Abstract
Objective
The purpose of the present study was to examine the relationship between thrombocytopenia at baseline and in-hospital outcomes in unselected patients undergoing elective percutaneous coronary intervention (PCI) in Japan.
Methods
Among a total of 1,247 consecutive elective PCI-treated patients, patients with a baseline platelet count 150,000-449,000/μL and 50,000-149,000/μL were assigned to the normal platelet (n=1,009) and thrombocytopenia (n=226) groups, respectively. The thrombocytopenia group was further divided into the mild thrombocytopenia (100,000-149,000/μL, n=187) and moderate thrombocytopenia (50,000-99,000/μL, n=39) groups.
Results
The angiographic success rate of PCI and in-hospital mortality rate did not differ to a statistically significant extent between the normal platelet and thrombocytopenia groups or between the mild thrombocytopenia and moderate thrombocytopenia groups, whereas the moderate thrombocytopenia group had a significantly higher rate of access site-related bleeding complications than the normal platelet group. According to a multivariate analysis, moderate thrombocytopenia was an independent predictor of access site-related bleeding complications.
Conclusion
Among patients with mild to moderate thrombocytopenia, elective PCI might be feasible and effective in the short term; however, more attention should be paid to access site-related bleeding complications, particularly in patients with moderate thrombocytopenia.
Keywords: thrombocytopenia, percutaneous coronary intervention, bleeding complications, antiplatelet therapy, hemodialysis
Introduction
Drug-eluting stent (DES) implantation has been a mainstay of the interventional treatment for coronary artery disease (CAD), and dual antiplatelet therapy (DAPT) with aspirin and thienopyridines such as clopidogrel or prasugrel for more than 6 months is a standard antiplatelet therapy for CAD patients undergoing DES implantation (1). Despite the efficacy of DESs in inhibiting in-stent restenosis (ISR), DAPT-induced bleeding complications have attracted more attention, particularly among patients with a high of hemorrhagic complications, such as those with thrombocytopenia. However, no guidelines and only several reports are available on the safety and tolerance of antiplatelet therapy/percutaneous coronary intervention (PCI) in CAD patients complicated with thrombocytopenia at baseline (2-6). The purpose of the present study was therefore to examine the prognostic impact of baseline thrombocytopenia on in-hospital outcomes in unselected Japanese patients undergoing elective PCI in a real-world setting.
Materials and Methods
Patient population
From January 2012 to December 2016, 1,247 consecutive patients undergoing elective PCI in our hospital were enrolled in the present study. Among the study population, patients with a baseline platelet count of ≥450,000/μL or <50,000/μL were excluded. Patients with baseline platelet counts of 150,000-449,000/μL and 50,000-149,000/μL were assigned to the normal platelet group (n=1,009) and thrombocytopenia group (n=226), respectively. The thrombocytopenia group was further divided into the mild thrombocytopenia (100,000-149,000/μL; n=187) and moderate thrombocytopenia (50,000-99,000/μL; n=39) groups. We retrospectively compared the clinical background, preprocedural antiplatelet/anticoagulation medications, acute results of PCI, and in-hospital prognosis between the normal platelet and thrombocytopenia group as well as between the mild thrombocytopenia and moderate thrombocytopenia groups. The baseline platelet count was defined as the platelet count of blood samples obtained before PCI. Elective PCI was performed according to standard techniques with the final decision about the appropriate strategy in each patient left to the judgment of the physician in charge. TR Band™ (TERUMO, Tokyo, Japan) was used as a hemostatic device for all patients undergoing transradial PCI. Manual compression, mechanical compression [CompressAR (Semler Technologies, Milwaukie, USA)], or a closure device [EXOSEAL (Cordis, Miami, USA), Angio-Seal™ STS Plus (TERUMO)] were employed to achieve hemostasis for each patient undergoing transfemoral PCI. Continuous compression using BLEED SAFE (Medikit, Tokyo, Japan) or manual compression was performed for each patient undergoing transbrachial PCI.
Data collection
The patients' demographic information and risk factors (i.e., hypertension, diabetes mellitus, dyslipidemia, and smoking) were recorded. Hypertension was defined as systemic blood pressure ≥140/90 mmHg and/or the use of antihypertensive treatment; diabetes mellitus was defined as fasting blood sugar ≥126 mg/dL, abnormal glycosylated hemoglobin (HbA1c) ≥6.5%, and/or the use of specific treatment. Dyslipidemia was defined as low-density lipoprotein cholesterol (LDL-C) >140 mg/dL, high-density lipoprotein cholesterol (HDL-C) <40 mg/dL, triglycerides (TG) >150 mg/dL, and/or the use of antidyslipidemic agents. Based on the WHO criteria, anemia was defined as a hemoglobin (Hb) concentration (on admission) of <13.0 g/dL for men and <12.0 g/dL for women. Obesity was defined as a body mass index (BMI) ≥25 kg/m2. Preprocedural antiplatelet/anticoagulation medications were defined as the regular intake of those medications at the time of PCI (the loading/restart of those medications during/after PCI was excluded). Dual antiplatelet therapy was defined as aspirin plus thienopyridine (ticlopidine, clopidogrel, or prasugrel). Anticoagulants included warfarin and direct oral anticoagulants. Triple therapy was defined as DAPT plus anticoagulants.
Clinical endpoint measurements
In-hospital death was defined as all-cause death during the admission period. Access site-related bleeding complications included pseudoaneurysm, retroperitoneal hematoma, or hematoma/recurrent bleeding at sheath-insertion sites requiring hemostasis to be achieved a second time, surgical repair, or prolonged admission period. Angiographic success of PCI was defined as <50% stenosis without flow delay on final CAG, evaluated according to the American Heart Association classification (7) and the Thrombolysis In Myocardial Infarction (TIMI) classification.
Statistical analysis
Data are expressed as the mean±SD for continuous variables and number (percentage) for categorical variables. The normal platelet and thrombocytopenia groups as well as the mild thrombocytopenia and moderate thrombocytopenia groups were compared using the chi-squared test for discrete variables and the unpaired Student's t-test for continuous variables, according to the standard statistical methods. The odds ratio (OR) and 95% confidence interval (CI) for the risk of access site-related bleeding complications were estimated by univariate and multivariate analyses with a logistic regression model. Significant variables with a p value <0.05 in the univariate analyses were entered into a multivariate analysis. The cut-off value for the baseline platelet count to predict access site-related bleeding complications was calculated by a receiver operating characteristics (ROC) curve analysis. Statistical analyses were performed using Statcel 3 (add-in software for Excel, Microsoft), StatView version 5.0 J (SAS Institution, Cary, USA) for univariate and multivariate analyses with logistic regression, and SPSS 22.0 (IBM, Tokyo, Japan) for the ROC analysis. In all analyses, p values of <0.05 were considered to indicate statistical significance.
Results
Patient characteristics and antiplatelet/Anticoagulant medications
The clinical characteristics and the preprocedural antiplatelet/anticoagulant agents of the normal platelet and thrombocytopenia groups are summarized in Table 1. The thrombocytopenia group had a higher age, higher frequencies of diabetes mellitus and hemodialysis (HD), and a lower Hb and BMI levels on admission than the normal platelet group. The thrombocytopenia group had a lower prevalence of thienopyridine and a higher prevalence of anticoagulants as preprocedural medications in comparison to the normal platelet group. The distribution of PCI indications differed significantly between the 2 groups. The clinical characteristics and the preprocedural antiplatelet/anticoagulant agents of the mild thrombocytopenia group and the moderate thrombocytopenia group are summarized in Table 2. The moderate thrombocytopenia group had lower Hb values and a larger prevalence of DAPT as preprocedural medication in comparison to the mild thrombocytopenia group.
Table 1.
Clinical Characteristics and Preprocedural Antiplatelet/anticoagulant Agents (Normal Platelet Group vs. Thrombocytopenia Group).
| Normal platelet | Thrombocytopenia | p | |
|---|---|---|---|
| n=1,009 | n=226 | ||
| Age (mean years±SD) | 72±10 | 74±9 | 0.024 |
| Male (%) | 741 (73.4) | 169 (74.8) | 0.679 |
| Platelets (×103/μL) | 222±55 | 122±23 | <0.001 |
| BMI (kg/m2) | 23.4±3.7 | 22.6±3.5 | 0.001 |
| Hypertension (%) | 766 (75.9) | 175 (77.4) | 0.628 |
| Diabetes mellitus (%) | 442 (43.8) | 120 (53.1) | 0.011 |
| Dyslipidemia (%) | 671 (66.5) | 137 (60.6) | 0.093 |
| Current smoking (%) | 163 (16.2) | 27 (11.9) | 0.113 |
| Hemodialysis (%) | 103 (10.2) | 73 (32.3) | <0.001 |
| Hb (g/dL) | 12.6±1.8 | 11.6±1.7 | <0.001 |
| PCI indication | |||
| Stable AP (%) | 507 (50.2) | 85 (37.6) | 0.003 |
| SMI (%) | 424 (42.0) | 118 (52.2) | |
| ACS (%) | 78 (7.7) | 23 (10.2) | |
| Preprocedural medication | |||
| Aspirin (%) | 875 (86.7) | 190 (84.1) | 0.296 |
| Thienopiridine (%) | 805 (79.8) | 165 (73.0) | 0.025 |
| DAPT (%) | 754 (74.7) | 155 (68.6) | 0.058 |
| Anticoagulant (%) | 86 (8.5) | 32 (14.2) | 0.009 |
| Triple therapy (%) | 58 (5.7) | 20 (8.8) | 0.083 |
BMI: body mass index, Hb: hemoglobin, PCI: percutaneous coronary intervention, AP: angina pectoris, SMI: silent myocardial ischemia, ACS: acute coronary syndrome, DAPT: dual antiplatelet therapy
Table 2.
Clinical Characteristics and Preprocedural Antiplatelet/anticoagulant Agents (Mild Thrombocytopenia Group vs. Moderate Thrombocytopenia Group).
| Mild thrombocytopenia | Moderate thrombocytopenia | p | |
|---|---|---|---|
| n=187 | n=39 | ||
| Age (mean years±SD) | 74±9 | 73±7 | 0.738 |
| Male (%) | 144 (77.0) | 25 (64.1) | 0.091 |
| Platelets (×103/μL) | 131±13 | 81±13 | <0.001 |
| BMI (kg/m2) | 22.5±3.5 | 23.1±3.2 | 0.284 |
| Hypertension (%) | 144 (77.0) | 31 (79.5) | 0.736 |
| Diabetes mellitus (%) | 99 (52.9) | 21 (53.8) | 0.918 |
| Dyslipidemia (%) | 108 (57.8) | 29 (74.4) | 0.054 |
| Current smoking (%) | 22 (11.8) | 5 (12.8) | 0.853 |
| Hemodialysis (%) | 59 (31.6) | 14 (35.9) | 0.597 |
| Hb (g/dL) | 11.7±1.7 | 11.1±1.8 | 0.023 |
| PCI indication | |||
| Stable AP (%) | 68 (36.4) | 17 (43.6) | 0.206 |
| SMI (%) | 97 (51.9) | 21 (53.8) | |
| ACS (%) | 22 (11.8) | 1 (2.6) | |
| Preprocedural medication | |||
| Aspirin (%) | 154 (82.4) | 36 (92.3) | 0.122 |
| Thienopiridine (%) | 132 (70.6) | 33 (84.6) | 0.073 |
| DAPT (%) | 120 (64.2) | 32 (82.1) | 0.030 |
| Anticoagulant (%) | 26 (13.9) | 6 (15.4) | 0.809 |
| Triple therapy (%) | 14 (7.5) | 6 (15.4) | 0.114 |
BMI: body mass index, Hb: hemoglobin, PCI: percutaneous coronary intervention, AP: angina pectoris, SMI: silent myocardial ischemia, ACS: acute coronary syndrome, DAPT: dual antiplatelet therapy
Results of coronary intervention and in-hospital outcomes
Table 3 shows the results of PCI and the in-hospital prognoses of the normal platelet and thrombocytopenia groups. A significant difference was seen between the two groups in the distribution of the approach sites, while the prevalence of DES usage, and bare metal stent (BMS) usage were similar between the 2 groups (Table 3). The thrombocytopenia group had a lower frequency of 6-Fr guiding catheter (GC) use than the normal platelet group. The angiographic success rate and in-hospital mortality rate of the 2 groups did not differ to a statistically significant extent (Table 3). Table 4 shows the results of PCI and the in-hospital prognoses of the mild thrombocytopenia and moderate thrombocytopenia groups. The moderate thrombocytopenia group had a higher prevalence of BMS usage and 6-Fr GC usage than the mild thrombocytopenia group. The angiographic success rate and in-hospital mortality rate of the 2 groups did not differ to a statistically significant extent (Table 4).
Table 3.
Results of Coronary Intervention and In-hospital Outcomes (Normal Platelet Group vs. Thrombocytopenia Group).
| Normal platelet | Thrombocytopenia | p | |
|---|---|---|---|
| n=1,009 | n=226 | ||
| DES (%) | 840 (83.3) | 176 (77.9) | 0.056 |
| BMS (%) | 42 (4.2) | 9 (4.0) | 0.902 |
| Approach site | |||
| Femoral (%) | 199 (19.7) | 88 (38.9) | <0.001 |
| Brachial (%) | 73 (7.2) | 13 (5.8) | |
| Radial (%) | 737 (73.0) | 125 (55.3) | |
| GC size | |||
| 6 Fr (%) | 694 (68.8) | 138 (61.1) | 0.025 |
| >6 Fr (%) | 315 (31.2) | 88 (38.9) | |
| Angiographic success (%) | 988 (97.9) | 219 (96.9) | 0.354 |
| In-hospital mortality (%) | 2 (0.2) | 0 (0.0) | 0.503 |
| Transfusion (%) | 4 (0.4) | 0 (0.0) | 0.343 |
DES: drug-eluting stent, BMS: bare metal stent, GC: guiding catheter
Table 4.
Results of Coronary Intervention and In-hospital Outcomes (Mild Thrombocytopenia Group vs. Moderate Thrombocytopenia Group).
| Mild Thrombocytopenia | Moderate Thrombocytopenia | p | |
|---|---|---|---|
| n=187 | n=39 | ||
| DES (%) | 148 (79.1) | 28 (71.8) | 0.314 |
| BMS (%) | 5 (2.7) | 4 (10.3) | 0.028 |
| Approach site | |||
| Femoral (%) | 73 (39.0) | 15 (38.5) | 0.848 |
| Brachial (%) | 10 (5.3) | 3 (7.7) | |
| Radial (%) | 104 (55.6) | 21 (53.8) | |
| GC size | |||
| 6 Fr (%) | 108 (57.8) | 30 (76.9) | 0.026 |
| >6 Fr (%) | 79 (42.2) | 9 (23.1) | |
| Angiographic success (%) | 182 (97.3) | 37 (94.9) | 0.421 |
| In-hospital mortality (%) | 0 (0.0) | 0 (0.0) |
BMI: body mass index, Hb: hemoglobin, PCI: percutaneous coronary intervention, AP: angina pectoris, SMI: silent myocardial ischemia, ACS: acute coronary syndrome, DAPT: dual antiplatelet therapy
Access site-related bleeding complications
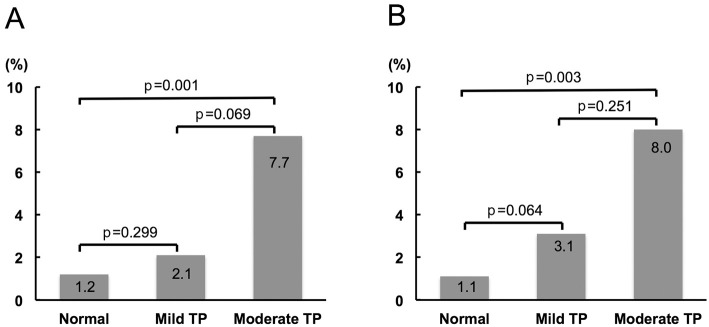
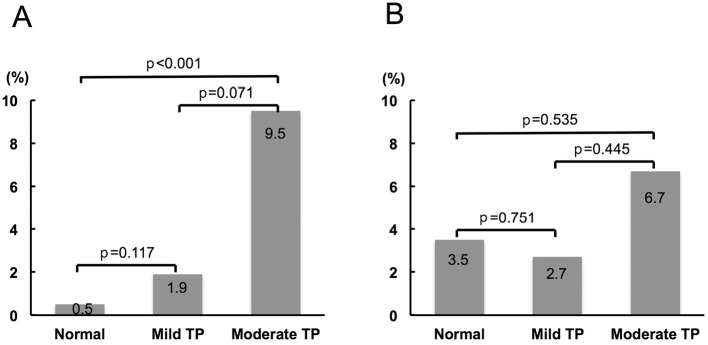
The frequency of access site-related bleeding complications increased according to the severity of thrombocytopenia (Fig. 1A). The moderate thrombocytopenia group had a significantly higher frequency of access site-related bleeding complications than the normal platelet group. Even after excluding patients on HD, the prevalence of access site-related bleeding complications increased in the same fashion (Fig. 1B). Moreover, in patients undergoing transradial PCI, the frequency of access site-related bleeding complications increased in a similar manner to that in the overall study population (Fig. 2A), while the tendency was not observed in patients undergoing transfemoral PCI (Fig. 2B). In order to assess the contribution of the clinical background, risk factors, preprocedural medications, and PCI procedure, univariate and multivariate logistic regression analyses were performed to identify the factors associated with access site-related bleeding complications during hospitalization. The significant variables included baseline moderate thrombocytopenia (platelet count 50,000-99,000/μL), age, gender, medical history (hypertension, diabetes mellitus, HD, anemia, and obesity), preprocedural medication (DAPT, anticoagulant, and triple therapy), 6-Fr GC usage, and transradial approach. On univariate testing, moderate baseline thrombocytopenia was positively associated with access site-related bleeding complications, while 6-Fr GC usage and a transradial approach were negatively associated with access site-related bleeding complications (Table 5). According to a multivariate analysis, baseline moderate thrombocytopenia was independently associated with access site-related bleeding complications (Table 5). Entering baseline thrombocytopenia (mild to moderate) instead of baseline moderate thrombocytopenia into a multivariate analysis indicated that mild to moderate thrombocytopenia at baseline tended to be related to the access site-related bleeding complications; however, the result was not statistically significant (OR 2.257, 95%CI 0.862-5.907, p=0.0972). With the exception of access site-related bleeding complications, there was 1 bleeding complication (intraabdominal bleeding of unknown origin) in the normal platelet group. The results of an ROC curve analysis to determine the baseline platelet count predicting access site-related bleeding complications are shown in Fig. 3. A baseline platelet count of 159,000/μL was the cut-off value for access site-related bleeding complications (area under the curve=0.625), with 52.6% sensitivity and 75.3% specificity.
Figure 1.
Access site-related bleeding complications according to the platelet count at baseline. A: The overall study population. B: The study population without hemodialysis. TP: thrombocytopenia
Figure 2.
Access site-related bleeding complications according to the platelet count at baseline. A: Patients undergoing transradial PCI. B: Patients undergoing transfemoral PCI. TP: thrombocytopenia
Table 5.
Predictors of Access Site-related Bleeding Complications in the Study Patients (Univariate/multivariate Logistic Regression Analysis).
| Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | ||
| Moderate thrombocytopenia | 6.146 | 1.714-22.039 | 0.0053 | 6.508 | 1.747-24.240 | 0.0052 | |
| DAPT | 1.954 | 0.565-6.749 | 0.2897 | ||||
| Anticoagulation agent | 1.795 | 0.515-6.254 | 0.3582 | ||||
| Triple therapy | 1.765 | 0.400-7.780 | 0.4530 | ||||
| Transradial approach | 0.381 | 0.154-0.947 | 0.0377 | 0.582 | 0.218-1.557 | 0.2814 | |
| 6Fr GC | 0.346 | 0.138-0.867 | 0.0235 | 0.388 | 0.143-1.053 | 0.0630 | |
| Hypertension | 2.686 | 0.617-11.697 | 0.1880 | ||||
| Diabetes mellitus | 1.079 | 0.435-2.674 | 0.8695 | ||||
| Hemodialysis | 1.130 | 0.326-3.921 | 0.8468 | ||||
| Anemia | 0.763 | 0.308-1.892 | 0.5595 | ||||
| Obesity | 1.137 | 0.429-3.017 | 0.7958 | ||||
| Male | 0.485 | 0.193-1.216 | 0.1229 | ||||
| Age | 1.006 | 0.959-1.056 | 0.7967 | ||||
OR: odds ratio, CI: confidence intervals, DAPT: dual antiplatelet therapy, GC: guiding catheter
Figure 3.
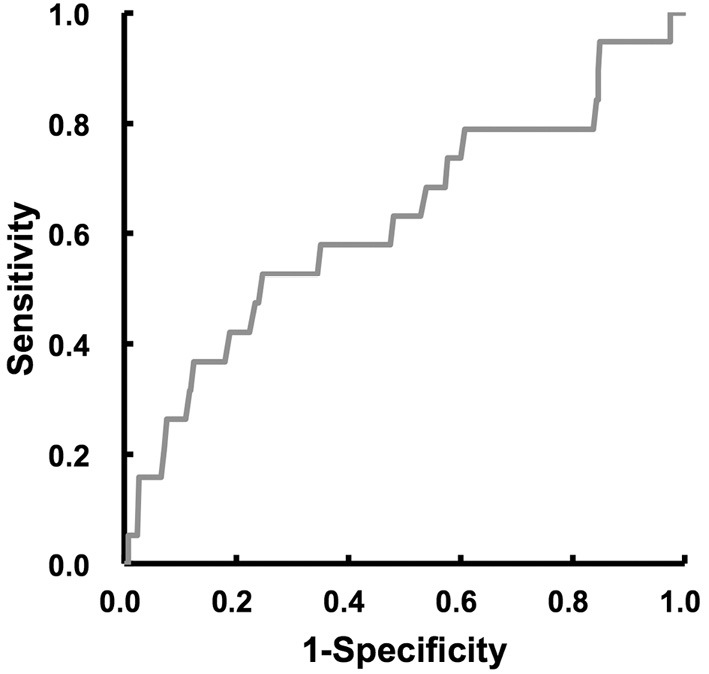
Receiver operating characteristics curves of the baseline platelet count for the prediction of access site-related bleeding complications in the study population.
Discussion
The major findings of the present study are as follows: 1) among patients undergoing elective PCI, the angiographic success rate and in-hospital mortality rate did not differ to a statistically significant extent between patients with mild to moderate thrombocytopenia at baseline and patients with a normal platelet count or between patients with mild thrombocytopenia and patients with moderate thrombocytopenia; 2) access site-related bleeding complications increased according to the severity of thrombocytopenia, and patients with baseline moderate thrombocytopenia had a significantly higher rate of access site-related bleeding complications than patients with a normal platelet count; and 3) baseline moderate thrombocytopenia was an independent predictor of access site-related bleeding complications.
This study is the first to investigate the prognostic impact of baseline thrombocytopenia on in-hospital outcomes among unselected Japanese patients undergoing elective PCI in the modern DES era in a real-world setting. A few previous reports have indicated that baseline thrombocytopenia was associated with in-hospital and 30-day mortality in patients undergoing PCI due to urgent indication or ST-elevation myocardial infarction (2, 3). Another recent study showed that baseline thrombocytopenia was related to death and cardiovascular events at 1 year, but not at 30 days in patients with acute coronary syndrome undergoing PCI (5). In contrast, among patients undergoing elective PCI, no significant relationship was observed between baseline thrombocytopenia and in-hospital death (2), which was consistent with the present report. A recent report based on the pooled data of 3 PCI studies (1 registry, 2 randomized trials) from Japanese investigators has demonstrated that baseline thrombocytopenia was associated with bleeding events and deaths, but not associated with composite events of myocardial infarction and ischemic stroke during 3 years of follow-up after PCI (6). Based on the data from the present and previous studies, patients with baseline thrombocytopenia tended to have more adverse prognostic factors, such as diabetes mellitus, anemia, or chronic renal insufficiency/HD, in comparison to those with a normal platelet count (2-6), and careful long-term observation would therefore be required, even for patients with stable CAD, when patients with thrombocytopenia undergo PCI.
Taking the higher frequency of HD in the thrombocytopenia group into consideration, HD might be an underlying clinical background factor associated with thrombocytopenia. A previous report from Japanese investigators indicated that thrombocytopenia was more frequent in patients on HD, particularly in the hepatitis C virus (HCV)-positive HD patients, and impaired megakaryocyte production in the bone marrow was an underlying cause of thrombocytopenia in HD patients (8). Moreover, among the HD patients, the serum platelet-associated IgG titers in the HCV-positive patients were significantly higher than those in the HCV-negative patients, suggesting that peripheral destruction of platelets might also contribute to thrombocytopenia, especially in HCV-positive HD patients (8). Indeed, HD patients (n=176) had significantly lower platelet counts than the patients who did not require HD (n=1,059) (178±69 vs. 208±62×103/μL, p<0.001), and the HCV-positive HD patients (n=18) had a significantly lower platelet counts than HCV-negative HD patients (n=158) (144±51 vs. 182±70×103/μL, p=0.030) among the our study population.
Based on the results of the multivariate logistic regression analysis in this study, moderate thrombocytopenia at baseline was tightly associated with access site-related bleeding complications, but mild to moderate thrombocytopenia at baseline (mean platelet count 122,000/μL) was not. The only available previous report (from Canadian investigators) also indicated that there was no significant relationship between baseline thrombocytopenia (mean platelet count, 124,000/μL) and in-hospital major bleeding events in patients undergoing elective PCI (2). In contrast, according to the present ROC curve analysis, despite the small AUC and low sensitivity, a baseline platelet count level of 159,000/μL was the cut-off value for predicting access site-related bleeding complications; this was nearly consistent with the lower limit of the normal platelet range. Taken together, it is reasonable to propose that moderate thrombocytopenia at baseline is a risk factor for access site-related bleeding complications, but that mild thrombocytopenia is not a conclusive risk factor.
From the point of view of hemorrhagic complications, antiplatelet therapy for CAD patients with thrombocytopenia remains a matter of debate. Contrary to the supposition, patients with moderate thrombocytopenia had a higher frequency of thienopyridine therapy or DAPT as a preprocedural treatment in comparison to patients with mild thrombocytopenia, while patients with mild to moderate thrombocytopenia received these medications less frequently in comparison to patients with a normal platelet count. Patients with moderate thrombocytopenia are considered to have not only a risk for bleeding complications, but also stent thrombosis-risk profiles, such as diabetes mellitus or HD (9, 10). Thus, the early discontinuation of DAPT after stent-based PCI might be inappropriate for patients with moderate thrombocytopenia, and physicians in charge might tend to check the durability of thienopyridine or DAPT tolerance among patients with moderate thrombocytopenia prior to the performance of elective PCI. Moreover, in the present study population, the preprocedural administration of DAPT or triple therapy was not tightly associated with access site-related bleeding complications in the univariate analysis. Taking the in-hospital mortality and angiographic success rates into consideration, DAPT usage might be acceptable for PCI-treated patients with mild to moderate thrombocytopenia, at least during the short term.
The safety and feasibility of DES implantation in patients with thrombocytopenia remains to be elucidated. A recent report proposed that the feasible elective PCI strategies might differ according to the magnitude of preprocedural thrombocytopenia, with the possible use of DESs or BMSs in patients with mild thrombocytopenia, BMSs in patients with moderate thrombocytopenia, and stent-less PCI in patients with severe thrombocytopenia (platelet count <50,000/μL) (11). Indeed, patients with moderate thrombocytopenia had a higher prevalence of BMS usage than patients with mild thrombocytopenia in the present study population. However, as shown in the present report, patients with thrombocytopenia are likely to have ISR-risk profiles, such as diabetes mellitus or chronic renal insufficiency/HD (2-6, 12), and even patients with moderate thrombocytopenia might therefore require DES implantation in real-world clinical practice. In the case of patients with severe thrombocytopenia, stent-less PCI is theoretically ideal, and transradial stent-less PCI using rotational atherectomy followed by drug-coated balloon dilation might be a revascularization therapy of choice (13, 14).
As far as access site-related bleeding complications are concerned, the advantages of a transradial approach over a transfemoral/transbrachial approach and that of 6-Fr GC over 7-Fr/8-Fr GC have already been established (15, 16). In the present report, the higher frequency of HD might account-at least in part-for the greater prevalence of the transfemoral approach in patients with mild-moderate thrombocytopenia. On the other hand, a thrombocytopenia severity-dependent increase in access site-related bleeding complications was observed in patients undergoing transradial PCI, but not in those undergoing transfemoral PCI. The number of patients undergoing transfemoral PCI was very small in comparison to the number undergoing transradial PCI; however, there is a possibility that other multiple factors, such as the method used to achieve hemostasis, peripheral artery disease, HD, anticoagulant therapy, obesity, the use of a larger guiding catheter, and female sex, rather than thrombocytopenia itself might play important roles in the pathogenesis of access site-related bleeding complications in patients undergoing transfemoral PCI.
Study limitations
The present study was associated with some limitations. First, this was a retrospective single-center observational study with a relatively small patient population. Second, we did not have detailed data regarding the etiology of thrombocytopenia, loading administration of thienopyridine derivatives before PCI, loading/additional administration of antiplatelet agents during/after PCI, platelet function tests, the amount of heparin used during PCI, or activated clotting time during and immediately after PCI, which may have affected PCI and in-hospital outcomes. Third, “elective PCI for de novo lesion” was not distinguished from “ad-hoc PCI for new lesion or ISR at the time of follow-up CAG.” Fourth, we did not have enough data regarding time between the completion of PCI (or the time of sheath removal) and the bleeding time. Finally, there were no data on the methods that were used to achieve hemostasis during transfemoral or transbrachial PCI.
Conclusion
The present study suggests that among patients with mild to moderate thrombocytopenia at baseline, elective PCI might be feasible and effective in the short term; however, more attention should be paid to access site-related bleeding complications, particularly in patients with moderate thrombocytopenia at baseline. The small sample size and short in-hospital follow-up period of our report are major limitations, and a larger study with a longer follow-up period should be performed to confirm our findings.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Levine GN, Bates ER, Bittl JA, et al. . 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation 134: e123-e155, 2016. [DOI] [PubMed] [Google Scholar]
- 2.Overgaard CB, Ivanov J, Seidelin PH, Todorov M, Mackie K, Dzavík V. Thrombocytopenia at baseline is a predictor of inhospital mortality in patients undergoing percutaneous coronary intervention. Am Heart J 156: 120-124, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Hakim DA, Dangas GD, Caixeta A, et al. . Impact of baseline thrombocytopenia on the early and late outcomes after ST-elevation myocardial infarction treated with primary angioplasty: analysis from the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial. Am Heart J 161: 391-396, 2011. [DOI] [PubMed] [Google Scholar]
- 4.Raphael CE, Spoon DB, Bell MR, et al. . Effect of preprocedural thrombocytopenia on prognosis after percutaneous coronary intervention. Mayo Clin Proc 91: 1035-1044, 2016. [DOI] [PubMed] [Google Scholar]
- 5.Yadav M, Généreux P, Giustino G, et al. . Effect of baseline thrombocytopenia on ischemic outcomes in patients with acute coronary syndromes who undergo percutaneous coronary intervention. Can J Cardiol 32: 226-233, 2016. [DOI] [PubMed] [Google Scholar]
- 6.Ito S, Watanabe H, Morimoto T, et al. . Impact of baseline thrombocytopenia on bleeding and mortality after percutaneous coronary intervention. Am J Cardiol 121: 1304-1314, 2018. [DOI] [PubMed] [Google Scholar]
- 7.Austen WG, Edwards JE, Frye RL, et al. . A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 51: 5-40, 1975. [DOI] [PubMed] [Google Scholar]
- 8.Ando M, Iwamoto Y, Suda A, Tsuchiya K, Nihei H. New insights into the thrombopoietic status of patients on dialysis through the evaluation of megakaryocytopoiesis in bone marrow and of endogenous thrombopoietin levels. Blood 97: 915-921, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Kimura T, Morimoto T, Kozuma K, RESTART Investigators, et al. . Comparisons of baseline demographics, clinical presentation, and long-term outcome among patients with early, late, and very late stent thrombosis of sirolimus-eluting stents: Observations from the Registry of Stent Thrombosis for Review and Reevaluation (RESTART). Circulation 122: 52-61, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Kimura T, Morimoto T, Nakagawa Y, j-Cypher Registry Investigators, et al. . Very late stent thrombosis and late target lesion revascularization after sirolimus-eluting stent implantation: five-year outcome of the j-Cypher Registry. Circulation 125: 584-591, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Morici N, Cantoni S, Savonitto S. Antiplatelet therapy for patients with stable ischemic heart disease and baseline thrombocytopenia: ask the hematologist. Platelets 25: 455-460, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Rathore S, Terashima M, Katoh O, et al. . Predictors of angiographic restenosis after drug eluting stents in the coronary arteries: contemporary practice in real world patients. EuroIntervention 5: 349-354, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Shiraishi J, Ohshiro M, Matsubara Y, et al. . Optical frequency domain imaging-guided rotational atherectomy followed by drug-coated balloon dilation to the non-calcified lesion in a patient with severe thrombocytopenia. Cardiovasc Interv Ther 33: 395-397, 2018. [DOI] [PubMed] [Google Scholar]
- 14.Shiraishi J, Koshi N, Matsubara Y, et al. . Stent-less percutaneous coronary intervention using rotational atherectomy and drug-coated balloon: a case series and a mini review. Cardiovasc Revasc Med 19: 705-711, 2018. [DOI] [PubMed] [Google Scholar]
- 15.Valgimigli M, Gagnor A, Calabró P, MATRIX Investigators, et al. . Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet 385: 2465-2476, 2015. [DOI] [PubMed] [Google Scholar]
- 16.Metz D, Meyer P, Touati C, et al. . Comparison of 6F with 7F and 8F guiding catheters for elective coronary angioplasty: results of a prospective, multicenter, randomized trial. Am Heart J 134: 131-137, 1997. [DOI] [PubMed] [Google Scholar]