Highlights
-
•
Historically, conventional radiotherapy has played a limited role in RCC treatment.
-
•
Radiotherapy with ablative doses can overcome RCC’s radioresistance.
-
•
SBRT for RCC results in excellent local control and cancer-specific survival rates.
-
•
MR-guided SBRT for localized RCC may be used for adaptive planning in the future.
Keywords: Renal cell carcinoma, Renal cell cancer, Radiotherapy, Stereotactic ablative radiotherapy (SABR), Stereotactic body radiotherapy (SBRT), MR-guided radiotherapy
Abstract
Renal cell carcinoma (RCC) has traditionally been regarded as radioresistant tumor based on preclinical data and negative clinical trials using conventional fractionated radiotherapy. However, there is emerging evidence that radiotherapy delivered in few fractions with high single-fraction and total doses may overcome RCC s radioresistance. Stereotactic radiotherapy (SRT) has been successfully used in the treatment of intra- and extracranial RCC metastases showing high local control rates accompanied by low toxicity. Although surgery is standard of care for non-metastasized RCC, a significant number of patients is medically inoperable or refuse surgery. Alternative local approaches such as radiofrequency ablation or cryoablation are invasive and often restricted to small RCC, so that there is a need for alternative local therapies such as stereotactic body radiotherapy (SBRT). Recently, both retrospective and prospective trials demonstrated that SBRT is an attractive treatment alternative for localized RCC. Here, we present a comprehensive review of the published data regarding SBRT for primary RCC. The radiobiological rationale to use higher radiation doses in few fractions is discussed, and technical aspects enabling the safe delivery of SBRT despite intra- and inter-fraction motion and the proximity to organs at risk are outlined.
1. Introduction
Renal cell carcinoma (RCC) is the 6th most frequent malignancy in men and the 10th in women, resulting in more than 140,000 RCC-attributed deaths per year worldwide [1]. RCC constitutes a heterogeneous group of different histological subtypes that comprises more than 90% of all primary kidney malignancies [2]. Among the various subtypes, clear cell RCC is most frequent followed by papillary RCC and chromophobe RCC [3]. RCC is more common in elderly people (median age of about 65 years) and in men (male-to-female-ratio of 1.65) [4], [5]. Due to the increasing use of abdominal imaging such as sonography, computer tomography and magnetic resonance imaging, RCC incidence has increased during the last decades [6]. The classical trias of RCC comprise flank pain, gross haematuria and palpable abdominal mass; however, more than half of RCC are detected incidentally [7]. Smoking, obesity, hypertension, and chronic kidney disease are known to be established risk factors for RCC [1]. Surgery including partial and radical nephrectomy is the gold standard for non-metastatic RCC; however, medical comorbidities may rule out surgical excision in a significant number of patients. Although radiofrequency ablation and cryoablation are alternative local treatment option, stereotactic body radiotherapy (SBRT) may be an advantageous treatment approach for larger, more centralized tumors with proximity to vessels or the ureter, as invasive locally ablative techniques may cause strictures, fistulas and bleeding [8]. Moreover, as SBRT is a non-invasive treatment modality, SBRT may have advantages over other alternative local treatment in frail patients and patients with anticoagulation.
2. Current state of the art in the treatment of local RCC
Based on a systematic review analyzing several retrospective trials and a randomized controlled trial, partial nephrectomy is the standard of care for T1 tumors (up to 7 cm) with normal contralateral kidney function and for local RCC with impaired contralateral kidney function, solitary kidney or bilateral kidney tumors without any size limitation [7], [9]. Comparison of partial and total nephrectomy revealed increased renal function and reduced overall mortality in patients receiving partial nephrectomy [10]. There are several scores such as the C-Index, PADUA score and RENAL nephrometry score which help quantifying the complexity of the renal mass and predicting perioperative outcomes [11], [12], [13], [14] (Table 1).
Table 1.
RENAL nephrometry score for describing renal tumor anatomy. Adapted from [11].
| RENAL nephrometry scoring system | ||
|---|---|---|
| Score | ||
| Radius (maximal diameter) | ≤4 cm | 1 |
| 4–7 cm | 2 | |
| ≥7 cm | 3 | |
| Exophytic/endophytic | ≥50% exophytic | 1 |
| <50% exophytic | 2 | |
| completely endophytic | 3 | |
| Nearness to collecting system/renal sinus | ≥7 mm | 1 |
| 4–7 mm | 2 | |
| ≤4 mm | 3 | |
| Anterior/posterior location | No points given. a, p or x as descriptor for mass location | |
| Location relative to the polar lines | Entirely below lower polar or above upper polar line | 1 |
| Mass crosses polar line | 2 | |
| 50% of mass is across polar line or mass is entirely between polar lines or mass crosses axial midline | 3 | |
| Scores | Group | |
| 4–6 | Low complexity | |
| 7–9 | Moderate complexity | |
| 10–12 | High complexity | |
In elderly patients with relevant comorbidities and decreased life expectancy presenting with small kidney tumors (up to 4 cm), active surveillance is an alternative, as most small tumors exhibit a slow growth rate and low metastasis rate leading to a low RCC-related mortality [7], [15]. For selected patients with T1a tumors (up to 4 cm), ablative techniques including radiofrequency ablation and cryoablation are additional alternatives [7], [15]. Albeit randomized controlled trials are lacking, systematic reviews suggest no different cancer-specific survival and metastasis rate after radiofrequency ablation and partial nephrectomy [16]. However, local recurrence rates appear to be higher after radiofrequency ablation compared to partial nephrectomy [7], [16]. By performing a meta-analysis, Kunkle et al. compared radiofrequency ablation and cryoablation for RCC and observed fewer retreatments and improved local tumor control in the cryoablation-group [17]. Incidence of metastatic progression did not differ between radiofrequency ablation, cryoablation and active surveillance. An important difference between both ablative approaches is the point that cryoablation is predominantly conducted laparoscopically, whereas radiofrequency ablation routinely is performed percutaneously [17]. Prior to ablative techniques, a renal tumor core biopsy is recommended, as a significant amount of small renal masses is benign (about 20% for masses up to 3 cm) [18].
Both the current ESMO and NCCN guidelines state that there is no evidence for the utilization of radiotherapy as neoadjuvant or adjuvant treatment in RCC [7], [15]. There are several scores accessing the risk for progression to metastatic RCC after nephrectomy (Table 2). Randomized studies investigating the effects of adjuvant systematic treatment in high-risk RCC after complete resection failed to detect an overall survival benefit for interleukin-2, interferon-α, sunitinib, sorafenib and pazobanib [19], [20], [21], [22]. Only the S-TRAC trial regarding adjuvant sunitinib therapy showed a superior disease-free survival compared to the placebo-group leading to FDA (United States Food and Drug Administration) but not EMA (European Medicines Agency) approval of adjuvant sunitinib treatment [7], [23]. Concerning these negative study results, observation is the standard of care after nephrectomy.
Table 2.
Scoring algorithm accessing the risk for progression to metastatic RCC after nephrectomy. Adapted from [103].
| Score | ||
|---|---|---|
| T category of primary tumor (based on TNM 2002) | pT1a | 0 |
| pT1b | 2 | |
| pT2 | 3 | |
| pT3a-4 | 4 | |
| Lymph node status (based on TNM 2002) | pNx or pN0 | 0 |
| pN1 or pN2 | 2 | |
| Tumor size | <10 cm | 0 |
| ≥10 cm | 1 | |
| Nuclear grade | 1–2 | 0 |
| 3 | 1 | |
| 4 | 3 | |
| Histological tumor necrosis | No | 0 |
| Yes | 1 | |
| Scores | Group | 5-year metastasis-free survival |
| 0–2 | Low risk | 97.1% |
| 3–5 | Intermediate risk | 73.8% |
| ≥6 | High risk | 31.2% |
3. Radiobiology of RCC
Traditionally, RCC is assumed to be a radioresistant tumor demonstrated both by in vitro and in vivo studies. Comparison of various cell lines in vitro indicated that RCC was amongst the most radioresistant cell lines [24]. As RCC cells are known to exhibit a low α/β-ratio, higher treatment doses which are delivered in hypofractionated radiotherapy or SBRT may overcome the intrinsic radioresistance of RCC. Performing clonogenic survival assays, the RCC cell lines Caki-1 and A498 exhibited α/β-ratios of 6.9 and 2.6, respectively [25]. Using higher radiation doses, alternative cell death mechanisms such as ceramide-induced apoptosis become more relevant in RCC cells [26]. Endothelial cell apoptosis based on the ceramide-pathway is believed to be an important mechanism how radiotherapy with high radiation doses acts in highly vascularized tumors such as RCC [26]. Molecularly, a secretory form of acid sphingomyelinase is translocated to the extracellular leaflet of the cell membrane and transforms sphingomyelin into the pro-apoptotic protein ceramide via enzymatic hydrolysis [27]. The fact that acid sphingomyelinase, especially its secretory form, is predominantly expressed in endothelial cells explains the high sensitivity of endothelium to ceramide-induced apoptosis [28]. In vivo studies comparing sphingomyelinase-knockout mice with wildtype mice demonstrated that sphingomyelinase-knockout mice exhibited an increased threshold to irradiation-induced endothelial apoptosis and were resistant to single-dose radiotherapy with 20 Gy [29]. The importance of sphingomyelinase activity regarding tumor response after SBRT was further underlined by the study of Sathishkumar and coworkers [30]: 75% of the patients with partial or complete tumor response after SBRT exhibited significantly increased both serum ceramide and serum sphingomyelinase levels, whereas none of the non-responders had increased levels of these proteins.
Considering the high immunogenicity of RCC, radiotherapy-induced immunogenic cell death may cause an abscopal effect leading to elimination of distant metastases. There are several case reports about abscopal effects in metastatic RCC after local radiotherapy [31], [32], [33]. In a small case series with 4 patients published by Wersäll and coworkers, all patients who exhibited an abscopal effect lived more than 5 years demonstrating the long-lasting anti-tumor effect in these cases [32]. A currently recruiting phase II study aims to investigate the safety profile and efficacy (overall survival, time to local progression, distant progression-free survival) of SBRT and the PD-1-antibody pembrolizumab in oligometastatic RCC (NCT02855203).
4. History of radiotherapy in the treatment of RCC
Both neoadjuvant and adjuvant approaches for radiotherapy have been investigated during the last decades. Two prospective clinical trials comparing nephrectomy alone versus neoadjuvant radiotherapy prior to nephrectomy failed to demonstrate a survival advantage of preoperative irradiation [34], [35]. In the study by van der Werf-Messing, 126 patients with non-metastasized RCC were randomized to either nephrectomy alone (n = 62) or neoadjuvant radiotherapy followed by nephrectomy (n = 64). Subgroup analyses were able to identify patients who benefitted from preoperative radiotherapy, namely patients in which intra- and extrarenal veins or lymph vessels were tumor-infiltrated, leading to a better survival at 18 months. Additionally, a lower metastasis incidence and delayed metastasis onset were observed in patients treated with preoperative radiotherapy. However, 5-year survival was not superior in the radiotherapy-group so that these results did not support neoadjuvant radiotherapy for localized RCC [34]. In the other study conducted by Juusela and colleagues, randomization between nephrectomy alone (n = 50) and neoadjuvant irradiation prior to nephrectomy (n = 38) was performed for 88 patients. Albeit not statistically significant, 5-year survival was inferior in patients who received preoperative radiotherapy (47%) compared to patients with nephrectomy only (63%). Contrary to the study by van der Werf-Messing, there were no subgroups who had a benefit through neoadjuvant radiotherapy [35]. In comparison to other tumor entities, the radiation dose was quite low in both studies (30 Gy in 15 fractions [34] and 33 Gy in 15 fractions [35]) which may have contributed to the negative study results.
Similarly to the neoadjuvant approach, two randomized trials investigating the role of postoperative irradiation after nephrectomy in selected patients could not show a survival benefit of additional radiotherapy [36], [37]. Finney and coworkers randomized 100 patients to either surgery followed by adjuvant radiotherapy (n = 51) or surgery alone (n = 49). Neither overall survival nor local recurrence rate were found to be improved in patients treated by adjuvant radiotherapy. Furthermore, a significant number in the radiotherapy-group suffered from radiotherapy-induced normal tissue injuries such as irradiation-induced liver damage and died from coincidental causes [36]. In the other randomized trial evaluating adjuvant radiotherapy after nephrectomy for localized RCC, 65 patients with stage II and III RCC were randomly treated by surgery followed by observation (n = 33) or adjuvant radiotherapy with 50 Gy delivered in 20 fractions (n = 32). While there was no difference regarding median survival, there was a significant number of patients in the postoperative radiotherapy-group who had radiotherapy-related complications (44%), especially gastrointestinal toxicity, and 19% of patients who received postoperative radiotherapy died from radiotherapy-induced complications [37]. In both studies, eligibility criteria were positive resection margins and vena cava infiltration, while in the study of the Copenhagen Renal Cell Cancer Study Group, positive lymph node status was an additional criterion [36], [37].
In a meta-analysis of 7 controlled trials with a total population of 735 patients, a significant reduction of locoregional failure with a pooled odds ratio of 0.47 was detected in patients with high-risk features (e.g. positive resection margins, positive lymph nodes) receiving postoperative radiotherapy; however, no difference in overall survival and disease-free survival was observed [38]. The authors concluded from these results that a prospective multicenter, randomized, controlled trial is needed to evaluate the role of postoperative radiotherapy for high-risk patients after nephrectomy.
It should be noted that these older randomized trials exhibit several limitations including insufficient radiation doses, non-conformal radiation techniques with large field sizes and parallel-opposed anteroposterior fields and inadequately low patient numbers, thereby limiting the value of these trials [38].
5. Role of stereotactic radiotherapy in RCC metastasis treatment
About 20%–40% of RCC patients develop metastases after nephrectomy, and lung is the second most frequent site of RCC metastases after liver [39]. While overall survival for nontreated metastatic RCC is poor with a 5-year survival of 0%–18% [40], both excellent local control and encouraging overall survival were reported for metastasectomy of pulmonary RCC metastases [41], [42]. In patients with technically resectable metastases and good prognostic factors such as metachronous disease with a long disease-free interval and limited metastases number (up to 6 metastases), a 5-year survival of 52% after metastasectomy is reported in the literature [41].
As a significant number of patients with pulmonary RCC metastases is medically or technically inoperable, alternative approaches such as SBRT came into the focus during the last years. The promising results of SBRT in the treatment of early-stage non-small-cell lung cancer (NSCLC) show the general feasibility and the safe toxicity profile of this technique in the treatment of pulmonary malignancies [43], [44], [45]. In a retrospective multicenter-analysis of the Working Group Stereotactic Radiotherapy of the German Society for Radiation Oncology (DEGRO) including 46 RCC patients with lung metastases, 1- and 3-year local control rates were 98.1% and 91.9% leading to 1- and 3-year overall survival rates of 84.3% and 43.8%, respectively [46]. Only 3 of 46 patients developed grade II + toxicity, and no treatment-related deaths were observed in this cohort. The investigators reported a trend towards improved local control rates using higher biologically effective doses (BED), although statistical significance was not reached (P = 0.054). Altoos et al. compared the effects of SBRT and conventional fractionated radiotherapy to thoracic, abdominal, skin and soft tissue RCC metastases analyzing 34 patients with 53 treated lesions [47]. With a radiographic local control of 93.4% at 36 months after SBRT and no grade III+ side effects, local control was found to be excellent with marginal toxicity. In a univariate analysis, BED ≥ 100 Gy and fraction size ≥ 9 Gy were reported to predict local control rate.
About 10% of RCC patients develop brain metastases leading to significant morbidity and mortality [48]. Even after the introduction of targeted therapies, overall survival after detection of RCC-derived brain metastases remains poor with a median survival time ranging between 6 and 12 months [49], [50]. In a phase II trial evaluating the role of sunitinib for RCC-derived brain metastases (n = 16), no objective response in the brain metastases was detected and overall survival was low with a median time of 6.3 months [51]. However, local therapies such as stereotactic radiotherapy (SRT) and resection have been shown promising results for selected patients in some studies [52], [53]. In a large retrospective analysis comprising 69 patients with a total of 146 RCC brain metastases, a local control rate of 96% and a median overall survival of 15 months was reported. Using this treatment approach, the majority of patients (83%) died of extracranial disease progression. In another retrospective study with 29 patients and 92 RCC brain metastases, gamma knife radiosurgery was used [54]. Especially in patients with Recursive Partitioning Analysis (RPA) class I, median survival of 18 months was promising. In contrast to these results using SRS, results of whole brain radiotherapy (WBRT) for RCC brain metastases are rather disappointing [55]. Comparing the results of SRT for brain metastases derived from different tumor entities, some studies observed similar local control rates, while others observed an enhanced radioresistance of RCC brain metastases [56]. Consideration of SRS with or without WBRT for good-prognosis patients with a single unresectable brain metastasis is recommended by the current ESMO guidelines; however, the increased cognitive dysfunction after the combined therapy is emphasized [7].
6. Current evidence of SBRT for primary RCC
There are several retrospective studies which analyzed the effects of SBRT for primary RCC and demonstrated high local control rates with minimal toxicity [57], [58], [59], [60], [61], [62], [63], [64], [65] (Table 3). Consequently, prospective phase I and II studies were conducted in order to investigate the feasibility and safety of SBRT for primary RCC and to find the optimal radiation dose and fractionation [66], [67], [68], [69], [70], [71] (Table 4). In 2012, Siva and colleagues performed a systematic review comprising 10 studies (3 prospective and 7 retrospective studies) with a total patient population of 126 patients [72]. Whereas local control rates ranged between 84% and 100% after SBRT, SBRT-related toxicity was low with a grade III+ toxicity rate of 3.8%.
Table 3.
Retrospective studies about SBRT for primary RCC.
| Study | Dose | Patient number | Results | Toxicity |
|---|---|---|---|---|
| Beitler [58] | 5 × 8 Gy or 6 × 7 Gy |
9 | LC 100% OS 44% (mean follow-up time 26.7 months) |
33% grade I-II No grade III+ |
| Chang [60] | 5 × 8 Gy (lowered up to 5 × 6 Gy to meet OAR constraints) | 16 | LC 100% (mean follow-up time 19 months) | 6% grade I 13% grade IV |
| Gilson [63] | 5 × 8 Gy (mean) | 33 | LC 88–94% (mean follow-up time 17 months) | Not reported |
| Lo [65] | 5 × 8 Gy | 3 | 100% (mean follow-up time 22 months) | 33% grade I No other toxicities |
| Nair [61] | 3 × 13 Gy | 3 | LC 100% (mean follow-up time 13 months) | Unknown |
| Nomiya [64] | 16 × 4.5 GyE (C12) | 10 | LC at 5 years 100% PFS at 5 years 100% OS at 5 years 74% |
10% grade IV No other toxicities |
| Svedman [57] | 4 × 10 Gy | 7 | LC 86% (mean follow-up time 39 months) | Not reported |
| Qian [62] | 5 × 8 Gy | 20 | LC 93% (mean follow-up time 12 months) | Not reported |
| Wersäll [59] | 5 × 8 Gy or 4 × 10 Gy or 3 × 15 Gy |
8 | LC 90–98% (for localized RCC and RCC metastases) Median survival >58 months |
For localized RCC and RCC metastases: 20% grade I-II 19% grade III |
Table 4.
Prospective trials about SBRT for localized RCC. FLP = freedom from local progression, FDP = freedom from distant progression, LC = local control, OS = overall survival.
| Study | Dose | Patient number | Results | Toxicity |
|---|---|---|---|---|
| Kaplan [70] | 3 × 7 Gy or 3 × 9.3 Gy or 3 × 10.6 Gy or 3 × 13 Gy |
12 | LC 91.7% (unknown follow-up time) | No grade I+ |
| Pham [69] | 3 × 14 Gy or 1 × 26 Gy |
20 | Not reported | 60% grade I-II No grade III+ |
| Ponsky [67] | 4 × 6 Gy or 4 × 8 Gy or 4 × 10 Gy or 4 × 12 Gy |
19 | OS at 3 years 72% | 10.6% grade II 15.8% grade IV |
| Siva [66] | 3 × 14 Gy or 1 × 26 Gy |
33 | FLP at 2 years 100% FDP at 2 years 89% OS at 2 years 92% |
78% grade I-II 3% grade III No grade IV+ |
| Staehler [68] | 1 × 25 Gy | 40 | LC at 9 months 98% | 13% grade I-II |
| Svedman [71] | 4 × 8 Gy or 4 × 10 Gy or 3 × 15 Gy |
5 | Primary and metastatic RCC: LC 98% (19% of lesions in patients with a follow-up < 6 months) OS 32 months |
Primary and metastatic RCC: 54% grade I-II |
Ponsky and colleagues performed a dose-escalation study comparing 24, 32, 40 and 48 Gy in 4 fractions, and showed that irradiation with 48 Gy in 4 fractions can be applied without dose-limiting toxicity [67]. Partial response and stable disease were observed in 20% and 80%, respectively [67]. Considering these promising results, the authors have conducted an escalation study and investigated SBRT with radiation dose up to 60 Gy in 3 fractions. This escalation study is completed, and results will be shown at the ASTRO's Annual Meeting in 2019 (personal communication).
Single fraction radiotherapy for RCC was prospectively investigated in a study by Staehler et al. [68]. In this cohort, 40 patients with 45 surgically untreatable renal tumors (15 transitional cell carcinoma and 30 RCC) were treated using a CyberKnife system with 25 Gy in a single fraction to the 70% isodose. Local control rate at 9 months after radiotherapy was 98%, and remission was observed in 39 of 45 lesions (86.7%) with a complete remission rate of 42.2%. Interestingly, renal function based on creatinine clearance was found to be unaffected after single fraction radiotherapy. However, median follow-up was quite short with 28.1 months and renal lesions larger than 4 cm were excluded from this study thereby limiting the transfer of these results to the treatment of larger tumors.
More recently, the International Radiosurgery Oncology Consortium for Kidney (IROCK) performed a pooled, multi-institutional analysis of 223 patients from 9 institutions after single-fraction and multi-fraction SBRT for RCC [6]. Single-fraction radiotherapy with a median dose of 25 Gy (median BED 87.5 Gy) was performed in 118 patients, while 105 patients received multi-fraction SBRT with a median dose of 40 Gy delivered in 2–10 fractions (median BED 80 Gy). At 4 years after treatment, local control rate, cancer-specific survival and overall survival were 97.8%, 91.9% and 70.7%, respectively. 35.6% had grade I or II toxicities, and only 1.3% suffered grade III or IV adverse reactions; additionally, a slight but significant decrease of median glomerular filtration rate (GFR) was observed (59.9–54.4 ml/min/1.73 m2). Comparing these results with thermal ablation, radical and partial nephrectomy, the relative GFR decrease after SBRT is similar to them after thermal ablation and partial nephrectomy; however, radical nephrectomy causes a significantly more severe GFR decline [73], [74]. Interestingly, 52 patients (26.5%) exhibited a higher GFR after SBRT compared to pre-treatment levels.
Whether SBRT is safe for patients with pre-existing renal dysfunction or single functioning kidney, was addressed in small case series and one larger multicenter analysis [57], [65], [75]. In one study including 3 patients with a GFR between 17.5 and 34.8 ml/min/1.73 m2 and stage I RCC were treated with 40 Gy delivered in 5 fractions using the CyberKnife system [65]. While local control was achieved in all patients, no patient required dialysis during follow-up. However, in 1 patient, GFR dropped from 17.5 ml/min/1.73 m2 to 12.3 ml/min/1.73 m2 at 26 months after SBRT thereby constituting renal failure. In another study comprising 7 patients with single functionating kidney, renal function was found to be unaffected in 5 patients after SBRT with 10 × 3 Gy or 10 × 4 Gy, and none of the treated patients required dialysis. Serum creatinine levels in 1 patient increased by about 30% und remained stable until a follow-up time of 52 months, while in another patient, serum creatine levels increased by about 20% during 6 years of follow-up. Local recurrence was observed in 1 patient after 54 months leading to re-irradiation and local control until to date [57]. Recently, the IROCK performed a multicenter analysis investigating SBRT for RCC in 81 patients with a solitary kidney [75]. Considering the local control, progression-free, cancer specific and overall survival of 98.0%, 77.5%, 98.2% and 81.5% after 2 years, SBRT for RCC in solitary kidney patients led to an excellent oncologic outcome. Mean GFR rate dropped from 64.6 ± 21.7 to 59.2 ± 23.9 ml/min/1.73 m2 after a median of 20.4 months, and no patient required dialysis after SBRT. Interestingly, 26.2% of patients exhibited even an increase in their GFR rate.
In order to further increase the evidence of SBRT for primary RCC, the Trans-Tasman Radiation Oncology Group (TROG) together with the Australian and New Zealand Urogenital and Prostate Cancer Trials Group (ANZUP) will perform a prospective, multi-institutional phase II study aiming to recruit 70 patients with biopsy-confirmed RCC and medical inoperability or refusal of surgery [76].
One limitation of many studies investigating SBRT for RCC is the fact that smaller RCCs were included. However, in a retrospective analysis comprising 11 patients, SBRT for renal tumors with a median tumor diameter of 9.5 cm was well tolerated with only 5 reported grade I toxicities and 1 grade II (diarrhea) and III (nausea) toxicity in 1 patient [77]. Based on these encouraging toxicity results, a prospective study is underway to further investigate SBRT for large RCC (NCT02264548).
In the vast majority of studies about radiotherapy for localized RCC, photon irradiation was used; however, few studies reported about the results using protons or heavy ions such as carbon ions for primary RCC [64], [78], [79]. The dosimetric advantages of protons and carbon ions compared to photon irradiation has been demonstrated in many studies [80], [81]. However, whether these advantages lead to improved clinical results in localized RCC needs to be studied in clinical trials.
In the largest cohort of RCC patients treated with carbon ion radiotherapy (n = 19), local control rate, disease-free-survival and overall survival at 5 years were 94.1%, 68.9% and 89.2% [78]. Patients were irradiated with several radiation doses and fractionation protocols including 16 × 4 GyE, 16 × 4.5 GyE, 16 × 5 GyE and 12 × 5.5 Gy. There was 1 grade IV dermatitis, but no other grade III+ non-renal toxicities. In the group of patients without preexisting renal comorbidities (n = 14), no one developed chronic kidney disease, whereas 4 of 5 patients with renal comorbidities prior to radiotherapy progressed to grade IV chronic kidney disease (GFR < 15 ml/min/1.73 m2 or dialysis) in a mean time of 5.6 years. The average reduction in GFR after carbon ion radiotherapy was 6.1 ml/min/1.73 m2 which is comparable to the results after SBRT. In summary, carbon ion radiotherapy for localized RCC seems to be a promising treatment modality regarding the favorable 5-year local control rate and overall survival accompanied by relatively low toxicity. In order to investigate the safety and efficacy of carbon ion radiotherapy in the treatment of RCC prospectively, Kasuya and colleagues performed a phase I/II study with 8 patients [82]. 5 patients were treated with 66 GyE delivered in 12 fractions and 3 patients with 72 Gy in 12 fractions. Excellent local control and cancer-specific survival rates (100% with a median follow-up time of 43.1 months) were reported, although 5 patients had tumors larger than 4 cm. No grade III+ toxicities were observed, and the GFR decreased by 10.8 ml/min/1.73 m2 during the follow-up.
So far, there is only one case report about proton radiotherapy for primary RCC [79]. In a 47-year-old female patient with multiple comorbidities and obesity (450 lb = 204 kg with a BMI > 90), bilateral synchronous RCCs were found incidentally. She was medically inoperable due to her multiple comorbidities including stage III chronic kidney disease. After radiotherapy with 30 Gy delivered in 5 fractions for both RCCs, the patient developed no significant clinical toxicity, although GFR slightly decreased by 5 ml/min/1.73 m2 over 1 year.
Taken together, there are encouraging results for particle radiotherapy, especially for carbon ion radiotherapy, in the treatment of RCC. However, further prospective trials with a longer follow-up and sufficient patient number are warranted to evaluate the role of particle irradiation for RCC.
7. Comparison with other non-surgical treatment approaches
Radiofrequency ablation and cryoablation are alternative local treatment options for small RCC with medical inoperability or refusal of surgery [83]. Other local ablative techniques such as microwave ablation, high‐intensity focused ultrasound and laser interstitial thermotherapy are experimentative therapies and only investigated in small case series [84], [85].
In contrast to these thermal ablation techniques in which tumor shrinkage can be observed shortly after treatment, long-term reduction in tumor size even years after SBRT has been reported [86]. As ionizing radiation may cause delayed cell death through certain mechanisms such as mitotic catastrophe, viable tumor cells may be found shortly after SBRT so that routine post-SBRT biopsy is not recommended [87]. This recommendation is different to recommendations for radiofrequency ablation where post-intervention biopsy is suggested as residual RCC cells can be found without radiographic evidence for treatment failure [88]. Contrary to thermal ablation therapies, contrast enhancement on CT can be found over a longer period after SBRT and thus is not a surrogate parameter for treatment failure [87], [89]. Dependence on technician experience in terms of a learning curve is less important for SBRT compared to thermal ablation treatments [90]. Radiofrequency ablation and microwave ablation are generally restricted to small RCC up to 4 cm diameter, whereas cryoablation may be an alternative for larger RCC [91]. However, both complication rate and tumor recurrence probability after cryoablation were found to be increased when tumor size exceeded 3.5 cm or 3 cm, respectively [92], [93].
As randomized controlled trials between thermal ablation techniques and SBRT for RCC are lacking, only indirect comparisons of retrospective and prospective series for both treatment modalities can give further evidence. Recent studies trying to compare different modalities for NSCLC or prostate cancer failed due to slow accrual and illustrate the difficulty of such studies [45], [94]. An attempt to compare SBRT and radiofrequency ablation for RCC was a trial sponsored by the University of Michigan Cancer Center (NCT02138578) which was prematurely closed for poor accrual and inability to follow patients. So far, neither the ESMO nor the NCCN or German S3 guideline recommend SBRT for primary RCC as an alternative to surgery or thermal ablation techniques [7], [15], [95]. In the German S3 guideline, SBRT is described as experimental treatment modality due to the significant lower number of treated patients compared to thermal ablation techniques [95], [96]. However, it is notable that the estimated 2-year local control rate of 94% (based on the systematic analysis by Siva et al. in 2012 [72]) is comparable to the results of radiofrequency ablation or cryoablation [95].
8. Technical aspects of SBRT for primary RCC
Intra-fraction respiratory-induced motion of the kidneys is a major challenge of renal radiotherapy. Besides intra-fraction motion, movement between different fractions (inter-fraction motion) especially of organs at risk such as small bowel and duodenum is another concern. Additionally, the anatomic adjacency to the small bowel, duodenum and liver is another critical point that needs to be considered. Several technical options to deal with these challenges are discussed in the following.
8.1. ITV-concept in four-dimensional computed tomography (4D-CT)
In free-breathing patients, kidneys move between 4.5 and 13.9 mm during breathing [97]. A 4D-CT can be used to define the internal target volume (ITV) which incorporates all tumor locations during a breathing cycle (Fig. 1). An additional margin due to random and systematic uncertainties including setup uncertainty, inaccuracy of the image guidance and changes in position should be added to the ITV to generate the planning target volume (PTV). Pham and colleagues used an ITV-PTV-margin of 5 mm in their prospective trial [69]. Similarly, in the prospective multicenter phase II clinical trial TROG 15.03 FASTRACK II, an ITV-PTV-margin of 5 mm must be used [76].
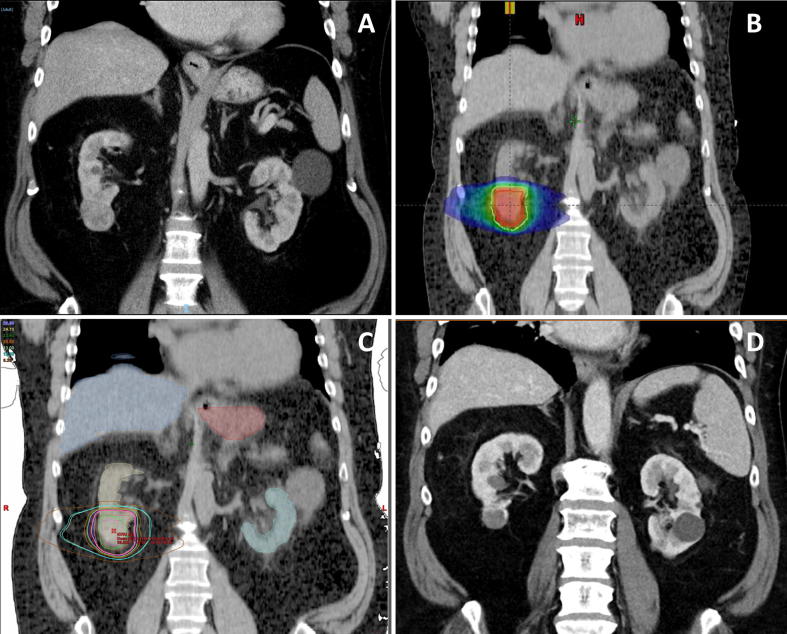
Fig. 1.
Single-fraction SBRT for a biopsy-confirmed RCC in a 64-year-old patient. A papillary T1b (4.2 × 3.8 × 3.5 cm) RCC was treated with single-fraction SBRT delivering 26 Gy at the 80% isodose in February 2013. The 64-year-old patient had a good performance status (ECOG 0) but suffered from some cardiac comorbidities such as cardiomyopathy and atrial fibrillation requiring warfarin. Furthermore, he exhibited a moderate chronic kidney disease with a GFR of 47 ml/min/1.73 m2 prior to SBRT. (A) CT image in January 2013 showing an RCC in the right kidney. (B) Treatment plan demonstrating the dose distribution. By using a 4D-CT, an ITV concept was used with an ITV-PTV-expansion of 5 mm. (C) Coronal CT image with some organs at risk (liver, contralateral kidney, stomach) and different isodoses for the SBRT. A cone beam CT was performed before, during and after SBRT. Immobilization was ensured using Elekta BodyFix® with a vacuum drape. (D) CT image at the 6-year follow-up (February 2019) showing tumor size reduction (2.8 × 2.3 cm) and central necrosis with no signs of recurrence. Blood tests revealed mild deterioration in kidney function (GFR of 39 ml/min/1.73 m2).
In one study, changing in breathing amplitude compared to the simulation 4D-CT led to 46% loss in PTV coverage [98]. In order to reduce tumor excursion during breathing, abdominal compression, shallow breathing coaching or dual vacuum stabilization devices are used. Usage of a dual vacuum stabilization device decreased kidney motion in 6 of 9 healthy volunteers with a median reduction ranging between 1.6 and 8 mm. Interestingly, in 1 participant, vacuum stabilization led to an increase in kidney motion of 8.2 mm [99]. In another study, average kidney motion was slightly reduced after abdominal compression [100].
8.2. Robotic SBRT
The CyberKnife system was invented by the neurosurgeon John Adler in the late 1980s and was initially used for the treatment of intracranial tumors, before extracranial [101]. It contains a 6 megavolt (MV) linear accelerator mounted on a robotic arm with 6 degrees of freedom of movement.
Usage of the CyberKnife system for localized RCC requires the insertion of tiny gold seeds (fiducial markers) which are placed via image guidance. As RCC is a highly vascularized tumor, the risk for hemorrhage during fiducial insertion may be increased, although there were no such reports in the largest study by Staehler et al. [68]. In this study, a CyberKnife system was used to treat patients with transitional cell cancer (11 patients) and RCC (29 patients) using 25 Gy in a single fraction which resulted in a local control rate of 98% after 9 months. Currently, a phase II study evaluates the efficacy of CyberKnife-based radiotherapy for localized primary RCC in terms of freedom from local tumor progression (NCT01890590). The study aims to enroll 46 patients with stage I (T1N0M0) who will be irradiated with 3–4 fractions.
8.3. MR-guided radiotherapy
In future, MR-guided radiotherapy may be used for adaptive radiotherapy of RCC. MR-guided radiotherapy enables direct visualization of the tumor and surrounding organs at risk. Due to the superior soft tissue contrast, improved anatomic visualization is achievable. Daily adaptive re-planning can be used to reduce toxicity, especially if small bowel is more adjacent to the treated RCC compared to the initial planning scan. Alternatively, online MR monitoring may give the opportunity to modify daily radiotherapy dose depending on the proximity to organs at risk. Compared to other tumor entities, usage of MR-guided radiotherapy as indicator for early tumor response is less important, as RCC size normally is unaffected during SBRT. So far, there are no clinical data regarding MR-guided SBRT for localized RCC. However, the theoretical advantages of MR-guided SBRT for RCC and the successful introduction of this new technology for other tumor entities such as prostate cancer are promising preconditions for clinical trials investigating MR-guided SBRT for RCC [102].
9. Summary
Historically, RCC is considered to be a radioresistant tumor entity based on preclinical studies and negative clinical trials using normofractionated radiotherapy. Considering the successful utilization of SBRT for the treatment of RCC metastases, SBRT has been investigated in retrospective and prospective trials for primary RCC. High local control rates accompanied by low toxicity rates were reported in these studies, so that SBRT for primary RCC may be an attractive local treatment option; however, additional prospective trials are warranted to further evaluate the role of SBRT for localized RCC.
10. Declarations of interests
The authors declare that they have no competing interests.
References
- 1.Capitanio U., Bensalah K., Bex A., Boorjian S.A., Bray F., Coleman J. Epidemiology of renal cell carcinoma. Eur Urol. 2019;75:74–84. doi: 10.1016/j.eururo.2018.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsieh J.J., Purdue M.P., Signoretti S., Swanton C., Albiges L., Schmidinger M. Renal cell carcinoma. Nat Rev Dis Primers. 2017;3:17009. doi: 10.1038/nrdp.2017.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nabi S., Kessler E.R., Bernard B., Flaig T.W., Lam E.T. Renal cell carcinoma: a review of biology and pathophysiology. F1000Res. 2018;7:307. doi: 10.12688/f1000research.13179.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woldrich J.M., Mallin K., Ritchey J., Carroll P.R., Kane C.J. Sex differences in renal cell cancer presentation and survival: an analysis of the National Cancer Database, 1993-2004. J Urol. 2008;179:1709–1713. doi: 10.1016/j.juro.2008.01.024. discussion 13. [DOI] [PubMed] [Google Scholar]
- 5.Thompson R.H., Ordonez M.A., Iasonos A., Secin F.P., Guillonneau B., Russo P. Renal cell carcinoma in young and old patients--is there a difference? J Urol. 2008;180:1262–1266. doi: 10.1016/j.juro.2008.06.037. discussion 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siva S., Louie A.V., Warner A., Muacevic A., Gandhidasan S., Ponsky L. Pooled analysis of stereotactic ablative radiotherapy for primary renal cell carcinoma: a report from the International Radiosurgery Oncology Consortium for Kidney (IROCK) Cancer. 2018;124:934–942. doi: 10.1002/cncr.31156. [DOI] [PubMed] [Google Scholar]
- 7.Escudier B., Porta C., Schmidinger M., Rioux-Leclercq N., Bex A., Khoo V. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019 doi: 10.1093/annonc/mdw328. [DOI] [PubMed] [Google Scholar]
- 8.Kothari G., Louie A.V., Pryor D., Vela I., Lo S.S., Teh B.S. Stereotactic body radiotherapy for primary renal cell carcinoma and adrenal metastases. Chin Clin Oncol. 2017 doi: 10.21037/cco.2017.06.30. [DOI] [PubMed] [Google Scholar]
- 9.MacLennan S., Imamura M., Lapitan M.C., Omar M.I., Lam T.B., Hilvano-Cabungcal A.M. Systematic review of oncological outcomes following surgical management of localised renal cancer. Eur Urol. 2012;61:972–993. doi: 10.1016/j.eururo.2012.02.039. [DOI] [PubMed] [Google Scholar]
- 10.Kim S.P., Thompson R.H., Boorjian S.A., Weight C.J., Han L.C., Murad M.H. Comparative effectiveness for survival and renal function of partial and radical nephrectomy for localized renal tumors: a systematic review and meta-analysis. J Urol. 2012;188:51–57. doi: 10.1016/j.juro.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Kutikov A., Uzzo R.G. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182:844–853. doi: 10.1016/j.juro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 12.Hew M., Baseskioglu B., Barwari K., Axwijk P., Can C., Horenblas S. Critical appraisal of the PADUA classification and assessment of the RENAL nephrometry score in patients undergoing partial nephrectomy. J Urol. 2011;186:42–46. doi: 10.1016/j.juro.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 13.Ficarra V., Novara G., Secco S., Macchi V., Porzionato A., De Caro R. Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumours in patients who are candidates for nephron-sparing surgery. Eur Urol. 2009;56:786–793. doi: 10.1016/j.eururo.2009.07.040. [DOI] [PubMed] [Google Scholar]
- 14.Simmons M.N., Ching C.B., Samplaski M.K., Park C.H., Gill I.S. Kidney tumor location measurement using the C index method. J Urol. 2010;183:1708–1713. doi: 10.1016/j.juro.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Motzer R.J., Jonasch E., Agarwal N., Bhayani S., Bro W.P., Chang S.S. version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:804–834. doi: 10.6004/jnccn.2017.0100. [DOI] [PubMed] [Google Scholar]
- 16.Pan X.W., Cui X.M., Huang H., Huang Y., Li L., Wang Z.J. Radiofrequency ablation versus partial nephrectomy for treatment of renal masses: a systematic review and meta-analysis. Kaohsiung J Med Sci. 2015;31:649–658. doi: 10.1016/j.kjms.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunkle D.A., Uzzo R.G. Cryoablation or radiofrequency ablation of the small renal mass: a meta-analysis. Cancer. 2008;113:2671–2680. doi: 10.1002/cncr.23896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volpe A., Kachura J.R., Geddie W.R., Evans A.J., Gharajeh A., Saravanan A. Techniques, safety and accuracy of sampling of renal tumors by fine needle aspiration and core biopsy. J Urol. 2007;178:379–386. doi: 10.1016/j.juro.2007.03.131. [DOI] [PubMed] [Google Scholar]
- 19.Passalacqua R., Caminiti C., Buti S., Porta C., Camisa R., Braglia L. Adjuvant low-dose interleukin-2 (IL-2) plus interferon-alpha (IFN-alpha) in operable renal cell carcinoma (RCC): a phase III, randomized, multicentre trial of the Italian Oncology Group for Clinical Research (GOIRC) J Immunother. 2014;37:440–447. doi: 10.1097/CJI.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 20.Pizzocaro G., Piva L., Colavita M., Ferri S., Artusi R., Boracchi P. Interferon adjuvant to radical nephrectomy in Robson stages II and III renal cell carcinoma: a multicentric randomized study. J Clin Oncol. 2001;19:425–431. doi: 10.1200/JCO.2001.19.2.425. [DOI] [PubMed] [Google Scholar]
- 21.Haas N.B., Manola J., Uzzo R.G., Flaherty K.T., Wood C.G., Kane C. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet. 2016;387:2008–2016. doi: 10.1016/S0140-6736(16)00559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Motzer R.J., Haas N.B., Donskov F., Gross-Goupil M., Varlamov S., Kopyltsov E. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma. J Clin Oncol. 2017;35:3916–3923. doi: 10.1200/JCO.2017.73.5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravaud A., Motzer R.J., Pandha H.S., George D.J., Pantuck A.J., Patel A. Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N Engl J Med. 2016;375:2246–2254. doi: 10.1056/NEJMoa1611406. [DOI] [PubMed] [Google Scholar]
- 24.Deschavanne P.J., Fertil B. A review of human cell radiosensitivity in vitro. Int J Radiat Oncol Biol Phys. 1996;34:251–266. doi: 10.1016/0360-3016(95)02029-2. [DOI] [PubMed] [Google Scholar]
- 25.Ning S., Trisler K., Wessels B.W., Knox S.J. Radiobiologic studies of radioimmunotherapy and external beam radiotherapy in Vitro and in Vivo in human renal cell carcinoma xenografts. Cancer. 1997;80:2519–2528. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2519::aid-cncr26>3.3.co;2-t. [DOI] [PubMed] [Google Scholar]
- 26.De Meerleer G., Khoo V., Escudier B., Joniau S., Bossi A., Ost P. Radiotherapy for renal-cell carcinoma. Lancet Oncol. 2014;15:e170–e177. doi: 10.1016/S1470-2045(13)70569-2. [DOI] [PubMed] [Google Scholar]
- 27.Kolesnick R., Fuks Z. Radiation and ceramide-induced apoptosis. Oncogene. 2003;22:5897–5906. doi: 10.1038/sj.onc.1206702. [DOI] [PubMed] [Google Scholar]
- 28.Marathe S., Schissel S.L., Yellin M.J., Beatini N., Mintzer R., Williams K.J. Human vascular endothelial cells are a rich and regulatable source of secretory sphingomyelinase. Implications for early atherogenesis and ceramide-mediated cell signaling. J Biol Chem. 1998;273:4081–4088. doi: 10.1074/jbc.273.7.4081. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Barros M., Paris F., Cordon-Cardo C., Lyden D., Rafii S., Haimovitz-Friedman A. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 30.Sathishkumar S., Boyanovsky B., Karakashian A.A., Rozenova K., Giltiay N.V., Kudrimoti M. Elevated sphingomyelinase activity and ceramide concentration in serum of patients undergoing high dose spatially fractionated radiation treatment: implications for endothelial apoptosis. Cancer Biol Ther. 2005;4:979–986. doi: 10.4161/cbt.4.9.1915. [DOI] [PubMed] [Google Scholar]
- 31.Van de Walle M., Demol J., Staelens L., Rottey S. Abscopal effect in metastatic renal cell carcinoma. Acta Clin Belg. 2017;72:245–249. doi: 10.1080/17843286.2016.1201614. [DOI] [PubMed] [Google Scholar]
- 32.Wersall P.J., Blomgren H., Pisa P., Lax I., Kalkner K.M., Svedman C. Regression of non-irradiated metastases after extracranial stereotactic radiotherapy in metastatic renal cell carcinoma. Acta Oncol. 2006;45:493–497. doi: 10.1080/02841860600604611. [DOI] [PubMed] [Google Scholar]
- 33.Fairlamb D.J. Spontaneous regression of metastases of renal cancer: a report of two cases including the first recorded regression following irradiation of a dominant metastasis and review of the world literature. Cancer. 1981;47:2102–2106. doi: 10.1002/1097-0142(19810415)47:8<2102::aid-cncr2820470833>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 34.van der Werf-Messing B. Proceedings: carcinoma of the kidney. Cancer. 1973;32:1056–1061. doi: 10.1002/1097-0142(197311)32:5<1056::aid-cncr2820320505>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 35.Juusela H., Malmio K., Alfthan O., Oravisto K.J. Preoperative irradiation in the treatment of renal adenocarcinoma. Scand J Urol Nephrol. 1977;11:277–281. doi: 10.3109/00365597709179965. [DOI] [PubMed] [Google Scholar]
- 36.Finney R. The value of radiotherapy in the treatment of hypernephroma–a clinical trial. Br J Urol. 1973;45:258–269. doi: 10.1111/j.1464-410x.1973.tb12152.x. [DOI] [PubMed] [Google Scholar]
- 37.Kjaer M., Iversen P., Hvidt V., Bruun E., Skaarup P., Bech Hansen J. A randomized trial of postoperative radiotherapy versus observation in stage II and III renal adenocarcinoma. A study by the Copenhagen Renal Cancer Study Group. Scand J Urol Nephrol. 1987;21:285–289. doi: 10.3109/00365598709180784. [DOI] [PubMed] [Google Scholar]
- 38.Tunio M.A., Hashmi A., Rafi M. Need for a new trial to evaluate postoperative radiotherapy in renal cell carcinoma: a meta-analysis of randomized controlled trials. Ann Oncol. 2010;21:1839–1845. doi: 10.1093/annonc/mdq028. [DOI] [PubMed] [Google Scholar]
- 39.Meacci E., Nachira D., Congedo M.T., Porziella V., Chiappetta M., Ferretti G. Lung metastasectomy following kidney tumors: outcomes and prognostic factors from a single-center experience. J Thorac Dis. 2017;9:S1267–S1272. doi: 10.21037/jtd.2017.05.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heinzer H., Huland E., Huland H. Treatment of metastatic renal cell carcinoma. Value of immunotherapy compared with surgery of metastases. Der Urologe Ausg A. 2000;39:356–361. doi: 10.1007/s001200050370. [DOI] [PubMed] [Google Scholar]
- 41.Hofmann H.S., Neef H., Krohe K., Andreev P., Silber R.E. Prognostic factors and survival after pulmonary resection of metastatic renal cell carcinoma. Eur Urol. 2005;48:77–81. doi: 10.1016/j.eururo.2005.03.004. discussion-2. [DOI] [PubMed] [Google Scholar]
- 42.Pfannschmidt J., Hoffmann H., Muley T., Krysa S., Trainer C., Dienemann H. Prognostic factors for survival after pulmonary resection of metastatic renal cell carcinoma. Ann Thoracic Surg. 2002;74:1653–1657. doi: 10.1016/s0003-4975(02)03803-1. [DOI] [PubMed] [Google Scholar]
- 43.Timmerman R., Paulus R., Galvin J., Michalski J., Straube W., Bradley J. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roesch J., Andratschke N., Guckenberger M. SBRT in operable early stage lung cancer patients. Transl Lung Cancer Res. 2014;3:212–224. doi: 10.3978/j.issn.2218-6751.2014.08.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang J.Y., Senan S., Paul M.A., Mehran R.J., Louie A.V., Balter P. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16:630–637. doi: 10.1016/S1470-2045(15)70168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoerner-Rieber J., Duma M., Blanck O., Hildebrandt G., Wittig A., Lohaus F. Stereotactic body radiotherapy (SBRT) for pulmonary metastases from renal cell carcinoma-a multicenter analysis of the German working group “Stereotactic Radiotherapy”. J Thorac Dis. 2017;9:4512–4522. doi: 10.21037/jtd.2017.10.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Altoos B., Amini A., Yacoub M., Bourlon M.T., Kessler E.E., Flaig T.W. Local control rates of metastatic renal cell carcinoma (rcc) to thoracic, abdominal, and soft tissue lesions using stereotactic body radiotherapy (SBRT) Radiat Oncol. 2015;10:218. doi: 10.1186/s13014-015-0528-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Briganti A., Montorsi F., Bianchi M., Sun M., Tian Z., Jeldres C. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol. 2011;23:973–980. doi: 10.1093/annonc/mdr362. [DOI] [PubMed] [Google Scholar]
- 49.Sperduto P.W., Deegan B.J., Li J., Jethwa K.R., Brown P.D., Lockney N.A. Prognostic factors in patients with renal cell carcinoma and brain metastases. Int J Radiat Oncol Biol Phys. 2017;99:S169–S170. doi: 10.1016/j.ijrobp.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi S.Y., Yoo S., You D., Jeong I.G., Song C., Hong B. Prognostic factors for survival of patients with synchronous or metachronous brain metastasis of renal cell carcinoma. Clin Genitourinary Cancer. 2017;15:717–723. doi: 10.1016/j.clgc.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 51.Chevreau C., Ravaud A., Escudier B., Amela E., Delva R., Rolland F. A phase II trial of sunitinib in patients with renal cell cancer and untreated brain metastases. Clin Genitourin Cancer. 2014;12:50–54. doi: 10.1016/j.clgc.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 52.Du Y., Pahernik S., Hadaschik B., Teber D., Duensing S., Jager D. Impact of resection and systemic therapy on the survival of patients with brain metastasis of metastatic renal cell carcinoma. J Neurooncol. 2016;130:221–228. doi: 10.1007/s11060-016-2238-2. [DOI] [PubMed] [Google Scholar]
- 53.Sheehan J.P., Sun M.H., Kondziolka D., Flickinger J., Lunsford L.D. Radiosurgery in patients with renal cell carcinoma metastasis to the brain: long-term outcomes and prognostic factors influencing survival and local tumor control. J Neurosurg. 2003;98:342–349. doi: 10.3171/jns.2003.98.2.0342. [DOI] [PubMed] [Google Scholar]
- 54.Hernandez L., Zamorano L., Sloan A., Fontanesi J., Lo S., Levin K. Gamma knife radiosurgery for renal cell carcinoma brain metastases. J Neurosurg. 2002;97:489–493. doi: 10.3171/jns.2002.97.supplement. [DOI] [PubMed] [Google Scholar]
- 55.Wronski M., Maor M.H., Davis B.J., Sawaya R., Levin V.A. External radiation of brain metastases from renal carcinoma: a retrospective study of 119 patients from the M. D. Anderson Cancer Center. Int J Radiat Oncol Biol Phys. 1997:37753–37759. doi: 10.1016/s0360-3016(97)00006-0. [DOI] [PubMed] [Google Scholar]
- 56.Ahmed K.A., Berglund A.E., Welsh E.A., Naghavi A.O., Kim Y., Yu M. The radiosensitivity of brain metastases based upon primary histology utilizing a multigene index of tumor radiosensitivity. Neuro Oncol. 2017;19:1145–1146. doi: 10.1093/neuonc/nox043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Svedman C., Karlsson K., Rutkowska E., Sandstrom P., Blomgren H., Lax I. Stereotactic body radiotherapy of primary and metastatic renal lesions for patients with only one functioning kidney. Acta Oncol. 2008;47:1578–1583. doi: 10.1080/02841860802123196. [DOI] [PubMed] [Google Scholar]
- 58.Beitler J.J., Makara D., Silverman P., Lederman G. Definitive, high-dose-per-fraction, conformal, stereotactic external radiation for renal cell carcinoma. Am J Clin Oncol. 2004;27:646–648. doi: 10.1097/01.coc.0000145289.57705.07. [DOI] [PubMed] [Google Scholar]
- 59.Wersall P.J., Blomgren H., Lax I., Kalkner K.M., Linder C., Lundell G. Extracranial stereotactic radiotherapy for primary and metastatic renal cell carcinoma. Radiother Oncol. 2005;77:88–95. doi: 10.1016/j.radonc.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 60.Chang J.H., Cheung P., Erler D., Sonier M., Korol R., Chu W. Stereotactic ablative body radiotherapy for primary renal cell carcinoma in non-surgical candidates: initial clinical experience. Clin Oncol (R Coll Radiol) 2016;28:e109–e114. doi: 10.1016/j.clon.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 61.Nair V.J., Szanto J., Vandervoort E., Cagiannos I., Breau R., Malone C. CyberKnife for inoperable renal tumors: Canadian pioneering experience. Can J Urol. 2013;20:6944–6949. [PubMed] [Google Scholar]
- 62.Qian G., Lowry J., Silverman P., Grosman I., Makara D., Lederman G. Stereotactic extra-cranial radiosurgery for renal cell carcinoma. Int J Radiat Oncol Biol Phys. 2003;57:S283. [Google Scholar]
- 63.Gilson B., Lederman G., Qian G., Fastaia M., Cangiane L. 2249: hypo-fractionated stereotactic extra-cranial radiosurgery (HFSR) for primary and metastatic renal cell carcinoma. Int J Radiat Oncol Biol Phys. 2006;66:S349. [Google Scholar]
- 64.Nomiya T., Tsuji H., Hirasawa N., Kato H., Kamada T., Mizoe J. Carbon ion radiation therapy for primary renal cell carcinoma: initial clinical experience. Int J Radiat Oncol Biol Phys. 2008;72:828–833. doi: 10.1016/j.ijrobp.2008.01.043. [DOI] [PubMed] [Google Scholar]
- 65.Lo C.H., Huang W.Y., Chao H.L., Lin K.T., Jen Y.M. Novel application of stereotactic ablative radiotherapy using CyberKnife((R)) for early-stage renal cell carcinoma in patients with pre-existing chronic kidney disease: Initial clinical experiences. Oncol Lett. 2014;8:355–360. doi: 10.3892/ol.2014.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Siva S., Pham D., Kron T., Bressel M., Lam J., Tan T.H. Stereotactic ablative body radiotherapy for inoperable primary kidney cancer: a prospective clinical trial. BJU Int. 2017;120:623–630. doi: 10.1111/bju.13811. [DOI] [PubMed] [Google Scholar]
- 67.Ponsky L., Lo S.S., Zhang Y., Schluchter M., Liu Y., Patel R. Phase I dose-escalation study of stereotactic body radiotherapy (SBRT) for poor surgical candidates with localized renal cell carcinoma. Radiother Oncol. 2015;117:183–187. doi: 10.1016/j.radonc.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 68.Staehler M., Bader M., Schlenker B., Casuscelli J., Karl A., Roosen A. Single fraction radiosurgery for the treatment of renal tumors. J Urol. 2015;193:771–775. doi: 10.1016/j.juro.2014.08.044. [DOI] [PubMed] [Google Scholar]
- 69.Pham D., Thompson A., Kron T., Foroudi F., Kolsky M.S., Devereux T. Stereotactic ablative body radiation therapy for primary kidney cancer: a 3-dimensional conformal technique associated with low rates of early toxicity. Int J Radiat Oncol Biol Phys. 2014;90:1061–1068. doi: 10.1016/j.ijrobp.2014.07.043. [DOI] [PubMed] [Google Scholar]
- 70.Kaplan I.D., Redrosa I., Martin C., Collins C., Wagner A. Results of a phase I dose escalation study of stereotactic radiosurgery for primary renal tumors. Int J Radiat Oncol Biol Phys. 2010;78:S191. [Google Scholar]
- 71.Svedman C., Sandstrom P., Pisa P., Blomgren H., Lax I., Kalkner K.M. A prospective Phase II trial of using extracranial stereotactic radiotherapy in primary and metastatic renal cell carcinoma. Acta Oncol. 2006;45:870–875. doi: 10.1080/02841860600954875. [DOI] [PubMed] [Google Scholar]
- 72.Siva S., Pham D., Gill S., Corcoran N.M., Foroudi F. A systematic review of stereotactic radiotherapy ablation for primary renal cell carcinoma. BJU Int. 2012;110:E737–E743. doi: 10.1111/j.1464-410X.2012.11550.x. [DOI] [PubMed] [Google Scholar]
- 73.Clark A.T., Breau R.H., Morash C., Fergusson D., Doucette S., Cagiannos I. Preservation of renal function following partial or radical nephrectomy using 24-hour creatinine clearance. Eur Urol. 2008;54:143–149. doi: 10.1016/j.eururo.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 74.Patel H.D., Pierorazio P.M., Johnson M.H., Sharma R., Iyoha E., Allaf M.E. Renal functional outcomes after surgery, ablation, and active surveillance of localized renal tumors: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2017;12:1057–1069. doi: 10.2215/CJN.11941116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Correa R.J.M., Louie A.V., Staehler M., Warner A., Gandhidasan S., Ponsky L. Stereotactic radiotherapy as a treatment option for renal tumors in the solitary kidney: a multicenter analysis from the IROCK. J Urol. 2019 doi: 10.1097/JU.0000000000000111. 101097JU0000000000000111. [DOI] [PubMed] [Google Scholar]
- 76.Siva S., Chesson B., Bressel M., Pryor D., Higgs B., Reynolds H.M. 15.03 phase II clinical trial of focal ablative stereotactic radiosurgery for cancers of the kidney – FASTRACK II. BMC Cancer. 2018;18:1030. doi: 10.1186/s12885-018-4916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Correa R.J.M., Rodrigues G.B., Chen H., Warner A., Ahmad B., Louie A.V. Stereotactic ablative radiotherapy (SABR) for large renal tumors: a retrospective case series evaluating clinical outcomes, toxicity, and technical considerations. Am J Clin Oncol. 2018;41:568–575. doi: 10.1097/COC.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 78.Kasuya G., Tsuji H., Nomiya T., Makishima H., Haruyama Y., Kobashi G. Updated long-term outcomes after carbon-ion radiotherapy for primary renal cell carcinoma. Cancer Sci. 2018;109:2873–2880. doi: 10.1111/cas.13727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Frick M.A., Chhabra A.M., Lin L., Simone C.B., 2nd First ever use of proton stereotactic body radiation therapy delivered with curative intent to bilateral synchronous primary renal cell carcinomas. Cureus. 2017;9 doi: 10.7759/cureus.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Adeberg S., Harrabi S., Bougatf N., Verma V., Windisch P., Bernhardt D. Dosimetric comparison of proton radiation therapy, volumetric modulated arc therapy, and three-dimensional conformal radiotherapy based on intracranial tumor location. Cancers. 2018;10:401. doi: 10.3390/cancers10110401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abe T., J-i Saitoh, Kobayashi D., Shibuya K., Koyama Y., Shimada H. Dosimetric comparison of carbon ion radiotherapy and stereotactic body radiotherapy with photon beams for the treatment of hepatocellular carcinoma. Radiat Oncol. 2015;10:187. doi: 10.1186/s13014-015-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kasuya G., Tsuji H., Nomiya T., Makishima H., Haruyama Y., Kobashi G. Prospective clinical trial of 12-fraction carbon-ion radiotherapy for primary renal cell carcinoma. Oncotarget. 2019;10:76–81. doi: 10.18632/oncotarget.26539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wagstaff P., Ingels A., Zondervan P., de la Rosette J.J., Laguna M.P. Thermal ablation in renal cell carcinoma management: a comprehensive review. Curr Opin Urol. 2014;24:474–482. doi: 10.1097/MOU.0000000000000084. [DOI] [PubMed] [Google Scholar]
- 84.Cornelis F.H., Marcelin C., Bernhard J.C. Microwave ablation of renal tumors: a narrative review of technical considerations and clinical results. Diagn Interventional Imaging. 2017;98:287–297. doi: 10.1016/j.diii.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 85.Ritchie R.W., Leslie T., Phillips R., Wu F., Illing R., ter Haar G. Extracorporeal high intensity focused ultrasound for renal tumours: a 3-year follow-up. BJU Int. 2010;106:1004–1009. doi: 10.1111/j.1464-410X.2010.09289.x. [DOI] [PubMed] [Google Scholar]
- 86.Sun M.R., Brook A., Powell M.F., Kaliannan K., Wagner A.A., Kaplan I.D. Effect of stereotactic body radiotherapy on the growth kinetics and enhancement pattern of primary renal tumors. AJR Am J Roentgenol. 2016;206:544–553. doi: 10.2214/AJR.14.14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Siva S., Ellis R.J., Ponsky L., Teh B.S., Mahadevan A., Muacevic A. Consensus statement from the International Radiosurgery Oncology Consortium for Kidney for primary renal cell carcinoma. Future Oncol. 2016;12:637–645. doi: 10.2217/fon.16.2. [DOI] [PubMed] [Google Scholar]
- 88.Weight C.J., Kaouk J.H., Hegarty N.J., Remer E.M., O'Malley C.M., Lane B.R. Correlation of radiographic imaging and histopathology following cryoablation and radio frequency ablation for renal tumors. J Urol. 2008;179:1277–12781. doi: 10.1016/j.juro.2007.11.075. discussion 81-3. [DOI] [PubMed] [Google Scholar]
- 89.Ortiz-Alvarado O., Anderson J.K. The role of radiologic imaging and biopsy in renal tumor ablation. World J Urol. 2010;28:551–557. doi: 10.1007/s00345-010-0549-z. [DOI] [PubMed] [Google Scholar]
- 90.Psutka S.P., Feldman A.S., McDougal W.S., McGovern F.J., Mueller P., Gervais D.A. Long-term oncologic outcomes after radiofrequency ablation for T1 renal cell carcinoma. Eur Urol. 2013;63:486–492. doi: 10.1016/j.eururo.2012.08.062. [DOI] [PubMed] [Google Scholar]
- 91.Buy X., Lang H., Garnon J., Sauleau E., Roy C., Gangi A. Percutaneous renal cryoablation: prospective experience treating 120 consecutive tumors. AJR Am J Roentgenol. 2013;201:1353–1361. doi: 10.2214/AJR.13.11084. [DOI] [PubMed] [Google Scholar]
- 92.Lagerveld B.W., Brenninkmeijer M., van der Zee J.A., van Haarst E.P. Can RENAL and PADUA nephrometry indices predict complications of laparoscopic cryoablation for clinical stage T1 renal tumors? J Endourol. 2014;28:464–471. doi: 10.1089/end.2013.0498. [DOI] [PubMed] [Google Scholar]
- 93.Kim E.H., Tanagho Y.S., Bhayani S.B., Saad N.E., Benway B.M., Figenshau R.S. Percutaneous cryoablation of renal masses: Washington University experience of treating 129 tumours. BJU Int. 2013;111:872–879. doi: 10.1111/j.1464-410X.2012.11432.x. [DOI] [PubMed] [Google Scholar]
- 94.Stockle M., Bussar-Maatz R. Localised prostate cancer: the PREFERE trial. Z Evid Fortbild Qual Gesundhwes. 2012;106:333–335. doi: 10.1016/j.zefq.2012.05.004. discussion 5. [DOI] [PubMed] [Google Scholar]
- 95.Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): Diagnostik, Therapie und Nachsorge des Nierenzellkarzinoms, Langversion 1.2, 2017, AWMF Registernummer: 043/017OL. 2017.
- 96.Muller A.C., van Oorschot B., Micke O., Guckenberger M. German S3 guideline for renal cell carcinoma: presentation and discussion of essential aspects for the radiation oncologist. Strahlenther Onkol. 2018;194:1–8. doi: 10.1007/s00066-017-1185-y. [DOI] [PubMed] [Google Scholar]
- 97.Pham D., Kron T., Foroudi F., Schneider M., Siva S. A review of kidney motion under free, deep and forced-shallow breathing conditions: implications for stereotactic ablative body radiotherapy treatment. Technol Cancer Res Treat. 2014;13:315–323. doi: 10.7785/tcrt.2012.500387. [DOI] [PubMed] [Google Scholar]
- 98.Pham D., Kron T., Foroudi F., Siva S. Effect of different breathing patterns in the same patient on stereotactic ablative body radiotherapy dosimetry for primary renal cell carcinoma: a case study. Med Dosim. 2013;38:304–308. doi: 10.1016/j.meddos.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 99.Pham D., Kron T., Styles C., Whitaker M., Bressel M., Foroudi F. The use of dual vacuum stabilization device to reduce kidney motion for stereotactic radiotherapy planning. Technol Cancer Res Treat. 2015;14:149–157. doi: 10.7785/tcrt.2012.500410. [DOI] [PubMed] [Google Scholar]
- 100.Heinzerling J.H., Anderson J.F., Papiez L., Boike T., Chien S., Zhang G. Four-dimensional computed tomography scan analysis of tumor and organ motion at varying levels of abdominal compression during stereotactic treatment of lung and liver. Int J Radiat Oncol Biol Phys. 2008;70:1571–1578. doi: 10.1016/j.ijrobp.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 101.Adler J.R., Jr., Chang S.D., Murphy M.J., Doty J., Geis P., Hancock S.L. The Cyberknife: a frameless robotic system for radiosurgery. Stereotact Funct Neurosurg. 1997;69:124–128. doi: 10.1159/000099863. [DOI] [PubMed] [Google Scholar]
- 102.Pathmanathan A.U., van As N.J., Kerkmeijer L.G., Christodouleas J., Lawton C.A., Vesprini D. Magnetic resonance imaging-guided adaptive radiation therapy: a “game changer” for prostate treatment? Int J Radiat Oncol Biol Phys. 2018;100:361–373. doi: 10.1016/j.ijrobp.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 103.Leibovich B.C., Blute M.L., Cheville J.C., Lohse C.M., Frank I., Kwon E.D. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer. 2003;97:1663–1671. doi: 10.1002/cncr.11234. [DOI] [PubMed] [Google Scholar]