Abstract
Purpose
To compare Descemet membrane endothelial keratoplasty (DMEK) outcomes using nondiabetic grafts in diabetic and nondiabetic recipients.
Methods
All eyes that underwent DMEK between February 2013 and October 2016 (follow-up ≥3 months, without prior keratoplasty) were included. Recipients were divided into diabetic (insulin dependent [IDDM] or noninsulin dependent [NIDDM]) and nondiabetic groups. Main outcome measures included postoperative visual acuity, rebubble procedure rates, and graft failure rates.
Results
Of 334 eyes (243 subjects) included for analysis, 63 eyes (18.8%) were from diabetic recipients. At each timepoint, best-corrected visual acuity trended lower for IDDM recipients compared to NIDDM and nondiabetic recipients. There were no statistically significant differences in rebubble rates of diabetic compared to nondiabetic recipients (20.6% vs. 12.9%, p = 0.17), or IDDM compared to nondiabetic recipients (27.3% vs. 12.9%, p = 0.08; hazard ratio 2.26). Overall, 13 grafts (3.9%) failed (mean follow-up, 565 days; range, 90–1293 days). Graft failures did not differ between diabetic and nondiabetic recipients (4.0% vs. 4.9%, p = 0.15) regardless of subgroup (p = 0.36).
Conclusions
DMEK provides excellent outcomes for patients with and without diabetes. DMEK outcomes were excellent with improvements in visual acuity and low rates of graft failure. Our findings were unable to determine differences between rebubble procedure rates but do emphasize the need for further research using stratified groups based on diabetes severity.
Keywords: Corneal endothelium, DMEK, Diabetes mellitus, Descemet membrane, Endothelial keratoplasty, Corneal transplantation
1. Introduction
Diabetes mellitus is prevalent in the United States and causes ocular manifestations including retinopathy and neurotrophic keratitis. Recently, there has been growing interest into how the diabetic corneal endothelium responds to surgical stress including keratoplasty. A recent study by Price et al. found that Descemet membrane endothelial keratoplasty (DMEK) recipients with diabetes had increased rebubble rates.1 That study utilized both nondiabetic and diabetic donor tissue and as a result did not isolate the effect of recipient diabetes. To evaluate this relationship further, we conducted a study evaluating DMEK outcomes in diabetic recipients using exclusively nondiabetic donor tissue.
2. Materials and methods
The University of Iowa Institutional Review Board approved this study and all research described herein adhered to the tenets of the Declaration of Helsinki. A retrospective review was performed on all eyes that underwent DMEK at the University of Iowa between February 2013 and October 2016. All eyes received nondiabetic donor tissue as established by screening protocols at Iowa Lions Eye Bank. Eyes with a previous corneal transplant procedure or inadequate follow-up (<90 days) were excluded. No cases had prior trabeculectomy or glaucoma tube shunt placement. Recipients were grouped into nondiabetic and diabetic recipients, and further subgrouped into noninsulin dependent (NIDDM) and insulin dependent (IDDM) diabetes mellitus.
Cases were performed using a standardized protocol of pre-stripped pre-cut donor corneal tissue, a modified DMEK Jones tube (Gunther Weiss Scientific Glassblowing, Portland, OR) to insert the tissue, corneal tapping in a shallow anterior chamber to unscroll the graft, and sulfur hexafluoride 20% to tamponade the tissue. Examinations were performed 1 day, 1 week, 1, 3, 6 and 12 months, and annually after surgery.
Main outcomes measures included postoperative visual acuity, rebubble procedure rate, and graft failure rate. Graft failure was defined as an irreversibly cloudy cornea that would require regrafting for any reason, regardless of whether or not a regrafting procedure was performed subsequently. Clinical indications for a rebubble procedure were progressive graft edge lift threatening the central visual axis or a graft edge lift causing a decrease in visual acuity.
Kaplan-Meier curves were constructed for rebubble rates and compared using the log rank test. Cox proportional hazard regression model was used to obtain hazard ratio estimates. To account for recipients contributing outcomes for both eyes, the sandwich aggregate method was used to estimate the covariance matrix for testing Cox model parameters. The differences in visual acuity between groups were assessed using linear mixed model analysis for repeated measures. The mixed model included a random effect to account for recipients contributing visual acuity from both eyes. For tests comparing between groups at multiple time points, p-values were Bonferroni adjusted.
3. Results
In total, 334 eyes from 243 patients undergoing DMEK surgery using nondiabetic donor tissue were analyzed, with a mean follow-up of 565 days (range, 90–1293 days). This included 271 eyes from 195 nondiabetic patients and 63 eyes from 48 diabetic patients. In the diabetes subgroups, there were 41 eyes from 31 NIDDM patients and 22 eyes from 17 IDDM patients. Fuchs endothelial corneal dystrophy was the most common indication for diabetic (93%) and nondiabetic (87%) recipients.
The Snellen equivalent for preoperative visual acuity was approximately 20/50 in both diabetic and nondiabetic groups and by 12 months after DMEK, the mean best-corrected visual acuity improved to 20/25-2 in diabetic and 20/25+1 in nondiabetic recipients. Preoperative visual acuity did not differ significantly among study groups (p = 0.57) but at all postoperative time points, nondiabetic recipients had better mean visual acuity measurements than NIDDM recipients, which in turn had better acuity than IDDM recipients; none of these differences reached statistical significance (all p-values > 0.12).
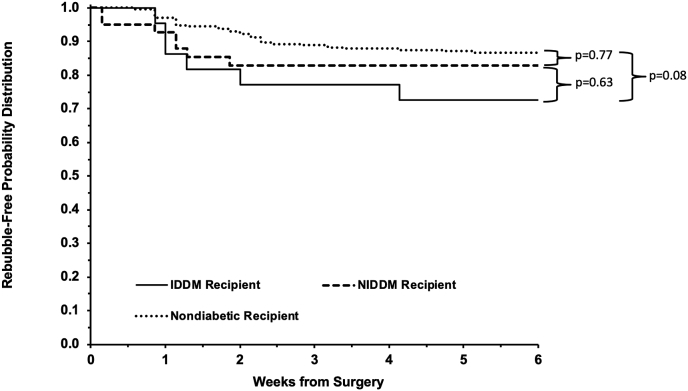
There was no statically significant difference in rebubble procedure rates between diabetic recipients (20.6%, n = 13) and nondiabetic recipients (12.9%, n = 36) (p = 0.17). In the comparison of rebubble rates among the stratified diabetic versus nondiabetic recipient groups (Fig. 1), the differences approached but did not reach statistical significance (p = 0.08) between IDDM (27.3%, n = 6) and nondiabetic (12.9%, n = 36) DMEK recipients (hazard ratio 2.26; 95% CI: 0.95, 5.46). In the comparison of graft survival, there were no differences (p = 0.36) in graft failure rates between NIDDM (5.9%, n = 1), IDDM (0%, n = 0), and nondiabetic eyes (4.9%, n = 11) but the retrospective review may underrepresent late graft failures.
Fig. 1.
Rebubble-free survival of insulin-stratified diabetic and nondiabetic DMEK graft recipients. Kaplan-Meier survival curve for rebubble-free survival after DMEK grafting indicating increased probability for recipients with insulin dependent diabetes mellitus (IDDM) to require a rubble procedure after DMEK compared to nondiabetic recipients.
4. Discussion
Overall, DMEK surgery provided excellent outcomes for all recipients. There were significant improvements in visual acuity in all groups with low rates of graft failures. Both the current study and Price's 2017 study share similar rates of rebubble procedures for nondiabetic (12.9% vs. 17%), NIDDM (17.1% vs. 19%), and IDDM (27.3% vs. 33%) recipients, respectively.1 Comparing both investigations, our study analyzed 334 total cases including 41 NIDDM and 22 IDDM cases, while the Price study was larger and analyzed 2104 total cases including 226 NIDDM and 75 IDDM cases. Although our study was unable to answer the question of higher rebubble rate in diabetics to a level of statistical significance, the parallel findings in our study and Price's 2017 study does raise interesting questions surrounding an underlying trend that needs further investigation. If there is a relationship between diabetes and rebubble rates, the reason for this possible increased rate of rebubble procedures in diabetic recipients is unclear. Diabetic aqueous may be less supportive of corneal endothelial cell function due to increased inflammatory proteins as observed in other diseases, such as surgically treated glaucoma.2 We hypothesize that diabetic aqueous accumulates inflammatory proteins due to blood-aqueous barrier breakdown and poses a similar risk for inflammation mediated impairment in corneal endothelial cell health and poor graft function in this disease impaired anterior segment environment. Diabetes has already been shown to increase the total protein and alter the protein profile of aqueous humor.3,4 While there are supportive clinical findings both in our study and the Price study, this hypothesis remains speculative and needs further investigation.
The rate of primary graft failure in this series was low with an overall failure rate of 3.9% and was comparable to the Price study.1 However, the role of diabetes in graft failure is far from clear based on conflicting results in other long-term studies of penetrating keratoplasty.5,6 However, the importance of studying the influence of diabetes is becoming more clear. Based on the results of the CPTS, donor diabetes was a risk factor for graft dislocation, lower endothelial cell density at 3 years, and graft failure at 3 years after DSAEK.7, 8, 9 These CPTS studies did not stratify based on diabetic severity or analyze nondiabetic tissue transplanted into diabetic and nondiabetic recipients. However, they do highlight the impact of diabetes on donor corneal tissue health and outcomes. Based on the comparison to our study and the Price study, it is possible that both donor and recipient diabetes is detrimental to corneal endothelial cell health. Prospective long-term studies are needed to determine whether recipient diabetes status affects endothelial keratoplasty survival.
The coarse nature of diabetes stratification based on insulin use was a significant study limitation. While a helpful marker of advanced disease, insulin use does not account for variations in A1c, diabetes duration, or the presence of organ complications. Investigation of both recipient and donor status with multicenter prospective trials to evaluate the influence of diabetes severity, with attention to markers of disease severity, are indicated. Another significant limitation was the small population size, which was underpowered to validate small but potentially significant outcome differences. Finally, while donor screening is rigorous it is possible that some of the tissues used for surgery could have come from undiagnosed diabetic donors; therefore, our findings may be impacted by the inability to isolate the influence of recipient diabetes completely.
5. Conclusions
In conclusion, DMEK outcomes were excellent in all recipient groups studied with improvements in visual acuity and low rates of graft failure. The small study population size was underpowered to evaluate fully any potential differences in rebubble rates and diabetic severity. This study emphasizes the need for stratified groups based on diabetes severity in outcomes research as well as the need for future, larger studies to identify potentially important differences in caring for diabetic patients after DMEK surgery.
6. Patient consent
Per IRB review, patient consent was not required for this retrospective review. This report does not contain any personal information that could lead to the identification of the patients studied.
Acknowledgments and disclosures
No funding or grant support. All authors have no financial disclosures. All authors attest that they meet the current ICMJE criteria for Authorship.
References
- 1.Price M.O., Lisek M., Feng M.T., Price F.W., Jr. Effect of donor and recipient diabetes status on descemet membrane endothelial keratoplasty adherence and survival. Cornea. 2017;36:1184–1188. doi: 10.1097/ICO.0000000000001305. [DOI] [PubMed] [Google Scholar]
- 2.Janson B.J., Alward W.L., Kwon Y.H. Glaucoma-associated corneal endothelial cell damage: a review. Surv Ophthalmol. 2018;63:500–506. doi: 10.1016/j.survophthal.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Chiang S.Y., Tsai M.L., Wang C.Y. Proteomic analysis and identification of aqueous humor proteins with a pathophysiological role in diabetic retinopathy. J Proteomics. 2012;75:2950–2959. doi: 10.1016/j.jprot.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Balaiya S., Zhou Z., Chalam K.V. Characterization of vitreous and aqueous proteome in humans with proliferative diabetic retinopathy and its clinical correlation. Proteomics Insights. 2017;8 doi: 10.1177/1178641816686078. 1178641816686078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lass J.H., Riddlesworth T.D., Gal R.L. The effect of donor diabetes history on graft failure and endothelial cell density 10 years after penetrating keratoplasty. Ophthalmology. 2015;122:448–456. doi: 10.1016/j.ophtha.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Price M.O., Thompson R.W., Jr., Price F.W., Jr. Risk factors for various causes of failure in initial corneal grafts. Arch Ophthalmol. 2003;121:1087–1092. doi: 10.1001/archopht.121.8.1087. [DOI] [PubMed] [Google Scholar]
- 7.Lass J.H., Benetz B.A., Patel S.V. Donor, recipient, and operative factors associated with increased endothelial cell loss in the cornea preservation time study. JAMA Ophthalmol. 2018 Oct 26 doi: 10.1001/jamaophthalmol.2018.5669. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aldave A.J., Terry M.A., Szczotka-Flynn L.B. Effect of graft attachment status and intraocular pressure on descemet stripping automated endothelial keratoplasty outcomes in the cornea preservation time study. Am J Ophthalmol. 2019;203:78–88. doi: 10.1016/j.ajo.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terry M.A., Aldave A.J., Szczotka-Flynn L.B. Donor, recipient, and operative factors associated with graft success in the cornea preservation time study. Ophthalmology. 2018;125:1700–1709. doi: 10.1016/j.ophtha.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]