Abstract
We described three severe asthmatics whose asthma symptoms were rapidly improved by benralizumab following favorable response to mepolizumab. Benralizumab-induced eosinophil depletion contributed to clinical improvement of severe asthma after mepolizumab-induced eosinophil reduction; thus, prior favorable responses to mepolizumab may predict benralizumab efficacy.
Keywords: Benralizumab, Mepolizumab, Severe asthma, Eosinophil
Abbreviations: ICS, inhaled corticosteroids; LABA, long-acting beta-agonist; LAMA, long-acting muscarinic antagonist; LTRA, leukotriene antagonist; BT, bronchial thermoplasty; ACT, Asthma control test; FeNO, fraction of exhaled nitric oxide; FVC, forced vital capacity; FEV1, expiratory volume in 1 second; CT, computed tomography
1. Introduction
Targeting airway eosinophila-related immunological processes could improve severe asthma control [1]. Blood eosinophil counts are the main biomarker used to identify responders to benralizuamab and mepolizumab [2]. Benralizumab is an anti-IL5 receptor antibody that depletes eosinophils via antibody-dependent cell-medicated cytotoxicity [3], whereas mepolizumab that is an anti-IL-5 antibody that reduces eosinophils via a passive mechanism [1,2]. The superior drug in term of initial therapy for severe eosinophilic asthma remains unclear. We present three cases of severe asthmatics who responded favorably to mepolizumab and exhibited clinical improvement after switching to benralizumab.
2. Case report
2.1. Case1
A 61-year-old man presented with a 7-year history of chronic sinusitis and asthma. The patient quit smoking 34 years prior. He was treated with Flutiform125® (Fluticasone/Formoterol) 8 puffs/day, tiotropium 2 puffs/day, montelukast 10 mg/day, and short bursts of systemic corticosteroids. However, these treatments were insufficient. He requested bronchial thermoplasty (BT) treatment, and referred to our hospital.
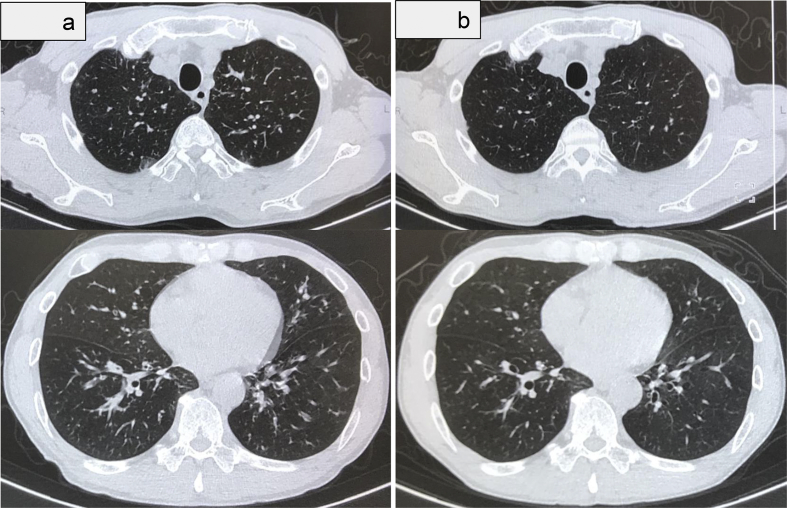
Chest auscultation revealed wheezing. Chest radiography revealed no abnormalities. The Asthma control test (ACT) was 20. The serum total IgE level was 1908 IU/mL. Inflammatory markers indicated high blood eosinophil counts (770/μL) and an elevated fraction of exhaled nitric oxide (FeNO: 117 ppb). The forced vital capacity (FVC) and expiratory volume in 1 second (FEV1) were 4.12L (%FVC 110.5%) and 2.60 L (%FEV1 84.1%), respectively. BT was performed in three treatment sessions within different regions of the lung. After an initial improvement, the asthma severity returned to the baseline level within 4 months. Nine months later, his ACT, FVC, and FEV1 declined to 16, 2.99 L, and 1.47 L, respectively. Chest computed tomography (CT) revealed thickening of the bronchial walls and mucus plugging (Fig. 1a).
Fig. 1.
Chest computed tomography before and after mepolizumab therapy. a: Chest computed tomography (CT) revealed thickening of the bronchial walls and mucus plugging prior to mepolizumab treatment. b: Chest CT revealed regression of thickening of the bronchial walls and mucus plugging after 10 months of mepolizumab treatment.
Mepolizumab was started (100 mg every 4 weeks) 9 months after BT. Ten months later, the peripheral eosinophil count decreased from 1790/μL to 50/μL. Spirometry revealed lung function improvement (%FVC 116.4%; FVC 4.25 L; %FEV1 97.7%; FEV1: 2.95 L). Chest CT revealed regression of the thickening of the bronchial walls and mucus plugging (Fig. 1b). However, the wheezing and dyspnea continued with exercise. The ACT was 24.
Benralizumab was started (30 mg subcutaneously every 4 weeks for the initial three doses and then every 8 weeks). The eosinophil count decreased from 50 to 0/μL after 1 month. After 6 months, the pulmonary wheezing and dyspnea with exercise disappeared. ACT was 25. The FVC and FEV1 were further improved (Table 1).
Table 1.
Demografic and clinical data for three patients whose severe asthma symptoms were rapidly improved by benralizumab after a good response to mepolizumab.
| Sex | Age (yr) | BMI | Smoking status | Duration of<> Mepo treatment (months) | ACT |
Eo (/μl) |
FEV1 (L) |
Complications | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| before Mepo | before Ben | 6 months after Ben | before Mepo | before Ben | 6 months after Ben | before Mepo | before Ben | 6 months after Ben | |||||||
| Case1 | M | 61 | 20.7 | ES | 10 | 17 | 24 | 25 | 1790 | 50 | 0 | 1.47 | 2.95 | 3.32 | AR, CRS |
| Case2 | F | 60 | 18.5 | CS | 12 | 11 | 20 | 25 | 830 | 90 | 10 | 1.03 | 1.23 | 1.91 | AR, CRS, EP |
| Case3 | F | 57 | 18.7 | NS | 16 | NA | 24 | 23 | 1350 | 160 | 0 | 0.42 | 0.95 | 1.68 | type I DM |
AR, allergic rhinitis; Ben, benralizumab; BMI, body mass index; CRS; chronic sinusitis; CS, current smoker; DM, diabetes mellitus; EP, eosinophilic pneumonia; ES, ex-smoker; Mepo, mepolizumab; NS, non-smoker.
2.2. Case2
A 60-year-old woman presented with a 21-year history of asthma. The patient was a non-smoker with a history of chronic sinusitis and eosinophilic pneumonia. Her treatment consisted of Symbicort® 4 puffs/day and 5 mg prednisone.
Chest auscultation revealed wheezing. Chest radiography revealed no abnormalities. The ACT was 11. The serum total IgE level was 100 IU/mL. Inflammatory markers indicated high blood eosinophil counts (830/μL) and an elevated FeNO (92 ppb). The FVC and FEV1 were 1.73 L (%FVC 60.7%) and 1.03 L (%FEV1 44.6%), respectively.
Mepolizumab was started (100 mg every 4 weeks). After an initial improvement, the corticosteroids were then gradually reduced, but the asthma attacks recurred. The peripheral eosinophil count decreased from 880/μL to 90/μL after 1 year. Spirometry revealed improved lung function (%FVC 78.1%; FVC 2.21 L; %FEV1 53.9%; FEV1: 1.23 L). ACT was 19. However, the wheezing and asthma attacks continued.
Benralizumab was started (30 mg subcutaneously every 4 weeks for the initial three doses and then every 8 weeks). The eosinophil count decreased from 90 to 0/μL and wheezing disappeared after 1 month. The ACT was 25 after 6 months. The FVC and FEV1 were further improved (Table 1).
2.3. Case3
A 57-year-old woman was admitted to our hospital for an asthma attack. She was a heavy smoker with a history of asthma, insulin‐dependent diabetes mellitus, and pulmonary tuberculosis. Chest auscultation revealed wheezing and rhonchi. Laboratory findings revealed peripheral blood eosinophilia (655/μL) and a total IgE value of 163 IU/mL. Chest radiography revealed scar lesion. High-dose ICS, LABA, and systemic corticosteroid (prednisolone 40mg/day) therapy induced symptom improvement. Six months later, she required Symbicort 8 puffs/day, QVAR100® (beclometasone) 4 puffs/day, theophylline 400 mg/day, and montelukast 10 mg/day. The wheezing and asthma attacks continued. Omalizumab (300 mg every 4 weeks) was started; her symptoms improved after 24 weeks, but then worsened. After 15 months, the FVC and FEV1 were 0.86 L (%FVC 32.2%) and 0.42 L (%FEV1 19.4%), respectively. Mepolizumab treatment (100 mg every 4 weeks) was started.
Ten months later, the peripheral eosinophil count decreased from 1350/μl to 60/μL. Spirometry revealed improved lung function (%FVC 86.7%; FVC 2.29 L; %FEV1 44.6%; FEV1: 0.95 L). The FeNO increased from 41 ppb to 75 ppb. The ACT was 24. However, the asthma attaks continued. Benralizumab was started (30 mg subcutaneously every 4 weeks for the initial three doses and then every 8 weeks). The eosinophil count decreased from 60 to 0/μL after 1 month. Asthma flares were markedly decreased after 6 months. The ACT was 23. The FVC and FEV1 were further improved (Table 1).
3. Discussion
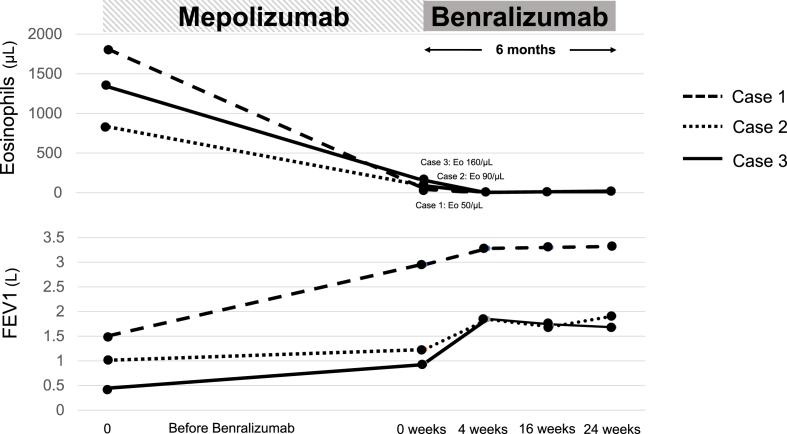
There are a number of unresolved issues related to the anti-IL-5 strategy in the treatment of eosinophilic asthma. Yancey et al. reported that mepolizumab is an effective treatment for patients with blood eosinophil counts ≥150 cells/μl [4]. We demonstrated that blood eosinophil reduction after mepolizumab therapy does not confirm full disease control (Fig. 2). Recently, Kurosawa et al. reported a case of severe eosinophilic asthma that responded to benralizumab after not responding to mepolizumab [5]. Unlike circulating eosinophils, low-dose mepolizumab did not attenuate sputum eosinophils [1]. Ojanguren et al. also reported that low blood eosinophil counts do not preclude from the presence of a significant airway eosinophilia after mepolizumab treatment [6]. However, high-dose of mepolizumab reduced airway eosinophils but had a limited effect on airway eosinophil activation markers, suggesting that these cells retain functionality [7]. Patients with severe eosinophilic asthma have exaggerated eosinophilopoiesis in the bronchial mucosa that is not affected by treatment with low-dose of mepolizumab [1]. The above observations may explain why our patients did not fully responsd to mepolizumab despite diminished circulating eosinophils.
Fig. 2.
The clinical courses of the three patients. The peripheral eosinophil level decreased rapidly in response to treatment with benralizumab. Lung function rapidly improved with benralizumab treatment following favorable results with mepolizumab. Abbreviations: Forced expiratory volume in 1 s (FEV1).
Eosinophils are known to participate in both immune homeostasis and immunity. Mensnil et al. reported that the normal mouse lungs contains resident eosinophils (rEos) and lungs lacking rEos exhibit increased Th2 cell responses to inhaled allergens [8]. Although rEos express the IL-5 receptors, this cell population is IL-5-independent and is not reduced in the presence of anti-IL-5. They also reported phenotypically distinct but related eosinophils in human lungs. If human rEos are a homeostasis-maintaining population, eosinophil depletion therapy may not be effective in severe asthmatics who respond to mepolizumab. However, eosinophil depletion by benralizumab resulted in further clinical improvement in our patients, suggesting that the remaining eosinophils contributed to clinical worsening of severe asthma after eosinophil reduction by mepolizumab.
We described three patients whose asthma symptom control was improved by benralizumab following favorable responses to mepolizumab. Our findings are limited to three cases, and prospective large-scale randomized studies comparing these drugs are needed to confirm the efficacy of eosinophil depletion therapy.
Consent for publication
Written consent to publish this report was obtained from the patient. A copy can be made available if required.
Ethics approval and consent to participate
The presented data are part of our clinical work and there is no ethical conflict.
Funding
No external funding was obtained.
Conflicts of interest
The authors declare that they have no competing interests.
Acknowledgements
We thank Kyoko Uekawa for assisting in the preparation of this manuscript.We would like to thank Editage (www.editage.jp) for English language editing.
References
- 1.Sehmi R., Smith S.G., Kjarsgaard M., Radford K., Boulet L.P., Lemiere C., Prazma C.M., Ortega H., Martin J.G., Nair P. Clin. Exp. Allergy. 2016;46:793–802. doi: 10.1111/cea.12695. [DOI] [PubMed] [Google Scholar]
- 2.Pavord I.D., Korn S., Howarth P., Bleecker E.R., Buhl R., Keene O.N., Ortega H., Chanez P. Lancet. 2012 Aug 18;380(9842):651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 3.Pham T.H., Damera G., Newbold P., Ranade K. Respir. Med. 2016;111:21–29. doi: 10.1016/j.rmed.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Yancey S.W., Bradford E.S., Keene O.N. Respir. Med. 2019;151:139–141. doi: 10.1016/j.rmed.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Kurosawa M., Sutoh E. Ann. Allergy Asthma Immunol. 2018;S1081–1206(18):31534–31535. [Google Scholar]
- 6.Ojanguren I., Chaboillez S., Lemiere C. J Allergy Clin Immunol Pract. 2018;6:2151–2153. doi: 10.1016/j.jaip.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Kelly E.A., Esnault S., Liu L.Y., Evans M.D., Johansson M.W., Mathur S. Am. J. Respir. Crit. Care Med. 2017;196:1385–1395. doi: 10.1164/rccm.201611-2234OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mesnil C., Raulier S., Paulissen G., Xiao X., Birrell M.A., Pirottin D., Janss T., Starkl P., Ramery E., Henket M., Schleich F.N., Radermecker M., Thielemans K., Gillet L., Thiry M., Belvisi M.G., Louis R., Desmet C., Marichal T., Bureau F. J. Clin. Investig. 2016;126:3279–3295. doi: 10.1172/JCI85664. [DOI] [PMC free article] [PubMed] [Google Scholar]