Abstract
A 66-year-old man was admitted to our hospital because of multiple refractory skin ulcers. Based on his severe systemic arterial calcification and severe calcium-phosphate imbalance due to severe kidney dysfunction, we initially considered calciphylaxis. However, a skin biopsy provided a diagnosis of cholesterol crystal embolization. Although we initiated hemodialysis, steroid treatment, and low-density lipoprotein-cholesterol apheresis, he died of multiple intestinal perforation. An autopsy showed cholesterol crystals occluding multiple organ arterioles. This case suggests that skin ulcers in patients with chronic kidney disease may be an important diagnostic hallmark and may be associated with several serious diseases.
Keywords: arteriolosclerosis, cholesterol crystal embolism, calciphylaxis, chronic kidney disease, multiple intestinal perforation, skin ulcer
Introduction
Skin disease is a common complication for patients with chronic kidney disease (CKD) (1), especially for dialysis patients (2). CKD patients tend to have peripheral vascular diseases mainly because of calcification and CKD mineral and bone disorders, which can prolong skin remodeling and worsen the skin disease (3). CKD patients are also in a chronic inflammatory state and are relatively immunodeficient (4), and their skin diseases can easily become infectious (5). Furthermore, skin diseases in CKD patients often occur as an aspect of systemic diseases, such as arteriosclerosis, cholesterol crystal embolism (CCE), microbiome infection, and collagen disturbances (6), which often develop multiple lethal complications. To avoid these complications, the treatment of the skin and systemic management, including fluid control, mineral metabolism, and dietary management, is important for CKD patients (6). Unfortunately, there are no systematic diagnostic criteria or treatment guidelines for skin ulcers in CKD patients, and the survival rate for CKD patients is rather low compared with the general population (6, 7).
We herein report a case of a severe refractory skin ulcers with instant development of renal failure and lethal gastrointestinal perforation that was subsequently diagnosed as CCE by an autopsy. We emphasize the difficulties in making a definitive diagnosis of skin diseases, as CKD patients often have multiple systemic complications, some of the symptoms of which are not particular for their diagnosis.
Case Report
A 66-year-old man with a severe skin ulcer on his lower limb was admitted to our hospital. He had received routine health checkups for years because of hypertension and hyperlipidemia since his 40s, although he refused treatments until his skin ulcer appeared. He had a family history of hypertension and cerebral infarction but no family history of CKD. The skin ulcer first appeared on his left lower limb four years prior to admission and was treated at a local dermatology clinic. The subject was also previously diagnosed with hypertension (blood pressure approximately 150/80 mmHg) and CKD (estimated glomerular filtration of approximately 30 mL/min/1.73 m2). Based on his history of hypertension and CKD, he was clinically diagnosed with benign nephrosclerosis, and he started anti-hypertensive medication.
Despite years of treatment of his skin ulcer with steroid ointments, antibiotics, and partial debridement with repeated biopsies, the ulcer did not reach complete remission, although biopsy results showed only non-specified inflammatory changes. Two years prior to admission, he received left lower limb varicose vein ablation without any complications. At that time, his C-reactive protein (CRP) and eosinophil counts remained at 2-4 mg/dL and 1,000-1,500/mm3, respectively, but his skin ulcer had been in partial remission. Five months prior to admission, his ulcer started to expand, with worsening pain and increased analgesic use. He was eventually referred to a local hospital for intensive treatment as the ulcer expanded rapidly and the pain became untreatable. At the local hospital admission, he exhibited severe kidney dysfunction, with a serum creatinine level of 7.9 mg/dL. Based on the requirement for renal replacement therapy and multidisciplinary treatment by a dermatologist, he was transferred to our hospital (day 0).
On admission, his height was 170.0 cm, weight was 70.9 kg, body mass index was 24.5 kg/m2, blood pressure was 130/80 mmHg, and heart rate was 86 beats/min in regular rhythm. There was no evidence of heart murmur or vascular murmur at his cervical and abdomen levels. The dorsal arteries in both feet were palpable. Multiple skin ulcers were present bilaterally on his thigh, buttocks, hip, and back. The skin ulcers consisted of viscous pus and a necrotizing area with poor granulation (Fig. 1). The skin ulcers were also surrounded by partial cornification regions, indicative of sites that had previously cured, and mild cyanosis. He was on several medications including aspirin, atorvastatin, and various anti-hypertensive drugs.
Figure 1.
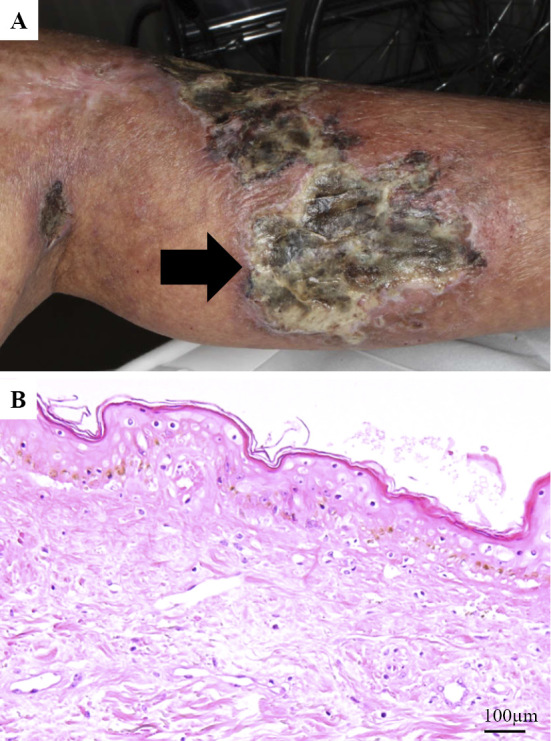
(A) Skin ulcer of the left lower thigh on admission day. (B) Hematoxylin and Eosin staining of the thigh skin ulcer.
His laboratory findings showed severe kidney dysfunction, and inflammation with particular elevation of eosinophils (Table). Cryoglobulins and most autoantibodies, including myeloperoxidase antineutrophil cytoplasmic antibody and proteinase 3 antineutrophil cytoplasmic antibody, were negative. We regarded his anti-double strand DNA antibodies as false positives, as he did not show any manifestation of systemic lupus erythematosus, and a repeated test after one month was negative with no immunosuppressant therapy. His coagulation examination showed high fibrinogen degradation products but a normal range of prothrombin time and activated partial thromboplastin time with no protein C or protein S deficiencies. Chest X ray showed no evidence of pleural effusion. His ankle-brachial index was 1.04 for his right limb, while we were unable to collect left limb data because of severe pain. Computed tomography (CT) showed severe calcification and shaggy thickening of his aortic wall. His lower limb artery had severe calcification, and contrast-enhanced CT revealed no complete occlusion of his peripheral arteries.
Table.
Laboratory Findings and Reference Ranges of Complete Blood Count, Biochemistry, Coagulation, Immunological Studies, and Urinalyses.
| <Complete blood cell count> | Reference Range, Male Adults | On admission, this patient |
|---|---|---|
| Hematocrit | 40.0-52.0 | 29.7 |
| Hemoglobin (g/dL) | 14.0-18.0 | 10.1 |
| White cell count (per mm3) | 4,000-9,000 | 7,700 |
| Differntial count (%) | ||
| Neutrophils | 28.0-68.0 | 77 |
| Lympocytes | 17.0-57.0 | 6.1 |
| Monocytes | 0.0-10.0 | 1.4 |
| Eosinophils | 0.0-10.0 | 15.4 |
| Platelet count (per mm3) | 150,000-350,000 | 179,000 |
| Erythrocyte count (per mm3) | 4,270,000-5,700,000 | 3,390,000 |
| <Coagulation> | Reference Range, Male Adults | On admission, this patient |
| Prothrombin time (sec) | >70.1 | 85.5 |
| International normalized ratio for prothrombin time | <1.15 | 1.06 |
| Activated partial thromboplastin time (sec) | <30 | 28.6 |
| Fibrin and fibrinogen degradation products (µg/mL) | <4.9 | 144.7 |
| Fibrinogen (mg/dL) | 200-400 | 456 |
| Protein C activation (%) | 74-132 | 86 |
| Protein S activation (%) | 64-135 | >151 |
| <Immnunological studies> | Reference Range, Male Adults | On admission, this patient |
| Immunogloblin G (mg/dL) | 870-1,700 | 1,501 |
| Immunogloblin A (mg/dL) | 110-410 | 356 |
| Immunogloblin M (mg/dL) | 35-220 | 55 |
| Complement C3 (mg/dL) | 65-135 | 88 |
| Complement C4 (mg/dL) | 13-35 | 26.5 |
| 50% hemolytic unit of complement (U/mL) | 31.6-57.6 | 59.8 |
| Anti-nucleus antibody (times) | <80 | <40 |
| Anti double strand DNA antibody (IU/mL) | <1.0 | 34.2 |
| Anti SS-A/Ro antibody (U/mL) | <1.0 | <1.0 |
| Anti SS-B/La antibody (U/mL) | <1.0 | <1.0 |
| Myeloperoxidase-anti-neutrophil cytoplasmic antibody (U/mL) | <1.0 | <1.0 |
| Proteinase3-anti-neutrophil cytoplasmic antibody (U/mL) | <1.0 | <1.0 |
| Rhumatoid factor (IgG) | negative | negative |
| Cryoglobulin | negative | negative |
| <Biochemical> | Reference Range, male adults | On admission, this patient |
| Sodium (mmol/L) | 136-145 | 131 |
| Potassium (mmol/L) | 3.5-5.1 | 3.7 |
| Chloride (mmol/L) | 98-107 | 93 |
| Urea nitrogen (mg/dL) | 8-20 | 100 |
| Creatinine (mg/dL) | 0.44-1.15 | 12.39 |
| Estimated glomerular filtration rate (mL/min/1.73m2) | >60 | 4 |
| Aspartate transaminase (IU/L) | 8-38 | 14 |
| Alanine transaminase (IU/L) | 4-43 | 12 |
| Lactate dehydrogenase (IU/L) | 124-222 | 609 |
| Alkalinephosphatase (IU/L) | 115-359 | 131 |
| Creatine phospokinase (IU/L) | 59-248 | 122 |
| Total protein (g/dL) | 6.7-8.1 | 6.8 |
| Albumin (g/dL) | 3.8-5.3 | 3.3 |
| Calcium (mg/dL) | 8.6-10.1 | 7.2 |
| Inorganic phosphorus (mg/dL) | 2.2-4.1 | 12.9 |
| Uric Acid (mg/dL) | 4.0-7.0 | 9.1 |
| C reactive protein (mg/dL) | 0.0-0.3 | 9 |
| Total cholesterol (mg/dL) | 130-220 | 151 |
| LDL cholesterol (mg/dL) | 0-139 | 73 |
| Triglycelyde(mg/dL) | 30-150 | 196 |
| Glucose (mg/dL) | 73-109 | 113 |
| Hemoglobin A1c (%) | 4.6-6.2 | 5.7 |
| Urinalysis | Reference range | On admission, this patient |
| Urine specfic gravity | 1.005-1.030 | 1.009 |
| Urine pH | 5.0-7.5 | 5 |
| Urine proteinuria cretinine ratio (g/gCr) | <0.3 | 0.89 |
| Urine red blood cell (per high power field) | <4 | <4 |
| Urinary casts (per high power field) | 1-9 epitherial casts |
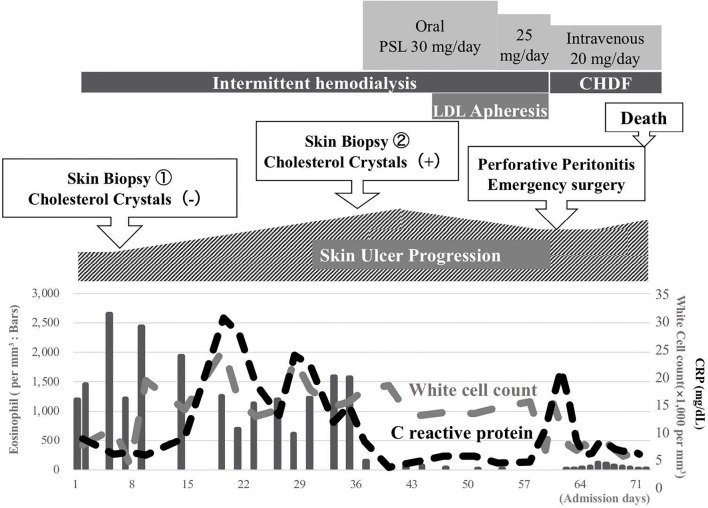
A timeline of events during his hospitalization is shown in Fig. 2. Because of his severe mineral disorders, a urine volume of 200-300 mL/day, and his uremic status, we initiated intermittent hemodialysis. On day 6, after three intermittent hemodialysis sessions, his platelet count decreased to 28,000/mm3. Since we suspected heparin-induced thrombocytopenia, we switched the anticoagulant for hemodialysis from heparin to nafamostat mesylate. His platelet count gradually improved to the normal range, though anti-heparin/platelet factor 4 antibody was negative. We performed a skin biopsy of his left thigh ulcer, although we were unable to diagnose his skin ulcer disease (Fig. 1B). We continued conservative therapy including partial debridement. However, his skin ulcer further expanded and worsened. As his skin ulcer became hemorrhagic, we withdrew aspirin. At three weeks after admission, livedo reticularis appeared on the tip of his toe, similar to ‘blue toe’ (Fig. 3A). A second skin biopsy was performed on day 33, and we found cholesterol crystals inside the arterioles of this toe skin. Finally, we diagnosed the subject with CCE. We initiated oral prednisolone treatment starting at 30 mg per day along with low-density lipoprotein apheresis combined with intermittent hemodialysis. His ulcer and complaints of pain gradually improved, with decreasing inflammatory serum markers.
Figure 2.
Clinical course of hospitalization. PSL: prednisolone, LDL: low density lipoprotein, CHDF: continuous hemodiafiltration, CRP: C reactive protein
Figure 3.
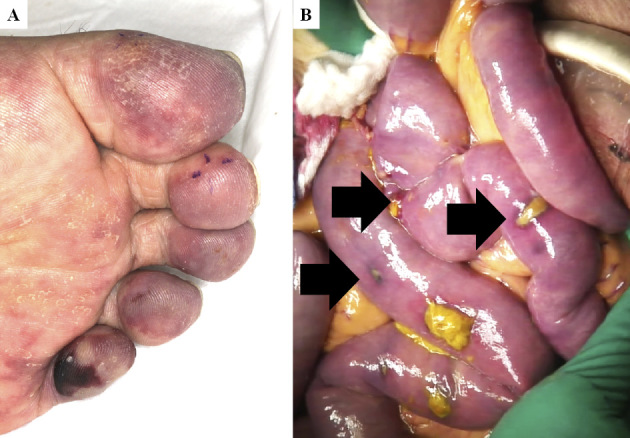
(A) Livedo reticularis and cyanosis of the left toe. (B) Surgical image of emergency ileectomy. Arrows show the multiple perforation sites.
However, on day 60 he complained of severe abdominal pain. A radiological examination revealed diffuse peritonitis because of gastrointestinal perforation. Emergency abdominal surgery was performed, and operative findings showed multiple perforations of necrotizing small intestinal ulcers over 120 cm starting from the ligament of Treitz, which were assumed to be caused by ischemia (Fig. 3B). The necrotized small intestine was resected, and an ileostomy was formed. Pathological findings showed multi-arteriole occlusion with sharp clefts, indicative of cholesterol crystals (Fig. 4A). He was transferred to the intensive-care unit, and we converted his renal replacement therapy to continuous hemodiafiltration. However, despite receiving multidiscipline treatment, he died on day 72.
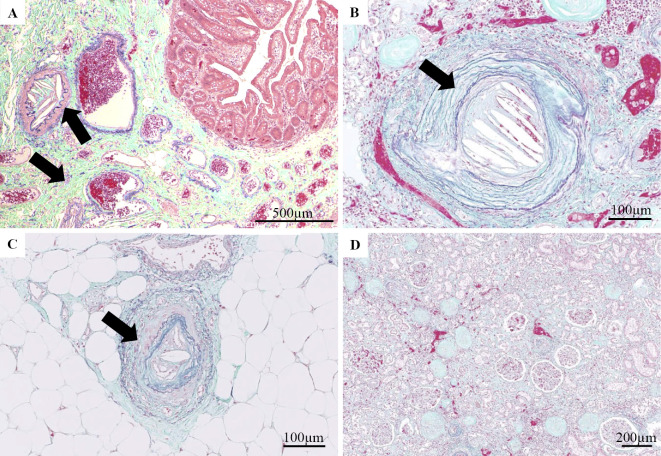
Figure 4.
(A) Surgical specimen of the small intestine resected on day 60. Cholesterol crystal clefts occluded arterioles (arrow). (B, D) Elastica Masson-Goldner (EMG) staining of the kidney from the autopsy specimen. (C) EMG staining of the toe skin from the autopsy specimen. Cholesterol crystal clefts occluding arterioles (arrow).
An autopsy showed atherosclerosis with moderate calcification of the aorta, and the bilateral internal iliac artery was occluded with thrombi. His bilateral kidneys were atrophic, with a distorted surface. Microscopic findings revealed that the small arteries of the kidney (Fig. 4B), skin of the toe (Fig. 4C), liver, spleen, and thyroid were occluded, with obliterated lumens containing numerous empty clefts, suggesting the presence of cholesterol crystals. These clefts were abundant at the atheroma of the aorta, suggesting that this was the origin of the multiple embolisms. His kidney specimen showed many glomeruli with global sclerosis, severe tubulointerstitial fibrosis with tubular atrophy, and severe arteriolosclerosis (Fig. 4D). However, we were unable to clearly diagnose his primary skin ulcer, as cholesterol crystals were absent in his thigh and surrounding arteries. Thus, the cause may be related to several different diseases.
Discussion
CCE is formed by diffusion of cholesterol atheroma debris from the great arteries and can be triggered by intraarterial interventions, including intracranial artery stenting and percutaneous coronary artery treatment (8, 9), as well as anticoagulant therapies (8, 10). However, there are some reports of ‘idiopathic’ cases that did not receive medication or arterial intervention (10, 11). Most of those cases had a history of severe atherosclerosis, and it is likely that the atheroma plaque was spontaneously dislodged following changes in the hemodynamics or coagulative state inside the great arteries (8, 10).
As atheroma can occlude all types of small arteries in all organs, the disease has many clinical manifestations. Previous reports have shown that the initial manifestations can develop in the brain (11), eye (12), kidney (10), gastrointestinal system (13, 14), and skin (1-5). Typical skin manifestations include skin livedo reticularis and cyanosis on the foot (15). Laboratory findings typically show elevation of the eosinophil count, kidney dysfunction, and elevation of inflammation markers, such as C-reactive protein (10, 15). Generally, the outcomes of CCE are improving worldwide, although it still has a poor renal survival rate, with 44-61% of CCE patients requiring dialysis, and a 1-year mortality rate of 13-64% (10). CCE cases with gastrointestinal manifestations also have an extremely poor prognosis, with a 50-90% mortality rate according to case reviews (14, 16). Statins and corticosteroids are the first-line treatment for CCE (8-10), while combination therapy with low-density lipoprotein apheresis and corticosteroids further improve the survival rate and renal outcome compared with corticosteroids alone (17). Withdrawal of any form of anticoagulation and forbidding any new radiologic or vascular surgery are highly recommended (15, 18). Although antiplatelets, such as aspirin, are thought to suppress platelet aggregation after cholesterol crystal lodging in blood vessels, such attempts in some studies have demonstrated no effects (18).
In the present case, CCE first presented as a skin manifestation, with the gradual development of multiple complications and fatal perforation of the small intestine within two months. To our knowledge, there have been no other reported cases of rapid progressive CCE. We have therefore termed this case one of ‘catastrophic’ CCE. We consider that this CCE patient had superimposed calciphylaxis. Skin ulcers were observed bilaterally on the thigh, buttocks, and back, which are atypical sites for CCE. Radiological and serological findings also showed severe CKD mineral and bone disorders with severe peripheral calcification. Thus, our initial diagnosis was calciphylaxis. Calciphylaxis is a skin disease that occurs in CKD patients with a high uremic state and severe calcium-phosphate imbalances (19). Calcium deposition is observed in the arteriole walls, causing arteriole obstruction, severe pain, and refractory wounds, as observed in our case. A study of Japanese calciphylaxis patients described three clinical features and one pathological feature that could be used to establish consensus criteria (20), all of which were noted in our case. The first-line therapy for calciphylaxis involves adequate dialysis and sodium thiosulfate treatment (19). Previous reports have also reported the successful treatment of calciphylaxis with corticosteroids (21), although corticosteroids are also considered a risk factor for calciphylaxis (19, 20). Taken together, these findings suggest that our therapies may have actually worsened the skin ulcer in our case, and the delay in an appropriate diagnosis was a major problem.
The obstruction of blood vessels was also a potential cause of the symptoms in our case. Given that the internal iliac artery in our patient was completely occluded by calcification, the ischemic state may have delayed the wound healing. Similar to a previously reported case of necrosis of the skin of the buttocks following internal iliac artery embolization for the treatment of massive bleeding (22), our case also had severe ulcers on the buttocks as well as the lower thigh. The blood supply to the thigh skin is derived from the femoral artery, a branch of the external iliac artery. CT images and an ankle-brachial index examination indicated the apparent preservation of the blood supply of the lower limb. However, the patient's severe pain induced a loss of appetite and malnutrition, which are unfavorable conditions for wound healing. Our case may also have had relatively vulnerable small blood vessels and chronic heart failure, which accelerates hypoperfusion of the skin. Indeed, the blood flow in his thigh skin was low enough to allow debris of cholesterol crystals to invade the arterioles of the proximal thigh, which may have caused occlusion, leading to necrosis and refractory skin ulcers of the thigh.
However, we lacked definitive evidence of ischemia of the thigh skin. Thus, the definitive diagnosis for the skin ulcer of his thigh remains unclear. Nevertheless, we concluded that the main pathophysiology was CCE rather than an ischemic state because specific treatment for CCE, such as steroids and plasma apheresis, was partially effective for the skin ulcer on the thigh. This case reminds us of the difficulties of diagnosing and treating skin ulcers of CKD patients, since the etiology is complicated by these patients' comorbidities.
Conclusion
We herein report a catastrophic case of CCE with rapid progression of multiple complications. Skin ulcers in CKD patients have multiple superimposing pathophysiologies and often show abnormal manifestations, making misdiagnoses common. Clinicians should be aware that skin ulcers in patients with CKD are an important diagnostic hallmark and may be associated with several serious diseases.
We explained the situation and obtained written consent to present these data from the patient's family at the time of performing the autopsy. All data, including images, were collected and handled in accordance with the guidelines of the Declaration of Helsinki.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Robles-Mendez JC, Vazquez-Martinez O, Ocampo-Candiani J. Skin manifestations of chronic kidney disease. Actas Dermosifiliogr 106: 609-622, 2015. [DOI] [PubMed] [Google Scholar]
- 2. Markova A, Lester J, Wang J, Robinson-Bostom L. Diagnosis of common dermopathies in dialysis patients: a review and update. Semin Dial 25: 408-418, 2012. [DOI] [PubMed] [Google Scholar]
- 3. Kato S, Chmielewski M, Honda H, et al. . Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol 3: 1526-1533, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Amdur RL, Feldman HI, Gupta J, et al. . Inflammation and progression of CKD: the CRIC study. Clin J Am Soc Nephrol 11: 1546-1556, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zoccali C, Vanholder R, Massy ZA, et al. . The systemic nature of CKD. Nat Rev Nephrol 13: 344-358, 2017. [DOI] [PubMed] [Google Scholar]
- 6. Kuypers DR. Skin problems in chronic kidney disease. Nat Clin Pract Nephrol 5: 157-170, 2009. [DOI] [PubMed] [Google Scholar]
- 7. Galperin TA, Cronin AJ, Leslie KS. Cutaneous manifestations of ESRD. Clin J Am Soc Nephrol 9: 201-218, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kronzon I, Saric M. Cholesterol embolization syndrome. Circulation 122: 631-641, 2010. [DOI] [PubMed] [Google Scholar]
- 9. Martin JB, Pache JC, Treggiari-Venzi M, et al. . Role of the distal balloon protection technique in the prevention of cerebral embolic events during carotid stent placement. Stroke 32: 479-484, 2001. [DOI] [PubMed] [Google Scholar]
- 10. Scolari F, Ravani P. Atheroembolic renal disease. Lancet 375: 1650-1660, 2010. [DOI] [PubMed] [Google Scholar]
- 11. Tashiro M, Ito K, Saito T. An autopsy case of idiopathic cholesterol embolism. Clin Exp Nephrol 17: 142-143, 2013. [DOI] [PubMed] [Google Scholar]
- 12. Iardino A, Garner O, Ramirez A, Lotta F. Cholesterol embolism: it's always a good idea to look into the eye. BMJ Case Rep 2017. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kawamura T, Amamiya K, Wada H, et al. . Multiple small intestinal ulcers associated with protein-losing enteropathy secondary to cholesterol crystal embolism: a case report. Nihon Shokakibyo Gakkai Zasshi 114: 1436-1445, 2017(in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 14. Ben-Horin S, Bardan E, Barshack I, Zaks N, Livneh A. Cholesterol crystal embolization to the digestive system: characterization of a common, yet overlooked presentation of atheroembolism. Am J Gastroenterol 98: 1471-1479, 2003. [DOI] [PubMed] [Google Scholar]
- 15. Meyrier A. Cholesterol crystal embolism: diagnosis and treatment. Kidney Int 69: 1308-1312, 2006. [DOI] [PubMed] [Google Scholar]
- 16. Shinozuka E, Yamada T, Kan H, et al. . A case of repeated small bowel perforations in a short period in a patient with cholesterol crystal embolism. Nihon Shokakibyo Gakkai Zasshi 113: 804-812, 2016(in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 17. Ishiyama K, Sato T, Yamaguchi T, Taguma Y. Efficacy of low-density lipoprotein apheresis combined with corticosteroids for cholesterol crystal embolism. Clin Exp Nephrol 21: 228-235, 2017. [DOI] [PubMed] [Google Scholar]
- 18. Scolari F, Tardanico R, Zani R, et al. . Cholesterol crystal embolism: a recognizable cause of renal disease. Am J Kidney Dis 36: 1089-1109, 2000. [DOI] [PubMed] [Google Scholar]
- 19. Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med 378: 1704-1714, 2018. [DOI] [PubMed] [Google Scholar]
- 20. Hayashi M. Calciphylaxis: diagnosis and clinical features. Clin Exp Nephrol 17: 498-503, 2013. [DOI] [PubMed] [Google Scholar]
- 21. Biswas A, Walsh NM, Tremaine R. A case of nonuremic calciphylaxis treated effectively with systemic corticosteroids. J Cutan Med Surg 20: 275-278, 2016. [DOI] [PubMed] [Google Scholar]
- 22. Al-Thunyan A, Al-Meshal O, Al-Hussainan H, Al-Qahtani MH, El-Sayed AA, Al-Qattan MM. Buttock necrosis and paraplegia after bilateral internal iliac artery embolization for postpartum hemorrhage. Obstet Gynecol 120: 468-470, 2012. [DOI] [PubMed] [Google Scholar]