Abstract
External beam radiotherapy for prostate cancer is an optimal treatment choice for men with localised prostate cancer and is associated with long term disease control in most patients. Image-guided prostate radiotherapy is standard of care, however, current techniques can include invasive procedures with imaging of poor soft tissue resolution, thus limiting accuracy.
MRI is the imaging of choice for local prostate cancer staging and in radiotherapy planning has been shown to reduce target volume and reduce inter-observer prostate contouring variability. The ultimate aim would be to have a MR-only workflow for prostate radiotherapy.
Within this article, we discuss these opportunities and challenges, relevant due to the increasing availability of MR-guided radiotherapy. Prospective multi-centre studies are underway to determine the feasibility of MR-guided prostate radiotherapy and daily adaptive replanning. In parallel, development and adaptation of the existing radiotherapy multidisciplinary workforce is essential to enable an efficient and effective MR-guided radiotherapy workflow. This technology potentially provides us with the anatomical and biological information to further improve outcomes for our patients.
Abbreviations: ADT, androgen deprivation therapy; CBCT, cone beam CT; CTV, clinical target volume; GI, gastrointestinal; GU, genitourinary; IGRT, image-guided radiotherapy; mpMRI, multi-parametric MRI; MRI, magnetic resonance imaging; OAR, organ at risk; PTV, planning target volume; RTOG, radiation therapy oncology group
Keywords: Prostate cancer, Radiotherapy, MRI, Daily adaptive replanning
1. Introduction and background
In the European Union, prostate cancer is the most frequently diagnosed male cancer, with around 365,000 new cases estimated in 2015 [1]. Around 1.3 million men in Europe have received a diagnosis of prostate cancer in the last 5 years [1], emphasising the vast potential impact of improvements in prostate cancer treatment.
External beam radiotherapy is the commonest treatment for localised prostate cancer in the UK and has become safer and more effective over the last 20 years, with successive randomised studies showing augmentation of cure rates and decreases in toxicity rates over time [2], [3], [4]. For example, the RT01 trial [2], comparing 64–74 Gy to the target noted a cumulative rate of RTOG Grade 2+ gastrointestinal (GI) toxicity of 24% at 5 years for those receiving 74 Gy. In contrast, patients in the CHHiP trial [3] receiving the same 74 Gy dose had a cumulative RTOG Grade 2+ GI toxicity rate of 13.7% at 5 years. This almost halving of the toxicity rate is likely due to technical innovations including the implementation of IMRT, standardised target definitions and development and adherence to strict dose constraints for the rectum which are known to predict for toxicity [5]. Additionally, the 5 year biochemical progression free survival was 71% in patients treated within the RT01 study with 74 Gy and 88.3% in patients treated with 74 Gy in the CHHiP trial.
The advent of prostate-targeted image-guided radiotherapy (IGRT) is likely to further improve both oncological and toxicity outcomes. Because of the low levels of significant toxicity seen in recent studies, there is no conclusive Level one evidence of a reduction in side effects with IGRT. Despite this, IGRT is now considered standard of care for prostate radiotherapy, with gold fiducials or CBCT as the most common methods employed. Both have disadvantages – principally, with fiducials and planar kV imaging no volumetric information is obtained and with CBCT alone the poor soft tissue resolution limits the accuracy of the prostate match [6]. The combination of fiducials and CBCT overcomes most of these limitations but cannot account for deformations or differential motion of two targets (eg prostate and pelvic lymph nodes).
With the move towards more profound hypofractionation, accurate delivery of every fraction becomes critical. Therefore, improved methods of radiation delivery are increasingly important as we push the boundaries of extreme hypofractionation. This article will review the prospects of MR-guided radiotherapy improving radical treatment for men with prostate cancer.
2. The role of MR in prostate cancer
The prostate is seen more clearly on MRI, compared to other forms of imaging, and multiparametric MRI (mpMRI) can give additional information on intraprostatic disease, increasing specificity and sensitivity for diagnosis. The PROMIS trial tested mpMRI against trans-rectal ultrasound-guided biopsy as an initial diagnostic tool for the detection of clinically significant prostate cancer and showed that mpMRI was more sensitive. The EAU-ESTRO-SIOG guidelines on prostate cancer [7] recommend mpMRI for all patients as the primary modality for staging localised disease.
MR for radiotherapy planning has been shown to decrease target volume, although latterly some studies in MR-experienced centres have shown no difference [8]. MR also reduces inter-observer variability in target contouring [9]. The ESTRO ACROP consensus guidelines on CT- and MRI-based target volume delineation for primary radiotherapy of localised prostate cancer [10] aim to improve consistency and reliability of prostate contours in what remains the weak link in radiation therapy.
The ultimate aim is to move towards an MR-only workflow for prostate radiotherapy. The opportunities and challenges of this have been recently reviewed [11] and are outlined in Table 1.
Table 1.
Opportunities and challenges of MR-only workflow for prostate radiotherapy.
| Opportunities | Challenges |
|---|---|
| Removes inaccuracies of MR-CT fusion | Need for electron density information |
| Improved soft tissue contrast of the prostate on MR | Need pelvic wide field of view MR (to skin surface) |
| Improved efficiency | Geometric fidelity of wide-field MRI |
| Ability to contour dominant lesion boost on MR | Lack of MR capacity in radiotherapy departments |
| Remove requirement for fiducials if delivery with MRgRT | Need for back up plan (and fiducials) if only one MR-Linac in department |
3. Potential advantages of MRgRT
The MR Linac systems are of value in three main ways, each of which will be discussed in turn below. The currently available systems can be used either to improve the accuracy of delivery (3.1 and 3.2) or to combine this with daily adaptive replanning (3.1, 3.2 and 3.3 below).
3.1. Augmented soft tissue contrast allowing more accurate delivery to the prostate
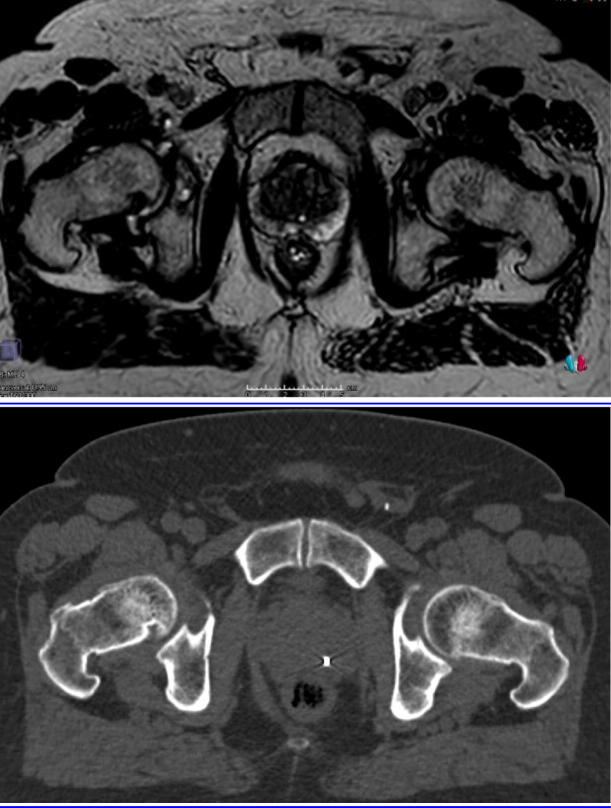
The prostate moves relative to bone and therefore IGRT, centred on the prostate, is mandatory to improve accuracy and reduce margins. The improvement in prostate “capsule” visualisation is shown in Fig. 1. MR improves prostate visibility at the apex and bladder interface, both of which are difficult to see on cone beam CT and impossible to see with planar kV fiducial matching. This increased accuracy may allow a reduction in CTV-PTV margins, hence decrease risk of toxicity, but the improvements to the margin size are likely to be small (of the order of 2–3 mm).
Fig. 1.
Improved soft tissue contrast with MR (top panel) compared to CT (bottom panel). The MR image shown was taken prior to treatment on the MR Linac.
3.2. Intrafraction cine MRI to monitor prostate position during dose delivery
The prostate is known to move during a timeframe relevant to delivery of a single fraction of radiotherapy. Motion can be erratic and unpredictable, but as a population average, is seen as a slow drift in the postero-inferior direction [12]. For most patients, over a 3–4 min treatment time, motion larger than the PTV margins is unlikely. Based on Calypso data, margins of 3 mm would achieve a 93.1% geometric coverage, over a median treatment time of 7 min [13]. For margins less than 3 mm, coverage drops off sharply, to 35.6% for a 1 mm margin. Langen et al found that the prostate was displaced >3 mm 13.2% of the time during treatment [12].
A recent study has used cine MRI to measure prostate motion based on centre of mass of fiducials and notes that motion >2 mm is seen in 43% scans by 5 min [14].
The use of cine MRI during beam delivery affords the option to intervene in the event of extreme anatomical changes. The ViewRay system has the capability of real-time soft tissue tracking and gating. The team from Amsterdam University medical centre (VUmc) have recently reported on the use of the MRIdian system for localised prostate cancer. They performed motion monitoring using a single sagittal plane at 4 frames-per-second and used a gating boundary of 3 mm on the prostate during the beam on time (which averaged 10 min). They found that 2D shifts (cranio-caudal and/or antero-posterior direction) were needed in >20% of all delivered fractions (149/700 fractions) [15].
3.3. Allowing daily adaptive replanning radiotherapy
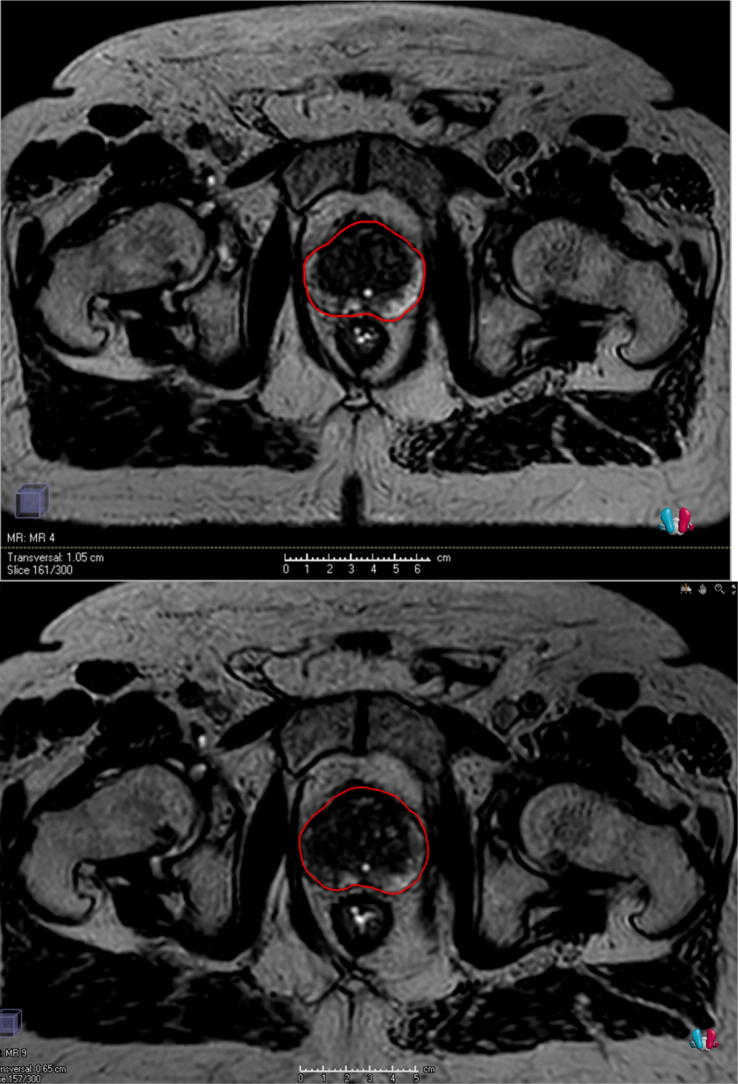
It has been interesting to see the inter- and intra-patient variation in anatomy visible during MRgRT for prostate cancer. Some patients (see Fig. 2) have had a very stable prostate, bowel and rectal anatomy and in these patients it is likely that daily adaptive replanning does not gain much dosimetrically over delivering the reference plan with a shift. Visibility of the prostate, and certainly the dominant lesion, may also change over the course of treatment.
Fig. 2.
Daily MR images (axial slice) used for replanning on two separate days (top and bottom panels) showing little change of the prostate and rectum. The prostate is contoured in red. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
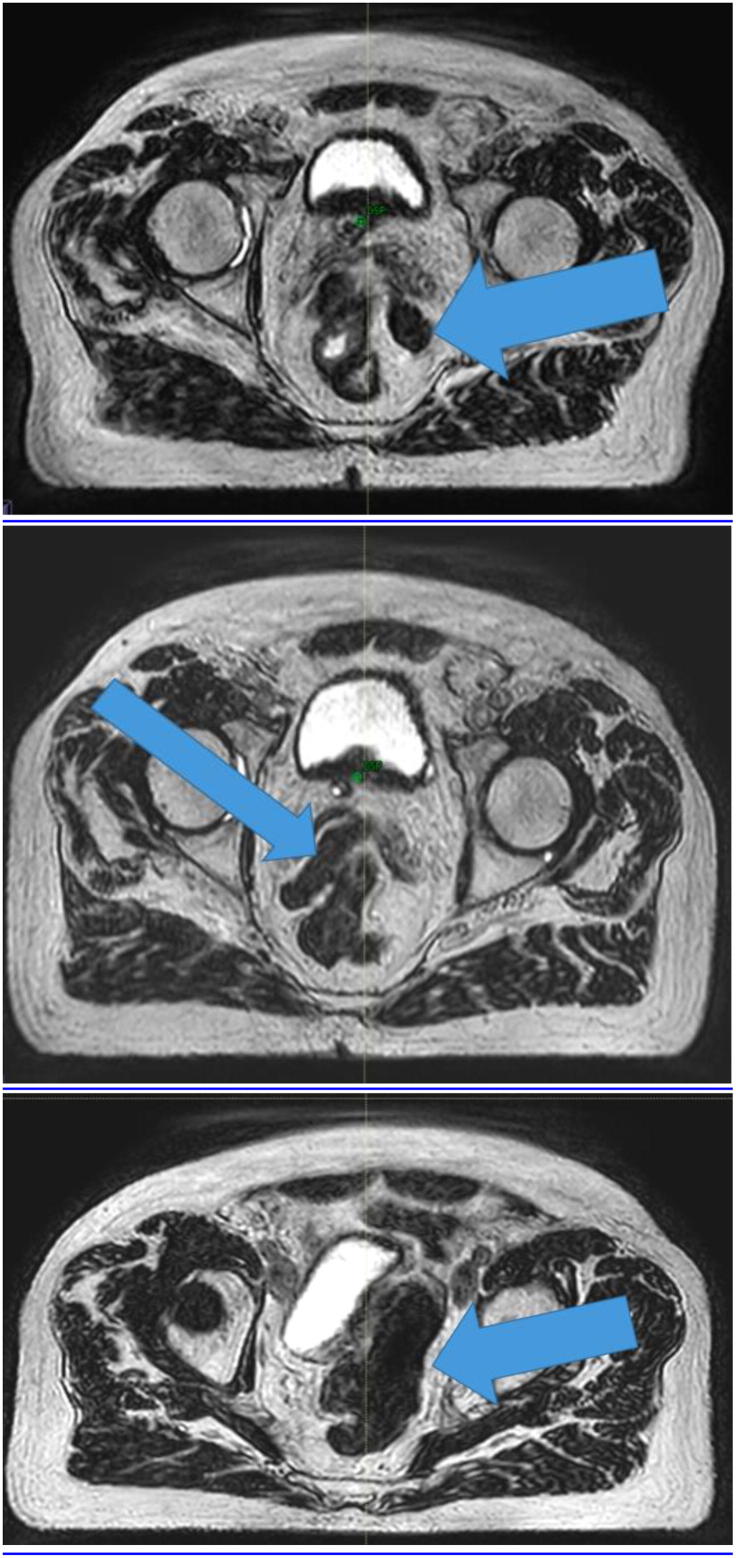
For other patients, there have been extreme anatomical changes (see Fig. 3) which have necessitated daily recontouring of the target, bowel and rectum to ensure the optimal OAR sparing and maximisation of target dose.
Fig. 3.
Daily axial MR images for online replanning showing bowel to the left of the rectum on one day (top panel, mid femoral head axial level), bowel inserting between the seminal vesicles on another day (middle panel, mid femoral head level) and bowel displacing the bladder to the right (bottom panel, above femoral head level).
4. Current experience with MRgRT in prostate cancer
Global experience of MRgRT is in its infancy but this will change rapidly with forthcoming expansion in MR Linac system numbers. With prostate cancer accounting for around 30% of most departmental workloads, it will be key to establish feasibility and potential benefits of MR-guided prostate radiotherapy.
There are two MRgRT systems currently available, both of which have been discussed in this special edition [insert references to the [16] and [17] article in this edition of CtRO]. The Washington university team have published their experience over the first 2.5 years of operation of their ViewRay systems (latterly, the MRIdian, ViewRay Inc., Oakwood Village, OH) and note that 21% of the 316 patients treated had pelvic malignancies. Specific experience with prostate cancer was not mentioned, except to highlight the ability of MRgRT to dispense with fiducials [18].
As mentioned in Section 3.2, the team from Amsterdam have recently reported on their use of the MRIdian system for localised prostate cancer. Of the patients treated with MR guided radiotherapy between May 2016 and June 2018, 130 patients were treated with the tri-60Co system and ten with MR-Linac. Their clinical workflow included daily plan re-optimisation prior to treatment delivery with partial OAR recontouring within the first 2 cm outside the PTV. The average duration of an uneventful fraction of MRgRT was 45 min. Patient experiences with MRgRT were assessed using a patient reported outcome questionnaire after the last fraction (N = 89) and showed that MRgRT was generally well tolerated, with disturbing noise sensations being most commonly reported [15].
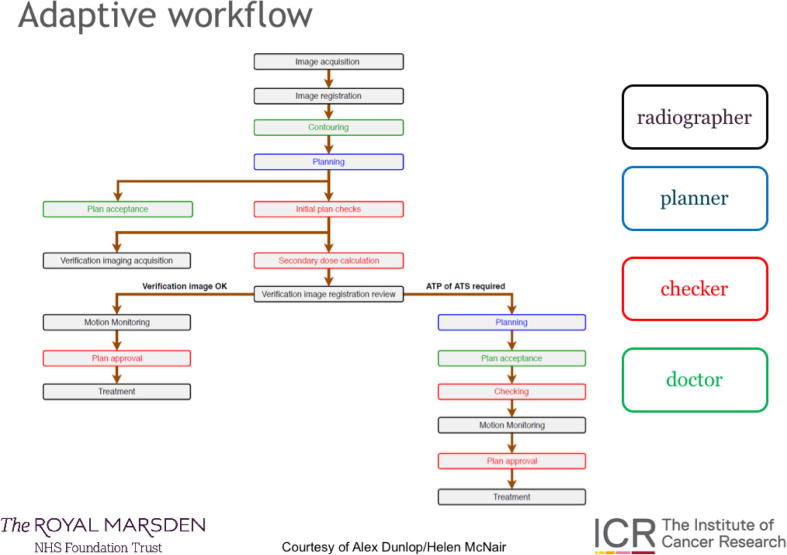
A workflow for MRgRT with adaptive replanning on the Elekta Unity is presented in Fig. 4. This is not the only workflow possible with this system, but is described as an example.
Fig. 4.
Workflow outline for the Unity at the Royal Marsden, UK (image courtesy of Helen McNair and Alex Dunlop).
In brief, a session MR image is acquired which is then fused with the image on which the reference plan was acquired. The clinician recontours the prostate (if applying an ‘adapt-to-shape’ workflow) prior to a reoptimisation of the plan (on the MR scan of the day) which can be warm start (optimising segment shape only) or cold start (full re-optimisation).
Once an acceptable new plan has been created, a second MRI is taken to ensure no prostate motion during planning – if motion is seen a simple shift (‘Adapt-To-Position apostrophe’) can be effected prior to beam on. During planning a secondary independent dose check occurs for quality assurance. Cine-MRI monitoring of prostate and OAR position occurs during treatment. The manpower-intense and multidisciplinary nature of MRgRT is clear to see.
Across the Elekta MR Linac consortium several sites have commenced an MRgRT programme for prostate on the Unity (Elekta AB, Stockholm, Sweden). For most sites this is being done in line with a synchronous clinical trial protocol called PRISM, Prostate Radiotherapy Integrated with Simultaneous MRI (UK trial NCT https://clinicaltrials.gov/ct2/show/NCT03658525), with the intention of data sharing and joint publication in due course. PRISM is delivering 60 Gy in 20 fractions over 4 weeks with MRgRT, using margins of 5 mm except 3 mm posteriorly. Daily adaptive replanning is permitted but not mandated. Patients receive frequent RTOG, CTCAE and QOL measurements whilst on radiotherapy and during follow up.
5. Implications of implementing MRgRT
There are challenges associated with fundamentally changing the workflow and the treatment we deliver. With constant MR imaging and the ability to change the plan daily, there is a risk of over-intervention. For example, if the small bowel sits close to the target on the daily image we may reduce coverage to the PTV in order to keep within our usual reference plan constraints. However, by the time the treatment is delivered the bladder will have filled and the bowel may have lifted, resulting in unnecessary under-dose to the target. We must remember that we have been safely delivering a single plan across a whole treatment course since the inception of fractionated radiotherapy and toxicity rates from prostate external beam radiotherapy are already low.
Replanning daily has significant implications for the workforce. Our current workflow requires two physicists, two radiographers and a clinician to be present for each fraction. Current research is focussed on streamlining this process and stratifying patients into those who do versus those who do not require daily adaptive replanning. We are also investigating the dosimetric impact (or otherwise) of radiographer-led contouring. Our early work in an offline environment indicates a high concordance between radiographer and clinician contours [19].
6. Where could MRgRT take us in prostate cancer?
MRgRT is currently more resource intensive to deliver compared with standard radiotherapy. In an arena where there are multiple effective ways to irradiate a prostate (LDR brachytherapy, HDR brachytherapy, SBRT, Cyberknife®), MRgRT will have to prove its worth. Hence research is needed to both prove the added value of this technology in prostate cancer and to streamline processes to reduce treatment times and workforce requirements.
At the most practical level, the ability to dispense with CT, and have a MR-only workflow to produce a complete plan, from contouring to checking, in minutes, paves the way for a paradigm shift in our departmental structures. Patients could be scanned, contoured and planned, all while waiting on the bed. With session times of around 45 min at present, and scope to reduce this, a streamlined workflow could eliminate patient waits for planning and protracted radiotherapy planning pathways.
There are exciting opportunities which are only possible using MR-guidance. Many centres are exploring delivering a simultaneous boost to the dominant tumour lesion within the prostate, visualised on a staging MRI [20], [21], [22]. It would be very attractive to deliver this boost with direct visualisation of the tumour bulk, rather than relying on surrogates. One key hurdle to overcome is the effect of Androgen Deprivation Therapy (ADT) on dominant tumour lesions; ADT results in changes in diffusion and structure which lead to the tumour nodule becoming less distinct [23], [24]. As ADT remains a key part of the treatment schedule for most men with prostate cancer, further research is needed to maximse tumour visibility even when on ADT.
Finally, the direction of travel for prostate irradiation is unmistakably towards hypofractionation. Several trials have shown that moderate hypofractionation (around 3 Gy per fraction) is equivalent to standard fractionation [3], [4], [25], [26]. The HYPO trial has been reported, but not published, to show an identical biochemical relapse-free survival for 78 Gy in 39 fractions and 42.7 Gy in 7 fractions. The PACE B trial (NCT 01584258) has completed accrual of 874 men, predominantly with intermediate risk prostate cancer, randomising to conventional or moderate hypofractionation vs 5 fraction SBRT to a dose of 36.25 Gy and has reported recently similar rates of acute GI and GU toxicity between the two groups, with efficacy data expected within the next couple of years.
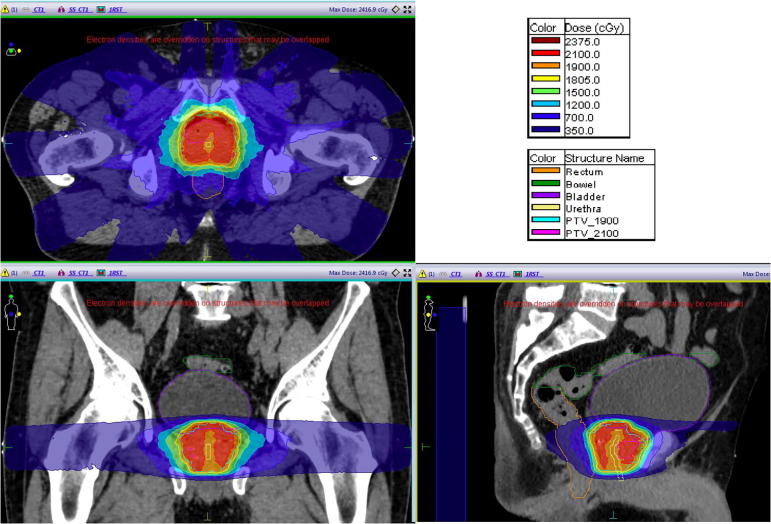
The ultimate question asks whether we can reduce fraction number below five, even to a single treatment? This has been done by several groups with HDR brachytherapy, mostly treating to 19 or 20 Gy [27], [28], [29] although recent reports have shown disappointing biochemical control rates [30]. Early clinical testing is in process to establish whether similar doses can be given with external beam radiotherapy (see illustrative plan in Fig. 5). However, the more accurate IGRT, intra-beam monitoring and the ability to rapidly produce a plan corresponding to the anatomy of the moment, would make MRgRT the optimal way to test this.
Fig. 5.
Images of a test plan for the MR Linac delivering 19 Gy to the whole prostate with 21 Gy to the dominant tumour lesion, whilst respecting HDR rectal and bladder constraints (image courtesy of Jonathan Mohajer).
7. Conclusions
MRgRT represents an exciting new horizon for prostate radiotherapy delivery. Clinical experience is gathering but treatments seem feasible and tolerable to patients. This innovative technology will allow us to test the limits of profound hypofractionation and biological targeting and we hope this will further improve outcomes for our patients.
Conflicts of interest
JM – Travel/honoraria – Astellas, Janssen, Ferring.
AT – research funding Elekta, MSD, Accuray, Rosetrees’s Trust, Travel/honoraria – Elekta, Genesis Healthcare, Ferring, Bayer, Astellas, Janssen.
Acknowledgments
Acknowledgements
This paper represents independent research part funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at the Royal Marsden NHS Foundation Trust and the Institute of Cancer Research, London, UK. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health, UK.
AT would like to acknowledge the support of the Rosetrees Trust.
References
- 1.EU. Epidemiology of prostate cancer in Europe [Internet]. EU science hub. Available from: https://ec.europa.eu/jrc/en/publication/epidemiology-prostate-cancer-europe.
- 2.Dearnaley D.P., Sydes M.R., Graham J.D., Aird E.G., Bottomley D., Cowan R.A. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol. [Internet] 2007;8(6):475–487. doi: 10.1016/S1470-2045(07)70143-2. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17482880. [DOI] [PubMed] [Google Scholar]
- 3.Dearnaley D., Syndikus I., Mossop H., Khoo V., Birtle A., Bloomfield D. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(8):1047–1060. doi: 10.1016/S1470-2045(16)30102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catton C.N., Lukka H., Gu C.S., Martin J.M., Supiot S., Chung P.W.M. Trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. Int. J. Clin. Oncol. 2017;35(17):1884–1890. doi: 10.1200/JCO.2016.71.7397. [DOI] [PubMed] [Google Scholar]
- 5.Gulliford S.L., Foo K., Morgan R.C., Aird E.G., Bidmead A.M., Critchley H. Dose-volume constraints to reduce rectal side effects from prostate radiotherapy: evidence from MRC RT01 Trial ISRCTN 47772397. Int J Radiat Oncol Biol Phys [Internet] 2010;76(3):747–754. doi: 10.1016/j.ijrobp.2009.02.025. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19540054. [DOI] [PubMed] [Google Scholar]
- 6.Deegan T., Owen R., Holt T., Fielding A., Biggs J., Parfitt M. Assessment of cone beam CT registration for prostate radiation therapy: fiducial marker and soft tissue methods. J. Med. Imaging Radiat. Oncol. 2015;59(1):91–98. doi: 10.1111/1754-9485.12197. [DOI] [PubMed] [Google Scholar]
- 7.Mottet N., Bellmunt J., Bolla M., Briers E., Cumberbatch M.G., De Santis M. Guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur. Urol. 2017;71(4):618–629. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Henderson D., Tree A., Harrington K., van As N. Dosimetric implications of computerised tomography-only versus magnetic resonance-fusion contouring in stereotactic body radiotherapy for prostate cancer. Medicines. 2018;5(2):32. doi: 10.3390/medicines5020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pathmanathan A., Schmidt M., Brand Kousi D.E., van As N.J., Tree A.C. Improving fiducial and prostate capsule visualization for radiotherapy planning using MRI. Radiat. Oncol. Phys. 2019 doi: 10.1002/acm2.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salembier C., Villeirs G., De Bari B., Hoskin P., Pieters B.R., Van Vulpen M. ESTRO ACROP consensus guideline on CT- and MRI-based target volume delineation for primary radiation therapy of localized prostate cancer. Radiother. Oncol. 2018;127(1):49–61. doi: 10.1016/j.radonc.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Kerkmeijer L.G.W., Maspero M., Meijer G.J., van der Voort van Zyp J.R.N., de Boer H.C.J., van den Berg C.A.T. Magnetic resonance imaging only workflow for radiotherapy simulation and planning in prostate cancer. Oncology. 2018;30(11):692–701. doi: 10.1016/j.clon.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Langen K.M., Willoughby T.R., Meeks S.L., Santhanam A., Cunningham A., Levine L. Observations on real-time prostate gland motion using electromagnetic tracking. Int. J. Radiat. Oncol. Biol. Phys. [Internet] 2008;71(4):1084–1090. doi: 10.1016/j.ijrobp.2007.11.054. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18280057. [DOI] [PubMed] [Google Scholar]
- 13.Curtis W., Khan M., Magnelli A., Stephans K., Tendulkar R., Xia P. Relationship of imaging frequency and planning margin to account for intrafraction prostate motion: analysis based on real-time monitoring data. Int. J. Radiat. Oncol. Biol. Phys. [Internet] 2013;85(3):700–706. doi: 10.1016/j.ijrobp.2012.05.044. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22795802. [DOI] [PubMed] [Google Scholar]
- 14.de D.M., Muinck Keizer, Pathmanathan A.U., Andreychenko A., Kerkmeijer L.G.W., van J.R.N., der, Voort, van, Zyp, Tree A.C. Fiducial marker based intra-fraction motion assessment on cine-MR for MR-Linac treatment of prostate cancer. Phys. Med. Biol. 2019 doi: 10.1088/1361-6560/ab09a6. [DOI] [PubMed] [Google Scholar]
- 15.Tetar Shyama, Bruynzeel Anna L.F. Clinical implementation of magnetic resonance imaging guided adaptive radiotherapy for localized prostate cancer. Phys. Imaging Radiat. Oncol. 2019;9:69–76. doi: 10.1016/j.phro.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winkel, D., Bol, G.H., Kroon, P.S., van Asselen, B., Hackett, S.S., Werensteijn-Honingh, A.M., et al. Adaptive radiotherapy: The Elkta Unity MR-linac concept. Clinical and Translational Radiation Oncology. 10.1016/j.ctrol.2019.04.001. [DOI] [PMC free article] [PubMed]
- 17.Klüter, S. Technical design and concept of a 0.35 T MR-Linac. Clinical and Translational Radiation Oncology. 10.1016/j.ctro.2019.03.005. [DOI] [PMC free article] [PubMed]
- 18.Fischer-valuck B.W., Henke L., Bradley J.D., Robinson C.G., Thomas M., Zoberi I. Two-and-a-half-year clinical experience with the world’s first magnetic resonance image guided radiation therapy system. Adv. Radiat. Oncol. [Internet] 2017;2(3):485–493. doi: 10.1016/j.adro.2017.05.006. Available from: http://dx.doi.org/10.1016/j.adro.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pathmanathan A.U., Mcnair H.A., Schmidt M.A., Brand D.H., Delacroix L., Eccles C.L. Comparison of prostate delineation on multimodality imaging for MR-guided radiotherapy. Br. J. Radiol. 2019;92(1095) doi: 10.1259/bjr.20180948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lips I.M., van der Heide U.A., Haustermans K., van Lin E.N., Pos F., Franken S.P. Single blind randomized phase III trial to investigate the benefit of a focal lesion ablative microboost in prostate cancer (FLAME-trial): study protocol for a randomized controlled trial. Trials [Internet] 2011;12:255. doi: 10.1186/1745-6215-12-255. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22141598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monninkhof E.M., van Loon J.W.L., van Vulpen M., Kerkmeijer L.G.W., Pos F.J., Haustermans K. Standard whole prostate gland radiotherapy with and without lesion boost in prostate cancer: Toxicity in the FLAME randomized controlled trial. Radiother. Oncol. [Internet] 2018;127(1):74–80. doi: 10.1016/j.radonc.2017.12.022. Available from: https://doi.org/10.1016/j.radonc.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 22.Onjukka E., Uzan J., Baker C., Howard L., Nahum A., Syndikus I. Twenty fraction prostate radiotherapy with intra-prostatic boost: results of a pilot study. Clin. Oncol. [Internet] 2017;29(1):6–14. doi: 10.1016/j.clon.2016.09.009. Available from: http://dx.doi.org/10.1016/j.clon.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Hotker A., Mazaheri Y., Zheng J., Chaya S., Berkowitz J., Lantos J.E. Prostate cancer: assessing the effects of androgen-deprivation therapy using quantitative multi-parametric MRI. Eur. Radiol. 2016;25(9):2665–2672. doi: 10.1007/s00330-015-3688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groenendaal G., van Vulpen M., Pereboom S.R., Poelma-Tap D., Korporaal J.G., Monninkhof E. The effect of hormonal treatment on conspicuity of prostate cancer: implications for focal boosting radiotherapy. Radiother. Oncol. [Internet] 2012;103(2):233–238. doi: 10.1016/j.radonc.2011.12.007. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22265733. [DOI] [PubMed] [Google Scholar]
- 25.Lee W.R., Dignam J.J., Amin M.B., Bruner D.W., Low D., Swanson G.P. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J. Clin. Oncol. [Internet] 2016;34(20):2325–2332. doi: 10.1200/JCO.2016.67.0448. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27044935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Incrocci L., Wortel R.C., Alemayehu W.G., Aluwini S., Schimmel E., Krol S. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17(8):1061–1069. doi: 10.1016/S1470-2045(16)30070-5. [DOI] [PubMed] [Google Scholar]
- 27.Hoskin P., Rojas A., Ostler P., Hughes R., Alonzi R., Lowe G. Single-dose high-dose-rate brachytherapy compared to two and three fractions for locally advanced prostate cancer. Radiother. Oncol. [Internet] 2017;124(1):56–60. doi: 10.1016/j.radonc.2017.06.014. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28666552. [DOI] [PubMed] [Google Scholar]
- 28.Krauss D.J., Ye H., Martinez A.A., Mitchell B., Sebastian E., Limbacher A. Favorable preliminary outcomes for men with low- and intermediate-risk prostate cancer treated with 19-Gy single-fraction high-dose-rate brachytherapy. Int. J. Radiat. Oncol. [Internet] 2017;97(1):98–106. doi: 10.1016/j.ijrobp.2016.08.011. Available from http://linkinghub.elsevier.com/retrieve/pii/S0360301616330966. [DOI] [PubMed] [Google Scholar]
- 29.Prada P.J., Anchuelo J., Cardenal J., Garc A. High-dose-rate interstitial brachytherapy as monotherapy in one fraction of 20.5 Gy for the treatment of localized prostate cancer: toxicity and 6-year biochemical results. Int. J. Radiat. Oncol. Biol. Phys. 2018;17:845–851. doi: 10.1016/j.brachy.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Siddiqui Z.A., Gustafson G.S., Ye H., Martinez A.A., Mitchell B., Sebastian E. Five-year outcomes of a single-institution prospective trial of 19-Gy single-fraction high-dose-rate brachytherapy for low- and intermediate-risk prostate cancer. Radiat. Oncol. Biol. [Internet] 2019;248:1–7. doi: 10.1016/j.ijrobp.2019.02.010. Available from: https://doi.org/10.1016/j.ijrobp.2019.02.010. [DOI] [PubMed] [Google Scholar]