Highlights
-
•
Issues of MRI that are relevant for radiation oncologists are addressed.
-
•
Radiation oncology requires dedicated scan protocols.
-
•
Use of diagnostic protocols is not recommended for radiotherapy.
-
•
MR images must be made in treatment position with the standard positioning devices.
-
•
Safety screening prior to entering the MRI room is crucial.
Keywords: MRI, Geometrical fidelity, Radiotherapy planning, MR-guided radiotherapy
Abstract
MRI is increasingly used in radiation oncology to facilitate tumor and organ-at-risk delineation and image guidance. In this review, we address issues of MRI that are relevant for radiation oncologists when interpreting MR images offered for radiotherapy. Whether MRI is used in combination with CT or in an MRI-only workflow, it is generally necessary to ensure that MR images are acquired in treatment position, using the positioning and fixation devices that are commonly applied in radiotherapy. For target delineation, often a series of separate image sets are used with distinct image contrasts, acquired within a single exam. MR images can suffer from image distortions. While this can be avoided with dedicated scan protocols, in a diagnostic setting geometrical fidelity is less relevant and is therefore less accounted for. Since geometrical fidelity is of utmost importance in radiation oncology, it requires dedicated scan protocols. The strong magnetic field of an MRI scanner and the use of radiofrequency radiation can cause safety hazards if not properly addressed. Safety screening is crucial for every patient and every operator prior to entering the MRI room.
1. Introduction
MRI is a versatile technique that is capable of producing images with a variety of tissue contrasts. Because its soft-tissue contrast is superior to CT, it is increasingly used in the radiation oncology practice to facilitate tumor and organ-at-risk delineation [1], [2], [3]. Recently MRI-guided radiotherapy systems have become available that allow MR imaging of a patient during a radiotherapy treatment fraction [4], [5], [6], [7]. A number of papers have been published on the use of MRI for radiotherapy simulation, in combination with CT or in an MR-only workflow [8], [9], [10]. Review papers are available on which image contrast to use for particular tumor sites [11], [12]. Physics studies addressing the specific issues of MRI for radiation oncology are published [13], [14], [15]. Particularly geometrical fidelity has been widely addressed and methods to ensure appropriate and reliable use of MRI for RT are available [16]. A consensus opinion on the use of MRI for radiotherapy simulation was published [15].
In this review, we specifically identify issues of MRI that are relevant for radiation oncologists when interpreting MR images offered for radiotherapy. We discuss the limitations of diagnostic MRI scans and the reason why MRI protocols specifically designed for use in radiotherapy are necessary. To this end some background on the generation of MRI will be given, but for a more complete handling of MRI physics the reader is referred to publications such as a special issue on MRI for radiotherapy published in Seminars in Radiation Oncology [13], [17], and training courses of the European Society for Radiotherapy and Oncology (ESTRO) and the International Society for Magnetic Resonance in Medicine (ISMRM).
2. MR in RT
MRI is used within the radiotherapy workflow in different scenarios. In external-beam radiotherapy MRI is used mostly in combination with CT to facilitate delineation of targets and organs at risk. The CT with delineated structures is then used for treatment planning and dose calculation. MRI is used mostly to provide anatomic information on a structure (tumor or organ at risk) that is not or poorly visible on CT. The challenge here lies in the validation of the registration [18]. Since the most relevant structures are not equally visible on both imaging modalities, the registration must rely on the correspondence of surrounding structures. In particular when deformation and internal movement of organs occurs between the MRI and CT exam, an accurate registration can be challenging. To deal with this, substantial effort can be given to ensure optimal correspondence between the two exams, for example by using identical positioning devices and by applying strict protocols to control rectal and bladder filling. Alternatively, the registration is only directed at the volume of interest and its immediate surroundings. Different strategies for several tumor sites are described by Paulson et al. [15].
To avoid the problem of registration between MRI and CT altogether, an MRI-only workflow can be chosen. The challenge here is to derive a pseudo-CT from MR images, needed for dose calculations. Several methods have been proposed, as reviewed by Nyholm and Jonsson [8]. Commercial solutions are now available for some tumor sites [19], [20].
Whether MRI is used in combination with CT or in an MRI-only workflow, it is generally necessary to ensure that MR images are acquired in treatment position, using the positioning and fixation devices that are commonly applied in radiotherapy. Often the standard diagnostic receive coils, that collect the MRI signal from the patient, cannot be used. For example for head and neck or brain imaging patients are scanned in a dedicated head coil for diagnostic purposes, which is a fixed construction with many coil elements positioned around the head. Since this leaves no space for a fixation mask, this prohibits scanning of patients in treatment position. Alternative solutions are widely available now, often using configurations of flexible coils, wrapped around the mask. However, the resulting images typically have a lower signal to noise than diagnostic images. For head-neck cancer patients, scanning in a mask is essential to reproduce the flexing of the neck between MRI and planning CT. For brain a rigid registration may be sufficient to provide an accurate correspondence between the two image sets, although a different angulation of the image planes may be confusing.
MRI is also used in the context of brachytherapy. For the treatment of cervical cancer the use of MRI guidance has created a new paradigm, allowing individual tailoring of the brachytherapy boost dose [21]. Here often an MRI-only workflow is followed, without registration of images to CT. For brachytherapy of prostate cancer, the use of MRI has helped to improve the quality of delineation of the target and organs at risk. Because of its superior soft-tissue contrast, compared to CT and ultrasound, it has been incorporated in the radiotherapy workflow for real-time image guidance, fusion with ultrasound during ultra-sound guidance, and for low dose-rate post implant dosimetry [21]. A particular benefit of MRI arises when it is used to identify the intra-prostatic lesion as a target for focal boost high dose rate brachytherapy [22].
3. Image contrast
One of the most useful features of MRI is its versatility in image contrast. MRI imaging is based on a signal that arises from hydrogen atoms that are placed in a strong magnetic field (usually 1.5 or 3.0 T for diagnostic scanners) and are irradiated with radio waves. Upon irradiation with a radio pulse, a radio signal will arise from the hydrogen atoms in the tissue. It decays however via two distinct processes called longitudinal relaxation and transversal relaxation. Longitudinal relaxation happens in a characteristic time called T1, whereas transversal relaxation is characterized by T2 relaxation time. The values of T1 and T2 depend on the surrounding of the hydrogen atom [23]. In a patient, most hydrogen is found in water and fat and therefore most signal arises from these compounds. The T1 and T2 values of water and fat are quite distinct but also other differences in composition of tissue give rise to variations in the T1 and T2 values.
The most commonly used contrasts are achieved by T1 and T2 weighting of the images. By simply tuning parameters on the console of an MRI scanner, the contrast of an image can be changed. For example, on a T1-weighted image water, with a long T1, will appear dark. However, it will appear bright on a T2-weighted image, due to its long T2. Fat will be displayed at intermediary grey values (Fig. 1). It is also possible to suppress the signal of fat specifically, which sometimes is useful to highlight pathology. As the specific T1 or T2 relaxation times vary between tissues, it is possible to highlight their differences.
Fig. 1.
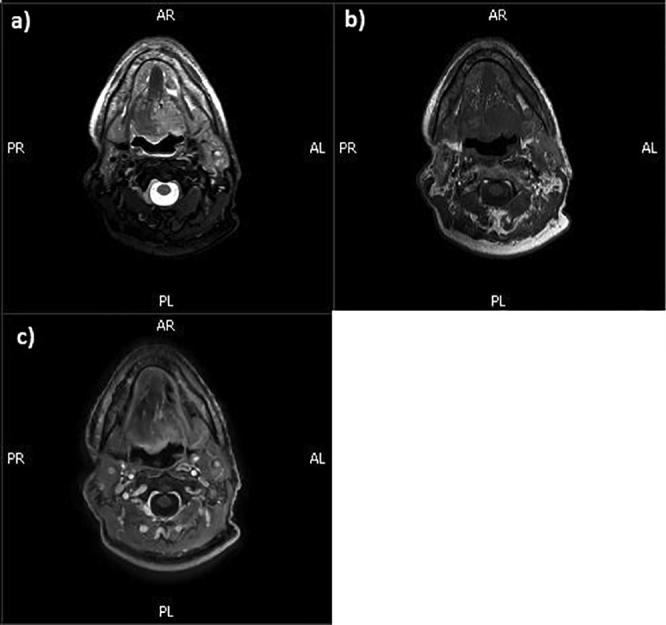
MR images of a patient with head-neck cancer showing a) T2-weighted with fat suppression; b) T1-weighted and c) T1-weigthed after administration of gadolinium contrast agent.
For target delineation in radiation oncology, often more than one type of image contrast is used. Separate image sets are acquired after each other, with different scan parameters. In this way, an MRI exam can consist for example of a T1-weighted, a T2-weighted image set and a T2-weighted image set with fat suppression.
Although MRI can produce a large variety of contrasts, simply by tuning scan parameters, it is sometimes useful to administer a contrast agent to improve the conspicuity of pathology. By administering gadolinium contrast agent, the T1 and T2 values are shortened in those areas where the contrast agent is taken up by tissue. On a T1-weighted imaging, this shortening of T1 results in an increase in signal. As contrast is mostly taken up by cancerous tissue, this enhances the conspicuity of the disease compared to surrounding tissue. Several studies review the optimal combination of sequences that is used for target delineation in radiotherapy [24], [25], [26], [27], [28].
Beyond anatomical imaging of T1 and T2 contrast, MRI also has the capacity to image tissue characteristics such as micro-anatomy, perfusion and metabolism. The concepts of functional techniques such as dynamic contrast enhanced (DCE), diffusion-weighted (DWI) MRI sequences and magnetic resonance spectroscopic imaging (MRSI) and their increasing application in radiation oncology, are discussed in a number of topical reviews [29], [30], [31].
4. Geometrical fidelity
To identify the origin of the MRI signal in the body, a process called ‘spatial encoding’ is applied. This process relies on magnetic field gradients. With these gradients, the radio signals from the hydrogen nuclei are slightly modified, depending on their location. The frequency of the radio signals is proportional to the local magnetic field. Thus, a small local change in the magnetic field results in a local off-set of the radiofrequency. This is used to trace back where the signal came from. Gradients can be applied separately in the three main directions of the scanner, but also in combination. In this way the slice orientation can be selected either in the axial, sagittal or coronal plane but also angulated along an arbitrary plane.
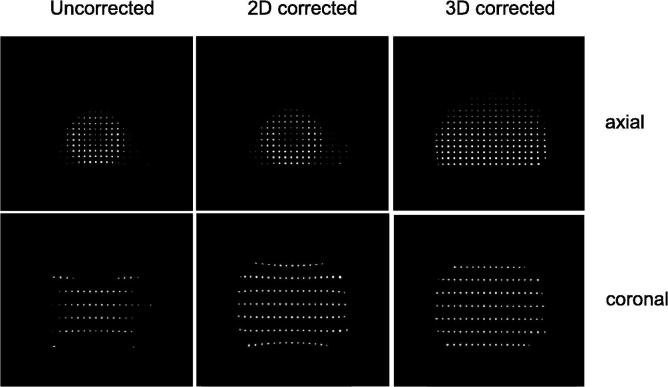
As the spatial encoding of the signal, that is used to identify from what location a signal arises, depends on magnetic field gradients, this process is sensitive to distortions in these field gradients. Unfortunately, in practice, these field gradients are not perfectly linear. In fact they flatten off towards the edges of the scanner. As a consequence, the signal encoding gets distorted. When this is not considered during reconstruction of the image, the origin of the signal will appear to be shifted. This can be corrected [16]. The main MRI vendors have integrated a software-based gradient non-linearity correction into their products. There are however some caveats: first of all, these corrections can be switched on and off. It is not easily recognized from an image itself if the correction was applied or not. Furthermore, the most commonly used sequences for T2-weighted imaging are multi-slice 2D sequences. This means that each slice is acquired one after the other. In some scanners, only a 2D correction of the gradient distortion is applied. This means that the geometry is corrected within the image plane. However, the image plane itself will still be warped when it is located outside of the iso-center of the MRI scanner [12]. In Fig. 2 the effect of these gradient non-linearities is demonstrated in the image of a grid. The distortions are minimal in the center but grow larger, up to 1 cm, towards the edges.
Fig. 2.
3D T1-weighted MRI of a grid phantom. No gradient non-linearity correction (left), a 2D gradient non-linearity correction (middle) and a 3D gradient non-linearity correction (right). Top row: axial view, bottom row: coronal view.
In 3D sequences the entire image is acquired simultaneously rather than slice by slice. In this case a 3D distortion correction can be applied. A drawback of particularly T2-weighted 3D sequences is that their image contrast differs from that of T2-weighted 2D sequences [32], [33]. When the 2D sequences are still preferred, the impact of the associated geometrical inaccuracy in those cases needs to be considered. For high-precision stereotactic treatments, the use of 2D sequences is undesirable.
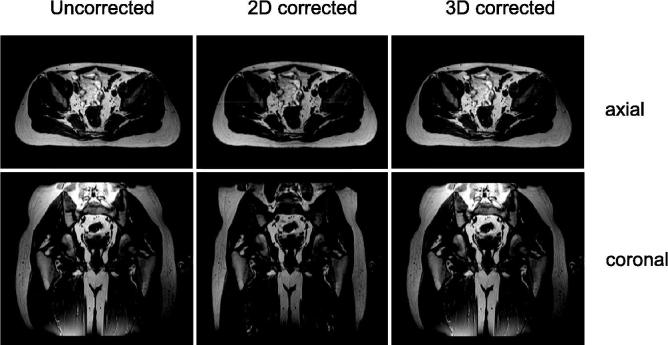
The previous argument brings to light an intrinsic problem with geometrical fidelity in clinical practice. It is very difficult, if not impossible, to assess by looking at an image if it is geometrically accurate or not. For example, in Fig. 3, the images of a healthy volunteer suffer from the same distortions as the grid. This illustrates that diagnostic images that may exhibit exquisite image contrast and signal to noise, may display a tumor and surrounding normal tissue with great clarity. Nonetheless, the actual location of these structures may be misrepresented. For this reason, it is generally recommended not to use MRI images that are made for diagnostic purposes or come from a source outside the radiation oncology department. Only when the geometrical fidelity of the specific acquisition protocol has been verified, should the images be used in a radiotherapy workflow. Accurate sequences should have a geometrical distortions smaller than 2 mm. For stereotactic applications, geometrical distortions should be smaller than 1 mm [15].
Fig. 3.
3D T1-weighted MRI of a healthy volunteer. No gradient non-linearity correction (left), a 2D gradient non-linearity correction (middle) and a 3D gradient non-linearity correction (right). Top row: axial view, bottom row: coronal view.
A consequence of the requirement of geometrical fidelity is that in the development of MRI sequences for radiation oncology different choices are optimal than for diagnostic use. Even though the image contrast of a T2-weighted 2D sequence may be superior to the 3D variation, it may be better to use the latter.
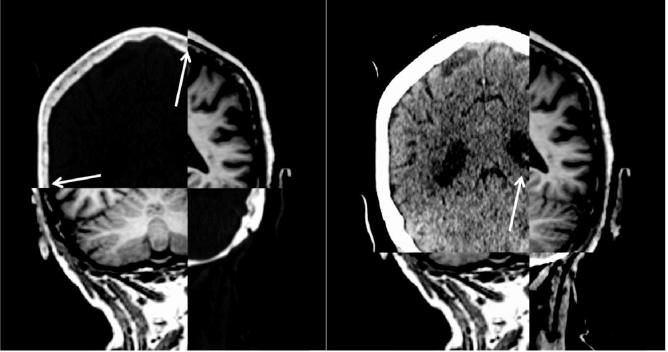
The geometrical fidelity is also affected by the so-called ‘water-fat shift’. The hydrogen atoms in water and fat produce radio signals at slightly different frequencies. The difference is only 3.4 parts per million, but this is sufficient to influence the spatial encoding. By this difference in frequency, the position of water and fat will be shifted slightly. The size of this shift is typically of the range of 0.5–2 image pixels with a typical size of 0.5–1.0 mm. For a geometrically accurate representation, a small water-fat shift is desirable and this can be tuned during sequence optimization. However, this reduction results in a loss of signal to noise. For this reason, diagnostic images are often acquired with a maximum water fat shift, compromising their usability for radiation oncology. This is another reason why radiation oncology requires dedicated scan protocols. An example is shown in Fig. 4. A diagnostic 3D T1-weighted sequence with a water-fat shift of 2 pixels of 1 × 1 mm2 is registered to a planning CT. The cortical bone is bright on CT, dark on MRI. The bone marrow has an intermediate intensity on both. The left image shows that the bone marrow in the skull is aligned correctly. On the right, the same images are shown but the window-level settings for CT are adjusted to show better soft-tissue contrast. Here the ventricles on MRI appear shifted in the cranio-caudal direction by about 2 mm. This shows that the water content of the brain tissue is shifted relative to the fatty content of the bone marrow.
Fig. 4.
A diagnostic 3D T1-weighted sequence of the brain with a water-fat shift of 2 pixels (size 1 × 1 mm2), registered to a planning CT. Left: window-level of the CT scan set to show cortical bone and bone marrow. The cortical bone is bright on CT, dark on MRI (arrows). Right: window-level of the CT scan set to show soft tissue contrast in the brain. The registration of MRI and CT is identical between left and right. The arrow points at the ventricle that appears shifted on MRI relative to CT.
5. Image artifacts
Imaging artifacts are features in an image that do not represent the underlying reality. Geometrical distortions are a particular type of artifact. The images seem of high quality and may be appropriate for diagnostic reading, although structures are represented in a wrong location, as discussed above. Another category of imaging artifacts show features that are not there. They may obscure pathology that is present or show characteristics that erroneously are interpreted as cancer.
Metal objects can cause severe image artifacts. Ferro-magnetic materials are not safe in an MRI as they may be attracted by the strong magnetic field of the scanner. Examples are some types of hip and knee prostheses. While this may be safe under certain conditions, these objects can have a profound effect on image quality [34]. First of all, the metal object itself will not produce an MRI signal, leaving a dark image void. Depending on the magnetic properties of the metal, the object will also distort the magnetic field in its surroundings. This means that the spatial encoding gets perturbed, resulting in warping of structures in the vicinity of the object. The degree of image distortion depends on the specifics of the MRI sequence and the magnetic susceptibility of the implant. The distortion tends to be larger at high-field (3 T) than at lower field (1.5 T) MRI systems. In general T2-TSE sequences are less affected than T1 gradient echo sequences. Diffusion-weighted MRI is mostly based on a so-called echo planar imaging (EPI) technique. These images are particularly sensitive to distortions of the magnetic field. While new techniques to minimize EPI distortion around metal implants are being proposed [35], diffusion-weighted MRI with standard EPI techniques is often not useful in patients with metal implants.
Although to a much smaller degree, interfaces between tissue and air/gas also distort the magnetic field. For most sequences this is a relatively small effect. It can be further reduced when a small water-fat shift is selected. However, again diffusion-weighted images are much more sensitive to this effect and severe image distortions may occur for example around rectal gas or air cavities in the head-neck area. Contouring of tumors on diffusion-weighted MRI should therefore be handled with caution. If no air pockets are present, the images may be reliable. However, near air pockets it is safer to contour on the standard T1 or T2-weighted images.
A particular class of imaging artifacts arises from motion. Periodic motion can show up as ‘ghosting’, a faint repetition of the structures in the image at periodic distances [36]. To avoid the ghosting artifacts caused by periodic breathing motion, a breath-hold acquisition can be applied. A breath-hold sequence is typically applied in the maximum inhale phase of the breathing cycle. Since this only works reliably with very fast acquisitions, for slower acquisitions a navigator-triggered sequence is often used. The breathing signal can be derived from the MRI signal in the form of a ‘navigator’. A navigator is a fast 1-dimensional acquisition, typically positioned in the cranio-caudal direction across the liver dome to detect the maximum exhale position of the breathing cycle. At this point, the regular sequence is acquired. This results in artifact-free images of the anatomy that can be used for diagnostic purposes. For radiotherapy contouring this may however be inappropriate if the treatment is done under deep inspiration breath-hold (the maximum inhale phase of the breathing cycle), or during free breathing, taking the mid-ventilation or mid-position of the tumor as reference. While standard solutions are not readily available, 4D-MRI solutions have been proposed that map multiple phases of the breathing motion and allow contouring in a mid-ventilation or mid-position phase that is more representative of the mean position during free breathing [37], [38], [39].
6. Safety
The strong magnetic field of an MRI scanner and the use of radiofrequency radiation can cause safety hazards if not properly addressed [40], [41]. The most obvious risk is that ferro-magnetic objects are attracted to the scanner. The forces involved are huge and can cause serious harm to people. This is one of the reasons why safety screening is crucial for every patient and every operator prior to entering the MRI room. Another risk is the malfunctioning of devices such as pacemakers. In some cases, modern pacemakers and implanted cardioverter defibrillators (ICDs) are compatible with use in an MRI scanner, but this needs to be checked carefully and often an operator from a cardiology department needs to be involved to verify the proper functioning of the device during and after the MRI exam. The radiofrequency waves that generate the MRI signal, can also cause heating of the patient. For this reason, strict limits on specific absorption ratio are in place on a scanner. To avoid loop currents in the body, crossing of arms and legs needs to be avoided when positioning the patient. Nonetheless, when implanted devices and wires are in position in the patient, while in the scanner, these may heat up locally and cause harm. Screening of devices for safety is therefore necessary. The same holds for fixation materials, such as carbon base plates. Increasingly, fixation devices are becoming available that are made of MRI compatible materials such as glass fiber.
To remove the risk of an MRI exam, every person and every object entering the MRI room needs to be screened for safety. Every radiology department with an MRI facility has screening procedures implemented that need to be observed. When referring a patient for an MRI exam or a treatment on an MRI-guided radiotherapy device, the radiation oncologist is responsible for this screening and has to make sure that no contra-indications exist. When in doubt, consultation with the responsible MRI safety officer, usually a medical physicist, is required. A regular updated list of implants and their safety implications can be found at http://www.mrisafety.com/ [42].
7. Conclusions
MRI has the capacity to generate images with a superior and versatile image contrast compared to CT, beneficial for the consistency of contouring of target volumes and organs at risk. For contouring often more than one type of MRI sequence is applied. Several studies have described the combinations of sequences for a range of tumor sites.
The geometrical fidelity of MRI is easily compromised. This doesn’t usually influence the quality of diagnostic reading of the images. As a consequence, the geometrical fidelity of standard MR images from a radiology department is not carefully secured. Often these images are therefore not suitable for radiotherapy contouring. In particular in the presence of breathing motion, MRI can result in image artifacts such as ghosting. Navigator-triggered sequences produce high-quality images without motion artifacts. However, as these images typically are acquired in the maximum exhale phase of the breathing cycle, they may not be appropriate for use in a radiotherapy workflow. For this reason, the use of images optimized specifically for use in a radiotherapy workflow is recommended.
The safety of the patient and operator is observed through a series of working practices. Careful screening of patients prior to referral for an MRI exam is critical. With the increased use of MRI within the radiation oncology treatment, it is necessary to collaborate closely with the experts in a radiology department. Importantly, as the specific requirements of MRI for radiation oncology may lead to different choices for image acquisition, it is important for the RTTs, physicists and radiation oncologists in a radiation oncology department to be aware of the specific issues and discuss them with the colleagues in a radiology department.
Increasingly, MRI is used in the various steps of a radiotherapy treatment. Although MRI can have limitations and risks, when operated with care and expertise both on MRI physics and the radiotherapy workflow, it has proven to be of great benefit for target delineation and treatment guidance both in brachytherapy and external-beam radiotherapy.
Declaration of interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2019.04.008.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Rasch C., Steenbakkers R., van Herk M. Target definition in prostate, head, and neck. Semin Radiat Oncol. 2005;15(3):136–145. doi: 10.1016/j.semradonc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Khoo V.S., Joon D.L. New developments in MRI for target volume delineation in radiotherapy. Br J Radiol. 2006;79(Spec No 1):S2–S15. doi: 10.1259/bjr/41321492. [DOI] [PubMed] [Google Scholar]
- 3.Gurney-Champion O.J., Versteijne E., van der Horst A., Lens E., Rütten H., Heerkens H.D., Paardekooper G.M.R.M., Berbee M., Rasch C.R.N., Stoker J., Engelbrecht M.R.W., van Herk M., Nederveen A.J., Klaassen R., van Laarhoven H.W.M., van Tienhoven G., Bel A. Addition of MRI for CT-based pancreatic tumor delineation: a feasibility study. Acta Oncol. 2017;56(7):923–930. doi: 10.1080/0284186X.2017.1304654. [DOI] [PubMed] [Google Scholar]
- 4.Ménard C., van der Heide U. Introduction: systems for magnetic resonance image guided radiation therapy. Semin Radiat Oncol. 2014;24(3):192. doi: 10.1016/j.semradonc.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Pollard J.M., Wen Z., Sadagopan R., Wang J., Ibbott G.S. The future of image-guided radiotherapy will be MR guided. Br J Radiol. 2017;90(1073):20160667. doi: 10.1259/bjr.20160667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kashani R., Olsen J.R. Magnetic resonance imaging for target delineation and daily treatment modification. Semin Radiat Oncol. 2018;28(3):178–184. doi: 10.1016/j.semradonc.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Liney G.P., Whelan B., Oborn B., Barton M., Keall P. MRI-linear accelerator radiotherapy systems. Clin Oncol (R Coll Radiol) 2018;30(11):686–691. doi: 10.1016/j.clon.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Nyholm T., Jonsson J. Counterpoint: opportunities and challenges of a magnetic resonance imaging-only radiotherapy work flow. Semin Radiat Oncol. 2014;24(3):175–180. doi: 10.1016/j.semradonc.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Kerkmeijer L.G.W., Maspero M., Meijer G.J., van der Voort van Zyp J.R.N., de Boer H.C.J., van den Berg C.A.T. Magnetic resonance imaging only workflow for radiotherapy simulation and planning in prostate cancer. Clin Oncol (R Coll Radiol) 2018;30(11):692–701. doi: 10.1016/j.clon.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Chandarana H., Wang H., Tijssen R.H.N., Das I.J. Emerging role of MRI in radiation therapy. J Magn Reson Imaging. 2018;48(6):1468–1478. doi: 10.1002/jmri.26271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dirix P., Haustermans K., Vandecaveye V. The value of magnetic resonance imaging for radiotherapy planning. Semin Radiat Oncol. 2014;24(3):151–159. doi: 10.1016/j.semradonc.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Paulson E.S., Erickson B., Schultz C., Allen Li X. Comprehensive MRI simulation methodology using a dedicated MRI scanner in radiation oncology for external beam radiation treatment planning. Med Phys. 2015;42(1):28–39. doi: 10.1118/1.4896096. [DOI] [PubMed] [Google Scholar]
- 13.Liney G.P., Moerland M.A. Magnetic resonance imaging acquisition techniques for radiotherapy planning. Semin Radiat Oncol. 2014;24(3):160–168. doi: 10.1016/j.semradonc.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt M.A., Payne G.S. Radiotherapy planning using MRI. Phys Med Biol. 2015;60(22):R323–R361. doi: 10.1088/0031-9155/60/22/R323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paulson E.S., Crijns S.P., Keller B.M., Wang J., Schmidt M.A., Coutts G., van der Heide U.A. Consensus opinion on MRI simulation for external beam radiation treatment planning. Radiother Oncol. 2016;121(2):187–192. doi: 10.1016/j.radonc.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 16.Doran S.J., Charles-Edwards L., Reinsberg S.A., Leach M.O. A complete distortion correction for MR images: I Gradient warp correction. Phys Med Biol. 2005;50(7):1343–1361. doi: 10.1088/0031-9155/50/7/001. [DOI] [PubMed] [Google Scholar]
- 17.Ménard C., van der Heide U.A. Introduction: magnetic resonance imaging comes of age in radiation oncology. Semin Radiat Oncol. 2014;24(3):149–150. doi: 10.1016/j.semradonc.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Brock K.K., Dawson L.A. Point: principles of magnetic resonance imaging integration in a computed tomography-based radiotherapy workflow. Semin Radiat Oncol. 2014;24(3):169–174. doi: 10.1016/j.semradonc.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Tyagi N., Fontenla S., Zelefsky M., Chong-Ton M., Ostergren K., Shah N. Clinical workflow for MR-only simulation and planning in prostate. Radiat Oncol. 2017;12(1):119. doi: 10.1186/s13014-017-0854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Persson E., Gustafsson C., Nordström F., Sohlin M., Gunnlaugsson A., Petruson K. A Multicenter/multivendor validation of magnetic resonance imaging-only prostate treatment planning using synthetic computed tomography images. Int J Radiat Oncol Biol Phys. 2017;99(3):692–700. doi: 10.1016/j.ijrobp.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Tanderup K., Lindegaard J.C., Kirisits C., Haie-Meder C., Kirchheiner K., de Leeuw A. Image guided adaptive brachytherapy in cervix cancer: a new paradigm changing clinical practice and outcome. Radiother Oncol. 2016;120(3):365–369. doi: 10.1016/j.radonc.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Mason J., Bownes P., Carey B., Henry A. Comparison of focal boost high dose rate prostate brachytherapy optimisation methods. Radiother Oncol. 2015;117(3):521–524. doi: 10.1016/j.radonc.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 23.Pooley R.A. AAPM/RSNA physics tutorial for residents: fundamental physics of MR imaging. Radiographics. 2005;25(4):1087–1099. doi: 10.1148/rg.254055027. [DOI] [PubMed] [Google Scholar]
- 24.Pech M., Mohnike K., Wieners G., Bialek E., Dudeck O., Seidensticker M. Radiotherapy of liver metastases. Comparison of target volumes and dose-volume histograms employing CT- or MRI-based treatment planning. Strahlenther Onkol. 2008;184(5):256–261. doi: 10.1007/s00066-008-1849-8. [DOI] [PubMed] [Google Scholar]
- 25.Whitfield G.A., Kennedy S.R., Djoukhadar I.K., Jackson A. Imaging and target volume delineation in glioma. Clin Oncol (R Coll Radiol) 2014;26(7):364–376. doi: 10.1016/j.clon.2014.04.026. Epub 2014 May 11. [DOI] [PubMed] [Google Scholar]
- 26.Popovtzer A., Ibrahim M., Tatro D., Feng F.Y., Ten Haken R.K., Eisbruch A. MRI to delineate the gross tumor volume of nasopharyngeal cancers: which sequences and planes should be used? Radiol Oncol. 2014;48(3):323–330. doi: 10.2478/raon-2014-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heerkens H.D., Hall W.A., Li X.A., Knechtges P., Dalah E., Paulson E.S. Recommendations for MRI-based contouring of gross tumor volume and organs at risk for radiation therapy of pancreatic cancer. Pract Radiat Oncol. 2017;7(2):126–136. doi: 10.1016/j.prro.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Kulkarni B.S.N., Bajwa H., Chandrashekhar M., Sharma S.D., Singareddy R., Gudipudi D. CT- and MRI-based gross target volume comparison in vestibular schwannomas. Rep Pract Oncol Radiother. 2017;22(3):201–208. doi: 10.1016/j.rpor.2017.02.002. Epub 2017 Apr 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Metcalfe P., Liney G.P., Holloway L., Walker A., Barton M., Delaney G.P., Vinod S., Tome W. The potential for an enhanced role for MRI in radiation-therapy treatment planning. Technol Cancer Res Treat. 2013;12(5):429–446. doi: 10.7785/tcrt.2012.500342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsuo M., Matsumoto S., Mitchell J.B., Krishna M.C., Camphausen K. Magnetic resonance imaging of the tumor microenvironment in radiotherapy: perfusion, hypoxia, and metabolism. Semin Radiat Oncol. 2014;24(3):210–217. doi: 10.1016/j.semradonc.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leibfarth S., Winter R.M., Lyng H., Zips D., Thorwarth D. Potentials and challenges of diffusion-weighted magnetic resonance imaging in radiotherapy. Clin Transl Radiat Oncol. 2018;20(13):29–37. doi: 10.1016/j.ctro.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hori M., Kim T., Onishi H., Ueguchi T., Tatsumi M., Nakamoto A., Tsuboyama T., Tomoda K., Tomiyama N. Uterine tumors: comparison of 3D versus 2D T2-weighted turbo spin-echo MR imaging at 3.0 T–initial experience. Radiology. 2011;258(1):154–163. doi: 10.1148/radiol.10100866. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka U., Ueno Y., Morinaga Y., Miyake H., Kyotani K., Ueda Y., Kitajima K., Sofue K., Suenaga Y., Sugimura K., Takahashi S. Value of three-dimensional T2-weighted turbo spin-echo imaging with tissue-specific variable refocusing flip angle for 3-T magnetic resonance imaging of prostate cancer: comparison with conventional two- and three-dimensional T2-weighted turbo spin-echo imaging. Jpn J Radiol. 2017;35(12):707–717. doi: 10.1007/s11604-017-0684-1. [DOI] [PubMed] [Google Scholar]
- 34.Dillenseger J.P., Molière S., Choquet P., Goetz C., Ehlinger M., Bierry G. An illustrative review to understand and manage metal-induced artifacts in musculoskeletal MRI: a primer and updates. Skeletal Radiol. 2016;45(5):677–688. doi: 10.1007/s00256-016-2338-2. [DOI] [PubMed] [Google Scholar]
- 35.Koch K.M., Bhave S., Gaddipati A., Hargreaves B.A., Gui D., Peters R., Bedi M., Mannem R., Kaushik S.S. Multispectral diffusion-weighted imaging near metal implants. Magn Reson Med. 2018;79(2):987–993. doi: 10.1002/mrm.26737. [DOI] [PubMed] [Google Scholar]
- 36.Zaitsev M., Maclaren J., Herbst M. Motion artifacts in MRI: A complex problem with many partial solutions. J Magn Reson Imaging. 2015;42(4):887–901. doi: 10.1002/jmri.24850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van de Lindt T.N., Schubert G., van der Heide U.A., Sonke J.J. An MRI-based mid-ventilation approach for radiotherapy of the liver. Radiother Oncol. 2016;121(2):276–280. doi: 10.1016/j.radonc.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 38.van de Lindt T.N., Fast M.F., van der Heide U.A., Sonke J.J. Retrospective self-sorted 4D-MRI for the liver. Radiother Oncol. 2018;127(3):474–480. doi: 10.1016/j.radonc.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 39.Stemkens B., Paulson E.S., Tijssen R.H.N. Nuts and bolts of 4D-MRI for radiotherapy. Phys Med Biol. 2018;63(21):21TR01. doi: 10.1088/1361-6560/aae56d. [DOI] [PubMed] [Google Scholar]
- 40.Weidman E.K., Dean K.E., Rivera W., Loftus M.L., Stokes T.W., Min R.J. MRI safety: a report of current practice and advancements in patient preparation and screening. Clin Imaging. 2015;39(6):935–937. doi: 10.1016/j.clinimag.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Panych L.P., Madore B.J. The physics of MRI safety. Magn Reson Imaging. 2018;47(1):28–43. doi: 10.1002/jmri.25761. [DOI] [PubMed] [Google Scholar]
- 42.Shellock FG. Reference Manual for Magnetic Resonance Safety, Implants, and Devices: 2019 edition (ISBN 978-0-9891632-6-2).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.