Highlights
-
•
This study represents one of the first reports of online MRgRT.
-
•
Integrated Low-field MR provides better anatomical visualization than CBCT or MVCT.
-
•
Better visualization of the target can help to reduce the margins from CTV to PTV.
-
•
MRgRT appears a feasible option in rectal cancer treatment offering potential benefits.
-
•
MRgRT represents a promising technology for rectal cancer management.
1. Introduction
During the last decades, neoadjuvant radiotherapy with or without concomitant chemotherapy followed by Total Mesorectum Excision (TME), has become the gold standard in Locally Advanced Rectal Cancer (LARC) treatment resulting in a significant improvement of local control (LC) and giving a better prognosis to these patients [1], [2].
Furthermore, neoadjuvant chemoradiotherapy (nCRT) followed by delayed surgery (long course treatment) resulted in an advantage in downsizing and downstaging the disease if compared with short course radiotherapy immediately followed by surgery with a higher complete response’s (CR) rates [1]. The possibility to detect patients with better prognosis could allow to select those who may benefit from treatment modulation, influencing the next therapeutic options and giving a possible improvement in terms of quality of life [3], [4], [5].
Experiences of dose escalation have been published during the years showing a better LC and tumor regression grade (TRG) for radiotherapy doses higher than 55–60 Gy [6], [7]. It was also proved that a boost on macroscopic disease is a potential strategy to optimize the effect of nCRT before resection [8], [9], [10], [11].
A randomized trial supports the benefits of radiotherapy dose intensification compared to a chemotherapy one, particularly in terms of side effects with a consequential higher compliance to the treatment [12].
Therefore, the goal is nowadays to find new approaches to intensify the treatment without increasing toxicities.
These attempts to escalate the dose have been certainly supported by the usage of both Intensity Modulated Radiation Therapy (IMRT), which grants a more efficient sparing of the surrounding normal tissues [13], [14], [15], [16] and Simultaneous Integrated Boost (SIB) that allows to boost the bulky tumor (Gross Tumor Volume, GTV) during the treatment sessions, in the same overall treatment time.
However, pelvic organs have high reproducibility uncertainties due mostly to the different degree of filling which need appropriate geometrical margins in order to benefit of these advanced techniques.
Therefore, any attempt to increase the dose needs improved accuracy to be safely applied. Image Guided RadioTherapy (IGRT) strategies appear to be mandatory in visualizing the target during daily treatment, allowing to reduce as far as possible the risk of missing target or involving the surrounding Organs at Risk (OaRs).
In this context, new scenarios are opening up with the increasing use of low and high field Magnetic Resonance (MR) images on board during treatment and hybrid MR-guided Radiation Therapy (MRgRT) can be a game changer in radiation oncology [17], [18].
It has been observed that low-field MR provides better anatomical visualization for many anatomical regions, including the pelvic one, in comparison with Cone Beam CT (CBCT) or MegaVoltage CT (MVCT) that currently represent the clinical standard for volumetric localization, [19].
Fig. 1 shows a low field MR alignment image of a locally advanced rectal cancer patient.
Fig. 1.
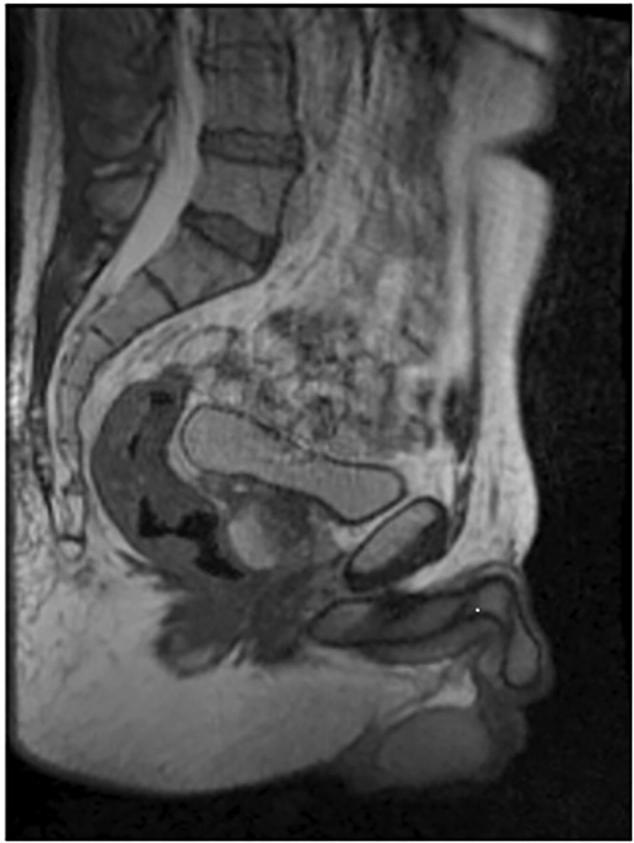
Low T alignment image.
Furthermore, the real time visualization and the gating solutions of the target allows to deliver the treatment in higher safety, leading to the possibility of reducing planning target volume margins.
Unfortunately, only very limited experiences about the clinical role of hybrid MRgRT are to date available. Boldrini et al. firstly proposed an in silico planning study with patients affected by locally advanced rectal cancer and showed that in all the considered cases, the hybrid tri-Co-60 step and shoot plans met the dose-volumetric objectives for both the target coverage and the OARs sparing, even if overall plan quality was slightly worse as compared to linac VMAT and step and shoot IMRT standard plans, with larger volumes of normal tissue receiving higher low-moderated doses [20]. The main aim of this retrospective study was to evaluate the feasibility of long-course rectal cancer’s treatments performed with a MRIdian® system (in its tri-60Co-60 version) giving an overview about the potentiality of MRgRT in rectal cancer management.
2. Materials and methods
Patients affected by histologically documented extra-peritoneal rectal cancer and treated in our center were retrospectively analyzed.
Every patient was studied at diagnosis with clinical history and a physical examination.
Endoscopy procedures were performed with biopsies and diagnostic MRI was performed for disease staging. A thoracic and abdominal CT or a PET-CT, were acquired for globally evaluation.
All cases were discussed in the frame of the institutional multidisciplinary tumor board (MTB), consisted of radiation oncologists, radiologists, pathologists and surgeons to share the best therapeutic options, both at the diagnosis and at presurgical restaging.
In the phase of clinical enrollment, we decided to treat a population of rectal cancer patients that was as representative as possible of the largest sample of patients usually treated with standard linear accelerators, with different clinical stage and tumor rectal location.
We included in our analysis patients >18 years old, with an ECOG Performance Status 0–1, a resectable disease and no previously treated with pelvic irradiation. Patients with evidence of metastatic disease at staging CT were excluded from analysis.
Specific informed consent and MRI safety screening forms were administered to all eligible patients. Patients denying specific consent to MRgRT or presenting clinical contraindications to MRI (e.g. the presence of non MRI-compatible implanted cardiac devices, claustrophobia or major psychiatric disorders) were excluded from the study.
2.1. Radiation treatment
Neoadjuvant long-course CRT was prescribed to the selected patients, according to a SIB2 delivery protocol. 55 Gy in fractions of 2.2 Gy were prescribed to planning target volume (PTV) 1, while 45 Gy in fractions of 1.8 Gy to PTV2. A total number of 25 fractions were therefore scheduled.
PTV1 was considered as primary tumour (GTV) and correspondent mesorectum with a 5-mm isotropic margin; PTV2 as mesorectum in toto and selected lymphatic drainage stations according to disease stage with a 7-mm isotropic margin [20].
Anal canal was included in CTV2 in case of infiltration of the elevator ani muscle or in case of anal canal involvement. The external iliac nodes were included in cT4 cases for major infiltration of anterior organs [21].
In case of extra-mesorectal nodal involvement, a boost on Nodal-GTV could be contemplated.
Patients were immobilized in the supine position, using the Fluxboard device (Fluxboard™, MacroMedics, The Netherlands) in an appropriate, fully personalized and comfortable configuration.
A first acquisition of a 25-seconds (sec.) sequence was performed as alignment image and used for the definition of the irradiation field. A second 175 s. sequence with higher morphological definition was then acquired for planning purposes and fused with a standard simulation CT, since the MR does not have relative electron density information need for plan calculation [22].
To improve the reproducibility, an internal protocol was used to achieve stable conditions of bladder filling: patients were instructed to drink 500 cc of water 30 min before simulation and before each treatment session.
A cine-MRI gating protocol was performed on PTV1, setting a 5% region of interest (ROI) value in a 3 mm boundary from the CTV1, ensuring therefore that the target volume was always in the proper position. Delay time value was set at 0 sec, so that the movement of the ROI outside the boundary would immediately trigger the beam off.
Fig. 2 reports an example of volume gating on a rectal cancer case.
Fig. 2.
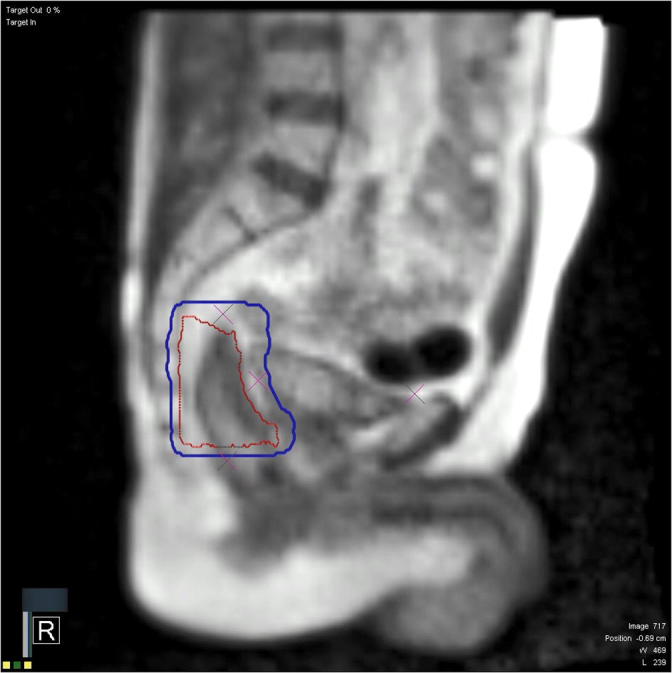
Volume gating: CTV1 (red) inside the 5 mm boundary gating ROI (blue). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.2. Concomitant chemotherapy
Concomitant chemotherapy with capecitabine chronomodulate (1650 mg/mq), 5-fluorouracil (5-FU) c.i. or an intensification schedule with capecitabine (1300 mg/mq) plus oxaliplatin (60 mg/mq) was prescribed, depending on the clinical stage and general conditions of the single patient [23].
In cases of toxicities higher than grade 3, it was considered a reduction or suspension of concomitant chemotherapy. For patients presenting acute and critical symptomatology or with important comorbidities, hospitalization was provided.
In case of extramesorectal nodes involvement a chemotherapy in presurgical pause should be considered depending on the patient’s compliance to the just completed nCRT.
2.3. Re-staging and surgery
According to our institutional guidelines and clinical practice, clinical restaging was assessed between 6 and 8 weeks after the end of nCRT by digital rectal examination (DRE) and pelvic MR. In case of major or complete clinical response at restaging imaging, endoscopic examination was performed with random biopsies to confirm absence of disease.
Surgery was performed at least 7 weeks after the end of nCRT.
If restaging exams showed a clinical complete response, a watch and wait approach and a close follow-up were considered after a collegial discussion.
In case of resectable disease, the choice between abdominoperineal resection and anterior resection was left to the discretion of the attending surgeon.
Based on nomograms prediction and clinical and pathological risk factors, adjuvant chemotherapy could be contemplated [24].
2.4. Endpoints
Treatment’s efficacy was determined by the obtained CR’s rate, both clinical and pathological.
Clinical complete response (cCR) was defined by the MTD independent review, as the presence of all the following three criteria:
-
A.
Complete absence of palpable lesions at DRE;
-
B.
Restaging MRI findings:
-
•
No lymph nodes detected or lymph nodes with short axis <5 mm [25];
-
•
No primary tumour residual at morphological and diffusion weighted imaging (DWI) series with complete integrity of rectal wall layers;
-
•
Hypointense parietal thickening in T2 sequences without evidence of hyperintense residual lesions in DWI sequences or hypointense lesions in apparent diffusion coefficient (ADC) map.
-
C.
No detection of residual lesions or presence of a fat scar at endoscopic examination.
Pathological complete response was defined as no evidence of persistent disease in the pathological specimen (ypT0ypN0, TRG1 Mandard scale).
Technique’s feasibility was investigated as the acute toxicity observed during the treatment. Weekly clinical evaluations were planned to assess toxicity and compliance to the treatment. Referred side effects were recorded according to Common Terminology Criteria for Adverse Events (CTCAE) v.4.0 scale.
3. Results
3.1. Patients’ selection
From March 2017 to May 2018, 35 pts affected by locally advanced rectal cancer were selected in MTB to undergo a radiochemotherapy treatment on primary disease.
Five patients were not included in the current analysis due to either a relapsed presentation.
In four pts, a short-course schedule was preferred for general clinical conditions.
Considering the remaining 26 patients, four pts were addressed to linac treatment.
In three of them MR contraindications were declared during the medical’s interview (claustrophobia in two pts and for panic attacks in one); in a fourth case, the treatment plan elaborated after MR simulation was not considered optimal in terms of dosimetric parameters due to the patient’s high Body Mass Index (BMI > 25).
The other 22 patients were considered suitable for this analysis.
3.2. Patients’ characteristics
Patients characteristics are described in Table1: nodal involvement was observed in 86% of the cases, 12 (54,5%) cases presented mesorectal fascia involvement either by tumor (5 pts) or by tumor and nodes simultaneously (5 pts). Extra-mesorectal nodes were present in 6 pts (27,3%).
Table 1.
Patient’s Characteristics.
| Characteristics | N° | (%) |
|---|---|---|
| Median Age At Diagnosis | 64 (range 41–86) |
|
| Sex | ||
| Male | 15 | (68.2) |
| Female | 7 | (31.8) |
| Location of the tumor | ||
| Upper | 1 | (4.5) |
| Middle | 12 | (54.5) |
| Lower | 9 | (40.9) |
| Clinical tumor stage | ||
| cT2 | 2 | (9.1) |
| cT3 | 13 | (59.02) |
| cT4 | 7 |
(31.8) |
| Clinical nodal stage | ||
| cN0 | 3 | (13.6) |
| cN1 | 8 | (36.4) |
| cN2 | 11 | (50.0) |
| Mesorectal fascia involvement | ||
| No | 10 | (45.5) |
| Yes | 12 | (54.5) |
| Chemotherapy | ||
| Capecitabine7 5- Fluorouracile | 12 | (54.5) |
| CAPOX | 9 | (41) |
| None | 1 | (4.5) |
| Chemotherapy in pre-surgical pause | ||
| No | 19 | (87.0) |
| Yes | 3 | (13.0) |
3.3. Treatment planning and chemoradiation treatment
Presacral space and posterior lateral nodes were included in all treatment planes, whereas anterior lateral nodes and external iliac nodes were comprehended in 16 and 3 cases (72,7% and 13,6%), respectively.
In two cases a boost on positive extromesorectal nodes was performed, to reach a total dose of 57,4 Gy and 59,4 Gy, respectively.
Twenty patients (90,9%) completed chemo-radiation as planned.
In one case a switch to a sequential boost was preferred due to a G3 gastrointestinal (GI) toxicity presented during the treatment. In this case a total dose of 55.8 Gy/1.8 Gy was reached after an EqD2 conversion.
Concomitant chemotherapy was considered not indicated because of comorbidities in one patient. In all the remaining cases a suspension of chemotherapy, often temporarily, was needed due to GI or hematological toxicity.
3.4. Acute toxicity
Gastrointestinal toxicities, as expected, were the most observed: all pts except one presented at least G1 GI toxicities (95,5%), mostly proctitis and diarrhea.
Five of them (22,7%) reached a G3 GI toxicities and required hospitalization for supportive care.
A G1-G2 hematological toxicity was recorded in 18 patients (81,8%).
Five patients (22,7%) presented a G1-G2 non-infective cystitis.
No G3 hematologic or genitourinary toxicities were registered.
Toxicities are reported in Table 2.
Table 2.
Acute Toxicities events according to CTCAE v 4.0.
| Toxicity | Grade |
Overall | (%) | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||||
| Blood and lymphatic system disorders |
White blood cell decreased | 4 | 7 | – | – | – | 11 | (50) |
| Neutrophil count decreased | 5 | 2 | – | – | – | 7 | (31.8) | |
| Platelets count decreased | 13 | 1 | – | – | – | 14 | (63.6) | |
| Anemia | 10 | 1 | – | – | – | 11 | (50) | |
| Gastrointestinal disorders | Abdominal distension | 2 | – | – | – | – | 2 | (9.1) |
| Abdominal pain | 1 | 1 | 1 | – | – | 3 | (13) | |
| Diarrhea | 7 | 2 | 5 | – | – | 14 | (63.6%) | |
| Vomiting | 1 | 1 | – | – | – | 2 | (9.1) | |
| Proctitis | 10 | 7 | – | – | – | 17 | (77.3) | |
| Rectal hemorrhage | 3 | 1 | – | – | – | 4 | (18.2) | |
| Nausea | 2 | 2 | – | – | – | 4 | (18.2) | |
| Flatulence | 2 | – | – | – | – | 2 | (9.1) | |
| Renal and urinary desorder | Urinary tract pain | 4 | – | – | – | – | 4 | (18.2) |
| Cystitis noninfective | 1 | – | – | – | – | 1 | (4.5) | |
3.5. Neoadjuvant chemotherapy and surgery
Chemotherapy in the presurgical pause was administered in 3 cases (13,6%).
Surgery was performed on average at 14,2 weeks (range 9,4–20,9) from the end of radiotherapy, after clinical re-evalutation and imaging restaging.
Three patients (13,6%) with a cCR did not receive surgery, because a watch and wait (WW) approach was preferred and are alive at the moment of this analysis without evidence of local or distant relapse.
Among the 19 pts who underwent surgery, 17 pts (89,5%) received anterior resection (AR) and 1 patient (5,3%) received an abdominoperineal resection (APR).
In the remaining case a Transanal Endoscopic Microsurgery (TEM) was preferred (Table 3).
Table 3.
Surgical and pathological characteristics.
| Characteristic | N° | (%) |
|---|---|---|
| Median Interval Between End of Radiochemotherapy and Surgery (Weeks) | 14.3 (range 9.4–20.9) |
|
| Type of surgery | ||
| None | 3 | (13) |
| TEM | 1 | (4.5) |
| Anterior Resection | 17 | (77.3) |
| Abdominal perineal resection | 1 | (4.5) |
| Trg sec. Mandard | ||
| TRG 1 | 3 | (13) |
| TRG 2 | 3 | (13) |
| TRG 3 | 9 | (40.9) |
| TRG 4 | 4 | (4.5) |
| TRG 5 | 0 | 0 |
3.6. Pathological analysis
Three pts (15,8%) resulted in a pathological Complete Response (pCR) at the pathological exam, while 4 pts (21%) reached a pathological major response (pT0-pT1). Tumor Regression Grade 1 or 2 was found in 6 pathological specimens (26%) (3 pts TRG1 and 3 pts TRG2). Considering downstaging, 11 pts (57,8%) presented a reduction of at least 1 tumor staging grade in respect to the clinical one, while 15 pts with clinical nodal involvement showed some pathological nodal response, as described in Table 4a and b, respectively.
Table 4.
a) Pathological tumor down staging according to clinical stage b) Pathological nodal down staging according to clinical stage. (All cases in whom there were some kind of response are highlighted).
 |
3.7. Complete response
Considering all the analyzed patients, a total of 6 pts (27,3%) showed no evidence of disease at either restaging or pathological exam.
Most cases presented a clinical nodal involvement at diagnosis and in a half of patients an initial cT4 tumor stage.
4. Discussion
In the last decades, the usage of the IMRT has increased the possibility to spare the surrounding normal structures with a consequent reduction of the side effects. This has been possible thanks to accurate IGRT solutions, able to avoid either target missing or unwanted dose to OARs.
In this process, although the increasing usage of CBCT during the time, 2-D kilovoltage (kV) images are still not the optimum, considering that most of the time they allow the alignment only on bony anatomy.
Furthermore, IGRT systems result in an increase of ionizing radiation administered to the patient; daily use of electronic portal imaging device (EPID) has been associated with an increase of 1–2% for prescribed doses of 70–80 Gy [26].
Therefore, the introduction of MR imaging in this setting seemed the most appropriate “logical next step” [27].
Magnetic Resonance is considered the gold standard in both initial staging and clinical response evaluation for patients affected by rectal cancer [28], [29]. Magnetic Resonances provides better image quality, especially in terms of soft tissue contrast, than current on-board imaging modalities (planar megavoltage and kV or CBCT), as an example low T MR imaging of the MRIdian® System (resolution 1.5 × 1.5 mm, slice thickness 1.5 or 3 mm) were compared in different sites in terms of visualization of targets and OARs to Trilogy CBCT imaging (resolution 0.7 × 1.2 mm, slice thickness 2–2.5 mm) for 14 patients with lesions. Despite the limitations of the low magnetic field and the lower resolution, MR was judged better than CT in 71% of the structures analyzed [18], [30].
Based on the superior soft-tissue contrast compared to standard ionizing imaging used in IGRT, the MR images provided by these hybrid machines can be indeed used mainly in three clinical tiers: alignment imaging, real time cine imaging for gating purposes and online adaptive applications [31].
Moreover, MR offers the possibility of integration with “functional” sequences, providing imaging biomarkers of therapy response of tumor or normal tissue or even both, using traditional and radiomics based approaches [32]. Subsequently, these imaging biomarkers can drive adaptive plan modifications to account for the observed therapy response.
Biological-based adaptive protocols typically aim to differentiate between responders and non-responders, redistributing the target dose to radioresistant regions, or lower dose constraints to OARs.
Between these, DWI seem to have greater potentials in providing predictive data on the response to treatment. For example Sun et al. have seen that early increase of mean tumor ADC as early as 1 week after initiating nCRT and low pretherapy mean ADC in rectal carcinoma correlates with good response to CRT and even first experiences of DWI applications during treatment in MRgRT seem promising [33], [34]
From a patient-safety perspective, daily image guidance with MR also avoids undesirable radiation exposure inherent to the use of CT imaging guidance such as CBCT [35]. On the other hand, an accurate patients’ selection is needed to verify compatibility with the MR device.
Further studies are definitely needed to to exploit MRgRT potentialities in this clinical setting. Considering the possibility to directly follow the target and OAR’s motion during the treatment, it could be interesting analyzing the feasibility and, in case, identifying the best timing of an additional boost.
In the current sample the pCR rate reached 27,3%, slightly higher than the value reported in the experimental arm of the INTERACT study (23,5%) [12]. Similar pCR were reported by other IMRT-SIB series with doses ranging from 47.5 to 57.5 Gy and pCR rates of 13–31% [36], [37], [38].
Furthermore, only one patient with a low rectum disease in our data set, underwent an abdominal-perineal resection.
Different dose-response models have shown as the increase of the dose is correlated to a higher rate of pCR [7] and long-term survival [39], [40].
For that reason, in recent years research has been focused on new ways of dose intensification’s programs [39], [40].
Furthermore, if the best visualization of the target can bring to the aforesaid attempts in increase dose aiming to higher tumor control, on the other hand it could help to reduce the margins from CTV to PTV, decreasing the involvement of healthy surrounding organs.
Park et al. compared tri-Co-60 IMRT plans with standard practice VMAT ones for cervical cancer long courses and they observed a higher overall quality of the tri-Co-60 IMRT plans, with several advantages with regards to normal tissue irradiation mainly related IMRT plans [41]. These significant advantages were mainly related to the reduced margin used in the tri-Co-60 IMRT plan for creating PTV (1 mm isotropic margin from the CTV).
In our cohort lower margins than those in use in our institution for standard linac treatments (0.5 mm versus 0.8 mm) were used.
Considering GI toxicity, almost all patients presented at least G1 GI toxicities (95,5%) and in five cases (22,7%) they required an hospitalization for supportive care because of G3 toxicities. In a meta-analysis Burbach showed that acute toxicity ≥ 3 reported between the studies was 10.3% (95% CI 5.4–18.6%) [6]. In preoperative standard treatment studies with concomitant 5-FU chemotherapy the rate of proctitis and G3 diarrhea was variable from 15 to 40% [2], [42], [43]. The data obtained from our retrospective analysis result therefore in line with what reported in the literature, but we believe that they can significantly improve after performing some protocols of margins’ reduction and thanks to the recent upgrade of the technology from 60-Co sources to 6 MV linac that will significantly improve the dose distributions, significantly reducing the low dose spread.
5. Conclusions
Although the small number of the analyzed patients, this is the first study reporting clinical data on patients affected by rectal cancer undergoing a long course MRgRT and it can be considered as a basis for further investigations about the potential advantages of this new technology in rectal cancer management.
MRgRT appears a feasible option in the treatment of rectal cancer showing several benefits mainly related to the possibility of better defining the target, reducing both the inter- and intra-fraction error.
6. Conflict of interest statement
Dr. Cellini, Dr. Boldrini and Dr. Cusumano received Speaker honorarium from Viewray Inc.
Prof. Valentini has an ongoing research agreement with Viewray Inc.
The other authors declare that they do not have any affiliation with industries or organizations with a financial interest, direct or indirect, that may affect the conduct or reporting of the work submitted.
References
- 1.Valentini V., Glimelius B., Haustermans K., Marijnen C.A.M., Rödel C., Gambacorta M.A. EURECCA consensus conference highlights about rectal cancer clinical management: the radiation oncologist’s expert review. Radiother Oncol. 2014;110:195–198. doi: 10.1016/j.radonc.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 2.Sauer R., Becker H., Hohenberger W., Rödel C., Wittekind C., Fietkau R. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 3.Vecchio F.M., Valentini V., Minsky B.D., Padula G.D.A., Venkatraman E.S., Balducci M. The relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancer. Int J Radiat Oncol Biol Phys. 2005;62:752–760. doi: 10.1016/j.ijrobp.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 4.Capirci C., Valentini V., Cionini L., De Paoli A., Rodel C., Glynne-Jones R. Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys. 2008;72:99–107. doi: 10.1016/j.ijrobp.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 5.Zorcolo L., Rosman A.S., Restivo A., Pisano M., Nigri G.R., Fancellu A. Complete pathologic response after combined modality treatment for rectal cancer and long-term survival: a meta-analysis. Ann Surg Oncol. 2012;19:2822–2832. doi: 10.1245/s10434-011-2209-y. [DOI] [PubMed] [Google Scholar]
- 6.Burbach J.P.M., Den Harder A.M., Intven M., Van Vulpen M., Verkooijen H.M., Reerink O. Impact of radiotherapy boost on pathological complete response in patients with locally advanced rectal cancer: a systematic review and meta-analysis. Radiother Oncol. 2014;113:1–9. doi: 10.1016/j.radonc.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 7.Appelt A.L., Ploen J., Vogelius I.R., Bentzen S.M., Jakobsen A. Radiation dose-response model for locally advanced rectal cancer after preoperative chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2013;85:74–80. doi: 10.1016/j.ijrobp.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jakobsen A., Ploen J., Vuong T., Appelt A., Lindebjerg J., Rafaelsen S.R. Dose-effect relationship in chemoradiotherapy for locally advanced rectal cancer: a randomized trial comparing two radiation doses. Int J Radiat Oncol Biol Phys. 2012;84:949–954. doi: 10.1016/j.ijrobp.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Mohiuddin M., Regine W.F., John W.J., Hagihara P.F., McGrath P.C., Kenady D.E. Preoperative chemoradiation in fixed distal rectal cancer: dose time factors for pathological complete response. Int J Radiat Oncol Biol Phys. 2000;46:883–888. doi: 10.1016/s0360-3016(99)00486-1. [DOI] [PubMed] [Google Scholar]
- 10.Myerson R.J., Valentini V., Birnbaum E.H., Cellini N., Coco C., Fleshman J.W. A phase I/II trial of three-dimensionally planned concurrent boost radiotherapy and protracted venous infusion of 5-FU chemotherapy for locally advanced rectal carcinoma. Int J Radiat Oncol Biol Phys. 2001;50:1299–1308. doi: 10.1016/s0360-3016(01)01540-1. [DOI] [PubMed] [Google Scholar]
- 11.Wiltshire K.L., Ward I.G., Swallow C., Oza A.M., Cummings B., Pond G.R. Preoperative radiation with concurrent chemotherapy for resectable rectal cancer: effect of dose escalation on pathologic complete response, local recurrence-free survival, disease-free survival, and overall survival. Int J Radiat Oncol Biol Phys. 2006;64:709–716. doi: 10.1016/j.ijrobp.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Valentini V., De Paoli A., Barba M.C., Friso M.L., Lupattelli M., Rossi R. Capecitabine based preoperative chemo-RT in rectal cancer intensified by RT or oxaliplatin: the INTERACT trial. Radiother Oncol. 2014;111:S193. [Google Scholar]
- 13.Samuelian J.M., Callister M.D., Ashman J.B., Young-Fadok T.M., Borad M.J., Gunderson L.L. Reduced acute bowel toxicity in patients treated with intensity-modulated radiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2012;82:1981–1987. doi: 10.1016/j.ijrobp.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 14.Urbano M.T.G., Henrys A.J., Adams E.J., Norman A.R., Bedford J.L., Harrington K.J. Intensity-modulated radiotherapy in patients with locally advanced rectal cancer reduces volume of bowel treated to high dose levels. Int J Radiat Oncol Biol Phys. 2006;65:907–916. doi: 10.1016/j.ijrobp.2005.12.056. [DOI] [PubMed] [Google Scholar]
- 15.Arbea L., Ramos L., Martínez-Monge R., Moreno M., Aristu J. Intensity-modulated radiation therapy (IMRT) vs. 3D conformal radiotherapy (3DCRT) in locally advanced rectal cancer (LARC): dosimetric comparison and clinical implications. Radiat Oncol. 2010;5:17. doi: 10.1186/1748-717X-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mok H., Crane C.H., Palmer M.B., Briere T.M., Beddar S., Delclos M.E. Intensity modulated radiation therapy (IMRT): differences in target volumes and improvement in clinically relevant doses to small bowel in rectal carcinoma. Radiat Oncol. 2011;6:1–9. doi: 10.1186/1748-717X-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mutic S., Dempsey J.F. The ViewRay system: magnetic resonance-guided and controlled radiotherapy. Semin Radiat Oncol. 2014;24:196–199. doi: 10.1016/j.semradonc.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Raaymakers B.W., Lagendijk J.J.W., Overweg J., Kok J.G.M., Raaijmakers A.J.E., Kerkhof E.M. Integrating a 1.5 T MRI scanner with a 6 MV accelerator: proof of concept. Phys Med Biol. 2009;54:N229–N237. doi: 10.1088/0031-9155/54/12/N01. [DOI] [PubMed] [Google Scholar]
- 19.Noel C.E., Parikh P.J., Spencer C.R., Green O.L., Hu Y., Mutic S. Comparison of onboard low-field magnetic resonance imaging versus onboard computed tomography for anatomy visualization in radiotherapy. Acta Oncol (Madr) 2015;54:1474–1482. doi: 10.3109/0284186X.2015.1062541. [DOI] [PubMed] [Google Scholar]
- 20.Boldrini L., Placidi E., Dinapoli N., Azario L., Cellini F., Massaccesi M. Technical innovations & patient support in radiation oncology hybrid tri-co-60 MRI radiotherapy for locally advanced rectal cancer: an in silico evaluation. Tech Innov Patient Support Radiat Oncol. 2018;6:5–10. doi: 10.1016/j.tipsro.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valentini V., Gambacorta M.A., Barbaro B., Chiloiro G., Coco C., Das P. International consensus guidelines on Clinical Target Volume delineation in rectal cancer. Radiother Oncol. 2016;120:195–201. doi: 10.1016/j.radonc.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 22.Uh J., Merchant T.E., Li Y., Li X., Hua C. MRI-based treatment planning with pseudo CT generated through atlas registration. Med Phys. 2014;41 doi: 10.1118/1.4873315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rödel C., Graeven U., Fietkau R., Hohenberger W., Hothorn T., Arnold D. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): Final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16:979–989. doi: 10.1016/S1470-2045(15)00159-X. [DOI] [PubMed] [Google Scholar]
- 24.Valentini V., Van Stiphout R.G.P.M., Lammering G., Gambacorta M.A., Barba M.C., Bebenek M. Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of european randomized clinical trials. J Clin Oncol. 2011;29:3163–3172. doi: 10.1200/JCO.2010.33.1595. [DOI] [PubMed] [Google Scholar]
- 25.Lahaye M.J., Beets G.L., Engelen S.M., Kessels A.G., de Bruïne A.P., Kwee H.W. Locally advanced rectal cancer: MR imaging for restaging after neoadjuvant radiation therapy with concomitant chemotherapy. Part II. What are the criteria to predict involved lymph nodes? Radiology. 2009;252(1):81–91. doi: 10.1148/radiol.2521081364. [DOI] [PubMed] [Google Scholar]
- 26.Verellen D., De Ridder M., Tournel K., Duchateau M., Reynders T., Gevaert T. An overview of volumetric imaging technologies and their quality assurance for IGRT. Acta Oncol (Madr) 2008;47:1271–1278. doi: 10.1080/02841860802244182. [DOI] [PubMed] [Google Scholar]
- 27.Verellen D., De Ridder M., Linthout N., Tournel K., Soete G., Storme G. Innovations in image-guided radiotherapy. Nat Rev Cancer. 2007;7:949–960. doi: 10.1038/nrc2288. [DOI] [PubMed] [Google Scholar]
- 28.Brown G., Davies S., Williams G.T., Bourne M.W., Newcombe R.G., Radcliffe A.G. Effectiveness of preoperative staging in rectal cancer: digital rectal examination, endoluminal ultrasound or magnetic resonance imaging? Br J Cancer. 2004;91:23–29. doi: 10.1038/sj.bjc.6601871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LeBlanc J.K. Imaging and management of rectal cancer. Nat Clin Pract Gastroenterol Hepatol. 2007;4:665–676. doi: 10.1038/ncpgasthep0977. [DOI] [PubMed] [Google Scholar]
- 30.Matsuoka H., Nakamura A., Masaki T., Sugiyama M., Takahara T., Hachiya J. A prospective comparison between multidetector-row computed tomography and magnetic resonance imaging in the preoperative evaluation of rectal carcinoma. Am J Surg. 2003;185:556–559. doi: 10.1016/s0002-9610(03)00067-9. [DOI] [PubMed] [Google Scholar]
- 31.Boldrini L., Cellini F., Manfrida S., Chiloiro G., Teodoli S., Cusumano D. Use of indirect target gating in magnetic resonance-guided liver stereotactic body radiotherapy: case report of an oligometastatic patient. Cureus. 2018;10(3) doi: 10.7759/cureus.2292. e2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boldrini L., Cusumano D., Chiloiro G., Casà C., Masciocchi C., Lenkowicz J. Radiomics for rectal cancer response prediction with hybrid 0.35 T Magnetic Resonance guided Radiotherapy (MRgRT): a hypothesis generating study for an innovative personalized medicine approach. Radiol Med. 2018 doi: 10.1007/s11547-018-0951-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun Y.-S., Zhang X.-P., Tang L., Ji J.-F., Gu J., Cai Y. Locally advanced rectal carcinoma treated with preoperative chemotherapy and radiation therapy: preliminary analysis of diffusion-weighted mr imaging for early detection of tumor histopathologic downstaging. Radiology. 2010;254:170–178. doi: 10.1148/radiol.2541082230. [DOI] [PubMed] [Google Scholar]
- 34.Shaverdian N., Yang Y., Hu P., Hart S., Sheng K., Lamb J. Feasibility evaluation of diffusion-weighted imaging using an integrated MRIradiotherapysystem for response assessment to neoadjuvant therapy in rectal cancer. Br J Radiol. 2017;90:20160739. doi: 10.1259/bjr.20160739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hauri P., Hälg R.A., Schneider U. Technical Note: comparison of peripheral patient dose from MR-guided 60 Co therapy and 6 MV linear accelerator IGRT. Med Phys. 2017;44:3788–3793. doi: 10.1002/mp.12293. [DOI] [PubMed] [Google Scholar]
- 36.Lupattelli M., Matrone F., Gambacorta M.A., Osti M., Macchia G., Palazzari E. Preoperative intensity-modulated radiotherapy with a simultaneous integrated boost combined with Capecitabine in locally advanced rectal cancer: short-term results of a multicentric study. Radiat Oncol. 2017;12(1):139. doi: 10.1186/s13014-017-0870-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hernando-Requejo O., López M., Cubillo A., Rodriguez A., Ciervide R., Valero J. Complete pathological responses in locally advanced rectal cancer after preoperative IMRT and integrated-boost chemoradiation. Strahlentherapie Und Onkol. 2014;190:515–520. doi: 10.1007/s00066-014-0650-0. [DOI] [PubMed] [Google Scholar]
- 38.Arbea L., Martínez-Monge R., Díaz-González J.A., Moreno M., Rodríguez J., Hernández J.L. Four-week neoadjuvant intensity-modulated radiation therapy with concurrent capecitabine and oxaliplatin in locally advanced rectal cancer patients: a validation phase II trial. Int J Radiat Oncol Biol Phys. 2012;83:587–593. doi: 10.1016/j.ijrobp.2011.06.2008. [DOI] [PubMed] [Google Scholar]
- 39.Chan A.K., Wong A.O., Langevin J., Jenken D., Heine J., Buie D. Preoperative chemotherapy and pelvic radiation for tethered or fixed rectal cancer: a phase II dose escalation study. Int J Radiat Oncol Biol Phys. 2000;48:843–856. doi: 10.1016/s0360-3016(00)00692-1. [DOI] [PubMed] [Google Scholar]
- 40.Van Wickle J.D., Paulson E.S., Landry J.C., Erickson B.A., Hall W.A. Adaptive radiation dose escalation in rectal adenocarcinoma: a review. J Gastrointest Oncol. 2017;8:902–914. doi: 10.21037/jgo.2017.07.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park J.M., Park S.-Y., Kim J.-I., Kang H.-C., Choi C.H. A comparison of treatment plan quality between Tri-Co-60 intensity modulated radiation therapy and volumetric modulated arc therapy for cervical cancer. Phys Med. 2017;40:11–16. doi: 10.1016/j.ejmp.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 42.Bosset J.F., Magnin V., Maingon P., Mantion G., Pelissier E.P., Mercier M. Preoperative radiochemotherapy in rectal cancer: long-term results of a phase II trial. Int J Radiat Oncol Biol Phys. 2000;46:323–327. doi: 10.1016/s0360-3016(99)00411-3. [DOI] [PubMed] [Google Scholar]
- 43.Bosset J.-F., Collette L., Calais G., Mineur L., Maingon P., Radosevic-Jelic L. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]


