Highlights
-
•
MR-only treatment planning could improve the spatial accuracy of radiotherapy.
-
•
The benefit compared to a mixed MR-CT workflow will vary between patient groups.
-
•
Further development of QA tools is needed before the procedure will save resources.
1. Introduction
In radiotherapy, images used for treatment planning serve three main purposes; representation of the anatomy for target and organ at risk segmentation, information for dose calculation and optimization, and as the positioning reference at treatment. CT and MR are two imaging modalities that both depict the patient in three dimensions with high spatial resolution, adequate for precision radiotherapy planning. The tissue properties that determine the appearance of the images are however fundamentally different between the two. CT provides a map of the attenuation properties of tissue represented by Hounsfield units (HU). The contrast in MR is determined by a multitude of factors such as proton density, relaxivity of the tissue, flow, diffusion and chemical composition. The weighting between these factors is determined by the acquisition sequence.
One strength of MR compared to CT is that MR can be optimized to enhance different aspects of the tissue. Anatomical MR sequences have superior soft tissue contrast compared to CT and MR is the diagnostic imaging modality of choice to visualize most of the tumors treated with definitive radiotherapy [1], [2], [3], [4]. Potential for significant benefits has been described for many indications, for example in prostate [5], head and neck [6], bladder [7]. MR also allows full flexibility of orientation of the imaging planes. Sagittal, coronal or anatomically determined imaging planes are often used in combination with standard transversal planes to achieve the best possible visualization of anatomical features. Certain MR sequences can give information about physiological or chemical properties of the tumor, many of which are explored as noninvasive biomarkers for adaptive treatment schedules and dose painting [8], [9], [10], [11]. CT is superior regarding dose calculation accuracy as it provides a direct measurement of the attenuation in tissue.
Integration of MR in the radiotherapy workflow could be either as a secondary information source co-registered to a planning CT and used for delineation of selected structures, or as a primary image set used both for delineation and optimization of the treatment. The latter alternative is often referred to as MR-only radiotherapy planning as it does not include a CT image set. To date only a few centers have treated patients with MR-only based treatment planning and only three described the implementation in a publication [12], [13], [14].
1.1. Rational for MR-only radiotherapy
There are three main advantages put forward in the literature in favor of MR-only radiotherapy planning compared to a workflow where CT is the primary imaging modality and MR a secondary (CT-MR workflow). The advantage for MR-only most often mentioned is that the registration between CT and MR will introduce geometrical uncertainties [3], [13], [15], [16], [17], [18], [19]. These errors introduced in the planning stage will affect each fraction and are systematic. In an MR-only workflow, image registration with the introduction of geometrical uncertainties is avoided. Secondly, it is hypothesized that MR-only is more efficient and cost-effective as no CT acquisition is necessary [3]. This would be of special importance in the scenario of adaptive radiotherapy based on MR data. Finally, the unspecific exposure of ionizing radiation will be reduced with the exclusion of CT. Although this statement is correct, the dose reduction is marginal as compared to the treatment dose the patients will receive, and we will therefore not examine this argument in depth in this review. These advantages apply to traditional linac based treatments. The introduction of combined MR and treatment units, MR-linacs, could bring further advantages in areas such as adaptive treatment, where re-planning could be executed daily on the acquired MR image set.
1.2. Challenges
MR is inherently not a quantitative imaging modality. This means that the pixel values in a standard MR image does not have a unit and the magnitude has no particular meaning. There are some underlying tissue properties that can be quantified with MR, such as the T1 and T2 relaxation times, but it is not possible to acquire an image that directly can be used for dose calculations. Therefore, effort has been put into creating methods to translate MR image data into CT like images, so called synthetic CTs (S-CT). The S-CT is used for dose calculations and in some scenarios to generate positioning references such as digitally reconstructed radiographs (DRR’s). Two reviews focused on the S-CT generation techniques have recently been published [20], [21]. The reported dosimetric difference between planning on CT and the S-CT were typically less than 1% in publications using atlas- or voxel-based techniques [21].
Another challenge in an MR-only workflow is the geometrical distortions that MR images are subject to as a result of the way the spatial position of signal is encoded. There are ways to reduce and mitigate distortions, and modern MR scanners have efficient corrections that can be applied. Characterization of distortions and methods to monitor the geomatical performance of the MR scanners are pivotal in the introduction of MR-only radiation treatment planning. The subject has attracted a lot of scientific attention over the last decade, but there is still work to be done [22], [23], [24], [25]. In particular, the area of appropriate effective QA measures to guarantee the performance for each and every patient is not sufficiently studied.
Finally, reliable quality control systems must be in place prior to implementation of MR-only treatment planning. The methods used for converting MR into S-CT are often abstract and there is a risk that atypical anatomy or artifacts in the MR data is misinterpreted resulting in faulty S-CTs. E.g. the use of S-CT for positioning of prostate cancer patients with fiducial markers is a scenario which highlighted in literature. The markers are not directly visualized with MR, but appear as a signal void, which may be confused with for example calcifications [13], [26], [27], [28], [29]. The challenges connected to MR-only treatment planning have been described in a recent review [18].
The use and future potential of MRI in radiation oncology has been reviewed in several recent publications [2], [3], [30], [31], [32]. MR-only radiation treatment planning is a tool which could facilitate improved utilization of the potential with MRI in radiotherapy. The introduction of MR-only treatment planning must be based on a careful evaluation of investment versus gain. Therefore, the aim of this work was to critically examine the arguments for MR-only treatment planning. Which are the current indications that MR-only treatment planning will improve the accuracy of the treatment? Is it correct that MR-only treatment planning will save resources with the tools that are available today?
2. Methods
The present text is not a systematic review, rather an overview of the research area. A literature search was performed with focus on the clinical opportunities. The Pubmed search terms used for the identification of articles describing clinical implementation of MR-only treatment planning were variants of “MR-only treatment planning” and “MR-only workflow” in title or abstract combined with a citation search. The findings were divided into two groups. 1) Target definition and margins, 2) benefits from a department logistics perspective. The analysis presented are based on both the findings from literature and our own long experience in a combined MR-CT workflow as well as the introduction of MR-only radiotherapy planning.
3. Results and discussion
We could only identify 3 publications describing clinical implementation of MR-only radiotherapy (all for prostate cancer). With two commercial solutions recently put on the market the clinical utilization could be expected to grow over the coming years.
3.1. Target definition and margins
The main rationale for including MR imaging into the radiotherapy workflow is the increased soft tissue contrast, leading to better tumor volume definitions. This has also been studied quite extensively and demonstrated for a variety of tumor sites. Several studies have been published on prostate delineations [33], [34], [35], all showing smaller volume definitions on MR as compared to CT, ranging from a 19–32% reduction in delineated volume. Other groups have focused on the physician variability in delineations [36], [37] and demonstrated significant reductions in both inter- and intra-observer variability when using MR instead of CT. In a recent consensus paper Ménard et al. argued that the main gain with MRI in the handling of prostate cancer patients is not the delineation of the prostate itself, but rather the potential to identify intra-prostatic lesions for boost [38]. Brain tumors have also been studied, showing that MR does not reduce the interobserver variability and increased the size of the delineated volumes, indicating that MR is indispensable for radiotherapy treatment planning in the brain by resolving conspicuous anatomy which was not seen using CT imaging alone [39], [40], [41]. In the head and neck area, the results of the studies have varied due to the type of tumor which has been studied, but in general the conclusions have stated the MR is more accurate for tumor delineation [42], [43], [44].
The impact of these findings could affect the actual volume that is irradiated. The gross tumor volume (GTV) is simply the visible tumor on the modality used as a basis for the decision [45], [46]. The GTV will be dependent on several factors which are of interest in this context, e.g. the visibility of the tumor (which is modality and image quality dependent) and delineation noise (i.e. inter- and intra-observer variability). On the GTV, a margin based on general oncologic principles will be added, creating the clinical target volume (CTV). This margin should account only for the local invasive potential and capacity to spread of the tumor and will vary with tumor type and anatomic location. Finally, another margin is added creating the planning target volume (PTV), which should account for all geometric uncertainties associated with the treatment, e.g. tumor movement and shape variations, set-up uncertainties, mechanical uncertainties and so on. Looking at the definitions of these margins, it becomes evident that adding an MR to the CT based workflow will likely improve the GTV definition but will add geometrical uncertainty due to image registration which must be accounted for in the PTV concept.
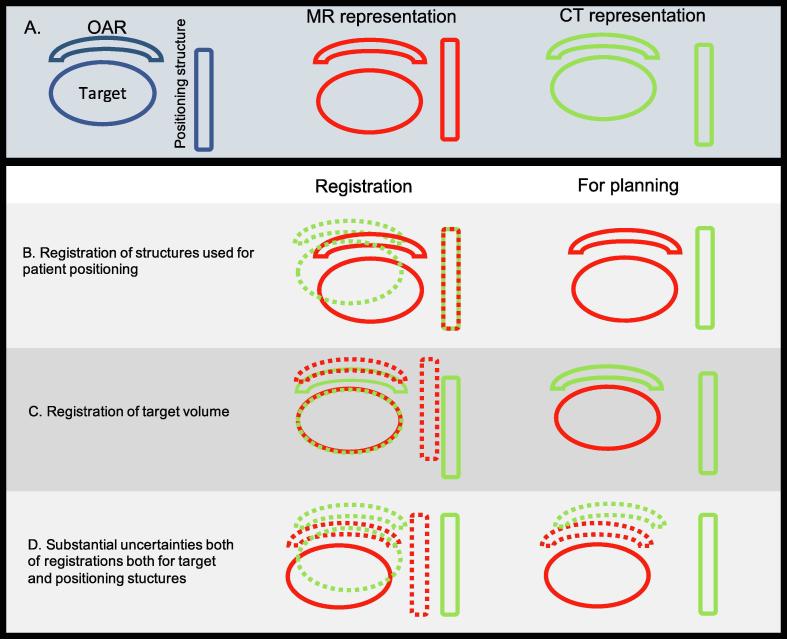
What is then the magnitude of uncertainty in image registration? This is not an easy thing to answer, keeping in mind that a mere difference in position of a certain landmark within a patient needs to not necessarily stem from registration uncertainty. Such a difference could also stem from anatomic movement between two imaging sessions, which is already accounted for in the PTV margin. Ulin et al. [47] investigated the registration accuracy in images of the brain (the skull and brain can be viewed as a rigid body which is preferable in this case) by sending MR and CT datasets to 45 different clinics and having them register the image sets in whatever fashion they preferred. The average inherent uncertainty of the registrations where 2.2 mm (1 S.D.), which should be accounted for in the PTV margin. Another example was published by Roberson et al. [48], where they compared a subvolume registration of the prostate (i.e. extracting a small volume around the prostate and considering it a rigid body) to manual landmark registration using implanted brachytherapy seeds. They estimated the error of the registration to be around 2 mm. The implication of registration uncertainties in the MR-CT workflow will depend how the MR and CT image information is used in the workflow. Fig. 1 provides a schematic representation of three different scenarios given in row B–D. With exception of the intra-cranial anatomy, there is a substantial risk for differences in the anatomical configuration of the patient in the MR and CT examination. This makes registrations challenging. In the scenario where MR and CT could be registered with high accuracy and precision based on the structures used for positioning the patient, then both target and OAR should be delineated based on the MR information as illustrated in Fig. 1. A MR-CT workflow for prostate cancer is an example of this scenario. For prostate cancer patients there are examples where a registration based on fiducial markers will result in large discrepancies in the positions of the organs at risk in the MR and CT examination. This could be distracting, but as long as all delineations of target and OAR are done based on the MR data the plan will be consistent. A more hypothetical scenario is a high accuracy registration between the target volumes as represented by MR and CT (see Fig. 1, row C). If this is possible, which also implies that the target volume should be rigid, then the target could be delineated based on MR information and organ at risk based on the CT information. The plan will be based on the observed patient anatomy from the CT. This scenario may be applicable and useful for larynx cancer patients where very focused MR imaging may be used and larynx registration between MR and CT may give sufficient accuracy [49].
Fig. 1.
Schematic representation of three different registration scenarios in a MR-CT radiotherapy workflow. The anatomy of the patient as visualized with MR is given in red and visualized with CT in green. In the registration column registrations between MR and CT are visualized. Structure positions that should not be used when planning the treatment are dotted. Row B shows the situation where the registration has been focused on the anatomical structures used for positioning of the patient at treatment. In this situation all structures should be delineated on MR, and the plan will be based on the observation of the anatomy as given in the MR examination. Row C shows a situation where a very accurate registration of the target volume has been achieved, while the registration of the structures used for positioning of the patient are of low quality. In this scenario all structures except the target should be delineated on CT, and the plan will be based on the observation of the anatomy as given in the CT examination. The final row D, illustrates the scenario where the registration has resulted in errors for both the target volume and the structure used for patient positioning. In this case, it will be impossible to get an unambiguous representation of the patient anatomy unless the positioning of the patient is done using the MR information. The position of the target relative the positioning structure will be a mixture between the MR and CT observation and delineations of the OAR can be consistent with the target or the positioning structure, but not with both. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
With variations in anatomy between MR and CT and using an unfocused registration approach it is likely that the result will be as illustrated in Fig. 1 row D. The registrations can be poor for both the target and positioning structures. In this case it is not possible to achieve an unambiguous representation of the patient for planning using both MR and CT. This is the scenario where MR-only radiotherapy planning will have the largest impact increasing the spatial accuracy of the treatment.
To summarize, in patient groups where MRI is superior to CT and where variations in the anatomical configuration between imaging sessions are present, achieving accurate registration between MR and CT is difficult. If it is not possible to achieve high quality registrations focused on the structures used to position the patient at treatment in a MR-CT workflow, then MR-only treatment planning could lead to significant reduction in systematic spatial uncertainty. This could be translated into reduced exposure of healthy tissue, either through reduced PTV margin or reduced uncertainty in a probabilistic planning optimization. The relative effect would be most pronounced for small target volumes and when highly heterogenous dose distributions are prescribed.
3.2. Logistics and adaptive treatment
Logistic considerations are often mentioned as an argument for MR-only treatment planning. The removal of the CT from the workflow will mean one examination less for the patient, and in addition, the removal of the registration of MR to CT in the workflow will also reduce the workload. However, there are also additional, mostly QA related, steps that need to be taken. Current S-CT generation methods have limitations with regard to implants, body size, atypical anatomy and body position [13]. e.g. atlas based S-CT generation methods won’t be able to correctly convert patients which have undergone brain surgery and removed a part of the skull, and at present, no method can correctly convert a patient with hip implants due to the large metallic artifacts generated in the MR. The generation of an S-CT from MR images is a process, at least with the present solutions, which is largely a black box, and therefore requires patient specific QA. Even though the published dosimetric results are convincing, there is still a lack of knowledge about the failure modes and no real consensus on how the QA should be conducted. In the MR-OPERA study including 170 patients, 24 had to be excluded most commonly because of MR acquisition errors [50]. It should also be mentioned that while the dosimetric properties of various S-CT solutions have been extensively characterized, substantially less attention has been payed to the accuracy and precision of positioning based on S-CT or MR data. Positioning of prostate cancer patients using fiducial markers has been subject to a relatively large number of studies. These markers are visible on MR, but not with 100% sensitivity or specificity [13], [26], [27], [28], [29]. There is therefore an obvious need for patient specific quality control measures. Positioning based on bony structures has been studied to a lesser extent, and typically on small patient cohorts for brain: [51], [52], [53] and pelvis [54]. MR-only radiotherapy is still in an early implementation phase, where the QA should be more extensive than what would be expected for a fully established procedure. It is therefore not surprising that the clinical procedures that have been described in literature are characterized by multiple QC steps and do likely not reduce the workload at the department. Under these circumstances it is also not surprising that there are still no publications that are able to show a reduced workload or health economic benefits of MR-only treatment planning. When the technology has matured, likely, there will still be some specific QA tests for MR-only radiotherapy, i.e. regular phantom scanning to ensure that the MRI scanner is not changing with regards to geometry (for example if metal has been introduced into the scanner bore) and constancy checks for the S-CT generation software after software updates.
A vast majority of the patients treated with external beam radiotherapy are subject to a fractionated treatment strategy where the entire treatment is planned prior to the first fraction. In this workflow, care should be taken to avoid introduction of systematic spatial uncertainties or ambiguities. MR-only treatment planning could play a role in this aspect as described above. It is likely that adaptive radiation therapy (ART) will be increasingly used in the future [55]. The idea is to be able to react to changes in anatomy or biology observed during treatment or to systematic or random daily variations using imaging [32]. At present, the number of success stories have been limited, which at least to some extent could be explained by lack of software support and integrated solutions. A great exception is cervical brachy therapy where an adaptive approach driven by MR imaging in connection to each fraction is established and has been used to benefit of patients for a long time [56]. Anatomical changes are best visualized using MRI and there are strong indications that functional MRI could serve as a basis for adaptation of the treatment for example for head and neck cancer [6], rectal cancer [57], and with integrate MR-Linac solutions a whole range of indications [31], [58]. In every scenario where the treatment is adapted for a patient for whom anatomical changes of significance have occurred, a re-optimization has to be performed. For patients where the adaptation has been driven by MR, there are three options; either an MR-only workflow with generation of an S-CT based on the MR examination that triggered the adaptation, a deformable registration of the planning CT or finally the possibility of acquiring a new CT followed by registration. MR driven ART will most likely be accompanied by broad introduction of MR-only treatment planning.
In case the patient is positioned using visualization of the target volume for example with MR in a MR-Linac, the scenario is different. Possible errors in the registration between MR and CT will then not introduce a risk for geographic miss, but rather introduce possible minor dosimetic errors. This has the somewhat counterintuitive effect that MR-only treatment planning may be less important when treating with an MR-Linac compared to treatment on a standard treatment unit with X-ray based positioning. The electron return effect [59], which is pronounced for high field MR-Linacs is a viable argument for MR-only treatment planning also for MR-Linacs. As the dose distribution will depend on the occurrence of air cavities, the position of these cavities needs to be correctly depicted and accounted for in the optimization of the treatment.
4. Conclusions
MR-only treatment planning is feasible and has been demonstrated clinically. The geometrical accuracy benefits of MR-only treatment planning compared to standard MR-CT workflow depend on the quality of the MR-CT registration. The smallest benefits are expected when it is possible to achieve high accuracy registrations of the structures used for positioning the patient at treatment, such as for prostate cancer patients using fiducial markers. The biggest advantage of MR-only treatment planning will be for patients where registrations of the positioning structures are difficult which could be the case for example for head and neck cancer patients. Still, MR-only treatment planning is a very natural development for all patient groups where MR images are the primary information carriers and will likely be standard procedure for several of the large patient groups in the coming years.
Conflicts of interest
The authors have no conflict of interest in relation to the work.
Acknowledgement
Cancer Research Foundation of Northern Sweden and Västerbotten County.
References
- 1.Khoo V.S., Joon D.L. New developments in MRI for target volume delineation in radiotherapy. Br J Radiol. 2006;79:S2–S15. doi: 10.1259/bjr/41321492. [DOI] [PubMed] [Google Scholar]
- 2.Metcalfe P., Liney G.P., Holloway L., Walker A., Barton M., Delaney G.P. The potential for an enhanced role for MRI in radiation-therapy treatment planning. Technol Cancer Res Treat. 2013 doi: 10.7785/tcrt.2012.500342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chandarana H., Wang H., Tijssen R.H.N., Das I.J. Emerging role of MRI in radiation therapy. J Magn Reson Imaging. 2018:1468–1478. doi: 10.1002/jmri.26271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dirix P., Haustermans K. The value of magnetic resonance imaging for radiotherapy planning. Semin Radiat Oncol. 2014;24:151–159. doi: 10.1016/j.semradonc.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 5.McPartlin A.J., Li X.A., Kershaw L.E., Heide U., Kerkmeijer L., Lawton C. MRI-guided prostate adaptive radiotherapy – a systematic review. Radiother Oncol. 2016;119:371–380. doi: 10.1016/j.radonc.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Wong K.H., Panek R., Bhide S.A., Nutting C.M., Harrington K.J., Newbold K.L. The emerging potential of magnetic resonance imaging in personalizing radiotherapy for head and neck cancer: an oncologist’s perspective. Br J Radiol. 2017;90 doi: 10.1259/bjr.20160768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vestergaard A., Hafeez S., Muren L.P., Nill S., Høyer M., Hansen V.N. The potential of MRI-guided online adaptive re-optimisation in radiotherapy of urinary bladder cancer. Radiother Oncol. 2016;118:154–159. doi: 10.1016/j.radonc.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Olsson L.E., Johansson M., Zackrisson B., Blomqvist L.K. Basic concepts and applications of functional magnetic resonance imaging for radiotherapy of prostate cancer. Phys Imaging Radiat Oncol. 2019;9:50–57. doi: 10.1016/j.phro.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grönlund E., Johansson S., Nyholm T., Thellenberg C., Ahnesjö A. Dose painting of prostate cancer based on Gleason score correlations with apparent diffusion coefficients. Acta Oncol (Madr) 2017 doi: 10.1080/0284186X.2017.1415457. [DOI] [PubMed] [Google Scholar]
- 10.van der Heide U.A., Korporaal J.G., Groenendaal G., Franken S., van Vulpen M. Functional MRI for tumor delineation in prostate radiation therapy. Future Med. 2011;3:219–231. [Google Scholar]
- 11.Georg P., Andrzejewski P., Baltzer P., Daniel M., Wadsak W., Mitterhauser M. Changes in tumor biology during chemoradiation of cervix cancer assessed by multiparametric MRI and hypoxia PET. Mol Imaging Biol. 2018;20:160–169. doi: 10.1007/s11307-017-1087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christiansen R.L., Jensen H.R., Brink C. Magnetic resonance only workflow and validation of dose calculations for radiotherapy of prostate cancer. Acta Oncol (Madr) 2017;56:787–791. doi: 10.1080/0284186X.2017.1290275. [DOI] [PubMed] [Google Scholar]
- 13.Tyagi N., Fontenla S., Zelefsky M., Chong-Ton M., Ostergren K., Shah N. Clinical workflow for MR-only simulation and planning in prostate. Radiat Oncol. 2017;12:1–12. doi: 10.1186/s13014-017-0854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korhonen J., Kapanen M., Collan J., Saarilahti K., Visapää H., Seppälä T. MRI-only based radiation therapy of prostate cancer: workflow and early clinical experience. Acta Oncol (Madr) 2018;57:902–907. doi: 10.1080/0284186X.2018.1445284. [DOI] [PubMed] [Google Scholar]
- 15.Nyholm T., Nyberg M., Karlsson M.G., Karlsson M. Systematisation of spatial uncertainties for comparison between a MR and a CT-based radiotherapy workflow for prostate treatments. Radiat Oncol. 2009;4:54. doi: 10.1186/1748-717X-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nyholm T., Jonsson J. Counterpoint: opportunities and challenges of a magnetic resonance imaging-only radiotherapy work flow. Semin Radiat Oncol. 2014;24:175–180. doi: 10.1016/j.semradonc.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Intven M.P.W., Tyyger M.D., van den Berg C.A.T., Maspero M., Tijssen R.H.N., Seevinck P.R. Feasibility of magnetic resonance imaging-only rectum radiotherapy with a commercial synthetic computed tomography generation solution. Phys Imaging Radiat Oncol. 2018;7:58–64. doi: 10.1016/j.phro.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Owrangi A.M., Greer P.B., Glide-Hurst C.K. MRI-only treatment planning: benefits and challenges. Phys Med Biol. 2018;63:aaaca4. doi: 10.1088/1361-6560/aaaca4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kerkmeijer L.G.W., Maspero M., Meijer G.J., van der Voort van Zyp J.R.N., de Boer H.C.J., van den Berg C.A.T. Magnetic resonance imaging only workflow for radiotherapy simulation and planning in prostate cancer. Clin Oncol. 2018;30:692–701. doi: 10.1016/j.clon.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Edmund J.M., Nyholm T. A review of substitute CT generation for MRI-only radiation therapy. Radiat Oncol. 2017;12:28. doi: 10.1186/s13014-016-0747-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnstone E., Wyatt J.J., Henry A.M., Short S.C., Sebag-Montefiore D., Murray L. Systematic review of synthetic computed tomography generation methodologies for use in magnetic resonance imaging-only radiation therapy. Int J Radiat Oncol. 2018;100:199–217. doi: 10.1016/j.ijrobp.2017.08.043. [DOI] [PubMed] [Google Scholar]
- 22.Walker A., Liney G., Metcalfe P., Holloway L. MRI distortion: Considerations for MRI based radiotherapy treatment planning. Australas Phys Eng Sci Med. 2014;37:103–113. doi: 10.1007/s13246-014-0252-2. [DOI] [PubMed] [Google Scholar]
- 23.Crijns S.P.M., Bakker C.J.G., Seevinck P.R., De Leeuw H., Lagendijk J.J.W., Raaymakers B.W. Towards inherently distortion-free MR images for image-guided radiotherapy on an MRI accelerator. Phys Med Biol. 2012;57:1349–1358. doi: 10.1088/0031-9155/57/5/1349. [DOI] [PubMed] [Google Scholar]
- 24.Adjeiwaah M., Bylund M., Lundman J.A., Söderström K., Zackrisson B., Jonsson J.H. Dosimetric impact of MRI distortions: a study on head and neck cancers. Int J Radiat Oncol. 2019;103:994–1003. doi: 10.1016/j.ijrobp.2018.11.037. [DOI] [PubMed] [Google Scholar]
- 25.Adjeiwaah M., Bylund M., Lundman J.A., Karlsson C.T., Jonsson J.H., Nyholm T. Quantifying the effect of 3T magnetic resonance imaging residual system distortions and patient-induced susceptibility distortions on radiation therapy treatment planning for prostate cancer. Int J Radiat Oncol Biol Phys. 2018;100 doi: 10.1016/j.ijrobp.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 26.Gustafsson C., Korhonen J., Persson E., Gunnlaugsson A., Nyholm T., Olsson L.E. Registration free automatic identification of gold fiducial markers in MRI target delineation images for prostate radiotherapy. Med Phys. 2017;44 doi: 10.1002/mp.12516. [DOI] [PubMed] [Google Scholar]
- 27.van den Ende R.P.J., Rigter L.S., Kerkhof E.M., van Persijn van Meerten E.L., Rijkmans E.C., Lambregts D.M.J. MRI visibility of gold fiducial markers for image-guided radiotherapy of rectal cancer. Radiother Oncol. 2019;132:93–99. doi: 10.1016/j.radonc.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 28.Maspero M., Van Den Berg C.A.T., Zijlstra F., Sikkes G.G., De Boer H.C.J., Meijer G.J. Evaluation of an automatic MR-based gold fiducial marker localisation method for MR-only prostate radiotherapy. Phys Med Biol. 2017;62:7981–8002. doi: 10.1088/1361-6560/aa875f. [DOI] [PubMed] [Google Scholar]
- 29.Jonsson J.H., Garpebring A., Karlsson M.G., Nyholm T. Internal fiducial markers and susceptibility effects in MRI - Simulation and measurement of spatial accuracy. Int J Radiat Oncol Biol Phys. 2012;82 doi: 10.1016/j.ijrobp.2011.01.046. [DOI] [PubMed] [Google Scholar]
- 30.Das I.J., McGee K.P., Tyagi N., Wang H. Role and future of MRI in radiation oncology. Br J Radiol. 2018;92:20180505. doi: 10.1259/bjr.20180505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McPartlin A.J., Li X.A., Kershaw L.E., Mahmood U., Kerkmeijer L., van der Voort van Zyp J. MRI-guided prostate adaptive radiotherapy – a systematic review. Radiother Oncol. 2016;119:371–380. doi: 10.1016/j.radonc.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 32.Kupelian P., Sonke J.J. Magnetic resonance-guided adaptive radiotherapy: a solution to the future. Semin Radiat Oncol. 2014;24:227–232. doi: 10.1016/j.semradonc.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 33.Rasch C., Barillot I., Remeijer P., Touw A., van Herk M., Lebesque J.V. Definition of the prostate in CT and MRI: a multi-observer study. Int J Radiat Oncol Biol Phys. 1999;43:57–66. doi: 10.1016/s0360-3016(98)00351-4. [DOI] [PubMed] [Google Scholar]
- 34.Debois M., Oyen R., Maes F., Verswijvel G., Gatti G., Bosmans H. The contribution of magnetic resonance imaging to the three-dimensional treatment planning of localized prostate cancer. Int J Radiat Oncol Biol Phys. 1999;45:857–865. doi: 10.1016/s0360-3016(99)00288-6. S0360-3016(99)00288-6 [pii] [DOI] [PubMed] [Google Scholar]
- 35.Kagawa K., Lee W.R., Schultheiss T.E., Hunt M.A., Shaer A.H., Hanks G.E. Initial clinical assessment of CT-MRI image fusion software in localization of the prostate for 3D conformal radiation therapy. Int J Radiat Oncol Biol Phys. 1997;38:319–325. doi: 10.1016/s0360-3016(96)00620-7. [DOI] [PubMed] [Google Scholar]
- 36.Parker C.C., Damyanovich A., Haycocks T., Haider M., Bayley A., Catton C.N. Magnetic resonance imaging in the radiation treatment planning of localized prostate cancer using intra-prostatic fiducial markers for computed tomography co-registration. Radiother Oncol. 2003;66:217–224. doi: 10.1016/s0167-8140(02)00407-3. [DOI] [PubMed] [Google Scholar]
- 37.Milosevic M., Voruganti S., Blend R., Alasti H., Warde P., McLean M. Magnetic resonance imaging (MRI) for localization of the prostatic apex: comparison to computed tomography (CT) and urethrography. Radiother Oncol. 1998;47:277–284. doi: 10.1016/s0167-8140(97)00232-6. [DOI] [PubMed] [Google Scholar]
- 38.Ménard C., Paulson E., Nyholm T., McLaughlin P., Liney G., Dirix P. Role of prostate MR imaging in radiation oncology. Radiol Clin North Am. 2018;56:319–325. doi: 10.1016/j.rcl.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 39.Weltens C., Menten J., Feron M., Bellon E., Demaerel P., Maes F. Interobserver variations in gross tumor volume delineation of brain tumors on computed tomography and impact of magnetic resonance imaging. Radiother Oncol. 2001;60:49–59. doi: 10.1016/s0167-8140(01)00371-1. [DOI] [PubMed] [Google Scholar]
- 40.Datta N.R., David R., Gupta R.K., Lal P. Implications of contrast-enhanced CT-based and MRI-based target volume delineations in radiotherapy treatment planning for brain tumors. J Cancer Res Ther. 2008;4:9–13. doi: 10.4103/0973-1482.39598. [DOI] [PubMed] [Google Scholar]
- 41.Prabhakar R., Haresh K.P., Ganesh T., Joshi R.C., Julka P.K., Rath G.K. Comparison of computed tomography and magnetic resonance based target volume in brain tumors. J Cancer Res Ther. 2007;3:121–123. doi: 10.4103/0973-1482.34694. [DOI] [PubMed] [Google Scholar]
- 42.Chung N.-N., Ting L.-L., Hsu W.-C., Lui L.T., Wang P.-M. Impact of magnetic resonance imaging versus CT on nasopharyngeal carcinoma: primary tumor target delineation for radiotherapy. Head Neck. 2004;26:241–246. doi: 10.1002/hed.10378. [DOI] [PubMed] [Google Scholar]
- 43.Rasch C., Keus R., Pameijer F.A., Koops W., De Ru V., Muller S. The potential impact of CT-MRI matching on tumor volume delineation in advanced head and neck cancer. Int J Radiat Oncol Biol Phys. 1997;39:841–848. doi: 10.1016/s0360-3016(97)00465-3. [DOI] [PubMed] [Google Scholar]
- 44.Rasch C.R.N., Steenbakkers R.J.H.M., Fitton I., Duppen J.C., Nowak P.J.C.M., Pameijer F.A. Decreased 3D observer variation with matched CT-MRI , for target delineation in Nasopharynx cancer. Radiat Oncol. 2010:4–9. doi: 10.1186/1748-717X-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.ICRU 50. Prescribing, Recording, and Reporting Photon Beam Therapy. 1993.
- 46.ICRU 62. Prescribing, Recording, and Reporting Photon Beam Therapy (Supplement to ICRU Report 50). 1999.
- 47.Ulin K., Urie M.M., Cherlow J.M. Results of a multi-institutional benchmark test for cranial CT/MR image registration. Int J Radiat Oncol Biol Phys. 2010;77:1584–1589. doi: 10.1016/j.ijrobp.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberson P.L., McLaughlin P.W., Narayana V., Troyer S., Hixson G.V., Kessler M.L. Use and uncertainties of mutual information for computed tomography/ magnetic resonance (CT/MR) registration post permanent implant of the prostate. Med Phys. 2005;32:473–482. doi: 10.1118/1.1851920. [DOI] [PubMed] [Google Scholar]
- 49.Verduijn G.M., Bartels L.W., Raaijmakers C.P.J., Terhaard C.H.J., Pameijer F.A., van den Berg C.A.T. Magnetic resonance imaging protocol optimization for delineation of gross tumor volume in hypopharyngeal and laryngeal tumors. Int J Radiat Oncol. 2009;74:630–636. doi: 10.1016/j.ijrobp.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 50.Persson E., Gustafsson C., Nordström F., Sohlin M., Gunnlaugsson A., Petruson K. A multicenter/multivendor validation of magnetic resonance imaging-only prostate treatment planning using synthetic computed tomography images. Int J Radiat Oncol Biol Phys. 2017;99. doi: 10.1016/j.ijrobp.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 51.Yang Y., Cao M., Kaprealian T., Sheng K., Gao Y., Han F. Accuracy of UTE-MRI-based patient setup for brain cancer radiation therapy. Med Phys. 2015;43:262–267. doi: 10.1118/1.4938266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu H., Caldwell C., Balogh J., Mah K. Toward magnetic resonance-only simulation: segmentation of bone in MR for radiation therapy verification of the head. Int J Radiat Oncol Biol Phys. 2014;89:649–657. doi: 10.1016/j.ijrobp.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 53.Price R.G., Kim J.P., Zheng W., Chetty I.J., Glide-Hurst C. Image guided radiation therapy using synthetic computed tomography images in brain cancer. Int J Radiat Oncol Biol Phys. 2016;95:1281–1289. doi: 10.1016/j.ijrobp.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kemppainen R., Vaara T., Joensuu T., Kiljunen T. Accuracy and precision of patient positioning for pelvic MR-only radiation therapy using digitally reconstructed radiographs. Phys Med Biol. 2018;63 doi: 10.1088/1361-6560/aaad21. [DOI] [PubMed] [Google Scholar]
- 55.Yan D, Vicini F, Wong J, Martinez A. Physics in Medicine & Biology Adaptive radiation therapy. 1997. [DOI] [PubMed]
- 56.Dimopoulos J.C.A., Petrow P., Tanderup K., Petric P., Berger D., Kirisits C. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (IV): basic principles and parameters for MR imaging within the frame of image based adaptive cervix cancer brachytherapy. Radiother Oncol. 2012;103:113–122. doi: 10.1016/j.radonc.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Herk M., Nijkamp J., Sonke J.-J., Marijnen C., van Triest B. Adaptive radiotherapy for long course neo-adjuvant treatment of rectal cancer. Radiother Oncol. 2012;103:353–359. doi: 10.1016/j.radonc.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 58.Lim-Reinders S., Keller B.M., Al-Ward S., Sahgal A., Kim A. Online adaptive radiation therapy. Int J Radiat Oncol Biol Phys. 2017;99:994–1003. doi: 10.1016/j.ijrobp.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 59.Raaijmakers A.J.E., Raaymakers B.W., Lagendijk J.J.W. Integrating a MRI scanner with a 6 MV radiotherapy accelerator: dose increase at tissue-air interfaces in a lateral magnetic field due to returning electrons. Phys Med Biol. 2005;50:1363–1376. doi: 10.1088/0031-9155/50/7/002. [DOI] [PubMed] [Google Scholar]