaceans stranded on the coast of England and Wales during the period 1990–1994. J. Zool. 1998;244:563–574. [Google Scholar]
- Gnezdilov V.G. The golden hamster (Mesocricetus auratus) as potential definitive host of broad tapeworm (Diphyllobothrium latum) Dok. Akad. Nauk SSSR. Otdel Biol. 1957;114:1328–1330. [Google Scholar]
- Grove D.I. C.A.B. International; Woodville, South Australia: 1990. A History of Human Helminthology. [Google Scholar]
- Hatsushika R., Maejima J., Kamo H. Experimental studies on the development of Diphyllobothrium macroovatum Jurakhno, 1973 from the minke whale, Balaenoptera acutorostrata II. Experimental infection of the coracidia to marine copepods. Jpn. J. Parasitol. 1981;30:417–427. [Google Scholar]
- Hébert F.O., Grambauer S., Barber I., Landry C.R., Aubin-Horth N. Transcriptome sequences spanning key developmental states as a resource for the study of the cestode Schistocephalus solidus, a threespine stickleback parasite. GigaScience. 2016;5:24. doi: 10.1186/s13742-016-0128-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermosilla C., Hirzmann J., Silva L.M.R., Brotons J.M., Cerdà M., Prenger-Berninghoff E., Ewers C., Taubert A. Occurrence of anthropozoonotic parasitic infections and faecal microbes in free-ranging sperm whales (Physeter macrocephalus) from the Mediterranean Sea. Parasitol. Res. 2018;117:2531–2541. doi: 10.1007/s00436-018-5942-3. [DOI] [PubMed] [Google Scholar]
- Hernández-Orts J.S., Scholz T., Brabec J., Kuzmina T., Kuchta R. High morphological plasticity and global geographical distribution of the Pacific broad tapeworm Adenocephalus pacificus (syn. Diphyllobothrium pacificum): molecular and morphological survey. Acta Trop. 2015;149:168–178. doi: 10.1016/j.actatropica.2015.05.017. [DOI] [PubMed] [Google Scholar]
- Hernández-Orts J.S., Scholz T., Brabec J., Kuzmina T., Kuchta R. Does the number of genital organs matter? Case of the seal tapeworm Diphyllobothrium (syn. Diplogonoporus) tetrapterum (Cestoda: Diphyllobothriidea) Can. J. Zool. 2017;96:193–204. [Google Scholar]
- Herreras M.V., Kaarstad S.E., Balbuena J.A., Kinze C.C., Raga J.A. Helminth parasites of the digestive tract of the harbour porpoise Phocoena phocoena in Danish waters: a comparative geographical analysis. Dis. Aquat. Org. 1997;28:163–167. [Google Scholar]
- Hong Q., Feng J., Liu H., Li X., Gong L., Yang Z., Yang W., Liang X., Zheng R., Cui Z., Wang W., Chen D. Prevalence of Spirometra mansoni in dogs, cats, and frogs and its medical relevance in Guangzhou, China. Int. J. Infect. Dis. 2016;53:41–45. doi: 10.1016/j.ijid.2016.10.013. [DOI] [PubMed] [Google Scholar]
- Howe K.L., Bolt B.J., Shafie M., Kersey P., Berriman M. WormBase ParaSite − a comprehensive resource for helminth genomics. Mol. Biochem. Parasitol. 2017;215:2–10. doi: 10.1016/j.molbiopara.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata S. Some experimental studies on the regeneration of the plerocercoid of Manson's tapeworm, Diphyllobothrium erinacei (Rudolphi), Sparganum proliferum Ijima. Jpn. J. Zool. 1934;6:209–247. [Google Scholar]
- Iwata S. Experimental and morphological studies of Manson's tapeworm Diphyllobothrium erinacei (Rudolphi) – special reference with its scientific name and relationship with Sparganum proliferum Ijima. Prog. Med. Parasitol. Jpn. 1972;4:535–590. [Google Scholar]
- Jakobsen P.J., Scharsack J.P., Hammerschmidt K., Deines P., Kalbe M., Milinski M. In vitro transition of Schistocephalus solidus (Cestoda) from coracidium to procercoid and from procercoid to plerocercoid. Exp. Parasitol. 2012;130:267–273. doi: 10.1016/j.exppara.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Janicki C., Rosen F. Le cycle évolutif du Dibothriocephalus latus L. Recherches expérimentales et observations. Bull. Soc. Sci. Nat. Neuch. 1917;42:19–53. [Google Scholar]
- Jeon H.-K., Park H., Lee D., Choe S., Kang Y., Bia M.M., Lee S.-H., Sohn W.-M., Hong S.-J., Chai J.-Y., Eom K.S. Genetic and morphologic identification of Spirometra ranarum in Myanmar. Kor. J. Parasitol. 2018;56:275–280. doi: 10.3347/kjp.2018.56.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon H.-K., Park H., Lee D., Choe S., Kim K.-H., Huh S., Sohn W.-M., Chai J.-Y., Eom K.S. Human infections with Spirometra decipiens plerocercoids identified by morphologic and genetic analyses in Korea. Kor. J. Parasitol. 2015;53:299–305. doi: 10.3347/kjp.2015.53.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamo H. 1999. Guide to Identification of Diphyllobothriid Cestodes, Tokyo, Japan. [Google Scholar]
- Kamo H., Iwata S., Hatsushika R., Maejima J. Experimental studies on the life-cycle of Diplogonoporus grandis. II. Experimental infection of marine copepods with coracidia. Jpn. J. Parasitol. 1973;22:21–28. [Google Scholar]
- Keeling N.G., Roelke M.E., Forrester D.J. Subcutaneous helminths of the raccoon Procyon lotor in southern Florida. J. Helminthol. Soc. Wash. 1993;60:115–117. [Google Scholar]
- Kim D.-W., Kim D.-W., Yoo W., Nam S.-H., Lee M.-R., Yang H.-W., Park J., Lee K., Lee S., Cho S.-H., Lee W.-J., Park H.-S., Ju J.-W. SpiroESTdb: a transcriptome database and online tool for sparganum expressed sequences tags. BMC Res. Notes. 2012;5:1–5. doi: 10.1186/1756-0500-5-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.-W., Yoo W., Lee M.-R., Yang H.-W., Kim Y.-J., Cho S.-H., Lee W.-J., Ju J.-W. Transcriptome sequencing and analysis of the zoonotic parasite Spirometra erinacei spargana (plerocercoids) Parasites Vectors. 2014;7:368. doi: 10.1186/1756-3305-7-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinertz S., Hermosilla C., Ziltener A., Kreicker S., Hirzmann J., Abdel-Ghaffar F., Taubert A. Gastrointestinal parasites of free-living Indo-Pacific bottlenose dolphins (Tursiops aduncus) in the northern red sea, Egypt. Parasitol. Res. 2014;113:1405–1415. doi: 10.1007/s00436-014-3781-4. [DOI] [PubMed] [Google Scholar]
- Kołodziej-Sobocińska M., Miniuk M. Sparganosis – neglected zoonosis and its reservoir in wildlife. Med. Weter. 2018;74:224–227. [Google Scholar]
- Kołodziej-Sobocińska M., Tokarska M., Kowalczyk R. The first report of sparganosis (Spirometra sp.) in Eurasian badger (Meles meles) Parasitol. Int. 2014;63:397–399. doi: 10.1016/j.parint.2013.12.011. [DOI] [PubMed] [Google Scholar]
- Kornyushin V., Malyshko (Varodi) E.I., Malega A.M. The helminths of wild predatory mammals of Ukraine. Cestodes. Vest. Zool. 2011;45 e-1–e-8. [Google Scholar]
- Koziol U., Koziol M., Preza M., Costábile A., Brehm K., Castillo E. De novo discovery of neuropeptides in the genomes of parasitic flatworms using a novel comparative approach. Int. J. Parasitol. 2016;46:709–721. doi: 10.1016/j.ijpara.2016.05.007. [DOI] [PubMed] [Google Scholar]
- Krivokhizhyn S.V. Pravda Severa; Arkhangelsk, Arkhangelsk, Russia: 2000. New Opinion about the Helminth Fauna of Black Sea Dolphins, Marine Mammals of Holarctic; pp. 192–197. 21–23 Sep 2000. [Google Scholar]
- Kuchta R., Brabec J., Kubáčková P., Scholz T. Tapeworm Diphyllobothrium dendriticum (Cestoda) – neglected or emerging human parasite? PLoS Neglected Trop. Dis. 2013;7:e2535. doi: 10.1371/journal.pntd.0002535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchta R., Scholz T. Diphyllobothriidea. In: Caira J.N., Jensen K., editors. Planetary Biodiversity Inventory (2008–2017): Tapeworms from Vertebrate Bowels of the Earth. University of Kansas, Natural History Museum, Special Publication No. 25; Lawrence: 2017. pp. 167–189. [Google Scholar]
- Kuchta R., Scholz T., Brabec J., Narduzzi-Wicht B. Chapter 17. Diphyllobothrium, Diplogonoporus and Spirometra. In: Xiao L., Ryan U., Feng F., editors. Biology of Foodborne Parasites. Section III. Important Foodborne Helminths. CRC Press; Boca Raton: 2015. pp. 299–326. [Google Scholar]
- Kuntz R.E. Intestinal protozoa and helminths in school children of Dacca, East Pakistan (East Bengal) Am. J. Trop. Med. Hyg. 1960;9:168–172. doi: 10.4269/ajtmh.1960.9.168. [DOI] [PubMed] [Google Scholar]
- Kuzmina T.A., Hernández-Orts J.S., Lyons E.T., Spraker T.R., Kornyushyn V.V., Kuchta R. The cestode community in northern Fur seals (Callorhinus ursinus) on St. Paul Island, Alaska. Int. J. Parasitol.: Par. Wildl. 2015;4:256–263. doi: 10.1016/j.ijppaw.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmina T.A., Spraker T.R., Kudlai O., Lisitsyna O.I., Zabludovskaja S.O., Karbowiak G., Fontaine C., Kuchta R. Metazoan parasites of California sea lions (Zalophus californianus): a new data and review. Int. J. Parasitol.: Par. Wildl. 2018;7:326–334. doi: 10.1016/j.ijppaw.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le A.T., Do L.-Q.T., Nguyen H.-B.T., Nguyen H.-N.T., Do A.N. Case report: the first case of human infection by adult of Spirometra erinaceieuropaei in Vietnam. BMC Infect. Dis. 2017;17:669. doi: 10.1186/s12879-017-2786-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Chai J.Y., Seo B.S., Cho S.Y. Two cases of human infection by adult of Spirometra erinacei. Kor. J. Parasitol. 1984;22:66–71. doi: 10.3347/kjp.1984.22.1.66. [DOI] [PubMed] [Google Scholar]
- Linnaeus C. tenth ed. Laurentius Salvius; Stockholm: 1758. Systema Naturae Per Regna Tria Naturae: Secundum Classes, Ordines, Genera, Species, Cum Characteribus, Differentiis, Synonymis, Locis. [Google Scholar]
- Liu Q., Li M.-W., Wang Z.-D., Zhao G.-H., Zhu X.-Q. Human sparganosis, a neglected food borne zoonosis. Lancet Infect. Dis. 2015;15:1226–1235. doi: 10.1016/S1473-3099(15)00133-4. [DOI] [PubMed] [Google Scholar]
- Magnino S., Colin P., Dei-Cas E., Madsen M., McLauchlin J., Nockler K., Maradona M.P., Tsigarida E., Vanopdenbosch E., Van Peteghem C. Biological risks associated with consumption of reptile products. Int. J. Food Microbiol. 2009;134:163–175. doi: 10.1016/j.ijfoodmicro.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Markowski S. On the species of Diphyllobothrium occurring in birds and their relation to man and other hosts. J. Helminthol. 1949;23:107–126. doi: 10.1017/s0022149x00032454. [DOI] [PubMed] [Google Scholar]
- Mitchell P.D. The origins of human parasites: exploring the evidence for endoparasitism throughout human evolution. Int. J. Paleopathol. 2013;3:191–198. doi: 10.1016/j.ijpp.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Moulinier R., Martinez E., Torres J., Noya O., Denoya B.A., Reyes O. Human proliferative sparganosis in Venezuela: report of a case. Am. J. Trop. Med. Hyg. 1982;31:358–363. doi: 10.4269/ajtmh.1982.31.358. [DOI] [PubMed] [Google Scholar]
- Mueller J.F. The biology of Spirometra. J. Parasitol. 1974;60:3–14. [PubMed] [Google Scholar]
- Müller O.F. typis Hallageriis; Havniae: 1776. Zoologiae Danicae prodromus: seu Animalium Daniae et Norvegiae indigenarum; characteres, nomina, et synonyma imprimis popularium. [Google Scholar]
- Muratov I.V. Predatory terrestrial mammals as the definitive hosts of Diphyllobothrium klebanovskii. Med. Parazitol. 1993:3–5. 1993. [PubMed] [Google Scholar]
- Nobrega-Lee M., Hubbard G., LoVerde P., Carvalho-Queiroz C., Conn D.B., Rohde K., Dick E.J., Nathanielsz P., Martin D., Siler-Khodr T., Schlabritz-Loutsevitch N. Sparganosis in wild-caught baboons (Papio cynocephalus anubis) J. Med. Primatol. 2007;36:47–54. doi: 10.1111/j.1600-0684.2006.00177.x. [DOI] [PubMed] [Google Scholar]
- Oda F.H., Borteiro C., da Graça R.J., Tavares L.E.R., Crampet A., Guerra V., Lima F.S., Bellay S., Karling L.C., Castro O., Takemoto R.M., Pavanelli G.C. Parasitism by larval tapeworms genus Spirometra in South American amphibians and reptiles: new records from Brazil and Uruguay, and a review of current knowledge in the region. Acta Trop. 2016;164:150–164. doi: 10.1016/j.actatropica.2016.09.005. [DOI] [PubMed] [Google Scholar]
- Odening K. Neue Erkenntnisse zu Ökographie, Bionomie und Taxonomie von Spirometra (Cestoda: Pseudophyllidea) Milu: Wissenschaft. K. Mitteil. Tierpark. 1985;6:277–294. [Google Scholar]
- Odening K., Bockhardt I. Two European Spirometra forms (Cestoidea: Diphyllobothriidae) with different sparganum growth factors. Angew. Parasitol. 1982;23:15–27. [PubMed] [Google Scholar]
- Okino T., Ushirogawa H., Matoba K., Nishimatsu S.-i., Saito M. Establishment of the complete life cycle of Spirometra (Cestoda: Diphyllobothriidae) in the laboratory using a newly isolated triploid clone. Parasitol. Int. 2017;66:116–118. doi: 10.1016/j.parint.2016.12.011. [DOI] [PubMed] [Google Scholar]
- Okumura T. An experimental study of the life-history of Sparganum mansoni Cobbold. Kitasato Arch. Exp. Med. 1919;3:190–197. [Google Scholar]
- Otake Sato M., Sato M., Yoonuan T., Pongvongsa T., Sanguankiat S., Kounnavong S., Maipanich W., Chigusa Y., Moji K., Waikagul J. The role of domestic dogs in the transmission of zoonotic helminthes in a rural area of Mekong river basin. Acta Parasitol. 2017;62:393–400. doi: 10.1515/ap-2017-0047. [DOI] [PubMed] [Google Scholar]
- Phares C. An unusual host-parasite relationship: the growth hormone-like factor from plerocercoids of spirometrid tapeworms. Int. J. Parasitol. 1996;26:575–588. doi: 10.1016/0020-7519(96)00025-2. [DOI] [PubMed] [Google Scholar]
- Preza M., Montagne J., Costábile A., Iriarte A., Castillo E., Koziol U. Analysis of classical neurotransmitter markers in tapeworms: evidence for extensive loss of neurotransmitter pathways. Int. J. Parasitol. 2018;48:979–992. doi: 10.1016/j.ijpara.2018.06.004. [DOI] [PubMed] [Google Scholar]
- Pronin N.M., Zhaltsanova D.S.D. Parasites of the Baikal seal: a systematic analysis. In: Korsunov V.M., Pronin N.M., Gonchikov G., editors. Biodiversity of Baikal Siberia, Novosibirsk. 1999. pp. 163–166. [Google Scholar]
- Qiu M.H., Qiu M.D. Human plerocercoidosis and sparganosis: I. A historical review on aetiology. Chin. J. Parasitol. Parasit. Dis. 2009;27:54–60. [PubMed] [Google Scholar]
- Ramana K.V., Rao S., Vinaykumar M., Krishnappa M., Reddy R., Sarfaraz M., Kondle V., Ratnamani M.S., Rao R. Diphyllobothriasis in a nine-year-old child in India: a case report. J. Med. Case Rep. 2011;5:1–3. doi: 10.1186/1752-1947-5-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch R.L., Hilliard D.K. Studies on the helminth fauna of Alaska. XLIX. The occurrence of Diphyllobothrium latum (Linnaeus, 1758) (Cestoda: Diphyllobothriidae) in Alaska, with notes on other species. Can. J. Zool. 1970;48:1201–1212. doi: 10.1139/z70-210. [DOI] [PubMed] [Google Scholar]
- Rausch R.L., Scott E.M., Rausch V.R. Helminths in eskimos in Western Alaska with particular reference to Diphyllobothrium infection and anaemia. Trans. R. Soc. Trop. Med. Hyg. 1967;61:351–357. [Google Scholar]
- Reif J.S., Kliks M.M., Aguirre A.A., Borjesson D.L., Kashinsky L., Braun R.C., Antonelis G.A. Gastrointestinal helminths in the Hawaiian monk seal (Monachus schauinslandi): associations with body size, hematology, and serum chemistry. Aquat. Mamm. 2006;32:157–167. [Google Scholar]
- Rojekittikhun W., Mahittikorn A., Prummongkol S., Puangsa-art S., Chaisiri K., Kusolsuk T. Prevalence of gastrointestinal parasitic infections in refuge dogs and cats and evaluation of two conventional examination techniques. J. Trop. Med. Parasitol. 2013;36:58–67. [Google Scholar]
- Rosenberg A.I. Karelia publ; Petrozavodsk: 1977. Diphyllobothriides and Diphyllobothrioses of Medical Veterinary Significance: Index of Native and Foreign Literature 1558–1972. [Google Scholar]
- Rukavina J., Džumurov N., Delič S. Larvae of Diphyllobothrium erinacei europaei in pigs. Vet. Sarajevo. 1957;6:46–55. [Google Scholar]
- Ryan G.E. Gastro-intestinal parasites of feral cats in New South Wales. Aust. Vet. J. 1976;52:224–227. doi: 10.1111/j.1751-0813.1976.tb00072.x. [DOI] [PubMed] [Google Scholar]
- Ryzhenko G.F. Experimental determination of the reservoir hosts of Spirometra erinacei-europaci. Tr. Vses. Inst. Gelmintol. 1969;15:252–256. [Google Scholar]
- Salb A.L., Barkema H.W., Elkin B.T., Thompson R.C.A., Whiteside D.P., Black S.R., Dubey J.R., Kutz S.J. Dogs as sources and sentinels of parasites in humans and wildlife, northern Canada. Emerg. Infect. Dis. 2008;14:60–63. doi: 10.3201/eid1401.071113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasikala M., Yogespriya R., Jayalakshmi K., Dhivya B., Arulkumar T. Rare incidence of diphyllobothriosis in a dog. Indian Vet. J. 2018;95:74–75. [Google Scholar]
- Shulman O.N., Popov V.N. AN USSR; Astrakhan, USSR: 1982. Study of Morphological Variations in Cestode Diphyllobothrium lanceolatus – Parasite of Bearded Seal, Thezisy Dokladov 8 Vsesoyuznogo Sovescheniya; pp. 412–414. [Google Scholar]
- Schaeffner B.C., Ditrich O., Kuchta R. A century of taxonomic uncertainty: re-description of two species of tapeworms (Diphyllobothriidea) from Arctic seals. Polar Biol. 2018;41:2543–2559. [Google Scholar]
- Scholz T., Garcia H.H., Kuchta R., Wicht B. Update on the human broad tapeworm (genus Diphyllobothrium), including clinical relevance. Clin. Microbiol. Rev. 2009;22:146–160. doi: 10.1128/CMR.00033-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz T., Kuchta R. Fish-borne, zoonotic cestodes (Diphyllobothrium and relatives) in cold climates: a never-ending story of neglected and (re)-emergent parasites. Food Waterb. Parasitol. 2016;4:23–38. [Google Scholar]
- Scholz T., Uhlířová M., Ditrich O. Helminth parasites of cats from the Vientiane Province, Laos, as indicators of the occurrence of causative agents of human parasitoses. Parasite. 2003;10:343–350. doi: 10.1051/parasite/2003104343. [DOI] [PubMed] [Google Scholar]
- Schurer J.M., Ndao M., Skinner S., Irvine J., Elmore S.A., Epp T., Jenkins E.J. Parasitic zoonoses: one health surveillance in northern Saskatchewan. PLoS Neglected Trop. Dis. 2013;7:e2141. doi: 10.1371/journal.pntd.0002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simão F.A., Waterhouse R.M., Ioannidis P., Kriventseva E.V., Zdobnov E.M. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31:3210–3212. doi: 10.1093/bioinformatics/btv351. [DOI] [PubMed] [Google Scholar]
- Sinisalo T., Kunnasranta M., Valtonen E.T. Intestinal helminths of a landlocked ringed seal (Phoca hispida saimensis) population in eastern Finland. Parasitol. Res. 2003;91:40–45. doi: 10.1007/s00436-003-0893-7. [DOI] [PubMed] [Google Scholar]
- Spöring H.D. Berättelse. Om en Qvinna, hos bvilken et ftycke af Binnike Mafken kommit utur en bålde i liumfkan. K. Svensk. Vet. Acad. Handl. 1747;8:103–112. [Google Scholar]
- Stief B., Enge A. Proliferative peritonitis with larval and cystic parasitic stages in a dog. Vet. Pathol. 2011;48:911–914. doi: 10.1177/0300985810382092. [DOI] [PubMed] [Google Scholar]
- Umar Y.A. Intestinal helminthoses in dogs in Kaduna metropolis, Kaduna state, Nigeria. Iran. J. Parasitol. 2009;4:34–39. [Google Scholar]
- Vik R. Investigations on the pseudophyllidean cestodes of the fish, birds and mammals in the Anøya water system in Trøndelag. Part I. Cyathocephalus truncatus and Schistocephalus solidus. Nytt Magasin Zool. 1954;2:5–51. [Google Scholar]
- Waeschenbach A., Brabec J., Scholz T., Littlewood D.T.J., Kuchta R. The catholic taste of broad tapeworms – multiple routes to human infection. Int. J. Parasitol. 2017;47:831‒843. doi: 10.1016/j.ijpara.2017.06.004. [DOI] [PubMed] [Google Scholar]
- Wang H., Tang Y., Yang Y. A case of Spirometra mansoni infection with both plerocercoid larvae and adult worm. Chin. J. Parasitol. Parasit. Dis. 2012;30:40. [PubMed] [Google Scholar]
- Wardle R.A., McLeod J.A., Radinovsky S. University of Minnesota Press; 1974. Advances in the Zoology of Tapeworms, 1950–1970. [Google Scholar]
- Woldemeskel M. Subcutaneous sparganosis, a zoonotic cestodiasis, in two cats. J. Vet. Diagn. Investig. 2014;26:316–319. doi: 10.1177/1040638713517697. [DOI] [PubMed] [Google Scholar]
- Yamane Y., Shiwaku K., Fukushima T., Isobe A., Qiang G.T., Yoneyama T. The taxonomic study of diphyllobothriid cestodes with special reference to Diphyllobothrium nihonkaiense in Japan. In: Ishikura H., Aikawa M., editors. Host Response to International Parasitic Zoonoses. Springer; 1998. pp. 25–38. [Google Scholar]
- Yurakhno M.V. On the taxonomy and phylogeny of some groups of cestodes of the order Pseudophyllidea. Parazitologiya. 1992;26:449–460. [Google Scholar]
- Yurakhno M.V., Maltsev V.N. An infection of seals from Antarctica with cestodes. Parazitologiya. 1997;31:81–89. [Google Scholar]
- Zhang X., Cui J., Liu L.N., Jiang P., Wang H., Qi X., Wu X.Q., Wang Z.Q. Genetic structure analysis of Spirometra erinaceieuropaei isolates from Central and Southern China. PLoS One. 2015;10:e0119295. doi: 10.1371/journal.pone.0119295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Duan J.Y., Shi Y.L., Jiang P., Zeng D.J., Wang Z.Q., Cui J. Comparative mitochondrial genomics among Spirometra (Cestoda: Diphyllobothriidae) and the molecular phylogeny of related tapeworms. Mol. Phylogenet. Evol. 2017;117:75–82. doi: 10.1016/j.ympev.2017.06.003. [DOI] [PubMed] [Google Scholar]
- Zhang X., Wang H., Cui J., Jiang P., Lin M.L., Zhang Y.L., Liu R.D., Wang Z.Q. The phylogenetic diversity of Spirometra erinaceieuropaei isolates from southwest China revealed by multi genes. Acta Trop. 2016;156:108–114. doi: 10.1016/j.actatropica.2016.01.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Host-parasite list of diphyllobothriid tapeworms in marine mammals (only most pertinent references are provided, whereas doubtful records or records needing verification are usually not included; type hosts of a given tapeworm are marked with an asterisk).