Key Teaching Points.
-
•
The HD Grid catheter (Abbott Technologies, Minneapolis, MN) has 16 equidistant electrodes, allowing for assessment of conduction directionality as well as omnipolar electrogram assessment.
-
•
This allows for the differentiation of far-field and near-field signals using a single catheter, which can be helpful for determination of an endpoint in pulmonary vein isolation.
-
•
The unique shape and predictable electrode location allows for rapid mapping of connections between the left atrium and the pulmonary veins as well as the diagnosis of complex post–pulmonary vein isolation atrial tachycardias.
-
•
This catheter can also be used easily to assess bidirectional conduction block along a line of ablation.
Introduction
Pulmonary vein isolation (PVI) remains the most important strategy for the management of paroxysmal atrial fibrillation (AF) in the electrophysiology laboratory, with reconnections resulting in recurrences of AF or atrial tachycardia (AT).1, 2 Conventionally assessment of conduction between the left atrium (LA) and the pulmonary veins (PVs) is performed using a circular multielectrode catheter positioned within the PV in which signals from the catheter are used to localize the approximate regions of conduction and pacing is performed from the catheter in order to assess for exit block. Although the use of these catheters has been shown to result in lower AF recurrence rates when compared with using only a radiofrequency (RF) catheter,3 the complexity and orientation of PV to LA may not always be accurately assessed, leading to the need for more RF energy delivery than may be required.
Recently a high-definition mapping catheter has been introduced that has 16 equidistant electrodes (HD Grid Mapping Catheter Sensor Enabled, Abbott Technologies, Minneapolis, MN), which allows the rapid assessment of voltage, activation, and directionality of conduction.
The unique grid pattern design and 3-3-3-mm spacing allows for bipolar recording along and across the splines, and the catheter is the first of its kind designed to facilitate substrate mapping and account for directionality. When used in conjunction with the Precision Mapping System (Abbott Technologies), this catheter is designed to reduce variability in electrogram characteristics associated with differential orientations relative to the propagating wavefront. The Best Duplicate algorithm of the Precision Mapping System uses orthogonal bipoles to compare signal amplitude for collocated mapping data to more accurately display the highest-amplitude signal.
In order to utilize this catheter fully, data can be collected from the LA and the antra around the PVs where ablation lesions should be targeted.
We describe the use of this catheter in the rapid acquisition of electrical and anatomic data for a first-time PVI, the ability to use the direction of the wavefront to help distinguish between near-field PV signals and far-field atrial signals, and its use in the accurate mapping of the antra around the PV in patients with prior PVI who present with a recurrence of AF or AT.
Case report
Case 1
A 64-year-old man presented with a 6-month history of symptomatic paroxysmal AF not amenable to drug therapy. Following wide antral circumferential ablation around the left superior pulmonary vein (LSPV) and left inferior pulmonary vein (LIPV), signals were still evident in both veins. Although these signals appeared to be in keeping with the LA appendage far-field, this can sometimes be difficult to differentiate. By positioning the HD Grid catheter along the coumadin ridge, as shown in Figure 1, the activation sequence along the catheter clearly shows the signals originating from the LA appendage to the left-sided PVs. This provided a simple immediate solution in demonstrating the exact direct of conduction and thus differentiating near-field from far-field. We have also demonstrated this use in the right superior pulmonary vein (RSPV), in which the HD Grid can be positioned from anterior to posterior and demonstrate far-field conduction from the right atrium. Unlike other currently available multielectrode catheters, the predictable spacing and orientation of the electrodes as well as the orientation allows directionality of conduction, which is also useful for focal and reentrant tachycardias. This is being further automated in order to compute and record omnipolar electrograms.
Figure 1.
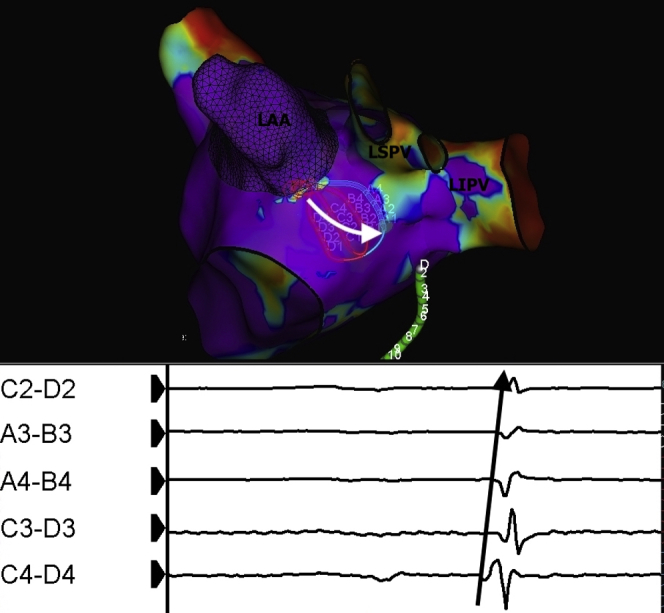
Voltage map of the left atrium showing positioning of the HD Grid (Abbott Technologies, Minneapolis, MN) at the lower coumadin ridge between the left atrial appendage (LAA) and the left superior pulmonary vein (LSPV) and left inferior pulmonary vein (LIPV). Electrograms selected from the catheter show that signals propagate from the LAA to the pulmonary veins, demonstrating that the signals recorded originate from the LAA.
Case 2
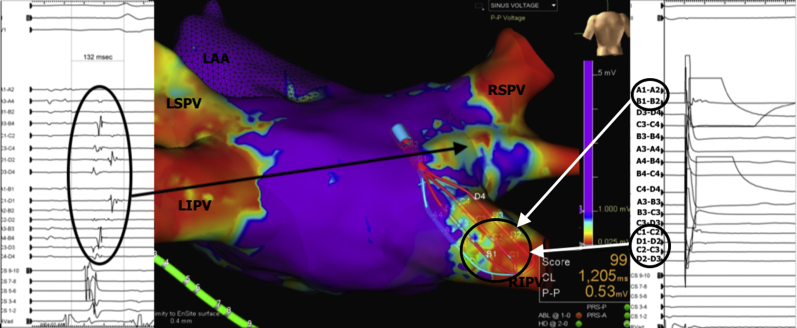
A 70-year-old man presented with multiple clinical recurrences of AF 12 months following a prior PVI for paroxysmal AF. The HD Grid catheter was used to map the LA in sinus rhythm. As shown in Figure 2, this demonstrated isolation of the LSPV and LIPVs. Mapping around the posterior carina at the level of the RSPV showed significant fractionation (total duration of fractionation 132 ms) in this region, suggestive of slow conduction from the LA to the RSPV. Catheter ablation in this region using RF energy duration (2 applications, each lasting 30 seconds) at 30 W resulted in isolation of the RSPV.
Figure 2.
Voltage mapping of the left atrium (LA) using the HD Grid (Abbott Technologies, Minneapolis, MN) with evidence of electrical conduction through the right posterior carina connecting into the right superior pulmonary vein (RSPV). Local electrograms recorded from this region showed significant fractionation (total duration of fractionation 132 ms) in this region, suggestive of slow conduction from the LA to the RSPV. Pacing from the right inferior pulmonary vein (RIPV) from the distal electrodes (A1–A2) demonstrated evidence of local capture within the pulmonary vein on poles B1–B2, C1–C2, D1–D2, C2–C3, and D2–D3, which did not result in local LA depolarization on poles A3–A4. LAA = left atrial appendage; LIPV = left inferior pulmonary vein; LSPV = left superior pulmonary vein.
We were then able to use this catheter to easily check for bidirectional block along the ablation line. Although several catheters can be used to perform this, the shape of the grid allows for simple positioning and contact on both sides of the ablation line using a single catheter with pacing on either side and detection of signals on the opposite side of the line. As shown in Figure 2, pacing from the distal electrodes (A1–A2) with evidence of local capture within the PV (B1–B2, C1–C2, D1–D2, C2–C3, D2–D3) did not result in local LA depolarization (A3–A4). This provides a very accurate assessment of local conduction block over the line of prior RF ablation.
Case 3
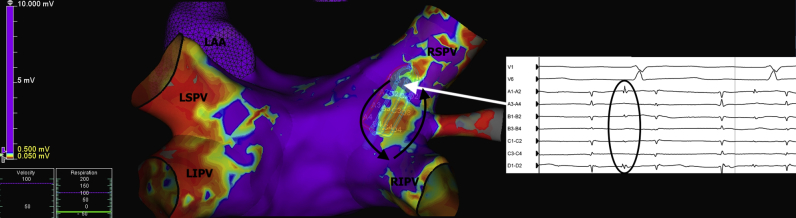
A 64-year-old woman presented with highly symptomatic palpitations 5 months following a PVI for paroxysmal AF. A 12-lead electrocardiogram revealed this to be a left -sided AT and the patient was brought to the electrophysiology laboratory for mapping and further ablation. The HD Grid was used to map the AT. A voltage map (Figure 3) revealed 2 regions of reconnection along the posterior region of the RSPV and RIPV. The activation of this tachycardia is shown in the Supplemental Video.
Figure 3.
Voltage mapping of the left atrium in a patient with a post–pulmonary vein isolation atrial tachycardia showing 2 regions of reconnection along the posterior region of the right superior pulmonary vein (RSPV) and right inferior pulmonary vein (RIPV). Entrainment confirmed involvement of these regions during tachycardia. Local electrograms recorded from the distal electrodes on the HD Grid (Abbott Technologies, Minneapolis, MN) at the superior region of reconnection revealed 2:1 mid-diastolic potentials, which indicated slow conduction through a critical isthmus. LAA = left atrial appendage; LIPV = left inferior pulmonary vein; LSPV = left superior pulmonary vein.
Entrainment confirmed involvement of these regions during tachycardia. Local electrograms recorded from the distal electrodes on the HD Grid at the superior region of reconnection revealed 2:1 mid-diastolic potentials, which indicated slow conduction through a critical isthmus. Ablation in this region at a power setting of 30 W resulted in termination of the tachycardia. Further ablation was performed along the inferior component of the circuit. During this case over 16,000 electrical points were automatically collected over a period of 12 minutes and a procedure time of 60 minutes. Of additional note, no catheter-induced ectopy has been noted with this catheter during atrial mapping cases. Following this, no further tachycardia could be induced.
Conclusion
High-resolution mapping of the atria can be performed rapidly and accurately using the HD Grid for the catheter ablation of AF, redo AF ablations, and post-PVI ATs. As well as providing rapid voltage and propagation maps, this catheter can be positioned in such a way as to rapidly localize gaps and provide information on the direction of conduction. In particular, omnipolar mapping will allow for accurate directionality as well as more detailed substrate mapping in the presence of changes in the direction of conduction. This will enhance the accuracy of mapping in atrial arrhythmias. Further algorithms are being studied in order to rapidly automate conduction directionality in focal and reentry circuits.
Footnotes
Dr Glover received research support and consulting fees from Abbott Technologies. Dr Redfearn received research support and consulting fees from Abbott Technologies. Mr Baley is an employee of Abbott Technologies.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrcr.2018.12.012.
Appendix. Supplementary data
Activation map superimposed on voltage map showing re-entry circuit involving regions of slow conduction posterior to the RSPV and RIPV separated by a region of scar. The distal electrograms (A1-A2, A1-B1, A2-B2) demonstrate highly fractionated electrograms indicative of slow conduction in the superior region of the re-entry circuit.
References
- 1.Suleiman M., Koestler C., Lerman A. Atorvastatin for prevention of atrial fibrillation recurrence following pulmonary vein isolation: a double-blind, placebo-controlled, randomized trial. Heart Rhythm. 2012;9:172–178. doi: 10.1016/j.hrthm.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 2.Gerstenfeld E.P., Callans D.J., Dixit S. Mechanisms of organized left atrial tachycardias occurring after pulmonary vein isolation. Circulation. 2004;110:1351–1357. doi: 10.1161/01.CIR.0000141369.50476.D3. [DOI] [PubMed] [Google Scholar]
- 3.Tamborero D., Mont L., Berruezo A. Circumferential pulmonary vein ablation: does use of a circular mapping catheter improve results? A prospective randomized study. Heart Rhythm. 2010;7:612–618. doi: 10.1016/j.hrthm.2010.01.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Activation map superimposed on voltage map showing re-entry circuit involving regions of slow conduction posterior to the RSPV and RIPV separated by a region of scar. The distal electrograms (A1-A2, A1-B1, A2-B2) demonstrate highly fractionated electrograms indicative of slow conduction in the superior region of the re-entry circuit.