Abstract
Background
Morphea (morphoea) is an immune‐mediated disease in which excess synthesis and deposition of collagen in the skin and underlying connective tissues results in hardened cutaneous areas. Morphea has different clinical features according to the subtype and stage of evolution of the disease. There is currently no consensus on optimal interventions for morphea.
Objectives
To assess the effects of treatments for people with any form of morphea.
Search methods
We searched the following databases up to July 2018: the Cochrane Skin Specialised Register, CENTRAL, MEDLINE, Embase, LILACS, and five trial registers. We checked the reference lists of included studies for further references to relevant randomised controlled trials.
Selection criteria
Randomised controlled trials of topical, intralesional, or systemic treatments (isolated or combined) in anyone who has been clinically diagnosed by a medical practitioner with any form of morphea. Eligible controls were placebo, no intervention, any other treatment, or different doses or duration of a treatment.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. The primary outcomes were global improvement of disease activity or damage assessed by a medical practitioner or by participants, and adverse effects. Secondary outcomes were improvement of disease activity and improvement of disease damage. We used GRADE to assess the quality of the evidence for each outcome.
Main results
We included 14 trials, with a total of 429 randomised participants, aged between 3 and 76 years. There were juvenile and adult participants; over half were female, and the majority had circumscribed morphea, followed by linear scleroderma. The settings of the studies (where described) included a dermatologic centre, a national laboratory centre, paediatric rheumatology and dermatology centres, and a university hospital or medical centre.
The studies evaluated heterogenous therapies for different types of morphea, covering a wide range of comparisons. We were unable to conduct any meta‐analyses. Seven studies investigated topical medications, two evaluated intralesional medications, and five investigated systemic medications. The study duration ranged from seven weeks to 15 months from baseline.
We present here results for our primary outcomes for our four key comparisons. All of these results are based on low‐quality evidence.
The included studies were at high risk of performance, detection, attrition, and reporting bias.
Global improvement of disease activity or damage after treatment may be higher with oral methotrexate (15 mg/m², maximum 20 mg, once a week, for 12 months or until disease flare) plus oral prednisone (1 mg/kg a day, maximum of 50 mg, in a single morning dose, for three months, and one month with gradually decreased dose until discontinuation) than with placebo plus oral prednisone in children and adolescents with active morphea (linear scleroderma, generalised morphea or mixed morphea: linear and circumscribed) (risk ratio (RR) 2.31, 95% confidence interval (CI) 1.20 to 4.45; number needed to treat for an additional beneficial outcome (NNTB) 3; 1 randomised controlled trial (RCT); 70 participants, all juvenile). This outcome was measured 12 months from the start of treatment or until flare of the disease. Data were not available separately for each morphea type. There may be little or no difference in the number of participants experiencing at least one adverse event with oral methotrexate (26/46) or placebo (11/24) (RR 1.23, 95% CI 0.75 to 2.04; 1 RCT; 70 participants assessed during the 12‐month follow‐up). Adverse events related to methotrexate included alopecia, nausea, headache, fatigue and hepatotoxicity, whilst adverse events related to prednisone (given in both groups) included weight gain (more than 5% of body weight) and striae rubrae.
One three‐armed RCT compared the following treatments: medium‐dose (50 J/cm²) UVA‐1; low‐dose (20 J/cm²) UVA‐1; and narrowband UVB phototherapy. There may be little or no difference between treatments in global improvement of disease activity or damage, as assessed through the modified skin score (where high values represent a worse outcome): medium‐dose UVA‐1 phototherapy versus low‐dose UVA‐1 group: MD 1.60, 95% CI −1.70 to 4.90 (44 participants); narrowband UVB phototherapy versus medium‐dose UVA‐1 group: MD −1.70, 95% CI −5.27 to 1.87 (35 participants); and narrowband UVB versus low‐dose UVA‐1 group: MD −0.10, 95% CI −2.49 to 2.29 (45 participants). This RCT included children and adults with active morphea (circumscribed morphea, linear scleroderma (with trunk/limb variant and head variant), generalised morphea, or mixed morphea), who received phototherapy five times a week, for eight weeks. Outcomes were measured at eight weeks from the start of treatment.
Safety data, measured throughout treatment, from the same RCT (62 participants) showed that treatment with UVA‐1 phototherapy may cause mild tanning compared to narrowband UVB: narrowband UVB versus medium‐dose UVA‐1: RR 0.03, 95% CI 0.00 to 0.42; 35 participants; narrowband UVB versus low‐dose UVA‐1: RR 0.03, 95% CI 0.00 to 0.41; 45 participants. However, there may be no difference in the number of participants reporting mild tanning when comparing medium and low dose UVA‐1 phototherapy (RR 1.00, 95% CI 0.91 to 1.10; 44 participants). Transient erythema was reported in three participants with narrowband UVB and no participants in the low‐ or medium‐dose UVA‐1 groups.
Authors' conclusions
Compared to placebo plus oral prednisone, oral methotrexate plus oral prednisone may improve disease activity or damage in juvenile active morphea (linear scleroderma, generalised morphea or mixed morphea: linear and circumscribed), but there may be a slightly increased chance of experiencing at least one adverse event.
When medium‐dose UVA‐1 (50 J/cm²), low‐dose UVA‐1 (20 J/cm²), and narrowband UVB were compared against each other in treating children and adults with active morphea (circumscribed morphea, linear scleroderma, generalised morphea and mixed morphea), there may be little or no difference between these treatments on global improvement of disease activity or damage. UVA‐1 phototherapy may cause more mild tanning than narrowband UVB, but there may be no difference between medium‐ and low‐dose UVA‐1 phototherapy. These results are based on low‐quality evidence.
Limitations of data and analyses include risk of bias and imprecision (small number of participants or events and wide confidence intervals). We encourage multicentre RCTs to increase sample size and evaluate, with validated tools, different treatment responses according to the subtypes of morphea and age groups.
Plain language summary
Interventions for morphea
Review question
The aim of this Cochrane Review was to assess the effects of treatments, either given in isolation or combination, for people with morphea (morphoea), when compared with an inactive substance (placebo), no intervention, any other treatment, or different doses or duration of a treatment. We collected and analysed all relevant studies published up to July 2018.
Background
Morphea is a rare disease that causes skin hardening. It affects adults and children equally, and is more common in females. There are different subtypes of morphea, with different characteristics: circumscribed morphea is generally less severe than the other subtypes; linear scleroderma can cause significant body differences, possibly affecting growth in children; generalised morphea is a severe type involving multiple areas of the body; pansclerotic morphea is a severe and progressive type of generalised morphea; and mixed morphea is the presence of two or more disease types. Recurrence rates are high, and even when disease activity reduces, a person can be left with permanent effects. This review intended to assess the safety and effectiveness of different treatments for morphea.
Study characteristics
We found 14 relevant studies, with a total of 429 participants, including children and adults aged from three to 76 years. Over half of the participants were female. Most participants had circumscribed morphea, followed by linear scleroderma. Six studies did not describe their setting, but the rest were set in university hospital, medical centre, or national laboratory centre. Seven studies received funding from either universities, government or association scholarships, or the pharmaceutical industry. Six studies had no funding, and one study did not report this information.
Seven studies compared topical medications: phototherapy; an immunosuppressive (suppresses immune system activity); an antiallergic drug; and a corticosteroid (an anti‐inflammatory). Two studies compared medications within the lesion itself: collagen, and an immunomodulator (modifies the immune response). Five studies compared systemic medications (meaning they affect the whole body): an immunosuppressive; traditional Chinese medicine therapies; and a vitamin D analogue (a form of vitamin D). These treatments were compared with either no treatment; placebo; differing doses of phototherapy; hydroxychloroquine (an immune system regulator); emollient petrolatum (moisturising treatment); corticosteroids; an anticoagulant agent (blood thinner) taken with a medicinal plant extract and vitamin E tablet; or antibiotic with base cream. The studies lasted between seven weeks and 15 months.
Key results
The results we present in this summary are based on low‐quality evidence.
Children and teenagers with active morphea (linear scleroderma, generalised morphea and mixed morphea: linear and circumscribed) may experience greater improvement of disease activity or damage with oral methotrexate plus prednisone than with placebo plus prednisone. We would expect that out of 100 children and teenagers, 67 would experience improvement with methotrexate, compared with 29 given placebo; this is based on results measured either 12 months after start of treatment or until flare of the disease. In addition, there may be little or no difference in the number of participants experiencing at least one side effect during treatment (such as hair loss, headache, sickness, tiredness, or liver damage) between those given methotrexate and those given placebo. Side effects from prednisone (given in both groups) included weight gain and stretch marks. We would expect that out of 100 children and teenagers, 56 would experience at least one side effect with methotrexate, compared with 46 given placebo.
Children and adults with active morphea (circumscribed morphea, linear scleroderma, generalised morphea, or mixed morphea) may present similar reduction in disease activity or damage with medium‐dose (50 J/cm²) UVA‐1, low‐dose (20 J/cm²) UVA‐1, or narrowband UVB phototherapy. Those treated with medium‐dose (50 J/cm²) UVA‐1 or low‐dose (20 J/cm²) UVA‐1 phototherapy may have mild tanning after the treatment compared to those treated with narrowband UVB phototherapy. However, there may be no difference in the number of participants reporting mild tanning when comparing medium‐ and low‐dose UVA‐1 phototherapy. Temporary redness was reported in three participants given narrowband UVB and none of the participants in either the low‐ or medium‐dose UVA‐1 groups.
Quality of the evidence
We considered the quality of evidence as low because most studies included few participants and there were concerns over the design of some studies, such as no treatment masking and incomplete analysis.
Summary of findings
Background
Please note that we include a glossary in Table 5 to explain the abbreviated terms we use.
1. Glossary.
| Term | Definition |
| ALA | 5‐aminolaevulinic acid |
| BB | broad‐band |
| CDLQI | Children's Dermatology Life Quality Index |
| C‐HAQ | Childhood Health Assessment Questionnaire |
| CI | confidence interval |
| CO₂ | carbon dioxide |
| DIET | dyspigmentation, induration, erythema, telangiectasia |
| DLQI | Dermatology Life Quality Index |
| DNA | deoxyribonucleic acid |
| HCQ | hydroxychloroquine |
| IFN‐γ | interferon gamma |
| ITT | intention‐to‐treat |
| ISDL | Impact of Chronic Skin Disease on Daily Life scale |
| LoSCAT | Localized Scleroderma Cutaneous Assessment Tool |
| LoSDI | Localized Scleroderma Skin Damage Index |
| LoSSI | Localized Scleroderma Skin Severity Index |
| mLoSSI | Modified Localized Scleroderma Skin Severity Index |
| mRSS | Modified Rodnan Skin Score |
| MD | mean difference |
| MHz | megahertz |
| MSS | modified skin score |
| MTX | methotrexate |
| NNT | number needed to treat |
| PDT | photodynamic therapy |
| PGA‐D | Physician Global Assessment of disease Damage |
| PtGA‐S | Patient Global Assessment of disease Severity |
| PUVA | psoralen plus ultraviolet A |
| RCT | randomised controlled trial |
| RR | risk ratio |
| RSS | Rodnan skin score |
| SMD | standardised mean differences |
| SSc | systemic sclerosis |
| SSR | skin score rate |
| UV | ultraviolet |
| UVA | ultraviolet A |
| UVB | ultraviolet B |
| VAS | visual analogue scale |
| ΔTh% | percentage thermal change from baseline |
Description of the condition
Morphea (morphoea) is a chronic inflammatory and fibrosing (thickening of tissue) disorder usually limited to the skin and underlying tissues: subcutaneous tissues, underlying bone, muscles, synovia and — on extremely rare occasions — the central nervous system (when the lesion is present on the face and head) (Badea 2009; Hawk 2001; Kroft 2009b; Rook 2010; Valanciene 2010; Zwischenberger 2011). It is an immune‐mediated disease related to autoimmune phenomena in which excess synthesis and deposition of collagen in the skin and connective tissues results in hardened cutaneous areas (Badea 2009; Hawk 2001; Hunzelmann 1998; Vasquez 2012).
Morphea is sometimes referred to as localised scleroderma — confusingly, as the term scleroderma may link the diagnosis to systemic sclerosis (SSc). Both diseases are characterised by skin hardening; however, it is important to distinguish morphea from SSc. In SSc, excessive deposition of collagen occurs not only in the skin but also in internal organs, such as the lungs, heart, and gastrointestinal tract, leading to high morbidity and mortality (Barnes 2012; Beyer 2012; Harding 1998; Pope 1998a; Pope 1998b; Tingey 1998). SSc may also affect the peripheral circulation and the extremities (Vasquez 2012). The linear and generalised forms of morphea are linked to significant morbidity but do not affect mortality, whilst the cardiopulmonary involvement of SSc leads to high disease‐specific mortality. Thus the term morphea is preferred for the adult population, but the term localised scleroderma is used particularly for the paediatric population to emphasise the morbidity linked to linear scleroderma, which is most common in children (Fett 2013).
Morphea is a diverse condition that presents different clinical features according to the subtype and stage of evolution of the disease (Fett 2013; Vasquez 2012). The initial morphea lesion appears as one or several inflamed or slightly erythematous and oedematous patches or plaques, usually on the trunk, that become fibrotic and sclerotic with an ivory‐coloured centre (Hawk 2001; Valanciene 2010). The edge of the lesion becomes reddish, indurated, or violaceous when the disease is in an active stage (Valanciene 2010; Vasquez 2012).
In the course of time atrophic skin changes appear, with pigmentation disorders (hypo‐ or hyperpigmentation), shiny skin, indentations, numbness, visible blood vessels, fat loss, loss of hair, loss of sweat glands, and in some cases, disabling joint contractures and restriction of movement of one or more limbs (Arkachaisri 2010; Saxton‐Daniels 2010; Valanciene 2010).
It is important to consider the signs of activity of morphea, such as lesions with marked or moderate erythema or violaceous colour change, new lesions, or expanding lesions, because skin changes in the active stage of the disease have better chances of improvement, whilst inactive sclerotic or atrophic lesions often have poor response to treatments (Fett 2013; Li 2012; Vasquez 2012). Other indicators of disease activity include: lesion warmth; mild erythema; marked or moderate induration of the lesion’s border; worsening hair loss in the scalp, eyebrow or eyelashes; elevated creatine kinase; and lesion histopathological findings (Knobler 2017; Li 2012).
Morphea can be present in several different forms that vary from each other in terms of age, possible causes, propensity to underlying sclerosis and hence treatment aims and need for systemic therapy. The classification systems proposed until now use different parameters and characteristics to distinguish clinical presentations, as the boundaries between the morphea phenotypes are not always clear and the presence of more than one subtype is common (Careta 2015; Fett 2011a; Knobler 2017; Kreuter 2015; Li 2012; Marsol 2013). When morphea develops during childhood or adolescence, it is called juvenile morphea. However, although it is characterised by high morbidity (as its most common type is linear scleroderma), juvenile morphea is not a clinical subtype, because it presents the same manifestations as in adults (Fett 2013).
The Padua Consensus Classification, published in 2004 by the Paediatric Rheumatology European Society, describes five disease types based on clinical and histopathological aspects (Asano 2018; Laxer 2006).
Circumscribed morphea (with superficial and deep variants): one to few patches of well‐circumscribed, circular to oblong lesions scattered on the trunk or limbs, that are usually restricted to the dermis and sometimes to the upper fat layer of the skin (superficial panniculus).
Linear scleroderma (with trunk/limb variant and head variant): linear streak of fibrosis that can involve the underlying tissues (bones, muscles, synovia, and central nervous system), inducing disability and joint contracture. The lesions on the limbs may affect growth in children, and the lesions on the face can cause deformity, facial asymmetry and dentition deformity.
Generalised morphea: a severe type of morphea characterized by the presence of four or more indurated lesions involving more than two body areas of the seven anatomic sites (head‐neck, each extremity, anterior trunk, and posterior trunk).
Pansclerotic morphea: a type of severe and progressive generalised morphea, in which the lesions may infiltrate the skin of the whole body and involve the underlying tissues, causing joint contracture, deformity, ulceration and calcification.
Mixed morphea: presence of two or more disease types, including circumscribed morphea, linear scleroderma, generalised morphea and pansclerotic morphea.
Figure 1 and Figure 2 illustrate some cases of circumscribed morphea, linear scleroderma, and generalised morphea.
1.

A ‐ Confluent sclero‐atrophic lesions, with hypochromic, achromic and brownish areas on the thighs, generalised morphea; B ‐ sclero‐atrophic oval lesion with dyschromic areas and halo erythematosus in its right and inferior portion, active circumscribed morphea; C ‐ brown macula with discretely erythematous areas and irregular borders, circumscribed morphea in involution. Copyright © 2019 Monica RA Vasconcellos: reproduced with permission.
2.

Linear scleroderma. A ‐ Sclero‐atrophic lesion involving the back of the hand and fingers, with deviation in the fourth and fifth chirodactyls; B – Streak of atrophy in the tongue (left) and dental implant defect (right); C – segmental sclero‐atrophic lesions in the trunk and limbs interspersed by hyper pigmented maculae. Copyright © 2019 Monica RA Vasconcellos: reproduced with permission.
A definitive diagnosis of morphea is based on the appearance and texture of the skin, with skin biopsies used to support the clinical hypothesis and rule out other disorders (Hawk 2001). The histopathological examination does not distinguish morphea from SSc. Generalised morphea could plausibly be confused with SSc, and the differentiation is based on careful direct examination of the patient, on serologic examinations and capillaroscopic changes, typical of SSc (Vasquez 2012).
Epidemiological data
Epidemiological studies have suggested the incidence of morphea is 0.4 to 2.7 per 100,000 people per year (Arkachaisri 2010; Fett 2011a). This data is from a population in the United States between 1960 and 1993 (Peterson 1997). As survival was not adversely affected by the development of the disease, Peterson 1997 observed that the prevalence of morphea increased with the age of the population under consideration, affecting 50 people per 100,000 at 18 years of age, rising to 220 people per 100,000 at 80 years of age (Mayes 1998; Valanciene 2010). Morphea affects adults and children equally, with the average age of adult onset being 40 years, and the average age of onset in children varying between two and 14 years (Fett 2011a; Valanciene 2010). The disease is more prevalent in women (the ratio of morphea in females to males is 2.6:1) (Fett 2011a; Hawk 2001). The most common clinical presentation in adults is circumscribed morphea (43.9%), whilst linear scleroderma is the most common subtype in children (41.8%) (Leitenberger 2009; Saxton‐Daniels 2010; Vasquez 2012). The mixed type of morphea affects around 15% of individuals, with juvenile morphea and pansclerotic morphea also predominantly affecting children (Asano 2018).
Causes
Despite numerous studies trying to elucidate the mechanisms of morphea, its causes are still unknown (Chen 2002; Hunzelmann 1998; Valanciene 2010). Morphea is probably caused by genetic, autoimmune, and environmental factors, such as trauma, radiation, hormones, medications, and infections (Chen 2002; Hawk 2001; Hunzelmann 1998; Valanciene 2010; Vasquez 2012).
Prognosis
Data on the long‐term clinical course of morphea is limited (Knobler 2017). Medical monitoring should start as early as possible, with a preventive approach. However, the decision to start or indicate a specific treatment can be problematic due to the difficulty in determining if the lesions are clinically active and progressing, or if the disease is stable and whether the damage will respond (Marsol 2013).
The disease severity and prognosis vary according to its subtype: circumscribed morphea generally has a more benign course when compared with linear scleroderma and generalised morphea. Circumscribed morphea usually results in atrophy and loss of collagen and adnexal structure rather than scarring due to scar tissue or excess collagen. Linear scleroderma can cause significant disfigurement and, in case of involvement of the underlying bone and growth plates, aesthetic and functional disability, muscular spasms, changes in the length of the arms and legs, reduced joint movement, and psychological disabilities (Fett 2013; Hawk 2001; Johnson 2012; Saxton‐Daniels 2010; Zwischenberger 2011).
Juvenile linear scleroderma often presents a more severe clinical course compared with adult linear scleroderma, and the considerable atrophy of the skin, fat tissue, fascia and muscle is linked to substantial functional, physical, and mental disability. Skin lesions on the limbs can induce disability and joint contracture, preventing symmetrical growth; and lesions on the head can lead to deformity, facial asymmetry and dentition deformity (Asano 2018). Thus, effective systemic therapy in the active stage of juvenile linear scleroderma is recommend as early as possible to prevent persistent damage (Knobler 2017).
Superficial linear scleroderma without involvement of underlying tissues is usually treated with local therapies or phototherapy, and generalised morphea tends to be initially treated with phototherapy (Fett 2013). However, systemic treatments may be required, and individuals with linear scleroderma and generalised morphea receive more aggressive treatment and have higher chances of adverse events when compared to those receiving treatment for superficial circumscribed morphea (Bielsa Marsol 2013; Vasquez 2012). The treatment of superficial circumscribed morphea usually includes topical therapy or local phototherapy, and circumscribed deep morphea may require local phototherapy or systemic immunosuppression (Fett 2013).
Although morphea usually has a self‐limited evolution, with frequent spontaneous regressions within 3 to 5 years, recurrence rates are high, even after many years of inactive disease, particularly in children with linear scleroderma or mixed morphea, which require a careful long‐term follow‐up (Asano 2018; Careta 2015; Mertens 2015). Disease activity remits, but permanent sequelae (consequent anatomical or functional abnormalities) may occur due to prolonged disease activity.
Description of the intervention
Because of its rarity (incidence of 0.4 to 2.7 people per 100,000 per year), there are few randomised controlled trials of therapeutic agents (Hawk 2001; Kroft 2009b; Valanciene 2010; Zulian 2011); and to date different medications for treating the various forms of morphea have been used. However, evidence of the effectiveness and safety of these medications is still unclear, and it is unknown which drug is best suited for each subtype of morphea (Bielsa Marsol 2013; Zulian 2011; Zwischenberger 2011).
The literature describes the following main treatments for the disease, which can be used alone or in combination.
Phototherapy (treatment with nature‐identical ultraviolet radiation sources that can be combined with certain medications called psoralens, which make people more sensitive to the effects of light): ultraviolet (UV) A‐1, broad‐band UVA, narrowband UVB, psoralen with UVA (PUVA) bath, PUVA cream, and extracorporeal photochemotherapy, which are used in adults and children. The phototherapy decreases the synthesis of collagen (Hawk 2001; Kroft 2009b; Valanciene 2010; Zwischenberger 2011). The side effects of these treatments are hyperpigmentation, pruritus, burning, recurrences of herpes simplex, photoaging, and increased risk of skin cancers (Zandi 2012).
Vitamin D derivatives: oral calcitriol, topical calcipotriene, and calcipotriol ointments, which are used in both adults and children. They inhibit the proliferation of fibroblasts (responsible for collagen production) and also have anti‐inflammatory effects (Hawk 2001; Kroft 2009b; Valanciene 2010). The side effects of these treatments include local irritation, contact allergy, and elevated serum calcium (hypercalcaemia) (Rook 2010).
Immunosuppressors (reduce DNA synthesis and cell division) and immunomodulators (act on the immune response): methotrexate, tacrolimus, antimalarials (chloroquine and hydroxychloroquine), interferon gamma, interferon alpha, mycophenolate mofetil, ciclosporin, cyclophosphamide, imiquimod, colchicine, Salazopyrine, and D‐penicillamine. These agents are used in both adults and children. The side effects of these treatments include teratogenicity (foetal malformation); ulcerative stomatitis; mucositis; nausea; vomiting; diarrhoea; anorexia; gonadal suppression; liver, kidney, and nerve damage; hypersensitivity reactions (allergies); hair loss; skin pigmentation changes; photosensitivity; bone marrow damage; leukopaenia (reduction in the number of leukocytes); and thrombocytopaenia (reduction in the number of platelets) (Hawk 2001; Hunzelmann 1998; Kroft 2009b; Rook 2010).
Oral and topical steroids, which are used in adults and children, act to reduce the inflammation of active lesions. The side effects of these treatments include cutaneous thinning, acne, facial hair growth, striae distensae, weight gain, glaucoma, osteoporosis, growth suppression in children, opportunistic infections, hypertension, diabetes, and Cushing's syndrome (Hawk 2001; Kroft 2009b; Rook 2010; Valanciene 2010).
Physical therapy, heat treatment and massage, to prevent joint disability and contracture and maintain movement (Hunzelmann 1998).
Other less commonly used therapies that have been described include anti‐infective agents (penicillin, aminobenzoate potassium), vitamin A derivatives (etretinate, acitretin), vitamin E, anticoagulant agents (heparin and heparinoids), lidocaine, anticonvulsants (phenytoin), bosentan, surgical interventions, photodynamic therapy, laser, and autologous fat implants (Fett 2011b; Hawk 2001; Kroft 2009b; Valanciene 2010; Vilela 2010; Zwischenberger 2011).
How the intervention might work
There is no causal treatment for morphea, but several therapeutic options are available, particularly for the active, inflammatory stage of disease (Knobler 2017). The aims of these interventions are to stop disease activity, prevent the appearance of new lesions, and improve any existing lesions. Most interventions reduce the inflammatory process or modulate the production of collagen (Hunzelmann 1998; Valanciene 2010; Vasquez 2012). It usually takes between eight and 12 weeks to achieve reduction of skin sclerosis after initiation of therapy (Knobler 2017).
Phototherapy decreases collagen synthesis; topical steroids and tacrolimus have anti‐inflammatory and immunomodulatory effects; topical vitamin D derivatives inhibit the proliferation of fibroblasts (cells that produce collagen fibres); and methotrexate has immunosuppressive effects — all of which result in the suppression of disease progression. Oral calcitriol is an anti‐inflammatory agent and fibroblast modulator. Interferon gamma and alpha normalise abnormal fibroblast production. When there is established damage, surgery is performed followed by physical and occupational therapy to promote any subsequent improvement (Hunzelmann 1998; Valanciene 2010).
The aims of treatment may be very different according to the subtype, extent and severity of morphea. It is also essential to evaluate the disease activity, as the aim for lesions at an early inflammatory stage is to inhibit disease activity; and at a late fibrotic stage the objective is to treat functional disorders and cosmetic issues (Asano 2018; Knobler 2017). Active morphea can be treated with topical or systemic immunosuppressives, whereas the damage stage is not treatable with immunosuppression (Fett 2013). Options for treating functional disorders, such as joint flexion contracture and restriction of motion, and cosmetic problems caused by inactive skin lesions, include physiotherapy and surgical interventions (these should not be considered in the active, inflammatory stage of morphea) (Asano 2018; Knobler 2017).
Topical therapy and phototherapy are not indicated for lesions involving the underlying tissue, as these interventions act on the deep dermis; thus morphea subtypes affecting deeper structures (e.g. fat tissue, fascia, muscle, or bone) require systemic therapy (Knobler 2017). However, when systemic therapy is indicated the extent of adverse events and the therapeutic effect must be taken into account (Asano 2018).
Linear scleroderma tends to be treated aggressively to prevent underlying dermal, muscle and bone atrophy whereas circumscribed morphea is often treated topically for global improvement. Superficial circumscribed morphea, restricted to the skin, tends to be treated topically if a single site or few sites are present, or with UV phototherapy in case of several sites; whereas systemic therapy is indicated for deep circumscribed morphea, linear scleroderma and generalised morphea (Fett 2013). In lesions crossing the joints or with the potential to disfigure, methotrexate is the first choice of treatment (Knobler 2017). In the treatment of juvenile circumscribed morphea, topical therapies can be used or even no intervention to avoid medicalisation, whereas in juvenile linear scleroderma and generalised morphea the treatment should start as early as possible to prevent underlying tissue damage.
There is no validation and consensual tool to assess the severity of morphea (Asano 2018). Clinical tools validated and widely used in SSc are inappropriate for the measurement of morphea skin involvement, and clinical scores specifically designed for morphea are relatively new because of the difficulties of defining clinical improvement (Knobler 2017). The use of non‐validated outcome measures to assess disease improvement limits the evaluation of the effectiveness of treatments (Fett 2013). The Localised Scleroderma Cutaneous Assessment Tool (LoSCAT) could become a standard tool for morphea as it measures both activity and damage (Knobler 2017). The LoSCAT is a combination of: the modified Localized Scleroderma Skin Severity Index (mLoSSI), the first validated skin score for morphea which evaluates erythema, skin thickness, new lesions and lesional extension in 18 anatomic regions; the Localized Scleroderma Skin Damage Index (LoSDI), a score later developed to assess skin damage in the same anatomic regions; and the Physician’s Global Assessment (PGA) (Arkachaisri 2010). There are other available tools to assess disease activity in morphea, such as ultrasound scans, cutometer, durometer, thermography, and contrast MRI; however, these usually account for secondary outcome measures (Asano 2018; Knobler 2017). Tools to assess quality of life include the Dermatology Life Quality Index (DLQI) and the Hospital Anxiety and Depression Scale (Knobler 2017).
Why it is important to do this review
Morphea is extremely varied clinically, with heterogenous subtypes. The alterations to the skin caused by morphea have different degrees of impact on a person's quality of life and can lead to irreversible physical and psychological damage (Arkachaisri 2010; Bielsa Marsol 2013; Kroft 2009b). Superficial circumscribed morphea has the potential to cause itching, pain and aesthetic concerns, whilst linear scleroderma can cause, particularly in children in their growth stage, muscle spasms, disabilities, disfigurement and limb length discrepancies (Arkachaisri 2010; Hawk 2001; Valanciene 2010; Zwischenberger 2011). Pain, fatigue, stigmatisation, the negative impact of the disease on daily life, and less social acceptance and support make those with morphea more susceptible to psychological problems, such as anxiety and depression (Kroft 2009b).
Despite numerous proposed treatments for morphea, each disease subtype presents with a different response to treatment, and there is no consensus on which therapies, dosages and treatment periods are widely tolerated, have fewer side effects, and offer the greatest benefit to people with morphea (Asano 2018; Knobler 2017; Zwischenberger 2011).
The plans for this review were published as a protocol 'Interventions for morphea' (Ravelli 2014).
Objectives
To assess the effects of treatments for people with any form of morphea.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) assessing treatments for morphea (isolated or combined). We included within‐participant trials (e.g. left‐side, right‐side comparisons) in the review, but only the first part of cross‐over trials to avoid carry‐over and period effects.
Types of participants
Anyone who has been clinically diagnosed with any form of morphea (e.g. limited, generalised, linear, deep, or mixed morphea) by a medical practitioner.
In case of studies with only a subset of relevant participants (e.g. treatment groups including participants with different diseases), we analysed only data from eligible participants (individuals with morphea). If these data were not available in the published study, we contacted the first author of the study to obtain it.
Types of interventions
Any topical, intralesional, or systemic medications including radiation therapy, photodynamic therapy, laser therapy, surgery, and physical therapy. Interventions could be isolated or combined. Controls could be placebo, no intervention, any other treatment, or different doses or duration of a treatment.
Types of outcome measures
Primary outcomes
Global improvement of disease activity or damage, assessed by a medical practitioner or by participants.
Adverse effects of the interventions, including local and systemic reactions.
Secondary outcomes
Improvement of disease activity defined as including skin softening, reduction in lesion size, reduction in skin thickness, fading signs of inflammation, or halting the progression of the disease.
Improvement of disease damage defined as including improvement in pigmentation (hypo‐ or hyperchromia), and improved range of motion, restriction of movement, disabilities, or any functional impairment of motor activity.
Timing of outcomes
We determined which treatments for morphea demonstrated the best outcomes up to six months and also from six to 12 months.
Assessment tools
We analysed the outcomes independently of the assessment tools, which include, among others, the following: LoSSI, mLoSSI, LoSDI, Physician Global Assessment of Damage (PGA‐D), Patient Global Assessment of Disease Severity (PtGA‐S), DLQI, Children's Dermatology Life Quality Index (CDLQI), LoSCAT, Rodnan skin score (RSS), modified Rodnan skin score (mRSS), Modified Skin Score (MSS), Impact of Chronic Skin Disease on Daily Life (ISDL), visual analog scale (VAS), Cutometer®, durometer, thermography, and ultrasound (see Table 5).
Search methods for identification of studies
We aimed to identify all relevant RCTs regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
The Cochrane Skin Information Specialist searched the following databases up to 17 July 2018 using strategies based on the draft strategy for MEDLINE in our published protocol (Ravelli 2014).
Cochrane Skin Group Specialised Register using the search strategy in Appendix 1.
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 6) in the Cochrane Library using the strategy in Appendix 2.
MEDLINE via Ovid (from 1946) using the strategy in Appendix 3.
Embase via Ovid (from 1974) using the strategy in Appendix 4.
LILACS (Latin American and Caribbean Health Science Information database, from 1982), using the strategy in Appendix 5.
Trials registers
We (JVA and BNGS) searched the following trial registers up to 17 July 2018 using the strategies in Appendix 6.
ISRCTN registry (www.isrctn.com).
ClinicalTrials.gov (www.clinicaltrials.gov).
the Australian New Zealand Clinical Trials Registry (www.anzctr.org.au).
the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch).
the EU Clinical Trials Register (www.clinicaltrialsregister.eu).
Searching other resources
References from included studies
We (JVA and BNGS) checked the bibliographies of included studies for further references to relevant trials.
Adverse Effects
We did not perform a separate search for adverse effects of interventions used for the treatment of morphea. We considered adverse effects and side effects described in included studies only.
Data collection and analysis
We include four 'Summary of findings' tables in this review, summarising the primary and secondary outcomes for the most clinically important comparisons.
Oral methotrexate and oral prednisone compared to placebo and oral prednisone for juvenile morphea.
Medium‐dose UVA‐1 phototherapy (50 J/cm²) compared to low‐dose UVA‐1 phototherapy (20 J/cm²) for morphea
Narrowband UVB phototherapy compared to medium‐dose UVA‐1 phototherapy (50 J/cm²) for morphea
Narrowband UVB compared to low‐dose UVA‐1 phototherapy (20 J/cm²) for morphea
Selection of studies
Initially, we assessed only the titles and abstracts of selected articles. After this first assessment, we obtained in full‐text form those studies that appeared to match the inclusion criteria. Two authors (JVA and BNGS) independently selected studies for inclusion in this review; they consulted a third author (VMFT) to settle any disagreements.
Data extraction and management
Two authors (JVA and BNGS) used a data collection form to independently extract data from the included studies. They piloted the data collection form initially on two included studies. The form contained the following essential items: population and intervention characteristics, methods, outcomes, and results. These were used to populate a 'Characteristics of included studies' table. In case of disagreements, they consulted a third author (VFMT). Then two authors (JVA and VTC) entered data into Review Manager 5 (Review Manager 2014).
We obtained translations of studies written in languages other than English, Portuguese, Spanish and French.
Assessment of risk of bias in included studies
Two authors (JVA and BNGS) examined and assessed the risk of bias independently, using a domain‐based evaluation. They evaluated the method of randomisation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other potential sources of bias. Each domain could be considered at high, low or unclear risk of bias. The authors resolved disagreements regarding risk of bias with a third author (VFMT), and used the assessment of 'Risk of bias' criteria available in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Measures of treatment effect
For dichotomous outcomes, we expressed the results as risk ratios (RR) with 95% confidence intervals (CI) and as number needed to treat for an additional beneficial outcome (NNTB) figures, where appropriate, with 95% CI and the baseline risk to which it applies. For continuous outcomes, we expressed the results as difference in means (MD) with 95% CI, and would have expressed the results as standardised mean differences (SMD) with 95% CI for the use of diverging assessment tools.
Unit of analysis issues
We based the unit of analysis on the individual participant, the morphea lesion or body region.
We analysed studies that randomised different parts of the body for different interventions similarly to cross‐over trials: we accounted the multiple interventions at different sites (intra‐individual studies) as if they were multiple interventions at different times (cross‐over studies), and compared the measurements of each pair‐wise comparison as if they were a parallel group. We included the mean and standard deviation values of the paired analyses in Review Manager 5 (Review Manager 2014), using the generic inverse variance method. If these data were not available and if studies did not report a paired t‐test, we contacted the authors for individual participant data.
In case of cross‐over trials, we would have analysed only data from the first period, to avoid carry‐over and period effects, considering the clinical evolution of the disease.
We analysed studies with multiple intervention groups through all possible pair‐wise comparisons between the intervention groups. In cases where we required the meta‐analysis to include more than two groups from one study, we would combine groups to create a single pair‐wise comparison in order to overcome a unit‐of‐analysis error of participants of the same study being included twice in the same meta‐analysis.
Dealing with missing data
For missing data, we contacted the first author of the primary study to obtain all the necessary information. We listed the information requested of the study authors in Table 6.
2. Contact with authors.
| Study ID | Date contacted | Information requested | Date of reply |
| El‐Mofty 2004 | 26 April 2016, 4 July 2016 | The register of the trial, ethics committee approval and funding source; If it was a single‐centre or double‐centre study; The methods used to generate the random sequence and to conceal it; If the outcome assessor was blinded from knowledge of which intervention a participant received; If authors could provide separate data for children and adults; What was the type of morphea and the sex of the four participants who discontinued the treatment. |
26 April 2016, 5 July 2016 |
| Furuzawa‐Carballeda 2012 | 26 April 2016, 4 July 2016 | If the study was conducted both at the Dermatologic Centre Ladislado de la Pascua and at the department of Immunology and Rheumatology of the National Institute of Medical Sciences and Nutrition Salvador Zubirán; If the trial was registered and received funding; If there were significant baseline differences between the intervention groups; The method used to conceal the random sequence; If authors could provide the baseline mRSS for the intervention groups separately; If authors could provide the numerical data for adverse events. |
26 April 2016, 7 July 2016 |
| Kreuter 2006 | 26 April 2016, 3 October 2016 | The register of the trial, ethics committee approval, funding source and declaration of interests; The method used to conceal the random sequence; If the outcome assessor of skin score was blinded from knowledge of which intervention a participant received; What was the duration of the follow‐up after treatment; If authors could provide VAS data with standard deviation. |
4 October 2016 |
| Noakes 2018 | 10 June 2017, 22 July 2018, 26 July 2018, 28 July 2018 | If this study was completed, and if authors have published the results or could provide data; If the trial received funding; The method used to conceal the random sequence; If outcome assessors were blinded from knowledge of which intervention a participant received; If the LoSCAT, PGA‐A and the PGA‐D included only participants with morphea; If there were baseline differences between treatment sites; If any participant left the study before completion; If authors could provide standard deviation values or raw data from the treatment sites. |
11 June 2017, 23 July 2018 27 July 2018 |
| Tang 2006 | 26 April 2016, 3 October 2016 | The register of the trial, ethics committee approval, funding source and declaration of interests; The methods used to generate the random sequence and to conceal it; If outcome assessors were blinded from knowledge of which intervention a participant received; If authors included the four participants who experienced side effects in the analysis after treatment; If authors could provide data of the planimetry and skin thickness assessments with standard deviation. |
28 April 2016 |
| NCT01799174 | 4 July 2016 | If this study was completed, and if authors have published the results or could provide data. | 7 July 2016 |
Assessment of heterogeneity
We would have assumed statistical heterogeneity to be present when the I² statistic was greater than 50% according to the criteria below. We intended to explore potential sources of discrepancy when substantial heterogeneity was apparent. However, we could not pool data in a meta‐analysis. A rough guide to interpretation is as follows.
0% to 40% = might not be important;
30% to 60% = may represent moderate heterogeneity*;
50% to 90% = may represent substantial heterogeneity*; and
75% to 100% = considerable heterogeneity*.
*The importance of the observed value of the I² statistic depends on (i) the magnitude and direction of effects and (ii) the strength of evidence for heterogeneity (e.g. P value from the Chi² test or a confidence interval for the I² statistic).
Assessment of reporting biases
We had planned to assess reporting biases through funnel plots only if we had 10 or more studies in a meta‐analysis.
Data synthesis
We followed the advice given in section 9.1.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and analysed our data in Review Manager 5 (Review Manager 2014), using the random‐effects model. We would have used a fixed‐effect analysis if heterogeneity between studies was not detected.
We followed the GRADE Handbook to assess the certainty of the body of evidence (risk of bias, inconsistency, indirectness, imprecision, and publication bias) for each outcome in our 'Summary of findings' tables (as high, moderate, low or very low certainty). We used GRADEpro GDT to create the 'Summary of findings' tables.
Subgroup analysis and investigation of heterogeneity
We had planned to undertake an interaction test across the following subgroups, in case of substantial heterogeneity on our primary outcomes.
Children versus adults.
Subtype and severity of morphea.
Lesions less than six months old versus lesions more than six months old.
Plaque morphea versus other forms of morphea.
However, we could not perform any meta‐analysis.
Sensitivity analysis
We had planned to perform sensitivity analyses to examine if studies with high risk of bias were under‐ or overestimating the effects of the interventions for our primary outcomes, but we could only analyse studies individually.
Results
Description of studies
We present information about each study in the Characteristics of included studies tables, Characteristics of excluded studies tables, and Characteristics of ongoing studies table.
Results of the search
Our electronic searches identified 339 records. We removed duplicates and assessed 278 records for eligibility. After screening titles and abstracts, we excluded 254 records and selected 24 articles and trial records to read in full text. We included in this review 14 RCTs that met our eligibility criteria (Azimi 2013; Batchelor 2008; El‐Mofty 2004; Furuzawa‐Carballeda 2012; Hulshof 2000; Hunzelmann 1997; Kreuter 2006; Kroft 2009a; Noakes 2018; Sator 2009; Shalaby 2016; Tang 2006; Yan 2013; Zulian 2011). We excluded seven studies (Bodemer 1999; Didenko 1978; Dortu 1974; Dytoc 2014; Hu 1996; Smirnov 1998; Wang 2008), plus the trial register of the excluded study Wang 2008, which was not an RCT. We list the reasons for exclusions in the Characteristics of excluded studies table. We also identified two trial records that met our eligibility criteria but are not yet published (NCT00812188; NCT01799174); we listed these trial records as awaiting classification and contacted the authors for further information. No ongoing studies were identified. For a further description of our screening process, see the study flow diagram Figure 3.
3.

Study flow diagram.
We requested translations of two excluded studies, one written in Russian and the other in Chinese (Didenko 1978 and Hu 1996 respectively); and translations of three included studies, one written in Chinese, one in German and one in Persian (Yan 2013, Tang 2006 and Azimi 2013, respectively).
Included studies
This review includes 14 RCTs, with a total of 429 participants. We present further details in the Characteristics of included studies tables.
Design
Most included studies had two trial arms (Azimi 2013; Batchelor 2008; Hulshof 2000; Hunzelmann 1997; Kroft 2009a; Noakes 2018; Shalaby 2016; Tang 2006; Yan 2013; Zulian 2011), and four studies had three trial arms (El‐Mofty 2004; Furuzawa‐Carballeda 2012; Kreuter 2006; Sator 2009). Five studies had within‐individual design (Batchelor 2008; Kroft 2009a; Noakes 2018; Sator 2009; Shalaby 2016). Three RCTs were pilot studies (Batchelor 2008; Kroft 2009a; Tang 2006). The included studies compared an active intervention to placebo (Hulshof 2000; Hunzelmann 1997; Kroft 2009a; Zulian 2011), to another active intervention (Azimi 2013; Furuzawa‐Carballeda 2012; Noakes 2018; Shalaby 2016; Tang 2006; Yan 2013), to two other active interventions (El‐Mofty 2004; Kreuter 2006), to no treatment (Batchelor 2008), or to both an active intervention and no treatment (Sator 2009). One study included a third group of healthy untreated individuals as control in immunohistochemistry assessments (Furuzawa‐Carballeda 2012), but we excluded these participants from our review.
Three studies investigated participants with a diagnosis of morphea and also participants with SSc (El‐Mofty 2004; Hulshof 2000; Noakes 2018); we included in the review only data from participants with morphea. Whilst El‐Mofty 2004 and Noakes 2018 reported separate data for all outcomes, Hulshof 2000 reported separate efficacy data but accounted adverse events data for participants with morphea and SSc together.
The duration of the included studies ranged from seven weeks to 15 months from baseline (El‐Mofty 2004 and Hulshof 2000 respectively). The other studies lasted 10 weeks (Shalaby 2016), 12 weeks (Azimi 2013; Batchelor 2008; Kroft 2009a; Noakes 2018), 20 weeks (Kreuter 2006), 24 weeks (Hunzelmann 1997; Tang 2006; Yan 2013), nine months (Furuzawa‐Carballeda 2012), 12 months (Zulian 2011), and 12 months plus 10 weeks (Sator 2009).
Setting
Two included studies were set in Africa (El‐Mofty 2004; Shalaby 2016), two were conducted in Asia (Azimi 2013; Yan 2013), eight in Europe (Batchelor 2008; Hulshof 2000; Hunzelmann 1997; Kreuter 2006; Kroft 2009a; Sator 2009; Tang 2006; Zulian 2011), one in North America (Furuzawa‐Carballeda 2012), and one in Oceania (Noakes 2018).
Three studies were multicentre RCTs (Furuzawa‐Carballeda 2012; Hunzelmann 1997; Zulian 2011).
The settings of the included studies were a dermatologic centre and a national laboratory centre (Furuzawa‐Carballeda 2012), paediatric rheumatology and dermatology centres (Zulian 2011), and university hospitals or medical centres (El‐Mofty 2004; Hulshof 2000; Kreuter 2006; Kroft 2009a; Noakes 2018; Shalaby 2016). Six studies did not describe their setting (Azimi 2013; Batchelor 2008; Hunzelmann 1997; Sator 2009; Tang 2006; Yan 2013).
The date of publication of included studies ranged from 1997 to 2018.
Participants
All included studies investigated individuals with a clinical diagnosis of morphea, and five studies performed histological confirmation (Azimi 2013; Furuzawa‐Carballeda 2012; Kreuter 2006; Tang 2006; Yan 2013). Six studies selected participants with a diagnosis of active morphea, defined as presenting with inflammation, increased size and/or new lesions (Azimi 2013; Hunzelmann 1997; Kreuter 2006; Kroft 2009a; Sator 2009; Zulian 2011). Four studies included participants with at least two morphea lesions (Batchelor 2008; Kroft 2009a; Noakes 2018; Shalaby 2016), and one study included participants with at least three morphea lesions (Sator 2009). Six studies excluded participants with Borrelia burgdorferi infection (Furuzawa‐Carballeda 2012; Hulshof 2000; Hunzelmann 1997; Kreuter 2006; Kroft 2009a; Tang 2006). The sample size of the included studies ranged from three to 70 individuals (Noakes 2018 and Zulian 2011, respectively), and the mean sample size was 31 individuals.
This review includes 429 participants: 294 females, 113 males, and 22 unclear, because one study did not report this information (Batchelor 2008) and four studies only reported the sex of the participants who completed the study (Azimi 2013; El‐Mofty 2004; Sator 2009; Shalaby 2016).
The age of the included participants ranged from 3 to 76 years, but Batchelor 2008 did not report the participants' age, and Hunzelmann 1997 only reported the median age of the groups. Six studies investigated adults aged 18 years or older (Batchelor 2008; Furuzawa‐Carballeda 2012; Kroft 2009a; Noakes 2018; Tang 2006; Yan 2013), one study selected juvenile participants aged 17 years or younger (Zulian 2011), one study did not describe this information (Hunzelmann 1997), and six studies investigated both juvenile and adult participants (Azimi 2013; El‐Mofty 2004; Hulshof 2000; Kreuter 2006; Sator 2009; Shalaby 2016). The study with children included 70 participants (Zulian 2011), but the number of juvenile participants in this review is unclear because the studies with children and adults did not present separate data for each age group.
The participants included in this review had circumscribed morphea (n = 197), linear scleroderma (n = 77; 6 with head variant), generalised morphea (n = 46), and mixed morphea (n = 9). Twenty participants had circumscribed or generalised morphea (Hulshof 2000 did not report the number of participants with each morphea type), and 80 participants had unclear morphea type (El‐Mofty 2004 and Shalaby 2016 only reported the morphea type of the participants who completed the study; Azimi 2013 and Yan 2013 did not report this information). Four studies included participants with circumscribed morphea (Batchelor 2008; Hunzelmann 1997; Kroft 2009a; Sator 2009). Eight studies included participants with more than one type of morphea and reported data for all types combined, thus it was not possible to analyse the subtypes separately (El‐Mofty 2004; Furuzawa‐Carballeda 2012; Hulshof 2000; Kreuter 2006; Noakes 2018; Shalaby 2016; Tang 2006; Zulian 2011).
Ten studies reported the length of the disease evolution, which was up to 10 years in six studies (El‐Mofty 2004; Hulshof 2000; Kroft 2009a; Sator 2009; Shalaby 2016; Tang 2006), and more than 10 years in three studies (Furuzawa‐Carballeda 2012; Kreuter 2006; Yan 2013). In the study with juvenile participants, the mean participants' length of disease was 2.3 years (Zulian 2011).
Five studies reported the treatment participants had previously received (Kreuter 2006; Kroft 2009a; Sator 2009; Tang 2006; Zulian 2011), which included UVB and PUVA phototherapy, topical calcipotriol, topical steroids, topical and oral corticosteroids, systemic steroids, methotrexate, cyclophosphamide, azathioprine, cyclosporin A, chloroquine, penicillamine, and systemic antibiotics. One study only reported that 71% of the participants had previously tried other therapies (Shalaby 2016).
Interventions
The studies included in this review covered heterogenous therapies for morphea. Seven studies investigated topical medications (Batchelor 2008; El‐Mofty 2004; Kreuter 2006; Kroft 2009a; Noakes 2018; Sator 2009; Shalaby 2016), two evaluated intralesional medications (Furuzawa‐Carballeda 2012; Hunzelmann 1997), and five investigated systemic medications (Azimi 2013; Hulshof 2000; Tang 2006; Yan 2013; Zulian 2011). We list details of the interventions in the Characteristics of included studies tables.
1. Topical medications
Five studies investigated phototherapy (Batchelor 2008; El‐Mofty 2004; Kreuter 2006; Sator 2009; Shalaby 2016), but performed different interventions. Thus, we could not pool data from these studies in a meta‐analysis. Batchelor 2008 selected two morphea lesions in each participant, and compared photodynamic therapy (PDT) using 5‐aminolaevulinic acid (ALA), once a week, for six weeks, to no treatment. El‐Mofty 2004 investigated different low doses of UVA phototherapy (5 J/cm², 10 J/cm² and 20 J/cm²), three sessions a week, totalling 20 sessions. Kreuter 2006 assessed treatment with low‐dose UVA‐1 (20 J/cm²), medium‐dose UVA‐1 (50 J/cm²), and narrowband UVB phototherapy, five times a week, during eight weeks. Sator 2009 compared medium‐dose UVA‐1 (70 J/cm²) whole body exposure (including a selected target plaque) with low‐dose UVA‐1 (20 J/cm²) on a second target plaque, four times a week for five weeks and two times a week for another five weeks, and no treatment on a third plaque (unirradiated control). Shalaby 2016 compared fractional carbon dioxide (CO₂) laser therapy, three sessions separated by 1‐month intervals, with low‐dose UVA‐1 (30 J/cm²) phototherapy, three sessions a week for 10 weeks.
One study selected two morphea lesions in each participant, and compared treatment with tacrolimus 0.1% ointment to emollient petrolatum, both applied twice a day, during 12 weeks (Kroft 2009a).
One study selected pairs of treatment sites in each participant, and compared treatment with tranilast plus topical betamethasone valerate 0.1% versus topical betamethasone valerate 0.1%, both applied twice a day, during three months (Noakes 2018). However, participants continued with their routine therapy, which included no adjuvant therapy, methotrexate 20 mg weekly plus hydroxychloroquine 400 mg daily, and methotrexate 10 mg weekly.
2. Intralesional medications
One study compared treatment with polymerised type I collagen (weekly intralesional injections of 0.2 to 1.0 mL polymerised type I collagen/1.66 to 8.3 mg collagen) to methylprednisolone (monthly subcutaneous injections of 0.1 mL ‒ maximum dose of 20 mg or 5.0 mL/month) plus placebo (weekly subcutaneous injections of 0.1 mL placebo), for three months (Furuzawa‐Carballeda 2012).
One study investigated treatment with intralesional injections of interferon gamma (IFN‐γ; 100 µg) and placebo (0.9% sodium chloride) on five consecutive days in the first two weeks and once a week for four more weeks (Hunzelmann 1997).
3. Systemic medications
Two studies investigated treatment with methotrexate (MTX), but performed different interventions (Azimi 2013; Zulian 2011). Thus, we could not pool data from these studies in a meta‐analysis. Azimi 2013 compared hydroxychloroquine (HCQ; 200 mg twice a day) plus topical corticosteroid (authors did not mention the type nor dosage) with methotrexate (15 mg once a week, on Fridays), plus folic acid (1 mg daily, except for Fridays) and topical corticosteroid, for three months. Zulian 2011 investigated treatment with oral MTX (15 mg/m², maximum 20 mg, once a week) compared to placebo, once a week, for 12 months or until disease flare. Both groups also received folic acid supplementation (2.5 mg, 48 hours after each treatment dose), and oral prednisone (1 mg/kg a day, maximum of 50 mg in the morning, during three months, plus one month with gradually decreased dose until discontinuation).
Two studies investigated Traditional Chinese Medicine therapies, but performed different interventions (Tang 2006; Yan 2013). Thus, we could not pool data from these studies in a meta‐analysis. Tang 2006 evaluated treatment with Traditional Chinese Medicine herbs (a 200 ml tea, twice a day), application of a herbal oil to the affected areas for five minutes a day, plus vitamin B6 (three tablets of 20 mg each), for 12 weeks. The ingredients of the tea and oil are specified in the Characteristics of included studies. The control group received phenoxymethylpenicillin 1.2 mg (three times a day) for six weeks, and applied DAC base cream (a standard moisturising base cream from the Deutsche Arzneimittel‐Codex (DAC, German Pharmaceutical Codex)) in the affected areas for five minutes a day, for 12 weeks. Yan 2013 assessed treatment with surrounding needles at the affected areas and the Hegu, Zusanli, Yanglingquan and Waiguan areas (for 30 minutes, every other day), application of a hot herbal compress to the affected areas (for 30 minutes, twice a day), and moxibustion at the affected areas and the Hegu and Zusanli areas (for 30 minutes a day), for six months, plus Centella triterpenes (four tablets of 6 mg, three times a day) and one vitamin E tablet (0.1 g, three times a day). The control group applied a heparin sodium cream to the affected areas, twice a day, for six months, plus Centella triterpenes (four tablets of 6 mg, three times a day) and one vitamin E tablet (0.1 g, three times a day).
One study compared treatment with oral calcitriol (0.75 μg/day before sleeping, for six months, and 1.25 μg/day for three more months) to placebo (before sleeping, for nine months; Hulshof 2000).
Outcomes
The studies included in this review used heterogeneous assessment tools. Ten studies reported outcomes up to six months (Azimi 2013; Batchelor 2008; El‐Mofty 2004; Hunzelmann 1997; Kreuter 2006; Kroft 2009a; Noakes 2018; Shalaby 2016; Tang 2006; Yan 2013), and four studies reported outcomes from six to 12 months (Furuzawa‐Carballeda 2012; Hulshof 2000; Sator 2009; Zulian 2011) from baseline.
Primary outcomes
Only four studies evaluated the global improvement of disease activity or damage assessed by a medical practitioner or by participants (Azimi 2013; Kreuter 2006; Yan 2013; Zulian 2011). Two studies evaluated the clinical response through the modified skin score (MSS), a score (from 0 to 42) developed for SSc, assessing skin thickness and pliability (from 0, normal skin, to 3, unable to pinch or move skin) plus involved area (from 0, no involvement, to 3 more than 67% involvement) in seven body regions (Zachariae 1994): Azimi 2013 after the 3‐month treatment; and Kreuter 2006 after the eight‐week treatment and at the 3‐month follow‐up after treatment. Yan 2013 assessed the clinical response after the 6‐month treatment based on Traditional Chinese Medicine criteria, considering skin hardening, pigmentation, dysfunction and hair loss (not effective: less than 30% improvement; effective: 30% to 70%; remarkably effective: 70% to 95%; clinical recovery: more than 95% improvement). Zulian 2011 calculated the rate of response to treatment at the end of the 12‐month treatment based on participants who met all three response criteria (skin score rate equal to or less than 1, indicating decreased lesion extension; at least a 10% decrease in the percentage thermal change from baseline, indicating decreased lesion inflammation; and absence of new lesions). Zulian 2011 also evaluated the physician's global assessment of disease severity and the parents' global assessment of the participant’s overall well‐being through the Visual Analogue Scale (VAS; 100 mm).
All included studies addressed adverse effects of the interventions (Azimi 2013; Batchelor 2008; El‐Mofty 2004; Furuzawa‐Carballeda 2012; Hulshof 2000; Hunzelmann 1997; Kreuter 2006; Kroft 2009a; Noakes 2018; Sator 2009; Shalaby 2016; Tang 2006; Yan 2013; Zulian 2011).
Secondary outcomes
All included studies evaluated the improvement of disease activity, using heterogeneous assessment tools. Six studies evaluated skin softening (Azimi 2013; Batchelor 2008; El‐Mofty 2004; Kreuter 2006; Kroft 2009a; Yan 2013), four studies assessed reduction in lesion size (Hunzelmann 1997; Kroft 2009a; Tang 2006; Zulian 2011), eight studies assessed reduction in skin thickness (Batchelor 2008; Furuzawa‐Carballeda 2012; Hulshof 2000; Hunzelmann 1997; Kreuter 2006; Sator 2009; Shalaby 2016; Tang 2006), three studies investigated fading signs of inflammation (Azimi 2013; Kreuter 2006; Zulian 2011), and three studies investigated the progression of the disease (Furuzawa‐Carballeda 2012; Hunzelmann 1997; Zulian 2011).
Two studies assessed the participants’ estimate of skin tightness in the morphea lesions through the VAS (from 0 to 10, maximum): Azimi 2013 after the 3‐month treatment, and Kreuter 2006 after the eight‐week treatment. Kreuter 2006 also evaluated skin density with a digital 20‐MHz ultrasound scanner after the eight‐week treatment. Two studies evaluated skin hardness through a durometer score: Batchelor 2008 (score from 0 to 25) at the 12‐week follow‐up, and Kroft 2009a (score from 0 to 1000, maximum) after 12‐week treatment. El‐Mofty 2004 graded by palpation of the morphea lesions the clinical response based on skin softening at the end of the seven‐week treatment (very good response: marked skin softening, almost normal skin texture; good response: moderate softening; fair response: mild softening; and poor response: no change in skin texture). Yan 2013 assessed skin sclerosis after the 6‐month treatment based on Steen criteria, a score used to evaluate SSc (from 0 to 90, maximum: sclerosis of 30 body regions rated from 0, none, to 3, severe; Steen 1982).
Three studies measured the size of the lesions manually: Hunzelmann 1997 at the 24‐week follow‐up, Kroft 2009a after the 12‐week treatment, and Tang 2006 after the 12‐week treatment and at the 12‐week follow‐up after treatment. Zulian 2011 measured the morphea lesions through a computerised skin scoring system, and calculated a skin score rate (SSR) based on the ratio between lesion and body surface area at baseline and after the 12‐month treatment (SSR equal to or less than 1 indicates decreased lesion extension; SSR greater than 1, increase).
Four studies evaluated skin thickness through a clinical score: Batchelor 2008 at the 12‐week follow‐up (score from 0, normal skin, to 3, unable to pinch or move skin ‒ hidebound), Furuzawa‐Carballeda 2012 after the 3‐month treatment and at the 6‐month follow‐up after treatment (score from 0, normal, to 4, extreme thickening; adapted from the modified Rodnan Skin Score (mRSS), used to assess SSc; Clements 1995), Hulshof 2000 after the 9‐month treatment and at the 6‐month follow‐up after treatment (a semi‐quantitative measure from 0 to 66, maximum thickening, developed for SSc, assessing skin thickness of 22 body regions from 0, normal, to 3, hidebound skin; Kahaleh 1985), and Hunzelmann 1997 at the 24‐week follow‐up (score from 0, normal, to 3, severe thickening or hidebound skin). One study evaluated skin thickness via ultrasound biomicroscopy with very high frequency (50 MHz) at the 10‐week follow‐up (Shalaby 2016), and three studies assessed skin thickness via high‐frequency ultrasound (20 MHz): Kreuter 2006 after the eight‐week treatment; Sator 2009 at the 3‐month, 6‐month, and 12‐month follow‐up after treatment; and Tang 2006 after the 12‐week treatment and at the 12‐week follow‐up after treatment).
Two studies assessed the participants' estimate of pruritus on the morphea lesions through the VAS (from 0 to 10, maximum): Azimi 2013 after the 3‐month treatment, and Kreuter 2006 after the 8‐week treatment. Zulian 2011 evaluated the degree of inflammation through infrared thermography, and calculated the percentage thermal change from baseline after the 12‐month treatment (ΔTh%; negative value indicates improvement and positive value, worsening).
Three studies assessed the occurrence of new morphea lesions: Furuzawa‐Carballeda 2012 after the 3‐month treatment and at the 6‐month follow‐up after treatment, Hunzelmann 1997 after the six‐week treatment and at the 18‐week follow‐up after treatment, and Zulian 2011 during the 12‐month follow‐up.
Four intra‐individual studies adapted scores of global evaluation of improvement of disease activity and damage to assess individual lesions (Kroft 2009a; Noakes 2018; Sator 2009; Shalaby 2016). Thus, we analysed this data as an indicator of disease activity. Kroft 2009a adapted the DIET score (dyspigmentation, induration, erythema, telangiectasia; Dytoc 2005) to assess the 12‐week treatment (from 0 to 15, maximum; dyspigmentation, induration, erythema, telangiectasia and atrophy of the lesions scored from 0, none, to 3, severe); Sator 2009 used a score (from 0 to 7) assessing atrophy (0, absent, or 1, present) and intensity of erythema and induration (each from 0, absent, to 3, maximum), at the 3‐month, 6‐month, and 12‐month follow‐up after treatment; Noakes 2018 used the LoSCAT (Arkachaisri 2010), which is the combination of the mLoSSI (it rates new or enlarged lesions within one month from 0 = none to 3 = new/enlarged; erythema from 0 = none to 3 = dark red/violaceous; and skin thickness from 0 = none to 3 = marked) and the LoSDI (it rates dermal atrophy from 0 = none to 3 = obvious ‘cliff drop’; subcutaneous atrophy from 0 = none to 3 = marked; and dyspigmentation from 0 = none to 3 = marked). However, the LoSCAT assesses these measures for 18 body sites, and Noakes 2018 used this tool to compare individual sites. Noakes 2018 also used the Physician Global Assessment of Activity (PGA‐A; from 0 = inactive, to 100 = markedly active), included in the LoSCAT, to evaluate individual lesions. Shalaby 2016 adapted the LoSCAT to assess the treatments (from 0 to 12; thickness, dermal atrophy, dyschromia, and erythema scored each from 0 to 3, maximum). Shalaby 2016 graded the improvement (decrease) on LoSCAT at the 10‐week follow‐up as: poor = no improvement; fair = less than 40% decrease from baseline; good = between 40% and 59% decrease from baseline; very good = 60% or more decrease from baseline. Shalaby 2016 also evaluated the participants’ satisfaction with both treatments (intra‐individual control) at the 10‐week follow‐up (regarding overall improvement, feasibility, and side effects) through a standardised satisfaction score (from 0 to 3, satisfied, best possible cosmetic result; Leheta 2013).
Two studies evaluated the improvement of disease damage through functional impairment of motor activity (Yan 2013; Zulian 2011). Yan 2013 assessed, after the 6‐month treatment, scores of joint function based on Kahan criteria (from 0 to 66, maximum impact; developed for SSc, assessing disease impact in 11 daily activities from 0, no difficulty, to 3, unable to perform; Guillevin 1983; Kahan 1989), and joint pain based on Traditional Chinese Medicine syndromes criteria (unclear total score). Zulian 2011 used the validated translated version of the Childhood Health Assessment Questionnaire (C‐HAQ) disability index, which presents a 3‐point scale (from 0, no difficulty, to 3, unable to perform) for 30 items evaluating eight areas of daily functioning (total score from 0 to 90; Ruperto 2001).
One study used the Physician Global Assessment of Damage (PGA‐D; from 0 = no damage, to 100 = marked damage), which is included in the LoSCAT, to evaluate individual lesions (Noakes 2018).
In case of missing data, we contacted the first author of the primary study to obtain all the necessary information. Some authors responded to our e‐mail contact (El‐Mofty 2004; Furuzawa‐Carballeda 2012; Kreuter 2006; Tang 2006), but not all could provide us with the missing data.
Funding sources
Two included studies received funding from universities (Azimi 2013; Shalaby 2016), four studies had government or association scholarships (Hunzelmann 1997; Tang 2006; Zulian 2011; Yan 2013), one study received medication from the pharmaceutical industry (Hulshof 2000), six studies had no funding (El‐Mofty 2004; Furuzawa‐Carballeda 2012; Kreuter 2006; Kroft 2009a; Noakes 2018; Sator 2009), and one study did not report this information (Batchelor 2008).
Excluded studies
After reading 24 articles and trial records in full text, we excluded five that were not RCTs: Bodemer 1999 investigated calcitriol for treating morphea in seven young participants; Didenko 1978 reported experiences with ultrasonics and lidase phonophoresis for treating various forms of scleroderma; Dortu 1974 reported experiences with the anti‐inflammatory agent Elarzone‐Dausse in disorders affecting the venous system; Hu 1996 investigated prostaglandin E1 and propylene glycol alginate sodium for treating urticarial vasculitis, which appears in the progression of SSc; and the abstract Smirnov 1998 reported a non‐randomised trial assessing Climen (combined estrogenic and gestagenic drug) for treating localised scleroderma in 17 postmenopausal women aged from 45 to 56, and 20 women in the control group.
We also excluded two trials that were not randomised, but we could only conclude that after reading the full‐text: Dytoc 2014 investigated imiquimod 5% cream for treating plaque morphea in 25 participants, and applied vehicle to a control plaque (intra‐individual control); and Wang 2008 assessed UVA‐1 phototherapy for treating four participants with morphea, 10 participants with scleroderma and four participants with sclerodermatous graft‐vs‐host disease, and also the pigmentation response of UVA‐1 phototherapy in eight healthy participants. We also identified a trial record of a randomised cross‐over study assessing the effectiveness of UVA1 phototherapy in the treatment of skin conditions with altered dermal matrix, including morphea (NCT00476801). However, we contacted the principal investigator and this was the trial register of the excluded study Wang 2008, which was not an RCT.
We list further details in the Characteristics of excluded studies tables.
Studies awaiting classification
We found two relevant trials records of studies evaluating the treatment of morphea (NCT00812188; NCT01799174). The studies have not been published yet and we include them as ‘studies awaiting classification’. NCT00812188 is an open‐label randomised trial of adults with plaque morphea comparing high‐dose UVA‐1 (120 J/cm²) three times a week for 12 weeks to one morphea plaque and fluocinonide 0.05% cream to another morphea plaque twice daily during the same period; or medium‐dose UVA‐1 (60 J/cm²) three times a week for 12 weeks to one morphea plaque and fluocinonide 0.05% cream to another morphea plaque twice daily during the same period. Authors described a 5‐year follow‐up but did not indicate any outcome measures. NCT01799174 is a double‐blind randomised trial of adults and children with active morphea (all types) comparing medium‐dose (70 J/cm²) phototherapy three times a week during 10 weeks versus "sham" UVA1 (0 J/cm²) phototherapy three times a week during 10 weeks. Authors described a 3‐year follow‐up and selected, as primary outcome measure, the Localized Scleroderma Severity Index (LoSSI), and as secondary measures, the physician's global assessment of disease activity (PGA‐A).
We list further details in the Characteristics of studies awaiting classification tables.
Risk of bias in included studies
We independently assessed the risk of bias in each included study. We represented each 'Risk of bias' item as percentages across all included studies in Figure 4, and each 'Risk of bias' item for each included study in Figure 5. We listed further details of the judgment of risk of bias in the Characteristics of included studies tables.
4.

Risk of bias graph: review authors' judgements about each 'Risk of bias' item represented as percentages across all included studies.
5.
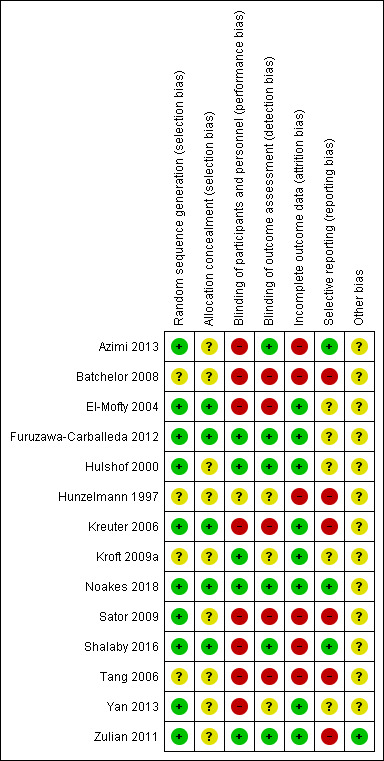
Risk of bias summary: review authors' judgements about each 'Risk of bias' item for each included study.
Allocation
Ten studies used adequate methods to generate the random sequence, producing comparable groups, thus we rated the majority of studies at low risk of bias for this domain (Figure 4; Figure 5). The methods included: randomisation lists (Azimi 2013; Hulshof 2000; Noakes 2018; Sator 2009; Zulian 2011), random number generation and block randomisation (Furuzawa‐Carballeda 2012), random number table (Yan 2013), shuffling envelopes (El‐Mofty 2004), and asking the participants to throw dice or pick a sealed envelope (Kreuter 2006 and Shalaby 2016, respectively). One of these studies informed us the randomisation method in a personal communication (El‐Mofty 2004). The other four studies did not describe the randomisation method in sufficient detail to assess whether the allocation produced comparable groups, thus presenting unclear risk of bias (Batchelor 2008; Hunzelmann 1997; Kroft 2009a; Tang 2006).
Nine studies provided insufficient information to judge if allocation was concealed (Azimi 2013; Batchelor 2008; Hulshof 2000; Hunzelmann 1997; Kroft 2009a; Sator 2009; Tang 2006; Yan 2013; Zulian 2011), thus we considered the majority of studies at unclear risk of bias for this domain (Figure 4; Figure 5). We rated the other five studies at low risk of bias: three studies used sealed envelopes (El‐Mofty 2004; Furuzawa‐Carballeda 2012; Shalaby 2016), one study used dice to assign participants (Kreuter 2006), and one intra‐individual study provided the list to only one investigator (Noakes 2018). Three of these studies informed us of the allocation concealment method in a personal communication (El‐Mofty 2004; Furuzawa‐Carballeda 2012; Noakes 2018).
Blinding
Eight studies reported no blinding of participants and personnel to the knowledge of which intervention a participant received, thus we considered the majority of studies at high risk of performance bias (Figure 4; Figure 5). Two studies had an untreated lesion as control (Batchelor 2008; Sator 2009), two studies performed different phototherapy interventions (El‐Mofty 2004; Kreuter 2006), and three studies performed heterogenous interventions (Shalaby 2016; Tang 2006; Yan 2013). One study did have identical capsules; however, the intervention group took one capsule once a week whilst the control group took a capsule twice a day, thus, presenting high risk of bias (Azimi 2013). We rated five studies who reported blinding of participants and personnel at low risk of performance bias: four studies used placebo (Furuzawa‐Carballeda 2012; Hulshof 2000; Kroft 2009a; Zulian 2011), and one study had the intervention drugs prepared as identical ointments (Noakes 2018). We considered one study, which reported a double‐blind method but provided insufficient information to judge if participants and personnel were blinded, at unclear risk of performance bias (Hunzelmann 1997).
We rated five studies which reported unblinded outcome assessment at high risk of detection bias (Batchelor 2008; El‐Mofty 2004; Kreuter 2006; Sator 2009; Tang 2006). One study attempted to blind outcome assessors, but could not maintain it because of the pigmentation induced by the PDT treatment (Batchelor 2008). We considered five studies which reported blinded outcome assessment at low risk of detection bias (Azimi 2013; Furuzawa‐Carballeda 2012; Hulshof 2000; Shalaby 2016; Zulian 2011). We rated the other three studies, which provided insufficient information to judge if the outcome assessors were effectively blinded from knowledge of which intervention a participant received, at unclear risk of detection bias (Hunzelmann 1997; Kroft 2009a; Yan 2013). Three studies informed us about blinding of outcome assessors in a personal communication (El‐Mofty 2004; Noakes 2018; Tang 2006).
Incomplete outcome data
We rated eight studies at low risk of attrition bias (Figure 4; Figure 5). Two studies had a null dropout rate (Kroft 2009a; Noakes 2018), three studies performed intention‐to‐treat (ITT) analysis (Furuzawa‐Carballeda 2012; Hulshof 2000; Zulian 2011), and three studies did not perform ITT analysis but the drop‐out rates probably do not represent serious threats to validity of the results (El‐Mofty 2004; Kreuter 2006; Yan 2013).
We considered the other six included studies at high risk of attrition bias (Figure 4; Figure 5). Five studies did not perform ITT analysis, analysing only participants who completed the treatment (Azimi 2013; Batchelor 2008; Sator 2009; Shalaby 2016; Tang 2006); and one study provided insufficient information to judge if the analysis included the participants who withdrew from the study (Hunzelmann 1997).
Selective reporting
We rated three studies which reported all outcomes pre‐specified in the protocol at low risk of reporting bias (Azimi 2013; Noakes 2018; Shalaby 2016). We considered six studies at high risk of reporting bias: Batchelor 2008 reported durometer readings data only for treated lesions; Kreuter 2006, Sator 2009 and Tang 2006 reported no standard deviation values; Zulian 2011 reported no numerical data for the C‐HAQ assessment; and Hunzelmann 1997 reported neither numerical data for outcomes nor information regarding the number of participants randomised and the number of participants in each group. We rated the other five studies at unclear risk of reporting bias (Figure 4; Figure 5), because authors did not register a protocol (El‐Mofty 2004; Furuzawa‐Carballeda 2012; Hulshof 2000; Kroft 2009a; Yan 2013).
Three studies provided us outcome data in a personal communication: Furuzawa‐Carballeda 2012 (adverse events data), Kreuter 2006 (length of the follow‐up after treatment), and Noakes 2018 (standard deviation values).
Other potential sources of bias
We considered the majority of studies at unclear risk of other potential sources of bias (Figure 4; Figure 5) because they either used global tools of assessment developed and validated for SSc (Azimi 2013; Hulshof 2000; Kreuter 2006), non‐validated global tools of assessment (El‐Mofty 2004; Yan 2013), or adapted clinical scores to assess individual lesions (Batchelor 2008; Furuzawa‐Carballeda 2012; Hunzelmann 1997; Kroft 2009a; Noakes 2018; Sator 2009; Shalaby 2016; Tang 2006). One study used a global tool of assessment which is not validated but considers the disease activity and also the disease progression in children at a growing stage; hence, it was considered at low risk of bias (Zulian 2011).
All studies reported no significant differences in baseline measurements (Azimi 2013; Batchelor 2008; El‐Mofty 2004; Furuzawa‐Carballeda 2012; Hulshof 2000; Hunzelmann 1997; Kreuter 2006; Kroft 2009a; Noakes 2018; Sator 2009; Shalaby 2016; Tang 2006; Yan 2013; Zulian 2011). Two studies provided us this data in a personal communication (Furuzawa‐Carballeda 2012; Noakes 2018).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Oral methotrexate and oral prednisone compared to placebo and oral prednisone for juvenile morphea.
| Oral methotrexate and oral prednisone compared to placebo and oral prednisone for juvenile morphea | ||||||
| Patient or population: children and adolescents with active morphea (linear scleroderma, generalised morphea and mixed subtype: linear and circumscribed). Setting: paediatric rheumatology and dermatology centres in Italy. Intervention: oral methotrexate (15 mg/m², maximum 20 mg a week for 12 months or until flare of the disease) plus folic acid supplementation (2.5 mg, 48 hours after MTX) and oral prednisone (1 mg/kg a day, maximum 50 mg, in a single morning dose for 3 months plus 1 month with gradually decreased dose until discontinuation) Comparison: placebo plus folic acid supplementation (2.5 mg, 48 hours after placebo) and oral prednisone (1 mg/kg a day, maximum 50 mg, in a single morning dose for 3 months plus 1 month with gradually decreased dose until discontinuation) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo plus folic acid supplementation and oral prednisone | Risk with oral methotrexate plus folic acid supplementation and oral prednisone | |||||
| Primary outcome: Global improvement of disease activity or damage assessed by a medical practitioner or by participants Assessed with: rate of response to treatment, based on participants who met all 3 response criteria Follow‐up: 12 months or until flare of the disease | Children and adolescents with morphea | RR 2.31 (1.20 to 4.45) | 70 (1 RCT) | ⊕⊕⊝⊝ Low a | ||
| 292 per 1000 | 674 per 1000 (350 to 1000) | |||||
| Primary outcome: Adverse effects Assessed with: number of participants with at least 1 adverse event Follow‐up: 12 months or until flare of the disease | Children and adolescents with morphea | RR 1.23 (0.75 to 2.04) | 70 (1 RCT) | ⊕⊝⊝⊝ Low b | ||
| 458 per 1000 | 564 per 1000 (344 to 935) | |||||
| Secondary outcome: Improvement of disease activity (reduction in lesion size) Assessed with: Skin Score Rate (SSR) scale from: ≤1, decreased extension of the lesion, to >1, increased extension of the lesion. Follow‐up: 12 months or until flare of the disease | The mean SSR was 1.1 | MD 0.31 lower (0.35 lower to 0.27 lower) | ‐ | 70 (1 RCT) | ⊕⊕⊝⊝ Lowc | |
| Secondary outcome: Improvement of disease damage | See comment | ‐ | 70 (1 RCT) | ‐ | Authors reported no significant differences between groups in the Childhood Health Assessment Questionnaire (C‐HAQ) disability index, but reported no numerical data. | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded by 2 levels to low quality evidence. 1 level due to unclear risk of selection bias (allocation concealment) and high risk of reporting bias (selective reporting). Downgraded by 1 level due to small number of events (less than 300 events).
bDowngraded by 2 levels to low quality evidence. 1 level due to unclear risk of selection bias (allocation concealment) and high risk of reporting bias (selective reporting). Downgraded by 1 level due to small number of events (less than 300 events) and wide confidence interval (includes both null effect and appreciable harm).
cDowngraded by 2 levels to low quality evidence. 1 level due to unclear risk of selection bias (allocation concealment) and high risk of reporting bias (selective reporting). Downgraded by 1 level due to small number of participants (less than 400 participants).
Summary of findings 2. Medium‐dose UVA‐1 phototherapy (50 J/cm²) compared to low‐dose UVA‐1 phototherapy (20 J/cm²) for morphea.
| Medium‐dose UVA‐1 phototherapy (50 J/cm²) compared to low‐dose UVA‐1 phototherapy (20 J/cm²) for morphea | ||||||
| Patient or population: children and adults with active morphea (circumscribed morphea, linear scleroderma (with trunk/limb variant and head variant), generalised morphea, or mixed morphea). Setting: university hospital in Germany. Intervention: medium‐dose UVA‐1 phototherapy (50 J/cm²) 5 times a week for a total of 40 treatment sessions. Comparison: low‐dose UVA‐1 phototherapy (20 J/cm²) 5 times a week for a total of 40 treatment sessions. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with low‐dose UVA‐1 phototherapy (20 J/cm²) | Risk with Medium‐dose UVA‐1 phototherapy (50 J/cm²) | |||||
| Primary outcome: Global improvement of disease activity or damage assessed by a medical practitioner or by participants Assessed with: Modified Skin Score (MSS) Scale from: 0, no affected skin, to 42, extreme involvement in all areas Follow up: 8 weeks | The mean score (MSS) was 5 | MD 1.60 more (1.70 fewer to 4.90 more) | ‐ | 44 (1 RCT) | ⊕⊝⊝⊝ Low a | |
| Primary outcome: Adverse effects Assessed with: number of participants who had mild tanning Follow up: 8 weeks | Individuals with morphea | RR 1.00 (0.91 to 1.10) | 44 (1 RCT) | ⊕⊝⊝⊝ Low b | ||
| 1000 per 1000 | 1000 per 1000 (910 to 1000) | |||||
| Secondary outcome: Improvement of disease activity (skin softening) Assessed with: dermal density with a digital 20‐MHz ultrasound scanner (lower values indicate improvement of disease activity). Follow up: 8 weeks | The mean ultrasound score was 69 | MD 16.43 lower (34.87 lower to 2.01 higher) | ‐ | 36 (1 RCT) | ⊕⊝⊝⊝ Low c | |
| Secondary outcome: Improvement of disease damage ‒ not measured | See comment | ‐ | ‐ | ‐ | There was no measure of this outcome for this comparison. | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded by 2 levels to low quality evidence. 1 level due to high risk of performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment) and reporting bias (selective reporting). Downgraded by 1 level due to small number of participants (less than 400 participants).
bDowngraded by 2 levels to low quality evidence. 1 level due to high risk of performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment) and reporting bias (selective reporting). Downgraded by 1 level due to small number of events (less than 300 events).
cDowngraded by 2 levels to low quality evidence. 1 level due to high risk of performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment) and reporting bias (selective reporting). Downgraded by 1 level due to small number of participants (less than 400 participants) and wide confidence interval (includes both null effect and appreciable benefit).
Summary of findings 3. Narrowband UVB phototherapy compared to medium‐dose UVA‐1 phototherapy (50 J/cm²) for morphea.
| Narrowband UVB phototherapy compared to medium‐dose UVA‐1 phototherapy (50 J/cm2) for morphea | ||||||
| Patient or population: children and adults with active morphea (circumscribed morphea, linear scleroderma (with trunk/limb variant and head variant), generalised morphea, or mixed morphea). Setting: university hospital in Germany. Intervention: narrowband UVB phototherapy 5 times a week for a total of 40 treatment sessions. Comparison: medium‐dose UVA‐1 phototherapy (50 J/cm2) 5 times a week for a total of 40 treatment sessions. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with medium‐dose UVA‐1 phototherapy (50 J/cm2) | Risk with Narrowband UVB phototherapy | |||||
| Primary outcome: Global improvement of disease activity or damage assessed by a medical practitioner or by participants Assessed with: Modified Skin Score (MSS) Scale from: 0, no affected skin, to 42, extreme involvement in all areas Follow up: 8 weeks | The mean score (MSS) was 6.6 | MD 1.70 lower (5.27 lower to 1.87 higher) | ‐ | 35 (1 RCT) | ⊕⊝⊝⊝ Low a | |
| Primary outcome: Adverse effects Assessed with: number of participants who had mild tanning Follow up: 8 weeks | Individuals with morphea | RR 0.03 (0.00 to 0.42) | 35 (1 RCT) | ⊕⊝⊝⊝ Low b | ||
| 1000 per 1000 | 30 per 1000 (0 to 420) | |||||
| Secondary outcome: Improvement of disease activity (skin softening) Assessed with: dermal density with a digital 20‐MHz ultrasound scanner (lower values indicate improvement of disease activity) Follow up: 8 weeks | The mean ultrasound score was 52.57 | MD 17.78 higher (6.08 lower to 41.64 higher) | ‐ | 28 (1 RCT) | ⊕⊝⊝⊝ Low c | |
| Secondary outcome: Improvement of disease damage ‐ not measured | See comment | ‐ | ‐ | ‐ | There was no measure of this outcome for this comparison. | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded by 2 levels to low quality evidence. 1 level due to high risk of performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment) and reporting bias (selective reporting). Downgraded by 1 level due to small number of participants (less than 400 participants).
bDowngraded by 2 levels to low quality evidence. 1 level due to high risk of performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment) and reporting bias (selective reporting). Downgraded by 1 level due to small number of events (less than 300 events).
cDowngraded by 2 levels to low quality evidence. 1 level due to high risk of performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment) and reporting bias (selective reporting). Downgraded by 1 level due to small number of participants (less than 400 participants) and wide confidence interval (includes both null effect and appreciable benefit).
Summary of findings 4. Narrowband UVB phototherapy compared to low‐dose UVA‐1 phototherapy (20 J/cm²) for morphea.
| Narrowband UVB compared to low‐dose UVA‐1 phototherapy (20 J/cm2) for morphea | ||||||
| Patient or population: children and adults with active morphea (circumscribed morphea, linear scleroderma (with trunk/limb variant and head variant), generalised morphea, or mixed morphea). Setting: university hospital in Germany. Intervention: narrowband UVB phototherapy 5 times a week for a total of 40 treatment sessions. Comparison: low‐dose UVA‐1 phototherapy (20 J/cm2) 5 times a week for a total of 40 treatment sessions. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with low‐dose UVA‐1 phototherapy (20 J/cm2) | Risk with Narrowband UVB | |||||
| Primary outcome: Global improvement of disease activity or damage assessed by a medical practitioner or by participants Assessed with: Modified Skin Score (MSS) Scale from: 0, no affected skin, to 42, extreme involvement in all areas Follow up: 8 weeks | The mean score (MSS) was 5 | MD 0.10 lower (2.49 lower to 2.29 higher) | ‐ | 45 (1 RCT) | ⊕⊝⊝⊝ Low a | |
| Primary outcome: Adverse effects Assessed with: number of participants who had mild tanning Follow up: 8 weeks | Individuals with morphea | RR 0.03 (0.00 to 0.41) | 45 (1 RCT) | ⊕⊝⊝⊝ Low b |
||
| 1000 per 1000 | 30 per 1000 (0 to 410) | |||||
| Secondary outcome: Improvement of disease activity (skin softening) Assessed with: dermal density with a digital 20‐MHz ultrasound scanner (lower values indicate improvement of disease activity) Follow up: 8 weeks | The mean ultrasound score was 69 | MD 1.35 higher (19.39 lower to 22.09 higher) | ‐ | 32 (1 RCT) | ⊕⊝⊝⊝ Very low c | |
| Secondary outcome: Improvement of disease damage ‐ not measured | See comment | ‐ | ‐ | ‐ | There was no measure of this outcome for this comparison. | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded by 2 levels to low quality evidence. 1 level due to high risk of performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment) and reporting bias (selective reporting). Downgraded by 1 level due to small number of participants (less than 400 participants).
bDowngraded by 2 levels to low quality evidence. 1 level due to high risk of performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment) and reporting bias (selective reporting). Downgraded by 1 level due to small number of events (less than 300 events).
cDowngraded by 3 levels to very low quality evidence. 1 level due to high risk of performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment) and reporting bias (selective reporting). Downgraded by 2 levels due to small number of participants (less than 400 participants) and wide confidence interval (includes both null effect and appreciable benefit).
We assessed the outcomes prespecified in our protocol to determine which treatments for morphea lead to the best outcomes up to six months and from six to 12 months. We followed the GRADE criteria to assess the certainty of the body of evidence for each outcome.
This review includes 20 pair‐wise comparisons. The comparisons of the studies were heterogenous, and we could not pool data in a meta‐analysis.
We summarise the outcomes for the most important comparisons in the Table 1, Table 2, Table 3, and Table 4
1. Medium‐dose UVA‐1 (50 J/cm²) versus low‐dose UVA‐1 (20 J/cm²) phototherapy
See Table 2.
Participants: children and adults with active morphea (circumscribed morphea, linear scleroderma (with trunk/limb variant and head variant), generalised morphea, or mixed morphea).
Primary outcome 1: Global improvement of disease activity or damage assessed by a medical practitioner or by participants
The modified skin score (MSS; from 0 to 42) evaluates skin thickness and pliability (from 0, normal skin, to 3, unable to pinch or move skin) plus involved area (from 0, no involvement, to 3 = more than 67% involvement) in seven body regions. A decrease in the MSS indicates global improvement of disease activity. The medium‐dose UVA‐1 (50 J/cm²) phototherapy group had a higher MSS score than the low‐dose UVA‐1 (20 J/cm²) group after the eight‐week treatment (MD 1.60, 95% CI −1.70 to 4.90; 44 participants; low‐certainty evidence; Analysis 1.1), and at the 3‐month follow‐up after treatment (MD 2.50, 95% CI −1.90 to 6.90; 44 participants; low‐certainty evidence; Analysis 1.2) (Kreuter 2006). However, the confidence intervals include the null effect and appreciable benefit.
1.1. Analysis.

Comparison 1 Medium‐dose UVA‐1 (50 J/cm²) x Low‐dose UVA‐1 (20 J/cm²) phototherapy, Outcome 1 Modified Skin Score (MSS) at the end of the eight‐week treatment.
1.2. Analysis.

Comparison 1 Medium‐dose UVA‐1 (50 J/cm²) x Low‐dose UVA‐1 (20 J/cm²) phototherapy, Outcome 2 Modified Skin Score (MSS) at last follow‐up visit (week 20).
Primary outcome 2: Adverse effects of the interventions, including local and systemic reactions
All 44 participants treated with UVA‐1 had mild tanning after the eight‐week treatment (RR 1.00, 95% CI 0.91 to 1.10; low‐certainty evidence; Analysis 1.3) (Kreuter 2006).
1.3. Analysis.

Comparison 1 Medium‐dose UVA‐1 (50 J/cm²) x Low‐dose UVA‐1 (20 J/cm²) phototherapy, Outcome 3 Number of participants with mild tanning.
Secondary outcome 1: Improvement of disease activity
Only 36 of the 44 participants completed the 20 MHz ultrasound examinations, for which lower values indicate improvement of disease activity (Kreuter 2006). The skin density of the morphea lesions after the eight‐week treatment was lower in the medium‐dose UVA‐1 phototherapy (50 J/cm²) group than in the low‐dose UVA‐1 (20 J/cm²) group (MD −16.43 µm, 95% CI −34.87 to 2.01; low‐certainty evidence; Analysis 1.4). However, the corium thickness of the morphea lesions after the eight‐week treatment was higher in the medium‐dose UVA‐1 (50 J/cm²) group (MD 196.29 µm, 95% CI −162.28 to 554.86; very low certainty evidence; Analysis 1.5). Nevertheless, we are uncertain of these results due to the wide confidence intervals, which include the null effect and appreciable benefit and harm.
1.4. Analysis.

Comparison 1 Medium‐dose UVA‐1 (50 J/cm²) x Low‐dose UVA‐1 (20 J/cm²) phototherapy, Outcome 4 Ultrasound dermal density at the end of the eight‐week treatment.
1.5. Analysis.
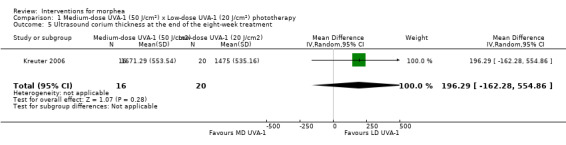
Comparison 1 Medium‐dose UVA‐1 (50 J/cm²) x Low‐dose UVA‐1 (20 J/cm²) phototherapy, Outcome 5 Ultrasound corium thickness at the end of the eight‐week treatment.
Authors reported that both groups had a significant improvement in the VAS of the participants’ estimate of skin tightness on the morphea lesions (from 0 to 10, maximum) after the eight‐week treatment, decreasing from 6.1 to 4.3 (P < 0.05) in the medium‐dose UVA‐1 phototherapy group; and from 4.4 to 2.7 (P = 0.05) in the low‐dose UVA‐1 phototherapy group. Only the medium‐dose UVA‐1 phototherapy group had a significant improvement in the VAS of the participants’ estimate of pruritus, decreasing from 4.0 to 2.4 (P < 0.05) in the medium‐dose UVA‐1 phototherapy group; and from 2.8 to 2.1 (P = 0.15) in the low‐dose UVA‐1 phototherapy group. However, authors reported mean values without standard deviations (Kreuter 2006).
Secondary outcome 2: Improvement of disease damage
Not measured.
2. Narrowband UVB versus MD UVA‐1 (50 J/cm²) phototherapy
See Table 3.
Participants: children and adults with active morphea (circumscribed morphea, linear scleroderma (with trunk/limb variant and head variant), generalised morphea, or mixed morphea).
Primary outcome 1: Global improvement of disease activity or damage assessed by a medical practitioner or by participants
The narrowband UVB phototherapy group had a lower MSS score (where high values represent a worse outcome) than the medium‐dose UVA‐1 (50 J/cm²) group after the eight‐week treatment (MD −1.70, 95% CI −5.27 to 1.87; 35 participants; low‐certainty evidence; Analysis 2.1), and at the 3‐month follow‐up after treatment (MD −2.10, 95% CI −6.73 to 2.53; 35 participants; low‐certainty evidence; Analysis 2.2) (Kreuter 2006). However, the confidence intervals include the null effect and appreciable harm.
2.1. Analysis.

Comparison 2 Narrowband UVB x Medium‐dose UVA‐1 (50 J/cm²) phototherapy, Outcome 1 Modified Skin Score (MSS) at the end of the eight‐week treatment.
2.2. Analysis.

Comparison 2 Narrowband UVB x Medium‐dose UVA‐1 (50 J/cm²) phototherapy, Outcome 2 Modified Skin Score (MSS) at last follow‐up visit (week 20).
Primary outcome 2: Adverse effects of the interventions, including local and systemic reactions
All 17 participants treated with medium‐dose UVA‐1 (50 J/cm²) phototherapy had mild tanning after the eight‐week treatment, whilst no participant from the narrowband UVB group (18 participants) had this adverse event (RR 0.03, 95% CI 0.00 to 0.42; low‐certainty evidence; Analysis 2.3) (Kreuter 2006). Three participants treated with narrowband UVB phototherapy had transient erythema whilst no participant from the medium‐dose UVA‐1 group had this adverse event (RR 6.63, 95% CI 0.37 to 119.59; low‐certainty evidence; Analysis 2.4). However, we are uncertain of this result due to the wide confidence intervals, which includes the null effect and appreciable benefit.
2.3. Analysis.

Comparison 2 Narrowband UVB x Medium‐dose UVA‐1 (50 J/cm²) phototherapy, Outcome 3 Number of participants with mild tanning.
2.4. Analysis.

Comparison 2 Narrowband UVB x Medium‐dose UVA‐1 (50 J/cm²) phototherapy, Outcome 4 Number of participants with transient erythema.
Secondary outcome 1: Improvement of disease activity
Only 28 of the 35 participants completed the 20 MHz ultrasound examinations, for which lower values indicate improvement of disease activity (Kreuter 2006). The skin density of the morphea lesions after the eight‐week treatment was higher in the narrowband UVB phototherapy group than in the medium‐dose UVA‐1 (50 J/cm²) group (MD 17.78 µm, 95% CI −6.08 to 41.64; low‐certainty evidence; Analysis 2.5). However, the corium thickness of the morphea lesions after the eight‐week treatment was lower in the narrowband UVB group (MD −78.35 µm, 95% CI −528.59 to 371.89; very low certainty evidence; Analysis 2.6). Nevertheless, we are uncertain of these results due to the wide confidence intervals, which include the null effect and appreciable benefit and harm.
2.5. Analysis.

Comparison 2 Narrowband UVB x Medium‐dose UVA‐1 (50 J/cm²) phototherapy, Outcome 5 Ultrasound dermal density at the end of the eight‐week treatment.
2.6. Analysis.

Comparison 2 Narrowband UVB x Medium‐dose UVA‐1 (50 J/cm²) phototherapy, Outcome 6 Ultrasound corium thickness at the end of the eight‐week treatment.
Authors reported that both groups had a significant improvement in the VAS of the participants’ estimate of skin tightness on the morphea lesions (from 0 to 10, maximum) after the eight‐week treatment, decreasing: from 3.3 to 2.8 (P < 0.05) in the narrowband UVB group; and 6.1 to 4.3 (P < 0.05) in the medium‐dose UVA‐1 phototherapy group. Only the medium‐dose UVA‐1 phototherapy group had a significant improvement in the VAS of the participants’ estimate of pruritus, decreasing from 4.0 to 2.4 (P < 0.05) in the medium‐dose UVA‐1 phototherapy group; and from 2.3 to 1.8 (P = 0.16) in the narrowband UVB group. However, authors reported mean values without standard deviations (Kreuter 2006).
Secondary outcome 2: Improvement of disease damage
Not measured.
3. Narrowband UVB versus LD UVA‐1 phototherapy (20 J/cm²)
See Table 4.
Participants: children and adults with active morphea (circumscribed morphea, linear scleroderma (with trunk/limb variant and head variant), generalised morphea, or mixed morphea).
Primary outcome 1: Global improvement of disease activity or damage assessed by a medical practitioner or by participants
The narrowband UVB phototherapy group had a lower MSS score (where high values represent a worse outcome) than the low‐dose UVA‐1 (20 J/cm²) group after the eight‐week treatment (MD −0.10, 95% CI −2.49 to 2.29; 45 participants; low‐certainty evidence; Analysis 3.1) ( Kreuter 2006). However, the narrowband UVB phototherapy group had a higher MSS score at the 3‐month follow‐up after treatment (MD 0.40, 95% CI −2.17 to 2.97; low‐certainty evidence; Analysis 3.2). Nevertheless, the confidence intervals include the null effect and appreciable benefit and harm.
3.1. Analysis.

Comparison 3 Narrowband UVB X Low‐dose UVA‐1 (20 J/cm²) phototherapy, Outcome 1 Modified Skin Score (MSS) at the end of the eight‐week treatment.
3.2. Analysis.

Comparison 3 Narrowband UVB X Low‐dose UVA‐1 (20 J/cm²) phototherapy, Outcome 2 Modified Skin Score (MSS) at last follow‐up visit (week 20).
Primary outcome 2: Adverse effects of the interventions, including local and systemic reactions
All 27 participants treated with low‐dose UVA‐1 had mild tanning after the eight‐week treatment, whilst no participant from the narrowband UVB group (18 participants) had this adverse event (RR 0.03, 95% CI 0.00 to 0.41; low‐certainty evidence; Analysis 3.3) (Kreuter 2006). Three participants treated with narrowband UVB phototherapy had transient erythema whilst no participant from the low‐dose UVA‐1 group had this adverse event (RR 10.32, 95% CI 0.56 to 188.49; low‐certainty evidence; Analysis 3.4). However, we are uncertain of this result due to the wide confidence interval, which includes the null effect.
3.3. Analysis.

Comparison 3 Narrowband UVB X Low‐dose UVA‐1 (20 J/cm²) phototherapy, Outcome 3 Number of participants with mild tanning.
3.4. Analysis.

Comparison 3 Narrowband UVB X Low‐dose UVA‐1 (20 J/cm²) phototherapy, Outcome 4 Number of participants with transient erythema.
Secondary outcome 1: Improvement of disease activity
Only 32 of the 45 participants completed the 20 MHz ultrasound examinations, for which lower values indicate improvement of disease activity (Kreuter 2006). The skin density of the morphea lesions after the eight‐week treatment was higher in the narrowband UVB phototherapy group than in the low‐dose UVA‐1 (20 J/cm²) (MD 1.35 µm, 95% CI −19.39 to 22.09; very low certainty evidence; Analysis 3.5). The corium thickness of the morphea lesions after the eight‐week treatment was also higher in the narrowband UVB phototherapy group (MD 117.94 µm, 95% CI −311.20 to 547.08; very low certainty evidence; Analysis 3.6). However, we are uncertain of these results due to the wide confidence intervals, which include the null effect and appreciable benefit and harm.
3.5. Analysis.

Comparison 3 Narrowband UVB X Low‐dose UVA‐1 (20 J/cm²) phototherapy, Outcome 5 Ultrasound dermal density at the end of the eight‐week treatment.
3.6. Analysis.

Comparison 3 Narrowband UVB X Low‐dose UVA‐1 (20 J/cm²) phototherapy, Outcome 6 Ultrasound corium thickness at the end of the eight‐week treatment.
Authors reported that both groups had a significant improvement in the VAS of the participants’ estimate of skin tightness on the morphea lesions (from 0 to 10, maximum) after the eight‐week treatment, decreasing from 3.3 to 2.8 (P < 0.05) in the narrowband UVB group; and 4.4 to 2.7 (P = 0.05) in the medium‐dose UVA‐1 phototherapy group. Authors reported no changes in the VAS of the participants’ estimate of pruritus, decreasing: from 2.3 to 1.8 (P = 0.16) in the narrowband UVB group; and from 2.8 to 2.1 (P = 0.15) in the low‐dose UVA‐1 phototherapy group. However, authors reported mean values without standard deviation (Kreuter 2006).
Secondary outcome 2: Improvement of disease damage
Not measured.
4. Medium‐dose (70 J/cm²) UVA‐1 phototherapy versus no treatment
Participants: children and adults with active circumscribed morphea.
Primary outcome 1: Global improvement of disease activity or damage assessed by a medical practitioner or by participants
Not measured.
Primary outcome 2: Adverse effects of the interventions, including local and systemic reactions
In the intra‐individual study comparing medium‐dose (70 J/cm²) UVA‐1 versus no treatment, all 14 participants had tanning, except on the plaque shielded from irradiation (RR 29.00, 95% CI 1.90 to 443.28; very low certainty evidence; Analysis 4.1) (Sator 2009). Two participants reported painless UVA‐1 erythema (RR 4.41, 95% CI 0.23 to 84.79; very low certainty evidence; Analysis 4.2); and two other participants reported pruritus during the first week of treatment (RR 4.41, 95% CI 0.23 to 84.79; very low certainty evidence; Analysis 4.3).
4.1. Analysis.

Comparison 4 Medium‐dose (70 J/cm²) or Low‐dose (20 J/cm²) UVA‐1 phototherapy x No treatment, Outcome 1 Number of plaques with moderate to significant tanning.
4.2. Analysis.

Comparison 4 Medium‐dose (70 J/cm²) or Low‐dose (20 J/cm²) UVA‐1 phototherapy x No treatment, Outcome 2 Number of plaques with painless erythema.
4.3. Analysis.

Comparison 4 Medium‐dose (70 J/cm²) or Low‐dose (20 J/cm²) UVA‐1 phototherapy x No treatment, Outcome 3 Number of plaques with pruritus.
Secondary outcome 1: Improvement of disease activity
Sator 2009 reported a significantly greater reduction of skin thickness (measured through 20 MHz ultrasound, where high values represent a worse outcome) of lesions treated with medium‐dose (70 J/cm²) UVA‐1 than untreated lesions after treatment and at the 3‐month, 6‐month and 12‐month follow‐up after treatment. However, authors reported median values without standard deviations and did not use the paired t‐test to compare groups. The lesions treated with medium‐dose (70 J/cm²) UVA‐1 reported a median skin thickness of 2.32 mm (1.92 to 2.55) at baseline, which reduced: −0.27 mm (−0.5 to −0.2) immediately after UVA‐1; −0.50 mm (−0.6 to −0.3) three months after UVA‐1; −0.53 mm (−0.7 to −0.4) six months after UVA‐1; and −0.64 mm (−0.8 to −0.5) one year after UVA‐1. The untreated lesions had a median skin thickness of 1.82 mm (1.49 to 2.38) at baseline, which reduced: −0.09 mm (−0.2 to −0.1) immediately after UVA‐1; −0.19 mm (−0.2 to −0.1) three months after UVA‐1; −0.21 mm (−0.3 to −0.1) six months after UVA‐1; and −0.225 mm (−0.7 to −0.1) one year after UVA‐1.
Sator 2009 reported that the lesions irradiated with medium‐dose (70 J/cm²) UVA‐1 phototherapy had a decreased median skin score (from 0 to 7: atrophy rated as 0 = absent or 1 = present, and intensity of erythema and induration, each from 0 to 3, maximum) during the study period compared to the untreated lesions. However, authors reported median values without standard deviation and did not use the paired t‐test to compare groups. The lesions treated with medium‐dose (70 J/cm²) UVA‐1 phototherapy had a median skin score of 4.1 at baseline; 2.1 immediately after UVA‐1; 1.9 three months after UVA‐1; 1.6 six months after UVA‐1; and 1.1 one year after UVA‐1. The untreated lesions had a median skin score of 3.4 at baseline; 3.2 immediately after UVA‐1; 3.1 three months after UVA‐1; 2.9 six months after UVA‐1; and 2.8 one year after UVA‐1.
Secondary outcome 2: Improvement of disease damage
Not measured.
5. Medium‐dose (70 J/cm²) UVA‐1 versus low‐dose (20 J/cm²) UVA‐1 phototherapy
Participants: children and adults with active circumscribed morphea.
Primary outcome 1: Global improvement of disease activity or damage assessed by a medical practitioner or by participants
Not measured.
Primary outcome 2: Adverse effects of the interventions, including local and systemic reactions
In the intra‐individual study comparing medium‐dose (70 J/cm²) UVA‐1 versus low‐dose (20 J/cm²) UVA‐1 phototherapy, all 14 participants had tanning (Sator 2009). Two participants reported painless UVA‐1 erythema and two other participants reported pruritus during the first week of treatment. However, authors did not present separate adverse events data for lesions treated with medium‐dose (70 J/cm²) and low‐dose (20 J/cm²) UVA‐1 phototherapy.
Secondary outcome 1: Improvement of disease activity
Sator 2009 reported a significantly greater reduction of skin thickness (measured through 20 MHz ultrasound, where high values represent a worse outcome) of lesions treated with medium‐dose (70 J/cm²) UVA‐1 than lesions treated with low‐dose (20 J/cm²) UVA‐1 at the 3‐month, 6‐month and 12‐month follow‐up after treatment. However, authors reported median values without standard deviation and did not use the paired t‐test to compare groups. The lesions treated with medium‐dose (70 J/cm²) UVA‐1 had a median skin thickness of 2.32 mm (1.92 to 2.55) at baseline, which reduced: −0.27 mm (−0.5 to −0.2) immediately after UVA‐1; −0.50 mm (−0.6 to −0.3) three months after UVA‐1; −0.53 mm (−0.7 to −0.4) six months after UVA‐1; and −0.64 mm (−0.8 to −0.5) one year after UVA‐1. The lesions treated with low‐dose (20 J/cm²) UVA‐1 had a median skin thickness of 1.71 mm (1.44 to 2.14) at baseline, which reduced: −0.195 mm (−0.2 to −0.1) immediately after UVA‐1; −0.28 mm (−0.4 to −0.2) three months after UVA‐1; −0.30 mm (−0.4 to −0.2) six months after UVA‐1; and −0.385 mm (−0.5 to −0.2) one year after UVA‐1.
Sator 2009 reported that both lesions irradiated with medium‐dose (70 J/cm²) and low‐dose (20 J/cm²) UVA‐1 phototherapy had a decreased median skin score (where high values represent a worse outcome) during the study period, and found no significant differences between the two dose regimens. However, authors reported median values without standard deviation and did not use the paired t‐test to compare groups. The lesions treated with medium‐dose (70 J/cm²) UVA‐1 phototherapy had a median skin score of: 4.1 at baseline; 2.1 immediately after UVA‐1; 1.9 three months after UVA‐1; 1.6 six months after UVA‐1; and 1.1 one year after UVA‐1. The lesions treated with low‐dose (20 J/cm²) UVA‐1 phototherapy had a median skin score of: 3.4 at baseline; 1.8 immediately after UVA‐1; 1.4 three months after UVA‐1; 1.2 six months after UVA‐1; and 1.0 one year after UVA‐1.
Secondary outcome 2: Improvement of disease damage
Not measured.
6. Low‐dose (20 J/cm²) UVA‐1 phototherapy versus no treatment
Participants: children and adults with active circumscribed morphea.
Primary outcome 1: Global improvement of disease activity or damage assessed by a medical practitioner or by participants
Not measured.
Primary outcome 2: Adverse effects of the interventions, including local and systemic reactions
Authors from the intra‐individual study comparing low‐dose (20 J/cm²) UVA‐1 versus no treatment in a plaque shielded from irradiation (14 participants) did not present separate adverse events data for lesions treated with medium‐dose (70 J/cm²) and low‐dose (20 J/cm²) UVA‐1 phototherapy (Sator 2009). Thus, we accounted the same adverse data when comparing the two active intervention groups with the control. All participants had tanning (RR 29.00, 95% CI 1.90 to 443.28; very low certainty evidence; Analysis 4.1). Two participants reported painless UVA‐1 erythema (RR 4.41, 95% CI 0.23 to 84.79; very low certainty evidence; Analysis 4.2); and two other participants reported pruritus during the first week of treatment (RR 4.41, 95% CI 0.23 to 84.79; very low certainty evidence; Analysis 4.3).
Secondary outcome 1: Improvement of disease activity
Sator 2009 reported a significantly bigger reduction of skin thickness (measured through 20 MHz ultrasound, where high values represent a worse outcome) of lesions treated with low‐dose (20 J/cm²) UVA‐1 than untreated lesions at the 6‐month follow‐up after treatment. However, authors reported median values without standard deviation and did not use the paired t‐test to compare groups. The lesions treated with low‐dose (20 J/cm²) UVA‐1 had a median skin thickness of 1.71 mm (1.44 to 2.14) at baseline, which reduced: −0.195 mm (−0.2 to −0.1) immediately after UVA‐1; −0.28 mm (−0.4 to −0.2) three months after UVA‐1; −0.30 mm (−0.4 to −0.2) six months after UVA‐1; and −0.385 mm (−0.5 to −0.2) one year after UVA‐1. The untreated lesions had a median skin thickness of 1.82 mm (1.49 to 2.38) at baseline, which reduced: −0.09 mm (−0.2 to −0.1) immediately after UVA‐1; −0.19 mm (−0.2 to −0.1) three months after UVA‐1; −0.21 mm (−0.3 to −0.1) six months after UVA‐1; and −0.225 mm (−0.7 to −0.1) one year after UVA‐1.
Sator 2009 reported that the lesions irradiated with medium‐dose (70 J/cm²) UVA‐1 phototherapy had a decreased median skin score (where high values represent a worse outcome) during the study period compared to the untreated lesions. However, authors reported median values without standard deviation and did not use the paired t‐test to compare groups. The lesions treated with low‐dose (20 J/cm²) UVA‐1 phototherapy had a median skin score of: 3.4 at baseline; 1.8 immediately after UVA‐1; 1.4 three months after UVA‐1; 1.2 six months after UVA‐1; and 1.0 one year after UVA‐1. The untreated lesions had a median skin score of: 3.4 at baseline; 3.2 immediately after UVA‐1; 3.1 three months after UVA‐1; 2.9 six months after UVA‐1; and 2.8 one year after UVA‐1.
Secondary outcome 2: Improvement of disease damage
Not measured.
7. 20 J/cm² versus 10 J/cm² UVA phototherapy
Participants: children and adults with circumscribed morphea, linear scleroderma or generalised morphea.
Primary outcome 1: Global improvement of disease activity or damage assessed by a medical practitioner or by participants
Not measured.
Primary outcome 2: Adverse effects of the interventions, including local and systemic reactions
All 47 participants treated with low‐dose (20 J/cm² or 10 J/cm²) UVA phototherapy had generalised tanning (RR 1.00, 95% CI 0.92 to 1.09; low‐certainty evidence; Analysis 5.1) (El‐Mofty 2004).
5.1. Analysis.

Comparison 5 20 J/cm2 UVA x 10 J/cm² UVA phototherapy, Outcome 1 Number of participants with generalised tanning.
In addition, three participants from each group reported temporary pruritus (RR 0.81, 95% CI 0.18 to 3.60; low‐certainty evidence; Analysis 5.2), and one of 26 participants in the 20 J/cm² group had increased erythema and exacerbated pain (RR 2.44, 95% CI 0.10 to 57.08; very low certainty evidence; Analysis 5.3). However, the wide confidence intervals include the null effect and appreciable benefit.
5.2. Analysis.

Comparison 5 20 J/cm2 UVA x 10 J/cm² UVA phototherapy, Outcome 2 Number of participants with temporary pruritus.
5.3. Analysis.
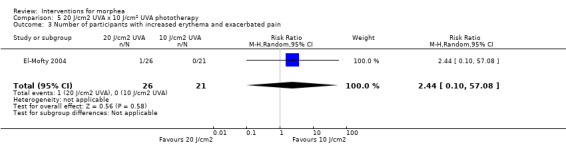
Comparison 5 20 J/cm2 UVA x 10 J/cm² UVA phototherapy, Outcome 3 Number of participants with increased erythema and exacerbated pain.
Secondary outcome 1: Improvement of disease activity
The number of participants with very good and good clinical response (marked and moderate skin softening) after the seven‐week treatment was higher in the 20 J/cm² UVA phototherapy group than in the 10 J/cm² UVA group (RR 1.21, 95% CI 0.69 to 2.11; very low certainty evidence; Analysis 5.4) (El‐Mofty 2004). However, the wide confidence interval includes the null effect and appreciable harm.
5.4. Analysis.
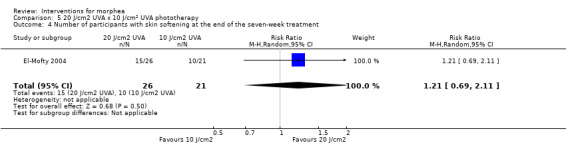
Comparison 5 20 J/cm2 UVA x 10 J/cm² UVA phototherapy, Outcome 4 Number of participants with skin softening at the end of the seven‐week treatment.
Secondary outcome 2: Improvement of disease damage
Not measured.
8. 20 J/cm² versus 5 J/cm² UVA phototherapy
Participants: children and adults with circumscribed morphea, linear scleroderma or generalised morphea.
Primary outcome 1: Global improvement of disease activity or damage assessed by a medical practitioner or by participants
Not measured.
Primary outcome 2: Adverse effects of the interventions, including local and systemic reactions
All 42 participants treated with low‐dose (20 J/cm² or 5 J/cm²) UVA phototherapy had generalised tanning (RR 1.00, 95% CI 0.91 to 1.10; low‐certainty evidence; Analysis 6.1) (El‐Mofty 2004).
6.1. Analysis.

Comparison 6 20 J/cm2 UVA x 5 J/cm² UVA phototherapy, Outcome 1 Number of participants with generalised tanning.
In addition, three participants from each group reported temporary pruritus (RR 0.62, 95% CI 0.14 to 2.69; low‐certainty evidence; Analysis 6.2); and one of 26 participants in the 20 J/cm² group had increased erythema and exacerbated pain (RR 1.89, 95% CI 0.08 to 43.75; very low certainty evidence; Analysis 6.3). However, the wide confidence intervals include the null effect and benefit.
6.2. Analysis.

Comparison 6 20 J/cm2 UVA x 5 J/cm² UVA phototherapy, Outcome 2 Number of participants with temporary pruritus.
6.3. Analysis.

Comparison 6 20 J/cm2 UVA x 5 J/cm² UVA phototherapy, Outcome 3 Number of participants with increased erythema and exacerbated pain.
Secondary outcome 1: Improvement of disease activity
The number of participants with very good and good clinical response (marked and moderate skin softening) after the seven‐week treatment was higher in the 20 J/cm² UVA phototherapy group than in the 5 J/cm² UVA group (RR 1.54, 95% CI 0.75 to 3.14; low‐certainty evidence; Analysis 6.4) (El‐Mofty 2004). However, the confidence interval includes the null effect and appreciable harm.
6.4. Analysis.

Comparison 6 20 J/cm2 UVA x 5 J/cm² UVA phototherapy, Outcome 4 Number of participants with skin softening at the end of the seven‐week treatment.
Secondary outcome 2: Improvement of disease damage
Not measured.
9. 10 J/cm² versus 5 J/cm² UVA phototherapy
Participants: children and adults with circumscribed morphea, linear scleroderma or generalised morphea.
Primary outcome 1: Global improvement of disease activity or damage assessed by a medical practitioner or by participants
Not measured.
Primary outcome 2: Adverse effects of the interventions, including local and systemic reactions
All 37 participants treated with low‐dose (10 J/cm² or 5 J/cm²) UVA phototherapy had generalised tanning (RR 1.00, 95% CI 0.90 to 1.11; low‐certainty evidence; Analysis 7.1) (El‐Mofty 2004). In addition, three participants from each group reported temporary pruritus (RR 0.76, 95% CI 0.18 to 3.29; low‐certainty evidence; Analysis 7.2).
7.1. Analysis.

Comparison 7 10 J/cm2 UVA x 5 J/cm² UVA phototherapy, Outcome 1 Number of participants with generalised tanning.
7.2. Analysis.

Comparison 7 10 J/cm2 UVA x 5 J/cm² UVA phototherapy, Outcome 2 Number of participants with temporary pruritus.
Secondary outcome 1: Improvement of disease activity
The number of participants with very good and good clinical response (marked and moderate skin softening) after the seven‐week treatment was higher in the 10 J/cm² UVA phototherapy group than in the 5 J/cm² UVA group (RR 1.27, 95% CI 0.58 to 2.76; very low certainty evidence; Analysis 7.3) (El‐Mofty 2004). However, the confidence interval includes the null effect and appreciable harm.
7.3. Analysis.

Comparison 7 10 J/cm2 UVA x 5 J/cm² UVA phototherapy, Outcome 3 Number of participants with skin softening at the end of the seven‐week treatment.
Secondary outcome 2: Improvement of disease damage
Not measured.
10. Photodynamic therapy using ALA versus no treatment
Participants: individuals with circumscribed morphea.
Primary outcome 1: Global improvement of disease activity or damage assessed by a medical practitioner or by participants
Not measured.
Primary outcome 2: Adverse effects of the interventions, including local and systemic reactions
In the intra‐individual study comparing photodynamic therapy using ALA versus no treatment (Batchelor 2008), all participants had pigmentation in the lesions treated with photodynamic therapy and no pigmentation in the untreated lesions after the six‐week treatment (RR 13.00, 95% CI 0.89 to 189.38; very low certainty evidence; Analysis 8.1). Other adverse events reported during and after phototherapy included a burning sensation during the phototherapy treatment (RR 9.00, 95% CI 0.59 to 137.65; 4 participants; very low certainty evidence; Analysis 8.2), dryness in the treated lesion (RR 3.00, 95% CI 0.15 to 61.73; 1 participant; very low certainty evidence; Analysis 8.3), erythema in the treated lesion (RR 5.00, 95% CI 0.29 to 86.43; 2 participants; very low certainty evidence; Analysis 8.4), and pruritus in the treated lesion (RR 3.00, 95% CI 0.15 to 61.73; 1 participant; very low certainty evidence; Analysis 8.5). However, we are uncertain of these results due to the wide confidence intervals, which include the null effect.
8.1. Analysis.

Comparison 8 Photodynamic therapy × No Treatment, Outcome 1 Number of plaques with pigmentation.
8.2. Analysis.
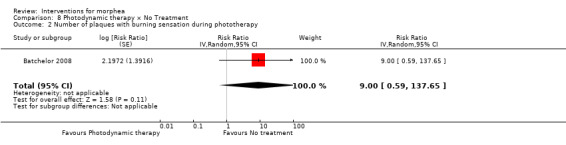
Comparison 8 Photodynamic therapy × No Treatment, Outcome 2 Number of plaques with burning sensation during phototherapy.
8.3. Analysis.

Comparison 8 Photodynamic therapy × No Treatment, Outcome 3 Number of plaques with dryness.
8.4. Analysis.

Comparison 8 Photodynamic therapy × No Treatment, Outcome 4 Number of plaques with erythema.
8.5. Analysis.

Comparison 8 Photodynamic therapy × No Treatment, Outcome 5 Number of plaques with pruritus.
Secondary outcome 1: Improvement of disease activity
Authors reported durometer score values only for treated lesions, excluding data from the control lesions (Batchelor 2008).
Regarding the skin score for thickness (where high values represent a worse outcome), 4 out of 6 treated lesions had improvement (reduction) at the 12‐week follow‐up, but also 4 out of 6 control lesions had improvement (RR 1.00, 95% CI 0.45 to 2.23; very low certainty evidence; Analysis 8.6) (Batchelor 2008).
8.6. Analysis.

Comparison 8 Photodynamic therapy × No Treatment, Outcome 6 Number of plaques with reduction in the skin score at the 12‐week follow‐up.
Secondary outcome 2: Improvement of disease damage
Not measured.
11. Fractional CO₂ laser therapy versus low‐dose UVA‐1 phototherapy (30 J/cm²)
Participants: children and adults with circumscribed morphea or linear scleroderma (with trunk/limb variant and head variant).
Primary outcome 1: Global improvement of disease activity or damage assessed by a medical practitioner or by participants
Not measured.
Primary outcome 2: Adverse effects of the interventions, including local and systemic reactions
All participants reported mild to moderate pain during fractional CO₂ laser therapy (RR 35.00, 95% CI 2.27 to 538.86; low‐certainty evidence; Analysis 9.1), 10 participants reported marked pain (RR 21.00, 95% CI 1.33 to 332.06; low‐certainty evidence; Analysis 9.2), and eight participants reported pruritus in the first 24 hours after treatment (RR 17.00, 95% CI 1.06 to 273.00; low‐certainty evidence; Analysis 9.3) whilst no participant experienced these adverse events with low‐dose UVA‐1 (30 J/cm²) phototherapy (Shalaby 2016).
9.1. Analysis.
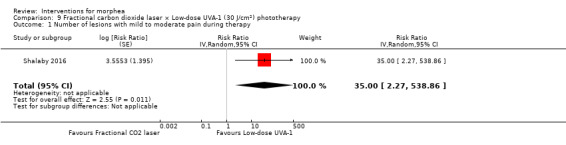
Comparison 9 Fractional carbon dioxide laser × Low‐dose UVA‐1 (30 J/cm²) phototherapy, Outcome 1 Number of lesions with mild to moderate pain during therapy.
9.2. Analysis.

Comparison 9 Fractional carbon dioxide laser × Low‐dose UVA‐1 (30 J/cm²) phototherapy, Outcome 2 Number of lesions with marked pain during therapy.
9.3. Analysis.

Comparison 9 Fractional carbon dioxide laser × Low‐dose UVA‐1 (30 J/cm²) phototherapy, Outcome 3 Number of lesions with pruritus in first 24h.
One lesion treated with fractional CO₂ laser had hyperpigmentation versus four lesions treated with LD UVA‐1 (RR 0.25, 95% CI 0.03 to 2.01; low‐certainty evidence; Analysis 9.4). One lesion treated with fractional CO₂ laser had persistent erythema (RR 3.00, 95% CI 0.13 to 68.84; very low certainty evidence; Analysis 9.5). However, the confidence intervals include the null effect and appreciable benefit and harm.
9.4. Analysis.
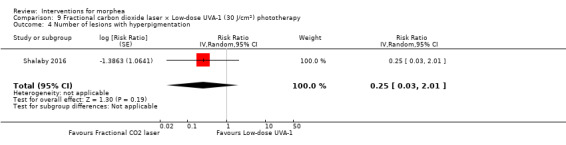
Comparison 9 Fractional carbon dioxide laser × Low‐dose UVA‐1 (30 J/cm²) phototherapy, Outcome 4 Number of lesions with hyperpigmentation.
9.5. Analysis.

Comparison 9 Fractional carbon dioxide laser × Low‐dose UVA‐1 (30 J/cm²) phototherapy, Outcome 5 Number of lesions with persistent erythema.
Secondary outcome 1: Improvement of disease activity
In the intra‐individual study Shalaby 2016, only nine of the 17 participants completed the 50 MHz ultrasound biomicroscopy. The skin thickness assessed through ultrasound biomicroscopy (where high values represent a worse outcome) at the 10‐week follow‐up was lower in the lesions treated with fractional CO₂ laser therapy than the lesions treated with low‐dose UVA‐1 (30 J/cm²) phototherapy (MD −0.15 mm, 95% CI −0.33 to 0.03; low‐certainty evidence; Analysis 9.6). However, the confidence interval includes the null effect and appreciable harm.
9.6. Analysis.

Comparison 9 Fractional carbon dioxide laser × Low‐dose UVA‐1 (30 J/cm²) phototherapy, Outcome 6 Ultrasound biomicroscopy dermal thickness at the 10‐week follow‐up.
The clinical score (adapted LoSCAT: from 0 to 12; thickness, dermal atrophy, dyschromia, and erythema scored each from 0 to 3, maximum) at the 10‐week follow‐up was also lower in the lesions treated with fractional CO₂ laser therapy than the lesions treated with low‐dose UVA‐1 (30 J/cm²) phototherapy (MD −1.59, 95% CI −2.82 to −0.36; low certainty evidence; Analysis 9.7).
9.7. Analysis.

Comparison 9 Fractional carbon dioxide laser × Low‐dose UVA‐1 (30 J/cm²) phototherapy, Outcome 7 Clinical score at the 10‐week follow‐up.
The participants' satisfaction score (from 0 to 3, satisfied, best possible cosmetic result) at the 10‐week follow‐up was higher with fractional CO₂ laser therapy than with low‐dose UVA‐1 (30 J/cm²) phototherapy (MD 1.12, 95% CI 0.80 to 1.44; low‐certainty evidence; Analysis 9.8).
9.8. Analysis.

Comparison 9 Fractional carbon dioxide laser × Low‐dose UVA‐1 (30 J/cm²) phototherapy, Outcome 8 Participant satisfaction score at the 10‐week follow‐up.
The number of lesions that had good or very good improvement in the clinical score (between 40% and 59% decrease or more than 60% decrease from baseline) at the 10‐week follow‐up was higher in the lesions treated with fractional CO₂ laser therapy (16/17) than the lesions treated with low‐dose UVA‐1 (30 J/cm²) phototherapy (6/17), indicating more improvement of disease activity with fractional CO₂ laser therapy (RR 2.67, 95% CI 1.39 to 5.13; low‐certainty evidence; Analysis 9.9).
9.9. Analysis.

Comparison 9 Fractional carbon dioxide laser × Low‐dose UVA‐1 (30 J/cm²) phototherapy, Outcome 9 Number of lesions with good or very good improvement in the clinical score at the 10‐week follow‐up.
Secondary outcome 2: Improvement of disease damage
Not measured.
12. Oral calcitriol (0.75 μg increased to 1.25 μg/day) versus placebo
Participants: adults and children with circumscribed or generalised morphea.
Primary outcome 1: Global improvement of disease activity or damage assessed by a medical practitioner or by participants
Not measured.
Primary outcome 2: Adverse effects of the interventions, including local and systemic reactions
In the study comparing the 9‐month treatment with oral calcitriol versus placebo (Hulshof 2000), authors reported adverse events for participants with morphea and SSc together (20 participants with morphea and 7 withSSc included in the study): of the 13 participants treated with calcitriol, three had transient hypercalciuria, whilst no participant treated with placebo had this event (RR 7.50, 95% CI 0.42 to 132.58; very low certainty evidence; Analysis 14.1).
14.1. Analysis.

Comparison 14 Oral calcitriol (0.75 μg increased to 1.25 μg/day) × Placebo, Outcome 1 Number of participants with transient hypercalciuria.
Secondary outcome 1: Improvement of disease activity
The skin score for thickness of the morphea lesions (from 0 to 66, maximum, rating 22 body regions from 0, normal to 3, hidebound skin) was higher in the oral calcitriol group than in the placebo group after the 9‐month treatment (MD 1.10, 95% CI −2.98 to 5.18; very low certainty evidence; Analysis 14.2) and at the 15‐month follow‐up (MD 3.70, 95% CI −1.49 to 8.89; very low certainty evidence; Analysis 14.3), indicating more disease activity in the oral calcitriol group (Hulshof 2000). However, the wide confidence intervals include the null effect and appreciable benefit.
14.2. Analysis.

Comparison 14 Oral calcitriol (0.75 μg increased to 1.25 μg/day) × Placebo, Outcome 2 Skin score at the end of the 9‐month treatment.
14.3. Analysis.

Comparison 14 Oral calcitriol (0.75 μg increased to 1.25 μg/day) × Placebo, Outcome 3 Skin score at the end of the 15‐month follow‐up.
Secondary outcome 2: Improvement of disease damage
Not measured.
13. Oral methotrexate (15 mg/m², maximum 20 mg a week) plus oral prednisone versus placebo plus oral prednisone
See Table 1
Participants: children with active morphea (linear scleroderma, generalised morphea, or mixed morphea).
Primary outcome 1: Global improvement of disease activity or damage assessed by a medical practitioner or by participants
The juvenile study Zulian 2011 considered the presence of all three response criteria for a significant clinical response: skin score rate equal or less than 1, indicating decreased lesion extension; at least a 10% decrease in the percentage thermal change from baseline, indicating decreased lesion inflammation; and absence of new lesions. The participants treated with oral MTX plus oral prednisone had a higher significant clinical response (31/46) than the participants in the placebo plus oral prednisone group (7/24) after the treatment (RR 2.31, 95% CI 1.20 to 4.45, NNTB 3; low‐certainty evidence; Analysis 11.1).
11.1. Analysis.

Comparison 11 Oral methotrexate (15 mg/m², maximum 20 mg a week) plus oral prednisone × Placebo plus oral prednisone, Outcome 1 Clinical response at the end of the 12‐month treatment.
Authors reported no differences between groups in the VAS of both the physician's global assessment of disease severity and the parents' global assessment of the participants' overall well‐being, but reported no numerical data for these outcomes (Zulian 2011).
Primary outcome 2: Adverse effects of the interventions, including local and systemic reactions
The number of participants with at least one adverse event was higher in the oral MTX plus oral prednisone group (26/46) than in the placebo plus oral prednisone group (11/24) during the 12‐month treatment (RR 1.23, 95% CI 0.75 to 2.04; low‐certainty evidence; Analysis 11.2) (Zulian 2011). However, the confidence interval includes the null effect and appreciable benefit.
11.2. Analysis.

Comparison 11 Oral methotrexate (15 mg/m², maximum 20 mg a week) plus oral prednisone × Placebo plus oral prednisone, Outcome 2 Number of participants with at least one adverse event.
Adverse events related to MTX treatment included alopecia (two participants), nausea (eight participants), headache (five participants), fatigue (two participants), and hepatotoxicity (three participants), whilst adverse events related to corticosteroid treatment included weight gain (more than 5% of body weight) and striae rubrae.
Secondary outcome 1: Improvement of disease activity
The participants treated with oral MTX plus oral prednisone had a lower skin score rate (SSR) than the control group (placebo plus oral prednisone) after the 12‐month treatment, indicating a greater reduction of lesion size in the MTX group (MD −0.31, 95% CI −0.35 to −0.27; low certainty evidence; Analysis 11.3) (Zulian 2011). The SSR was based on the ratio between lesion and body surface area at baseline and after the 12‐month treatment (SSR equal or < 1 indicates decreased lesion extension; SSR > 1 indicates an increased extension of the lesion).
11.3. Analysis.

Comparison 11 Oral methotrexate (15 mg/m², maximum 20 mg a week) plus oral prednisone × Placebo plus oral prednisone, Outcome 3 Skin Score Rate (SSR) at the end of the 12‐month treatment.
The participants treated with oral MTX plus oral prednisone also had a lower percentage thermal change from baseline after the 12‐month treatment compared with the placebo plus oral prednisone group (MD −32.30, 95% CI −37.92 to −26.68; low‐certainty evidence; Analysis 11.4) (Zulian 2011). As a negative percentage thermal change value indicates improvement, and a positive indicates worsening, there was a greater reduction of lesion inflammation in the MTX plus oral prednisone group.
11.4. Analysis.

Comparison 11 Oral methotrexate (15 mg/m², maximum 20 mg a week) plus oral prednisone × Placebo plus oral prednisone, Outcome 4 Percentage thermal change at the end of the 12‐month treatment.
The MTX plus oral prednisone group had a lower number of participants with new morphea lesions after the 12‐month treatment: three out of 46 participants in the treated grouped versus four out of 24 in the control (RR 0.39, 95% CI 0.10 to 1.61, Analysis 11.5; very low certainty evidence; Zulian 2011). However, the confidence interval includes the null effect and appreciable harm.
11.5. Analysis.

Comparison 11 Oral methotrexate (15 mg/m², maximum 20 mg a week) plus oral prednisone × Placebo plus oral prednisone, Outcome 5 Number of participants with new lesions at the end of the 12‐month treatment.
Secondary outcome 2: Improvement of disease damage
Authors reported no differences between groups in the C‐HAQ disability index after the 12‐month treatment, but reported no numerical data for this outcome (Zulian 2011).
14. Oral hydroxychloroquine (200 mg twice a day) plus topical corticosteroid versus oral methotrexate (15 mg a week) plus topical corticosteroid
Participants: children and adults with active morphea (unclear type).
Primary outcome 1: Global improvement of disease activity or damage assessed by a medical practitioner or by participants
The modified skin score (MSS; from 0 to 42, where 0 = no affected skin, 42 = extreme involvement in all areas) evaluates skin thickness and pliability (from 0, normal skin, to 3, unable to pinch or move skin) plus involved area (from 0, no involvement, to 3 = more than 67% involvement) in seven body regions. The participants treated with HCQ plus topical corticosteroid had a higher MSS score than the participants treated with oral MTX plus topical corticosteroid (25 participants) after the 3‐month treatment (MD 0.50, 95% CI −1.93 to 2.93; low‐certainty evidence; Analysis 12.1) (Azimi 2013). However, the confidence interval includes the null effect and appreciable benefit.
12.1. Analysis.

Comparison 12 Oral hydroxychloroquine (200 mg twice a day) plus topical corticosteroid x Oral methotrexate (15 mg a week) plus topical corticosteroid, Outcome 1 Modified Skin Score (MSS) at the end of the three‐month treatment.
Primary outcome 2: Adverse effects of the interventions, including local and systemic reactions
No participants treated with HCQ reported serious side effects after the 3‐month treatment, whilst three out of 12 participants had liver enzymes rise at least three times in the MTX group (RR 0.13, 95% CI 0.01 to 2.33; very low certainty evidence; Analysis 12.2) (Azimi 2013). However, the wide confidence interval includes the null effect and appreciable harm.
12.2. Analysis.

Comparison 12 Oral hydroxychloroquine (200 mg twice a day) plus topical corticosteroid x Oral methotrexate (15 mg a week) plus topical corticosteroid, Outcome 2 Number of participants with liver enzymes rise more than three times during the three‐month treatment.
Secondary outcome 1: Improvement of disease activity
The participants treated with HCQ plus topical corticosteroid had a higher VAS of the participants’ estimate of skin tightness (from 0 to 10, maximum) on the morphea lesions than the participants treated with oral MTX plus topical corticosteroid after the 3‐month treatment (MD 0.30, 95% CI −0.49 to 1.09; very low certainty evidence; Analysis 12.3) (Azimi 2013). However, the wide confidence interval includes the null effect and appreciable benefit.
12.3. Analysis.

Comparison 12 Oral hydroxychloroquine (200 mg twice a day) plus topical corticosteroid x Oral methotrexate (15 mg a week) plus topical corticosteroid, Outcome 3 VAS for participants' estimate of skin tightness at the end of the three‐month treatment.
The HCQ plus topical corticosteroid group had a lower score in the VAS of the participants’ estimate of pruritus (from 0 to 10, maximum) on the morphea lesions (indicating less disease activity) after the 3‐month treatment compared to the MTX plus topical corticosteroid group (MD −2.30, 95% CI −3.25 to −1.35; low‐certainty evidence; Analysis 12.4).
12.4. Analysis.

Comparison 12 Oral hydroxychloroquine (200 mg twice a day) plus topical corticosteroid x Oral methotrexate (15 mg a week) plus topical corticosteroid, Outcome 4 VAS for participants' estimate of pruritus at the end of the three‐month treatment.
Secondary outcome 2: Improvement of disease damage
Not measured.
15. Topical tacrolimus 0.1% versus placebo
Participants: adults with active circumscribed morphea.
Primary outcome 1: Global improvement of disease activity or damage assessed by a medical practitioner or by participants
Not measured.
Primary outcome 2: Adverse effects of the interventions, including local and systemic reactions
In the intra‐individual study comparing topical tacrolimus 0.1% versus placebo (10 participants), one participant reported pruritus on the plaque treated with tacrolimus (RR 3.00, 95% CI 0.14 to 65.90; very low certainty evidence; Analysis 13.1) (Kroft 2009a). However, the wide confidence interval includes the null effect and appreciable benefit. One participant also reported a mild headache.
13.1. Analysis.

Comparison 13 Topical tacrolimus 0.1% × Placebo, Outcome 1 Number of plaques with pruritus.
Secondary outcome 1: Improvement of disease activity
The durometer score (from 0 to 1000, maximum, where high values represent a worse outcome) was higher in the lesions treated with tacrolimus 0.1% than in the lesions treated with placebo after the 12‐week treatment (MD 47.20, 95% CI −44.55 to 138.95; low‐certainty evidence; Analysis 13.2) (Kroft 2009a). However, the wide confidence interval includes the null effect and appreciable benefit.
13.2. Analysis.
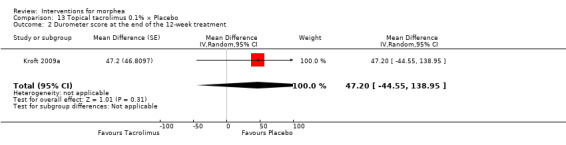
Comparison 13 Topical tacrolimus 0.1% × Placebo, Outcome 2 Durometer score at the end of the 12‐week treatment.
The lesion size was slightly higher in the lesions treated with tacrolimus 0.1% than in the lesions treated with placebo after the 12‐week treatment (MD 0.50, 95% CI −38.35 to 39.35; very low certainty evidence; Analysis 13.3). However, the wide confidence interval includes the null effect and appreciable benefit.
13.3. Analysis.
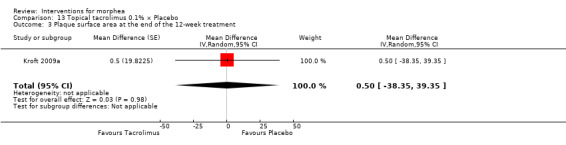
Comparison 13 Topical tacrolimus 0.1% × Placebo, Outcome 3 Plaque surface area at the end of the 12‐week treatment.
The number of lesions that had a decrease in the modified DIET score (from 0 to 15, maximum; dyspigmentation, induration, erythema, telangiectasia and atrophy of the lesions scored from 0, none, to 3, severe) was higher with tacrolimus 0.1% (8/10) than with placebo (7/10) after the 12‐week treatment (RR 1.14, 95% CI 0.69 to 1.90; low‐certainty evidence; Analysis 13.4). However, the confidence interval includes the null effect and appreciable benefit.
13.4. Analysis.

Comparison 13 Topical tacrolimus 0.1% × Placebo, Outcome 4 Number of plaques with a reduced modified DIET score at the end of the 12‐week treatment.
Although the number of lesions that had a decrease was similar, the modified DIET score was lower in the lesions treated with tacrolimus 0.1% than in the lesions treated with placebo after the 12‐week treatment (MD −1.70, 95% CI −3.11 to −0.29; low‐certainty evidence; Analysis 13.5), indicating more improvement of disease activity with tacrolimus 0.1%.
13.5. Analysis.

Comparison 13 Topical tacrolimus 0.1% × Placebo, Outcome 5 Modified DIET score at the end of the 12‐week treatment.
Secondary outcome 2: Improvement of disease damage
Not measured.
16. Intralesional injections of interferon‐γ (100 µg) versus placebo
Participants: individuals with active circumscribed morphea.
Primary outcome 1: Global improvement of disease activity or damage assessed by a medical practitioner or by participants
Not measured.
Primary outcome 2: Adverse effects of the interventions, including local and systemic reactions
In the study comparing intralesional injections of interferon‐γ versus placebo (24 participants, 24‐week follow‐up; Hunzelmann 1997), three participants treated with IFN‐γ reported arthralgia, fatigue and dizziness, whilst one participant from the placebo group reported headache and temperature elevation. However, authors informed only the total number of participants without the number of participants in each intervention group.
Secondary outcome 1: Improvement of disease activity
Authors reported there were no changes in size of the lesions and skin score for thickness at the 24‐week follow‐up, but reported no numerical data. In addition, there were no differences between groups in the number of participants with new morphea lesions at the 24‐week follow‐up (1 participant in the interferon‐γ group and 7 participants in the control group), but authors did not present the number of participants in each intervention group.
Secondary outcome 2: Improvement of disease damage
Not measured.
17. Polymerised collagen intralesional injection versus methylprednisolone subcutaneous injection plus placebo intralesional injection
Participants: adults with circumscribed morphea or linear scleroderma.
Primary outcome 1: Global improvement of disease activity or damage assessed by a medical practitioner or by participants
Not measured.
Primary outcome 2: Adverse effects of the interventions, including local and systemic reactions
In the study evaluating the 3‐month treatment with polymerised collagen intralesional injection versus methylprednisolone subcutaneous injection plus placebo intralesional injection (9‐month follow‐up; Furuzawa‐Carballeda 2012), all 13 participants treated with polymerised collagen reported short‐duration (less than five minutes) pain at the injection site versus two out of 14 participants treated with methylprednisolone and placebo (RR 5.79, 95% CI 1.86 to 18.02; moderate‐certainty evidence; Analysis 10.1).
10.1. Analysis.

Comparison 10 Polymerized collagen intralesional injection (0.2 to 1.0 mL / 1.66 to 8.3 mg weekly) × Methylprednisolone subcutaneous injection (maximum dose of 20 mg or 5.0 mL monthly) plus placebo intralesional injection, Outcome 1 Number of participants with short‐duration pain at injection site.
Participants treated with polymerised collagen reported no other adverse events, whilst seven out of 14 participants in the methylprednisolone group reported pruritus (RR 0.07, 95% CI 0.00 to 1.14; low‐certainty evidence; Analysis 10.2), and one of 14 developed sclerosis (RR 0.36, 95% CI 0.02 to 8.06; low‐certainty evidence; Analysis 10.3). However, the confidence intervals include the null effect and appreciable harm.
10.2. Analysis.

Comparison 10 Polymerized collagen intralesional injection (0.2 to 1.0 mL / 1.66 to 8.3 mg weekly) × Methylprednisolone subcutaneous injection (maximum dose of 20 mg or 5.0 mL monthly) plus placebo intralesional injection, Outcome 2 Number of participants with pruritus.
10.3. Analysis.

Comparison 10 Polymerized collagen intralesional injection (0.2 to 1.0 mL / 1.66 to 8.3 mg weekly) × Methylprednisolone subcutaneous injection (maximum dose of 20 mg or 5.0 mL monthly) plus placebo intralesional injection, Outcome 3 Number of participants with sclerosis.
Secondary outcome 1: Improvement of disease activity
Furuzawa‐Carballeda 2012 used an adapted skin score (from 0, normal, to 4, extreme thickening) to assess skin thickness of the morphea lesions, which indicates disease activity. The group treated with polymerised collagen had a higher skin score compared to the methylprednisolone group after the 3‐month treatment (MD 1.30, 95% CI 0.31 to 2.29; moderate‐certainty evidence; Analysis 10.4). The skin score at the 9‐month follow‐up was also higher in the polymerised collagen group (MD 0.50, 95% CI −0.25 to 1.25; low‐certainty evidence; Analysis 10.5); however, the confidence interval includes the null effect and appreciable benefit.
10.4. Analysis.

Comparison 10 Polymerized collagen intralesional injection (0.2 to 1.0 mL / 1.66 to 8.3 mg weekly) × Methylprednisolone subcutaneous injection (maximum dose of 20 mg or 5.0 mL monthly) plus placebo intralesional injection, Outcome 4 Skin score at the end of the three‐month treatment.
10.5. Analysis.

Comparison 10 Polymerized collagen intralesional injection (0.2 to 1.0 mL / 1.66 to 8.3 mg weekly) × Methylprednisolone subcutaneous injection (maximum dose of 20 mg or 5.0 mL monthly) plus placebo intralesional injection, Outcome 5 Skin score at last follow‐up visit (month nine).
The number of new morphea lesions at the 9‐month follow‐up was higher in the polymerised collagen group then in the methylprednisolone group (MD 0.20, 95% CI −0.16 to 0.56; low‐certainty evidence; Analysis 10.6). However, the confidence interval includes the null effect and appreciable benefit.
10.6. Analysis.

Comparison 10 Polymerized collagen intralesional injection (0.2 to 1.0 mL / 1.66 to 8.3 mg weekly) × Methylprednisolone subcutaneous injection (maximum dose of 20 mg or 5.0 mL monthly) plus placebo intralesional injection, Outcome 6 Number of morphea lesions at the end of the three‐month treatment.
Secondary outcome 2: Improvement of disease damage
Not measured.
18. Tranilast plus topical betamethasone valerate 0.1% versus topical betamethasone valerate 0.1%
Participants: adults with circumscribed morphea, linear scleroderma or generalised morphea.
Primary outcome 1: Global improvement of disease activity or damage assessed by a medical practitioner or by participants
Not measured.
Primary outcome 2: Adverse effects of the interventions, including local and systemic reactions
Authors reported no adverse events after the 3‐month treatment (Noakes 2018; three participants).
Secondary outcome 1: Improvement of disease activity
The intra‐individual study Noakes 2018 adapted the LoScat score to compare individual lesions (11 pairs) of the three participants (from 0, minimum, to 18, maximum enlargement, erythema, skin thickness, dermal atrophy, subcutaneous atrophy and dyspigmentation ‒ each rated from 0 to 3). The lesions treated with tranilast plus topical betamethasone valerate 0.1% had a lower clinical score than the lesions treated with only topical betamethasone valerate 0.1% after the 3‐month treatment (MD −1.50, 95% CI −3.88 to 0.88; low‐certainty evidence; Analysis 15.1). However, the wide confidence interval includes the null effect and appreciable harm.
15.1. Analysis.

Comparison 15 Tranilast plus topical betamethasone valerate 0.1% versus topical betamethasone valerate 0.1%, Outcome 1 Clinical score at the end of the three‐month treatment.
The lesions treated with tranilast plus topical betamethasone valerate 0.1% also had a lower Physician Global Assessment of Activity (from 0 = inactive, to 100 = markedly active) than the lesions treated with only topical betamethasone valerate 0.1% after the 3‐month treatment (MD −7.50, 95% CI −23.73 to 8.73; low‐certainty evidence; Analysis 15.2). However, the wide confidence interval includes the null effect and appreciable harm.
15.2. Analysis.

Comparison 15 Tranilast plus topical betamethasone valerate 0.1% versus topical betamethasone valerate 0.1%, Outcome 2 Physician Global Assessment of Activity at the end of the three‐month treatment.
Noakes 2018 noted no disease progression in the lesions treated with tranilast plus topical betamethasone valerate 0.1% (0/8), whilst two of eight lesions treated with topical betamethasone valerate 0.1% had disease progression (RR 0.24, 95% CI 0.01 to 4.47; low‐certainty evidence; Analysis 15.3). However, the wide confidence interval includes the null effect and appreciable harm.
15.3. Analysis.

Comparison 15 Tranilast plus topical betamethasone valerate 0.1% versus topical betamethasone valerate 0.1%, Outcome 3 Number of lesions with disease progression at the end of the three‐month treatment.
It is important to consider that the participants continued with their routine therapy, which included systemic medications.
Secondary outcome 2: Improvement of disease damage
The lesions treated with tranilast plus topical betamethasone valerate 0.1% also had a lower Physician Global Assessment of Damage (from 0 = no damage, to 100 = markedly damaged) than the lesions treated with only topical betamethasone valerate 0.1% after the 3‐month treatment (MD −6.00, 95% CI −24.90 to 12.90; low‐certainty evidence; Analysis 15.4). However, the wide confidence interval includes the null effect and appreciable harm.
15.4. Analysis.

Comparison 15 Tranilast plus topical betamethasone valerate 0.1% versus topical betamethasone valerate 0.1%, Outcome 4 Physician Global Assessment of Damage at the end of the three‐month treatment.
19. Traditional Chinese Medicine herbal tea plus herbal oil and vitamin B6 versus phenoxymethylpenicillin plus DAC base cream
Participants: adults with circumscribed morphea or linear scleroderma.
Primary outcome 1: Global improvement of disease activity or damage assessed by a medical practitioner or by participants
Not measured.
Primary outcome 2: Adverse effects of the interventions, including local and systemic reactions
In the study comparing Traditional Chinese Medicine herbal tea plus herbal oil and vitamin B6 versus phenoxymethylpenicillin plus DAC base cream (Tang 2006), two out of 12 participants in the control group prematurely terminated the study due to adverse events (exanthema and Quincke edema), and two of 12 participants in the Traditional Chinese Medicine group had contact dermatitis (RR 1.00, 95% CI 0.17 to 5.98; very low certainty evidence; Analysis 16.1). However, the wide confidence interval includes the null effect and appreciable benefit.
16.1. Analysis.

Comparison 16 Traditional Chinese Medicine herbal tea plus herbal oil and vitamin B6 x Phenoxymethylpenicillin plus DAC base cream, Outcome 1 Number of participants with adverse events.
Secondary outcome 1: Improvement of disease activity
Authors reported a significantly greater reduction of lesion size in the Traditional Chinese Medicine group compared to the control after the 12‐week treatment (70.9 cm² versus 126.1 cm²; P < 0.0001) and at the 24‐week follow‐up (52.5 cm² versus 126.2 cm²; P < 0.0001), but reported mean values without standard deviation (Tang 2006).
Authors also reported a significantly greater reduction of skin thickness (measured through 20 MHz ultrasound) in the Traditional Chinese Medicine group compared to the control after the 12‐week treatment (1.417 mm versus 1.785 mm; P < 0.0001) and at the 24‐week follow‐up (1.277 mm versus 1.862 mm; P < 0.0001), but reported mean values without standard deviation (Tang 2006).
Secondary outcome 2: Improvement of disease damage
Not measured.
20. Acupuncture, hot herbal compress and moxibustion plus Centella triterpenes and vitamin E versus heparin sodium cream plus Centella triterpenes and vitamin E
Participants: adults with morphea (unclear type).
Primary outcome 1: Global improvement of disease activity or damage assessed by a medical practitioner or by participants
In the study evaluating the 6‐month treatment with acupuncture, hot herbal compress and moxibustion plus Centella triterpenes tablets and vitamin E versus heparin sodium cream plus Centella triterpenes tablets and vitamin E (Yan 2013), 19 out of 22 participants treated with Traditional Chinese Medicine had a significant clinical response versus 10 out of 19 participants in the control group (RR 1.64, 95% CI 1.04 to 2.59, NNTB 3; low‐certainty evidence; Analysis 17.1). Authors based the clinical response on Traditional Chinese Medicine criteria and considered more than 30% improvement as a significant clinical response.
17.1. Analysis.

Comparison 17 Acupuncture, hot herbal compress, and moxibustion plus Centella triterpenes tablets and vitamin E × Heparin sodium cream plus Centella triterpenes tablets and vitamin E, Outcome 1 Number of participants with significant clinical response at the end of the six‐month treatment.
Primary outcome 2: Adverse effects of the interventions, including local and systemic reactions
One participant reported pain during acupuncture versus zero events in the control group (RR 2.61, 95% CI 0.11 to 60.51; very low certainty evidence; Analysis 17.2) (Yan 2013). However, the wide confidence interval includes the null effect and appreciable benefit.
17.2. Analysis.

Comparison 17 Acupuncture, hot herbal compress, and moxibustion plus Centella triterpenes tablets and vitamin E × Heparin sodium cream plus Centella triterpenes tablets and vitamin E, Outcome 2 Number of participants with pain during treatment.
Secondary outcome 1: Improvement of disease activity
The group treated with Traditional Chinese Medicine had a lower skin sclerosis score compared with the control group (MD −10.34, 95% CI −16.83 to −3.85; low‐certainty evidence; Analysis 17.3). Authors based the skin score on Steen criteria (from 0 to 90, maximum), which rates sclerosis of 30 body regions from 0, none, to 3, severe.
17.3. Analysis.

Comparison 17 Acupuncture, hot herbal compress, and moxibustion plus Centella triterpenes tablets and vitamin E × Heparin sodium cream plus Centella triterpenes tablets and vitamin E, Outcome 3 Skin sclerosis score at the end of the six‐month treatment.
Secondary outcome 2: Improvement of disease damage
The group treated with Traditional Chinese Medicine also had a lower joint function score compared with the control group (MD −1.65, 95% CI −2.95 to −0.35; low‐certainty evidence; Analysis 17.4), and a lower joint pain score (MD −6.63, 95% CI −11.20 to −2.06; low certainty evidence; Analysis 17.5), indicating less disease impact in daily activities. Authors based the joint function score on Kahan criteria (from 0 to 66, maximum impact; assessing disease impact in 11 daily activities from 0, no difficulty, to 3, unable to perform) and the joint pain score on Traditional Chinese Medicine syndromes criteria (unclear total score).
17.4. Analysis.

Comparison 17 Acupuncture, hot herbal compress, and moxibustion plus Centella triterpenes tablets and vitamin E × Heparin sodium cream plus Centella triterpenes tablets and vitamin E, Outcome 4 Joint function score at the end of the six‐month treatment period.
17.5. Analysis.

Comparison 17 Acupuncture, hot herbal compress, and moxibustion plus Centella triterpenes tablets and vitamin E × Heparin sodium cream plus Centella triterpenes tablets and vitamin E, Outcome 5 Joint pain score at the end of the six‐month treatment.
Discussion
Summary of main results
Morphea is an immune‐mediated disease with multiple subtypes that have different clinical presentations, aetiology, prognosis and treatment. We aimed to assess the effectiveness and safety of treatments for morphea, and we found 14 RCTs.
Various interventions have been used to treat morphea, and the response to treatment is variable according to the subtype, extent, severity, and activity of the condition. The study participants had various subtypes of morphea, which limited our conclusions; and less than half of the studies included participants with a diagnosis of active morphea. Furthermore, only four studies evaluated our primary outcome ‘Global improvement of disease activity or damage assessed by a medical practitioner or by participants’, and few used validated outcome measurement tools for morphea.
All included studies addressed adverse effects of the interventions and evaluated the improvement of disease activity, although they used heterogeneous assessment tools. Only three studies assessed our secondary outcome ‘Improvement of disease damage’. The quality of the evidence in this review was mainly low, indicating potential uncertainty in the results.
Based on one study (70 participants), we found that the treatment of active morphea (linear scleroderma, generalised morphea, or mixed subtype: linear and circumscribed) in children and teenagers with oral MTX (given once per week for 12 months or until time of flare) plus oral prednisone (given once per day for three months plus one month with gradually decreased dose until discontinuation) may result in better global improvement of disease activity or damage when compared with placebo plus oral prednisone. Both groups also received a folic acid supplement. There may be little or no difference in the likelihood of experiencing at least one adverse event with oral MTX or placebo. Adverse events related to methotrexate included alopecia, nausea, headache, fatigue, and hepatotoxicity; whilst adverse events related to the corticosteroid (which was given in both groups) included weight gain (more than 5% of body weight) and striae rubrae. These results are based on low‐certainty evidence (Table 1). As both groups received oral prednisone as an adjuvant therapy, it is unclear whether the initial corticosteroid treatment should be given in combination to MTX or if the treatment with MTX alone could have the same effectiveness.
Based on one three‐armed study in which 62 participants (children and adults) had active morphea (circumscribed morphea, linear scleroderma (with trunk/limb variant and head variant), generalised morphea, or mixed morphea, we found that the global improvement of disease activity or damage after treatment may be similar between low‐dose UVA‐1 (20 J/cm²), medium‐dose UVA‐1 (50 J/cm²), and narrowband UVB phototherapy given five times a week, for eight weeks. Treatment with UVA‐1 phototherapy may cause mild tanning compared to narrowband UVB phototherapy, but there may be no difference in mild tanning when comparing medium‐ and low‐dose UVA‐1 phototherapy. Transient erythema was reported in three participants with narrowband UVB and none of the participants in either the low‐ or medium‐dose UVA‐1 groups. These results are based on low‐certainty evidence (Table 2; Table 3; Table 4).
Overall completeness and applicability of evidence
Our included studies were not sufficient to address all of the objectives of the review: to assess the effects of treatments for people with any form of morphea. Their relevance was limited by the diversity of both the morphea subtypes and included participants; the lack of distinction regarding the stage of the condition (active or inactive); and heterogeneous and non‐validated tools of assessment.
Participants
Six studies included both children and adults, but did not report separate data for each age group; thus the number of children included in this review is unclear. Most studies investigated more than one type of morphea, but reported data for all subtypes combined. Some trials did not report the participants' type of morphea at all. Therefore, it was not possible to analyse the treatment response differences between subtypes and between children and adults, and so we are not able to generalise this result for all age groups and morphea types.
The different forms of morphea included in this review can be very different from each other in terms of age, possible causes, propensity to underlying sclerosis and hence treatment aims and need for systemic therapy. Most participants had circumscribed morphea (n = 197), followed by linear scleroderma (n = 77; 6 with head variant), generalised morphea (n = 46), and mixed morphea (n = 9). However, the exact number of participants with each type of morphea is also unclear, as two studies did not report this information, and one study included participants with circumscribed and generalised morphea, without disclosing the number of participants for each type.
Furthermore, most studies did not consider the active inflammatory stage versus inactive sclerotic or atrophic phase of morphea, which is a key concept directly related to response to treatment (treatments in the active stage of the disease have better chances of improvement). Less than half of the included studies investigated participants with a diagnosis of active morphea. Most studies which reported the length of the disease evolution investigated participants with up to 10 years of morphea diagnosis.
Interventions
An aspect to consider is the necessity or not of initial corticosteroid therapy combination (e.g. oral MTX plus oral prednisone). Although both groups used the same corticosteroid dosage, the group treated with MTX had a greater improvement, but we cannot exclude a synergic effect between these medications in the improvement of morphea lesions. It is unclear whether the initial corticosteroid therapy may induce a faster treatment response compared with MTX alone or not. Given the prevalence of morphea in children and the adverse effects of systemic corticosteroid therapy during the growth phase, it is important to explore steroid sparing agents. Topical corticosteroid therapy is also linked to adverse effects such as atrophy.
The treatment of active morphea (unclear type) with HCQ plus topical corticosteroid may occasionally be indicated for severe cases with contraindications to MTX or failure to standard therapy. We also cannot exclude the effect of the topical corticosteroid combined with HCQ or MTX, especially considering that the follow‐up period of this study was short (three months).
Outcomes
Only four studies evaluated our primary outcome ‘Global improvement of disease activity or damage assessed by a medical practitioner or by participants’, although all included studies addressed adverse effects of the interventions. Only three studies assessed our secondary outcome ‘Improvement of disease damage’, but all included studies evaluated the improvement of disease activity. However, most studies used non‐validated, as well as heterogeneous, tools of assessment, which hindered the evaluation of the effectiveness of treatments.
It is, furthermore, important to note that the assessment tool in most studies was inappropriate, such as scores developed and validated for SSc or scores adapted to assess individual lesions. Clinical tools developed and validated for SSc are inappropriate to assess skin involvement in morphea, which is a different disease with distinct clinical characteristics. It is essential to use a specific validated tool for morphea to assess outcome measure. Selecting lesions rather than globally assessing individuals and comparing two interventions in the same individual (intra‐individual control) also limited the evaluation of the effectiveness of treatments.
Most outcome measures of the included studies were considered as indicators of disease activity (secondary outcome), as the assessments were made mostly on individual lesions. Most studies used adapted clinical scores to assess skin lesions individually rather than globally evaluate the participants. Only two studies included the LoSCAT in the outcome measures; however, they had intra‐individual design and adapted the tool to assess individual lesions.
In addition, the follow‐up duration was generally short (less than six months) for a chronic disease with high recurrence rates. A long‐term assessment is required; however, the ideal period depends on the subtype of morphea, type of therapy and the expected result.
Quality of the evidence
We found several methodological problems in the included studies. These included a small number of randomised participants (inadequate statistical power), short‐term follow up, lack of validated tools, and some studies did not have a placebo control group. In addition, some studies adapted available tools of global evaluation to assess individual lesions, but the global assessment of the disease is more clinically relevant.
We downgraded all outcomes in the GRADE assessment by one level due to study limitations (risk of bias). Reasons included unclear risk of selection bias (allocation concealment), and high risk of performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data) and reporting bias (selective reporting). Allocation should be concealed and the concealment method should be reported. Studies ideally include a placebo or a sham intervention control group to effectively blind participants, personnel and outcome assessors, and avoid performance and detection bias. However, the nature of some interventions, such as phototherapy for example, may compromise blinding because of the tanning associated with the treatment. Studies should also analyse data from all randomised participants, regardless of study completion (ITT analysis). We also downgraded all outcomes by at least one level due to imprecision. All studies had a small number of participants, and most results had small numbers of events and wide confidence intervals, including both null effect and appreciable benefit and harm. Overall, we judged the evidence to be of low certainty, except for one judgement which we considered to be very low quality (Table 4).
Potential biases in the review process
Our inclusion of intra‐individual studies could be questioned, as interventions applied in a specific lesion (injected medications or phototherapy, for example) could have systemic effects and reverberate in other lesions, altering the final result. However, considering that morphea is a rare diverse condition treated with different medications, and there are few randomised controlled trials of therapeutic agents, it was necessary to assess all available evidence (from RCTs) on the treatment of morphea.
Regarding the implementation of protocol methods we decided to add cross‐over trials, as their analysis is similar to within‐participants trials, which were already included in the criteria for considering studies for the review. We included only the first part of the cross‐over trials to avoid any carry‐over effects from insufficient washout periods. However, we do not consider this departure from protocol a potential source of bias, and there were no cross‐over studies in our search results. Furthermore, we did not make any decisions about the analysis after seeing the data, as we had anticipated that the unit of analysis could be the individual participant, the morphea lesion, or body region.
Limitations of the search process include the lack of access of grey literature sources, and the incomplete correspondence with study investigators. We could not obtain all relevant data because some authors reported data inadequately. In an effort to address reporting bias, we contacted the first author of the primary study to obtain missing data. Although some authors responded to our e‐mail contact not all could provide us with the missing data.
Agreements and disagreements with other studies or reviews
There are some narrative reviews of interventions for morphea reporting different types of studies (Careta 2015; Fett 2012; Tratenberg 2017), and one systematic review of interventions for morphea (Zwischenberger 2011); however, these reviews include, besides RCTs, prospective uncontrolled trials, retrospective studies, and case series. Contrary to these reviews, we included only RCTs, because other types of studies, such as uncontrolled and retrospective trials, for example, would be inappropriate to evaluate the effectiveness of interventions.
We followed a rigorous methodology, performing assessment of risk of bias and certainty of evidence. Our search methods retrieved more studies than the other systematic review, as we included more databases and trial registers, and used a wider search strategy: we found 339 records and included 14 RCTs that met our eligibility criteria, compared to 66 reports found by Zwischenberger 2011, of which 47 fulfilled their criteria for inclusion (5 RCTs).
In concordance with our findings, Careta 2015, Fett 2012, Tratenberg 2017 and Zwischenberger 2011 found that methotrexate in combination with a short course of oral prednisone may be an effective therapy for treating active juvenile morphea (linear, generalised or mixed). Careta 2015 reported that the treatment of severe types of morphea with corticosteroids and MTX was similar compared to the treatment with MTX alone, but this conclusion was based on the results of non‐randomised and uncontrolled studies. Our findings regarding the lack of studies investigating the effectiveness of the treatment with MTX combined with steroids versus MTX alone are in accordance with the results of Fett 2012 and Zwischenberger 2011.
Our results concerning efficacy and safety of phototherapy correspond closely with Careta 2015, Fett 2012, Tratenberg 2017 and Zwischenberger 2011. Although phototherapy may be effective for treatment of widespread and progressive superficial forms of morphea, these reviews agree that there is no evidence of more effectiveness with one type of phototherapy over another, and that more studies are necessary to establish optimum dose and regimen.
With regard to the outcome measures, our findings are in accordance with Zwischenberger 2011: outcome measures across studies are often inconsistent and not fully validated, and there is little data on the improvement of disease damage. In concordance with our results, Zwischenberger 2011 noted that few studies differentiated active and inactive stages of the disease, and few trials reported response to treatment according to population (juvenile and adult) and morphea subtypes.
Authors' conclusions
Implications for practice.
There is a lack of high‐certainty evidence for the treatment of morphea, and more studies are necessary to establish the optimal treatment, dosage and period of treatment for each morphea subtype. Although the studies were too heterogeneous for a meta‐analysis, the results of this systematic review help to confirm current understandings regarding the effectiveness of different treatments which are used in practice to varying degrees, and identify opportunities for future research. It is important to analyse and balance the risks and benefits of the available treatments, considering the preferences and needs of each individual.
Low‐certainty evidence demonstrates that oral MTX plus prednisone may be more effective than placebo plus prednisone in the global improvement of disease activity or damage for treating active juvenile morphea (linear scleroderma, generalised morphea and mixed morphea: linear and circumscribed). There may be little or no difference in the number of participants experiencing at least one adverse event (e.g. alopecia, nausea, headache, fatigue) when comparing oral methotrexate with placebo.
Low‐certainty evidence indicates that there may be a similar effectiveness between treatment with low‐dose UVA‐1 (20 J/cm²), medium‐dose UVA‐1 (50 J/cm²) and narrowband UVB phototherapy in terms of reduction of disease activity or damage in children and adults with active morphea (circumscribed morphea, linear scleroderma (with trunk/limb variant and head variant), generalised morphea or mixed morphea). UVA‐1 phototherapy may cause mild tanning compared to narrowband UVB phototherapy, but there may be no difference when comparing medium‐ and low‐dose UVA‐1 phototherapy. Transient erythema was reported in three participants with narrowband UVB and in none of the participants in either the low‐ or medium‐dose UVA‐1 groups.
Implications for research.
Future studies could consider investigating the treatment of:
morphea characterised by superficial and multiple lesions with phototherapy combined with initial systemic corticosteroid treatment versus phototherapy alone;
linear scleroderma, generalised morphea, pansclerotic morphea or mixed subtype with MTX in combination with initial systemic corticosteroid treatment versus MTX alone;
refractory morphea previously treated with MTX: phototherapy in combination with MTX versus phototherapy alone; and
circumscribed morphea with topical tacrolimus versus topical corticosteroid versus placebo.
Different doses and regimens of phototherapy also need assessing. We encourage multicentre studies of national and international collaborative networks to increase sample sizes, which need to be much larger (e.g. 100 to 200). The report of RCTs must follow the CONSORT 2010 statement (Schulz 2010), and include a registered protocol. Studies ideally should have a triple‐blind design (participants, personnel, and outcome assessors), a long‐term follow‐up (more than one year), and use validated outcome measures such as the LoSCAT. Other technical procedures to access outcome measures should also be included, as well as quality of life and psychosocial outcomes. The development of a core outcomes set on morphea in international platforms such as the Cochrane Skin Core Outcome Set Initiative (CS‐COUSIN) may enable greater potential for studies to be pooled in future updates. Global individual assessments are preferable than intra‐individual comparisons. Considering the heterogeneity of the disease, it is important to evaluate, with validated tools, different treatment responses according to the subtypes of morphea and age groups. Studies should report both combined and separate data in case of multiple morphea subtypes or age groups.
Acknowledgements
We would like to thank Cochrane Brazil and the editorial team of Cochrane Skin (Emma Axon, Finola Delamere, Helen Scott, Laura Prescott, Liz Doney). We would also like to thank: Alireza Firooz, Daniel Heinl, Lu Ban, and Volha Shpadaruk, who translated the articles in Persian, German, Chinese and Russian, respectively.
Cochrane Skin would like to thank the peer referees: Hywel Williams (key editor); Laurence Le Cleach (methods editor); Matthew Grainge (statistical editor); Peggy A Wu and Heidi Jacobe (external content experts); and Jason Elliot‐Smith, who copy‐edited this review.
Appendices
Appendix 1. Cochrane Skin Group Specialised Register (CRS Web)
"en coup de sabre" or morphea* or scleroderma* or "parry romberg"
Appendix 2. CENTRAL (Cochrane Library) search strategy
#1 MeSH descriptor: [Scleroderma, Localized] explode all trees #2 morphea*:ti,ab,kw #3 en coup de sabre:ti,ab,kw #4 (circumscri* and scleroderma*):ti,ab,kw #5 parry romberg:ti,ab,kw #6 localised scleroderma*.ti,ab,kw #7 localized scleroderma*.ti,ab,kw #8 linear scleroderma*.ti,ab,kw #9 {or #1‐#8}
Appendix 3. MEDLINE (Ovid) search strategy
1. exp Scleroderma, Localized/ 2. morphea$1.mp. 3. en coup de sabre.mp. 4. circumscribed scleroderma$.mp. 5. parry romberg.mp. 6. localised scleroderma$.mp. 7. localized scleroderma$.mp. 8. linear scleroderma$.mp. 9. or/1‐8 10. randomised controlled trial.pt. 11. controlled clinical trial.pt. 12. randomised.ab. 13. placebo.ab. 14. clinical trials as topic.sh. 15. randomly.ab. 16. trial.ti. 17. 10 or 11 or 12 or 13 or 14 or 15 or 16 18. exp animals/ not humans.sh. 19. 17 not 18 20. 9 and 19
[Lines 10‐19: Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision)]
Appendix 4. Embase (Ovid) search strategy
1. morphea$1.mp. 2. en coup de sabre.mp. 3. circumscribed scleroderma$.mp. 4. parry romberg.mp. 5. localised scleroderma$.mp. 6. localized scleroderma$.mp. 7. linear scleroderma$.mp. 8. morphea/ 9. exp localized scleroderma/ 10. scleroderma circumscripta.mp. 11. or/1‐10 12. crossover procedure.sh. 13. double‐blind procedure.sh. 14. single‐blind procedure.sh. 15. (crossover$ or cross over$).tw. 16. placebo$.tw. 17. (doubl$ adj blind$).tw. 18. allocat$.tw. 19. trial.ti. 20. randomised controlled trial.sh. 21. random$.tw. 22. or/12‐21 23. exp animal/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 24. human/ or normal human/ 25. 23 and 24 26. 23 not 25 27. 22 not 26 28. 11 and 27
Appendix 5. LILACS search strategy
morphea$ or morfea$ or scleroderma$ or esclerodermia$
In LILACS we searched using the above terms and the Controlled clinical trials topic‐specific query filter.
Appendix 6. Trial registers search strategies
• ISRCTN registry
morphea OR ("localized scleroderma") OR ("localised scleroderma")
• US National Institutes of Health Ongoing Trials Register
morphea OR localized scleroderma OR localised scleroderma
• Australian New Zealand Clinical Trials Registry
morphea OR "localized scleroderma" OR "localised scleroderma"
• World Health Organization International Clinical Trials Registry platform
morphea OR locali scleroder*
• EU Clinical Trials Register
morphea OR "localized scleroderma" OR "localised scleroderma"
Data and analyses
Comparison 1. Medium‐dose UVA‐1 (50 J/cm²) x Low‐dose UVA‐1 (20 J/cm²) phototherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Modified Skin Score (MSS) at the end of the eight‐week treatment | 1 | 44 | Mean Difference (IV, Random, 95% CI) | 1.60 [‐1.70, 4.90] |
| 2 Modified Skin Score (MSS) at last follow‐up visit (week 20) | 1 | 44 | Mean Difference (IV, Random, 95% CI) | 2.5 [‐1.90, 6.90] |
| 3 Number of participants with mild tanning | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.91, 1.10] |
| 4 Ultrasound dermal density at the end of the eight‐week treatment | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐16.43 [‐34.87, 2.01] |
| 5 Ultrasound corium thickness at the end of the eight‐week treatment | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 196.29 [‐162.28, 554.86] |
Comparison 2. Narrowband UVB x Medium‐dose UVA‐1 (50 J/cm²) phototherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Modified Skin Score (MSS) at the end of the eight‐week treatment | 1 | 35 | Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐5.27, 1.87] |
| 2 Modified Skin Score (MSS) at last follow‐up visit (week 20) | 1 | 35 | Mean Difference (IV, Random, 95% CI) | ‐2.10 [‐6.73, 2.53] |
| 3 Number of participants with mild tanning | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 0.03 [0.00, 0.42] |
| 4 Number of participants with transient erythema | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 6.63 [0.37, 119.59] |
| 5 Ultrasound dermal density at the end of the eight‐week treatment | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 17.78 [‐6.08, 41.64] |
| 6 Ultrasound corium thickness at the end of the eight‐week treatment | 1 | 28 | Mean Difference (IV, Random, 95% CI) | ‐78.35 [‐528.59, 371.89] |
Comparison 3. Narrowband UVB X Low‐dose UVA‐1 (20 J/cm²) phototherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Modified Skin Score (MSS) at the end of the eight‐week treatment | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐2.49, 2.29] |
| 2 Modified Skin Score (MSS) at last follow‐up visit (week 20) | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐2.17, 2.97] |
| 3 Number of participants with mild tanning | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.03 [0.00, 0.41] |
| 4 Number of participants with transient erythema | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 10.32 [0.56, 188.49] |
| 5 Ultrasound dermal density at the end of the eight‐week treatment | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 1.35 [‐19.39, 22.09] |
| 6 Ultrasound corium thickness at the end of the eight‐week treatment | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 117.94 [‐311.20, 547.08] |
Comparison 4. Medium‐dose (70 J/cm²) or Low‐dose (20 J/cm²) UVA‐1 phototherapy x No treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of plaques with moderate to significant tanning | 1 | Risk Ratio (Random, 95% CI) | 29.00 [1.90, 443.28] | |
| 2 Number of plaques with painless erythema | 1 | Risk Ratio (Random, 95% CI) | 4.41 [0.23, 84.79] | |
| 3 Number of plaques with pruritus | 1 | Risk Ratio (Random, 95% CI) | 4.41 [0.23, 84.79] |
Comparison 5. 20 J/cm2 UVA x 10 J/cm² UVA phototherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants with generalised tanning | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.92, 1.09] |
| 2 Number of participants with temporary pruritus | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.18, 3.60] |
| 3 Number of participants with increased erythema and exacerbated pain | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [0.10, 57.08] |
| 4 Number of participants with skin softening at the end of the seven‐week treatment | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.69, 2.11] |
Comparison 6. 20 J/cm2 UVA x 5 J/cm² UVA phototherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants with generalised tanning | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.91, 1.10] |
| 2 Number of participants with temporary pruritus | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.14, 2.69] |
| 3 Number of participants with increased erythema and exacerbated pain | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [0.08, 43.75] |
| 4 Number of participants with skin softening at the end of the seven‐week treatment | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.75, 3.14] |
Comparison 7. 10 J/cm2 UVA x 5 J/cm² UVA phototherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants with generalised tanning | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.90, 1.11] |
| 2 Number of participants with temporary pruritus | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.18, 3.29] |
| 3 Number of participants with skin softening at the end of the seven‐week treatment | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.58, 2.76] |
Comparison 8. Photodynamic therapy × No Treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of plaques with pigmentation | 1 | Risk Ratio (Random, 95% CI) | 13.00 [0.89, 189.38] | |
| 2 Number of plaques with burning sensation during phototherapy | 1 | Risk Ratio (Random, 95% CI) | 9.00 [0.59, 137.65] | |
| 3 Number of plaques with dryness | 1 | Risk Ratio (Random, 95% CI) | 3.00 [0.15, 61.73] | |
| 4 Number of plaques with erythema | 1 | Risk Ratio (Random, 95% CI) | 5.00 [0.29, 86.43] | |
| 5 Number of plaques with pruritus | 1 | Risk Ratio (Random, 95% CI) | 3.00 [0.15, 61.73] | |
| 6 Number of plaques with reduction in the skin score at the 12‐week follow‐up | 1 | Risk Ratio (Random, 95% CI) | 1.0 [0.45, 2.23] |
Comparison 9. Fractional carbon dioxide laser × Low‐dose UVA‐1 (30 J/cm²) phototherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of lesions with mild to moderate pain during therapy | 1 | Risk Ratio (Random, 95% CI) | 35.00 [2.27, 538.86] | |
| 2 Number of lesions with marked pain during therapy | 1 | Risk Ratio (Random, 95% CI) | 21.00 [1.33, 332.06] | |
| 3 Number of lesions with pruritus in first 24h | 1 | Risk Ratio (Random, 95% CI) | 17.00 [1.06, 273.00] | |
| 4 Number of lesions with hyperpigmentation | 1 | Risk Ratio (Random, 95% CI) | 0.25 [0.03, 2.01] | |
| 5 Number of lesions with persistent erythema | 1 | Risk Ratio (Random, 95% CI) | 3.00 [0.13, 68.84] | |
| 6 Ultrasound biomicroscopy dermal thickness at the 10‐week follow‐up | 1 | Mean Difference (Random, 95% CI) | ‐0.15 [‐0.33, 0.03] | |
| 7 Clinical score at the 10‐week follow‐up | 1 | Mean Difference (Random, 95% CI) | ‐1.59 [‐2.82, ‐0.36] | |
| 8 Participant satisfaction score at the 10‐week follow‐up | 1 | Mean Difference (Random, 95% CI) | 1.12 [0.80, 1.44] | |
| 9 Number of lesions with good or very good improvement in the clinical score at the 10‐week follow‐up | 1 | Risk Ratio (Random, 95% CI) | 2.67 [1.39, 5.13] |
Comparison 10. Polymerized collagen intralesional injection (0.2 to 1.0 mL / 1.66 to 8.3 mg weekly) × Methylprednisolone subcutaneous injection (maximum dose of 20 mg or 5.0 mL monthly) plus placebo intralesional injection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants with short‐duration pain at injection site | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 5.79 [1.86, 18.02] |
| 2 Number of participants with pruritus | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.14] |
| 3 Number of participants with sclerosis | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.02, 8.06] |
| 4 Skin score at the end of the three‐month treatment | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 1.30 [0.31, 2.29] |
| 5 Skin score at last follow‐up visit (month nine) | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐0.25, 1.25] |
| 6 Number of morphea lesions at the end of the three‐month treatment | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.16, 0.56] |
Comparison 11. Oral methotrexate (15 mg/m², maximum 20 mg a week) plus oral prednisone × Placebo plus oral prednisone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical response at the end of the 12‐month treatment | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 2.31 [1.20, 4.45] |
| 2 Number of participants with at least one adverse event | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.75, 2.04] |
| 3 Skin Score Rate (SSR) at the end of the 12‐month treatment | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.35, ‐0.27] |
| 4 Percentage thermal change at the end of the 12‐month treatment | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐32.3 [‐37.92, ‐26.68] |
| 5 Number of participants with new lesions at the end of the 12‐month treatment | 1 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.10, 1.61] |
Comparison 12. Oral hydroxychloroquine (200 mg twice a day) plus topical corticosteroid x Oral methotrexate (15 mg a week) plus topical corticosteroid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Modified Skin Score (MSS) at the end of the three‐month treatment | 1 | 25 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐1.93, 2.93] |
| 2 Number of participants with liver enzymes rise more than three times during the three‐month treatment | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.01, 2.33] |
| 3 VAS for participants' estimate of skin tightness at the end of the three‐month treatment | 1 | 25 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.49, 1.09] |
| 4 VAS for participants' estimate of pruritus at the end of the three‐month treatment | 1 | 25 | Mean Difference (IV, Random, 95% CI) | ‐2.3 [‐3.25, ‐1.35] |
Comparison 13. Topical tacrolimus 0.1% × Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of plaques with pruritus | 1 | Risk Ratio (Random, 95% CI) | 3.00 [0.14, 65.90] | |
| 2 Durometer score at the end of the 12‐week treatment | 1 | Mean Difference (Random, 95% CI) | 47.2 [‐44.55, 138.95] | |
| 3 Plaque surface area at the end of the 12‐week treatment | 1 | Mean Difference (Random, 95% CI) | 0.5 [‐38.35, 39.35] | |
| 4 Number of plaques with a reduced modified DIET score at the end of the 12‐week treatment | 1 | Risk Ratio (Random, 95% CI) | 1.14 [0.69, 1.90] | |
| 5 Modified DIET score at the end of the 12‐week treatment | 1 | Mean Difference (Random, 95% CI) | ‐1.7 [‐3.11, ‐0.29] |
Comparison 14. Oral calcitriol (0.75 μg increased to 1.25 μg/day) × Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants with transient hypercalciuria | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 7.50 [0.42, 132.58] |
| 2 Skin score at the end of the 9‐month treatment | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 1.10 [‐2.98, 5.18] |
| 3 Skin score at the end of the 15‐month follow‐up | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 3.70 [‐1.49, 8.89] |
Comparison 15. Tranilast plus topical betamethasone valerate 0.1% versus topical betamethasone valerate 0.1%.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical score at the end of the three‐month treatment | 1 | Mean Difference (Random, 95% CI) | ‐1.5 [‐3.88, 0.88] | |
| 2 Physician Global Assessment of Activity at the end of the three‐month treatment | 1 | Mean Difference (Random, 95% CI) | ‐7.5 [‐23.73, 8.73] | |
| 3 Number of lesions with disease progression at the end of the three‐month treatment | 1 | Risk Ratio (Random, 95% CI) | 0.24 [0.01, 4.47] | |
| 4 Physician Global Assessment of Damage at the end of the three‐month treatment | 1 | Mean Difference (Random, 95% CI) | ‐6.00 [‐24.90, 12.90] |
Comparison 16. Traditional Chinese Medicine herbal tea plus herbal oil and vitamin B6 x Phenoxymethylpenicillin plus DAC base cream.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants with adverse events | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.17, 5.98] |
Comparison 17. Acupuncture, hot herbal compress, and moxibustion plus Centella triterpenes tablets and vitamin E × Heparin sodium cream plus Centella triterpenes tablets and vitamin E.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants with significant clinical response at the end of the six‐month treatment | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [1.04, 2.59] |
| 2 Number of participants with pain during treatment | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 2.61 [0.11, 60.51] |
| 3 Skin sclerosis score at the end of the six‐month treatment | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐10.34 [‐16.83, ‐3.85] |
| 4 Joint function score at the end of the six‐month treatment period | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐1.65 [‐2.95, ‐0.35] |
| 5 Joint pain score at the end of the six‐month treatment | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐6.63 [‐11.20, ‐2.06] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Azimi 2013.
| Methods | This was a double‐blind randomised clinical trial conducted in Tabriz, northwestern Iran. The aim of this study was to compare the effect of methotrexate and hydroxychloroquine in treatment of morphea. | |
| Participants |
Inclusion criteria: presence of morphea without any signs of systemic involvement, evidence of active and expanding lesions (evidence of the spreading lesions, the appearance of new lesions or clinical signs of inflammation include erythema and warmth over the past 3 months), morphea confirmed with a histological samples from all patients before treatment, any systemic treatment with effect on morphea had to be discontinued for at least 4 weeks and topical treatments for at least 2 weeks prior to the study. Exclusion criteria: acute or chronic infection, pregnancy or childbearing potential without an acceptable method to prevent, liver disease or elevated liver enzyme levels more than twice the normal, creatinine level of more than 130 mmol, known pulmonary disease, leukocyte count less than 3500 or platelet count less than 150000, active peptic ulcer or neoplasm, insulin‐dependent diabetes mellitus, use of other anti‐folate drugs such as sulphonamides, and allopurinol and Probenesid, presence of lupus or mixed connective tissue disease (MCTD), contraindication of ophthalmology to start hydroxychloroquine. Number of participants randomised: 30 (15 group 1 + 15 group 2) Number of participants analysed: 25 (13 group 1 + 12 group 2) Women: 16 (9 group 1 + 7 group 2) Men: 9 (4 group 1 + 5 group 2) Age: mean age in group 1 was 33.7 ± 11 years (13 to 57) and 40.2 ± 15.5 years (17 to 74) in group 2. Ethnicity: Iranian Morphea Type: authors did not report this information (probably circumscribed). Length of illness: authors did not report this information. |
|
| Interventions |
Group 1: a. Therapy and dosage: hydroxychloroquine (200 mg twice a day) plus topical corticosteroid (authors did not mention the type or dosage). b. Administration: oral and topical c. Duration of treatment: 3 months d. Follow‐up after treatment: none Group 2: a. Therapy and dosage: methotrexate (15 mg) once a week (on Fridays) plus folic acid 1mg daily (except for Friday) and topical corticosteroid (authors did not mention the type or dosage). b. Administration: oral and topical c. Duration of treatment: 3 months d. Follow‐up after treatment: none |
|
| Outcomes | The clinical score MSS, which is the numerical sum of the thickness and percentage of involvement in 7 regions of the body. Thickness and flexibility were scored from zero to 3, and the area with the most severe grade was recorded as the final grade. The extent of involvement was scored as: 0: no involvement; 1: less than 33% involvement; 2: 33% to 67% involvement; 3: more than 67% involvement. Participants also evaluated hardness and pruritus of the skin through the Visual Analogue Scale. Assessments: follow‐up at week 4,8 and 12 after initiating the treatment. |
|
| Notes |
Trial registration: authors registered the protocol of this study on the Iranian Registry of Clinical Trials (IRCT201110057712N2). Ethics committee approval: Tabriz University of Medical Sciences and Health Services Funding source: Tabriz University of Medical Sciences and Health Services Declarations of interest: authors did not report this information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The translator informed us that the authors used Randlist, a software which creates randomisation lists. Thus, the method used to generate the random sequence was considered adequate to produce comparable groups. |
| Allocation concealment (selection bias) | Unclear risk | Authors did not provide enough information to judge if participants and investigators enrolling participants could foresee assignment. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | The translator informed us that the pharmacist prepared the drugs in the form of identical capsules. However, the intervention group took 1 capsule once a week whilst the control group took a capsule twice a day. Thus, the outcome is likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The translator informed us that authors reported that the physician who registered the data was also blinded. Thus, the outcome assessor was effectively blinded from knowledge of which intervention a participant received. It is unlikely that the blinding could have been broken. |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Quote: "We studied 30 patients with localised scleroderma". Quote: "In our study, 9 (36%) of patients was male and 16 (64%) was female". Quote: "Three patients from group A withdrew due to severe side effects of MTX and two patients from group B did not return for follow‐up". Comment: authors excluded 5 participants and performed 'as treated' analysis, including data only from the participants who completed the treatment. |
| Selective reporting (reporting bias) | Low risk | Authors reported all outcomes pre‐specified in the study protocol. |
| Other bias | Unclear risk | The clinical tool used (MSS) is validated for SSc, and is inappropriate for the measurement of morphea skin involvement, which could affect the result. |
Batchelor 2008.
| Methods | This was a prospective, comparative, single‐centre pilot trial with intraindividual controls, conducted in England. The aim of this study was to investigate the treatment of morphea with topical photodynamic therapy (PDT) using 5‐aminolaevulinic acid (ALA). | |
| Participants |
Inclusion criteria: individuals aged > 18 years, with at least 2 areas of localised morphea affecting the trunk or limbs. Exclusion criteria: authors did not report this information. Number of participants: 7 Number of lesions randomised: 14; 7 Treatment/7 Control Number of lesions analysed: 12; 6 T/6 C Women: authors did not report this information. Men: authors did not report this information. Age: authors did not report this information (only adults). Ethnicity: authors did not report this information. Morphea Type: circumscribed morphea. Length of illness: authors did not report this information. |
|
| Interventions |
Lesion 1: a. Therapy and dosage: 5‐ALA 20% cream, applied under occlusion for 5 hours, followed by PDT with a non‐coherent, broadband halogen light source filtered to give 570 nm to 670 nm bandwidth (full width, half maximum), peak 635 nm, to an area of 55 mm in diameter. A dose of 25 J ⁄ cm² was given at a rate of 90 mW ⁄ cm², once a week. b. Administration: topical c. Duration of treatment: 6 weeks d. Follow‐up after treatment: 6 weeks Lesion 2: a. Therapy and dosage: no treatment b. Duration of treatment: six weeks d. Follow‐up after treatment: six weeks |
|
| Outcomes |
Primary outcomes: improvement in the clinical score, assessing the skin as: 0 = normal skin, 1 = thickened skin, 2 = decreased ability to pinch or move the skin and 3 = skin unable to be pinched or moved (hidebound). Secondary outcomes: improvement in durometer score and histological appearances of skin biopsies, and cutaneous tolerance of the treatment. Assessments: weekly outcomes assessments for both lesions, on the same days as intervention (6 days in total) and at follow‐up. |
|
| Notes |
Intervention product information/details: light source was PhotoCureTM CureLight 1301; PhotoCure ASA, PO Box 55, Montebello, N‐0310, Oslo, Norway. Trial registration: this was a pilot study and authors did not mention a registered protocol. Ethics committee approval: Leeds (West) Funding source: authors did not report this information. Declarations of interest: authors declared no conflict of interest. The author list of this study was incomplete, and an erratum with the name of all authors and their affiliations was published in November 2008. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "The selection of which of the two plaques was to be treated was made randomly by a technician who performed the PDT". Comment: authors did not describe the method used to generate the random sequence in sufficient detail to allow an assessment of whether the allocation was adequate to produce comparable groups. |
| Allocation concealment (selection bias) | Unclear risk | Authors did not provide enough information to judge if participants and investigators enrolling participants could foresee assignment. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Each patient had 1 of the randomised lesions treated and the other not, thus participants and personnel had knowledge of which lesion was treated. The outcome is likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Quote: "Although the investigator was initially blinded to the selection of the treated lesion, this was not maintained because the treated lesion became pigmented after the initial PDT treatment". Comment: the outcome assessor had knowledge of which lesion was treated due to pigmentation after treatment, thus the blinding of outcome assessment was broken. The outcome measurement is likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Quote: "One patient completed only three treatments of PDT then failed to attend any further appointments". Comment: authors excluded this participant and performed 'as treated' analysis, including data only from the participants who completed the treatment (6 out of 7 participants). |
| Selective reporting (reporting bias) | High risk | Authors presented durometer readings data only for treated lesions, thus there is no available comparison for this outcome. |
| Other bias | Unclear risk | The clinical tool used assessed individual lesions instead of all the lesions of the individual, which could affect the result. |
El‐Mofty 2004.
| Methods | This was a prospective, randomised, 3‐arm active comparison trial, conducted in Egypt. The aim of this study was to define the lowest effective broadband UVA dose in the treatment of morphea and SSc. | |
| Participants |
Inclusion criteria: individuals complaining of cutaneous sclerosis. Exclusion criteria: authors did not report this information. Number of participants randomised: 67 participants with morphea. This study also included 17 individuals with systemic scleroderma (their abbreviation = SS), who were randomised separately from the morphea group. Number of participants analysed: 63 participants with morphea (16 group 1 + 21 group 2 + 26 group 3) Women: 43 (6 group 1 + 16 group 2 + 21 group 3) Men: 20 (10 group 1 + 5 group 2 + 5 group 3) Age: mean age in group 1 was 17.88 ± 13.00 years (3 to 47), 22.14 ± 12.90 years (6 to 51) in group 2 and 20.85 ± 14.75 years (6 to 66) in group 3. Ethnicity: 9 participants were skin type III, 37 participants were skin type IV and 17 participants were skin type V, according to Fitzpatrick’s classification. Morphea Type: 27 participants presented with circumscribed morphea (7 group 1 + 12 group 2 + 8 group 3), 12 participants presented with linear scleroderma (3 group 1 + 1 group 2 + 8 group 3) and 24 participants presented with generalised morphea (6 group 1 + 8 group 2 + 10 group 3). Length of illness: between 1 and 10 years; 20.28 ± 22.69 months (2 to 8) in group 1, 25.48 ± 33.12 months (1 to 120) in group 2 and 23.54 ± 24.46 months (1 to 120) in group 3. |
|
| Interventions | Total body irradiation with different low doses of UVA (320 nm to 400 nm; broadband light source with a spectrum of 315 nm to 400 nm and a maximum at 365 nm). During therapy patients wore protective goggles and covered the genitals. Group 1: a. Therapy and dosage: 5 J/cm²/session, 3 times a week for 20 sessions. b. Administration: topical c. Duration of treatment: 7 weeks d. Follow‐up after treatment: none Group 2: a. Therapy and dosage: 10 J/cm²/session, 3 times a week for 20 sessions. b. Administration: topical c. Duration of treatment: 7 weeks d. Follow‐up after treatment: none Group 3: a. Therapy and dosage: 20 J/cm²/session, 3 times a week for 20 sessions. b. Administration: topical c. Duration of treatment: 7 weeks d. Follow‐up after treatment: none |
|
| Outcomes | Inspection of the skin lesions as regards site, colour (hypo‐ or hyperpigmented), pattern of lesions (circumscribed, linear or disseminated) and palpation of the lesions for skin thickening, induration, atrophy and sclerosis were performed. The clinical response was assessed subjectively by palpation of the skin lesions for skin softening, graded as very good response (marked skin softening, almost normal skin texture), good response (moderate softening), fair response (mild softening) and poor response (no change in skin texture). Investigators assessed clinically other associated manifestations such as trophic changes, grip strength, flexion deformity, joint mobility, by evaluating each point as present (+) or absent (−) before treatment. After treatment, the criteria with (+) or present, were evaluated for an improvement, without scaling for the degree of change, and recorded as such. Participants and physicians also assessed subjectively these other manifestations.
Pre‐ and post‐treatment skin specimens were obtained from some morphea participants (4 from group I; 5 from group II; and 7 from group III) from the same plaque, and stained (haematoxylin and eosin) for routine histopathologic examination. Assessments: before starting UVA therapy, every week and at the end of the study period (20 sessions). |
|
| Notes |
Intervention product information/details: source of UVA was a Waldmann Medizin technik UVA cabin 7001 equipped with 40 UVA lamps and PUVA 1000 cabin containing 26 lamps of Waldmann type F 85/100 W‐PUVA. Trial registration: authors did not register a protocol for this study. Ethics committee approval: a research ethics committee was not established at Cairo University. Funding source: authors declared no funding sources. Declarations of interest: authors declared no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Patients were randomly divided into three groups". Comment: authors did not describe the method used to generate the random sequence in sufficient detail to allow an assessment of whether the allocation was adequate to produce comparable groups. However, authors responded to our contact and informed that they generated the random sequence by shuffling envelopes. Thus the process was adequate to produce comparable groups. |
| Allocation concealment (selection bias) | Low risk | Authors did not provide enough information to judge if the intervention allocation could be foreseen before or during the recruitment of participants. However, authors responded to our contact and informed that the allocation was unsealed only on the admission to the first treatment session. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Authors did not describe blinding, but responded to our contact and confirmed there was no blinding to the knowledge of which intervention a participant received. Thus the outcome is likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Authors did not clearly describe whether the outcome assessors were effectively blinded from knowledge of which UVA dose each participant received. However, authors responded to our contact and informed that the outcome assessors were unblinded to the knowledge of which intervention a participant received; only the histopathological assessors were blinded. Thus, the outcome measurement of interest is likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote: "Of 67 M patients who started UVA therapy, 63 completed the study. Dropouts were not related to therapy". (M = circumscribed morphea) Quote: "Clinical data of the 63 patients were assessed". Comment: authors excluded 4 patients (without informing from which group they were) and performed 'as treated' analysis, including data only from the participants who completed the treatment. However, this probably does not represent serious threats to validity of the results. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available. |
| Other bias | Unclear risk | The clinical assessment was not a validated measure, which could affect the result. |
Furuzawa‐Carballeda 2012.
| Methods | This was a prospective, double‐blind, randomised controlled trial with 3‐arm comparisons, conducted in Mexico. The aim of this study was to evaluate the clinical effect of polymerised collagen (PC) vs. methylprednisolone (MP) in the treatment of morphea, and to determine the influence of PC on Th1, Th2, Th17 and Treg peripheral cells, and on the expression in skin of TGF‐b1, IL‐17A, IL‐22 and Foxp3+ cells. | |
| Participants | The participants were enrolled over a period of 2 years (2008 to 2010) at the Dermatologic Centre Ladislado de la Pascua. Histological, immunohistochemical and flow cytometric evaluation was conducted at the Department of Immunology and Rheumatology of the National Institute of Medical Sciences and Nutrition Salvador Zubirán. Inclusion criteria: individuals aged > 18 years with a diagnosis of morphea based on clinical findings and histological evaluation; at least 1 skin lesion; and a negative reaction to a standard forearm skin test for PC administration. This study also included 10 healthy, untreated individuals (control group) to compare subsets of CD4+ peripheral T cells. Exclusion criteria: pregnancy or breastfeeding; presence of lesions of lichen sclerosis et atrophicus; fibrosis induced by L‐tryptophan, bleomycin, vinyl to PC or its components; history of photosensitivity; use of topical steroids within the 2 months prior to the study, use of systemic corticosteroids or immunosuppressant drugs within 3 months prior to the study; concomitant chronic or malignant disease (melanoma or non‐melanoma skin cancer), any relevant abnormalities in baseline laboratory assessment at baseline, or serological evidence of Borrelia burgdorferi infection. Women of childbearing potential were required to use an acceptable means of contraception. Number of participants randomised: 31 (15 group 1 + 16 group 2) Number of participants analysed: 27 (13 group 1 + 14 group 2) Women: 25 (12 group 1 + 13 group 2) Men: 6 (3 group 1 + 3 group 2) Age: mean age was 38.6 ± 15.0 years, range 18 to 73 years (35.5 ± 14.2 in group 1, 18 to 62; 41.4 ± 15.6 in group 2, 18 to 73). Ethnicity: authors did not report this information. Morphea Type: 30 participants presented with circumscribed morphea and 1 participant in group 1 presented with linear scleroderma. Lenght of illness: 40.5 ± 37.8 months (median 24) |
|
| Interventions |
Group 1: a. Therapy and dosage: weekly injections of PC ranging from 0.2 mL (equivalent to 1.66 mg collagen) for a lesion of 50 mm in size, up to a maximum of 1.0 mL (8.3 mg collagen) for a lesion > 100 mm in size. b. Administration: subcutaneous (intralesional) c. Duration of treatment: 3 months d. Follow‐up after treatment: 6 months Group 2: a. Therapy and dosage: monthly subcutaneous injections of 0.1 mL methylprednisolone (MP, maximum dose of 20 mg or 5 mL ⁄ month) and weekly subcutaneous injections of 0.1 mL placebo (PVP citric ⁄ citrate buffer). b. Administration: subcutaneous (intralesional) c. Duration of treatment: 3 months d. Follow‐up after treatment: 6 months Groups 1 and 2: additional treatment was restricted to the use of emollients, but the composition was not specified. Group 3: no intervention in healthy participants (not included in the review), only a blood sample. |
|
| Outcomes | Complete blood cell count, serum analyses including glucose and electrolyte measurement, and liver function tests and urinalysis. Skin appearance was assessed using a score adapted from the modified Rodnan Scoring System (mRSS), which is an aggregate skin score (0 = normal; 1 = thickened skin (able to pinch skin fold); 2 = thickened skin (unable to pinch skin fold); or 3 = unable to move skin) assessed for each of 17 areas (face, anterior chest, abdomen plus each limb (comprising upper arm, forearm, dorsum of hand, fingers, thighs, lower legs, dorsum of foot)). The maximum potential score is 51. All individual lesions of morphea were graded from 0 to 4 (0 = normal thickness; 1 = mild thickening; 2 = moderate thickening; 3 = severe thickening; 4 = extreme thickening). The total score for all the lesions was then calculated. A decrease in the adapted mRSS exceeding 35% from baseline was considered to reflect a clinically significant improvement. A biopsy site was chosen according to clinical criteria, and was required to be an indurate and inflammatory area, excluding areas with close proximity to vasculature or tendons. The biopsy was observed and also study through Immunohistochemistry to determine expression of IL‐17A, IL‐22, TGF‐b1 and Foxp3. A sample (10 mL) of venous blood was obtained from each subject and a single blood sample was also collected from each of the 10 healthy controls. Peripheral blood mononuclear cells (PBMCs) were obtained by gradient centrifugation. Treatment safety was determined by the occurrence of systemic and local adverse events (AEs) related to administration of the study drug. Safety monitoring included records of vital signs and clinical laboratory tests (blood chemistry, urinalysis and liver function tests). Assessments: laboratory exams were performed at baseline, at the end of treatment and at the last follow‐up visit. At each subsequent visit, efficacy evaluations were conducted and AEs recorded before the scheduled administration of the study medication. Participants attended for follow‐up every 4 weeks for 6 months after the end of the treatment period. |
|
| Notes |
Trial registration: authors did not register a protocol for this study. Ethics committee approval: from both institutions, the National Institute of Medical Sciences and Nutrition Salvador Zubirán and the Dermatologic Centre Ladislado de la Pascua. Funding source: authors did not report this information. However, authors responded to our contact and informed there was no funding or grant. Declarations of interest: authors declared no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Patients were allocated using random number generation and block randomisation to two parallel groups, to receive either PC or steroid". Comment: the method used to generate the random sequence was adequate to produce comparable groups. |
| Allocation concealment (selection bias) | Low risk | Authors did not provide enough information to judge if the intervention allocation could be foreseen before or during the recruitment of participants. However, authors responded to our contact and reported using sealed envelopes to conceal the assignment. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk |
Quote: "Both researchers and patients were blinded to the study preparations and had no access to them". Quote: "The placebo experimental preparation was visually identical, and its viscosity was very similar to PC". Comment: participants and personnel were effectively blinded from knowledge of which intervention a participant received. It is unlikely that the blinding could have been broken. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Quote: "All patients were assessed before and after treatment by two dermatologists blinded to the treatment groups". Comment: outcome assessors were effectively blinded from knowledge of which intervention a participant received. It is unlikely that the blinding could have been broken. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote: "For the primary analysis, the means of the scores were compared between the two treatment groups on an intention‐to‐treat (ITT) basis (all patients who received a dose of study medication and had at least one efficacy observation recorded after treatment)". Quote: "In total, 13 patients (87%) in the PC group and 14 (88%) in the MP group completed the study, and were valid for ITT analysis" Comment: authors performed ITT analysis. In addition, authors reported no numerical data for adverse events, but responded to our contact and provided them. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available. |
| Other bias | Unclear risk | Authors reported the baseline mean clinical score for all participants, but responded to our contact and provided this data for each group (no statistical difference). However, the clinical tool used (adapted mRSS) assessed individual lesions instead of all the lesions of the individual, which could affect the result. |
Hulshof 2000.
| Methods | This was a randomised, double‐blind, placebo‐controlled trial conducted at the Leiden University Medical Center, in the Netherlands. The aim of this study was to assess the therapeutic effect and possible side effects of calcitriol in morphea and SSc. | |
| Participants |
Inclusion criteria: participants diagnosed with morphea (localised or generalised) or SSc according to the criteria in the literature. Exclusion criteria: use of any systemic or topical therapy for morphea less than 1 month before the start of the study; use of medications (such as calcium, vitamin D, vitamin D metabolites and analogues, multivitamin preparations containing vitamin D, calcium‐containing antacids, digitalis, thiazide diuretics, and D‐penicillamine) that are likely to interfere with the assessment of safety, tolerance, or efficacy of the test drug; any clinically relevant abnormalities in the laboratory assessment at baseline; and serologic evidence of Borrelia burgdorferi infection. Women with childbearing potential had to commit themselves to adequate contraception. Number of participants randomised: 20 participants with morphea (10 group 1 + 10 group 2). This study also included 7 individuals with SSc, who were randomised separately from the morphea group. Number of participants analysed: 20 participants with morphea (10 group 1 + 10 group 2) Women: 19 (10 group 1 + 9 group 2) Men: 1 (group 2) Age: mean age in group 1 was 41.8 ± 19.1 years (17 to 72) and 55.5 ± 14.6 years (30 to 76) in group 2. Ethnicity: authors did not report this information. Morphea Type: circumscribed and generalised morphea Length of illness: 2.3 ± 1.5 years (0.15 to 4.9) in group 1 and 2.6 ± 1.9 years (0.37 to 5.6) in group 2. |
|
| Interventions |
Group 1: a. Therapy and dosage: 0.75 μg calcitriol once a day for 6 months, followed by 1.25 μg calcitriol once a day for 3 months. b. Administration: single oral dose just before bedtime. Dietary calcium intake was moderated with assistance of a dietician. c. Duration of treatment: 9 months d. Follow‐up after treatment: 6 months Group 2: a. Therapy and dosage: placebo (composition not specified) once a day for 9 months. b. Administration: single oral dose just before bedtime. Dietary calcium intake was moderated with assistance of a dietician. c. Duration of treatment: 9 months d. Follow‐up after treatment: 6 months |
|
| Outcomes | A semi‐quantitative measure of cutaneous involvement (skin score) in which the body is divided into 22 regions and the degree of skin involvement in each region is quantified as follows: 0 = normal; 1 = mild thickening; 2 = moderate thickening; and 3 = hidebound skin. At examination the highest possible semi‐quantitative skin score in an individual region is recorded. The maximum possible skin score is 66 units. Plus, measurement of serum markers of collagen synthesis and degradation; the aminoterminal propeptide of type III procollagen (PIIINP); the carboxyterminal propeptide of type I procollagen (PICP); and the cross‐linked telopeptide of type I collagen (ICTP). Monitoring of side effects included: a) measurement of serum values of calcium, albumin, phosphate, and creatinine; b) 48‐hour urinary calcium/creatinine ratio; c) serum calcitriol (1,25[OH]2D3); and d) serum parathyroid hormone. Assessments: the participants were seen by 2 dermatologists at baseline and follow‐up visits: 1 dermatologist was the skin scores assessor, and the other informed the patients about laboratory values, medication, etc. Follow‐up visits were scheduled at every month for 9 months and then at 12 and 15 months. Skin score was interpreted at baseline, 3, 6, 9, 12, and 15 months. Serum markers measured at baseline and at 9 months. Side effects monitoring included the following measurements: serum values of calcium, albumin, phosphate, and creatinine monthly; 48‐hour urinary calcium/creatinine ratio monthly; serum calcitriol (1,25[OH]2D3) every 3 months; and serum parathyroid hormone at baseline and 9 months. |
|
| Notes |
Trial registration: authors did not mention a registered protocol. Ethics committee approval: Leiden University Medical Center. Fundind source: Roche Nederland BV, Mijdrecht, provided calcitriol. Declarations of interest: authors did not report this information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Participants who enrolled in the study were stratified according to the diagnoses morphea or SSc. The pharmaceutical chemist provided randomisation lists, with the patients grouped in blocks of 6 to ensure that the two groups were balanced within the strata". Comment: the method used to generate the random sequence was adequate to produce comparable groups. |
| Allocation concealment (selection bias) | Unclear risk | Authors did not provide enough information to judge if the intervention allocation could be foreseen before or during the recruitment of participants. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk |
Quote: "The pharmaceutical chemist of the LUMC provided placebo and arranged calcitriol and placebo in a blinded fashion". Comment: Participants and personnel were effectively blinded from knowledge of which intervention a participant received. It is unlikely that the blinding could have been broken. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Quote: "The pharmaceutical chemist of the LUMC provided placebo and arranged calcitriol and placebo in a blinded fashion". Comment: Outcome assessors were effectively blinded from knowledge of which intervention a participant received. It is unlikely that the blinding could have been broken. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote: "We analysed all assessable patients, including the patients who did not complete the 15‐month protocol, and used the last available data for the intent‐to‐treat analysis." Quote: "Three patients with morphea (1 receiving calcitriol and 2 receiving placebo) withdrew because of noncompliance, that is, they failed to present for outpatient monitoring after 3, 4, and 10 months of treatment, respectively. One patient with morphea receiving calcitriol withdrew after 13 months for personal reasons. The patients mentioned above, however, were included for analysis". Comment: Authors performed ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available. |
| Other bias | Unclear risk | The clinical tool used is validated for SSc, and is inappropriate for the measurement of morphea skin involvement, which could affect the result. |
Hunzelmann 1997.
| Methods | This was a double‐blind, randomised, placebo‐controlled, multi‐centre trial conducted in Germany. The aim of this study was to investigate the potential of interferon gamma (IFN‐γ) as an anti‐fibrotic agent in the treatment of morphea. | |
| Participants |
Inclusion criteria: all participants had evidence of progressive disease, that is, lesions showed evidence of an inflammatory reaction (lilac ring) and increase in size. Exclusion criteria: serologic evidence of Borrelia burgdorferi infection. Number of patients randomised: authors did not report this information. Number of participants analysed: 24 (authors did not report the number of participants in each group). Women: 14 Men: 10 Age: median age was 50 years in group 1, and 49.8 years in group 2. Ethnicity: authors did not report this information. Morphea Type: circumscribed morphea. Length of illness: 12 months (median) |
|
| Interventions |
Group 1: a. Therapy and dosage: 100 µg IFN‐γ in the periphery of 1 defined lesion on 5 consecutive days during the first 2 weeks and once a week for an additional 4 weeks. b. Administration: subcutaneous (intralesional). c. Duration of treatment: 6 weeks d. Follow‐up after treatment: 18 weeks Group 2: a. Therapy and dosage: placebo (0.9% sodium chloride) in the periphery of 1 defined lesion on 5 consecutive days during the first 2 weeks and once a week for an additional 4 weeks. b. Administration: subcutaneous (intralesional). c. Duration of treatment: 6 weeks d. Follow‐up after treatment 18 weeks |
|
| Outcomes | The unit of analysis was the individual, and the morphea lesion. Primary outcomes: changes of skin score, lesion size, and the appearance of new lesions. The skin score of the marker lesion was determined according to the following scale: 0 = normal, 1 = mild thickening, 2 = moderate thickening, 3 = severe thickening or hidebound skin. Assessment of the size of the lesions was achieved by covering it with a plastic sheet and marking the circumference. In a subset of patients (6 treated with IFN‐γ, 7 receiving placebo), lesion size before and after therapy was assessed by a computer‐aided scanning program calculating the surface area in square centimetres. In addition, it was noted whether new scleroderma lesions appeared during the study period in each patient. Secondary outcomes: to determine whether improvement could be related to an altered level of collagen messenger RNA (mRNA), biopsy specimens were obtained before the beginning of the study (week 0) from involved and uninvolved skin and at week 6 from involved skin only, for RNA extraction and dot blot analysis. Laboratory studies included a complete blood cell count, platelet count, determination of liver enzyme and creatinine levels, urinalysis, and antinuclear antibody titre. Assessments: lesion score was compared before and after therapy. Lesion size was compared at weeks 0 (baseline), 6, and 24 by overlaying the plastic sheets obtained at the different time points. Laboratory studies were performed before the study and at weeks 2, 6, and 24. |
|
| Notes |
Trial registration: authors did not mention a registered protocol. Ethics committee approval: University of Cologne. Funding source: The Bundesministerium fur Forschung und Technologie cooperative study "Lymphokine". Declarations of interest: authors did not report this information. Some parameters of this trial remain unknown because authors reported only the total number of participants and no information regarding the number of participants in each group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "After randomisation patients received..." Comment: authors have not described the method used to generate the random sequence in sufficient detail to allow an assessment of whether the allocation was adequate to produce comparable groups. |
| Allocation concealment (selection bias) | Unclear risk | Authors have not provided enough information to assess if the allocation sequence could have been foreseen in advance of, or during the enrolment of participants. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Authors described this study as double‐blind. However, authors did not provide enough information to assess if participants and personnel were effectively blinded from knowledge of which intervention a participant received. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Authors did not provide enough information to assess if the outcome assessors were effectively blinded from knowledge of which intervention a participant received. |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Quote: "Two patients withdrew from the study, one patient receiving IFN‐γ because of dizziness and dyspnoea after four weeks, and one patient in the placebo group from lack of compliance after six weeks". Comment: it is unclear whether authors included these participants in the analysis or performed 'as treated' analysis. |
| Selective reporting (reporting bias) | High risk | Authors reported only the total number of participants and no information regarding the number of participants randomised and the number of participants in each group. Authors also reported no numerical outcome data. |
| Other bias | Unclear risk | The clinical tool used assessed individual lesions instead of all the lesions of the individual, which could affect the result |
Kreuter 2006.
| Methods | This was a prospective, randomised, 3‐arm comparison trial with active controls, conducted at a German university hospital. The aim of this study was to compare the safety and efficacy of low‐band (LD) UVA1, medium‐band (MD) UVA1, and narrowband (NB) UVB phototherapy in the treatment of morphea. | |
| Participants | Participants were recruited from dermatologic outpatient clinics from 2004 to 2005. Inclusion criteria: diagnosis of morphea established according to accepted clinical and histopathologic features, plus signs of active disease expressed by increasing size of lesions, appearance of new lesions, and/or clinical signs of inflammation within 3 months prior to the study. Exclusion criteria: pregnancy or lactation, any internal immunomodulating or immunosuppressive therapy within the last 4 weeks before treatment, any topical therapy within the last 2 weeks before treatment except the use of emollients, use of potentially photosensitising drugs, and a history of photosensitising dermatoses. Number of patients randomised: 64 (27 group 1 + 18 group 2 + 19 group 3) Number of participants analysed: 62 (27 group 1 + 17 group 2 + 18 group 3) Women: 54 (22 group 1 + 17 group 2 + 15 group 3) Men: 10 (5 group 1 + 1 group 2 + 4 group 3) Age: from 5 to 73 years old; mean age in group 1 was 36.2 ± 21.7 years (5 to 73), 43.7 ± 16.1 years (19 to 73) in group 2 and 47.7 ± 19.8 years (15 to 73) in group 3. Ethnicity: all included participants were white and had skin type II or III. Morphea type: circumscribed morphea (52), linear scleroderma (4), en coup de sabre (4), and generalised (3) subtype. 1 participant had en coup de sabre with coexisting circumscribed morphea (mixed type). Length of illness: from 5 months to 39 years; 4.4 ± 3.8 years (1 to 14) in group 1, 4.9 ± 5.1 years (1 to 19) in group 2 and 7.9 ± 9.4 years (1 to 39) in group 3. Participant's prior treatments: In 43 participants, prior treatment (topical steroids, n = 20; topical calcipotriol, n = 5; systemic steroids, n = 13; methotrexate, n = 5; cyclophosphamide, n = 1; azathioprine, n = 1; hydroxychloroquine, n = 2; penicillin, n = 8) had not resulted in a sufficient improvement. A total of 21 participants had not previously been treated for morphea. |
|
| Interventions |
Group 1: a. Therapy and dosage: exposure of 20 J/cm² LD UVA1 in a bed emitting wavelengths mainly from 340 nm to 400 nm. The irradiation at body distance was 24 mW/cm² resulting in a dose of 1.44 J/min/cm². The average time of exposure for applying 20 J/cm² was about 15 minutes. Phototherapy was performed 5 times a week for a total of 40 treatment sessions, resulting in a cumulative dose of 800 J/cm². b. Administration: topical c. Duration of treatment: 8 weeks d. Follow‐up after treatment: 3 months Group 2: a. Therapy and dosage: exposure of 50 J/cm² MD UVA1 in a irradiation equipment emitting wavelengths from 340 nm to 530 nm. The irradiation at body distance was 28 mW/cm² resulting in a dose of 1.68 J/min/cm². The average time of exposure for applying 20 J/cm² was about 30 minutes. Whole body irradiation was performed 5 times a week for a total of 40 treatment sessions, resulting in a cumulative dose of 2000 J/cm². b. Administration: topical c. Duration of treatment: 8 weeks d. Follow‐up after treatment: 3 months Group 3: a. Therapy and dosage: exposure to NB UVB phototherapy in a cabin fitted with fluorescent lamps that emit wavelengths between 310 nm and 315 nm with a peak at 311 nm. Starting dose was 0.1 J/cm² NB UVB for skin type II and 0.2 J/cm² for skin type III. Depending on tolerability and skin type, NB UVB dosage was increased with 0.1 to 0.2 J/cm². Maximum NB UVB dose was considered 1.3 J/cm² for skin type II and 1.5 J/cm² for skin type III. Irradiation was performed 5 times a week. b. Administration: topical c. Duration of treatment: 8 weeks d. Follow‐up after treatment: 3 months Groups 1, 2 and 3: Participants wore eye goggles as protection against UV radiation. Additional therapy was restricted to the use of emollients (not specified) that had been used according to a standard protocol and had been applied once daily in the evening. Emollients were not applied shortly (1 hour) before or after phototherapy to avoid any alteration of UV transmission. |
|
| Outcomes |
Primary outcome: clinical evaluation was performed by using a previously reported modified skin score, the MSS, which divides the whole body into 7 regions: head and neck, trunk, arms, hands, fingers, legs, and feet. The degree of thickness is assessed on a 0 to 3 scale (0, normal skin; 1, slightly palpable thickened skin; 2, decreased ability to move skin; 3, skin that is unable to be pinched or moved). In addition, involvement of each area is assessed (0: no involvement, 1: less than 33% involvement, 2: between 33% and 67% involvement, 3: more than 67% involvement). The sum of both thickness and affected area is the MSS, with a score of 0 for skin not affected and 42 representing extreme involvement as the maximum score. Secondary outcomes: assessment of the participants' estimate of tightness and itching on a visual analogue scale (VAS) from 0 to 10, 0 representing the absence of symptoms and 10 maximal tightness or itch, and ultrasound measurements with a digital 20 MHz ultrasound scanner to measure both structure and thickness of the skin. Authors also performed skin biopsies to assess skin involvement by a histologic score. Assessments: clinical evaluation at baseline, after therapy and at follow‐up, VAS before and after therapy, ultrasound measurements before and after therapy (on the same skin site), skin biopsies before and after phototherapy. |
|
| Notes |
Intervention product details: LD UVA1 treatment was performed in a Sellas WL 20.000 bed (System Dr Sellmeier, Gevelsberg, Germany) and LD UVA1 measurements were performed with UV‐Meter (Waldmann, Villingen‐Schwenningen, Germany). MD UVA1 irradiation equipment consisted of a Photomed CL 300000 liquid (Photomed, Hamburg, Germany) and MD UVA1 measurements were performed with a calibrated photometer equipped with an MP‐136U photo detector (Photomed). NB UVB phototherapy was performed using the CosmedicoGP‐42 (Cosmedico Medizintechnik GmbH, Villingen‐Schwenningen, Germany) cabin fitted with Arimed 311 (Cosmedico Medizintechnik GmbH) fluorescent lamps. Digital 20‐MHz ultrasound scanner were performed using DUB 20 (Taberna pro medicum, Lüneburg, Germany) with a 8 mm usable depth of signal penetration.. Trial registration: authors did not register a protocol for this study. Ethics committee approval: local ethics review board. Funding sources: authors declared no funding sources. Declarations of interest: authors declared no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Randomised assignment of the 3 phototherapeutic arms was performed by asking the patients to throw dice without knowing the underlying allocation criteria (numbers 1 and 2 = LD UVA1; 3 and 4 = MD UVA1; 5 and 6 = NB UVB)". Comment: the method used to generate the random sequence was adequate to produce comparable groups. |
| Allocation concealment (selection bias) | Low risk | Participants and investigators enrolling participants could not foresee assignment (sequence generation process: throwing dice). |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk |
Quote: "Lack of a double‐blinded setting. The latter is difficult to perform in photo dermatologic studies because irradiation time, UV cabin and equipment, and to a certain degree tanning response vary in different phototherapies"; Comment: participants and personnel had knowledge of the treatment. The outcome is likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Quote: "Image analysis of the ultrasound measurements was performed by an investigator who was blinded to any treatment details". Comment: only the ultrasound assessor was blinded from knowledge of which intervention a participant received. Assessors of the skin score and investigators who guided the participants in the VAS evaluation were unblinded. Thus, the primary outcome measurement of interest is likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote: "Two patients discontinued phototherapy before finishing the treatment protocol because of reasons independent to the trial. These patients were excluded from further statistical evaluation, and 62 patients were included in the ITT analysis". Comment: authors have performed 'as treated' analysis; however, the dropout rate probably does not represent serious threats to validity of the results as the study included 64 participants. |
| Selective reporting (reporting bias) | High risk | Authors did not report the length of the follow‐up after treatment, but responded to our contact and provided these data. However, authors reported only mean value for VAS results, without standard deviation, and did not provide us these data. |
| Other bias | Unclear risk | The clinical tool used (MSS) is validated for SSc, and is inappropriate for the measurement of morphea skin involvement, which could affect the result. |
Kroft 2009a.
| Methods | This was a prospective, randomised, double‐blind, within‐patient, controlled pilot trial conducted in the Netherlands. The aim of this study was to evaluate the efficiency of tacrolimus 0.1% ointment versus emollient petrolatum on active plaque morphea. | |
| Participants | Participants were recruited from the outpatients' clinic of the Dermatology Department of the Radboud University Nijmegen Medical Centre. Inclusion criteria: 2 or more active morphea lesions separated by at least 15 cm, no concomitant topical treatment for at least 3 weeks, and no use of systemic immunosuppressants, penicillamine, interferon‐gamma or phototherapy for at least 3 months. An active morphea plaque was defined as a sclerotic plaque with an erythematous border. Exclusion criteria: SSc (this diagnosis was excluded by a rheumatologist for all patients according to American College of Rheumatology criteria) and pseudosclerosis, proven adverse reactions to tacrolimus in the past (e.g. hypersensitivity/intolerance), women who were pregnant, nursing, or planning to become pregnant during the study, an active skin infection at the site of the morphea plaque, and recent vaccination (within 28 days prior to the start of this study). Borrelia burgdorferi infection was excluded by serology. Criteria for therapy interruption: participants were discontinued from the study if they developed an active skin infection, became pregnant, initiated prohibited medication during study, experienced serious adverse events, or failed to comply with the treatment application protocols, evaluations, or other study requirements. Number of participants: 10 Number of lesions randomised: 20 (10 group 1 + 10 group 2) Number of lesions analysed: 20 (10 group 1 + 10 group 2) Women: 7 Men: 3 Age: over 18 years old; mean age was 44.3 ± 22.1 years. Ethnicity: 9 patients were Caucasians and 1 patient was an Indonesian Creole. Morphea type: plaque Locations of the lesions: trunk (n = 6) and arms (n = 4). Length of illness: 3.4 ± 2.8 years. Extensiveness of the morphea lesions (MSS): 4.1 ± 1.9 Participant's prior treatments: topical corticosteroids (n = 9), oral corticosteroids (n = 1), penicillin (n = 1), penicillamine (n = 1), UVB‐TL01 phototherapy (n = 2), PUVA phototherapy (n = 1), and methotrexate (n = 6). |
|
| Interventions |
Treated lesions: a. Therapy and dosage: participants were required to apply tacrolimus 0.1% ointment twice daily on 1 of the selected lesions. b. Administration: topical c. Duration of treatment: 12 weeks d. Follow‐up after treatment: none Control lesions: a. Therapy and dosage: participants were required to apply petrolatum ointment (placebo) twice daily on the other selected lesion, which served as a control. b. Administration: topical c. Duration of treatment: 12 weeks d. Follow‐up after treatment: none |
|
| Outcomes | Measurement of the surface area of the 2 lesions: an adhesive transparent film pre‐printed with squared centimetres was used to measure the surface area of the 2 lesions. The film was applied on the skin of the participant and the borders of the indurated lesions were marked with permanent colour. Objective measurements of the hardness of the skin were performed using a durometer. The durometer is fitted with a calibrated gauge that registers linearly divided units on a scale of 0 to 100. The hardness of the skin was measured by 1 investigator at 10 locations distributed equally on the morphea plaque, 3 times at each location, and the score was averaged. The averages of the 10 points were summed to give the total durometer score for 1 plaque (thus, in theory, the total durometer score ranged from 0 to 1000). The locations were selected preferentially on sites with no bony structures present directly beneath the skin. At the first visit, the measure points were photographed to guarantee measurements were taken at the same location on each subsequent visit. The clinical features of each plaque were also evaluated using the modified DIET score, that is, dyspigmentation (D), induration (I), erythema (E), telangiectasia (T) plus atrophy (A). Each clinical feature was rated on a scale of 0 (none), 1 (mild), 2 (moderate), and 3 (severe). The total clinical feature score for each plaque was determined by the sum of the separate features. The minimum score was 0 and the maximum score was 15. Adverse reactions of the applied ointments were recorded and the amount of medication applied was calculated by subtracting the remaining ointment from the total amount the patient started with. Assessments: follow‐up visits were performed every 4 weeks, and results analysed before and after treatment. |
|
| Notes |
Outcome measurement product information/details: the adhesive transparent film was from 3M Health Care (St Paul, MN, USA). The durometer was the 1600‐OO rex Gauge Co. (Glenview, IL, USA). When taking measurements, the durometer was used at a temperature of 24 °C and rested against the skin with the force of gravity, with a constant weight that did not allow generation of additional pressure. Trial registration: this was a pilot study and authors did not mention a registered protocol. Ethics committee approval: Radboud University Nijmegen. Funding sources: authors declared no funding. Declarations of interest: authors declared no conflicts of interested. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Tacrolimus 0.1% ointment and petrolatum were randomly assigned to the two plaques." Comment: authors did not describe the method used to generate the random sequence in sufficient detail to allow an assessment of whether the allocation was adequate to produce comparable groups. |
| Allocation concealment (selection bias) | Unclear risk | Authors did not provide enough information to judge if the intervention allocation could be foreseen before or during the recruitment of participants. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk |
Quote: "The ointments were blinded for patient and investigator". Comment: participants and personnel were effectively blinded from knowledge of which intervention a participant received. It is unlikely that the blinding could have been broken. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Authors did not provide enough information to assess if the outcome assessors were effectively blinded from knowledge of which intervention a participant received. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote: "All patients completed the study". Comment: authors included all participants in the analysis. There is no missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available. |
| Other bias | Unclear risk | The clinical tool used (modified DIET score) assessed individual lesions instead of all the lesions of the individual, which could affect the result |
Noakes 2018.
| Methods | This is a double‐blinded within‐individual randomised controlled trial with adult participants comparing tranilast plus topical corticosteroid betamethasone valerate 0.1% versus topical corticosteroid betamethasone valerate 0.1%. | |
| Participants |
Inclusion criteria: adults (individuals over 18 years of age) with morphea and no significant renal, hematological or hepatic disease. Only women not pregnant or lactating included. Exclusion criteria: less than 18 years of age, pregnant or lactating women, known sensitivity to the trial agent, and significant renal, hematological or hepatic disease Number of participants: 3 participants with morphea. This study also included 1 participant with limited scleroderma. Number of lesions randomised: 22 Number of lesions analysed: 22 Women: 2 Men: 1 Age: 21, 25 and 77 years old. Ethnicity: 9 participants were skin type III, 37 participants were skin type IV and 17 participants were skin type V, according to Fitzpatrick’s classification. Morphea Type: plaque (1), linear (1) and generalised morphea (1). |
|
| Interventions |
Treated lesions (B): a. Therapy and dosage: topical betamethasone valerate 0.1% applied twice daily. Dosage of 1 finger tip unit (0.5g) per hand sized region (125 cm²). b. Administration: topical c. Duration of treatment: 3 months d. Follow‐up after treatment: none Treated lesions (B+T): a. Therapy and dosage: topical betamethasone valerate 0.1% and tranilast 1% applied twice daily. Dosage of 1 finger tip unit (0.5g) per hand sized region (125 cm²). b. Administration: topical c. Duration of treatment: 3 months d. Follow‐up after treatment: none All patients continued with their routine therapy. This varied form sole therapy in plaque disease and limited scleroderma to methotrexate in generalized morphea and methotrexate and hydroxychloroquine in linear morphea. All systemic agents had been introduced prior to enrolment in the trial and doses remained unchanged during the trial. Participant's adjuvant therapy: Nil (1), Methotrexate 20 mg weekly plus hydroxychloroquine 400 mg daily (1), Methotrexate 10 mg weekly (1). |
|
| Outcomes | Protocol: monitoring to be done 3 months after commencing therapy, via the Localized Scleroderma Cutaneous Assessment Tool (LoSCAT) scores, and monthly, via laboratory tests and cream return. Methods: assessment was via Localized Scleroderma Assessment Tool (LoSCAT). assessments were performed by the same observer and photographs of the involved sites were taken. At the completion of the study LoScat scores and images were collated, and scores confirmed. Results: LoSCAT, The Physician Global Assessment of Activity (PGA‐A) and The Physician Global Assessment of Damage (PGA‐D). |
|
| Notes |
Trial registration: ACTRN12615001356550. Ethics committee approval: Greenslopes Private Hospital Ethics Committee. Funding sources: authors declared no funding. Declarations of interest: author declared no conflicts of interested. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Randomization was done via the MS Excel random number function. Number one was used to assign B/T application to the proximal/right treatment site with B to the comparator site and number two to assign B application to the proximal/right treatment site with B/T to the comparator site". Comment: the method used to generate the random sequence was adequate to produce comparable groups. |
| Allocation concealment (selection bias) | Low risk |
Quote: "The list was provided to the study coordinator who liaised with the patients". Comment: authors responded to our contact and confirmed the list was only provided to the nurse who provided the participants with the trial agents. Thus, intervention allocation could not be foreseen before or during the recruitment of participants. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk |
Quote: "The investigators were blinded to the randomisation protocol". Quote: "Betamethasone valerate 0.1% in pracisal (B) was used as a control with betamethasone valerate 0.1% and tranilast 1% in pracisal (B/T) at the contralateral treatment site. Both agents were prepared by a registered compounding pharmacy (Wickham House Compounding Pharmacy, Brisbane, QLD, Australia). Comment: participants and personnel were effectively blinded from knowledge of which intervention a participant received. It is unlikely that the blinding could have been broken. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Quote: "The investigators were blinded to the randomisation protocol". Comment: authors responded to our contact and confirmed the outcome assessors were effectively blinded from knowledge of which intervention a participant received. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors responded to our contact and confirmed that all participants completed the study, and also provided standard deviation values. |
| Selective reporting (reporting bias) | Low risk | Authors reported all outcomes pre‐specified in the study protocol. |
| Other bias | Unclear risk | Authors responded to our contact and confirmed that there were no significant baseline differences between groups. However, the clinical tool used (adapted LoSCAT) assessed individual lesions instead of all the lesions of the individual, which could affect the result. |
Sator 2009.
| Methods | This was a prospective, randomised, within‐patient, 3‐arm comparison trial conducted in Austria. The aim of this study was to evaluate the relative efficacy of low‐ versus medium‐dose UVA‐1 phototherapy for plaque‐type morphea, with a control plaque that remained unirradiated. The study extended during a follow‐up period of 1 year after treatment to assess the duration of the therapeutic response. | |
| Participants |
Inclusion criteria: presence of active plaque‐type morphea with at least 3 lesions of comparable localization and evolution. Exclusion criteria: other types of morphea or systemic scleroderma, pregnancy or lactation, age younger than 14 years, severe cardiac insufficiency, systemic or local corticosteroids or any other disease‐related therapies within 4 weeks before study entry, abnormal photosensitivity, or intake of photosensitising drugs. Number of participants: 16 Number of lesions randomised: 48 (16 group 1 + 16 group 2 + 16 group 3) Number of lesions analysed: 42 (14 group 1 + 14 group 2 + 14 group 3) Women: 10 Men: 4 Age: 15 to 69 years old (median 49 years) Ethnicity: Caucasian, 3 participants had skin type II and 11 participants had skin type III. Morphea type: plaque Length of illness: between 4 months and 10 years (median 30 months). Participant's prior treatments: all participants had been unresponsive to previous treatment with topical corticosteroids. |
|
| Interventions |
Lesion 1: a. Therapy and dosage: the participant's whole body (including a selected target plaque) was treated with medium‐dose UVA‐1 (70 J/cm²), 4 times a week for 5 weeks and 2 times a week for another 5 weeks (30 UVA‐1 exposures totalising 2100 J/cm²). b. Administration: topical c. Duration of treatment: 10 weeks d. Follow‐up after treatment: 12 months Lesion 2: a. Therapy and dosage: a second plaque only received 20 J/cm² (low‐dose UVA‐1), 4 times a week for 5 weeks and 2 times a week for another 5 weeks (30 UVA‐1 exposures totalising 600 J/cm²). b. Administration: topical c. Duration of treatment: 10 weeks d. Follow‐up after treatment: 12 months Lesion 3: a. Therapy and dosage: a third plaque was always shielded from irradiation (unirradiated control). b. Administration: topical c. Duration of treatment: 10 weeks d. Follow‐up after treatment: 12 months Additional therapy for all lesions was restricted to the use of emollients that were applied once every evening. |
|
| Outcomes |
Primary outcome: mean decrease in skin thickness at the end of the treatment as assessed by high‐frequency ultrasound. Secondary outcomes: mean decrease in skin thickness during the follow‐up period and the mean decrease in the clinical scleroderma score. Authors used a numeric scleroderma scoring systema according to a modified score of Hulshof and colleagues and Rodnan and colleagues. For each plaque, a sum score for the intensity of erythema and induration were each assessed on a 4‐point ordinal scale between 0 (absent) and 3 (maximum intensity). Atrophy was assessed as 0 (absent) or 1 (present). Assessments: the participants were examined by the same investigator at baseline, at the end of phototherapy, and 3, 6 and 12 months after treatment. The mean value of 3 ultrasound measurements was taken. All side effects of treatment were recorded during the study |
|
| Notes |
Intervention product information/details: 24‐kW dermalight ultrA1 unit (Dr Sellmeier, Gevelsberg, Germany) with an emission spectrum between 340 and 440 nm. The irradiance at skin level was measured with a double monochromator (Bentham DM 150, Bentham Instruments Ltd, Reading, Berkshire, UK) and ranged between 70 and 75 mW/cm². Outcome measurement product information/details: high‐frequency ultrasound system (Dermascan C, Cortex Technology, Hadsund, Denmark) with a long‐focusing transducer. The probe had a frequency of 20 MHz. The total thickness of epidermis and dermis was measured. The gain compensation curve was adjusted in the oblique position at 20 to 45 dB. Trial registration: authors did not mention a registered protocol. Ethics committee approval: Medical University of Vienna. Funding sources: authors declared no funding. Declarations of interest: authors declared no conflicts of interested. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Allocation to treatment with 70 J/cm², 20 J/cm², or no irradiation was done by using a computer‐generated randomisation list". Comment: The method used to generate the random sequence was adequate to produce comparable groups. |
| Allocation concealment (selection bias) | Unclear risk | Authors did not provide enough information to judge if the intervention allocation could be foreseen before or during the recruitment of participants. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk |
Quote: "Each time, the patient’s whole body (including a selected target plaque) was treated with medium‐dose UVA1 (70 J/cm²) with the exception of a second plaque that only received 20 J/cm² (low‐dose UVA1) and a third plaque that was always shielded from irradiation to rule out spontaneous remission (unirradiated control)." Comment: each participant had 1 lesion treated differently from the whole body, and 1 untreated lesion. Thus, participants and personnel had knowledge of the treatment. The outcome is likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Quote: "The patients were always examined by the same unblinded investigator. A blinded assessment was not performed because UVA1‐induced pigmentation in the periphery of the target lesions would have immediately allowed the investigator to distinguish between irradiated and control plaques". Comment: The outcome assessor was unblinded from knowledge of which intervention a participant received. The outcome is likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Quote: "Two patients were withdrawn from evaluation because of irregular attendance to treatment". Comment: authors excluded these 2 participants from the statistical evaluation, performing 'as treated' analysis. ITT analysis includes data from all randomised participants, regardless of study completion. |
| Selective reporting (reporting bias) | High risk | Authors reported only median or mean value (without standard deviation) for outcomes results. |
| Other bias | Unclear risk | The clinical tool used assessed individual lesions instead of all the lesions of the individual, which could affect the result |
Shalaby 2016.
| Methods | This was a parallel intra‐individual comparative randomised controlled clinical trial conducted in Egypt. The aim of this study was to evaluate the efficacy of the fractional carbon dioxide laser as a new modality for treatment of morphea and to compare its results with the well‐established method of UVA‐1 phototherapy. | |
| Participants | Patients with localized morphea who consecutively presented over 1 year to outpatient clinics of Dermatology Department, Kasr Al‐Aini Hospitals, Faculty of Medicine, Cairo University, were assessed for eligibility. Inclusion criteria: patients with plaque and linear morphea (limb variant and en coup de sabre), either newly diagnosed or discontinuing any treatments for at least 3 months before the study. Exclusion criteria: patients with deep and systemic types of morphea, contraindications to phototherapy and/or laser therapy. Number of participants: 21 Number of lesions randomised: 42 (21 group 1 + 21 group 2) Number of lesions analysed: 34 (17 group 1 + 17 group 2) Women: 15 Men: 2 Age: 7 to 47 years old; mean age was 25.6 ± 11.06 years. Ethnicity: 10 participants had skin type III and 7 participants had skin type IV. Morphea type: plaque (n = 12), linear (n = 3), en coup de sabre (n = 2). Stage of disease: active (n = 7), indurated (n = 9), atrophic (n = 1). Length of illness: between 6 and 96 months (mean 23.6 ± 29.3 months). Participant's prior treatments: 12 participants had tried previous therapies. |
|
| Interventions |
Lesion 1: a. Therapy and dosage: fractional CO₂ laser treatment, 3 sessions separated by 1‐month intervals. b. Administration: topical c. Duration of treatment: 10 weeks d. Follow‐up after treatment: none Lesion 2: a. Therapy and dosage: low‐dose UVA‐1 phototherapy (30 J/cm²), 3 sessions a week. b. Administration: topical c. Duration of treatment: 10 weeks d. Follow‐up after treatment: none |
|
| Outcomes |
Primary outcome: clinical improvement (regarding thickness, dermal atrophy, dyschromia, and erythema based on the scores adopted and modified from the Localised Scleroderma Cutaneous Assessment Tool ‐ LoSCAT), histopathological and immunohistochemical analysis, and patient satisfaction scores (regarding overall improvement, the feasibility of therapy, and side effects according to a standardised patient satisfaction score). Results were compared to those of the standard treatment with low‐dose UVA‐1 phototherapy. Secondary outcomes: assessing the complications and the mechanism of action of fractional CO₂ laser on collagen remodeling. Dermal thickness in mm was measured by ultrasound biomicroscopy (UBM). Assessments: clinical evaluation was done before, on monthly basis, and at the end of the study. Patient satisfaction scores were evaluated at the end point of the study. Improvement was graded as follows: poor: no improvement, fair: < 40 %, good: 40% to 59 %, very good: ≥ 60 % improvement. End of study (EoS) was at the last session in UVA‐1‐treated areas and 1 month after the last session in FAL‐treated areas. |
|
| Notes |
Intervention product information/details: for phototherapy, a hand lamp unit (Waldmann, UV 109 A,Germany) was used with a radiation spectrum ranging from 350 to 400 nm with a maximum peak at 370 nm. For laser therapy, a DEKA fractional CO₂ laser (SmartXideDOT, Italy) was used (power 25 W stack 2, dwelling time 500 msec, spacing 500 μm). Outcome measurement product information/details: paradigm ultrasound biomicroscopy plus Model P45 using very high frequency ultrasound (50 MHz). Trial registration: authors registered the protocol of this study on clinical trial.gov (ID: NCT02002897). Ethics committee approval: Dermatology Research Ethical Committee (Derma REC) at Kasr Al‐Aini Hospital. Funding sources: Cairo University (in partial). Declarations of interest: authors declared no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Randomization was carried out using the sealed envelope method where the patients drew lots between sealed envelopes, contact cards with treatment codes either UVA‐1 phototherapy for lesion A and fractional CO₂ laser therapy for lesion B". Comment: the method used to generate the random sequence was adequate to produce comparable groups. |
| Allocation concealment (selection bias) | Low risk | Participants and investigators enrolling participants could not foresee assignment (sequence generation process: sealed envelopes). |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Considering the nature of the interventions, the protocol determined an open‐label study. Participants and personnel had knowledge of the treatment. The outcome is likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Quote: "The first author generated the random allocation and enrolled the participants, whereas clinical assessment was done by the fourth author who was blinded to the used intervention". Comment: the outcome assessor was effectively blinded from knowledge of which intervention a participant received. It is unlikely that the blinding could have been broken. |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Quote: "Demographic and clinical data of the 17 patients are summarized in Table 2". Comment: authors excluded from the analysis 4 participants who did not complete the study. Thus, authors performed 'as‐treated' analysis. |
| Selective reporting (reporting bias) | Low risk | Authors reported all outcomes pre‐specified in the study protocol. |
| Other bias | Unclear risk | The clinical tool used (adapted LoSCAT) assessed individual lesions instead of all the lesions of the individual, which could affect the result. |
Tang 2006.
| Methods | This was a prospective, controlled, randomised pilot trial conducted in Germany. The aim of this study was to assess the efficacy and tolerability of a Traditional Chinese Medicine (TCM) treatment for localised scleroderma established in China in European participants. | |
| Participants | Participants were recruited in clinics from January to October 2004. Inclusion criteria: individuals between 18 and 75 years old with an histologically secured localised scleroderma, type plaque or linear morphea of any duration, presenting < 10 foci and a lesion between 10 cm² to 300 cm², who have not used any other local and/or systemic therapy for morphea 4 weeks prior to inclusion in the study. Exclusion criteria: punctuate lesions or lenticular spots; lesion extent > 500 cm²; systemic involvement or other special forms; systemic immunosuppressant or immunomodulatory medication; positive serology for Borrelia; heart or cardiovascular disease, hepatitis, asymptomatic elevation of renal retention values or renal disease; intolerance to penicillin or ingredients of the herbal oil; pregnant and breastfeeding women. Criteria for interruption of the therapy : patient's wish, local and systemic intolerance, or disease progression (growth of focus > 5 cm², more than 2 new foci). Number of participants randomised: 24 (12 group 1 + 12 group 2) Number of participants analysed: authors did not report this information. Women: 17 (9 group 1 + 8 group 2) Men: 7 (3 group 1 + 4 group 2) Age range: from 18 to 68 years old, mean 43 (from 18 to 68 years old, mean 43.5 group 1+ from 19 to 62 years old, mean 40.5 group 2) Ethnicity: European participants Morphea Type: 18 plaque, 6 linear; > 5 foci in all patients (6 to 10, mean 7) Length of illness: 5 years (from 0.5 to 40) Extent of selected marker lesions: between 10 and 300 cm²; mean 180.2 cm² group 1 + 129.5 cm² group 2) Participant's past treatments: systemic antibiotics (n = 5), systemic glucocorticoids (n = 4), local glucocorticoids (n = 19), chloroquine (n = 1), bath PUVA (n = 7) |
|
| Interventions |
Group 1: a. Therapy and dosage: 200ml of a TCM herbs solution (tea), twice a day. Additionally, vitamin B6 (3× 20 mg capsules per day) and manual application (with rubbing movements) of a herbal oil onto the affected body sites, once a day, during 5 minutes. b. Administration: oral intake and topical c. Duration of treatment: 12 weeks d. Follow‐up after treatment: 12 weeks Group 2: a. Therapy and dosage: phenoxymethylpenicillin 1.2 mega 3 times a day. Addditionally, manual application (with rubbing movements) of base cream DAC onto the affected body sites, once a day, during 5 minutes. b. Administration: oral intake and topical c. Duration of treatment: 6 weeks (penicillin) and 12 weeks (cream) d. Follow‐up after treatment: 12 weeks |
|
| Outcomes | Planimetric assessment of the extent of the marker lesion using a stencil, sonographic assessment of skin thickness, and laboratory assessments: differential blood count, GPT, GOT, Gamma‐GT, AP, CRP, creatinine, serology for ANA. Assessments: the area and thickness of the marker lesions was assessed at baseline and weeks 2, 4, 6, 8, 10, 12 and 24. Antinuclear antibody test at baseline and week 12, other laboratory assessments at baseline and weeks 4, 8, 12 and 24. |
|
| Notes |
Intervention product details: Composition of the TCM herbal tea (group 1): Radix astragali, Rhizoma Atractylodis Macrocephalae, Pachyma, Semen sinapis albae, Radix salviae miltiorrhizae, Flos carthami, Rhizoma zedoariae, Ramulus Cinnamomi, Caulis spatolobi, Bombyx batryticatus, Rhizoma dioscoreae, Herba Lycopodii, Semen coicis, Cortex eucommiae, Radix paeoniae rubra, Radix paeoniae alba, Fructus ligustri and Fructus corni. A laboratory tested the TCM used in this study for contaminations with hydrocortisone, lead, cadmium and mercury. Composition of the herbal oil (group 1): true dragon red flower oil, paraffin, cassia oil, camphor, cinnamon, turpentine, cinnamaldehyde and wintergreen oil. DAC base cream (group 2): a standard moisturizing base cream from the Deutsche Arzneimittel‐Codex (DAC, German Pharmaceutical Codex). Outcome measurement product details: sonography with a Esaote ultrasound (20 MHz). Trial registration: this was a pilot study and authors did not mention a registered protocol. Ethics committee approval: Charité Humboldt University Berlin. Funding sources: Verein zur Foerderung der Dermatologie e.v. Berlin. Declarations of interest: authors declared no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors did not describe the method used to generate the random sequence in sufficient detail to allow an assessment of whether the allocation was adequate to produce comparable groups. |
| Allocation concealment (selection bias) | Unclear risk | Authors did not provide enough information to judge if the intervention allocation could be foreseen before or during the recruitment of participants. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | The interventions had different forms of administration. Participants and personnel had knowledge of which intervention a participant received. The outcome is likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Authors did not clearly describe whether the outcome assessors were effectively blinded from knowledge of which UVA dose each participant received. However, authors responded to our contact and informed that the outcome assessors were unblinded to the knowledge of which intervention a participant received. Thus, the outcome measurement of interest is likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Authors responded to our contact and confirmed they excluded from the analysis 4 participants who experienced side effects ('as treated' analysis). Only 24 participants were randomised. |
| Selective reporting (reporting bias) | High risk | Authors reported only mean value for outcomes results, without standard deviation, and did not provide us this data. |
| Other bias | Unclear risk | The assessment was of individual lesions instead of all the lesions of the individual, which could affect the result |
Yan 2013.
| Methods | This was a randomised controlled trial conducted in China. The aim of this study was to assess the clinical efficacy and safety of surrounding needle, moxibustion and hot compress of Traditional Chinese Medicine (TCM) herbs for localised scleroderma. | |
| Participants | Participants were enrolled in dermatology outpatient clinics from March 2010 to June 2011. Inclusion criteria: individuals aged 18 to 65 years, with a confirmed diagnosis of localised scleroderma based on characteristics of skin lesions and tissue biopsy, voluntarily participating in the study and giving a written consent. Exclusion criteria: pregnant/breastfeeding women; allergy to Chinese medicine; having oral steroids in the prior 2 weeks and/or having oral or topical steroids in the prior week before treatment; individuals with co‐morbidities (cerebral cardiovascular disease, live, renal and hematopoietic system disease) or mental disorders. Number of participants randomised: 42 (23 group 1 + 19 group 2) Number of participants analysed: 41 (22 group 1 + 19 group 2) Women: 22 Men: 20 Age range: from 18 to 65 years old Ethnicity: authors did not report this information. Morphea Type: authors did not report this information. Length of illness (mean): 14.2 years group 1 +13.6 years group 2 Affected areas: 7 in head‐face, 11 in body and 5 in limbs group 1 + 6 in head‐face, 9 in body and 4 in limbs group 2 |
|
| Interventions |
Group 1: a. Therapy and dosage: surrounding needle at local area and Hegu, Zusanli, Yanglingquan and Waiguan area for 30 minutes every the other day. Additionally, hot external application of hot compress herbs at local area for 30 minutes, twice a day, and moxibustion at affected site as well as Hegu and Zusanli area for 30 minutes every day, plus 4 Centella triterpenes tablets (6 mg per tablet), 3 times a day (total 12 tablets), and 1 vitamin E tablet (0.1 g), 3 times a day (total 3 tablets). b. Administration: manual application (each treatment should be separated for at least 2 hours) and oral intake. c. Duration of treatment: 6 months d. Follow‐up after treatment: none Group 2: a. Therapy and dosage: external application of heparin sodium cream at affected areas, twice a day, plus 4 Centella triterpenes tablets (6 mg per tablet), 3 times a day (total 12 tablets), and 1 vitamin E tablet (0.1 g), 3 times a day (total 3 tablets). b. Administration: oral intake and topical c. Duration of treatment: 6 months d. Follow‐up after treatment: none |
|
| Outcomes |
Outcomes: authors have not clearly defined primary and secondary outcomes. Outcomes included clinical assessment according to TCM syndromes criteria, scores of skin sclerosis based on Steen criteria, scores of joint pain based on TCM syndromes criteria and scores of joint function based on Kahan criteria, as well as assessment of safety. Assessments: at baseline and after the treatment |
|
| Notes |
Trial registration: authors did not mention a registered protocol Ethics committee approval: yes Funding sources: governmental funding bodies Declarations of interest: authors did not report this information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The translator informed that the method used to generate the random sequence was a random number table. Thus, it was adequate to produce comparable groups. |
| Allocation concealment (selection bias) | Unclear risk | Authors did not provide enough information to judge if the intervention allocation could be foreseen before or during the recruitment of participants. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | The interventions had different forms of administration. Participants and personnel had knowledge of which intervention a participant received. The outcome is likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Authors did not provide enough information to assess if the outcome assessors were effectively blinded from knowledge of which intervention a participant received. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The translator informed that 1 participant from the intervention group dropped out because of pain due to acupuncture and authors excluded this participant from the data analysis. However, this probably does not represent serious threats to validity of the results. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available. |
| Other bias | Unclear risk | The outcome assessment followed criteria from TCM, which could affect the result. |
Zulian 2011.
| Methods | This was a multi‐centre, randomised, double‐blind, placebo‐controlled trial, conducted in Italy. The aim of this study was to assess the clinical efficacy and safety of methotrexate (MTX) compared with placebo in children and adolescents with active juvenile localized scleroderma. | |
| Participants | This study was a cooperative effort of the Italian Pediatric Rheumatology and Dermatology Study Groups, in which 14 paediatric rheumatology and dermatology centres participated. Informed consent was sought from the parents and patients according to legal requirements. After consent was obtained, patients were stratified according to juvenile localized scleroderma subtype to ensure comparable distribution between treatment groups. The recruitment period was from June 2005 to March 2009. Inclusion criteria: diagnosis of juvenile localised scleroderma (linear, generalised, or mixed subtype) disease in an active phase, onset before 16 years of age, and absence of immunosuppressive treatment during the 6 months prior to enrolment. Active disease was defined as the appearance of new lesions or increased size of preexisting lesions, with signs of active inflammation such as erythema and/or positive thermography findings. Exclusion criteria: SSc, major concomitant medical conditions, leukopenia < 3.0 × 10⁹/litre, thrombocytopenia 100 × 10⁹/litre, liver transaminase levels more than twice the upper limit of normal, or renal impairment defined as creatinine clearance < 90 ml/minute/1.73 m², or if the participant or parent was unwilling or unable to adhere to the protocol. Number of participants randomised: 70 (46 group 1 + 24 group 2) Number of participants analysed: 70 (46 group 1 + 24 group 2) Women: 50 (34 group 1 + 16 group 2) Men: 20 (12 group 1 + 8 group 2) Age (range; mean ± SD, years): 6–17 years (9 ± 4.9 group 1 + 10.2 ± 4.8 group 2) Age at disease onset (mean ± SD, years): 6.9 ± 3.8 group 1 + 8.0 ± 3.4 group 2 Ethnicity: authors did not report this information. Length of illness (mean ± SD, years): 2.3 years (2.4 ± 2.2 group 1 + 2.1 ± 2.8 group 2) Morphea type: linear scleroderma (n = 44; 32 group 1 + 12 group 2), generalised morphea (n = 18; 10 group 1 + 8 group 2) and mixed subtype (linear / circumscribed; n = 8; 4 group 1 + 4 group 2). Participant's prior treatments: 22 patients had been previously treated with topical steroids, 6 with D‐penicillamine, 1 with cyclosporin A, and 13 with other non‐immunomodulatory drugs before study entry. |
|
| Interventions |
Group 1: a. Therapy and dosage: oral MTX at a dosage of 15 mg/m² once per week (maximum 20 mg), plus folic acid supplementation (2.5 mg, 48 hours after MTX treatment) and oral prednisone (1 mg/kg/day, maximum 50 mg, in a single morning dose for 3 months, followed by gradual tapering for 1 month until discontinuation). b. Administration: oral c. Duration of treatment: 12 months or until flare of the disease d. Follow‐up after treatment: none Group 2: a. Therapy and dosage: oral placebo at a dosage of 15 mg/m² once per week (maximum 20 mg) plus folic acid supplementation (2.5 mg, 48 hours after placebo treatment) and oral prednisone (1 mg/kg/day, maximum 50 mg, in a single morning dose for 3 months, followed by gradual tapering for 1 month until discontinuation) b. Administration: oral c. Duration of treatment: 12 months or until flare of the disease. d. Follow‐up after treatment: none |
|
| Outcomes |
Primary outcomes: rate of response to treatment at the end of the 12‐month follow‐up. Responders were defined as participants who satisfied all 3 criteria, i.e., SSR ≤ 1, decreased ΔTh% of at least 10% compared to baseline, and absence of new lesions. At baseline, a single lesion judged as active by both clinical evaluation and thermography was selected as “target,” and its size was measured by a computerized skin scoring (CSS) system. Authors calculated the ratio between area of the lesion and body surface area, expressed as a percentage (standardized computerized skin score (S‐CSS)), to analyse the progression of the skin lesions, and considering that almost all patients were in a growing stage. This parameter helps to distinguish whether the enlargement of a lesion is related to disease progression or simply to the natural process of growth over the time. The values obtained at baseline (t0) and at the following visits (tn) allow calculation of the skin score rate (SSR), i.e. the ratio tn S‐CSS:t0 S‐CSS. In this way, the change of a lesion becomes independent of the natural growth of the body surface area. An SSR of ≤ 1 means decreased extension of the lesion, and an SSR of > 1 indicates an increase. The degree of inflammation was assessed by infrared thermography, according to a standardized procedure, and performed by the same investigator, using the same infrared camera. All patients were scanned 15 minutes after acclimatization, wearing underwear only. A lesion was considered positive on thermography (active lesion) when a substantial area > 0.5 °C warmer than the matching opposite limb or body area site was visible. Authors calculated the percentage thermal change from the baseline value (ΔTh%) according to the formula ([tn − t0]/t0) × 100, where tn was the temperature value at the time of evaluation and t0 was the value at baseline. A negative ΔTh% value meant improvement, and a positive ΔTh% value meant worsening of the target lesion. If there was doubt about characterization of a lesion in atrophic skin as active, a careful clinical evaluation was performed by 2 independent observers, and their assessment was compared to the thermography result, to avoid false‐positive results. Secondary outcomes: the proportion of responder patients who had disease flare, the changes from baseline in each of the 3 response parameters (SSR, ΔTh%, new lesions), and assessment of safety and tolerability. Disease flare was defined as the occurrence of at least 1 of the following: SSR > 1; < 10% improvement or positive ΔTh% value; or appearance of new lesions. Clinical assessment of general systems and of the skin lesions also included the physician’s global assessment of disease severity (100 mm visual analogue scale (VAS)), parents’ global assessment of the patient’s overall well‐being (100 mm VAS), and functional ability assessment using the validated translated version of the Childhood Health Assessment Questionnaire (C‐HAQ) disability index (0 to 3 point scale). The shape and size of the lesions and presence of new lesions were assessed by clinical examination and represented on a mannequin. A digital photograph was also taken at each visit. The following laboratory parameters were measured: haemoglobin level, mean red blood cell volume, white blood cell and differential cell count, platelet count, erythrocyte sedimentation rate, and levels of C‐reactive protein, serum creatinine, blood urea nitrogen and glucose, serum glutamic oxaloacetic transaminase, serum glutamic pyruvic transaminase, and gamma glutamyl transpeptidase. At study entry and after 3 and 12 months, levels of creatine kinase, aldolase, serum immunoglobulins (IgG, IgA, IgM), and complement fractions (C3 and C4) were also measured and urinalysis was performed. At study entry and after 12 months, the following autoantibodies were assessed: antinuclear antibody (ANA) (by indirect immunofluorescence on Hep‐2 cells), anti–double‐stranded DNA (anti‐dsDNA), anti–topoisomerase I (anti–Scl‐70), anti‐centromere antibody, anti–extractable nuclear antigen, anticardiolipin antibody, anti‐2‐glycoprotein I, lupus anticoagulant, and rheumatoid factor (RF). At each visit, adverse events were carefully monitored, specifically the occurrence of skin rash, anorexia, nausea, vomiting, gastrointestinal discomfort, diarrhoea, change in behavior, weight gain, and headache. Follow‐up duration: each patient was scheduled to be examined and to have laboratory tests during a total of 6 visits over a 12‐month period (at study entry and after 1, 3, 6, 9, and 12 months). |
|
| Notes | Participants were given the option to receive open‐label treatment with MTX in a 2‐year follow‐up treatment period if they had a flare of scleroderma in the double‐blind period or completed the double‐blind period without flare. Thermographer information/details: GM, infrared camera (ThermaCAM PM695; Flir Systems). Withdrawals: 32 participants (15 group 1 + 17 group 2) prematurely discontinued the study drug because of disease flare. Trial registration: authors did not mention a registered protocol. Ethics committee approval: University Hospital of Padua, where all patients were evaluated. Funding sources: supported by Mediafriends, ONLUS, Milan, Italy, and Il Volo, Association for Rheumatic Diseases in Children, ONLUS, Padua, Italy. Declarations of interest: authors did not report this information. ‐ Authors published this study 3 times. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "To reduce the risk of prolonged exposure to the placebo treatment and considering the difficulty for parents to accept a 9‐month period without active treatment if the patient was included in the placebo arm, a 2:1 MTX/placebo randomisation rate was adopted. A computer‐generated randomisation list was used to randomise patients to receive MTX or placebo. Patients were assigned numbers according to the sequence in which they entered the study". Comment: the method used to generate the random sequence was adequate to produce comparable groups. |
| Allocation concealment (selection bias) | Unclear risk | Authors did not provide enough information to judge if the intervention allocation could be foreseen before or during the recruitment of participants. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk |
Quote: "MTX was provided in the form of tablets in doses of 2.5 mg, with corresponding placebo tablets. The production of the study medications, packaging, and labelling were done by a certified pharmaceutical laboratory". Comment: participants and personnel were effectively blinded from knowledge of which intervention a participant received. It is unlikely that the blinding could have been broken. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
Quote: "The code was not broken until completion of the study, except in cases of medical emergency or relapse". Comment: outcome assessors were effectively blinded from knowledge of which intervention a participant received. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote: "Data were analysed according to the intent‐to‐treat principle; all patients were assessed at each of the 6 time points, whether or not they were still receiving treatment". Quote: "All 70 enrolled patients were qualified for the intent‐to‐treat analysis of efficacy. Among them, 38 (54.3%) completed the 12‐month trial, including 31 (67.4%) of the 46 patients in the MTX group and 7 (29.2%) of the 24 patients in the placebo group (P = 0.005). In all cases, the reason for premature discontinuation of the study drug was disease flare" Comment: Authors performed ITT analysis. |
| Selective reporting (reporting bias) | High risk |
Quote: "Although a trend toward improvement in the physician's and the patient's/parents' global assessment of disease severity and functional ability (evaluated using the C‐HAQ) was found in MTX treated patients (data not shown), the changes did not reach statistical significance". Comment: authors reported no numerical data for outcomes of interest. |
| Other bias | Low risk | Besides no baseline differences, the global tool of assessment, although not validated, considers the disease activity and also the disease progression in children at a growing stage. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bodemer 1999 | This study investigated calcitriol for treating morphea in 7 young participants (mean age: 7.3 years) and was not an RCT. This study was published in French and without an abstract in English. |
| Didenko 1978 | This descriptive study reported experiences with ultrasonics and lidase phonophoresis for various forms of scleroderma. This study was published in Russian and without an abstract in English. |
| Dortu 1974 | This non‐randomised, open trial investigated the anti‐inflammatory agent Elarzone‐Dausse in disorders affecting the venous system. This study was published in French and without an abstract in English. |
| Dytoc 2014 | This multicentre, prospective, non‐randomised, vehicle‐controlled trial evaluated the therapeutic potential and safety profile of imiquimod 5% cream in plaque‐type morphea. 25 adult participants from 2 Canadian centres with histologically confirmed plaque‐type morphea were enrolled. Imiquimod 5% was applied to a representative plaque and, if more than 1 lesion were present, vehicle was applied to a control plaque for 9 months (non‐randomised selection). Treatment efficacy was assessed with the dyspigmentation, induration, erythema, and telangiectasias (DIET) score, histology, and ultrasound evaluation. |
| Hu 1996 | This study investigated the efficacy of Prostaglandin E1 and propylene glycol alginate sodium in the treatment of urticarial vasculitis, which appears in the progression of SSc. This study was published in Chinese and without an abstract in English. |
| Smirnov 1998 | This abstract reported a prospective, controlled, non‐randomised study aiming to assess the efficacy, tolerance and safety of Climen (combined estrogenic and gestagenic drug) in the treatment of localised scleroderma. This study included 17 postmenopausal women aged from 45 to 56 without contraindications to the gonadotherapy, and a control group of 20 participants matched by age and clinical features. Climen was administered orally for 21 days a month during 3 to 6 months (mean 5 months) versus placebo in control group. No other therapy was used. The outcomes measures included scoring of the number and square of lesions (maximum worse score was 30). |
| Wang 2008 | This was an open, non‐randomised study investigating the efficacy, potential limitations, and biological mechanisms of UV‐A1 phototherapy for skin sclerosis due to collagen deposition disorders. 8 participants with scleroderma and 4 participants with sclerodermatous graft‐vs‐host disease were treated with high‐dose (130 J/cm²) UV‐A1 phototherapy 3 times per week for 14 weeks. 2 participants with scleroderma and 4 participants with morphea were treated with medium‐dose (65 J/cm²) UV‐A1 phototherapy 3 times per week for 14 weeks. Healthy volunteers without skin disease were treated with UV‐A1 irradiation at various doses and frequencies, with biopsies performed afterwards. In sclerotic skin, induration was clinically assessed using a scoring scale. In normal skin, quantitative polymerase chain reaction was used to assess anti‐fibrotic responses, defined as decreased type I and type III procollagen and increased matrix metalloproteinase levels. |
Characteristics of studies awaiting assessment [ordered by study ID]
NCT00812188.
| Methods | A prospective randomised open label trial comparing high‐dose UVA‐1 or medium‐dose UVA‐1 versus fluocinonide 0.05% cream in the treatment of morphea |
| Participants | Inclusion criteria: male and female subjects 18 years of age or older; symmetric limited morphea. Exclusion criteria: known sensitivity to fluocinonide 0.05% cream; clinical evidence of superinfected skin; immunocompromised state (including previously documented HIV); generalized scleroderma; previous history of skin cancer; non‐English speaking individuals. |
| Interventions | Interventions groups: 1: Medium‐dose (60 J/cm²) UVA‐1 3x/week for 12 weeks to 1 morphea plaque and fluocinonide 0.05% cream to another morphea plaque twice daily for twelve weeks; 2: High‐dose (120 J/cm²) UVA‐1 treatment 3x/week for 12 weeks to 1 morphea plaque and fluocinonide 0.05% cream twice daily for 12 weeks to another morphea plaque. |
| Outcomes | Authors only described that they would assess the efficacy of UVA‐1 treatment vs. topical steroid during 5 years, indicating no measures for the evaluations. |
| Notes |
NCT01799174.
| Methods | A randomised, blinded, and controlled trial assessing the efficacy and safety of UVA1 phototherapy in the treatment of active morphea in adults and children. |
| Participants | Presence of at least 1 active morphea lesion (linear, plaque, generalized, or mixed subtypes) confirmed by the primary investigator and/or by histopathological examination. |
| Interventions | 2 intervention groups: 1: medium dose (70 J/cm²) phototherapy (active UVA1 phototherapy) with an ultraviolet translucent acrylic screen 3 times per week for 10 weeks; 2: "sham" UVA1 (0 J/cm²) phototherapy with an ultraviolet opaque acrylic screen 3 times per week for 10 weeks. The phototherapists, patients, and principal investigator were blinded to whether the patients receive active or sham UVA1 phototherapy. Patients only allowed to apply emollients during the study. |
| Outcomes | The primary outcome measure is mean change in Localized Scleroderma Severity Index (LoSSI), a validated clinical score of morphea activity, from baseline versus after 30 treatments. Follow‐up: 3 years. The secondary outcome measure is the physician's global assessment of disease activity (PGA‐A). To identify UVA1‐induced molecular pathways that may account for the efficacy of UVA1 phototherapy in morphea, authors performed gene expression profiling using RNA derived from affected and non‐lesional whole skin from patients with morphea before and after UVA1 phototherapy. |
| Notes |
Differences between protocol and review
We updated the Background section.
We included cross‐over trials as a type of study (previously listed as reason for exclusion) but only the first part of the trial to avoid any carry‐over effects from insufficient washout periods.
We evaluated skin thickness as an indicator of disease activity (as in the LoSCAT).
We searched the ISRCTN registry because the metaRegister of Controlled Trials service is currently under review.
A new author (Albuquerque, JV de) entered the group in September 2015 after the former first author (Ravelli FN) relinquished responsibility for this review; JVA took over the roles of screening, data extraction and assessing risk of bias. A new author entered the group in June 2016 (Civile, VT).
Contributions of authors
JVA was the contact person with the editorial base. JVA and VFMT co‐ordinated contributions from the co‐authors and wrote the final draft of the review. JVA, BNGS and VFMT screened papers against eligibility criteria. JVA and BNGS obtained data on ongoing and unpublished studies. JVA, BNGS, MRAV, VTC and VFMT appraised the quality of papers. JVA and BNGS extracted data for the review and sought additional information about papers. JVA and VTC entered data into Review Manager 5. JVA, BNGS, MRAV, VTC and VFMT analysed and interpreted data. JVA, BNGS, MRAV, VTC and VFMT worked on the Methods sections. JVA, MRAV and VFMT drafted the clinical sections of the background and responded to the clinical comments of the referees. JVA, VTC and VFMT responded to the methodology and statistics comments of the referees. AL was the consumer co‐author and checked the review for readability and clarity, as well as ensuring outcomes are relevant to consumers. JVA is the guarantor of the update.
Disclaimer
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Skin. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Sources of support
Internal sources
No sources of support supplied
External sources
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
Declarations of interest
Julia V de Albuquerque: nothing to declare. Brenda NG Andriolo: nothing to declare. Monica RA Vasconcellos: nothing to declare. Anne Lyddiatt: nothing to declare. Vinicius T Civile: nothing to declare. Virginia FM Trevisani: nothing to declare.
New
References
References to studies included in this review
Azimi 2013 {published data only}
- Azimi H, Golfroushan F, Nasimi M. Comparison of hydroxychloroquine and methotrexate in treatment of patients with localized scleroderma. Medical Journal of the Egyptian Armed Forces 2013;35(3):60‐5. [Google Scholar]
Batchelor 2008 {published data only}
- Batchelor R, Lamb S, Goulden V, Stables G, Goodfield M, Merchant W. Photodynamic therapy for the treatment of morphoea. Clinical and Experimental Dermatology 2008;33(5):661‐3. [CENTRAL: CN‐00666991; PUBMED: 18627394] [DOI] [PubMed] [Google Scholar]
El‐Mofty 2004 {published data only}
- El‐Mofty M, Mostafa W, El‐Darouty M, Bosseila M, Nada H, Yousef R, et al. Different low doses of broad‐band UVA in the treatment of morphea and systemic sclerosis. Photodermatology, Photoimmunology & Photomedicine 2004;20(3):148‐56. [CENTRAL: CN‐00516067; PUBMED: 15144393] [DOI] [PubMed] [Google Scholar]
Furuzawa‐Carballeda 2012 {published data only}
- Furuzawa‐Carballeda J, Ortiz‐Avalos M, Lima G, Jurado‐Santa Cruz F, Llorente L. Subcutaneous administration of polymerized type I collagen downregulates interleukin (IL)‐17A, IL‐22 and transforming growth factor‐beta1 expression, and increases Foxp3‐expressing cells in localized scleroderma. Clinical and Experimental Dermatology 2012;37(6):599‐609. [CENTRAL: CN‐00968871] [DOI] [PubMed] [Google Scholar]
Hulshof 2000 {published data only}
- Hulshof MM, Bouwes Bavinck JN, Bergman W, Masclee AA, Heickendorff L, Breedveld FC, et al. Double‐blind, placebo‐controlled study of oral calcitriol for the treatment of localized and systemic scleroderma. Journal of the American Academy of Dermatology 2000;43(6):1017‐23. [CENTRAL: CN‐00330232] [DOI] [PubMed] [Google Scholar]
Hunzelmann 1997 {published data only}
- Hunzelmann N, Anders S, Fierlbeck G, Hein R, Herrmann K, Albrecht M, et al. Double‐blind, placebo‐controlled study of intralesional interferon gamma for the treatment of localized scleroderma. Journal of the American Academy of Dermatology 1997;36(3 Pt 1):433‐5. [CENTRAL: CN‐00137759] [DOI] [PubMed] [Google Scholar]
Kreuter 2006 {published data only}
- Kreuter A, Hyun J, Stücker M, Sommer A, Altmeyer P, Gambichler T. A randomized controlled study of low‐dose UVA1, medium‐dose UVA1, and narrowband UVB phototherapy in the treatment of localized scleroderma. Journal of the American Academy of Dermatology 2006;54(3):440‐7. [CENTRAL: CN‐00555244] [DOI] [PubMed] [Google Scholar]
Kroft 2009a {published data only}
- Kroft EB, Groeneveld TJ, Seyger MM, Jong EM. Efficacy of topical tacrolimus 0.1 in active plaque morphea: Randomized, double‐blind, emollient‐controlled pilot study. American Journal of Clinical Dermatology 2009;10(3):181‐7. [CENTRAL: CN‐00699723] [DOI] [PubMed] [Google Scholar]
Noakes 2018 {published data only}
- Noakes R. Assessing the response of morphea and limited scleroderma to tranilast: a small prospective study comparing topical corticosteroids to a combination of topical corticosteroids and tranilast. Clinical, Cosmetic and Investigational Dermatology 2018;11:321‐26. [CENTRAL: CN‐01616903] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sator 2009 {published data only}
- Sator PG, Radakovic S, Schulmeister K, Honigsmann H, Tanew A. Medium‐dose is more effective than low‐dose ultraviolet A1 phototherapy for localized scleroderma as shown by 20‐MHz ultrasound assessment. Journal of the American Academy of Dermatology 2009;60(5):786‐91. [PUBMED: 19211170 ] [DOI] [PubMed] [Google Scholar]
Shalaby 2016 {published data only}
- Shalaby SM, Bosseila M, Fawzy MM, Abdel Halim DM, Sayed SS, Allam RS. Fractional carbon dioxide laser versus low‐dose UVA‐1 phototherapy for treatment of localized scleroderma: a clinical and immunohistochemical randomized controlled study. Lasers in Medical Science 2016;31(8):1707‐15. [CENTRAL: CN‐01263458] [DOI] [PubMed] [Google Scholar]
Tang 2006 {published data only}
- Tang Y, Treudler R, Tebbe B, Orfanos C. Treatment of localized scleroderma with herbs of traditional chinese medicine [Einsatz von Krautern der traditionellen chinesischen Medizin (TCM) bei der zirkumskripten Sklerodermie]. Kosmetische Medizin 2006;27(3):100‐9. [CENTRAL: CN‐00612744] [Google Scholar]
Yan 2013 {published data only}
- Yan XN, Zhang JR, Zhang CQ, Tian Q, Chen L, Chen L. Efficacy observation on acupuncture and moxibustion combined with hot compress of TCM herbs for scleroderma. Zhongguo Zhen Jiu [Chinese Acupuncture & Moxibustion] 2013;33(5):403‐6. [CENTRAL: CN‐00918725] [PubMed] [Google Scholar]
Zulian 2011 {published data only}
- Zulian F, Martini G, Vallongo C, Vittadello F, Falcini F, Patrizi A, et al. Methotrexate treatment in juvenile localized scleroderma: a randomized, double‐blind, placebo‐controlled trial. Arthritis and Rheumatism 2011;63(7):1998‐2006. [CENTRAL: CN‐00801715] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bodemer 1999 {published data only}
- Bodemer C, et al. Localized scleroderma in childhood and therapeutic trial with calcitriol: A therapeutic option to define [[French] Sclerodermies localisees de l'enfant et tentative therapeutique par calcitriol: Une possibilite therapeutique a definir]. Annales de Dermatologie et de Venereologie 1999;126(10):725‐726. [PubMed] [Google Scholar]
Didenko 1978 {published data only}
- Didenko IG. Therapeutic effectiveness of ultrasonics and lidase phonophoresis in various forms of scleroderma. Vestnik Dermatologii i Venerologii 1978;6:76‐79. [CENTRAL: CN‐01131746] [PubMed] [Google Scholar]
Dortu 1974 {published data only}
- Dortu J. Evaluation of Elarzone‐Dausse phlebology. Phlebologie 1974;27(3):381‐384. [PUBMED: 4614284] [PubMed] [Google Scholar]
Dytoc 2014 {published data only}
- Dytoc M, Wat H, Cheung‐Lee M, Sawyer D, Ackerman T, Fiorillo L. Evaluation of the efficacy and safety of topical imiquimod 5% for plaque‐type morphea: a multicenter, prospective, vehicle‐controlled trial. Journal of Cutaneous Medicine & Surgery 2015;19(2):132‐139. [CENTRAL: CN‐01097258] [DOI] [PubMed] [Google Scholar]
Hu 1996 {published data only}
- Hu G, Zhu Y, Ran LH, Wang ZY. Clinical observation on the efficacy of Prostaglandin E1 and Propylene glycol alginate sodium in the treatment of scleroderma. Chinese journal of dermatology 1996;29(4):284‐285. [CENTRAL: CN‐00843757] [Google Scholar]
Smirnov 1998 {published data only}
- Smirnov A. Gonadotherapy as a treatment of patients with localized scleroderma. Journal of the European Academy of Dermatology & Venereology 7‐11 Ocober 1998;11(Suppl 2):S273. [Google Scholar]
Wang 2008 {published data only}
- UVA1 Light for Treatment of Scleroderma and Similar Conditions. clinicaltrials.gov/ct2/show/NCT00476801 (first received 22 May 2007).
- Wang F, Garza LA, Cho S, Kafi R, Hammerberg C, Quan T, et al. Effect of increased pigmentation on the antifibrotic response of human skin to UV‐A1 phototherapy. Archives of dermatology 2008;144(7):851‐58. [CENTRAL: CN‐00649884] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT00812188 {unpublished data only}
- NCT00812188. A prospective, open label trial of high dose UVA‐1, 3x/week or medium dose UVA‐1, 3x/week vs. fluocinonide 0.05% cream treatment of morphea. clinicaltrials.gov/ct2/show/record/NCT00812188 (first received 18 December 2008).
NCT01799174 {unpublished data only}
- NCT01799174. Treatment study comparing UVA‐1 phototherapy versus placebo treatment for morphea. clinicaltrials.gov/ct2/show/NCT01799174 (first received 26 February 2013).
Additional references
Arkachaisri 2010
- Arkachaisri T, Vilaiyuk S, Torok KS, Medsger TA. Development and initial validation of the Localized Scleroderma Skin Damage Index and Physician Global Assessment of disease Damage: a proof‐of‐concept study. Rheumatology 2010;49(2):373‐381. [PUBMED: 20008472] [DOI] [PMC free article] [PubMed] [Google Scholar]
Asano 2018
- Asano Y, Fujimoto M, Ishikawa O, Sato S, Jinnin M, Takehara K, et al. Diagnostic criteria, severity classification and guidelines of localized scleroderma. Journal of Dermatology 2018;45(7):755‐80. [DOI] [PubMed] [Google Scholar]
Badea 2009
- Badea I, Taylor M, Rosemberg A, Foldvari M. Pathogenesis and therapeutic approaches for improved topical treatment in localized scleroderma and systemic sclerosis. Rheumatology 2009;48(3):213‐21. [PUBMED: 19022832] [DOI] [PubMed] [Google Scholar]
Barnes 2012
- Barnes J, Mayer MD. Epidemiology of systemic sclerosis: incidence, prevalence, survival, risk factors, malignancy, and environmental triggers. Current Opinion in Rheumatology 2012;24(2):165‐70. [PUBMED: 22269658] [DOI] [PubMed] [Google Scholar]
Beyer 2012
- Beyer C, Distler O, Distler JH. Innovative antifibrotic therapies in systemic sclerosis. Current Opinion in Rheumatology 2012;24(3):274‐80. [PUBMED: 22450392] [DOI] [PubMed] [Google Scholar]
Bielsa Marsol 2013
- Bielsa Marsol I. Update on the classification and treatment of localized scleroderma [Actualización en la clasificación y el tratamiento de la esclerodermia localizada]. Actas Dermo‐Sifiliográficas 2013;104(8):654‐66. [DOI: 10.1016/j.ad.2012.10.003; PUBMED: 23948159] [DOI] [PubMed] [Google Scholar]
Careta 2015
- Careta MF, Romiti R. Localized scleroderma: clinical spectrum and therapeutic update. Anais Brasileiros de Dermatologia 2015;90(1):62‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2002
- Chen K, See A, Shumack S. Epidemiology and pathogenesis of scleroderma. Australasian Journal of Dermatology 2003;44(1):1‐9. [PUBMED: 12581091] [DOI] [PubMed] [Google Scholar]
Clements 1995
- Clements PH, Lachenbruch P, Siebold J, White B, Weiner S, Martin R, et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. Journal of Rheumatology 1995;22(7):1281‐5. [PUBMED: 7562759] [PubMed] [Google Scholar]
CS‐COUSIN
- Cochrane Skin ‐ Core Outcome Set Initiative. cs‐cousin.org/ (accessed 04 March 2019).
Dytoc 2005
- Dytoc M, Ting PT, Man J, Sawyer D, Fiorillo L. First case series on the use of imiquimod for morphoea. British Journal of Dermatology 2005;153(4):815‐20. [DOI] [PubMed] [Google Scholar]
Fett 2011a
- Fett N, Werth VP. Update on morphea. Part I. Epidemiology, clinical presentation, and pathogenesis. Journal of the American Academy of Dermatology 2011;64(2):217‐28. [PUBMED: 21238823] [DOI] [PubMed] [Google Scholar]
Fett 2011b
- Fett N, Werth VP. Update on morphea. Part II. Outcome measures and treatment. Journal of the American Academy of Dermatology 2011;64(2):231‐42. [PUBMED: 21238824] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fett 2012
- Fett NM. Morphea: Evidence‐based recommendations for treatment. Indian Journal of Dermatology, Venereology and Leprology 2012;78(2):135‐41. [PUBMED: 22421642] [DOI] [PubMed] [Google Scholar]
Fett 2013
- Fett, N. Scleroderma: nomenclature, etiology, pathogenesis, prognosis, and treatments: facts and controversies. Clinics in dermatology 2013;31.4:432‐437. [DOI] [PubMed] [Google Scholar]
GRADE Handbook
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 23 July 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guillevin 1983
- Guillevin L, Ortonne JP. Treatment of scleroderma [Traitement de la sclerodermie]. Annals of Internal Medicine 1983;134(8):754‐65. [PUBMED: 6364917] [PubMed] [Google Scholar]
Harding 1998
- Harding SE, Tingey PC, Pope J, Fenlon D, Furst D, Shea B, et al. Prazosin for Raynaud’s phenomenon in progressive systemic sclerosis. Cochrane Database of Systematic Reviews 1998, Issue 2. [DOI: 10.1002/14651858.CD000956] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hawk 2001
- Hawk A, English JC. Localized and systemic scleroderma. Seminars in Cutaneous Medicine and Surgery 2001;20(1):27‐37. [PUBMED: 11308134] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hunzelmann 1998
- Hunzelmann N, Scharffetter Kochanek K, Hager C, Krieg T. Management of localized scleroderma. Seminars in Cutaneous Medicine & Surgery March 1998;17(1):34‐40. [PUBMED: 9512105] [DOI] [PubMed] [Google Scholar]
Johnson 2012
- Johnson W, Jacobe H. Morphea in adults and children cohort II: Patients with morphea experience delay in diagnosis and large variation in treatment. Journal of the American Academy of Dermatology November 2012;67(5):881‐9. [PUBMED: 22382198] [DOI] [PubMed] [Google Scholar]
Kahaleh 1985
- Kahaleh MB, Sultany GL, Smith EA, Huffstutter JE, Loadholt CB, LeRoy EC. A modified scleroderma skin scoring method. Clinical and Experimental Rheumatology 1985;4(4):367‐9. [PubMed] [Google Scholar]
Kahan 1989
- Kahan A, Amor B, Menkes CJ, Strauch G. Recombinant interferon‐γ in the treatment of systemic sclerosis. American Journal of Medicine 1989;87(3):273‐7. [PUBMED: 2505614] [DOI] [PubMed] [Google Scholar]
Knobler 2017
- Knobler R, Moinzadeh P, Hunzelmann N, Kreuter A, Cozzio A, Mouthon L, et al. European Dermatology Forum S1‐guideline on the diagnosis and treatment of sclerosing diseases of the skin, Part 1: localized scleroderma, systemic sclerosis and overlap syndromes. Journal of the European Academy of Dermatology and Venereology : JEADV 2017;31(9):1401‐24. [DOI] [PubMed] [Google Scholar]
Kreuter 2015
- Kreuter A, Hunzelmann N. Recurrence rates in localized scleroderma (morphoea). British Journal of Dermatology 2015;172(3):562‐3. [PUBMED: 25776242] [DOI] [PubMed] [Google Scholar]
Kroft 2009b
- Kroft EB, Jong EM, Evers AW II. Psychological distress in patients with morphea and eosinophilic fasciitis. Archives of Dermatology 2009;145(9):1017‐22. [PUBMED: 19770441] [DOI] [PubMed] [Google Scholar]
Laxer 2006
- Laxer RM, Zulian F. Localized scleroderma. Current Opinion in Rheumatology 2006;18(6):606‐13. [PUBMED: 17053506] [DOI] [PubMed] [Google Scholar]
Leheta 2013
- Leheta T, Garem Y, Hegazy R, Abdel Hay RM, Abdel Halim D. Non‐ablative 1540 fractional laser: how far could it help injection lipolysis and dermal fillers in lower‐face rejuvenation? A randomized controlled trial. Journal of Cosmetic and Laser Therapy 2013;15(1):13‐20. [PUBMED: 23057533] [DOI] [PubMed] [Google Scholar]
Leitenberger 2009
- Leitenberger JJ, Cayce RL, Haley RW, Adams‐Huet B, Bergstresser PR, Jacobe HT. Distinct autoimmune syndromes in morphea: a review of 245 adult and pediatric cases. Archives of Dermatology 2009;145(5):545‐50. [PUBMED: 19451498] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2012
- Li SC, Torok KS, Pope E, Dedeoglu F, Hong S, Jacobe HT, et al. Development of consensus treatment plans for juvenile localized scleroderma: a roadmap toward comparative effectiveness studies in juvenile localized scleroderma. Arthritis Care & Research 2012;64(8):1175‐85. [PUBMED: 22505322] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marsol 2013
- Bielsa Marsol I. Update on the classification and treatment of localized scleroderma. Actas Dermo‐Sifiliográficas 2013;104(8):654‐66. [PUBMED: 23948159] [DOI] [PubMed] [Google Scholar]
Mayes 1998
- Mayes MD. Classification and epidemiology of scleroderma. Seminars in cutaneous medicine and surgery 1998;17(1):22‐26. [DOI] [PubMed] [Google Scholar]
Mertens 2015
- Mertens JS, Seyger MMB, Kievit W, Hoppenreijs EPAH, Jansen TL, Kerkhof PCM, et al. Disease recurrence in localized scleroderma: a retrospective analysis of 344 patients with paediatric‐or adult‐onset disease. British Journal of Dermatology 2015;172(3):722‐8. [PUBMED: 25381928 ] [DOI] [PubMed] [Google Scholar]
Peterson 1997
- Peterson LS, Nelson AM, Su WP, Mason T, O'Fallon W M, Gabriel SE. The epidemiology of morphea (localized scleroderma) in Olmsted County 1960‐1993. The Journal of rheumatology 1997;4(1):73‐80. [PubMed] [Google Scholar]
Pope 1998a
- Pope J, Fenlon D, Thompson A, Shea B, Furst D, Wells GA, et al. Ketanserin for Raynaud’s phenomenon in progressive systemic sclerosis. Cochrane Database of Systematic Reviews 1998, Issue 2. [DOI: 10.1002/14651858.CD000954] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pope 1998b
- Pope J, Fenlon D, Thompson A, Shea B, Furst D, Wells GA, et al. Iloprost and cisaprost for Raynaud’s phenomenon in progressive systemic sclerosis. Cochrane Database of Systematic Reviews 1998, Issue 2. [DOI: 10.1002/14651858.CD000953] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rook 2010
- Breathnach SM. 73. Drug Reactions. In: Burns T, Breathnach S, Cox N, Griffiths C editor(s). Rook's Textbook of Dermatology. 8th Edition. Vol. 4, Wiley‐Blackwell, 2010:73.1‐73.177. [Google Scholar]
Ruperto 2001
- Ruperto N, Ravelli A, Pistorio A, Malattia C, Viola S, Cavuto S, et al. Paediatric Rheumatology International Trials Organisation. The Italian version of the Childhood Health Assessment Questionnaire (CHAQ) and the Child Health Questionnaire (CHQ). Clinical and Experimental Rheumatology 2001;19(4 Suppl 23):S91‐5. [PUBMED: 11510339] [PubMed] [Google Scholar]
Saxton‐Daniels 2010
- Saxton‐Daniels S, Jacobe HT. An evaluation of long‐term outcomes in adults with pediatric‐onset morphea. Archives of Dermatology 2010;146(9):1044‐5. [PUBMED: 20855712] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Steen 1982
- Steen VD, Medsger TA, Rodnan GP. D‐Penicillamine therapy in progressive systemic sclerosis (scleroderma). Annals of Internal Medicine 1982;97(5):652‐9. [PUBMED: 7137731] [DOI] [PubMed] [Google Scholar]
Tingey 1998
- Tingey PC, Harding SE, Pope J, Fenlon D, Furst D, Shea B, et al. Cyclofenil for Raynaud’s phenomenon in progressive systemic sclerosis. Cochrane Database of Systematic Reviews 1998, Issue 2. [DOI: 10.1002/14651858.CD000955] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tratenberg 2017
- Tratenberg M, Gutwein F, Rao V, Sperber K, Wasserrman A, Ash J. Localized scleroderma: a clinical review. Current Rheumatology Reviews 2017;13(2):86‐92. [PUBMED: 27604889] [DOI] [PubMed] [Google Scholar]
Valanciene 2010
- Valanciene G, Jasaitiene D, Valiukeviciene S. Pathogenesis and treatment modalities of localized scleroderma. Medicina (Kaunas, Lithuania) 2010;46(10):649‐56. [PUBMED: 21393982] [PubMed] [Google Scholar]
Vasquez 2012
- Vasquez R, Sendejo C, Jacobe H. Morphea and other localized forms of scleroderma. Current Opinion in Rheumatology 2012;24(6):685‐93. [PUBMED: 23018858] [DOI] [PubMed] [Google Scholar]
Vilela 2010
- Vilela FA, Carneiro S, Ramos‐e‐Silva M. Treatment of morphea or localized scleroderma: review of the literature. Journal of Drugs in Dermatology 2010;9(10):1213‐19. [PUBMED: 20941945] [PubMed] [Google Scholar]
Zachariae 1994
- Zachariae H, Bjerring P, Halkier Sorensen L, Heickendorff L, Sondergaard K. Skin scoring in systemic sclerosis: a modification—relations to subtypes and the aminoterminalpropeptide of type III procollagen (PIIINP). Acta Dermato‐venereologica 1994;74(6):444‐6. [PUBMED: 7701875] [DOI] [PubMed] [Google Scholar]
Zandi 2012
- Zandi S, Kalia S, Lui H. UVA1 phototherapy: a concise and practical review. Skin Therapy Letter 2012;17(1):1‐4. [PUBMED: 22358227] [PubMed] [Google Scholar]
Zwischenberger 2011
- Zwischenberger BA, Jacobe HT. A systematic review of morphea treatments and therapeutic algorithm. Journal of the American Academy of Dermatology 2011;65(5):925‐41. [PUBMED: 21645943] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ravelli 2014
- Ravelli FN, Andriolo BNG, Vasconcellos MRA, Lyddiatt A, Fernandes Moça Trevisani V. Interventions for morphea. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD005027.pub4] [DOI] [Google Scholar]


