Abstract
An 80-year-old man was found to have a reddish depressed lesion on the middle thoracic esophagus. The morphology of the lesion had been almost unchanged for 3 years, but it transformed to a 2-cm depressed lesion with elevated margins and an irregular nodular surface. The lesion was resected endoscopically and ultimately diagnosed as a combined neuroendocrine carcinoma and squamous cell carcinoma with submucosal invasion. The patient was additionally treated with chemoradiotherapy but died of the primary disease eight months after the initial treatment. It is important to elucidate the natural history of this disease at an early stage.
Keywords: neuroendocrine carcinoma, superficial esophageal cancer, natural history, endoscopic resection
Introduction
Early-stage neuroendocrine carcinoma (NEC) of the esophagus is rarely encountered because the disease is most often discovered at an advanced stage (1). Owing to this rarity, standard therapy has not been established. This disease is thought to be rapidly progressive and highly metastatic with a poor prognosis (2), but the natural history of the early-stage disease has been unclear.
We herein report a case of superficial NEC of the esophagus. The patient was observed with annual endoscopy for three years before the diagnosis and died within eight months of rapid disease progression after treatment.
Case Report
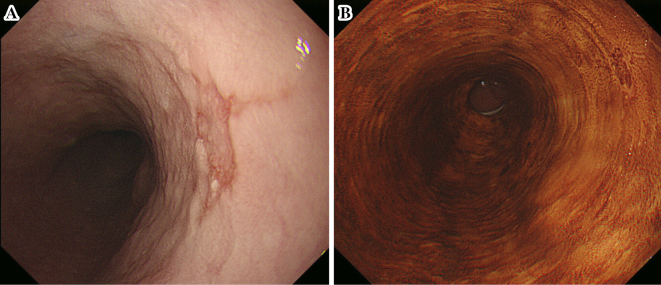
In 2012, an 80-year-old man with no history of smoking or alcohol consumption underwent surveillance endoscopy after total gastrectomy had been performed 15 years earlier for gastric cancer (pT2N0M0 according to the Union for International Cancer Control tumor/node/metastases classification for gastric cancer) (3). He had no symptoms and no family history. A reddish depressed lesion with unclear margins was found on the right wall of the middle thoracic esophagus, 28 cm from the incisors (Fig. 1A), and gastroesophageal reflux disease was not detected. The lesion stained weakly with iodine staining (Fig. 1B). A biopsy specimen showed no evidence of malignancy, and we diagnosed him with a benign lesion and recommended annual follow-up.
Figure 1.
Initial endoscopic findings in the lesion. (A) White-light imaging revealed a reddish depressed lesion with unclear margins on the right wall of the middle thoracic esophagus. (B) The lesion was weakly stained by iodine staining, and no areas were unstained.
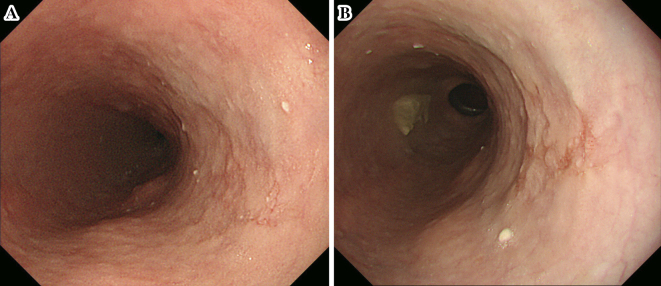
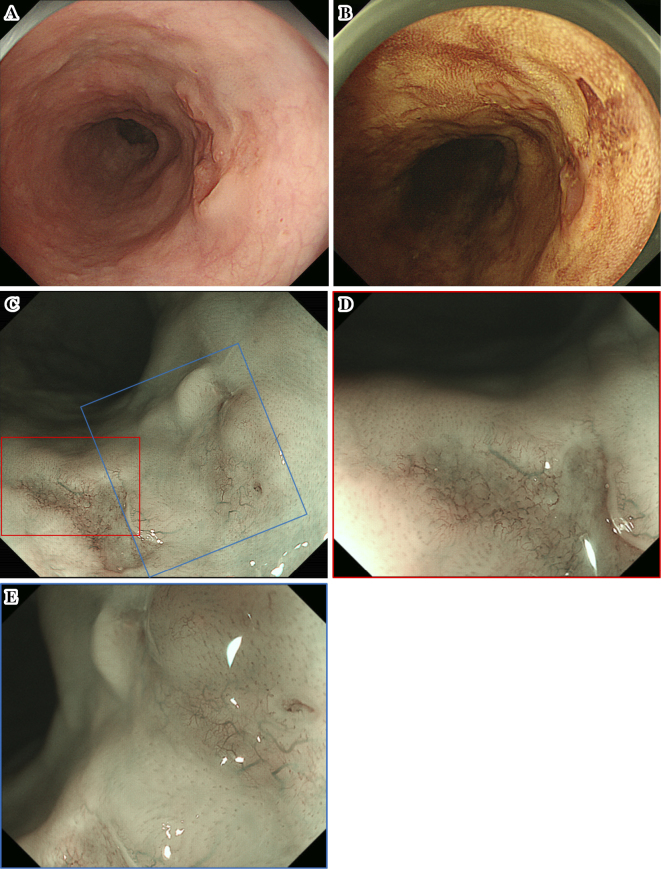
On subsequent endoscopy by white-light imaging in 2013 and again in 2014, although the mucosal unevenness of the lesion had become slightly stronger, the morphology of the lesion was almost unchanged with unclear margins at the same site (Fig. 2). A biopsy was not performed because we did not suspect the lesion to be neoplastic. However, endoscopy in 2015 showed that the lesion had transformed to a depressed lesion of approximately 2 cm with elevated margins and an irregular nodular surface (Fig. 3A). Iodine staining showed unstained and stained areas in the depressed area, which appeared to be partially covered by a non-neoplastic epithelium (Fig. 3B). The results of narrow-band imaging (NBI) showed that a portion of the depressed area was slightly brownish (Fig. 3C). Magnified endoscopy with NBI indicated the presence of irregular microvessels with plexiform microvessels and highly dilated irregular vessels (Fig. 3D and E). These microvessels were diagnosed as Type B3 and Type R based on the magnifying endoscopic classification of the Japan Esophageal Society (4). A biopsy specimen of this area indicated squamous cell carcinoma (SCC).
Figure 2.
Findings of the second and third endoscopic examinations. (A) The second endoscopy showed that the margins of the lesion remained unclear at the same site. (B) The third endoscopy showed no remarkable changes from the findings of the previous year.
Figure 3.
Findings of the fourth endoscopic examination. (A) White-light imaging showed that the lesion had developed elevated margins, and the central area had an irregular nodular surface. (B) Both stained and unstained areas were evident in the central area after iodine staining. (C) Narrow-band imaging (NBI) showed that a portion of the depressed area was slightly brownish. (D, E) Magnified endoscopy with NBI indicated the presence of irregular microvessels with plexiform microvessels and highly dilated irregular vessels.
Endoscopic ultrasound (20 MHz) showed a hypoechoic mass invading the third hyperechoic layer (submucosal layer). Computed tomography revealed neither lymph node nor distant metastasis. We diagnosed the patient with SCC, cT1bN0M0 according to the Union for International Cancer Control tumor/node/metastases classification for esophageal carcinoma (3) and recommended surgical resection. However, the patient declined surgery. The lesion was therefore resected en bloc with endoscopic submucosal dissection (ESD).
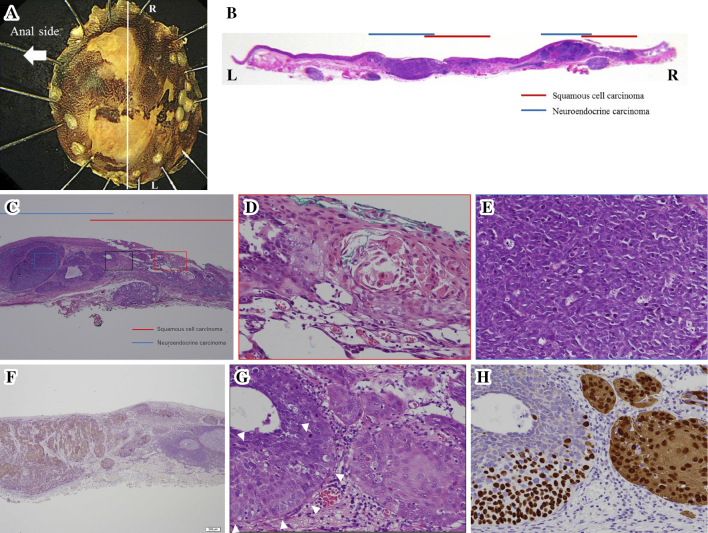
The gross findings of the resected specimen showed a nearly flat lesion with unclear margins. Iodine staining indicated unstained area in a part of the resected specimen (Fig. 4A). Microscopically, the tumor contained small round cells forming solid nests as well as SCC. The small round cells contained hyperchromatic nuclei and scant cytoplasm and were immunoreactive for CD56, chromogranin A, and synaptophysin, indicating that this tumor was NEC (Fig. 4B-F). In addition, lymphatic permeation by NEC was observed in the submucosa. The tumor that was stained by iodine staining was partially covered in non-neoplastic epithelium. The tumor was ultimately diagnosed as a combined NEC, small cell type, and SCC. The tumor was 22×15 mm, pT1b (invasive depth, 1,000 μm), ly1, v0, pHM0, pVM0.
Figure 4.
Histological findings of the resected specimen. (A) The specimen resected with endoscopic submucosal dissection. (B) The macroscopic appearance showed neuroendocrine carcinoma (NEC) mixed with squamous cell carcinoma (SCC). (C) The NEC and SCC components of the tumor were adjacent and partially intermingled. The tumor was partially covered by a non-neoplastic epithelium. (D) The SCC component observed in a high-power field. (E) The NEC component observed in a high-power field. (F) The NEC component was immunohistochemically positive for synaptophysin. (G) On Hematoxylin and Eosin staining, a transitional area of SCC and NEC was observed (white arrows). In the transitional area, SCC-like and NEC-like cells were observed. (H) p40 and CK14 staining. In the area of typical SCC, p40 was positive for nuclei, and CK14 was positive for cytoplasm. In the area of NEC, these were all negative. In the SCC-like region of the transition area, p40 was positive, but CK14 was negative.
On Hematoxylin and Eosin staining, in addition to the area of typical SCC and NEC, a transitional area of both histologies was observed (Fig. 4G). In the transitional area, SCC-like cells at the periphery and NEC-like cells in the center were observed. Immunohistologically, the area of typical SCC, p40 was positive for nuclei, while CK14 was positive for cytoplasm. In the area of NEC, these were all negative. In the SCC-like region of the transition area, p40 was positive, but CK14 was negative (Fig. 4H). These findings suggest that the NEC developed via dedifferentiation of SCC, as morphologically there is a transitional area between SCC and NEC, characterized by an immunohistochemically intermediate staining attitude.
The patient declined additional surgical resection. Chemoradiotherapy, consisting of cisplatin (80 mg/m2 on day 1), etoposide (80 mg/m2 on days 1-3), and 60 Gy of radiation, which is usually used for small cell carcinoma of the lung, was administered. However, positron emission tomography at three months after ESD revealed lymph node metastasis (No 106 rec R). The disease state gradually worsened without any additional treatment, and magnetic resonance imaging showed multiple liver metastases at six months after ESD. The patient died of the primary disease eight months after initial treatment.
Discussion
NECs of the esophagus are very rare, accounting for 0.5-5.9% of all esophageal cancers in Asia and 0.05-2.4% in Western countries (2, 5). Early-stage tumors are even rarer, with only a few case reports available. Therefore, the natural history of the early-stage disease is unknown. The optimal treatment strategy for early-stage NEC of the esophagus has not been established, although various combinations of endoscopic resection, surgery, radiotherapy, and chemotherapy have been described (6, 7). We encountered a rare case of superficial NEC of the esophagus. The patient was observed for three years before the initial treatment and died within eight months due to rapid disease progression.
The endoscopic diagnosis of this tumor at an early stage is critical. However, at present, the majority of cases are diagnosed at a late stage. In some cases of early-stage disease, the diagnosis may be missed based on the findings of endoscopy or an endoscopic biopsy (8). NEC at an early stage is generally a subepithelial growth and is frequently covered by non-neoplastic epithelium (9). In our patient, because the tumor was partially covered by non-neoplastic squamous epithelium, the initial biopsy specimen may have contained no tumor components. Therefore, we did not diagnose NEC of the esophagus at the initial biopsy and instead observed the lesion for more than three years before starting treatment.
Huang et al. (10) reported that NEC develops via dedifferentiation of SCC during the process of invasion. A serial histological examination of 42 primary high-grade NEC specimens of the esophagus indicated SCC in situ involvement in 50% of cases. In the present case, the presence of a transitional area between SCC and NEC supports this mechanism. In addition, the preoperative biopsy specimen was misdiagnosed as SCC, possibly due to the unique pathological features of this disease.
The study by Guanrei et al. in Chinese patients with esophageal cancer, mostly including cases of SCC that had gone untreated for various reasons and merely been observed, has provided substantial help in understanding the natural history of early esophageal cancer. The authors suggest that early esophageal cancer may remain a superficial early cancer for an extended period of time and that a natural history of 4-5 years is likely for the progression of carcinoma in situ to advanced cancer (11). In the present case, the morphology of the lesion had been almost unchanged for at least three years. In addition, there were no findings suggestive of submucosal invasion endoscopically, and the lesion might have remained a mucosal one for at least three years. Although it is difficult to determine the timing of the dedifferentiation to NEC retrospectively, the growth of mucosal cancer may be slow, even in cases of NEC, and submucosal invasion may ultimately increase the potential risk of malignancy.
To our knowledge, there are no previous reports of superficial NEC of the esophagus that were observed during the early stage of development. Thus, our case is exceptionally rare in elucidating the natural history of this disease at an early stage.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Tustumi F, Takeda FR, Uema RH, Pereira GL, Sallum RA, Cecconello I. Primary neuroendocrine neoplasm of the esophagus - report of 14 cases from a single institute and review of the literature. Arg Gastroenterol 54: 4-10, 2017. [DOI] [PubMed] [Google Scholar]
- 2. Maru DM, Khurana H, Rashid A, et al. Retrospective study of clinicopathologic features and prognosis of high-grade neuroendocrine carcinoma of the esophagus. Am J Surg Pathol 32: 1404-1411, 2008. [DOI] [PubMed] [Google Scholar]
- 3. James D, Mary K, Christian W. TNM Classification of Malignant Tumors. 8th ed. Wiley-Blackwell Sci Pub, Oxford, 2017: 66-78. [Google Scholar]
- 4. Oyama T, Monma K. A new classification of magnified endoscopy for superficial esophageal squamous cell carcinoma. Esophagus 8: 247-251, 2011. [Google Scholar]
- 5. Casas F, Ferrer F, Farrus B, Casals J, Biete A. Primary small cell carcinoma of the esophagus: a review of the literature with emphasis on therapy and prognosis. Cancer 80: 1366-1372, 1997. [PubMed] [Google Scholar]
- 6. Chin K, Baba S, Hosaka H, et al. Irinotecan plus cisplatin for therapy of small cell carcinoma of the esophagus: report of 12 cases from single instruction experience. Jpn J Clin Oncol 38: 426-431, 2008. [DOI] [PubMed] [Google Scholar]
- 7. Isoyama Y, Shioyama Y, Nomoto S, et al. Carboplatin and etoposide combined with radiotherapy for limited-stage small-cell esophageal carcinoma: three cases and review of the literature. Jpn J Radiol 28: 181-187, 2010. [DOI] [PubMed] [Google Scholar]
- 8. Chino O, Makuuchi H, Ozawa S, et al. Small cell type of esophageal neuroendocrine carcinoma resembling a submucosal tumor. Tokai J Exp Clin Med 40: 36-39, 2015. [PubMed] [Google Scholar]
- 9. Takubo K, Nakamura K, Sawabe M, et al. Primary undifferentiated small cell carcinoma of the esophagus. Hum Pathol 30: 216-221, 1999. [DOI] [PubMed] [Google Scholar]
- 10. Huang Q, Wu H, Nie L, et al. Primary high-grade neuroendocrine carcinoma of the esophagus: a clinicopathologic and immunohistochemical study of 42 resection cases. Am J Surg Pathol 37: 467-483, 2013. [DOI] [PubMed] [Google Scholar]
- 11. Guanrei Y, Songliang Q, He H, et al. Natural history of early esophageal squamous carcinoma and early adenocarcinoma of the gastric cardia in the People's Republic of China. Endoscopy 20: 95-98, 1988. [DOI] [PubMed] [Google Scholar]