Abstract
Purpose
Although pirfenidone (PFD) is a key drug for the treatment of idiopathic pulmonary fibrosis (IPF), differences in tolerability between elderly and young patients remain unclear. This study aimed to investigate age-related differences in adverse drug reactions to PFD and to evaluate whether patient age influences the safety and tolerability of PFD in clinical practice.
Patients and method
One hundred fifty-four patients with IPF were treated with PFD in our institution between May 2009 and April 2017; these patients were classified into 2 groups on the basis of age: ≥75 years of age (elderly patients) and <75 years of age (younger patients). In each group, the clinical course, laboratory data, radiographic findings, adverse events, and tolerability of PFD at 6 months and 1 year after administration were retrospectively analyzed.
Results
Among the 120 patients examined in this study, 31 patients (26%) were ≥75 years of age. The continuation rate of PFD at 1 year in the elderly patient group was significantly lower (n=11 [35%] vs 57 [64%], p=0.007) than in the younger patient group. Regarding adverse drug reactions to PFD, the incidence of gastrointestinal disorders including anorexia (n=24 [77%] vs 40 [45%], p=0.002) and the discontinuation caused by gastrointestinal disorders (n=11 [35%] vs 13 [15%], p=0.019) were significantly higher in elderly patients than those in younger patients. However, with the exception of gastrointestinal disorders, other adverse drug reactions did not significantly differ between elderly and younger patients.
Conclusions
Compared with younger patients, elderly patients with IPF had a higher incidence of gastrointestinal disorders, along with an increased discontinuation rate of PFD. More careful management of gastrointestinal disorders may be required to ensure continuation of PFD in elderly patients.
Keywords: idiopathic pulmonary fibrosis, pirfenidone, tolerability, adverse drug reactions
Introduction
Idiopathic pulmonary fibrosis (IPF) is a devastating chronic lung disease with a poor prognosis1 Although many clinical trials of medications for IPF have consistently failed to demonstrate a significant treatment effect, two novel anti-fibrotic agents, pirfenidone (PFD) and nintedanib, have shown positive effects in several recent clinical trials.2–5 PFD is the first oral anti-fibrotic and anti-inflammatory agent approved for the treatment of IPF in Japan (2008), in Europe (2011), and in the United States (2014). PFD was reported to reduce decline in vital capacity/forced vital capacity of patients with IPF in several randomized, placebo-controlled, phase III trials: Shionogi Phase 3,2 CAPACITY3 and ASCEND.4 Pooled analyses from CAPACITY and ASCEND trials demonstrated that PFD therapy reduced both IPF-related and all-cause mortality.6 In addition, these trials and several other real-world studies have shown that treatment with PFD is generally tolerable for patients with IPF.7–10
In clinical practice, however, we occasionally encounter patients with IPF who must discontinue PFD because of adverse drug reactions; this is particularly notable among elderly patients. Post-marketing surveillance in Japan revealed that 24.3% of patients discontinued PFD therapy because of adverse drug reactions.11 There have been many studies regarding adverse drug reactions to PFD; however, differences according to age are unclear. An important reason is that the above major trials specifically excluded elderly patients (CAPACITY and ASCEND trials excluded patients over 80 years of age; Shionogi Phase 3 excluded patients over 75 years of age). An open-label study from the United States, which did not exclude patients with advanced age, demonstrated that elderly patients had a slightly higher but comparable rate of adverse drug reactions leading to discontinuation, relative to younger patients (20.9% in patients ≥80 years, 18.0% in patients ≥75 to <80 years, 10.9% in patients ≥65–<75 years, and 7.5% in patients ≤65 years).12 However, the tolerability and adverse drug reactions of PFD in elderly patients have not been thoroughly assessed (eg, the incidence rate of each adverse drug reaction based on age); thus, an additional study was needed.
In this study, we investigated the differences in adverse drug reactions to PFD according to age and evaluated whether patient age is correlated with safety and tolerability of PFD in clinical practice.
Materials and methods
This single-center, retrospective study was performed in accordance with the ethical principles of 1964 Helsinki Declaration and subsequent amendments. All procedures involving human participants were approved by the Human Ethics Committee of the Graduate School of Medicine of Chiba University (approval number 2584). The requirement for informed consent was waived by the ethics committee because this retrospective analysis was limited to preexisting data collected as part of the standard-of-care by respiratory physicians, and data anonymization and privacy issues are protected.
Patients
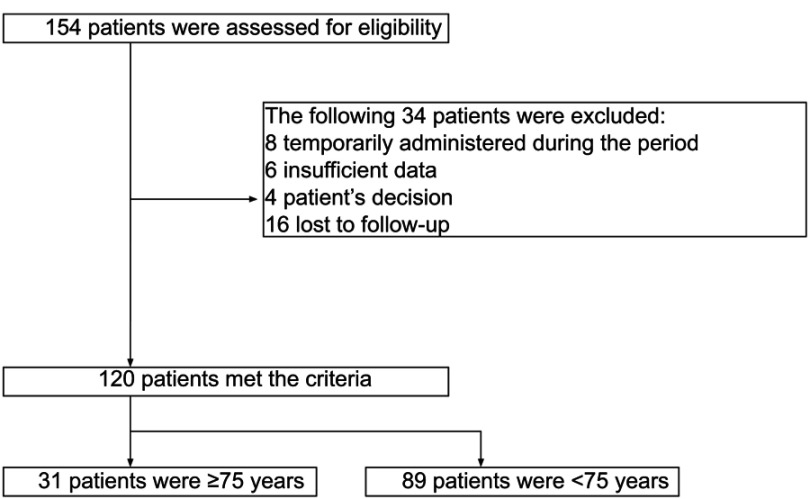
This study included 154 consecutive patients with IPF who received PFD between May 2009 and April 2017 in Chiba University Hospital. Patients were excluded on the basis of the following criteria: temporary administration of PFD during the perioperative period of lung cancer, insufficient data, patient’s decision or lost to follow-up (Figure 1). The remaining 120 patients were classified into 2 groups according to age: ≥75 years of age (elderly patients) and <75 years of age (younger patients); the clinical course, laboratory data, and radiographic findings of each group were reviewed. Diagnosis of IPF was made in accordance with the American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Association IPF guidelines of 2011.13 Patients participating in another clinical trial in our hospital, which evaluated the efficacy and safety of perioperative PFD for the prevention of acute exacerbation of IPF in lung cancer,14 were also included if they were administered PFD continuously after the perioperative period and did not receive postoperative chemotherapy. Additionally, this study included five IPF patients in whom the possibility of other chronic fibrosing pneumonia could not be denied.
Figure 1.
Study flow chart.
Administration of pirfenidone
Based on the Japanese guideline of IPF,15 the initial PFD dose was 600 mg/day for the first 2 weeks; it was then increased to 1200 mg/day, and if possible, further increased to 1800 mg/day. An experienced attending doctor determined whether to continue, reduce, stop temporarily, or stop permanently when adverse drug reactions occurred in relation to PFD.
Assessment of safety and tolerability
Tolerability of PFD and the causes of discontinuation of PFD at 6 months and 1 year after administration were assessed. Because PFD was shown to be effective at a dose of ≥1200 mg/day in the Japanese clinical trial,2 we designated patients continuing PFD at ≥1200 mg/day as the “continued” group, and patients continuing PFD with <1200 mg/day as the “dose reduction” group. Although administration of PFD with <1200 mg/day has not been shown to be effective in clinical trials, patients in the ”continued” group and “dose reduction” group were regarded as able to tolerate the drug because the dose can later be increased in the “dose reduction” group. The incidence of adverse drug reactions that occurred within 1 year in both elderly and younger patient groups was analyzed. Adverse drug reactions were classified based on the Common Terminology Criteria for Adverse Events version 4.0 (CTCAE v4.0).
Statistical analysis
All statistical analysis was performed by using JMP® pro 13.2.0 software (SAS Institute Inc. Cary, NC, USA). Fisher’s exact test was used for categorical data and the Mann-Whitney U test was used for continuous data. Univariate and multivariate analysis was performed by using logistic regression. A p-value of <0.05 was considered statistically significant for all analyses.
Results
Patient characteristics
Among 120 enrolled patients who received PFD for treatment of IPF, 31 patients (26%) were ≥75 years of age. The mean durations of PFD intake in the follow-up period were 248 and 286 days in elderly and younger patients, respectively. The baseline characteristics of the enrolled patients are shown in Table 1. There were significant differences in body weight (55 [44–62] vs 61 [56–69] kg, p=0.004) and body surface area (1.57 [1.39–1.68] vs 1.66 [1.56–1.75] m2, p=0.003) between elderly and younger patients. Although patients associated with lung cancer were included in both groups (see Patients in Methods section), there was no significant difference in the proportion of patients between the 2 groups (n=15 [48%] and n=41 [46%], respectively). Some patients were administered corticosteroid or immunosuppressant drugs (n=2 [6%] and n=14 [16%], respectively) as post-therapy after acute exacerbation of IPF, or because of other possible interstitial pneumonia.
Table 1.
Baseline characteristics of the 120 patients in this study
| ≥75 years (n=31) | <75 years (n=89) | p-value | |
|---|---|---|---|
| Median age, years (range) | 77 (75–85) | 69 (30–74) | |
| Male, no (%) | 22 (71) | 62 (70) | 1.0 |
| Smoking history, no (%) | 22 (71) | 72 (81) | 0.31 |
| Performance status, 0/1/2/3/4 | 2/15/11/3/0 | 21/27/35/6/0 | |
| Body weight (kg), median [IQR] | 55 [44–62] | 61 [56–69] | 0.004 |
| BMI (kg/m2), median [IQR] | 22.3 [18.5–24.8] | 23.7 [21.3–25.6] | 0.068 |
| BSA (m2), median [IQR] | 1.57 [1.39–1.68] | 1.66 [1.56–1.75] | 0.003 |
| Co-existing lung cancer, no (%) | 15 (48) | 41 (46) | 0.84 |
| KL-6 (U/mL), median [IQR] | 917 [698–1526] | 890 [617–1796] | 0.80 |
| SP-Da (ng/mL), median [IQR] | 203 [166–334] | 222 [136–366] | 0.99 |
| Maximum dose of PFD, no (%) | |||
| <1200 mg | 1 (3) | 10 (11) | 0.29 |
| ≥1200 to <1800 mg | 23 (74) | 56 (63) | 0.28 |
| ≥1800 mg | 7 (23) | 23 (26) | 0.81 |
| Pulmonary function test, median [IQR] | |||
| FVCb (L) | 2.07 [1.49–2.69] | 2.13 [1.70–3.28] | 0.29 |
| %FVCb (%) | 77.6 [57.6–90.3] | 71.1 [54.8–92.4] | 0.56 |
| %DLCOc (%) | 60.3 [46.6–80.8] | 52.8 [35.9–75.2] | 0.142 |
| GAP staged, I/II/III | 13/12/4 | 34/32/9 | |
| Supplemental oxygen, no (%) | 5 (16) | 26 (29) | 0.23 |
| Concomitant drug, no (%) | |||
| Corticosteroid | 2 (6) | 14 (16) | 0.24 |
| Immunosuppressant | 0 | 5 (6) | 0.33 |
| Proton pump inhibitor/H2-blocker | 19 (61) | 39 (44) | 0.101 |
Notes: an=22 and 65, respectively. bn=30 in over 75 years. cn=28 and 74, respectively. dn=29 and 75, respectively. Bold values indicate p<0.05.
Abbreviations: BMI, body mass index; BSA, body surface area; DLCO, diffusing capacity of the lung for carbon monoxide; FVC, forced vital capacity; GAP, gender, age and physiology; PFD, pirfenidone.
Tolerability
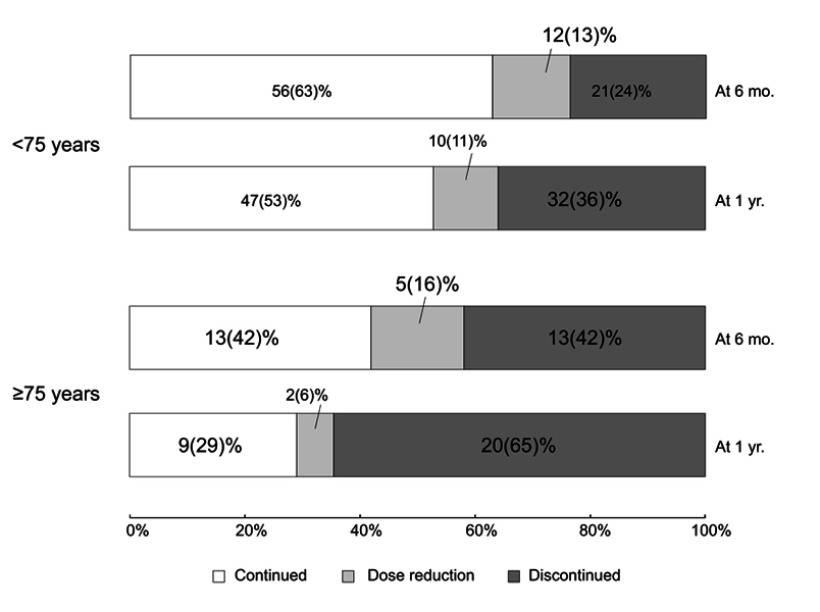
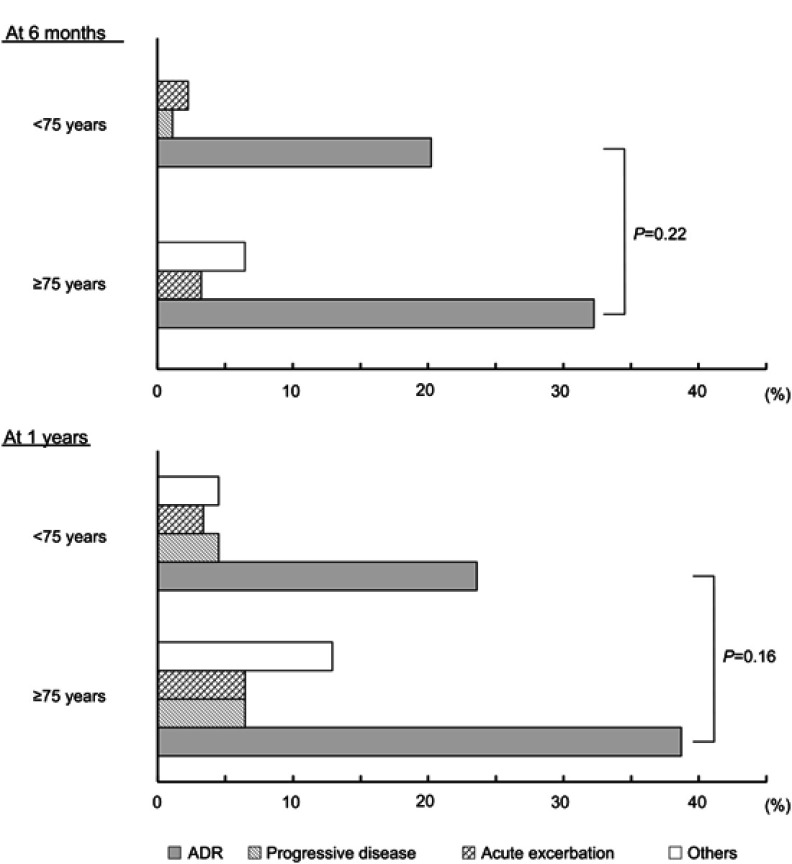
Outcomes at 6 months and 1 year after administration of PFD are shown in Figure 2. The continuation rate at 6 months in the elderly patient group tended to be low (n=18 [58%] vs 68 [76%], p=0.065), compared with the rate in the younger patient group, but was not significantly different. In contrast, the continuation rate at 1 year in the elderly patient group was significantly lower (n=11 [35%] vs 57 [64%], p=0.007) than in the younger patient group. An adverse drug reaction was the most common cause of discontinuation of PFD at 6 months and 1 year in both groups; however, discontinuation rates due to all adverse drug reactions between the 2 groups were not significantly different (at 6 months: n=10 [32%] vs 18 [20%], p=0.22; at 1 year: n=12 [39%] vs 21 [24%], p=0.16, Figure 3). Additionally, the discontinuation rates due to progressive disease (at 6 months: n=0 vs 1 [1%], p=1.0; at 1 year: n=2 [6%] vs 4 [4%], p=0.47) or acute exacerbation of IPF (at 6 months: n=1 [3%] vs 2 [2%], p=1.0; at 1 year: n=2 [6%] vs 3 [3%], p=0.60) were not significantly different between the 2 groups. Other reasons for discontinuation were factors not directly related to IPF (eg, lung cancer, pneumonia, and stroke); the discontinuation rates due to these factors were not significantly different between the 2 groups (at 6 months: n=2 [6%] vs 0, p=0.065; at 1 year: n=4 [13%] vs 4 [4%], p=0.20). Furthermore, the discontinuation rate due to adverse drug reactions at 1 year did not significantly differ from that at 6 months in both groups (elderly patients: n=12 [39%] vs 10 [32%]; younger patients: n=21 [24%] vs 18 [20%]).
Figure 2.
Outcomes at 6 months and 1 year after administration of pirfenidone. The discontinuation rate at 1 year was significantly higher in elderly patients than in younger patients (65% vs 36%, respectively, p=0.007). It did not significantly differ at 6 months (42% vs 24%, respectively, p=0.065).
Abbreviations: mo, months; yr, year.
Figure 3.
Reasons for discontinuation of pirfenidone. Discontinuation due to adverse drug reactions (ADR) was the most common cause in both elderly and younger patients; the discontinuration rate due to ADR did not significantly differ between the two groups at 6 months and 1 year.
Adverse effects
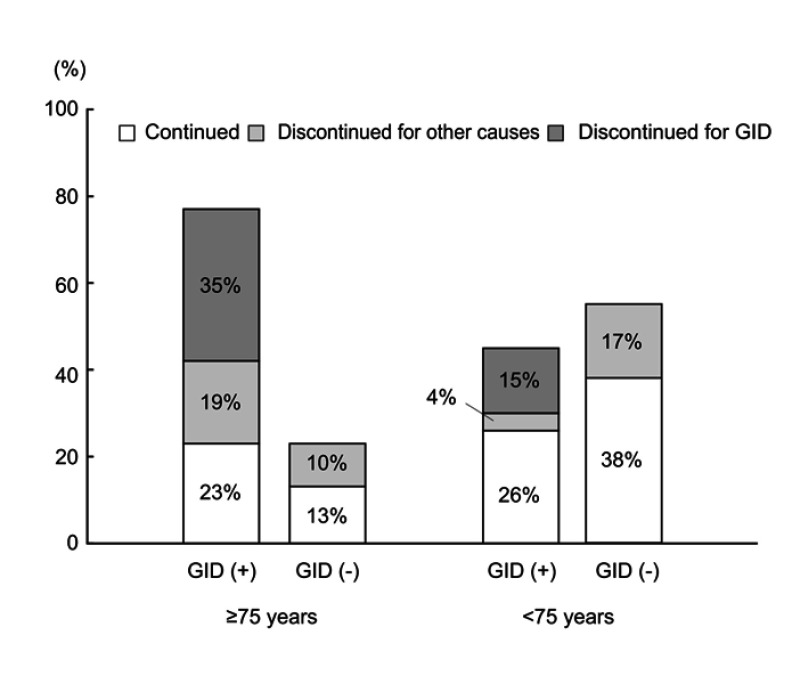
Table 2 shows the incidences of each adverse drug reaction to PFD that occurred within 1 year after the administration of PFD in the 2 groups. Anorexia was the most common event in both groups and the incidence of these disorders was significantly higher in the elderly patient group (n=20 [65%] vs 32 [36%], p=0.007). Similarly, gastrointestinal disorders, including anorexia, dyspepsia, gastroesophageal reflux syndrome, nausea, and constipation were significantly higher in the elderly patient group (n=24 [77%] vs 40 [45%], p=0.002, Table 2). The incidence of photosensitivity/rash was 19% (n=6) in the elderly patient group and 21% (n=19) in the younger patient group; there was no significant difference between the 2 groups. Additionally, nearly all adverse drug reactions were mild (Grade ≤2, except for one case). Furthermore, analysis of the outcome at 1 year after administration in patients with gastrointestinal disorders due to PFD demonstrated that the discontinuation rate due to gastrointestinal disorders was significantly higher in the elderly patient group, as shown in Figure 4 (n=11 [35%] vs 13 [15%], p=0.019).
Table 2.
Incidence of adverse drug reactions (ADR)
| ≥75 years (n=31) | <75 years (n=89) | p-value | |||
|---|---|---|---|---|---|
| No, % | Grade (1/2/3/4–5) |
No, % | Grade (1/2/3/4–5) |
||
| Any ADR | 27 (87) | - | 63 (71) | - | 0.092 |
| Any gastrointestinal disorders | 24 (77) | - | 40 (45) | - | 0.002 |
| Anorexia | 20 (65) | 9/10/1/0 | 32 (36) | 18/13/1/0 | 0.007 |
| Dyspepsia | 4 (13) | 4/0/0/0 | 12 (13) | 10/2/0/0 | 1.0 |
| Gastroesophageal reflux disease | 5 (16) | 1/4/0/0 | 3 (3.4) | 3/0/0/0 | 0.027 |
| Nausea | 1 (3.2) | 1/0/0/0 | 8 (9.0) | 2/6/0/0 | 0.44 |
| Constipation | 1 (3.2) | 1/0/0/0 | 0 | - | 0.26 |
| Photosensitivity/Rash | 6 (19) | 4/2/0/0 | 19 (21) | 14/5/0/0 | 1.0 |
| Dizziness | 2 (6.5) | 2/0/0/0 | 9 (10) | 8/1/0/0 | 0.73 |
| Fatigue | 3 (9.7) | 3/0/0/0 | 6 (6.8) | 5/1/0/0 | 0.69 |
| Hepatic dysfunction | 1 (3.2) | 1/0/0/0 | 7 (7.9) | 6/1/0/0 | 0.68 |
| Dysgeusia | 0 | - | 3 (3.3) | 2/1/0/0 | 0.57 |
| Mucositis oral | 0 | - | 2 (2.2) | 1/1/0/0 | 1.0 |
| Cough | 0 | - | 1 (1.1) | 1/0/0/0 | 1.0 |
| Myalgia | 0 | - | 1 (1.1) | 0/1/0/0 | 1.0 |
| Arthralgia | 0 | - | 1 (1.1) | 0/1/0/0 | 1.0 |
| Headache | 0 | - | 1 (1.1) | 0/1/0/0 | 1.0 |
| Abdominal pain | 0 | - | 1 (1.1) | 0/1/0/0 | 1.0 |
| Somnolence | 0 | - | 1 (1.1) | 1/0/0/0 | 1.0 |
Note: Bold values indicate p<0.05.
Figure 4.
Outcome at 1 year after administration in patients with gastrointestinal disorders due to pirfenidone. Of the patients with gastrointestinal disorders (GID) due to pirfenidone, the proportion of patients who discontinued pirfenidone due to GID was greatest in elderly patients; it was significantly higher than in younger patients (35% vs 15%, respectively, p=0.019).
Finally, using a set of variables that appeared to be important in gastrointestinal disorders related to PFD, we performed univariate and multivariate analyses to explore the determinants of these symptoms (Table 3). In univariate analysis, age and body surface area (BSA) were related to the incidence of gastrointestinal disorders. In multivariate analysis using these variables, age was most strongly related.
Table 3.
Logistic regression analysis of gastrointestinal disorders in patients with idiopathic pulmonary fibrosis received pirfenidone
| Parameter | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value | |
| Age (≥75 years) | 4.2 (1.64–10.8) | 0.003 | 3.42 (1.30–9.00) | 0.013 |
| Male | 0.88 (0.40–1.93) | 0.75 | ||
| PS (≥2) | 1.25 (0.61–2.58) | 0.54 | ||
| Co-existing lung cancer | 1.33 (0.65–2.74) | 0.43 | ||
| BSA (<1.65 m2) | 3.11 (1.41–6.85) | 0.005 | 2.51 (1.10–5.71) | 0.029 |
| GAP stage II or III | 0.63 (0.29–1.37) | 0.25 | ||
| GAP stage III | 1.25 (0.39–4.00) | 0.71 | ||
| %FVC (<50%) | 0.58 (0.19–1.75) | 0.33 | ||
| %DLCO (<35%) | 0.63 (0.24–1.72) | 0.37 | ||
Note: Bold values indicate p<0.05.
Abbreviations: BSA, body surface area; DLCO, diffusing capacity of lung for carbon monoxide; GAP, gender, age and physiology; FVC, forced vital capacity; PS, performance status.
Discussion
In the present study, we found that elderly patients had a significantly higher incidence of gastrointestinal disorders caused by the administration of PFD (77% vs 45%). In addition, elderly patients exhibited a significantly higher discontinuation rate (65% vs 36%) of PFD at 1 year than younger patients. Furthermore, the discontinuation rate by gastrointestinal disorders was significantly higher in elderly patients than in younger patients (35% vs 15%). In contrast, the incidences of adverse drug reactions other than gastrointestinal disorders did not significantly differ between elderly and younger patients.
A previous study demonstrated that gastrointestinal disorders due to PFD were related to the PFD Cmax (maximum plasma concentration), and that co-administration with food reduced Cmax.16 Another study reported that patients with adverse drug reactions had greater dosage of PFD to body surface area (BSA) or body mass index (BMI) than patients without adverse drug reactions;17 these results may also be related to differences in the PFD Cmax. Additionally, in our study, elderly patients exhibited lower BSA and body weight than younger patients; this could have partly contributed to the increased incidence of gastrointestinal disorders associated with PFD usage. However, a direct link between PFD Cmax and BSA or body weight was not identified in the present study. Furthermore, reduced gastrointestinal and metabolic function associated with aging might have been related to the incidence of gastrointestinal disorders. Furthermore, poor adherence to medication, reduced number of meals, and irregular meal times might have been a factor among the elderly patients; these might have had an impact on the high PFD Cmax. Additionally, drugs that can interact with PFD are few (eg, fluvoxamine maleate, ciprofloxacin); notably, combined use of PFD and drugs that potentially cause dyspepsia (eg, nonsteroidal anti-inflammatory drugs, calcium antagonists, angiotensin converting enzyme inhibitors, glucocorticoids)18 may exacerbate gastrointestinal disorders.
Regarding the incidence of adverse drug reactions other than gastrointestinal disorders in the present study, the incidence was not significantly different between elderly and younger patients. In addition, the severity of each adverse drug reaction was mild (Grade ≤2) in almost all cases in both elderly and younger patient groups as a previous study showed.7 This suggests that, if gastrointestinal symptoms are properly controlled, PFD can be continuously used, even in elderly patients. Therefore, unless adverse drug reactions that lead to discontinuation occur within the first few months after administration of PFD, it is unlikely that such reactions will occur later, even in elderly patients. This suggests that clinicians should not avoid prescription of PFD due to concern for adverse drug reactions, simply because patients are older.
When administering PFD to elderly patients, careful adjustment of dosage, administration interval, or frequency, according to dietary habits or symptoms, may be important. Indeed, an expert panel recommended taking PFD separately throughout the meal, according to the situation;19,20 consistent with this recommendation, in a rat model, the PFD Cmax and the PFD associated inhibition on gastric emptying was reduced by dividing the dose of PFD, compared to single-bolus dose.21 In addition, split dose administration at the start, middle, and end of a meal was reported to increase the continuation rate of PFD, in a monthly specialist nurse review of the first three months of treatment.8 Additionally, prokinetic agents such as domperidone, mosapride, metoclopramide, proton pump inhibitors, and some herbal medicines may help to reduce gastrointestinal disorders due to PFD;22 however, there is no established evidence for the use of these treatments.
Our study has some limitations. First, this study was retrospectively conducted; thus, some patients were excluded from the analysis for various reasons (Figure 1). Nevertheless, the incidences of adverse drug reactions among younger patients in this study were not significantly different from those in post-marketing surveillance in Japan.9 Second, analysis of the effects of PFD was not performed in this study; however, this analysis was not a primary objective of the study. Third, this study included many patients with lung cancer. However, nearly all patients with lung cancer were operable and did not receive chemotherapy. Furthermore, only one patient received postoperative chemotherapy because of comorbid idiopathic pulmonary fibrosis. In our study, the rate of discontinuation due to adverse drug reactions was slightly higher than that of a previous report,12 it was not considerably different from that of other reports.7,9,11 Therefore, although the surgery itself could have influenced adverse drug reactions to PFD, we found no major impact. Despite these limitations, we consider our results to be useful for elucidating the characteristics of adverse drug reactions to PFD. Furthermore, understanding adverse drug reactions to anti-fibrotic agents in detail may help physicians to determine which anti-fibrotic agent to administer to patients with IPF in clinical practice.
In conclusion, PFD might cause more frequent gastrointestinal disorders leading to discontinuation in elderly patients, compared to younger patients. Careful management of gastrointestinal disorders may be necessary to ensure the continuation of PFD in elderly patients with IPF.
Acknowledgments
We would like to thank Editage for English language editing. This study was supported in-part by a grant to The Intractable Respiratory Diseases and Pulmonary Hypertension Research Group, the Ministry of Health, Labor and Welfare, Japan (http://www.mhlw.go.jp/english/index.html); the Japan Agency for Medical Research and Development (AMED; https://www.amed.go.jp).
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. Lancet. 2017;389(10082):1941–1952. doi: 10.1016/S0140-6736(17)30866-8 [DOI] [PubMed] [Google Scholar]
- 2.Taniguchi H, Ebina M, Kondoh Y, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35(4):821–829. doi: 10.1183/09031936.00005209 [DOI] [PubMed] [Google Scholar]
- 3.Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377(9779):1760–1769. doi: 10.1016/S0140-6736(11)60405-4 [DOI] [PubMed] [Google Scholar]
- 4.King TE Jr., Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–2092. doi: 10.1056/NEJMoa1402582 [DOI] [PubMed] [Google Scholar]
- 5.Richeldi L. du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–2082. doi: 10.1056/NEJMoa1402584 [DOI] [PubMed] [Google Scholar]
- 6.Noble PW, Albera C, Bradford WZ, et al. Pirfenidone for idiopathic pulmonary fibrosis: analysis of pooled data from three multinational phase 3 trials. Eur Respir J. 2016;47(1):243–253. doi: 10.1183/13993003.00026-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okuda R, Hagiwara E, Baba T, Kitamura H, Kato T, Ogura T. Safety and efficacy of pirfenidone in idiopathic pulmonary fibrosis in clinical practice. Respir Med. 2013;107(9):1431–1437. doi: 10.1016/j.rmed.2013.06.011 [DOI] [PubMed] [Google Scholar]
- 8.Chaudhuri N, Duck A, Frank R, Holme J, Leonard C. Real world experiences: pirfenidone is well tolerated in patients with idiopathic pulmonary fibrosis. Respir Med. 2014;108(1):224–226. doi: 10.1016/j.rmed.2013.11.005 [DOI] [PubMed] [Google Scholar]
- 9.Tzouvelekis A, Ntolios P, Karampitsakos T, et al. Safety and efficacy of pirfenidone in severe idiopathic pulmonary fibrosis: a real-world observational study. Pulm Pharmacol Ther. 2017;46:48–53. doi: 10.1016/j.pupt.2017.08.011 [DOI] [PubMed] [Google Scholar]
- 10.Yoon HY, Kim DS, Song JW. Efficacy and safety of pirfenidone in advanced idiopathic pulmonary fibrosis. Respiration. 2019;97(3):242–251. doi: 10.1159/000492937 [DOI] [PubMed] [Google Scholar]
- 11.Ogura T, Azuma A, Inoue Y, et al. All-case post-marketing surveillance of 1371 patients treated with pirfenidone for idiopathic pulmonary fibrosis. Respir Investig. 2015;53(5):232–241. doi: 10.1016/j.resinv.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 12.Lancaster L, Morrison L, Auais A, et al. Safety of pirfenidone in patients with idiopathic pulmonary fibrosis: experience from 92 sites in an open-label US expanded access program. Pulm Ther. 2017;3(2):317–325. doi: 10.1007/s41030-017-0049-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwata T, Yoshino I, Yoshida S, et al. A phase II trial evaluating the efficacy and safety of perioperative pirfenidone for prevention of acute exacerbation of idiopathic pulmonary fibrosis in lung cancer patients undergoing pulmonary resection: West Japan oncology group 6711 L (PEOPLE Study). Respir Res. 2016;17(1):90. doi: 10.1186/s12931-016-0398-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Japanese Respiratory Society. Idiopathic Interstitial Pneumonias: Diagnosis and Treatment. 3rd ed. Tokyo: Nankodo;2016:54–55. [Google Scholar]
- 16.Rubino CM, Bhavnani SM, Ambrose PG, Forrest A, Loutit JS. Effect of food and antacids on the pharmacokinetics of pirfenidone in older healthy adults. Pulm Pharmacol Ther. 2009;22(4):279–285. doi: 10.1016/j.pupt.2009.03.003 [DOI] [PubMed] [Google Scholar]
- 17.Uehara M, Enomoto N, Oyama Y, et al. Body size-adjusted dose analysis of pirfenidone in patients with interstitial pneumonia. Respirology. 2018;23(3):318–324. doi: 10.1111/resp.13145 [DOI] [PubMed] [Google Scholar]
- 18.Talley NJ, Ford AC. Functional Dyspepsia. N Engl J Med. 2015;373(19):1853–1863. doi: 10.1056/NEJMra1501505 [DOI] [PubMed] [Google Scholar]
- 19.Costabel U, Bendstrup E, Cottin V, et al. Pirfenidone in idiopathic pulmonary fibrosis: expert panel discussion on the management of drug-related adverse events. Adv Ther. 2014;31(4):375–391. doi: 10.1007/s12325-014-0112-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lancaster LH, de Andrade JA, Zibrak JD, et al. Pirfenidone safety and adverse event management in idiopathic pulmonary fibrosis. Eur Respir Rev. 2017;26(146). doi: 10.1183/16000617.0057-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan L, Gelzleichter T, Chen Y, Burg C, Limb SL, Nguyen L. Effect of pirfenidone on gastric emptying in a rat model. Pulm Pharmacol Ther. 2018;51:41–47. doi: 10.1016/j.pupt.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 22.Itoh T, Koyabu K, Morimoto A, et al. Ameliorative effects of mosapride or rikkunshi-to on the suppression of gastrointestinal motility by pirfenidone in rats. Jpn Pharmacol Ther. 2012;40:405–411. [Google Scholar]