Highlights
-
•
The Elekta Unity MR-linac adaptive radiotherapy concept is explained.
-
•
The adapt to shape and adapt to position workflows are compared.
-
•
Different methods for dose re-calculation and optimization are discussed.
-
•
Full online re-planning is the most robust adaptive planning method for the Unity.
-
•
Faster methods are available, but should be dosimetrically explored per use case.
Keywords: Radiotherapy, MRI-guided radiotherapy, Online plan adaptation, MR-linac, Adaptive radiotherapy
Abstract
Background and purpose
The promise of the MR-linac is that one can visualize all anatomical changes during the course of radiotherapy and hence adapt the treatment plan in order to always have the optimal treatment. Yet, there is a trade-off to be made between the time spent for adapting the treatment plan against the dosimetric gain. In this work, the various daily plan adaptation methods will be presented and applied on a variety of tumour sites. The aim is to provide an insight in the behavior of the state-of-the-art 1.5 T MRI guided on-line adaptive radiotherapy methods.
Materials and methods
To explore the different available plan adaptation workflows and methods, we have simulated online plan adaptation for five cases with varying levels of inter-fraction motion, regions of interest and target sizes: prostate, rectum, esophagus and lymph node oligometastases (single and multiple target). The plans were evaluated based on the clinical dose constraints and the optimization time was measured.
Results
The time needed for plan adaptation ranged between 17 and 485 s. More advanced plan adaptation methods generally resulted in more plans that met the clinical dose criteria. Violations were often caused by insufficient PTV coverage or, for the multiple lymph node case, a too high dose to OAR in the vicinity of the PTV. With full online replanning it was possible to create plans that met all clinical dose constraints for all cases.
Conclusion
Daily full online replanning is the most robust adaptive planning method for Unity. It is feasible for specific sites in clinically acceptable times. Faster methods are available, but before applying these, the specific use cases should be explored dosimetrically.
1. Introduction
In the UMC Utrecht, together with Elekta AB. (Stockholm, Sweden) and Philips (Best, The Netherlands) a hybrid 1.5 T MRI linac (MR-linac) has been developed, built and clinically introduced [1], [2], [3]. This system has evolved to the Elekta Unity system and has been clinically introduced with the treatment of oligometastatic lymph nodes in 2018 [4].
The MR-linac design is a linear accelerator with integrated 1.5 T MRI functionality, the proof of concept of this system is presented in Raaymakers et al. (2009) [3]. The goal of such integration is to enable soft-tissue contrast MR imaging directly from the treatment table to visualize all anatomical changes during the course of radiotherapy. MRI can be used to capture both inter-fraction motion but also intra-fraction motion [5]. These data can be applied in many ways as input for adaptive radiotherapy. Daily MRI can be used for soft-tissue based position verification or daily replanning, beam-on MRI can be used for time resolved dose accumulation [6] or real-time replanning [7].Finally, repeated anatomical and functional MRI can be used for treatment response assessment [8], [9].
Using repeated MRI data for adaptive radiotherapy requires to interpret it, which typically means propagating the contours from the (pre-treatment) reference data to the latest timepoint by image registration. This image registration is often an ill-posed problem which requires tumour site specific quality assurance to warrant realistic results [10]. The first release of Unity provides different means for daily adaptive, MRI based radiotherapy, as the treatment is initialized daily, manual inspection is used to validate the contouring. Also, the concept of Unity is that every treatment fraction requires a treatment plan adaptation, so for every treatment fraction, a choice between the various adaptation modes has to be made. The treatment plan adaptation strategies will be detailed below in the methods section. They can be divided into two main categories, being ‘adapt-to-position’ (ATP) and ‘adapt-to-shape’ (ATS). For ATP no daily delineation is needed nor possible, only the (isocenter) position is updated in the pre-treatment CT, while for ATS the daily MRI can be re-contoured to be used for adapting the treatment plan.
The promise of the MR-linac is that one can visualize all anatomical changes during the course of radiotherapy and hence adapt the treatment plan in order to always have the optimal treatment. This should lead to better target conformality and lower normal tissue involvement. Yet, there is a trade-off to be made between the time spent for adapting the treatment plan against the dosimetric gain. In this work, the various daily plan adaptation methods will be presented and applied on a variety of tumour sites. Both the dosimetric consequences as well as the time such adaptation takes will be reported. The aim is to provide an insight in the current behavior of the state-of-the-art 1.5 T MRI guided on-line adaptive radiotherapy methods.
2. Methods
2.1. Pre-treatment preparation
Prior to treatment on the 1.5 T MR-linac a pre-treatment CT and MRI were acquired. A special table overlay was used for CT scan acquisition, to enable reproducible positioning of the RF coil and approximative patient set-up using specific couch index points. The target and organs at risk were contoured by a radiation oncologist on the pre-treatment imaging data. A pre-treatment plan is then generated using the Monaco 5.4 (Elekta AB, Stockholm, Sweden) treatment planning system (TPS).
2.2. Adaptive treatment planning
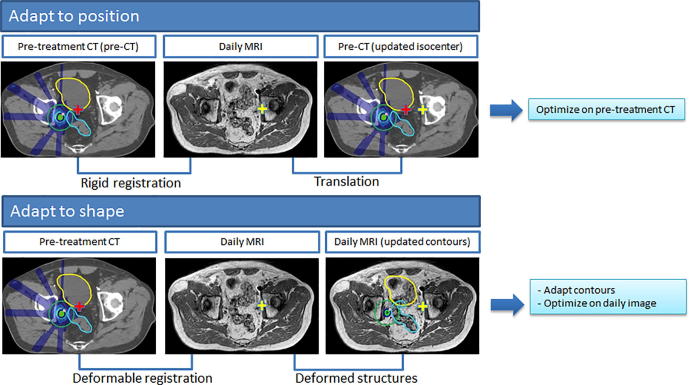
Each treatment fraction starts with the acquisition of an online MRI. The pre-treatment CT, contours and plan, together with the online MRI are used as input to adapt the plan for that specific session. Performing plan adaptation on the Unity system can be performed through two different workflows using the Monaco TPS: adapt to position (ATP) and adapt to shape (ATS) (Fig. 1).
Fig. 1.
Schematic overview of the differences between the MR-linac Unity “adapt to shape” method in which online plan adaptation is performed on the new patient anatomy and optimized on the daily MRI and adapted contours, and the “adapt to position” method in which online plan adaptation is performed based on the new patient position and optimized on the pre-treatment CT and contours. Using the “adapt to position” method, rigid registration can be performed on the entire image sets, or using a clipbox around a region of interest.
The ATP workflow allows for plan adaptation based on the online patient position. The pre-treatment CT is matched with the online MRI through rigid registration. Based on this rigid registration, the isocenter position in the reference data is updated. The pre-treatment plan is then recalculated or reoptimized to reproduce or improve the target coverage from the pre-treatment plan through one of the available plan adaptation methods, see Section 2.3. Recalculating or reoptimizing the plan is performed on the pre-treatment CT and contours. This implies, no contours need (and can) be edited, as the original contours will be used for the adapted plan.
The second workflow, adapt to shape (ATS), allows for plan adaptation based on the new patient anatomy and the plan is optimized on the daily MRI and adapted contours. Again, the first step is that the pre-treatment CT and online planning MRI are registered. The pre-treatment contours are then automatically propagated by deformable registration onto the online planning MRI. If deemed necessary, contours are edited by a radiation oncologist. Electron densities (ED) were assigned per structure based on the average ED value of the corresponding contour on the pre-treatment CT. This last step is important, because the plan is then recalculated or reoptimized on the online planning MRI and adjusted contours. Optimization in the ATS workflow is performed based on the pre-treatment planning objectives. Similar to ATP, the ATS workflow offers multiple options for plan recalculation or reoptimization.
2.3. Calculation and optimization methods
Overall, online MR-guided adaptive treatment planning on the 1.5 T MR-linac allows for six different plan adaptation methods:
-
A.
Original Segments
-
B.
Adapt Segments
-
C.
Optimize Weights from segments
-
D.
Optimize Weights from fluence
-
E.
Optimize Weights and Shapes from segments
-
F.
Optimize Weights and Shapes from fluence
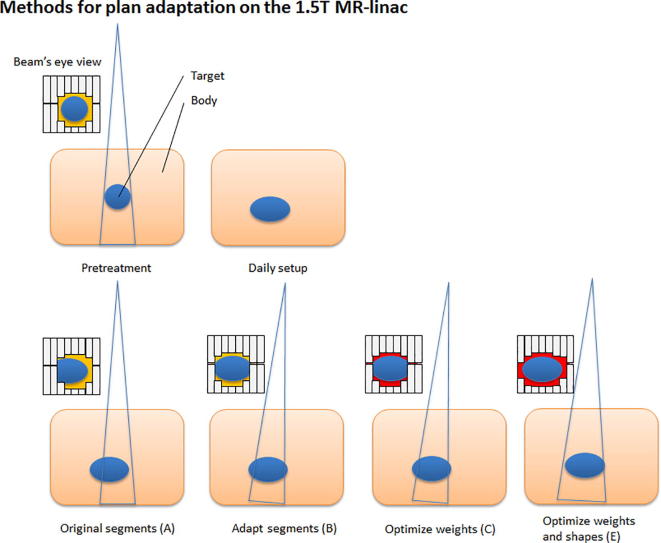
The Original Segments (A) method makes use of the segments and monitor units (MUs) from the pre-treatment plan. Herewith, the original plan is calculated on the pre-treatment CT (ATP) or daily MRI (ATS). The Adapt Segments (B) method shifts the segments from the pre-treatment plan relative to the isocenter, based on the registration between the pre-treatment and online images, using Segment Aperture Morphing (SAM) [10]. Using the resulting segments and the original segment weights, the dose is then recalculated. Both Optimize Weights (C,D) methods are based on optimizing the weights of the segments for the new patient position or daily anatomy by adjusting the amount of MUs. Method C optimizes the weights, using the set of segments from the pre-treatment plan after SAM. With method D the segments from the pre-treatment plan are discarded. The fluence is first reoptimized and a new set of segments is created. The new set of segments is then further optimized using segment weight optimization. The same distinction applies for both Weights and Shapes (E,F) methods. Either the pre-treatment segments are used (after SAM) for weight and shape optimization (method E), or the pre-treatment segments are discarded a dose fluence reoptimization is performed. The adjusted or new set of segments is then reoptimized for the new patient position or daily anatomy situation using both segment weight optimization. This method optimizes the amount of MUs per segment and further optimizes the segment shapes.
In general this means that for methods A, B, C and E, the segments from the pre-treatment plan are used for input (Fig. 2). For method D and F, a new set of segments is created based on the reoptimized fluence. Method F, in which first the fluence is reoptimized and segmented and after which segment weight and shape optimization is performed, is equal to full online replanning. The ATP workflow does not offer the option to start with a fluence optimization. This implies that the ATP workflow only offers methods A, B, C and E. Again, note that for ATP, the plan adaptation is done on the pre-treatment CT, i.e. for E the plan on the original CT with the original contours is reoptimized solely for the updated isocenter position.
Fig. 2.
Schematic overview of the segment changes for the different plan recalculation and reoptimization methods available in the treatment planning software for the 1.5 T MR-linac. A different background color (e.g. red or yellow) in the Beam’s eye view (BEV) indicates a different segment weighting. When performing plan adaptation methods using optimize weights (method D) or optimize weights and shapes (method F) starting with full fluence optimization, the original segments are discarded and new initial plan segmentation is performed. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.4. Plan exploration
To explore the different available plan adaptation workflows and methods, we have simulated online plan adaptation using the clinical system for five cases with varying levels of inter-fraction motion, regions of interest and target sizes: prostate, rectum, esophagus and lymph node (LN) oligometastases (single and multiple target). A pre-treatment CT and clinical plan were used to adapt the plan to the daily anatomy, as visible on MR-imaging. The datasets were registered to each other through rigid registration using a clipbox around the PTV. For the ATP workflow the optimization method to reproduce goal dose was used. The resulting plans were recalculated on the daily anatomy as visible on the online planning MRI. For the ATS workflow, the patient contours were manually corrected by a radiation oncologist. The plans were evaluated based on the clinical dose constraints and the optimization time was measured.
3. Results
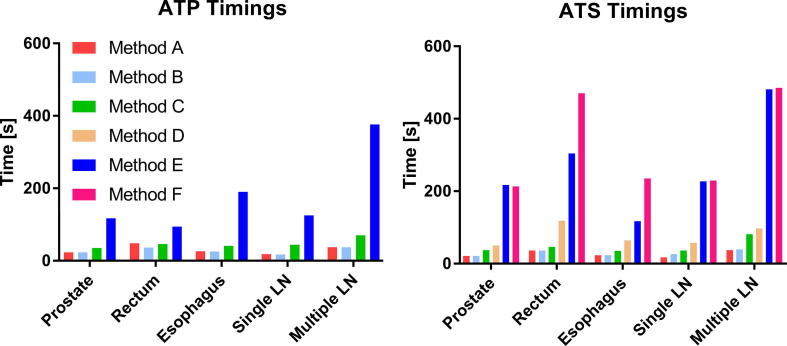
The time needed for reoptimization using the available methods in the ATP workflow ranged between the 18 and 376 s (Table 1). Generally, a more complex patient anatomy led to longer reoptimization times. Method A and B did not result in plans that met the clinical dose constraints, see Table 1. With method C, only for the single LN case the adapted plan met all dose criteria. For the rectum case, the criteria were met when evaluating on the pre-treatment contours, but after recalculating the dose on the daily anatomy, violations were observed. For method E, the rectum and esophagus plans also met all dose criteria when evaluating on the pre-treatment contours, but showed violations after recalculation on the daily MR anatomy. The single LN plan met all dose criteria.
Table 1.
Optimization time required for online plan optimization for the 1.5 T MR-linac. A red background indicates one or more dose constraints were violated. Orange depicts all criteria were met on the pre-treatment data, but not when evaluating on the daily anatomy. Green depicts all criteria were met. Method A – F describe: A the original segments, B adapt segments, C optimize weights from segments, D optimize weights from fluence, E optimize weights and shapes from segments and F optimize weights and shapes from fluence, respectively.
 |
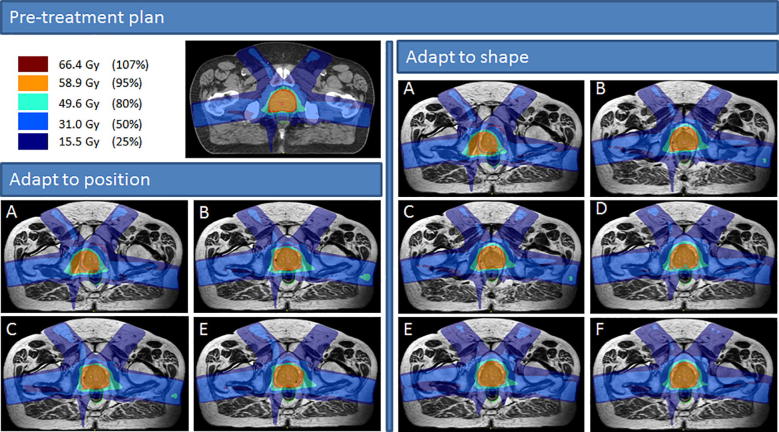
With the ATS workflow, reoptimization time was longer than for ATP and ranged between 17 and 485 s (Fig. 3). Method A did not result in any plans that met the clinical dose criteria. Using method B, C and D, it was possible to create plans that met all clinical dose criteria for the single LN case, but not for prostate, rectum, esophagus and the multiple LN case. Using method E it was possible to create plans that met all criteria for rectum, esophagus and the single LN case. With method F, which is essentially full online replanning, it was possible to create plans that met all clinical dose constraints for all cases used in this study. Violations for other methods were often caused by insufficient PTV coverage or, for the multiple lymph node case, a too high dose to OAR in the vicinity of the PTV. In Fig. 4 the dose distributions for the various adaptive modi are shown for the prostate case. It can be seen that, looking at the 80% dose-level, each results in different dose distributions.
Fig. 3.
Recalculation or optimization time required for plan adaptation for the methods available in the adapt to position (ATP) and adapt to shape (ATS) workflows. Method A – F describe: A the original segments, B adapt segments, C optimize weights from segments, D optimize weights from fluence, E optimize weights and shapes from segments and F optimize weights and shapes from fluence, respectively.
Fig. 4.
Prostate case with the resulting dose distributions for the adapt to position (ATP) and adapt to shape (ATS) workflows. Method A – F describe: A the original segments, B adapt segments, C optimize weights from segments, D optimize weights from fluence, E optimize weights and shapes from segments and F optimize weights and shapes from fluence, respectively.
4. Discussion
The ATP based plan adaptations are faster than ATS based methods. However, the ATP methods lack target coverage, even for small anatomical variations. For this study we have used the current clinical CBCT-linac PTV margins. Using smaller margins, target coverage after ATP based plan adaptation is expected to be further reduced. Moreover, the ATP results are standardly presented by calculations on the pre-treatment reference data and this might not be representative for the actual anatomy as can be seen for the rectum and esophagus examples by the orange boxes in Table 1; the ATP may report agreement with treatment planning constraints but when recalculating on the daily MRI, violations are seen.
The only method that passed all treatment planning constraints on the daily MRI was method F in the ATS workflow. This result was expected as this equals daily full replanning. One conclusion is that if the time for this method is considered acceptable, ATS with method F is the most robust adaptation. Yet, this approach requires on-line contour review or online contour editing which further adds to the overall required time. When only small anatomical changes are expected or relatively large intra-fraction motion could be induced by the increased initialization time, ATP might be preferred. Given the dosimetric results for the examples in this work, and the difference in time spent on method C, i.e. segment weight optimization, relative to A and B, ATP plus method C seems the minimal adaptation mode to use.
The choice between method C and E for ATP, i.e. reoptimizing just segment weights or also reoptimizing the segment shapes, depends on the tolerance for errors. Errors induced by any method can be quantified by recalculation using the daily MRI. If the error is too large, an offline replanning could be considered in order to mitigate, or at least minimize, the overall treatment dose violation. This approach might be acceptable for hyper-fractionated treatments. For hypo-fractionated treatments the investment into full daily replanning is better justified.
Within the Unity workflow, it is also possible to combine ATS and ATP. For instance, the treatment is initialized using full replanning, i.e. ATS using method F, but prior to beam delivery, a second MRI is acquired and if the anatomy is changed during the preparation, ATP is used to quickly adapt to the (probably small) anatomical change. In UMC Utrecht, this is the approach for our prostate treatments on Unity: ATS using method F is potentially followed by ATP with segment weight optimization if the prostate has shifted. Again, offline dose reconstruction can be done to verify accuracy of this approach.
A small disadvantage of ATS is that its MRI based reoptimization is done using bulk densities. However, this does not significantly influence the dose calculation accuracy when adequate bulk assignments are made [12]. For several cases this might have a larger effect in which case electron densities assignment should be considered more carefully. In particular structures with high densities or air cavities with the influence of the electron return effect [13], [14].
The examples in this work are quite heterogeneous to show the mechanisms of the various workflows and adaptation methods. Before introducing a new patient category with a specific fractionation scheme on the Unity, a careful evaluation of the various options has to be made to choose the appropriate trade-off. For oligometastatic lymph node treatments with 5 × 7 Gy, this is done in Winkel et al. (2019) [15], [16].
Future work is on intra-fraction plan adaptation, where the ultimate goal is to get to real-time plan adaptation while accounting for the dose delivered so far. Kontaxis et al. [17] presented a loop that enables such continuous reoptimization during beam delivery. Basically the approach mentioned above, where dosimetric errors are revealed by offline recalculation are a first, very simple, yet feasible, implementation of such loop. A next step could be to also allow, for instance, intra-fraction ATP.
The computational time associated with the different methods will decrease with increasing computer power and smarter algorithms. In a research setting, full replanning can already be done in approximately 1 min [7]. Solid contour propagation and reliable quality assurance for this are key to speed up full replanning approaches [11].
5. Conclusion
Daily full online replanning is the most robust adaptive planning method for Unity. It is for specific sites feasible in clinically acceptable times. Faster methods are available, but before applying these, the specific use case should be explored dosimetrically.
Acknowledgments
Acknowledgements
This work is part of a long standing research relation with, and is financially supported by, Elekta.
Conflict of interest statement
The University Medical Center Utrecht MR-linac scientific project, including employment of multiple authors, has been partly funded by Elekta AB (Stockholm, Sweden) and Philips Medical Systems (Best, The Netherlands). R.H.N. Tijssen receives research support from Philips Medical Systems (Best, The Netherlands). The other authors declared that there is no other conflict of interest.
References
- 1.Lagendijk J.J.W., Raaymakers B.W., van Vulpen M. The magnetic resonance imaging-linac system. Semin Radiat Oncol. 2014;24:207–209. doi: 10.1016/j.semradonc.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Raaymakers B.W., Jürgenliemk-Schulz I.M., Bol G.H., Glitzner M., Kotte A.N.T.J., van Asselen B. First patients treated with a 1.5 T MRI-Linac: clinical proof of concept of a high-precision, high-field MRI guided radiotherapy treatment. Phys Med Biol. 2017;62:L41–L50. doi: 10.1088/1361-6560/aa9517. [DOI] [PubMed] [Google Scholar]
- 3.Raaymakers B.W., Lagendijk J.J.W., Overweg J., Kok J.G., Raaijmakers A.J., Kerkhof E.M. Integrating a 1.5 T MRI scanner with a 6 MV accelerator: proof of concept. Phys Med Biol. 2009;54:N229–N237. doi: 10.1088/0031-9155/54/12/N01. [DOI] [PubMed] [Google Scholar]
- 4.Werensteijn-Honingh A.M., Kroon P.S., Winkel D., Aalbers E.M., Van Asselen B., Bol G.H. Feasibility of stereotactic radiotherapy using a 1.5 T MR-linac: multi-fraction treatment of pelvic lymph node oligometastases. Radiother Oncol. 2019;134:50–54. doi: 10.1016/j.radonc.2019.01.024. [DOI] [PubMed] [Google Scholar]
- 5.Kleijnen J.J.E., van Asselen B., Burbach J.P.M., Intven M.P.W., Philippens M.E.P., Reerink O. Evolution of motion uncertainty in rectal cancer: implications for adaptive radiotherapy. Phys Med Biol. 2016;61:1–11. doi: 10.1088/0031-9155/61/1/1. [DOI] [PubMed] [Google Scholar]
- 6.Stemkens B., Glitzner M., Kontaxis C., de Senneville B.D., Prins F.M., Crijns S.P.M. Effect of intra-fraction motion on the accumulated dose for free-breathing MR-guided stereotactic body radiation therapy of renal-cell carcinoma. Phys Med Biol. 2017;62:7407–7424. doi: 10.1088/1361-6560/aa83f7. [DOI] [PubMed] [Google Scholar]
- 7.Kontaxis C., Bol G.H., Kerkmeijer L.G.W., Lagendijk J.J.W., Raaymakers B.W. Fast online replanning for interfraction rotation correction in prostate radiotherapy. Med Phys. 2017;44:5034–5042. doi: 10.1002/mp.12467. [DOI] [PubMed] [Google Scholar]
- 8.van der Heide U.A., Houweling A.C., Groenendaal G., Beets-Tan R.G.H., Lambin P. Functional MRI for radiotherapy dose painting. Magn Reson Imaging. 2012;30:1216–1223. doi: 10.1016/j.mri.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Rossum P.S., van Lier A.L., van Vulpen M., Reerink O., Lagendijk J.J.W., Lin S.H. Diffusion-weighted magnetic resonance imaging for the prediction of pathologic response to neoadjuvant chemoradiotherapy in esophageal cancer. Radiother Oncol. 2015;115:163–170. doi: 10.1016/j.radonc.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 10.Ahunbay E.E., Peng C., Chen G.P., Narayanan S., Yu C., Lawton C. An on-line replanning scheme for interfractional variations. Med Phys. 2008;35:3607–3615. doi: 10.1118/1.2952443. [DOI] [PubMed] [Google Scholar]
- 11.Zachiu C., de Senneville B.D., Moonen C.T.W., Raaymakers B.W., Ries M. Anatomically plausible models and quality assurance criteria for online mono- and multi-modal medical image registration. Phys Med Biol. 2018;63 doi: 10.1088/1361-6560/aad109. [DOI] [PubMed] [Google Scholar]
- 12.Jonsson J.H., Karlsson M.G., Karlsson M., Nyholm T. Treatment planning using MRI data: an analysis of the dose calculation accuracy for different treatment regions. Radiat Oncol. 2015;5:62. doi: 10.1186/1748-717X-5-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoogcarspel S.J., Van der Velden J.M., Lagendijk J.J., van Vulpen M., Raaymakers B.W. The feasibility of utilizing pseudo CT-data for online MRI based treatment plan adaptation for a stereotactic radiotherapy treatment of spinal bone metastases. Phys Med Biol. 2014;7:7383–7391. doi: 10.1088/0031-9155/59/23/7383. [DOI] [PubMed] [Google Scholar]
- 14.Raaijmakers A.J., Raaymakers B.W., Lagendijk J.J.W. Integrating a MRI scanner with a 6 MV radiotherapy accelerator: dose increase at tissue-air interfaces in a lateral magnetic field due to returning electrons. Phys Med Biol. 2005;50:1363–1376. doi: 10.1088/0031-9155/50/7/002. [DOI] [PubMed] [Google Scholar]
- 15.Winkel D., Bol G., Werensteijn-Honingh A., Kiekebosch I., Van Asselen B., Intven M. Evaluation of plan adaptation strategies for stereotactic radiotherapy of lymph node oligometastases using online magnetic resonance image guidance. Phys Imaging Radiat Oncol. 2019;9:58–64. doi: 10.1016/j.phro.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winkel D., Werensteijn-Honingh A., Kroon P.S., Eppinga W.S.C., Bol G.H., Intven M.P.W. Individual Lymph Nodes: “See it and Zap it”. Clin Transl Radiat Oncol. 2019 doi: 10.1016/j.ctro.2019.03.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kontaxis C., Bol G.H., Lagendijk J.J.W., Raaymakers B.W. A new methodology for inter- and intrafraction plan adaptation for the MR-linac. Phys Med Biol. 2015;60:7485–7497. doi: 10.1088/0031-9155/60/19/7485. [DOI] [PubMed] [Google Scholar]