Highlights
-
•
The use of MRgRT in clinical practice requires thorough preparation.
-
•
MRgRT combining with daily plan adaptation indicates adjusting existing workflows.
-
•
Clinical implementation of adaptive MRgRT requires a multidisciplinary approach.
-
•
Role and responsibilities of RTT will expand with growing clinical experience.
Keywords: MR-guided radiation therapy (MRgRT); Clinical workflow, stereotactic body radiation therapy (SBRT); Stereotactic MR-guided adaptive radiation therapy (SMART); On-table adaptation; RTT perspective
Abstract
The latest development in radiation oncology departments towards high precision and adaptive radiation therapy is the clinical introduction of magnetic resonance image guided radiation therapy (MRgRT). Early 2016, patient treatment using MRgRT was started at Amsterdam UMC, location VU University Medical Center. Introducing this novel technique in clinical practice requires thorough preparation with regard to important topics, such as MR-safety and training, equipping the treatment vault and console room, development of MRgRT workflow and logistical issues. Certainly when MRgRT is combined with daily plan adaptation, this indicates adjusting existing workflows and protocols. The MRgRT workflow requires a multidisciplinary process, and while each discipline has had its own tasks and responsibilities, with growing clinical experience there has been a shift towards RTT responsibilities. In this overview we discuss preclinical training and preparation for the implementation of (adaptive) MRgRT, with a particular focus on the perspective of RTTs. Although the reviewed logistics are partly the result of the decision to perform daily plan re-optimization, our experience can be extrapolated to implementation of alternative approaches for MRgRT.
1. Introduction
The role of imaging in radiation therapy has been rapidly expanding over the last years. Commonly used image-guided radiation therapy (IGRT) techniques using on-board imaging systems such as kV-imaging and cone beam computed tomography (CBCT) prior to delivery, have shown to provide significant benefit for positioning of the target volume [1], [2], [3]. Daily generated set-up images are compared and co-registered with corresponding digitally reconstructed radiographs. IGRT is of particular importance in high-precision hypofractionated treatment in the form of stereotactic body radiation therapy (SBRT) [4], [5]. There is no clear international consensus on which discipline should be responsible to perform IGRT in daily clinical practice, with medical physicist and RTT being most frequently involved. Retrospective work shows that qualified and trained radiation therapist (RTT) are capable of interpreting IGRT, comparable to the interpretation of radiation oncologists [6]. A recent analysis stated that periodic evaluation and in-house training are required when pretreatment image-registration is routinely delegated to RTTs [7].
The latest development in IGRT in radiation oncology departments is the clinical introduction of magnetic resonance image guided radiation therapy (MRgRT). MRgRT provides superior soft-tissue information and the possibility to perform real-time imaging during treatment [8], [9]. Associated with the introduction of MRgRT, is the option to perform daily online plan re-optimization based on the anatomy of the day [9], [10]. MRgRT was introduced clinically in our department in early 2016 [MRIdian, ViewRay, Mountainview, USA]. The implementation of MRgRT combined with routine plan re-optimization, required multidisciplinary adjustment of the radiation delivery workflow, making it different from logistics of that on the other linacs. In this paper we describe the applied method and impact of introducing (adaptive) MRgRT in daily clinical practice, with a particular focus on the perspective of RTTs. The evolving role of RTTs with increasing clinical experience and the importance of RTT education and training during the preparation phase are discussed. Although the described logistics is partly a result of the decision to perform daily plan re-optimization, our experience can be extrapolated to implementation of alternative methods for MRgRT.
2. The preclinical phase of MRgRT
The use of MR studies performed at the department of diagnostic imaging for better target definition and radiotherapy contouring purposes has been routine for many years. However, introducing a hybrid system providing MR-imaging on the linear accelerator is a novelty, and requires thorough preparation. Well before the clinical implementation of MRgRT, a small multidisciplinary group consisting of RTT’s, medical physicists, engineers and radiation oncologists was formed, each with specific tasks. Important preparatory topics that were addressed were MR-safety and training, equipping the treatment vault and console room, development of a standardized MRgRT workflow and logistical issues. Some of these topics will be discussed below.
2.1. MR-safety
Although our MR-linac uses 0.35 T magnetic fields, MR-safety measures are similar to those of MR machines using higher strength magnetic fields. This indicates that all patients need to be specifically screened for contra-indications just as for diagnostic MR scans. Because of its importance, several lectures addressing the basic principles and awareness of MR-safety were provided by a physicist from the department of diagnostic Radiology, application specialists from the vendor and as part of a specific MR-imaging course. A brief MR-safety questionnaire, similar to that used at the Radiology department, was developed which has to be completed by all patients (and volunteers and visitors) prior to entering the treatment vault, addressing among others the presence of ferromagnetic metals, intracranial clips, electronic devices such as pacemakers or neurostimulators, and claustrophobia. It is always reassuring if the patient has previously undergone diagnostic MR-imaging, although changes in contraindications in the interval need to be ruled out for each instance. Most modern implants and prostheses, e.g. hip implants, from recent years are MR-safe, but certainly also for MRgRT the issue of image distortion and thereby contouring errors needs to be thoroughly assessed. Although many modern electronic devices such as pacemakers are nowadays denominated MR conditional, i.e. safe under well-characterized MR conditions, we have not treated patients with pacemakers or defibrillators with MRgRT. Main reasons for this have been the prolonged MR-imaging during delivery, and the presence of alternative radiation therapy approaches on standard linear accelerators. Before entering the treatment vault, patients are instructed to remove all jewelry, body piercing materials, partial dental plates, hearing aids and any other (potentially) metal containing accessories. The presence of a ferromagnetic detection system that is installed at the entrance of the MR-linac further enhances MR-safety. It is important to adhere to all MR-safety instructions and precautions, not only when initiating MRgRT, but also at a stage when this treatment modality has become routine in the radiotherapy department. Claustrophobia due to prolonged positioning within the MR-bore is another issue that needs attention when considering MRgRT. In addition to the safety questionnaire, simulation is also performed on the MR-linac in our center, selecting out patients with severe claustrophobia, a few percent of patients. Milder forms of claustrophobia can usually be resolved by audio reassurance of the patients, mounting a mirror within the MR-bore which extends the field of view of patients, or, occasionally, mild sedatives.
MR imaging involves radio-frequency pulses, which lead to exposing the patient’s body to electromagnetic radiation. The energy absorbed by the patient depends on their body weight and is indicated as the specific absorption rate (SAR), expressed as W/kg. Because of prolonged imaging (pretreatment and during delivery), the SAR is an important measure because exceeding safe SAR values may cause local or general heating problems for the patient. The MR-console alerts the user with a warning when high SAR values are expected. The SAR values according to the scan protocols in the MRIdian system operating at 0.35 T are well below the limits for normal mode which is up to 2 W/kg for whole-body exposure. Finally, general resuscitation equipment and emergency pharmaceutical kits are not MR-compatible and therefore, in case of an emergency, the patient has to be quickly removed from the vault. The latter can be accomplished using a MR compatible stretcher, which is routinely stored within the vault. Because this procedure differs from routine resuscitation, the MR-linac resuscitation protocol is repeated yearly as part of the training program with all RTT’s working at the MR-linac. An alternative approach, dependent on the patient selection and throughput, is to purchase and store MR compatible emergency resuscitation equipment in the treatment vault.
2.2. RTT role in equipping the treatment vault and console room for MRgRT
In addition to general advice regarding the design of the treatment console and the treatment vault, RTT’s were specifically involved in two key features of MRgRT. Firstly, to prepare patient treatment with MRgRT, a customized MR-compatible positioning board was developed and tested with volunteers in cooperation with a manufacturer for positioning products (Macromedics, Waddinxveen, The Netherlands), which includes foot-, knee- and arm support. The flux-board allows the plugs and cables of the body coils for MRI-imaging to be maneuvered in such a way that the patient remains in a comfortable position during treatment. Noise reduction headphones are supplied to patients, also enabling communication between patients and RTT’s during treatment, and if requested by patients music can be played via the headphones. A squeeze bulb allows patients to activate an auditory alert, to indicate that communication is wanted. Both the headphones and auditory communication are tested prior to each simulation and treatment.
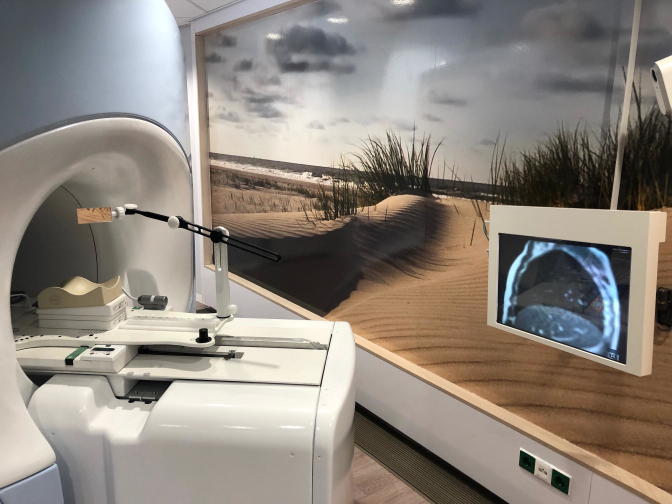
MR-imaging during radiation delivery allows for performing gated breath-hold delivery for lesions located in the thoracic and abdominal region. The MRIdian Linac has an automatic tumor tracking algorithm, allowing beam-on when the target volume is within the user-defined safety boundary, and triggering beam-hold when the target exceeds the boundary. An in-house developed system for visual feedback to patients was installed using an MR-compatible monitor at the head end of the MR bore, in combination with an adjustable mirror mounted on the positioning board, shown in Fig. 1. This real-time visual feedback system allows patients to manage their repeated breath-holds at the appropriate phase at their own pace, resulting in an efficient duty cycle [11]. Although most modern eyeglasses are MR-compatible, in case of non-compatibility, a pair of adjustable glasses were purchased and tested.
Fig. 1.
The visual feedback system, including an MR-compatible monitor at the head end of the MR bore and an adjustable mirror.
2.3. Development of the MRgRT workflow
Before treating the first patient, clinical MRgRT workflow for the different anatomical regions was tested in healthy volunteers, which proved to be extremely useful. This allowed testing and adjustment of positioning, developing experience with the MR-coils, headphones and other accessories, generating high-resolution scans with appropriate MR-settings, and in general, training with the MR-linac software. These generated scans were also used for treatment planning training based on simulated target volumes. Although our MR-linac uses a single balanced steady-state-free-precession acquisition (Siemens, True FISP), volunteer studies may offer the opportunity to test the different MR sequences and settings for other MR-linacs. As an example of the importance of volunteer testing, standard duration of the breath-hold MR scan initially was set at 25 s by the vendor. Several volunteers however, experienced difficulties with this breath-hold duration, resulting in the in-house development of an alternative MR protocol for breath-hold patients with a smaller field-of-view and a duration of 17 s, which is now used in everyday clinical practice.
2.4. Departmental logistics
At our center we elected to perform simulation on the MR-linac. Although logistically challenging, generating simulation MR scans in treatment position using the sequence that is also used for daily positioning plan adaptation greatly facilitates (re-) contouring, it produces better results after applying online deformable image registration and is preferred above importing diagnostic MR scans for this purpose. Until such time that MR-only planning will be clinically feasible, planning CT scans in treatment position with dummy coils are routinely generated. The baseline simulation CT is deformed to match the pretreatment MR-scan reflecting the anatomy of the day, generating electron density (ED) images which are used for dose calculation purposes. The interval between the simulation CT- and MR scan normally does not exceed 30 min. Because of the novelty of MRgRT, RTT’s were involved in the first consultation for completing the MR-contraindication questionnaire, illustrating the breath-hold instructions using generated movies and providing other logistic information on MRgRT.
In general, with the exception of a slightly longer time needed for patient positioning due to coils placement, MRgRT does not necessarily require longer treatment slots than other forms of IGRT. It is the special features of online plan adaptation with required re-contouring, and breath-hold delivery using step-and-shoot IMRT beams that make online adapted MRgRT a time-consuming procedure, as will be described below. At our center, we have opted to perform plan re-optimization or adaptation prior to each fraction to generate a new optimal plan at each fraction. In recognition that a re-optimized plan using the same number and direction of beams in combination with high quality robust baseline plans and online plan QA will at the very least be of similar quality than the baseline plan, we opted for routine re-optimization rather than deciding for each fraction whether to adapt or not. Our MRgRT workflow was specifically adjusted for fast daily plan re-optimization, with the patient waiting in treatment position on the couch. Our approach for partial organ at risk re-contouring and plan adaptation has been published previously, and for further details we refer to this publication [12].
3. Adaptive MRgRT in clinical practice
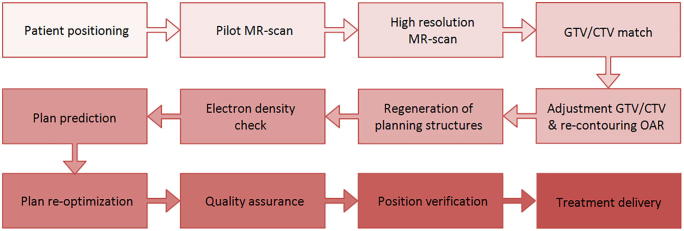
Because MRgRT without plan adaptation is similar to IGRT on other linacs, this paragraph is specifically directed to the role of RTT’s in MRgRT performed in combination with daily plan adaptation, which has been performed in more than 3000 fractions since early 2016 at our center. The workflow that has been used routinely is schematically shown in Fig. 2.
Fig. 2.
Flowchart of institutional MRgRT workflow with daily plan adaptation for each fraction.
Although MRgRT with plan adaptation is a multidisciplinary process, each discipline is responsible for some specific tasks and steps on the MRgRT wofkflow. However, the responsibilities and tasks have evolved over time and have shifted with growing clinical experience. The first steps, i.e. patient positioning, performing the pilot and high-resolution MR scans and aligning the target volume, usually the gross tumor volume (GTV; e.g. in oligometastatic disease) or clinical target volume (CTV; e.g. the prostate) are performed by dedicated MR-linac RTT’s. In the Netherlands, these initial steps for delivering IGRT are also performed by RTT’s at other linacs, and only in case of uncertainties medical physicists or Radiation Oncologists are consulted. After approval of the alignment by the present Radiation Oncologist, minor adjustments of the GTV or CTV are performed by the clinician. Our approach of daily plan adaptation has been described in detail previously [12]. Because the vast majority of patients treated with MRgRT with plan adaptation are treated with short courses of SBRT, tumor shrinkage or progressions hardly plays a role. Minor adjustment of the target volume is mainly required to correct for positional variations between the pre-treatment MR and the simulation MR resulting in rotations of the target volume and/or local deformations because of the changes in the surrounding anatomy (for instance prostate due to rectum distension). Initially, the relevant organs at risk (OARs) had been re-contoured by the clinicians on duty at the MR-linac. For the daily adaptive process, OARs only need to be adjusted manually after deformable contouring within the first 2 cm outside the PTV because this represents the area with the most relevant high doses.
With growing clinical experience and the availability of a dedicated RTT team at the MR-linac, for most indications the OAR re-contouring has been referred to RTTs currently, and only checked by the present Radiation Oncologist. The next steps of checking the electron density from the deformed baseline simulation CT on the basis of the daily high-resolution MR scan is briefly checked by the RTT’s and manually adjusted with an adequate density override (water, air or lung tissue) if a relevant mismatch is observed due to e.g. large air pockets in adjacent OARs. The generation of a non-optimized (predicted) and adapted plan is routinely performed within the software. In 99% of fractions at our center, the adapted plan is chosen for the actual treatment. This is an institutional policy with speed of treatment in mind, however, in case of equal plans a more detailed comparison of both plans in order to decide if the adapted plan is superior may be performed in other centers. If the adapted plan is selected for delivery, patient specific QA with secondary Monte Carlo-based dose calculation of the newly generated IMRT plan is required [13]. Although by definition, this is the responsibility of medical physicists, the nature of plan adaptation (only optimization of fluence, without changing the direction and number of beams) makes this QA relatively straightforward. After a routine review of QA by medical physicists in the first 500 fractions, this task has now been delegated to RTT’s with fixed rules when to contact the medical physicist for review of the QA results. At our center, this physics review is always performed for the first fraction for each patient.
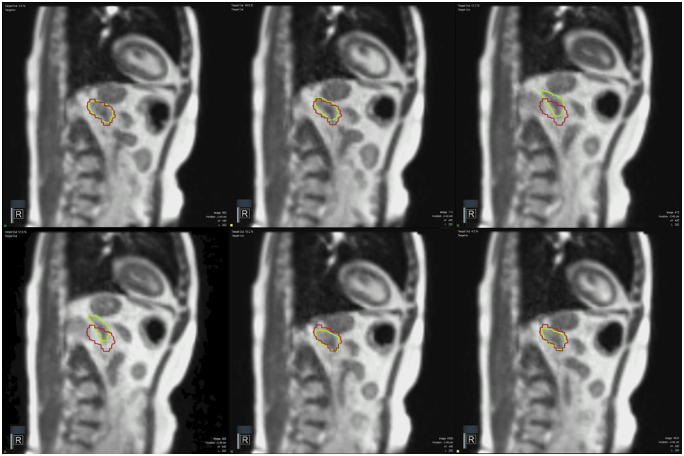
Obviously the specific role of RTT’s is the greatest during the delivery phase. MRgRT is currently performed using real-time tumor tracking and automated gated delivery. Guidance is performed on a single sagittal image on which the tracked target volume as well as the safety boundary are projected, shown in Fig. 3. The system uses an automatic tumor tracking algorithm resulting in triggering a beam-on when target volume is within the boundary, and a beam-hold when the target volume exceeds the boundary. In this way automated gated delivery is applied. Because MRgRT is delivered with small (3 mm) safety margins, adjustments of MRgRT delivery parameters are common. Although it would be too vendor specific to address all possible adjustments, it is observed that 2D (performed on the basis of the tracking image) or 3D (performed on the basis of a repeat MR scan) couch shifts with a required pause in treatment is needed in approximately 15–20% of fractions. The need for positioning shifts is indicated when the system provides a low correlation warning or when the duty cycle efficiency is low, and all required steps for adjustments are performed by RTT’s, albeit under supervision of the present clinician and/or physicist. On average, these adjustments add ten minutes to a MRgRT session.
Fig. 3.
Six sagittal images of real time tumor tracking with 4 frames per second of an MRgRT adrenal gland metastasis treatment. The target volume is shown in green, the boundary is shown in red. The automatic tumor tracking algorithm triggers a beam-on when target volume is within the boundary, and triggers a beam-hold when the target volume exceeds the boundary. This allows patients to hold their breath at their own pace by using an adjustable mirror to see the real-time sagittal projection on the in-room monitor. Patients are instructed to hold their breath once the green structure is within the red contour. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
As mentioned, MRgRT for lesions in the thorax and upper abdomen is performed using video-assisted gated breath-hold. For most patients, this can be performed with a high duty-cycle efficiency [11]. In addition to adjustments during delivery, auditory assistance and reassurance is regularly needed to ensure this duty-cycle efficiency, and RTT’s play an essential role in this aspect.
Patient notes are collected during each delivery, e.g. on positioning, breath-hold specifications and other patient-specific information. This information can be used for subsequent fractions of the same patient resulting in a more adequate workflow. On average, full MRgRT with daily plan adaptation delivery takes up to 45 min for pelvic lesions and up to 60 min for delivery in combination with breath-hold. This time includes all necessary steps from positioning to the end of delivery. Time-consuming MRgRT with adaptation, therefore, indicates that only a limited number of patients can be treated during working hours, and that the impact of prolongation of a single fraction, e.g. due to 2D or 3D positioning shifts has logistical consequences. Interrupted treatments due to software errors or patient tolerance reasons immediately have a consequence for the program of the day. It is also for this reason, that a comfortable and sustainable patient positioning which may take between 10 and 20 min remains key for MRgRT because failure to achieve this can lead to difficulties during treatment or to incompletely delivered fractions. Although dosimetrically superior, positioning patients with both arms up for thoracic or abdominal lesions cannot be tolerated for a prolonged period of time by all patients, therefore, during simulation special attention is required for this aspect. A similar issue in prostate cancer patients is bladder filling. Whereas generally for radiotherapy prostate cancer patients are instructed to have a full bladder during simulation and treatment, this proved to be too difficult for many patients, especially when the first signs of radiaton-induced urinary toxicity occurred after the first treatments. As this resulted in frequent interruption of MRgRT (and because of the availability of daily plan adaptation to correct for changes in bladder filling), patients are currently instructed to be treated with half full bladder.
Patient tolerance of MRgRT with plan adaptation was evaluated in the first 150 patients using an in-house developed patient-reported outcome questionnaires (PRO-Q), with a particular focus patient comfort, potential MR side effects, treatment duration and the active participation during gated delivery. The outcomes of this PRO-Q evaluation have been reported previously and indicate that MRgRT is a well-tolerated treatment by patients [14].
Once every six weeks, a multidisciplinary meeting with RTT’s, medical physicists and clinicians is scheduled for all those involved with daily MRgRT. Several planning-, delivery- and logistical issues as well as new developments and initiatives are discussed at this meeting. In addition, once every two months new training sessions are arranged to remain up-to-date on several processes of MRgRT. Although to date, our MR-linac has operated with a single sequence as described, other MR- sequences (T1-, T2-weighted and diffusion-weighted) will be introduced in clinical practice. For other vendors, these alternative sequences are already available. Of course, this will impact upon the basic and advanced MR training required for RTTs. For MRgRT, we think it is essential that treatment planners are aware of the adaptive process at the treatment machine and conversely that the therapists are aware of the planning approaches. Thus, regular rotation of all RTT’s between delivery and treatment planning and repeated periodic multidisciplinary training is a necessity to ensure optimal MRgRT.
In conclusion, MRgRT with daily plan adaptation constitutes a novel but challenging new treatment paradigm. Clinical implementation requires a multidisciplinary approach, and the use of MR-imaging prior to and during delivery is a novelty for radiotherapy departments. Early experience has shown that RTT’s role in MRgRT has been expanding, although clear agreements need to be in place on responsibilities.
Conflict of interest
None.
References
- 1.Dawson L.A., Sharpe M.B. Image-guided radiotherapy: rationale, benefits, and limitations. Lancet Oncol. 2006;7:848–858. doi: 10.1016/S1470-2045(06)70904-4. [DOI] [PubMed] [Google Scholar]
- 2.Ye J.C., Qureshi M.M., Clancy P., Dise L.N., Willins J., Hirsch A.E. Daily patient setup error in prostate image guided radiation therapy with fiducial-based kilovoltage onboard imaging and conebeam computed tomography. Quant Imaging Med Surg. 2015;5(5):665–672. doi: 10.3978/j.issn.2223-4292.2015.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis B.C., Snyder W.J., Kim S., Kim T. Monitoring frequency of intra-fraction patient motion using the ExacTrac system for LINAC-based SRS treatments. J Appl Clin Med Phys. 2018;19(3):58–63. doi: 10.1002/acm2.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zelefsky M.J., Kollmeier M., Cox B., Fidaleo A., Sperling D., Pei X. Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int J RadiatOncol Biol Phys. 2012;84:125–129. doi: 10.1016/j.ijrobp.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 5.Van Herk M. Different styles of image-guided radiotherapy. Semin Radiat Oncol. 2007;17:258–267. doi: 10.1016/j.semradonc.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Jereczek-Fossa B.A., Pobbiati C., Santoro L., Fodor C., Fanti P., Vigorito S. Prostate positioning using cone-beam computer tomography based on manual soft-tissue registration: interobserver agreement between radiation oncologists and therapists. Strahlenther Onkol. 2014;190(1):81–87. doi: 10.1007/s00066-013-0387-1. [DOI] [PubMed] [Google Scholar]
- 7.Lauche O., Castan F., de Forges H., Guillaumon C., Gourgou S., Fenoglietto P. Prospective medical analysis of radiation therapist image repositioning during image-guided radiotherapy. Cancer Radiother. 2018;22(1):25–30. doi: 10.1016/j.canrad.2017.07.048. [DOI] [PubMed] [Google Scholar]
- 8.Noel C.E., Parikh P.J., Spencer C.R., Green O.L., Hu Y., Mutic S. Comparison of onboard low-field magnetic resonance imaging versus onboard computed tomography for anatomy visualization in radiotherapy. Acta Oncol. 2015;54(9):1474–1482. doi: 10.3109/0284186X.2015.1062541. [DOI] [PubMed] [Google Scholar]
- 9.Kashani R., Olsen J.R. Magnetic resonance imaging for target delineation and daily treatment modification. Semin Radiat Oncol. 2018;28(3):178–184. doi: 10.1016/j.semradonc.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Tetar S.U., Bruynzeel A.M.E., Lagerwaard F.J. Clinical implementation of magnetic resonance imaging guided adaptive radiotherapy for localized prostate cancer. PhiRO. 2019;9(1):69–76. doi: 10.1016/j.phro.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Sörnsen de Koste J.R., Palacios M.A., Bruynzeel A.M.E., Slotman B.J., Senan S., Lagerwaard F.J. MR-guided gated stereotactic radiation therapy delivery for lung, adrenal, and pancreatic tumors: a geometric analysis. Int J Radiat Oncol Biol Phys. 2018;102(4):858–866. doi: 10.1016/j.ijrobp.2018.05.048. [DOI] [PubMed] [Google Scholar]
- 12.Bohoudi O., Bruynzeel A.M.E., Senan S., Cuijpers J.P., Slotman B.J., Lagerwaard F.J. Fast and robust online adaptive planning in stereotactic MR-guided adaptive radiation therapy (SMART) for pancreatic cancer. Radiother Oncol. 2017;125(3):439–444. doi: 10.1016/j.radonc.2017.07.028. [DOI] [PubMed] [Google Scholar]
- 13.Palacios M.A., Apicella T., Hoffmans D., Rosario T., Admiraal M., Kawrakow I. OC-0262: Implementation of patient specific QA for daily adaptive MR-guided radiation therapy. Radiother Oncol. 2017;123:S134–S135. [Google Scholar]
- 14.Tetar S., Bruynzeel A., Bakker R., Jeulink M., Slotman B., Oei S. Patient-reported outcome measurements on the tolerance of magnetic resonance imaging-guided radiation therapy. Cureus. 2018;10(2) doi: 10.7759/cureus.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]