Abstract
Background
Video-assisted thoracoscopy and atypical resection of lung parenchyma is a surgical procedure that is carried out very commonly around the world, mainly to determine the degree of malignancy of a suspect pulmonary nodule. A pleural drain is routinely inserted at the end of the procedure. The goal of our study was to evaluate the outcomes of this procedure with and without pleural drainage.
Methods
From June 2015 to January 2018, 74 patients were prospectively randomized to either the chest-tube group (CT group, 37 patients) or the no-chest-tube group (NCT group, 37 patients) and were followed up until the seventh day after surgery. The postoperative duration of hospital stay was the primary endpoint; the secondary endpoints were the rates of pneumothorax and repeated chest drainage, pain intensity, and analgesic consumption. Blinding was not possible. An intention-to-treat analysis was performed. (Study registration; DRKS00008194, www.drks.de/drks.)
Results
Hospital stays were significantly shorter in the NCT group (means and first and fourth quartiles: 1.5 [1.5; 1.5] versus 2.5 [2.5, 2.5] days, p<0.001). The two groups did not differ significantly with respect to the frequency of postoperative complications. There were two occurrences of postoperative pneumothorax in the NCT group, with one patient needing drainage via chest tube and the other needing no treatment. Pain intensity and analgesic consumption were markedly lower in the NCT group; the cumulative oral intake of metamizole and acetaminophen was also lower in the NCT group (mean ± standard deviation: 3.7 ± 2.2 g in the NCT group versus 10.0 ± 4.2 g in the CT group, p<0.001).
Conclusion
Not inserting a chest tube after video-assisted thoracoscopic lung biopsy significantly shortens the postoperative hospital stay, and the complications in the chest-tube and no-chest-tube groups are similar. Postoperative pain and analgesic consumption are markedly less when no chest tube is inserted.
For patients with a solitary pulmonary nodule that is suspicious for malignancy (larger than 8–10 mm), surgery should generally be performed to evaluate for malignancy (1, 2). The procedure of choice is the minimally invasive video-assisted thoracoscopic surgery (VATS) for atypical (or wedge) lung parenchymal resection, which is mainly performed using three thoracic ports (of 1-cm to 3-cm long incisions). Another indication for wedge resection is for histological clarification of unclear interstitial lung disease. At the end of surgery, a pleural drainage is usually inserted to drain air and fluid from the pleural cavity. Wedge lung resection is performed with endoscopic staplers. These are able to seal the lung parenchyma against air or blood leakage. After airtight sealing, the lung can be fully expanded intraoperatively by selective manual inflation. Thus, placement of an additional drainage of the pleural space is possibly no longer necessary. Previous studies have concluded that surgical operation without drainage is safe and can shorten the hospital stay (3– 6). The aim of our study was to carry out a prospective randomized evaluation of VATS lung resection with or without drainage.
Methods
A prospective, randomized, two-arm intervention study was initiated to evaluate the outcome (primary endpoint: length of postoperative [PO] hospital stay) of patients with pleural drainage (chest tube [CT] group) as compared to those without pleural drainage (non–chest tube [NCT] group) following VATS atypical lung parenchymal resection (Ethics Committee LÄK Thüringen, Germany: 23302/2015/65; DRKS 00008194).
From June 2015 to January 2018, all patients over the age of 18 who presented at the SRH Wald-Klinikum Gera for diagnostic lung resection were examined for suitability for study participation. Exclusion criteria for study participation were:
Lack of study consent
Pulmonary nodule greater than 3 cm in diameter
Nodule location deeper to visceral pleura than the nodule diameter
More than one wedge resection required
Bullous emphysema
Preoperative pleural effusion
Intraoperative air fistula
Diffuse pleural adhesions
Injury of the visceral pleura visible intraoperatively
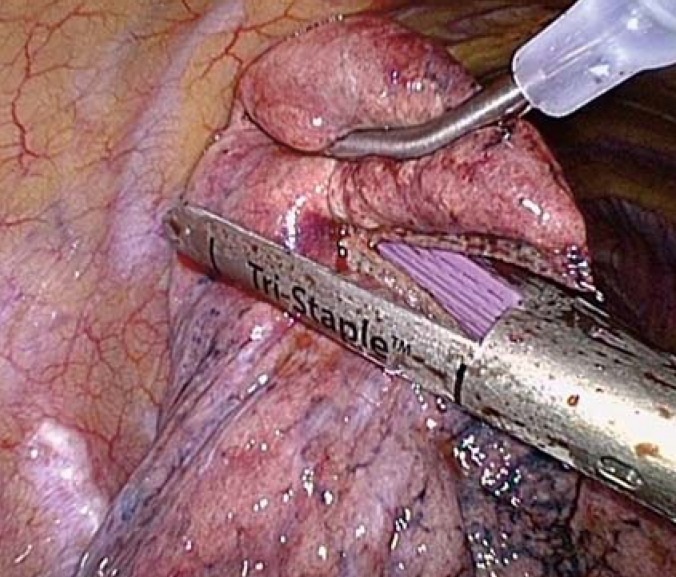
Under standardized conditions (one-lung ventilation, three-trocar technique), the pulmonary nodule was resected from the lung tissue using an endoscopic stapler (figure 1). This was followed by thoracoscopic control of potential sources of bleeding, air leakage testing along the staple line (underwater air-tightness test), and targeted insertion of a chest drain (size 24 Fr). With the patient in a reverse Trendelenburg posture, the pleural space was ventilated by manual inflation of the lungs under continuous suction (–20 cm H2O) to the chest drain. After air leakage was excluded, patients were randomized. Depending on the result, the pleural drainage was either left in place (CT group) or removed during intubation anesthesia (NCT group).
Figure 1.
Videothoracoscopic view of an atypical lung parenchymal resection (linear resection) with an endostapler, on the partially collapsed, non-ventilated lung
The primary endpoint of the study was the length of PO hospital stay. This was measured in half days after surgery (surgery day = 0). Secondary endpoints were: pneumothorax, rate of renewed drainage placement, pain severity, and analgesic consumption. Parameters were collected according to a standardized management plan. On the day of surgery, pleural ultrasonography was performed at bedside at three and six hours postoperatively, using a 4-quadrant examination (5–2 MHz C60e convex transducer, MicroMaxx, FUJIFILM SonoSite GmbH, Frankfurt/Main, Germany). Chest radiographs were taken at six hours PO, before and after drainage placement, and as an outpatient on PO day (POD) 7. Radiographs on the day of the surgery were taken with the patient in bed with the upper body raised, and on the other days, with the patient standing. Pain therapy was applied according to a standardized scheme: basic analgesics (metamizole or, in case of a metamizole allergy, paracetamol) of 1 g given intravenously as a short infusion every six hours at pain intensity 1–3 (numerical rating scale [NRS] from 0–10), with piritramide given additionally (7.5 mg subcutaneously, with a maximum of every six hours) at NRS values >3.
Patients were discharged if: pneumothorax requiring treatment was excluded by radiotherapy; wound healing was favorable; pain severity was NRS <3; and patient consent was given. Largely independent care at home also had to be guaranteed.
Continuous variables were presented as mean ± standard deviation (SD) or as median (25th percentile, 75th percentile) as a function of the normal distribution test using a Q-Q plot. Categorical variables were given as absolute and relative frequency (%). Continuous variables were compared between the CT and NCT groups using the Mann-Whitney U test for independent samples, and the Wilcoxon test for two connected, non-normally distributed samples. Categorical variables were examined for differences between the CT group and NCT group using Fisher’s exact test for expected case numbers <5, and using Chi-squared tests for expected case numbers >5. For p-values <0.05, a statistically significant difference was assumed. Statistical analysis was done using the SPSS software (version 25.0 Multilingual). Detailed information can be found in the eMethods section.
Results
Clinical characteristics
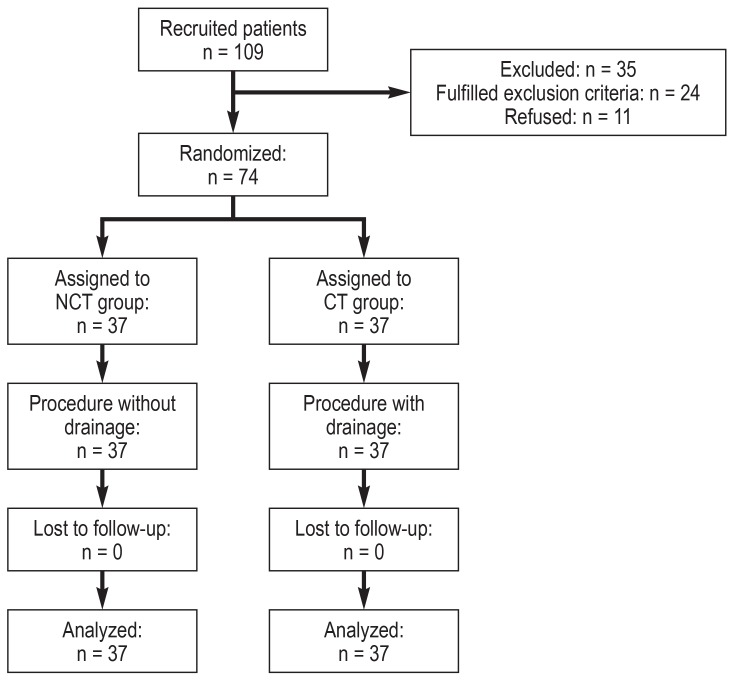
From June 2015 to January 2018, 109 patients were screened. Twenty-four patients were excluded from the study, and eleven patients did not consent to participate in the study. Of the 74 patients who were randomized, 37 patients were assigned to the CT group, and 37 to the NCT group. The intended treatment was completed for all patients without loss to follow-up, so that 37 patients per group could be evaluated (figure 2).
Figure 2.
Flow chart CT, chest tube; NCT, non–chest tube
No differences were observed for age, sex, height, weight, Karnofsky index, ASA classification, or comorbidities (such as COPD, diabetes, and chronic renal insufficiency) between the two groups (etable 1).
eTable 1. Patient characteristics.
| CT group (n = 37) | NCT group (n = 37) | P*1 | |
| Age (years) | 67.4 ± 11.3 | 63 ± 11.5 | 0.095 |
| Sex (M : W) | 21:16 | 25:12 | 0.341 |
| Height (cm) | 170 ± 9 | 172 ± 9 | 0.294 |
| Weight (kg) | 76.9 ± 15.5 | 83.7 ± 13.9 | 0.088 |
| Karnofsky Index (%) | 86.5 ± 7.7 | 90 ± 8.8 | 0.062 |
| ASA (n) | 0.108*2 | ||
| I | 3 (8) | 2 (5) | |
| II | 10 (27) | 19 (51) | |
| III | 24 (65) | 15 (41) | |
| IV | 0 | 1 (3) | |
| COPD (n) | 8 (22) | 5 (14) | 0.655 *2 |
| I | 3 | 2 | |
| II | 5 | 3 | |
| Diabetes mellitus (n) | 5 (14) | 7 (19) | 0.531 |
| Chronic renal insufficiency (n) | 4 (11) | 2 (5) | 0.398 |
Mean ± standard deviation or number (%),
*1Mann-Whitney U test, with exceptions
*2Chi-squared test
ASA, American Society of Anesthesiologists; COPD, chronic obstructive lung disease (according to Gold); CT, chest tube; M, men
NCT, non–chest tube; W, women
Likewise, no significant differences were observed for the size, localization, or stage of disease of the pulmonary findings between the two groups (etable 2).
eTable 2. Characteristics of lung findings*1.
|
CT group (n = 37) |
NCT group (n = 37) |
P*2 | |
| Amount | 0.351 | ||
| Solitary | 18 (49) | 14 (38) | |
| Multiple | 19 (51) | 23 (62) | |
| Side | 1 | ||
| Right | 21 (57) | 21 (57) | |
| Left | 16 (43) | 16 (43) | |
| Lung lobe | 0.389 | ||
| Lung upper lobe | 13 (35) | 16 (43) | |
| Lung middle lobe | 2 (5) | 3 (8) | |
| Lung lower lobe | 22 (60) | 18 (49) | |
| Size (mm) | 11.2 ± 5.2 | 12.6 ± 6.2 | 0.172 |
| 0–9 | 14 (38) | 10 (27) | |
| 10–20 | 19 (51) | 18 (49) | |
| > 20 | 2 (5) | 6 (16) | |
| “ground-glass” | 2 (5) | 3 (8) | |
| Location in parenchyma | 0.078 | ||
| Subpleural | 25 (68) | 31 (84) | |
| Intraparenchymal | 12 (32) | 6 (16) | |
|
Distance from visceral pleura (mm) (for intraparenchymal location) |
3 [3; 5] | 2.5 [1.7; 6.2] | 0.291 |
| Position with respect to facies (F) | 0.115 | ||
| F. mediastinalis | 1 (3) | 2 (5) | |
| F. costalis | 23 (62) | 30 (81) | |
| F. diaphragmatica | 3 (8) | 0 | |
| F. interlobaris | 10 (27) | 5 (14) | |
| Stage of disease of tumor | 0.38 | ||
| Metastasis | 17 (46) | 13 (35) | |
| Interstitial lung disease | 1 (3) | 1 (3) | |
| Lung carcinoma | 4 (11) | 5 (14) | |
| Benign nodule | 15 (40) | 18 (49) |
*1Median [25th; 75th percentile], mean ± standard deviation or amount (%)
*2Mann-Whitney U test
CT, chest tube; NCT, non–chest tube
Intra- and postoperative course
In more than 80% of cases, biopsies were removed with a linear resection technique in both groups. Except for one case in the CT group, the same type of stapler (Endo-GIA 45) was used, with median use in each group of three cartridges (EGIA 45 AMT) [2; 4]. For one patient in the CT group, and two patients in the NCT group, adhesions observed intraoperatively had to be detached. No intraoperative complications occurred for any patient (etable 3).
eTable 3. Intra- and postoperative course*1.
|
CT group (n = 37) |
NCT group (n = 37) |
P | |
| Intraoperative course | |||
| Type of atypical resection | 0.746*2 | ||
| Linear | 32 (86) | 31 (84) | |
| Wedge | 5 (14) | 6 (16) | |
| Stapler | |||
| EndoGia 45 Turple (EGIA 45 AMT) | 36 (97) | 37 (100) | 0.314 *3 |
| Other stapler | 1 (3) | 0 | 1*4 |
| Number of cartridges | 3 [2; 4] | 3 [2; 4] | 0.839*2 |
| Local adhesiolysis | 1 (3) | 2 (5) | 0.5*4 |
| Intraoperative complications | 0 | 0 | 1*4 |
| Postoperative course | |||
| Number of chest X-rays | 4 [4; 4] | 3 [3; 3] | < 0.001*2 |
| Skin emphysema | 0 | 2 (5) | 0.493*4 |
| Pneumothorax (within 48 h postoperative) | 0.493*4 | ||
| < 2 cm apical | 0 | 1 (3) | |
| > 2 cm apical | 0 | 1 (3) | |
| Air fistula | 1 (3) | ||
| Other complications | |||
| Pneumonia | 1 (3) | 0 | 1*4 |
| Heart arrhythmias | 0 | 1 (3) | 1*4 |
| Duration of pleural drainage (days) | 1.5 [1.5; 1.5] | ||
| Re-operation | 0 | 0 | 1*4 |
| Renewed pleural drainage | 0 | 1 (3) | 1*4 |
| Length of PO hospital stay (day) | 2.5 [2.5; 2.5] | 1.5 [1.5; 1.5] | < 0.001*2 |
*1Number (%), median [25th; 75th percentile]; *2Mann-Whitney U test; *3Chi-squared test;
*4Fisher’s exact test
CT, chest tube; NCT, non–chest tube
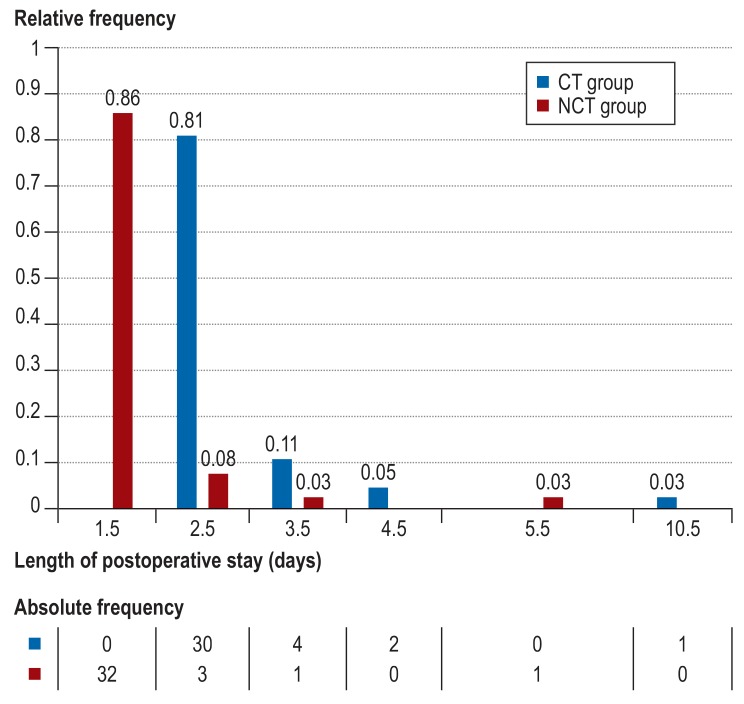
The primary endpoint (length of PO hospital stay) was significantly lower in the NCT group. Patients without pleural drainage were medically discharged one day earlier (CT versus NCT: 2.5 days [25th and 75th percentiles: 2.5, 2.5] versus 1.5 days [1.5, 1.5], p<0.001). In the NCT group, 86% of patients were discharged after 1.5 days, and in the CT group, 81% of patients were discharged after 2.5 days (figure 3).
Figure 3.
Frequency of length of postoperative hospital stay for both groups
CT, chest tube; NCT, non–chest tube
With respect to the secondary endpoints (pneumothorax, rate of renewed drainage), no significant differences were found between the groups. In the NCT group, pneumothorax was suspected in two patients based on pleural ultrasonography, and it was confirmed by radiograph (5.4%; 95% CI, 0.6% to 18.2%). One patients received conservation treatment; the other patient (apical pneumothorax >2 cm) required pleural drainage placement. The frequencies of other specific complications (air fistula, skin emphysema, reoperation) as well as general complications did not differ significantly between the two groups (etable 3).
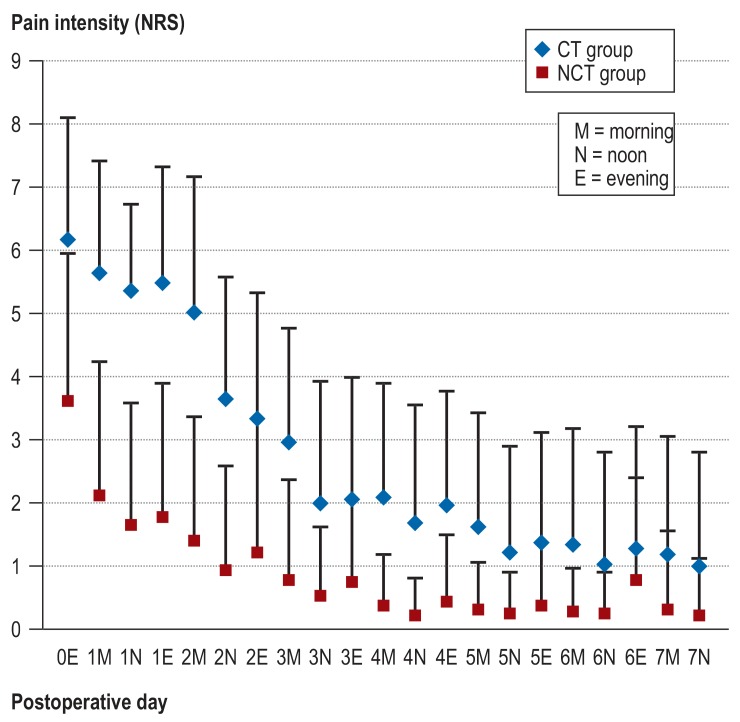
Postoperative pain and the need for analgesics were positively influenced by avoiding pleural drainage (figure 4). The pain intensity for the NCT group was lower on all days as compared to the CT group (p<0.05, except on POD 6 at noon and in the evening). On POD 1 (at noon), 40.5% of patients in the NCT group were pain-free, while no patient in the CT group was pain-free (NRS 0). Low pain scores (NRS 1–3) were reported by 48.6% of patients in the NCT group, and by 8.1% of patients in the CT group. The pain intensity in the CT group was 1.6 points lower on the following day after removal of the pleural drainage (mean ± SD of NRS prior to removal as compared to after removal: 3.6 ± 2.0 versus 2.0 ± 1.9; p<0.001).
Figure 4.
Postoperative pain intensity for both groups (according to NRS) measured daily (mornings, noon, and evenings) until POD 7 (mean ± SD; p<0.05 at every measuring point except for POD 6 at noon and in the evening).
CT, chest tube; NCT, non–chest tube; NRS, numerical rating scale; POD, postoperative day;
SD, standard deviation; 0 = day of surgery
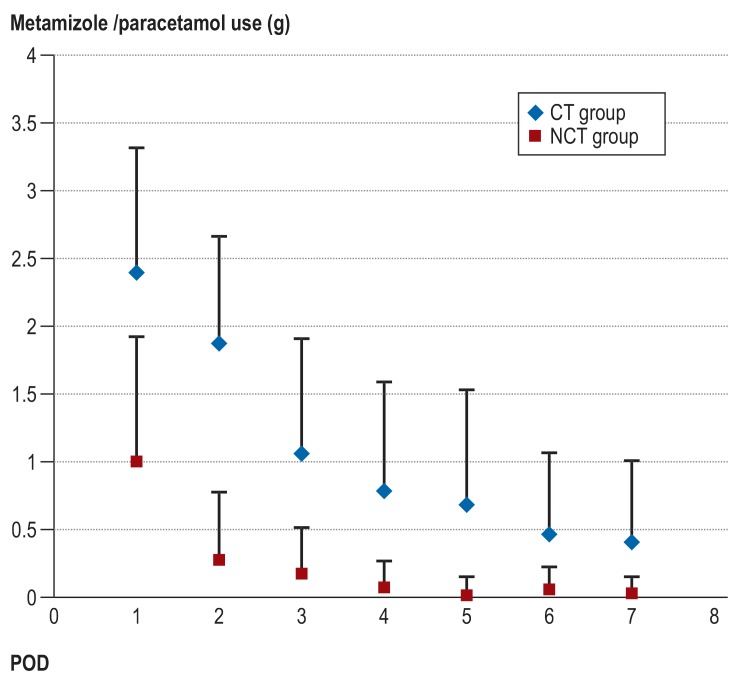
On POD 1, the analgesic use of metamizole or acetaminophen in the NCT group was 60% lower than in the CT group (mean ± SD: 1 g ± 0.9 versus 2.4 g ± 0.9; p<0.001). From POD 2 to the end of observation (POD 7), daily use in the NCT group was 80% to 96% lower (figure 5). Cumulatively, a 2.7-fold increase in analgesic requirement (with metamizole or paracetamol) in the CT group as compared to the NCT group was observed postoperatively (including the day of surgery and the POD 7) (10.0 g ± 4.2 for the CT group, versus 3.7 g ± 2.2 for the NCT group; p<0.001). The cumulative use of piritramide was lower in the NCT group than in the CT group (11.5 mg ± 19.3 versus 18.5 mg ± 19.1; p = 0.072).
Figure 5.
Postoperative consumption of the analgesics metamizole or paracetamol (in grams) for both groups (mean ± SD; p<0.05 at every measuring point). CT, chest tube; NCT, non–chest tube; POD, postoperative day; SD, standard deviation
Additionally, a positive effect on the number of chest radiographic images was observed for the NCT group: on average, fewer chest X-rays were taken for the NCT group as compared to the CT group (3 [3, 3] versus 4 [4, 4]; p<0.001). One patient in the CT group was X-rayed six times due to a persistent air fistula, and one patient in the NCT group was X-rayed 12 times due to recurrent pneumothorax.
Discussion
Length of hospital stay
Avoiding chest drain placement led to a significant reduction of the length of PO hospital stay in our study, in median by one day. Most patients (86%) in the NCT were discharged with subjective well-being and without suspicion of pneumothorax at 1.5 days after surgery. Three retrospective analyses and a randomized controlled study also showed a reduced length of hospital stay when pleural drainage was avoided (3, 7, 8). In a randomized trial from Wales (UK), patients without chest drain were discharged on the day of surgery (6). This would be currently inconceivable in the German hospital reimbursement system. In our study, with a median total hospital stay of three days in the NCT group, no deduction in the German diagnosis-related group (G-DRG) system would be expected. Our patients were discharged once independent care at home was guaranteed and always under consideration of the patient´s request. In a prospective randomized study of early chest tube removal (1 to 2 hours after surgery), no decrease in length of hospital stay could be demonstrated (9).
Complications
The rate of PO complications was not significantly different in the groups with or without a chest drain. We observed two cases (5.4%; 95% CI, [0.6%; 18.2%]) of patients with pneumothorax in the NCT group, one of whom required drainage (2.7% [0.1%; 14.2%]). Almost identical frequencies were reported in comparable studies: any pneumothorax: 7.6% and 9.5%, respectively; and pneumothorax requiring chest drain: 2.3% and 2.4%, respectively (3, 7). Comparable rates (4% to 7%) of patients with postoperative pneumothorax also have been observed for patients with chest drain placement (7, 9). Postoperative bleeding was not observed in either the CT group or the NCT group in our study. In comparable studies, one patient in the CT group experienced bleeding from a trocar incision and required treatment (7). For the patients in the NCT group, bleeding was ruled out by taking periodic bedside pleural sonography on the day of surgery. Pleural sonography can diagnose pneumothorax or hematothorax with high accuracy (10). This advantage is particularly useful for supine patients postoperatively. Thus, we see no reason for pleural drainage to remain in place postoperatively for 2 to 6 hours, as was the case in early drainage studies (9, 11). The rate of postoperative bleeding as a complication after VATS is very low (between 0.4% and 1.9%), independent of the type of intervention (12– 15). In VATS lung resection, bleeding can only result from pleural adhesions after adhesiolysis, parenchyma stapling, or trocar incisions (16). Therefore, a thorough thoracoscopic examination of the suture as well as of the trocar incisions is necessary at the end of surgery.
In addition, pleural effusion could be ruled out for patients in the NCT group using chest ultrasound. We would like to further evaluate the risk of postoperative complications with a larger observational study.
Pain and analgesic consumption
We were able to show that pain intensity and analgesic consumption were lower in the NCT group on all days in the postoperative phase (except for POD 6, due to placement of drainage for recurrent pneumothorax). In the few studies that examined the influence of pleural drainage on pain (3, 6, 9), only one observational study showed a significant reduction in opiate use in the group after early drainage removal (within 90 minutes of surgery) (11).
In general, VATS lung resection is a painless operation. Pain complaints are predominantly projected in the area of the entry point of the pleural drainage (17). We also found that patients in the NCT group had a lower pain intensity in follow-up than the CT group, even after pleural drainage removal in the CT group. This situations could be explained by a drainage-induced intercostal neuralgia.
To be able to identify differences with respect to pain between the two procedures, the surgical technique was standardized, with particular attention paid to certain pain-sparing techniques, such as minimal incision lengths, use of flexible plastic trocars, and avoidance of intercostal neuralgia due to instrument levering. Furthermore, pain therapy was adapted to the concept of a minimally invasive, painless procedure. The positive effect on pain reduction in the patient group without chest drain placement cannot be demonstrated if epidural anesthesia is used (3).
Chest X-rays
By avoiding chest drain placement during VATS lung resection, the number of PO X-rays taken was reduced by one. The early removal of the pleural drainage (on the day of surgery) also leads to a reduction in X-ray examinations (9, 11). Pneumothorax diagnostics were also performed on the day of surgery using pleural ultrasonography. According to our experience and to published literature, PO X-rays can be completely omitted if ultrasound detects ubiquitous evidence of lung gliding (18, 19).
Prerequisites for avoiding chest drainage
Avoiding placement of a chest drain in VATS atypical lung parenchymal resection is justifiable if the following conditions are met:
No bleeding or air leakage at staple closure of lung parenchyma
No injury to the visceral pleura
Careful thoracoscopic inspection of the trocar incisions after their removal has ruled out bleeding
Complete evacuation of pleural air has been done before definitive closure of thoracic incisions
The exclusion of air leakage can be done by thoracoscopic inspection of the staple line with inflation of the lungs after the instillation of a saline solution (“underwater air-tightness test”). As this technique can be difficult and uncertain, it is recommended to additionally digitally measure air leakage by connecting an active suction with –20 cm H2O and using mechanical ventilation with at least 15 cm H20 peak pressure over five minutes. The initial flow should drop to 0 mL/minute.
The main cause of a PO pneumothorax in the absence of chest drainage is incomplete de-airing of the pleural space at the end of surgery. For complete de-airing, the following measures are necessary from our point of view:
Thoracoscopic placement of drain tube apico-ventral
Thoracoscopic monitoring of complete re-expansion of lungs without atelectatic regions
Active suction of –20 cm H2O at the drain port
Inflation of the lung manually / mechanically with up to 20 cm H20 peak pressure
Removal of drainage tubes with intermittently increasing suction and constant inflation with the patient in a supine position (reverse Trendelenburg posture) under general anesthesia
After VATS lung resection without pleural drainage, it is recommended to perform periodic pleural ultrasounds on the day of surgery, in order to detect postoperative bleeding by sonographic detection of pleural fluid in a timely manner. This method can replace the lost diagnostic function of having a pleural drainage. Thus, we see no convincing reason to leave the drainage in place on the day of surgery for two to six hours, especially given the risk of air entering the pleural cavity during drainage removal on spontaneously breathing patients who are often not fully cooperative.
Conclusion
The study showed that thoracoscopic lung biopsy without pleural drainage in a selected patient population that meets certain prerequisites has advantages for length of PO hospital stay, the number of chest X-rays needed, and pain as compared to the classic surgery with drainage. No differences in complication rates were observed. These results should be tested in a larger observational study. If successful, it would be useful to introduce thoracoscopic lung biopsy without pleural drainage into routine care.
Supplementary Material
eMETHODS
Study design
A prospective, randomized, two-arm intervention study was initiated (Ethics Committee LÄK Thüringen, Germany: 23302/2015/65; DRKS 00008194). In one group of patients (chest tube [CT] group), pleural drainage was placed following a minimally invasive video-assisted thoracoscopic surgery (VATS) for atypical (or wedge) lung parenchymal resection; the other group did not receive pleural drainage (non–chest tube [NCT] group). Each group was assigned 37 patients, all of whom received the respective intervention; all patients were analyzed without loss to follow-up. The incidence of dropout, loss, or change of group was zero.
Patient selection
All patients over the age of 18 who presented for diagnostic lung parenchymal resection at the SRH Wald-Klinikum Gera (Germany) in the period from June 2015 to January 2018 were assessed for eligibility for study participation. An indication for VATS lung resection was based on the CT scan of the thorax and on functional operability. After providing information about the surgery and the study, written consent was obtained from each patient. Exclusion criteria for study participation were:
Intervention/treatment
The procedure was performed under general anesthesia after intubation with a double-lumen endotracheal tube (DLT), with the patient in a lateral side position on an operating table angled at 30°. After one-lung ventilation, three chest incisions were made: 10.5-mm trocar incision for a 10 mm, 30° fixed angle optic, a 7-mm trocar incision for a parenchymal clamp, and a 11.5-mm trocar incision for the endostapler (MICTEC Flexible Trocar Set, MICASEPT parenchymal clamp; Dufner, Tuttlingen, Germany). The endostapler Endo GIA with a Turple cartridge EGIA 45 AMT (Covidien, Norderstedt, Germany) was used for resection.
In the non-ventilated, partially collapsed lung, the pathological findings were located either visually or by instrumental palpation and then clamped with a parenchymal clamp below the nodule. Resection was performed with the endostapler using either a typical wedge or linearly below the clamp (figure 1). The resected lung parenchymal tissue was removed from the thorax via the stapler trocar incision (widened to a maximum of 3 cm, as required for the nodule) using a specimen pouch. The airtightness of the staple seam was tested by instillation of a saline solution along the stapling seam with re-ventilation of lung. Additionally, after removal of the trocars, the trocar incisions were examined by videothoracoscopy for blood dryness. Finally, using a videothoracoscopic view, a pleural drainage (silicone round drain, 24 Fr; PFM Medical, Nonnweiler-Otzenhausen, Germany) was inserted apico-ventrally, and full re-expansion of the lung was checked by manual ventilation. After airtight closure of the thorax incisions and placement of the pleural drainage and of the U-sutures, a suction of –20 cm H2O was applied to the chest drain using an electric pump (Thopaz, Medela Medizintechnik GmbH, Eching, Germany). After this point, the patient was in a supine position. After ruling out air leakage (flow: 0 mL/min) with double-lung ventilation for five minutes, patient randomization was performed.
For patients assigned to the NCT group, the chest drain was removed as follows:
For the CT group, a continuous suction of –20 cm H2O was applied to the chest drain on the day of surgery. On the first postoperative day (POD), a physiological negative air pressure was applied in the absence of air leakage (gravity mode). The drainage system was removed according to the following criteria:
The removal of the chest drain was carried out at the end of inspiration during a Valsalva maneuver. The skin incision was sealed and made airtight by tying a presented U-suction.
Radiographic criteria (performed in standing) for a renewed placement of drainage in the postoperative course in both groups were:
Pain therapy was provided on the day of surgery with regular administration of metamizole or paracetamol (in case of metamizole allergy) (using 1 g intravenously as a short infusion every six hours). Piritramide (7.5 mg intravenously, not more frequently than every six hours) was administered as a required medication if the patient reported a pain severity >3 (NRS, numeric rating scale). On the following days, metamizole or paracetamol was administered for NRS values of 1–3 (1 g intravenously as a short infusion, not more frequently than every six hours), and piritramide was administered at NRS values >3 (7.5 mg subcutaneously, not more frequently than every six hours). Decisions about pain therapy were taken after assessment of pain history by the visiting physician according to the above-mentioned protocol. After discharge, patients were advised to take 1 tablet of metamizole or paracetamol (500 mg/tablet, up to four times daily) for milder levels of pain (NRS 1–3). For NRS values of >3, patients in outpatient care were advised to visit the hospital. Patient records were retrieved on POD 7 for the outpatient examination.
Endpoints
The primary endpoint of the study was the length of the postoperative (PO) hospital stay. This was measured in half days after surgery (surgery day = 0). Secondary endpoints were: pneumothorax, rate of renewed drainage placement, pain severity, and analgesic consumption. Parameters were collected according to a standardized management plan. Twice daily during hospitalization, and on the POD 7, a diagnosis-oriented clinical examination was performed (physical lung examination, palpation for identifying a skin emphysema). On the day of surgery, pleural ultrasonography was performed at bedside at three and six hours postoperatively, using a 4-quadrant examination (5–2 MHz C60e convex transducer, MicroMaxx, FUJIFILM SonoSite GmbH, Frankfurt am Main, Germany). Chest radiographs were taken postoperatively at six hours, before and after drainage placement, and as an outpatient on POD 7 (taken on the day of the surgery with the patient in bed with the upper body raised, and on the other days, with the patient standing). An apical pneumothorax <2 cm was not considered to require treatment. Pain severity was recorded daily in the morning, at noon, and in the evening after prompting for a cough while sitting (using the NRS scale of 0–10). Patients were not explicitly informed about the expected benefit in terms of pain. After discharge, the pain intensity was independently self-documented by patients.
Patients were discharged if: pneumothorax requiring treatment was excluded by radiotherapy; wound healing was favorable; pain severity was NRS <3; and patient consent was given. Largely independent care at home also had to be guaranteed.
The planned secondary endpoint—pleural effusion—was not evaluated in the study, as objective measurement was not guaranteed.
Case number determination
An internal retrospective analysis (unpublished) of the length of PO hospital stay after VATS lung parenchymal resection with pleural drainage in our clinic in 2014 showed a mean length of PO hospital stay of 4.6days (± 1.22). It has been hypothesized that the length of PO hospital stay can be reduced by avoiding pleural drainage. The one-day difference between the CT and NCT groups was considered clinically relevant for this minimally invasive procedure that has a generally short PO hospital stay. To prove the difference with a two-tailed t-test for independent samples, 37 patients in each group need to be evaluated (a=5%, power: 90%, drop-out rate: 10%). The case number determination was carried out with the software PASS 2014.
Randomization
Block randomization was carried out (1:1 distribution, block size 4) and was performed by Dr. T. Lehmann (Institute of Medical Statistics, Computer Science and Data Science, University Hospital Jena, Germany) (software: nQuery 7.0). Anonymous numbered envelopes were opened by the surgical scrub nurse at the end of surgery (after exclusion of an air fistula) under anesthesia and with double-lung ventilation. Depending on the outcome of the randomization, pleural drainage was either left in place (CT group) or removed during intubation anesthesia (NCT group). Blinding was not possible, as patients in the CT group were identifiable by visible chest drain tubes.
Statistical methods
The continuous variables were presented as mean ± standard deviation (SD) or as median (25th percentile, 75th percentile) as a function of the normal distribution test using the Q-Q plot. Categorical variables were given as absolute and relative frequency (%). Continuous variables were compared between the CT and NCT groups by Mann-Whitney test (U test) for independent samples, and by Wilcoxon test for two connected, non-normally distributed samples. Categorical variables were examined for differences between the CT group and NCT group using Fisher’s exact test for expected case numbers <5, and using Chi-squared tests for expected case numbers >5.
For p-values below 0.05, a statistically significant difference was assumed. Statistical analyses were performed using the SPSS software (version 25.0 Multilingual).
Lack of study consent
Pulmonary nodules larger than 3 cm in diameter
Nodule location deeper to the visceral pleura than the nodule diameter
More than one resection required
Bullous emphysema
Preoperative pleural effusion
Intraoperative air fistula
Diffuse pleural adhesions
Injury of the visceral pleura, visible intraoperatively
Application of continuous suction to chest drain (–40 cm H2O), while maintaining constant inflation of lungs over the presented DLT, with a maximum of 20 cm H2O (1st assistant)
Simultaneously airtight skin closure of the drain incision using the presented U- sutures (2nd assistant)
Weaning and extubation after sufficient spontaneous breathing
No air leakage (0mL/min flow under gravity mode [–8cmH2O])
Secretion amount <200mL/24 hours
No pneumothorax or peak pneumothorax <2cm (based on chest X-ray in standing position)
An apical pneumothorax >2cm (cranio-caudal extension
A mantle (minor) pneumothorax
Key Messages.
Avoiding pleural drainage leads to a significant reduction of the length of postoperative hospital stay, as well as to a reduction in the number of chest X-rays performed.
No differences in postoperative complications were observed for surgery with or without drainage. Further evaluation of a larger observational study is required.
Postoperative pain and analgesic consumption were lower for each of the seven days following surgery in the group without pleural drainage.
Important prerequisites for performing video-thoracoscopic lung biopsy without pleural drainage include a thorough intraoperative control of potential sources of bleeding and air leakage, regular postoperative pleural sonography, and sufficient de-airing of the pleural cavity at the end of surgery.
Acknowledgments
Translated from the original German by Dr. Veronica A. Raker
Data sharing
The authors are willing to share data with other researchers for scientific purposes. Please direct enquires regarding the dataset to Jerar Mukdessi.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Hoffmann H, Dienemann H. [Pulmonary nodule The surgeon‘s approach] Zentralbl Chir. 1999,;124:128–135. [PubMed] [Google Scholar]
- 2.Hoffmann H, Dienemann H. Der pulmonale Rundherd: Prinzipien der Diagnostik. Dtsch Arztebl. 2000;97:A1065–A1071. [Google Scholar]
- 3.Watanabe A, Watanabe T, Ohsawa H, et al. Avoiding chest tube placement after video-assisted thoracoscopic wedge resection of the lung. Eur J Cardiothorac Surg. 2004,;25:872–876. doi: 10.1016/j.ejcts.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 4.Satherley LK, Luckraz H, Rammohan KS, et al. Routine placement of an intercostal chest drain during video-assisted thoracoscopic surgical lung biopsy unnecessarily prolongs in-hospital length of stay in selected patients. Eur J Cardiothorac Surg. 2009;36:737–740. doi: 10.1016/j.ejcts.2009.04.067. [DOI] [PubMed] [Google Scholar]
- 5.Holbek BL, Hansen HJ, Kehlet H, et al. Thoracoscopic pulmonary wedge resection without post-operative chest drain: an observational study. Gen Thorac Cardiovasc Surg. 2016;64:612–617. doi: 10.1007/s11748-016-0692-6. [DOI] [PubMed] [Google Scholar]
- 6.Luckraz H, Rammohan KS, Phillips M, et al. Is an intercostal chest drain necessary after video-assisted thoracoscopic (VATS)lung biopsy? Ann Thorac Surg. 2007;84:237–239. doi: 10.1016/j.athoracsur.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Nakashima S, Watanabe A, Mishina T, Obama T, Mawatari T, Higami T. Feasibility and safety of postoperative management without chest tube placement after thoracoscopic wedge resection of the lung. Surg Today. 2011,;41:774–779. doi: 10.1007/s00595-010-4346-5. [DOI] [PubMed] [Google Scholar]
- 8.Simbrey N, Leschber G. German Medical Science GMS Publishing House. Düsseldorf: 2016. Fast-Track Thoraxchirurgie - Drainage noch notwendig? Deutsche Gesellschaft für Chirurgie. 133. Kongress der Deutschen Gesellschaft für Chirurgie. Berlin, 26.-29.4.2016. [Google Scholar]
- 9.Sienel W, Mueller J, Eggeling S, Thetter O, Passlick B. [Early chest tube removal after video-assisted thoracoscopic surgery Results of a prospective randomized study] Chirurg. 2005;76:1155–1160. doi: 10.1007/s00104-005-1058-2. [DOI] [PubMed] [Google Scholar]
- 10.Staub LJ, Biscaro RRM, Kaszubowski E, Maurici R. Chest ultrasonography for the emergency diagnosis of traumatic pneumothorax and haemothorax: a systematic review and meta-analysis. Injury. 2018;49:457–466. doi: 10.1016/j.injury.2018.01.033. [DOI] [PubMed] [Google Scholar]
- 11.Russo L, Wiechmann RJ, Magovern JA, et al. Early chest tube removal after video-assisted thoracoscopic wedge resection of the lung. Ann Thorac Surg. 1998;66:1751–1754. doi: 10.1016/s0003-4975(98)00946-1. [DOI] [PubMed] [Google Scholar]
- 12.Yim AP, Liu HP. Complications and failures of video-assisted thoracic surgery: experience from two centers in Asia. Ann Thorac Surg. 1996;61:538–541. doi: 10.1016/0003-4975(95)01097-1. [DOI] [PubMed] [Google Scholar]
- 13.Krasna MJ, Deshmukh S, McLaughlin JS. Complications of thoracoscopy. Ann Thorac Surg. 1996;61:1066–1069. doi: 10.1016/0003-4975(96)00021-5. [DOI] [PubMed] [Google Scholar]
- 14.Downey RJ. Complication after video-assisted thoracic surgery. Chest Surg Clin N Am. 1998;8:907–915. [PubMed] [Google Scholar]
- 15.Imperatori A, Rotolo N, Gatti M, et al. Peri-operative complications of video-assisted thoracoscopic surgery. Int J Surg. 2008;6(Suppl 1):S78–S81. doi: 10.1016/j.ijsu.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Solaini L, Prusciano F, Bagioni P, et al. Video-assisted thoracic surgery (VATS) of the lung: analysis of intraoperative and postoperative complications over 15 years and review of the literature. Surg Endosc. 2008;22:298–310. doi: 10.1007/s00464-007-9586-0. [DOI] [PubMed] [Google Scholar]
- 17.Wildgaard K, Petersen RH, Hansen HJ, Møller-Sørensen H, Ringsted TK, Kehlet H. Multimodal analgesic treatment in video-assisted thoracic surgery lobectomy using an intraoperative intercostal catheter. Eur J Cardiothorac Surg. 2012;41:1072–1077. doi: 10.1093/ejcts/ezr151. [DOI] [PubMed] [Google Scholar]
- 18.Lavingia KS, Soult MC, Collins JN, et al. Basic ultrasound training can replace chest radiography for safe tube thoracostomy removal. Am Surg. 2014;80:783–786. [PubMed] [Google Scholar]
- 19.Soult MC, Collins JN, Novosel TJ, et al. Thoracic ultrasound can predict safe removal of thoracostomy tubes. J Trauma Acute Care Surg. 2014;77:256–261. doi: 10.1097/TA.0000000000000315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMETHODS
Study design
A prospective, randomized, two-arm intervention study was initiated (Ethics Committee LÄK Thüringen, Germany: 23302/2015/65; DRKS 00008194). In one group of patients (chest tube [CT] group), pleural drainage was placed following a minimally invasive video-assisted thoracoscopic surgery (VATS) for atypical (or wedge) lung parenchymal resection; the other group did not receive pleural drainage (non–chest tube [NCT] group). Each group was assigned 37 patients, all of whom received the respective intervention; all patients were analyzed without loss to follow-up. The incidence of dropout, loss, or change of group was zero.
Patient selection
All patients over the age of 18 who presented for diagnostic lung parenchymal resection at the SRH Wald-Klinikum Gera (Germany) in the period from June 2015 to January 2018 were assessed for eligibility for study participation. An indication for VATS lung resection was based on the CT scan of the thorax and on functional operability. After providing information about the surgery and the study, written consent was obtained from each patient. Exclusion criteria for study participation were:
Intervention/treatment
The procedure was performed under general anesthesia after intubation with a double-lumen endotracheal tube (DLT), with the patient in a lateral side position on an operating table angled at 30°. After one-lung ventilation, three chest incisions were made: 10.5-mm trocar incision for a 10 mm, 30° fixed angle optic, a 7-mm trocar incision for a parenchymal clamp, and a 11.5-mm trocar incision for the endostapler (MICTEC Flexible Trocar Set, MICASEPT parenchymal clamp; Dufner, Tuttlingen, Germany). The endostapler Endo GIA with a Turple cartridge EGIA 45 AMT (Covidien, Norderstedt, Germany) was used for resection.
In the non-ventilated, partially collapsed lung, the pathological findings were located either visually or by instrumental palpation and then clamped with a parenchymal clamp below the nodule. Resection was performed with the endostapler using either a typical wedge or linearly below the clamp (figure 1). The resected lung parenchymal tissue was removed from the thorax via the stapler trocar incision (widened to a maximum of 3 cm, as required for the nodule) using a specimen pouch. The airtightness of the staple seam was tested by instillation of a saline solution along the stapling seam with re-ventilation of lung. Additionally, after removal of the trocars, the trocar incisions were examined by videothoracoscopy for blood dryness. Finally, using a videothoracoscopic view, a pleural drainage (silicone round drain, 24 Fr; PFM Medical, Nonnweiler-Otzenhausen, Germany) was inserted apico-ventrally, and full re-expansion of the lung was checked by manual ventilation. After airtight closure of the thorax incisions and placement of the pleural drainage and of the U-sutures, a suction of –20 cm H2O was applied to the chest drain using an electric pump (Thopaz, Medela Medizintechnik GmbH, Eching, Germany). After this point, the patient was in a supine position. After ruling out air leakage (flow: 0 mL/min) with double-lung ventilation for five minutes, patient randomization was performed.
For patients assigned to the NCT group, the chest drain was removed as follows:
For the CT group, a continuous suction of –20 cm H2O was applied to the chest drain on the day of surgery. On the first postoperative day (POD), a physiological negative air pressure was applied in the absence of air leakage (gravity mode). The drainage system was removed according to the following criteria:
The removal of the chest drain was carried out at the end of inspiration during a Valsalva maneuver. The skin incision was sealed and made airtight by tying a presented U-suction.
Radiographic criteria (performed in standing) for a renewed placement of drainage in the postoperative course in both groups were:
Pain therapy was provided on the day of surgery with regular administration of metamizole or paracetamol (in case of metamizole allergy) (using 1 g intravenously as a short infusion every six hours). Piritramide (7.5 mg intravenously, not more frequently than every six hours) was administered as a required medication if the patient reported a pain severity >3 (NRS, numeric rating scale). On the following days, metamizole or paracetamol was administered for NRS values of 1–3 (1 g intravenously as a short infusion, not more frequently than every six hours), and piritramide was administered at NRS values >3 (7.5 mg subcutaneously, not more frequently than every six hours). Decisions about pain therapy were taken after assessment of pain history by the visiting physician according to the above-mentioned protocol. After discharge, patients were advised to take 1 tablet of metamizole or paracetamol (500 mg/tablet, up to four times daily) for milder levels of pain (NRS 1–3). For NRS values of >3, patients in outpatient care were advised to visit the hospital. Patient records were retrieved on POD 7 for the outpatient examination.
Endpoints
The primary endpoint of the study was the length of the postoperative (PO) hospital stay. This was measured in half days after surgery (surgery day = 0). Secondary endpoints were: pneumothorax, rate of renewed drainage placement, pain severity, and analgesic consumption. Parameters were collected according to a standardized management plan. Twice daily during hospitalization, and on the POD 7, a diagnosis-oriented clinical examination was performed (physical lung examination, palpation for identifying a skin emphysema). On the day of surgery, pleural ultrasonography was performed at bedside at three and six hours postoperatively, using a 4-quadrant examination (5–2 MHz C60e convex transducer, MicroMaxx, FUJIFILM SonoSite GmbH, Frankfurt am Main, Germany). Chest radiographs were taken postoperatively at six hours, before and after drainage placement, and as an outpatient on POD 7 (taken on the day of the surgery with the patient in bed with the upper body raised, and on the other days, with the patient standing). An apical pneumothorax <2 cm was not considered to require treatment. Pain severity was recorded daily in the morning, at noon, and in the evening after prompting for a cough while sitting (using the NRS scale of 0–10). Patients were not explicitly informed about the expected benefit in terms of pain. After discharge, the pain intensity was independently self-documented by patients.
Patients were discharged if: pneumothorax requiring treatment was excluded by radiotherapy; wound healing was favorable; pain severity was NRS <3; and patient consent was given. Largely independent care at home also had to be guaranteed.
The planned secondary endpoint—pleural effusion—was not evaluated in the study, as objective measurement was not guaranteed.
Case number determination
An internal retrospective analysis (unpublished) of the length of PO hospital stay after VATS lung parenchymal resection with pleural drainage in our clinic in 2014 showed a mean length of PO hospital stay of 4.6days (± 1.22). It has been hypothesized that the length of PO hospital stay can be reduced by avoiding pleural drainage. The one-day difference between the CT and NCT groups was considered clinically relevant for this minimally invasive procedure that has a generally short PO hospital stay. To prove the difference with a two-tailed t-test for independent samples, 37 patients in each group need to be evaluated (a=5%, power: 90%, drop-out rate: 10%). The case number determination was carried out with the software PASS 2014.
Randomization
Block randomization was carried out (1:1 distribution, block size 4) and was performed by Dr. T. Lehmann (Institute of Medical Statistics, Computer Science and Data Science, University Hospital Jena, Germany) (software: nQuery 7.0). Anonymous numbered envelopes were opened by the surgical scrub nurse at the end of surgery (after exclusion of an air fistula) under anesthesia and with double-lung ventilation. Depending on the outcome of the randomization, pleural drainage was either left in place (CT group) or removed during intubation anesthesia (NCT group). Blinding was not possible, as patients in the CT group were identifiable by visible chest drain tubes.
Statistical methods
The continuous variables were presented as mean ± standard deviation (SD) or as median (25th percentile, 75th percentile) as a function of the normal distribution test using the Q-Q plot. Categorical variables were given as absolute and relative frequency (%). Continuous variables were compared between the CT and NCT groups by Mann-Whitney test (U test) for independent samples, and by Wilcoxon test for two connected, non-normally distributed samples. Categorical variables were examined for differences between the CT group and NCT group using Fisher’s exact test for expected case numbers <5, and using Chi-squared tests for expected case numbers >5.
For p-values below 0.05, a statistically significant difference was assumed. Statistical analyses were performed using the SPSS software (version 25.0 Multilingual).
Lack of study consent
Pulmonary nodules larger than 3 cm in diameter
Nodule location deeper to the visceral pleura than the nodule diameter
More than one resection required
Bullous emphysema
Preoperative pleural effusion
Intraoperative air fistula
Diffuse pleural adhesions
Injury of the visceral pleura, visible intraoperatively
Application of continuous suction to chest drain (–40 cm H2O), while maintaining constant inflation of lungs over the presented DLT, with a maximum of 20 cm H2O (1st assistant)
Simultaneously airtight skin closure of the drain incision using the presented U- sutures (2nd assistant)
Weaning and extubation after sufficient spontaneous breathing
No air leakage (0mL/min flow under gravity mode [–8cmH2O])
Secretion amount <200mL/24 hours
No pneumothorax or peak pneumothorax <2cm (based on chest X-ray in standing position)
An apical pneumothorax >2cm (cranio-caudal extension
A mantle (minor) pneumothorax