Highlights
-
•
HIIT can benefits the HRV, especially in healthy and MetS subjects.
-
•
The HIIT can be used in cardiovascular rehabilitation and prevention.
-
•
Future randomized clinical trials are needed to confirm the effectiveness of HIIT.
Keywords: Heart rate, Cardiovascular oscillations, Autonomic nervous system
Abstract
Background
High intensity interval training (HIIT) has been used as a cardiovascular exercise strategy to promote greater adherence in cardiovascular rehabilitation. However, little is known about the effect of this training modality on cardiac autonomic control.
Objective
To perform a systematic review to evaluate the effects of HIIT on cardiac autonomic responses in humans.
Methods
PEDro, SCOPUS and PubMed were searched from the inception to March 29th, 2018. Moreover, the methodological quality and statistical reporting from all eligible clinical trials were assessed by the PEDro scale. The articles were eligible if: The primary objective was related to the effects of HIIT on the cardiac autonomic nervous system. Outcomes evaluated were indirect measures of cardiac autonomic control, represented by HRV indexes.
Results
The search strategies resulted in 339 citations and 2 additional citations were identified through other sources. After deleting the duplicate articles and revising the full text, 6 articles were included. Overall, the results showed an improvement in parasympathetic and/or sympathetic modulation after HIIT, when evaluated by linear and non-linear indexes of HRV.
Conclusions
HIIT is a promising tool to improve the cardiac autonomic control, with more recommendation in healthy individuals and patients with metabolic syndrome.
Introduction
Various sources of evidence suggest that regularly practicing physical exercise has an inverse relationship with premature mortality and the development of chronic cardiovascular diseases (CVD), as well as preventing major risk factors.1 This relationship occurs due to neurohumoral, vascular and structural changes in the cardiovascular system.1 Neural adaptations to aerobic physical exercise, such as decreased cardiac sympathetic and increased parasympathetic modulation in rest, are important cardioprotective factors as sympathetic hyperactivity is an integral part of the pathophysiology of various cardiac diseases.2, 3, 4
Therefore, the evaluation of autonomic control by indirect measures, such as heart rate variability (HRV), has been applied to clinical practice to provide information on cardiac regulation, as well as to evaluate neural adaptations to physical exercise.5, 6, 7, 8 HRV can be evaluated by linear methods, which quantify the sympatho-vagal balance or non-linear methods, which consider the complexity of the interaction of biological systems on the heart.7, 8, 9 Reduction in resting HRV is a sensitive indicator of cardiac autonomic impairment and is associated with a higher risk of CVD morbidity and mortality. This condition occurs due to the predominance of sympathetic modulation, consequently, enhancing the cardiac overload and/or reduction of cardiac parasympathetic modulation. Therefore, interventions to increase HRV, that is, increment of parasympathetic modulation at rest, should be investigated.10
Although regular physical exercise is a factor in increasing HRV, few patients are referred to and engaged into a physical training program.11 Low adherence is often associated with exercise intolerance caused by the pathological condition, in addition to the lack of time.12 In view of this situation, high intensity interval training (HIIT) may be a cardiovascular exercise strategy to promote greater adherence in cardiovascular rehabilitation as it covers shorter training sessions (20–25 min), comprising high intensity exercise intervals (85% to 95% of maximum HR and/or maximum oxygen consumption) interspersed with rest or active recovery, allowing patients to have complete periods at high intensity.13, 14 Moreover, the periods of high intensity stimulate more intense cardiovascular and muscular adaptations in cardiopathy patients, which favors mitochondrial function and calcium reabsorption rate into the sarcoplasmic reticulum, reducing skeletal muscle fatigue and increasing exercise capacity.15
Although some studies demonstrate better effects of HIIT, when compared to continuous moderate intensity training, on the aerobic capacity of patients with CVD, little is known about the effect of this training modality on cardiac autonomic control.15, 16 Thus, the purpose of this study was to conduct a systematic review to evaluate the effects of HIIT on cardiac autonomic responses in humans, evaluated by HRV, allowing the training application in the rehabilitation and prevention of pathologies that have autonomic imbalance as clinical characteristics.
Methods
This systematic review was reported following the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).17 Moreover, the State of the Art through Systematic Review (StArt)18 was used to help and systematize the search and data extraction (available from: http://lapes.dc.ufscar.br/tools/start_tool).
Data sources and search strategy
Electronic databases from inception to March 29th, 2018 were searched including: PEDro (Physiotherapy Evidence Database), SCOPUS (Elsevier) and PubMed (via National Library of Medicine). The following MeSH terms were used for the intervention type (“high intensity interval training”) and outcomes (“autonomic nervous system” OR “heart rate”). Furthermore, the search was limited to humans (population of interest) and the English language. Finally, the additional limits were used: clinical study, clinical trial, controlled clinical trial and randomized clinical trial.
Study criteria and selection
The eligibility criteria were determined using the Patient/Population–Intervention–Comparison/Comparator–Outcome (PICO) format. The selected studies had looked at: Adults with or without cardiovascular disease (P); who carried out a HIIT program (I); there were no comparisons with other interventions (C); our results of interest were measurements of cardiac autonomic control through HRV (O). Review articles, non-randomized clinical trials, short communications, letters with insufficient information to analyze the results, case studies, guidelines, theses, dissertations, qualitative studies, scientific conference abstracts, studies on animals, acute interventions, non-English language articles and articles addressing other exercises type (continuous aerobic training and resistance training) as primary intervention, were excluded.
Two authors (PRS and RMA) initially selected the articles by screening titles and abstracts, independently, according to the study criteria. Full-text screening was performed when considered potentially eligible by the two reviewers. If there were some disagreements between these reviewers, a third independent reviewer (RPS) was consulted to determine inclusion in the systematic review. In addition, when articles were not available, the authors were contacted and references of articles selected were verified to search for other relevant studies.
Data extraction
From each selected study, data were reported through descriptive analysis and complementary the deltas of HRV indexes (post minus pre) were calculated. Moreover, the data were extracted by two independent reviewers (PRS and RMA), using standardized forms that included information about characteristics of participants and groups (population, sample size, groups, gender, age, peak oxygen consumption [VO2PEAK] and body mass index [BMI] at baseline); characteristics of intervention (ergometer, high intensity interval, recovery interval, number and duration of sessions, intervention time, supervised intervention); measurements and main outcomes related to the effects of HIIT on HRV indexes. In addition, the secondary variables and other functional gains (blood pressure, functional capacity, corporal composition, cardiovascular function, cardiorespiratory fitness and metabolic changes) found in each study were also extracted.
Heart rate variability analysis
The comparison of interest were indexes of HRV assessment by linear methods (frequency and time domains), also non-linear methods (e.g., SampEn, Poincaré method and detrended fluctuation analysis) which are described below.
Linear methods
The frequency domain to quantify the short-term HRV provides two main frequency components: high frequency (HF), that represents an index of parasympathetic modulation and low frequency (LF), that when expressed in normalized units correspond to cardiac sympathetic modulation.8 However, some authors consider the LF, if expressed in absolute values, as a component that has sympathetic and parasympathetic influences19 while others showed that this component does not have cardiac sympathetic influence but reflects baroreceptor reflex activity.20, 21
In addition, the LF/HF ratio is applied as an indicator of “sympatho-vagal balance,” where possibly an increase is related to sympathetic predominance and a decrease indicates greater parasympathetic modulation.22 However, there is still questionable the interpretation of LF/HF, since the LF band does not correspond purely to sympathetic modulation, also the interpretation of this ratio depends on the environmental conditions and collection time (short or long term).23 In addition, the interactions of the autonomic nervous system pathways have a complex and non-linear dynamic, making controversial the “sympatho-vagal balance” theory.24
Moreover, components of very low frequency (VLF) and ultra-low frequency (ULF) are less used because, although there are some hypotheses, their physiological mechanisms are still being investigated. Furthermore, the analyses of ULF requires a recording period of at least 24 h and it seems to be related to very slow-acting biological processes, like metabolism, the renin-angiotensin-aldosterone system and thermoregulation.8, 19, 20, 25 While, the VLF ideally requires a long-term recording period (over 24 h) and appears to be related to heart's intrinsic nervous system and its oscillations is influenced by sympathetic nervous system.8, 26
Another linear approach, the time domain method identified the intervals between successive beats and carried out the numerical analyses such as: standard deviation of the RR interval (SDNN) and standard deviation of the means of the normal RR intervals, every 5 min (SDANN) that represents the conjoint sympathetic and parasympathetic modulation8; square root of the mean squared differences of successive RR intervals (RMSSD) and the percentage of adjacent RR intervals with a duration difference greater than 50 ms (pNN50) and both indicate cardiac parasympathetic modulation.10 Finally, triangular interpolation of RR intervals (TINN) express the global variability with joint action of the autonomic nervous system pathways.27
Non-linear methods
Complementarily, the non-linear methods such as: SampEn, Poincaré method and detrended fluctuation analysis (DFA), consider the complex dynamic of biological systems on RR interval series. The values of SampEn approaching 0 are considered highly regular and larger values mean greater complexity.28 The Poincaré method consists of constructed plots from the RR interval data for each participant and these plots display each RR interval as a function of the subsequent RR interval in that time-series. This analysis can visually search for patterns buried within a time series.29 Moreover, these plots can derive two main non-linear measurements, SD1 and SD2.
The SD1 represents the dispersion of points perpendicular to the identity line and measures of short-term HRV in ms. It characterizes cardiac parasympathetic modulation30, 31 and it correlates with baroreflex sensitivity,32 while SD2 represents the dispersion of points along the identity line and indicates the short and long-term HRV in ms (jointly cardiac sympathetic and parasympathetic modulation and correlates with LF power and baroreflex sensitivity).32 Finally, the slope α1 of DFA analysis, consists of brief fluctuations, while the slope α2 describes long-term fluctuations. The slope α1 reflect the baroreflex sensitivity, while the long-term reflects the mechanisms that limit oscillations of the beat cycle.8, 33
Quality assessment
The methodological quality of individual studies was assessed using the Physiotherapy Evidence Database (PEDro) scale34 and two researchers classified the articles independently (PRS and RMA). When there was any disagreement, the researchers discussed it to obtain a consensus between them concerning the final score. Articles were classified into two levels: high quality (score: ≥6) and low quality (score: <6).35 The PEDro scale included 11 questions related to methodological quality and statistical reporting: eligibility criteria were specified; subjects were randomly allocated to groups; allocation was concealed; the groups were similar at baseline regarding the most important prognostic indicators; there was blinding of all subjects; there was blinding of all therapists who administered the therapy; there was blinding of all assessors who measured at least one key outcome; measures of at least one key outcome were obtained from more than 85% of the subjects initially allocated to groups; all subjects for whom outcome measures were available received the treatment or control condition as allocated or, where this was not the case, data for at least one key outcome was analyzed by “intention to treat”; the results of between-group statistical comparisons are reported for at least one key outcome; the study provides both point measures and measures of variability for at least one key outcome. However, the maximum final score was 10 points because it did not include question 1 (specifying the origin of the subject and eligibility criteria) as it affects the external validity rather than internal validity.36 Moreover, for interpretation and discussion of the results, the impact of methodological quality was considered according to the PEDro scale total score.
Results
Study selection
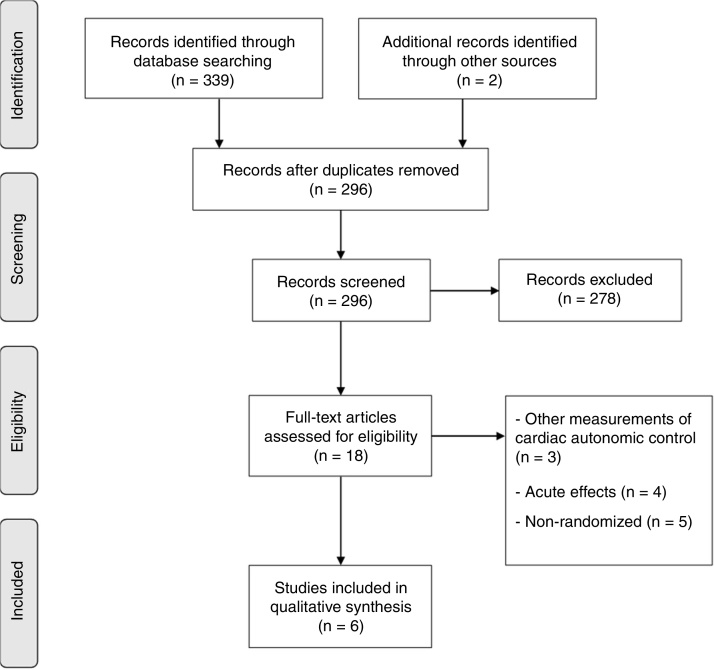
The search strategies in the electronic databases resulted in 339 citations and 2 additional citations were identified through other sources. After deleting duplicate articles and revising the full text, 6 articles were included. The main reasons for excluding the articles were: (a) assessment of acute effects37, 38, 39, 40; (b) other measurements of cardiac autonomic control and not by HRV41, 42, 43; (c) non-randomized clinical trials44, 45, 46, 47 (Fig. 1).
Figure 1.
Flow chart of search strategy and retrieval of articles.
Quality assessment
The methodological quality of the included studies is described in Table 1. The mean score of PEDro was 6 (ranging from 5 to 8), with 3 studies of high quality48, 49, 50 and 3 studies of low quality.51, 52, 53 Commonly, the items not performed or omissions in reporting information were: blinding of all subjects (6 studies), all therapists who administered the therapy (5 studies) and all assessors who measured at least one key outcome (5 studies) and the allocation was not concealed (4 studies).
Table 1.
Study and participants characteristics.
| First author, year | Population | Groups and sample size | Gender per group (M/W) | Age (years) | VO2peak baseline (ml kg−1 min−1) | BMI baseline (kg/m2)/%total body fat | PEDro total score |
|---|---|---|---|---|---|---|---|
| Heydari, 2013 | Healthy | HIIT (n = 20) CON (n = 18) |
ALL (38/0) | 18–35 (24.9 ± 4.3) | HIIT (34.2 ± 4.4) CON (29 ± 5) |
HIIT (28.4 ± 2.4) CON (29.0 ± 3.9) |
8/10 (High) |
| Kiviniemi, 2014 | Healthy | HIIT (n = 13) AET (n = 13) |
ALL (26/0) | HIIT (48 ± 5) AET (48 ± 5) |
HIIT (34.7 ± 3.9) AET (33.9 ± 4.6) |
HIIT (25.6 ± 2.7) AET (26.1 ± 2.0) |
5/10 (Low) |
| Koufaki, 2014 | CHF | HIIT (n = 8) CAT (n = 9) |
HIIT (14/2) CAT (13/4) |
HIIT (60 ± 7) CAT (60 ± 11) |
HIIT (14.6 ± 4.8) CAT (15.5 ± 15.9) |
HIIT (28.9 ± 4.7) CAT (29.5 ± 4.7) |
5/10 (Low) |
| Munk, 2009 | CAD | HIIT (n = 20) CON (n = 18) |
HIIT (17/3) CON (15/3) |
HIIT (58 ± 10) CON (60 ± 8) |
NR | HIIT (27.0 ± 4.0) CON (28.2 ± 3.4) |
5/10 (Low) |
| Piras, 2014 | Healthy | HIIT (n = 8) PHA (n = 10) |
HIIT (2/6) PHA (7/3) |
HIIT (25 ± 3) PHA (23 ± 2) |
HIIT (∼28) PHA (∼37) |
HIIT (23.6 ± 2.7) PHA (21.9 ± 4.2) |
6/10 (High) |
| Ramos, 2017 | MetS | 1 × 4 HIIT (n = 21) 4 × 4 HIIT (n = 19) MICT (n = 16) |
1 × 4 HIIT (13/8) 4 × 4 HIIT (9/10) MICT (9/7) |
1 × 4 HIIT (58 ± 7) 4 × 4 HIIT (56 ± 8) MICT (54 ± 11) |
1 × 4 HIIT (26.3 ± 6.8) 4 × 4HIIT (23.1 ± 4.7) MICT (27.3 ± 7.5) |
1 × 4 HIIT (39.6% ± 7.1) 4 × 4 HIIT (42.6% ± 6.4) MICT (40.4%± 9.5) |
7/10 (High) |
AET, aerobic endurance training; BMI, body mass index; CAD, coronary artery disease; CAT, continuous moderate intensity aerobic exercise; CON, control group; CHF, chronic heart failure; HIIT, high-intensity interval training; M, men; MetS, metabolic syndrome; MICT, moderate-intensity continuous training; NR, not report; PHA, peripheral heart action training; VO2peak, peak oxygen consumption; W, women; For age, VO2peak, and BMI at baseline, data are expressed as mean and standard deviation.
Characteristics of participants
The characteristics of participants and groups are described in Table 1. The total sample size was 193 individuals aged from 18 to 60 years old, with the BMI at baseline from 21.9 to 29.5 kg/m2. Five articles reported the peak oxygen consumption (VO2peak) ranging from 14.6 to 37 ml kg−1 min−1, while one study did not report this measurement.21 Regarding the groups, there was a range from 8 to 21 individuals per group, and two studies presented a control group,48, 53 three compared the HIIT with aerobic training of moderate intensity49, 51, 52 and one with peripheral heart action training.50 Three articles included a healthy population in their samples,48, 50, 51 while the other studies evaluated the effects of HIIT in patients with coronary artery disease (CAD),53 chronic heart failure52 and metabolic syndrome (MetS).49
Characteristics of interventions
The characteristics of HIIT interventions are summarized in Table 2. Most studies used the cycle ergometer to perform the training sessions while one also used the treadmill according to preference and physical limitation of the participants49 and one study trained the participants only on the treadmill.53 The time of the high intensity interval ranged from 8 s to 4 min, with intensity between 80% and 100% prescribed by the HRpeak, VO2max/peak or peak power output (PPO) reached in the incremental test. The cadence of the high intensity interval in cycle was maintained from 70 to 130 rpm, while four studies did not report this information.49, 51, 52, 53 Regarding recovery interval periods, the time ranged from 12 s to 4 min of active recovery without resistance. Moreover, most frequently, the training was performed three training sessions per week of 13–38 min per session and all were supervised in at least two of them. Furthermore, the mean of the duration of HIIT was 14 weeks, varying from 2 to 24 weeks.
Table 2.
Characteristics of HIIT interventions.
| First author, year | Ergometer | High intensity interval (time/intensity) | Recovery interval (time/intensity) | Sessions (days/week) | Session duration | Intervention time | Supervised intervention |
|---|---|---|---|---|---|---|---|
| Heydari, 2013 | Cycle | 8 s at 80–90% of HRmax with a cadence between 120 and 130 rpm | 12 s at 40 rpm with no change in resistance | 3 | 30 min | 12 weeks | All sessions |
| Kiviniemi, 2014 | Cycle | 4–6 repetitions of 30 s at maximal load | 4 min of passive recovery or without resistance | 3 | NR | 2 weeks | All sessions |
| Koufaki, 2014 | Cycle | 30 s at ∼100% of PPO | 1 min at 20–30% of PPO | 3 | 30 min | 24 weeks | All sessions |
| Munk, 2009 | Treadmill | 4 min at 90–95% of HRpeak | 3 min at 50% to 70% of HRpeak | 3 | 38 min | 24 weeks | 2 sessions/week |
| Piras, 2014 | Cycle | 1 min at 100% of VO2max with cadence of 70 rpm | 2 min at 70 rpm without resistance | 3 | 13 min | 12 weeks | All sessions |
| Ramos, 2017 | Cycle/Treadmill | 1 × 4 HIIT: 1 × 4 min of 85–95% of HRpeak 4 × 4 HIIT: 4 × 4 min of 85–95% of HRpeak |
1 × 4 HIIT: no recovery 4 × 4 HIIT: 3 min of 50–70% HRpeak |
3 | 1 × 4 HIIT: 38 min 4 × 4 HIIT: 17 min |
16 weeks | 2 sessions/week |
HIIT, high intensity interval training; HRmax, heart rate maximal; HRpeak, heart rate peak; NR, not reported; PPO, peak power output; Rpm, rounds per minute; VO2max, maximal oxygen consumption.
Assessment of heart rate variability
All information about the effects of HIIT on indexes of HRV is described in Table 3. The studies evaluated the cardiac autonomic control by linear and/or non-linear analysis, moreover, the HRV was acquired at rest condition during short (10 min) and long-term (30 min to 24 h) ECG recording.
Table 3.
Effects of HIIT on HRV indexes and secondary outcomes.
| First author, year | Linear HRV measures |
Non-linear HRV measures | Outcomes in HIIT group | Secondary outcomes | |
|---|---|---|---|---|---|
| Time domain | Frequency domains | ||||
| Heydari, 2013 | rMSSD, PNN50 | VLF (abs, ln), LF (abs, ln), HF (abs, ln) | NA | Increase in LF (ln), HF (ln), rMSSD and PNN50 | Decrease in BMI, WC, BP (SBP, DBP and MAP) and arterial stiffness. Increase in VO2max, SV and LVET |
| Kiviniemi, 2014 | NA | LF (ln), HF (ln), LF/HF (abs) | NA | Decrease in LF/HF (ln) in day period | Increase in VO2peak and load peak in the maximal exercise test |
| Koufaki, 2014 | TI24, TP, SDNN | LF/HF (abs) | NA | No changes in HRV indexes | Increase in VO2peak and functional capacity |
| Munk, 2009 | SDNN, SDANN, rMSSD TINN, TP | ULF (ln), VLF (ln), VHF (ln), LF (ln), HF (ln), LF/HF (ln) | NA | Increase in ULF (day), SDNN (24 h), rMSSD (24 h), SDANN (24 h) and TINN (24 h) | Increase in VO2peak and in anaerobic threshold, maximal workload and endothelial function |
| Piras, 2014 | rMSSD (ln, abs), SDNN (ln, abs), NN50 (ln, abs), pNN50(%, ln%) | LF (abs, ln, nu), HF (abs, ln, nu) LF/HF (abs, ln, nu) | NA | Increase in rMSSD, SDNN, pNN50, LF (abs, nu), HF (abs, nu) and LF/HF (nu) | Increase in VO2max and BRS, decrease in SBP and MAP |
| Ramos, 2017 | SDNN, rMSSD, PNN50 | LF (abs), HF (abs), LF/HF (abs) | SampEn, α1, α2, SD1, SD2 | 1 × 4 HIIT: No changes in HRV indexes 4 × 4 HIIT: Increase in SDNN, rMSSD, SD1, SD2 and HF (abs) |
Increase in VO2max, decrease of total body fat %, HOMA-IR, HDL-C, WC and BP |
interval, mean of all RR intervals; α1 and α2, detrended fluctuation analysis; abs, absolute; BMI, body mass index; BP, blood pressure; BRS, baroreflex sensitivity; DBP, diastolic blood pressure; HF, high frequency; HRV, heart rate variability; LVET, left ventricular ejection time; LF, low frequency; ln, logarithm natural; MAP, mean arterial pressure; NA, not assessed; NN50, number of sequential N–N intervals differing by longer than 50 ms; nu, normalized units; PNN50, percentage of successive differences between normal adjacent intervals >50 ms; rMSSD, square roost of the mean differences between consecutive high-squared RR interval; SampEn, sample entropy; SD1 and SD2, geometric parameter of the Poincaré plot; SDANN, the standard deviation of the average normal RR intervals for 288 5-minute segments of a 24-hour ECG recording; SBP, systolic blood pressure; SDNN, standard deviation of all normal RR interval; SV, stroke volume; TI24, triangular Index of a 24 h recording; TINN, the triangular interpolation of NN interval histogram; ULF, ultra low frequency; VHF, very high frequency; VLF, very low frequency; VO2max, maximal oxygen consumption; VO2peak, peak oxygen consumption; WC, waist circumference.
All studies used the frequency domain to quantify the short-term HRV, while five studies also adopted time domain approaches.48, 49, 50, 52, 53 On the other hand, one article49 performed the non-linear analyses including the sample entropy (SampEn), Poincaré method and DFA.
Linear methods
Regarding analysis of time and frequency domain, five studies showed improvements in parasympathetic and/or sympathetic modulation, observed in some HRV indexes. Since in the time domain, an increase of the SDNN (Δ: 5.3–27), PNN50 (Δ: 2.7–4.2), rMSSD (Δ: 0.3–7.1), SDANN (Δ: 27) and TINN (Δ: 108) components was observed.48, 49, 50, 53 While in the frequency domain, the HFabs (Δ: 44.1–416.13), HFln (Δ: 0.3), LF/HFnu (Δ: 0.13) and ULFln (Δ: 1.1) were increased48, 50 and the LF/HFln (Δ: −0.2) was decrease.51 However, the changes in linear indexes were not observed in one study after HIIT.52
Non-linear methods
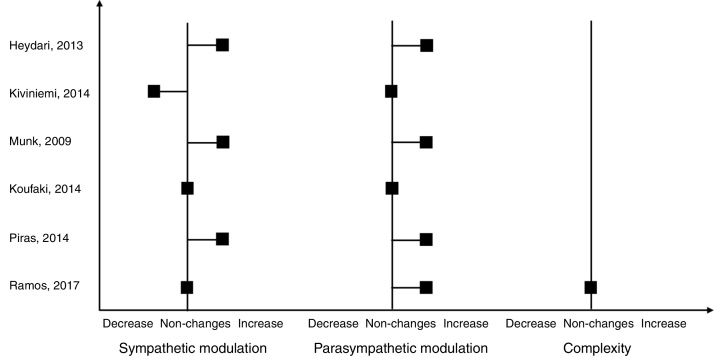
Among the nonlinear analysis of HRV, one study with patients with MetS identified an increase in SD1 (Δ: 3.3) and SD2 (Δ: 4.8), when evaluated by the Poincaré method, suggesting an improvement in nonlinear heart rate dynamics.49 Briefly, the results about effects of HIIT on cardiac autonomic modulation of each included article are expressed in Fig. 2 qualitatively.
Figure 2.
Qualitative results of HIIT on cardiac autonomic control.
Secondary outcomes
In addition, others secondary outcomes were observed post HIIT as described in Table 3. All included studies showed improvement of cardiorespiratory fitness, demonstrated by the increase in maximal or peak oxygen consumption (VO2max and VO2peak, respectively), load peak in the maximal exercise test and/or VO2peak at anaerobic threshold. Moreover, the functional capacity assessed by sit-to-stand and gait speed was improved post training.52 Regarding the cardiovascular benefits, were found decrease of blood pressure values,48, 49, 50 reduction of arterial stiffness,48 improvement in cardiac48 and endothelial function.53 Finally, other gains such as improvement in body composition,48, 49 lipid profile,49 insulin resistance,49 baroreflex sensitivity50 were observed.
Discussion
This present systematic review, through an evaluation of 6 randomized clinical trials, demonstrated that HIIT can be a training option to improve the cardiac autonomic control in healthy conditions and in patients who have CAD and MetS. The positive effects on HRV observed, were an increase of parasympathetic modulation at rest, represented by the linear indexes, such as: HF, rMSSD, pNN50 and decrease in LF/HF.48, 49, 50, 53 However, the LF/HF index, presented controversial effects between included studies, Kiviniemi51 observed that this index decreased after the HIIT, representing an increase in vagal modulation; while Piras50 found an increase of LF/HF, indicating a greater sympathetic modulation. On the other hand, when evaluated by non-linear methods, values of DFA did not modify, while increase in SD1 and SD2 indexes of the Poincare analysis were observed reflecting predominance of vagal modulation post training.49
These adaptations represent reductions of cardiovascular risk and overall mortality in cardiovascular rehabilitation, as well as prevention in the development of cardiovascular diseases and their risk factors in healthy individuals.21 Although there is no consensus for the prescription of a HIIT protocol, in general, when performed between 80% and 100% of HRmax, over 20 min for 3 days/week can be effective to promote responses on cardiac autonomic control, with effects observed shortly after two weeks.51
Furthermore, several physiological mechanisms support the application of this training in clinical practice. When compared to continuous training, HIIT has a greater ability to stimulate nitric oxide (NO) syntheses by endothelium, due to the shear stress promoted in the vessel wall during high intensity periods.54 The bioavailability of this oxide, promotes stimulus in vagal neurons which are also modulated by endothelial NO.55 Moreover, the HIIT induces a greater degree of distensibility of carotid artery, which is associated with improvements in baroreflex sensitivity.48, 56, 57 Some studies in this revision attributed these effects by an increase in LF power and its possible relation with baroreceptor reflex sensitivity,48, 50, 51 although there is still no consensus in the literature about this relationship.19, 20, 58 In addition, it is well defined that HIIT favors oxidative capacity by improving mitochondrial function, facilitating the diffusion capacity of skeletal muscle.15, 59 These mechanisms favor aerobic power (VO2max), which may be correlated to the predominance of rest vagal modulation after HIIT, as demonstrated by one study included.53
In contrast, one study did not identify changes in the indexes of HRV in patients with CHF52 post HIIT. One hypothesis is that these individuals worsened their overall health status during the study and underlying medical conditions, resulting in substantial pauses throughout the experimental protocol.52 Another possible hypothesis to non-changes in HRV indexes post HIIT is that the protocol was performed at a low volume of training. According to some authors, the autonomic control can be improved after high doses of endurance training, equivalent to 75% of HRmax for 200 min/wk and is able to restore the indexes of the HRV of elderly sedentary people. On the other hand, low doses of training, performed at 75% HRmax for 95–150 min/wk may improve baroreflex sensitivity indexes, but not HRV.60 Although HIIT generally has a lower training volume, the protocol performed by this study52 may not have been enough to promote adaptations in cardiac autonomic function or overlap the effects of medication in these patients.
In general, the quality of the included articles ranged from 5 to 8 points on the PEDro scale. Thus, the evidences that support the beneficial effects of HIIT on the cardiac autonomic control seem to be more reliable in healthy individuals48, 50 and in patients with MeTS,49 which presented high methodological quality. Moreover, this systematic review was limited by the heterogeneity of the included studies, in relation to training protocols, populations and methods of analysis. In addition, although individuals with cardiometabolic diseases had their medications monitored and controlled during the trials included in this review,49, 53 healthy individuals may have benefited more from HIIT compared to those who administer drugs that may influence the results on HRV.
Finally, despite the promising results of HIIT on cardiac autonomic control demonstrated in this systematic review, it is necessary to carry out future randomized clinical trials that consider the evaluation of the effects of HIIT on the HRV indices, as well as the investigation of the relationship between these analyses. Thus, making it possible to prescribe this method of training, with safety and effectiveness in clinical practice.
Conclusion
Although this systematic review was limited by different population and protocols of exercise, considering the current evidence, the HIIT is a promising tool to improve the HRV, favoring the increase of parasympathetic modulation at rest, mainly in healthy individuals and patients with MetS, according to the methodological quality and risk of bias of the included studies.
Conflicts of interest
The authors declare that there is no conflict of interest
Acknowledgments
The authors would like to acknowledge the São Paulo Research Foundation – FAPESP (2017/13402-0) for funding this study.
References
- 1.“ACSM's Resource Manual for Guidelines for Exercise Testing and Prescription” by David P. Swain, Clinton A. Brawner et al. [Internet]. Available from: http://digitalcommons.odu.edu/hms_books/3/ [cited 7.9.17].
- 2.Florea V.G., Cohn J.N. The autonomic nervous system and heart failure. Circ Res. 2014;114(11):1815–1826. doi: 10.1161/CIRCRESAHA.114.302589. [DOI] [PubMed] [Google Scholar]
- 3.Izzo L.J. Sympathoadrenal activity catecholamines, and the pathogenesis of vasculopathic hypertensive target-organ damage. Am J Hypertens. 1989;2(12_Pt_2):305S–312S. doi: 10.1093/ajh/2.12.305s. [DOI] [PubMed] [Google Scholar]
- 4.Saavedra M.J., Romero F., Roa J., Rodríguez-Núñez I. Exercise training to reduce sympathetic nerve activity in heart failure patients. A systematic review and meta-analysis. Braz J Phys Ther. 2018;22(2):97–104. doi: 10.1016/j.bjpt.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santos-Hiss M.D.B., Melo R.C., Neves V.R. Effects of progressive exercise during phase I cardiac rehabilitation on the heart rate variability of patients with acute myocardial infarction. Disabil Rehabil. 2011;33(10):835–842. doi: 10.3109/09638288.2010.514016. [DOI] [PubMed] [Google Scholar]
- 6.Murad K., Brubaker P.H., Fitzgerald D.M. Exercise training improves heart rate variability in older patients with heart failure: a randomized, controlled, single-blinded trial. Congest Heart Fail. 2012;18(4):192–197. doi: 10.1111/j.1751-7133.2011.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Negrão C.E., Irigoyen M.C., Moreira E.D., Brum P.C., Freire P.M., Krieger E.M. Effect of exercise training on RSNA, baroreflex control, and blood pressure responsiveness. Am J Physiol. 1993;265(2 Pt 2):R365–R370. doi: 10.1152/ajpregu.1993.265.2.R365. [DOI] [PubMed] [Google Scholar]
- 8.Shaffer F., Ginsberg J.P. An overview of heart rate variability metrics and norms. Front Public Health [Internet] 2017;28:5. doi: 10.3389/fpubh.2017.00258. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5624990/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17(3):354–381. [PubMed] [Google Scholar]
- 10.Vanderlei L.C.M., Pastre C.M., Hoshi R.A., Carvalho T.D.de., Godoy M.F.de. Basic notions of heart rate variability and its clinical applicability. Rev Bras Cir Cardiovasc. 2009;24(2):205–217. doi: 10.1590/s0102-76382009000200018. [DOI] [PubMed] [Google Scholar]
- 11.Routledge F.S., Campbell T.S., McFetridge-Durdle J.A., Bacon S.L. Improvements in heart rate variability with exercise therapy. Can J Cardiol. 2010;26(6):303–312. doi: 10.1016/s0828-282x(10)70395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herdy A.H., López-Jiménez F., Terzic C.P. South American Guidelines for cardiovascular disease prevention and rehabilitation. Arq Bras Cardiol. 2014;103(2):1–31. doi: 10.5935/abc.2014s003. [DOI] [PubMed] [Google Scholar]
- 13.Gaesser G.A., Angadi S.S. High-intensity interval training for health and fitness: can less be more? J Appl Physiol. 2011;111(6):1–1540. doi: 10.1152/japplphysiol.01237.2011. [DOI] [PubMed] [Google Scholar]
- 14.Ciolac E.G. High-intensity interval training and hypertension: maximizing the benefits of exercise? Am J Cardiovasc Dis. 2012;2(2):102–110. [PMC free article] [PubMed] [Google Scholar]
- 15.Wisløff U., Støylen A., Loennechen J.P. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115(24):3086–3094. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 16.Sheykhlouvand M., Gharaat M., Khalili E., Agha-Alinejad H., Rahmaninia F., Arazi H. Low-volume high-intensity interval versus continuous endurance training: effects on hematological and cardiorespiratory system adaptations in professional Canoe Polo athletes. J Strength Cond Res. 2017 doi: 10.1519/JSC.0000000000002112. [DOI] [PubMed] [Google Scholar]
- 17.Liberati A., Altman D.G., Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Fabbri S., Silva C., Hernandes E., Octaviano F., Di Thommazo A., Belgamo A. Improvements in the StArt tool to better support the systematic review process. Proceedings of the 20th International Conference on Evaluation and Assessment in Software Engineering [Internet]; New York, NY: ACM; 2016. p. 21:1–21:5. (EASE’16). Available from: http://doi.acm.org/10.1145/2915970.2916013. [Google Scholar]
- 19.Akselrod S., Gordon D., Ubel F.A., Shannon D.C., Berger A.C., Cohen R.J. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213(4504):220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 20.Rahman F., Pechnik S., Gross D., Sewell L., Goldstein D.S. Low frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Clin Auton Res. 2011;21(3):133–141. doi: 10.1007/s10286-010-0098-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mccraty R., Shaffer F. Heart rate variability: new perspectives on physiological mechanisms, assessment of self-regulatory capacity, and health risk. Glob Adv Health Med. 2015;4(1):46–61. doi: 10.7453/gahmj.2014.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michael S., Graham K.S., Davis G.M. Cardiac Autonomic Responses during Exercise and Post-exercise Recovery Using Heart Rate Variability and Systolic Time Intervals-A Review. Front Physiol. 2017;8:301. doi: 10.3389/fphys.2017.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lombardi F., Stein P.K. Origin of heart rate variability and turbulence: an appraisal of autonomic modulation of cardiovascular function. Front Physiol [Internet] 2011;2 doi: 10.3389/fphys.2011.00095. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3233900/ [cited 7.8.18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Billman G.E. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front Physiol [Internet] 2013;4 doi: 10.3389/fphys.2013.00026. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3576706/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akselrod S., Gordon D., Madwed J.B., Snidman N.C., Shannon D.C., Cohen R.J. Hemodynamic regulation: investigation by spectral analysis. Am J Physiol. 1985;249(4 Pt 2):H867–H875. doi: 10.1152/ajpheart.1985.249.4.H867. [DOI] [PubMed] [Google Scholar]
- 26.Shaffer F., McCraty R., Zerr C.L. A healthy heart is not a metronome: an integrative review of the heart's anatomy and heart rate variability. Front Psychol [Internet] 2014;5 doi: 10.3389/fpsyg.2014.01040. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4179748/ [cited 25.7.18] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanderlei L.C.M., Pastre C.M., Júnior F., Forte I., Godoy M.F de. Geometric indexes of heart rate variability in obese and eutrophic children. Arq Bras Cardiol. 2010;95(1):35–40. doi: 10.1590/s0066-782x2010005000082. [DOI] [PubMed] [Google Scholar]
- 28.Goldberger A.L., Amaral L.A., Glass L. PhysioBank PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation. 2000;101(23):E215–E220. doi: 10.1161/01.cir.101.23.e215. [DOI] [PubMed] [Google Scholar]
- 29.Carrasco S.O.Y., Gaitán M.J., González R. Correlation among Poincaréplot indexes and time and frequency domain measures of heart rate variability. J Med Eng Technol. 2001;25(6):240–248. doi: 10.1080/03091900110086651. [DOI] [PubMed] [Google Scholar]
- 30.Tulppo M.P., Mäkikallio T.H., Seppänen T., Airaksinen J.K.E., Huikuri H.V. Heart rate dynamics during accentuated sympathovagal interaction. Am J Physiol Heart Circul Physiol. 1998;274(3):H810–H816. doi: 10.1152/ajpheart.1998.274.3.H810. [DOI] [PubMed] [Google Scholar]
- 31.Brennan M., Palaniswami M., Kamen P. Poincaré plot interpretation using a physiological model of HRV based on a network of oscillators. Am J Physiol Heart Circ Physiol. 2002;283(5):H1873–H1886. doi: 10.1152/ajpheart.00405.2000. [DOI] [PubMed] [Google Scholar]
- 32.Tulppo M.P., Mäkikallio T.H., Takala T.E., Seppänen T., Huikuri H.V. Quantitative beat-to-beat analysis of heart rate dynamics during exercise. Am J Physiol. 1996;271(1 Pt 2):H244–H252. doi: 10.1152/ajpheart.1996.271.1.H244. [DOI] [PubMed] [Google Scholar]
- 33.Huikuri H.V., Perkiömäki J.S., Maestri R., Pinna G.D. Clinical impact of evaluation of cardiovascular control by novel methods of heart rate dynamics. Philos Trans A Math Phys Eng Sci. 2009;367(1892):1223–1238. doi: 10.1098/rsta.2008.0294. [DOI] [PubMed] [Google Scholar]
- 34.Moseley A., Sherrington C., Herbert R., Maher C. The extent and quality of evidence in neurological physiotherapy: an analysis of the physiotherapy evidence database (PEDro) Brain Impairment. 2000;1(2):130–140. [Google Scholar]
- 35.Moseley A.M., Herbert R.D., Sherrington C., Maher C.G. Evidence for physiotherapy practice: a survey of the Physiotherapy Evidence Database (PEDro) Aust J Physiother. 2002;48(1):43–49. doi: 10.1016/s0004-9514(14)60281-6. [DOI] [PubMed] [Google Scholar]
- 36.Maher C.G., Sherrington C., Herbert R.D., Moseley A.M., Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83(8):713–721. [PubMed] [Google Scholar]
- 37.Kiviniemi A.M., Tulppo M.P., Eskelinen J.J. Autonomic function predicts fitness response to short-term high-intensity interval training. Int J Sports Med. 2015;36(11):915–921. doi: 10.1055/s-0035-1549854. [DOI] [PubMed] [Google Scholar]
- 38.Cipryan L., Laursen P.B., Plews D.J. Cardiac autonomic response following high-intensity running work-to-rest interval manipulation. Eur J Sport Sci. 2016;16(7):808–817. doi: 10.1080/17461391.2015.1103317. [DOI] [PubMed] [Google Scholar]
- 39.Perkins S.E., Jelinek H.F., Al-Aubaidy H.A., de Jong B. Immediate and long term effects of endurance and high intensity interval exercise on linear and nonlinear heart rate variability. J Sci Med Sport. 2017;20(3):312–316. doi: 10.1016/j.jsams.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 40.Kaikkonen P., Hynynen E., Mann T., Rusko H., Nummela A. Heart rate variability is related to training load variables in interval running exercises. Eur J Appl Physiol. 2012;112(3):829–838. doi: 10.1007/s00421-011-2031-z. [DOI] [PubMed] [Google Scholar]
- 41.Matsuo T., Saotome K., Seino S. Low-volume, high-intensity, aerobic interval exercise for sedentary adults: VO2max, cardiac mass, and heart rate recovery. Eur J Appl Physiol. 2014;114(9):1963–1972. doi: 10.1007/s00421-014-2917-7. [DOI] [PubMed] [Google Scholar]
- 42.Ciolac E.G., Bocchi E.A., Greve J.M.D., Guimarães G.V. Heart rate response to exercise and cardiorespiratory fitness of young women at high familial risk for hypertension: effects of interval vs continuous training. Eur J Cardiovasc Prev Rehabil. 2011;18(6):824–830. doi: 10.1177/1741826711398426. [DOI] [PubMed] [Google Scholar]
- 43.Ciolac E.G., Bocchi E.A., Bortolotto L.A., Carvalho V.O., Greve J.M., Guimarães G.V. Effects of high-intensity aerobic interval training vs. moderate exercise on hemodynamic, metabolic and neuro-humoral abnormalities of young normotensive women at high familial risk for hypertension. Hypertens Res. 2010;33(8):836–843. doi: 10.1038/hr.2010.72. [DOI] [PubMed] [Google Scholar]
- 44.Bond B., Cockcroft E.J., Williams C.A. Two weeks of high-intensity interval training improves novel but not traditional cardiovascular disease risk factors in adolescents. Am J Physiol Heart Circ Physiol. 2015;309(6):H1039–H1047. doi: 10.1152/ajpheart.00360.2015. [DOI] [PubMed] [Google Scholar]
- 45.Boutcher S.H., Park Y., Dunn S.L., Boutcher Y.N. The relationship between cardiac autonomic function and maximal oxygen uptake response to high-intensity intermittent-exercise training. J Sports Sci. 2013;31(9):1024–1029. doi: 10.1080/02640414.2012.762984. [DOI] [PubMed] [Google Scholar]
- 46.Currie K.D., Rosen L.M., Millar P.J., McKelvie R.S., MacDonald M.J. Heart rate recovery and heart rate variability are unchanged in patients with coronary artery disease following 12 weeks of high-intensity interval and moderate-intensity endurance exercise training. Appl Physiol Nutr Metab. 2013;38(6):644–650. doi: 10.1139/apnm-2012-0354. [DOI] [PubMed] [Google Scholar]
- 47.Rakobowchuk M., Harris E., Taylor A., Cubbon R.M., Birch K.M. Moderate and heavy metabolic stress interval training improve arterial stiffness and heart rate dynamics in humans. Eur J Appl Physiol. 2013;113(4):839–849. doi: 10.1007/s00421-012-2486-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heydari M., Boutcher Y.N., Boutcher S.H. High-intensity intermittent exercise and cardiovascular and autonomic function. Clin Auton Res. 2013;23(1):57–65. doi: 10.1007/s10286-012-0179-1. [DOI] [PubMed] [Google Scholar]
- 49.Ramos J.S., Dalleck L.C., Borrani F. High-intensity interval training and cardiac autonomic control in individuals with metabolic syndrome: a randomised trial. Int J Cardiol. 2017;245:245–252. doi: 10.1016/j.ijcard.2017.07.063. [DOI] [PubMed] [Google Scholar]
- 50.Piras A., Persiani M., Damiani N., Perazzolo M., Raffi M. Peripheral heart action (PHA) training as a valid substitute to high intensity interval training to improve resting cardiovascular changes and autonomic adaptation. Eur J Appl Physiol. 2015;115(4):763–773. doi: 10.1007/s00421-014-3057-9. [DOI] [PubMed] [Google Scholar]
- 51.Kiviniemi A.M., Tulppo M.P., Eskelinen J.J. Cardiac autonomic function and high-intensity interval training in middle-age men. Med Sci Sports Exerc. 2014;46(10):1960–1967. doi: 10.1249/MSS.0000000000000307. [DOI] [PubMed] [Google Scholar]
- 52.Koufaki P., Mercer T.H., George K.P., Nolan J. Low-volume high-intensity interval training vs continuous aerobic cycling in patients with chronic heart failure: a pragmatic randomised clinical trial of feasibility and effectiveness. J Rehabil Med. 2014;46(4):348–356. doi: 10.2340/16501977-1278. [DOI] [PubMed] [Google Scholar]
- 53.Munk P.S., Butt N., Larsen A.I. High-intensity interval exercise training improves heart rate variability in patients following percutaneous coronary intervention for angina pectoris. Int J Cardiol. 2010;145(2):312–314. doi: 10.1016/j.ijcard.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 54.Green D.J., Spence A., Halliwill J.R., Cable N.T., Thijssen D.H.J. Exercise and vascular adaptation in asymptomatic humans. Exp Physiol. 2011;96(2):57–70. doi: 10.1113/expphysiol.2009.048694. [DOI] [PubMed] [Google Scholar]
- 55.Buch A.N., Coote J.H., Townend J.N. Mortality, cardiac vagal control and physical training – what's the link? Exp Physiol. 2002;87(4):423–435. doi: 10.1111/j.1469-445x.2002.tb00055.x. [DOI] [PubMed] [Google Scholar]
- 56.Guimarães G.V., Ciolac E.G., Carvalho V.O., D’Avila V.M., Bortolotto L.A., Bocchi E.A. Effects of continuous vs. interval exercise training on blood pressure and arterial stiffness in treated hypertension. Hypertens Res. 2010;33(6):627–632. doi: 10.1038/hr.2010.42. [DOI] [PubMed] [Google Scholar]
- 57.Lal C., Kaur M., Jaryal A.K., Deepak K.K., Bhowmik D., Agarwal S.K. Reduced baroreflex sensitivity decreased heart rate variability with increased arterial stiffness in predialysis. Indian J Nephrol. 2017;27(6):446–451. doi: 10.4103/ijn.IJN_63_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reyes del Paso G.A., Langewitz W., Mulder L.J.M., van Roon A., Duschek S. The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: a review with emphasis on a reanalysis of previous studies. Psychophysiology. 2013;50(5):477–487. doi: 10.1111/psyp.12027. [DOI] [PubMed] [Google Scholar]
- 59.Boutcher Y.N., Boutcher S.H. Exercise intensity and hypertension: what's new? J Hum Hypertens. 2017;31(3):157–164. doi: 10.1038/jhh.2016.62. [DOI] [PubMed] [Google Scholar]
- 60.Okazaki K., Iwasaki K., Prasad A. Dose–response relationship of endurance training for autonomic circulatory control in healthy seniors. J Appl Physiol. 2005;99(3):1041–1049. doi: 10.1152/japplphysiol.00085.2005. [DOI] [PubMed] [Google Scholar]