Abstract
We encountered a case of syndrome of inappropriate antidiuretic hormone secretion (SIADH) caused by duloxetine, serotonin and norepinephrine reuptake inhibitor (SNRI). A 74-year-old woman complaining of severe lethargy was transferred to our emergency department. Her serum sodium level was 109 mEq/L. Plasma hypo-osmolality with urine normo-osmolality was observed, indicating SIADH. Her essential hypertension had long been treated with telmisartan, and she had just started duloxetine 20 mg/day for chronic musculoskeletal pain 4 days prior to admission. On prescribing duloxetine in the primary care setting, clinicians should be aware of the possibility of duloxetine-induced hyponatremia, particularly in combination with telmisartan.
Keywords: hyponatremia, SIADH, duloxetine, chronic musculoskeletal pain, primary care setting
Introduction
Duloxetine is the first dual serotonin and noradrenaline re-uptake inhibitor (SNRI) indicated for chronic musculoskeletal pain (1). Recently, more and more physicians have been prescribing this medicine to elderly patients (2). However, many doctors are unaware that duloxetine can cause life-threating hyponatremia as a side-effect (3). This also, means that many medications potentially inducing hyponatremia may be prescribed along with duloxetine. Evidence surrounding the combination of this new analgesic agent with other drug is insufficient at present.
We herein report the first case of the combination of duloxetine and telmisartan causing severe and rapid-onset hyponatremia for a 74 year-old woman, 4 days after starting her prescription.
Case Report
A 74-year-old woman (154 centimeters in height, weighing 63 kg) with a history of hypertension was transferred to the emergency department of our hospital by emergency services because of severe lethargy at home. She had taken telmisartan 40 mg/day and benidipine hydrochloride 8 mg/day for hypertension for several years and had just started duloxetine 20 mg/day for chronic musculoskeletal pain 4 days prior to the emergency admission, as prescribed by her regular attending physician. She was not taking any diuretic. She did not have any other medical or particular family history that might imply a hereditary disease.
On arrival, she was complaining of headache and nausea but had not vomited. Her vital signs were in the normal range (blood pressure, 143/79 mmHg; heart rate, 66 bpm; respiratory rate, 18/min; oxygen saturation on ambient air, 99%, and body temperature, 36.5℃). A physical examination revealed no conjunctival pallor, indicating no anemia. Her thyroid was not palpable; neither crackles in the lungs nor heart murmurs were audible. There were no physical signs of systemic edema or dehydration. A neurological examination revealed no obvious muscle weakness, or any sensory abnormalities or abnormalities of deep tendon reflex. However, her chief complaint was severe lethargy, which made her unable to walk by herself. An emergent laboratory evaluation indicated the following serum sodium of 110 mEq/L, potassium 3.8 mEq/L, chloride 73 mEq/L, BUN 11.4 mg/dL, and creatinine 0.6 mg/dL. Her plasma osmolality was 230 mOsm/kg・H20, whereas urine osmolality was 493 mOsm/kg・H20. Urine sodium was found to be 59 mEq/L, and the plasma antidiuretic hormone (ADH) concentration was 2.1 pg/mL severe hyponatremia. Thyroid-stimulating hormone (TSH), free T3, and free T4 levels were found to be normal at 2.3 μIU/mL, 2.7 pg/mL, and 1.7 ng/dL, respectively. Computed tomography (CT) of her brain and chest revealed no abnormalities. Her level of serum sodium continued to drop, reaching 109 mEq/L after the infusion of 500 mL intravenous saline.
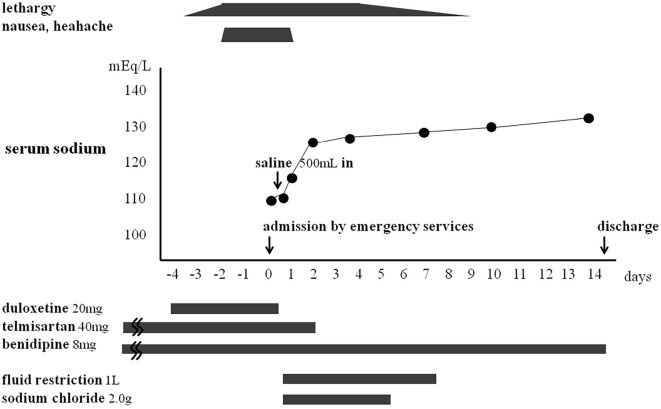
The patient was restricted to 1 L of water per day for 7 days and administered 2.0 g/day sodium chloride for 5 days. The duloxetine was discontinued immediately, and we stopped telmisartan on the third day of admission. Her serum sodium slowly increased from 109 mEq/L to 130 mEq/L over 10 days. She attained a full recovery without any complications and was discharged 14 days after admission (Figure).
Figure.
The patient’s clinical course. The change in the patient’s serum sodium levels in relation to duloxetine administration. Severe hyponatremia occurred 4 days after the initiation of duloxetine, and resolved 10 days after its discontinuation.
Discussion
We encountered an elderly patient who developed hyponatremia most likely related to SIADH caused by duloxetine. SIADH is defined by hyponatremia and hypo-osmolality resulting from the inappropriate continued secretion or action of ADH despite a normal or increased plasma volume, which results in impaired water excretion (4). In our patient, severe hyponatremia, plasma hypo-osmolality, urine normo-osmolality, and measurable levels of plasma ADH indicated SIADH.
There are four main categories of differential diagnoses for euvolemic hyponatremia (5). First, complication due to diuretics other medications should be considered. However, our patient had never been on other medications that result in SIADH aside from the duloxetine and telmisartan. Second, thyroid dysfunction and adrenal insufficiency should be considered, but this possibility was also dismissed because of her present illness and laboratory findings. Third, traumatic brain injury and brain tumor were should be considered, but we were able to rule them out based on her history and brain CT findings. Fourth, lung cancer and certain other types of cancer should also be considered. However, these were unlikely because of her normal chest CT findings. Her symptoms associated with hyponatremia emerged just 4 days after the initiation of duloxetine treatment and resolved with its discontinuation subsequent conservative care. Therefore, we concluded that she was suffering from the duloxetine and telmisartan-induced SIADH.
SIADH accounts for nearly 60% of cases of hyponatremia among geriatric ambulatory outpatients (6). One of the most common causes of SIADH is medications such as diuretics, antiepileptics, antipsychotics, and antidepressants. A case control study found that serotonergic antidepressants, such as selective serotonin reuptake inhibitors (SSRIs) and SNRIs increase the risk of hyponatremia 5.6-fold in elderly patients (7). According to the data of the French National Pharmacovigilance Database, a comparison of the incidence rates from spontaneous reports indicated a greater risk of hyponatremia with duloxetine than with other serotonergic antidepressants (8). Animal experiments showed that both serotonin and norepinephrine stimulate ADH secretion (9). Thus, duloxetine causes SIADH by increasing ADH secretion via the stimulation of serotonergic and norepinephrinergic receptors in the hypothalamus.
The following three characteristic features constitute the learning points of our case. First, female sex seems to be a particular risk factor for duloxetine-induced SIADH (10). Previous case reports have noted that most cases of duloxetine-induced SIADH occur in women (11-13). The effectiveness of duloxetine for chronic musculoskeletal pain is known to differ between men and women (14), and women are more likely than men to achieve pain reduction with the same dose of duloxetine. Furthermore, in an animal study, a sex-specific difference in the diuretic response to a water load caused by collecting duct nitrous oxide was noted (15). Second, this was the first report to declare the possibility of a bad combination between telmisartan and duloxetine. Angiotensin II type 2 receptor blockers inhibit aldosterone from causing renal tubules to increase the reabsorption of sodium. There is a high probability that this mechanism causes hyponatremia as a side effect. One previous study reported that angiotensin II type 2 receptor blockers increase the risk of hyponatremia 4.097-fold (16). Angiotensin II type 2 receptors also show sex-related differences in their expression, likely due to sexual dimorphism in the physiological mechanisms that regulate the renin-angiotensin system and sodium level (17). Previous studies have warned that duloxetin should not be used in combination with CYP1A2 and CYP2D6 (18, 19) However, telmisartan is not related to CYP metabolism, and benidipine hydrochloride is metabolized with CYP3A4. This result may suggest that no angiotensin-converting enzyme inhibitors or angiotensin II type 2 receptor blockers should be prescribed alongside duloxetine. Third, old age is a risk factor for hyponatremia induced by duloxetine (20), as elderly patients tend to develop hyponatremia due to a reduced renal function, increased level of antidiuretic hormone, and polypharmacy (21).
Treatment of symptomatic hyponatremia is guided by the level of clinical severity. The rapid onset of marked hyponatremia with unconsciousness may induce life-threatening encephalopathy, which requires an emergent infusion of intravenous hypertonic (3%) saline to reverse acute cerebral edema (22). However, sub-acute or chronic hyponatremia presenting with headache, nausea, and lethargy requires gradual correction (10, 11). Current guidelines call for a rate of correction with a maximal increase in sodium of 8-10 mEq/L in the first 24 hours to avoid complications of osmotic demyelination syndrome or central pontine myelinolysis (23). Conservative treatment of drug-induced SIADH includes removing the inciting agent and restricting fluids.
Our patient was successfully treated by the discontinuation of duloxetine and fluid restriction with salt intake. In addition, we stopped telmisartan because it affects the renin-angiotensin system, resulting in sodium depletion. Fluid restriction is considered the first-line therapy according to the recent published guidelines (23). However, it is ineffective or unfeasible in some patients with SIADH. Our patient showed a gradual improvement on her serum sodium level with fluid restriction, but did not recover to the normal level.
Tolvaptan therapy was recently reported able to improve and maintain the sodium level in an elderly SIADH patient (24). This drug can efficiently reverse the antidiuretic effect of ADH by competitively binding to vasopressin V2 receptors, thereby increasing the free water clearance and elevating the plasma sodium level. Vasopressin V2 receptor antagonists might therefore be useful as a therapeutic agent for the treatment of chronic dilutional hyponatremia caused by SIADH in the future (25).
Duloxetine is an SNRI, used mainly to treat major depressive disorder. However, duloxetine has recently been indicated for not only neuropathic pain disorders, such as diabetic neuropathy or fibromyalgia, but also the management of chronic musculoskeletal pain, as it helps inhibit pain signals by activating the descending pain inhibitory pathways (26). Duloxetine is increasingly being prescribed by doctors who are not familiar with psychopharmacology in the primary care setting (3). As elderly patients are easily predisposed to hyponatremia due to multiple factors, the use of SNRI may be more likely to aggravate hyponatremia in these patients than in younger ones. Doctors who prescribe duloxetine for chronic musculoskeletal pain should weigh the benefits against the side effects. Clinicians should be encouraged to monitor the serum sodium level, especially a few days after the prescription of duloxetine.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Howard SS, Eric JS, Benjamin RS. Duloxetine in the management of chronic musculoskeletal pain Smith. Ther Clin Risk Manag 8: 267-277, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gahimer J, Wernicke J, Yalcin I, Ossanna MJ, Wulster-Radcliffe M, Viktrup L. A retrospective pooled analysis of duloxetine safety in 23,983 subjects. Curr Med Res Opin 23: 175-184, 2007. [DOI] [PubMed] [Google Scholar]
- 3. Nagamine T. Responsibility of the doctor who prescribes serotonin and noradrenaline reuptake inhibitors for patients with chronic musculoskeletal pain. Psychiatry Clin Neurosci 72: 45-46, 2018. [DOI] [PubMed] [Google Scholar]
- 4. Esposito P, Piotti G, Bianzina S, Malul Y, Dal Canton A. The syndrome of inappropriate antidiuresis: pathophysiology, clinical management and new therapeutic options. Nephron Clin Pract 119: c62-c73, 2011. [DOI] [PubMed] [Google Scholar]
- 5. Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med 343: 886-887, 2000. [Google Scholar]
- 6. Miller M, Hecker MS, Friedlander DA, Carter JM. Apparent idiopathic hyponatremia in an ambulatory geriatric population. J Am Geriatr Soc 44: 404-408, 1996. [DOI] [PubMed] [Google Scholar]
- 7. Kirby D, Harrigan S, Ames D. Hyponatraemia in elderly psychiatric patients treated with Selective Serotonin Reuptake Inhibitors and venlafaxine: a retrospective controlled study in an inpatient unit. Int J Geriatr Psychiatry 17: 231-237, 2002. [DOI] [PubMed] [Google Scholar]
- 8. Revol R, Rault C, Polard E, Bellet F, Guy C. Hyponatremia associated with SSRI/NRSI: Descriptive and comparative epidemiological study of the incidence rates of the notified cases from the data of the French National Pharmacovigilance Database and the French National Health Insurance. Encephale 44: 291-296, 2018. [DOI] [PubMed] [Google Scholar]
- 9. Iovino M, Steardo L. Effect of substances influencing brain serotonergic transmission on plasma vasopressin levels in the rat. Eur J Pharmacol 113: 99-103, 1985. [DOI] [PubMed] [Google Scholar]
- 10. Kulkarni M. Duloxetine induced hyponatremia. Indian J Nephrol 25: 259, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nakamura M, Satoh Y, Hiraoka A, Fujita Y, Nagamine T. Duloxetine-induced hyponatremia in the elderly. Clin Neuropsychopharmacol Ther 3: 33-36, 2012. [Google Scholar]
- 12. Amoako AO, Brown C, Riley T. Syndrome of inappropriate antidiuretic hormone secretion: a story of duloxetine-induced hyponatraemia. BMJ Case Rep 2015: 208037, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Siegel AJ, Forte SS, Bhatti NA, Gelda SE. Drug-related hyponatremic encephalopathy: rapid clinical response averts life-threatening acute cerebral edema. Am J Case Rep 17: 150-153, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alev L, Fujikoshi S, Yoshikawa A, et al. Duloxetine 60 mg for chronic low back pain: post hoc responder analysis of double-blind, placebo-controlled trials. J Pain Res 10: 1723-1731, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gao Y, Stuart D, Pollock JS, Takahishi T, Kohan DE. Collecting duct-specific knockout of nitric oxide synthase 3 impairs water excretion in a sex-dependent manner. Am J Physiol Renal Physiol 311: F1074-F1083, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Correia L, Ferreira R, Correia I, et al. Severe hyponatremia in older patients at admission in an internal medicine department. Arch Gerontol Geriatr 59: 642-647, 2014. [DOI] [PubMed] [Google Scholar]
- 17. Hilliard LM, Mirabito KM, Denton KM. Unmasking the potential of the angiotensin AT2 receptor as a therapeutic target in hypertension in men and women: what we know and what we still need to find out. Clin Exp Pharmacol Physiol 40: 542-550, 2013. [DOI] [PubMed] [Google Scholar]
- 18. Knadler MP, Lobo E, Chappell J, Bergstrom R. Duloxetine: clinical pharmacokinetics and drug interactions. Clin Pharmacokinet 50: 281-294, 2011. [DOI] [PubMed] [Google Scholar]
- 19. Wernicke JF, Gahimer J, Yalcin I, Wulster-Radcliffe M, Viktrup L. Safety and adverse event profile of duloxetine. Expert Opin Drug Saf 4: 987-993, 2005. [DOI] [PubMed] [Google Scholar]
- 20. Kirby D, Ames D. Hyponatraemia and selective serotonin re-uptake inhibitors in elderly patients. Int J Geriatr Psychiatry 16: 484-493, 2001. [DOI] [PubMed] [Google Scholar]
- 21. Ganguli A, Mascarenhas RC, Jamshed N, Tefera E, Veis JH. Hyponatremia: incidence, risk factors, and consequences in the elderly in a home-based primary care program. Clin Nephrol 84: 75-85, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cuesta M, Garrahy A, Thompson CJ. SIAD: practical recommendations for diagnosis and management. J Endocrinol Invest 39: 991-1001, 2016. [DOI] [PubMed] [Google Scholar]
- 23. Siegel AJ. Hyponatremia in psychiatric patients: update on evaluation and management. Harv Rev Psychiatry 16: 13-24, 2008. [DOI] [PubMed] [Google Scholar]
- 24. Foppiani L. SIADH with severe hyponatremia in an elderly man with herpes zoster infection: a causal or casual association? Intern Med 57: 3393-3398, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Palmer BF. The role of V2 receptor antagonists in the treatment of hyponatremia. Electrolyte Blood Press 11: 1-8, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pergolizzi JV Jr, Raffa RB, Taylor R Jr, Rodriguez G, Nalamachu S, Langley P. A. Review of duloxetine 60 mg once-daily dosing for the management of diabetic peripheral neuropathic pain, fibromyalgia, and chronic musculoskeletal pain due to chronic osteoarthritis pain and low back pain. Pain Pract 13: 239-252, 2013. [DOI] [PubMed] [Google Scholar]