Abstract
The liquefaction of biomass is an important technology to converse the biomass into valuable biofuel. The common technologies for liquefaction of biomass are indirect liquefaction and direct liquefaction. The indirect liquefaction refers to the Fischer–Tropsch (F–T) process using the syngas of biomass as the raw material to produce the liquid fuel, including methyl alcohol, ethyl alcohol, and dimethyl ether. The direct liquefaction of biomass refers to the conversion biomass into bio-oil, and the main technologies are hydrolysis fermentation and thermodynamic liquefaction. For thermodynamic liquefaction, it could be divided into fast pyrolysis and hydrothermal liquefaction. In addition, this review provides an overview of the physicochemical properties and common upgrading methods of bio-oil.
Keywords: review, biomass, liquefaction, bio-oil, upgrading
1. Introduction
Fossil fuels, such as coal, oil, and natural gas, are non-renewable resources. They play a vital role in human life and social progress. Although, the total amount of fossil fuels on the earth would meet the needs of human beings for several decades, these non-renewable fossil fuels would eventually run out [1]. In addition, the excessive emission of greenhouse gases (such as CO, CO2, NOx, SOx, and CH4) into the atmosphere makes the global climate uncontrollable with the consumption of fossil fuels [2]. Hence, the renewable fuels (nuclear energy, solar energy, wind energy, and biomass energy) should be further developed [3,4]. Biomass has a high utilization potential and is one of the most important energy sources of the future.
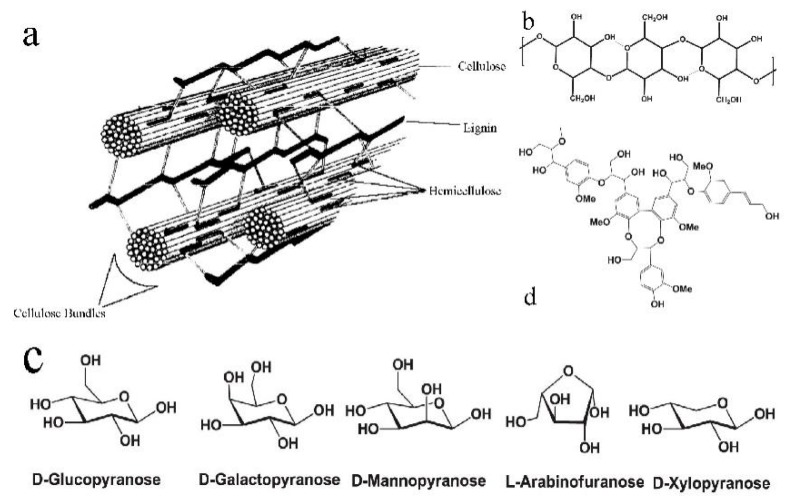
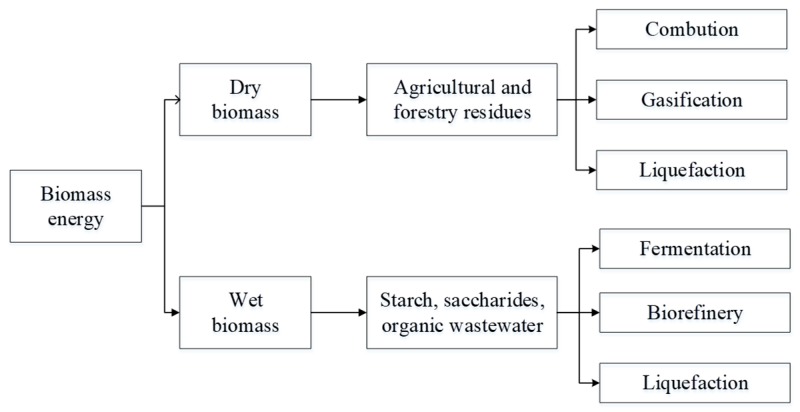
Generally, biomass is usually grouped as follows [5,6,7]: (1) Agricultural and forestry residues, (2) herbaceous crops, (3) aquatic and marine biomass, and (4) wastes. Biomass waste means the materials generated in the process of production or consumption of biomass, including wood, straw, animal dungs, and household garbage. Lignocellulose is a complex structure (Figure 1a), which is composed of a mixture of cellulose (Figure 1b), hemicellulose (Figure 1c), lignin (Figure 1d), and inorganic components. Cellulose and hemicellulose are tightly bound to lignin mainly by hydrogen and covalent bonds. Cellulose is usually represented by (C6H10O5)n, and the polymerization degree of the long polysaccharide chain is approximately 10,000, resulting in a high molecular weight (>500,000). Cellulose is formed by the β-1,4 glycosidic linkage of D-glucopyranose units. For hemicellulose, the content in dry biomass is usually about 25%, and it is a low degree of amorphous heteropolysaccharide with high degree branching of a straight-chain skeleton. The basic units in hemicellulose are xylan and gulucomannan. Lignin is an amorphous aromatic natural polymer, which is linked primarily via ether bonds with hydroxyl and methoxy groups. The solubility of lignin in water is very low. The main roles of lignin in a plant are as follows: Strengthen their structure, regulate the flow of fluids, protect against microorganisms, and store energy. As we know, biomass energy is the exclusive renewable organic carbon resource to produce liquid fuels. Biomass is the renewable organic materials. While the biomass waste still belongs to the macroscopic category of biomass. According to the statistics, the total amount of biomass waste could reach 2 × 109 t/Y. Nevertheless, only about 40% of biomass waste is used for fuel, building materials, feed, and power generation, and the residual parts are treated in an extensive mode, for instance incineration, landfill, and centralized stacking, resulting in a waste of resources and serious pollution of the environment. Therefore, it is the key component of sustainable development for biomass waste resources. Recently, the mainly technologies of the utilization of biomass resource are physical, thermochemical, and biological. Considering the type of products, it could be divided into gasification, liquefaction, biorefinery, and combustion. Figure 2 shows the current conversion technologies of biomass. For the dry biomass, the primary pathways are combustion, gasification, and liquefaction. Moreover, the products of gasification of biomass could also be conversed to liquid products. For the wet biomass, the primary pathways are hydrolysis fermentation, biorefinery, and liquefaction. The products of biomass conversion are abundant, including syngas, bio-oil, biodiesel, methyl alcohol, ethyl alcohol, etc.
Figure 1.
(a) Structure of plant cell walls [8]; (b) structure of cellulose [9]; (c) basic units of hemicellulose [9]; and (d) simple structure of lignin [9].
Figure 2.
Current conversion technologies of biomass.
This review paper aims to provide a solution of the current liquefaction technologies of biomass, including indirect liquefaction and direct liquefaction. In indirect liquefaction, we mainly discuss the synthesis pathway of ethyl alcohol and summarize the common catalysts. In direct liquefaction, we discuss the hydrolysis fermentation and thermodynamic liquefaction. In the end, the upgrading technologies of bio-oil have been provided.
2. Indirect Liquefaction
2.1. Reaction Process
Indirect liquefaction is a promising technology, which is divided into two stages. The first stage is a thermochemical gasification process [10,11]. In this process, the syngas is produced after the raw material reacts with air or steam. In the syngas, the primary substances are CO, CO2, H2, and H2O. The second stage is the well-established Fischer–Tropsch (F–T) process [12]. During the F–T process, the mixture would be used to produce a range of chemicals, including methyl alcohol, dimethyl ether, and ethyl alcohol, while there is little research on the higher alcohols derived from the biomass syngas. The biggest challenges are the design of the novel catalytic reactor for the typically smaller scale of biomass conversion processes and catalysts for specific chemicals according to the molar ratio of H2 to CO. We take the synthesis of ethyl alcohol as an example to introduce the indirect liquefaction process.
2.2. Reaction Mechanism
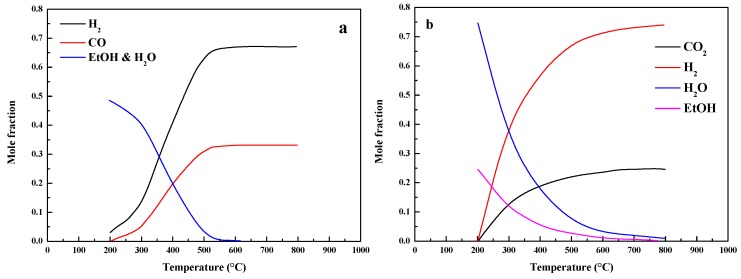
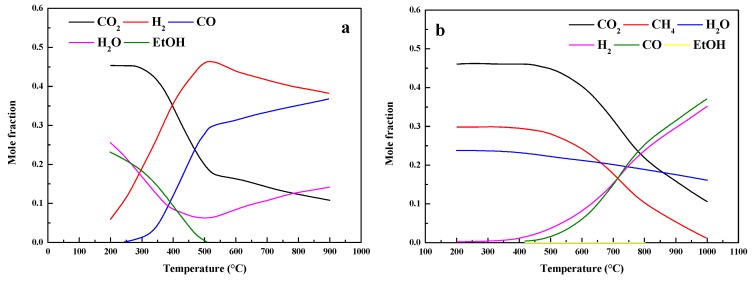
There are two approaches to produce ethyl alcohol [13]. The first pathway is hydrogenation of CO, and the reaction equation is shown in Equation (1). From the equation, it indicates that hydrogenation of CO is a highly exothermic and favorable reaction. Figure 3a shows the thermodynamic analysis of the hydrogenation of CO, and the reaction conditions (H2/CO = 2.0, 30 bar) are assumed. The results release that the concentrations of ethyl alcohol and water decrease with a temperature increase, while the concentrations of H2 and CO increase with a temperature increase. Hence, the suitable reaction temperature should be below 350 °C via the hydrogenation of CO to produce ethyl alcohol. The second pathway is hydrogenation of CO2, and the reaction equation is shown in Equation (2). From the equation, it indicates that hydrogenation of CO2 is also a highly exothermic and favorable reaction. The thermodynamic analysis of the hydrogenation of CO2 is shown in Figure 3b. From the results, the concentrations of ethyl alcohol and water decrease with a temperature increase, while the concentrations of CO2 and H2 increase with a temperature increase. The reasonable temperature for the synthesis of ethyl alcohol via the hydrogenation of CO2 should also be below 300 °C. However, the by-products would be formed during the F–T process. For hydrogenation of CO, the H2O product would react with CO quickly, and the by-products of CO2 and H2 would be formed, which refers to the water gas shift reaction (Equation (3)). On the contrary, the reverse water gas shift reaction may be occurred in the hydrogenation of the CO2 process, and the by-product is CO. It indicates that the two pathways proceed through a common intermediate. In addition, the methanation (Equations (4) and (5)) would occur along with the hydrogenation of CO or CO2, and CH4 is the most significant by-product [14]. Figure 4 shows the equilibrium concentrations of an initial mixture syngas. From the results, it indicates that the suitable temperature should be below 400 °C at 30 bar with no CH4 formation. However, if CH4 is allowed as a product under the same conditions, the content of ethyl alcohol is virtually zero. Therefore, CH4 must be kinetically limited to improve the yield of ethyl alcohol. Moreover, the equilibrium concentration of ethyl alcohol increases with the reaction pressure increase. As shown in the literature, there are little researches that studied the hydrogenation of CO2 or the mixture of CO and CO2, and the primary researches studied the hydrogenation of CO. The catalyst is the key factor for this reaction, and it could be divided into four groups, including Rh-based materials, modified CH3OH synthesis catalysts, modified F–T catalysts, and modified Mo-based catalysts [15,16].
| 2CO + 4H2 → C2H5OH, ∆H = −61.2 kcal·mol−1, ∆G = −29.3 kcal·mol−1, | (1) |
| 2CO2 + 4H2 → C2H5OH, ∆H = −41.5 kcal·mol−1, ∆G = −15.7 kcal·mol−1, | (2) |
| CO + H2O → CO2 + H2, | (3) |
| CO2 + 4H2 → CH4 + 2H2O, | (4) |
| CO + 4H2 → CH4 + H2O. | (5) |
Figure 3.
Thermodynamic analysis of the hydrogenation of CO (a) and CO2 (b; H2/CO = 2.0, H2/CO2 = 3.0, 30 bar).
Figure 4.
Equilibrium concentrations of mixture syngas: No CH4 allowed (a) and CH4 allowed (b; H2 = 49%, CO = 26%, CO2 = 21%, and H2O = 4%).
3. Direct Liquefaction
3.1. Hydrolysis-Fermentation Liquefaction
3.1.1. Main Steps
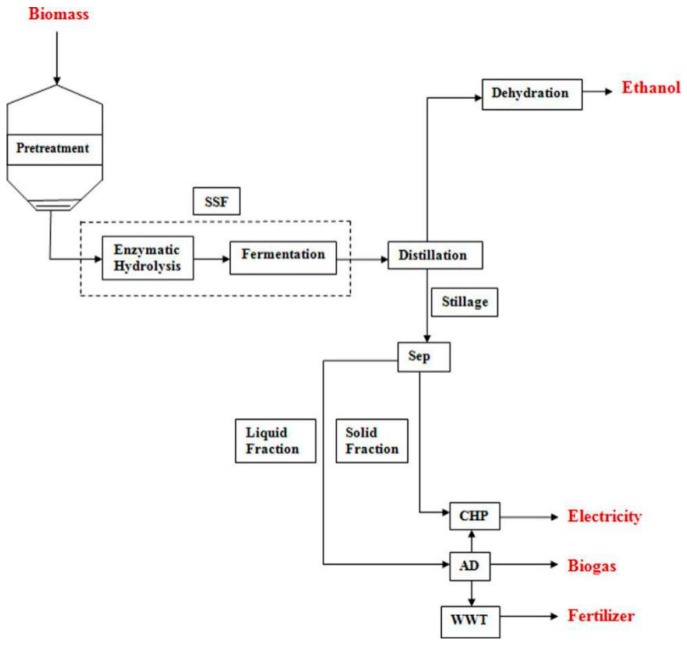
In the last few decades, ethyl alcohol has attracted a great deal of attention as a potential alternative to fossil fuels [9]. Currently, fermentation of biomass is the main industrial technology to produce ethyl alcohol, which the primary raw materials are glucose (obtained from corn) and sucrose (obtained from sugar cane and beets) [17]. While, there are the same negative effects on ethyl alcohol production using starch or sugar as the raw material, which would compete with food production directly [18,19]. Up to now, corn straw has been considered as possible raw material for ethyl alcohol production [20]. The flow diagram of enzymatic ethanol production process is shown in Figure 5. Once the biomass is transported to the production plant, it would be stored in the warehouse to prevent from fermentation and bacterial contamination. Then, the raw material would be pre-treated to make it more accessible for extraction. In the fermentation process, hydrolysate, yeasts, nutrients, and other ingredients would be added. The fermentation is usually executed at 25–30 °C and the suitable reaction time would last for 6–72 h. The parameters are primarily dependent on the components of hydrolysate, type, density, or activity of yeasts. The recycle yeasts are employed to improve the activity and productivity of fermentation. The concentration of ethyl alcohol in the broth typically contains 8–14%, while the activity of yeasts would be inhibited above this concentration. After distillation, a mixture may be obtained, which is often termed “hydrous” or “hydrated” ethyl alcohol (95% alcohol, 4% water). The hydrated ethyl alcohol would be then dehydrated to obtain “anhydrous” ethyl alcohol (99.6% alcohol, 0.4% water). The remaining vinasse or stillage from the distillation column could be valorized to produce process steam and electricity, products for feeding animals, fertilizer, and other valuable by-products. During the hydrolysis-fermentation process, pre-treatment has been recognized as a necessary upstream process.
Figure 5.
Flow diagram of the enzymatic ethanol production process [21].
3.1.2. Pre-Treatment
Cellulose in biomass has a straight chain structure composed of dehydrated glucose groups connected β-1,4 glucoside bonds resulting in a high degree of polymerization and stable property [22]. It is difficult to dissolve in water at room temperature, even at high temperature the dissolution is also very slow [23]. The representative pre-treatment includes a physical pre-treatment [24], chemical pre-treatment [25], and biological treatment pre-treatment [26]. The advantages and weaknesses of selected pre-treatment processes are listed in Table 1. Biological pre-treatment is an eco-friendly technology, while the efficiency is very low (residence time: 10–14 d) for industrial purposes [27]. In addition, careful growth conditions, a large amount of space, and a high cost of enzymes make it difficult to attract an entrepreneur’s attention [28]. Physical pre-treatment refers to the reduction of physical size of raw materials to increase enzyme-accessible surface areas [29], and the primary pathways of physical pre-treatments are mechanical comminution [30], pyrolysis [31], and steam explosion [32]. Chemical pre-treatment refers to the process of using chemicals to remove or modify hemicelluloses and lignin. The primary pathways of chemical pre-treatments are acidic pretreatment [25], alkali pre-treatment [33], sulfur dioxide [9], organosolv [34], liquid hot water (LHW) [35], and wet oxidation [36]. Currently, dilute acid pre-treatment, steam explosion, and liquid hot water (LHW) pre-treatment have the best performance.
Table 1.
Advantages and weaknesses of selected pretreatment processes.
| Pretreatment | Yield of FS * | Chemical Recycling | Wastes | Investment | |
|---|---|---|---|---|---|
| Physical | Mechanical | - | ++ | ++ | + |
| Physico-chemical | Steam explosion | + | ++ | + | - |
| Ammonia fiber explosion | +/- | -- | + | - | |
| Carbonic Acid | ++ | ++ | ++ | + | |
| Chemical | Dilute acid | ++ | -- | - | +/- |
| Concentrated acid | ++ | -- | - | - | |
| Alkaline extraction | ++/+ | -- | - | ++ | |
| Wet oxidation | +/- | ++ | + | + | |
| Organosolv | ++ | -- | + | -- | |
* FS: Fermentable sugars; ** ++: very good; +: good; -: bad; --: very bad.
(1) LHW Pre-Treatment
In the LHW pre-treatment process, the liquid water is used to promote disintegration and separation of lignocellulose, which the water could maintain a liquid phase at high temperature and pressure [9,35]. The typical temperature ranges from 160 °C to 240 °C, and the reaction time ranges from 5 min to 1 h. The parameters are dominated by the types of biomass and sugar formation [37]. During this pre-treatment, no acid and chemical catalysts are required, and there is a high xylose recovery (88–98%) [38]. Although this method is economically interesting and environmentally attractive, it requires higher energy due to high temperature and pressure and a large amount of water [39].
(2) Acidic Pre-Treatment
The most studied method is acidic pre-treatment for biomass, and it could provide the satisfactory cellulose conversion [25]. According to the literature, H2SO4, HCl, H3PO4, and HNO3 have been studied, and these acids would promote the hydrolysis of hemicelluloses and amorphous cellulose [25,40,41,42]. However, the concentrated acids are not favored due to the corrosion and toxicity in nature. In the industrial scale, dilute acid pre-treatment is more popular. Generally, dilute acid refers to the concentration of 0.5–1.5% [43]. The yield of sugars is high from hemicelluloses. In this pre-treatment process, the lignocellulose could be usually executed at high temperature (about 180 °C) for a shorter period of time (less than 15 min), while the materials could be also performed at low temperature (about 120 °C) for a long reaction time (about 30–90 min) [44]. For instance, the total sugar recovery from poplar wood is about 83% with a loading of 15 FPU/g cellulose [45]. During this process, different simple sugars are released from hydrolysis of hemicellulose, e.g., xylose, arabinose, mannose, and galactose, while it also releases some other compounds, which could inhibit the enzymatic hydrolysis and fermentation. Finally, part of the CH3COOH, H2SO4, and other inhibitors produced during the degradation are removed, and neutralization is formed. However, dilute acid pre-treatment only provides satisfactory enzymatic cellulose conversion for hardwood. Cellulose conversion is only about 40% when using softwood as the raw material [46].
(3) Steam Explosion
Steam explosion is another effective pre-treatment for biomass. During the process, steam would make the biomass reach to the target temperature rapidly without excessive dilution of sugars [32]. Generally, the raw material is treated by hot steam under high pressure, e.g., 180–240 °C, 1–3.5 MPa, and then the lignocellulosic structure would be destroyed [26]. The sudden release of pressure contributes to defibrillation of cellulose bundles, which improves the accessibility of cellulose for enzymatic hydrolysis and fermentation. There are two stages in the steam explosion process: Auto-hydrolysis and de-pressurization [47]. In the auto-hydrolysis process, the hydrolysis of hemicellulose occurs when acetic acid from acetyl groups connected with hemicellulose is formed at high temperature. The acetic acid would further catalyze the hydrolysis of hemicelluloses [48]. Then the particle size of raw materials would be reduced resulting in the high enzymatic accessibility of cellulose in the depressurization process. This method leads to a remarkable breakdown of lignocellulose, hydrolysis of hemicellulose, depolymerization of lignin, accessibility of enzymes [47]. The primary advantages of this method are less hazardous chemicals, high energy efficiency, and low environment pollution. In order to further improve the energy efficiency, some scientists reported that the addition of SO2, CO2, and NH3 would achieve this goal [49,50]. The method also names the acid-catalyzed steam pre-treatment. For instance, the raw materials are impregnated with acid catalyst either in the gas phase with SO2 or in the aqueous phase with H2SO4 before steam pre-treatment. We think that the acid-catalyzed steam pre-treatment is actually another form of the dilute acid pre-treatment. Typical acid or SO2 charge on biomass varies from 0% to 5%, and the temperature ranges from 190 to 220 °C. Reaction time varies from 1 to 10 min [49,50]. The addition of NH3 (ammonia fiber explosion) would also greatly improve the conversion efficiency [51]. In ammonia fiber explosion, lignocellulose biomass is exposed to liquid ammonia at a moderate temperature (60–100 °C) under a high pressure (1.5–2.0 MPa) for a period of time, and then the pressure is suddenly released [52,53]. However, the important limitation is that hemicellulose would not be removed significantly, leading to a lower enzyme accessibility and yield of sugars.
3.2. Thermodynamic Liquefaction
In general, there are two types for thermodynamic liquefaction of biomass depending on the operating conditions: Pyrolysis liquefaction [31] and hydrothermal liquefaction [54]. In pyrolysis liquefaction, it could be divided into slow pyrolysis, fast pyrolysis, and flash pyrolysis [55]. Bio-oil, which is also regarded as pyrolysis oil or pyrolytic oil, could be obtained from both of these two methods. As shown in the literature, bio-oil is the extremely complex substance and composed of hundreds of organic compounds, e.g., alkanes, aromatic hydrocarbons, phenol derivatives, ketones, esters, ethers, sugars, amines, and alcohols [56,57]. In addition, the molar ratio of H to C in bio-oil is higher than 1.5. The pyrolyze bio-oils could be directly burned in boilers, or upgraded to produce valuable fuels and chemicals using the following methods: Extraction [58], emulsification [59], esterification/alcoholysis [60], supercritical fluids [61], hydrotreating [62], catalytic cracking [63], and steam reforming [64].
3.2.1. Fast Pyrolysis
Slow pyrolysis is usually executed at a low reaction temperature, heating rate, and a long residence times, which produces a little of bio-oil. In the flash pyrolysis process, the reaction time is only or less than several seconds with a very high heating rate and small particle size, and the primary product is syngas [65]. Fast pyrolysis also proceeds at a high heating rate (less than in flash pyrolysis) and short residence time of the vapor [66]. The favorable product in the process is bio-oil. This technology has developed considerably in the last years, due to the low investment costs, high energy efficiencies, and environmental acceptability [67,68]. In the fast pyrolysis, the core technology is the reactor design. During the last decades, in order to realize rapid heat-transfer, several different reactor designs have been developed [68,69,70], such as fixed bed, bubbling fluidized bed, circulating fluidized bed, rotating cone reactor, Auger reactor, and vacuum reactor. The choice of the technology and quality of bio-oil are affected by many parameters, including raw material, reaction temperature/time, particle size of raw material, residence time of volatiles, heat transfer rate, and feed rate.
Operating Parameters
(1) Raw Material
The main components in biomass are cellulose, lignin, hemicellulose, and inorganic substance, and the percentage of these components is mainly influenced by the biomass species [71]. Even with the same biomass, the percentage of these components is also dependent on soil, age, or planting conditions [72,73]. The percentage of the components affects the yield and distribution of bio-oil. The higher yield of bio-oil would be obtained from the higher contents of cellulose and hemicellulose than that obtained from a higher content of lignin component [74,75]. As the greater structural stability, lignin component is difficult to be decomposed and the primary pyrolysis product is biochar [76]. Quan et al. [77] reported that the yield of bio-oil obtained from cellulose is 18.67%, the yield of bio-oil obtained from hemicellulose is 30.83%, and the yield of bio-oil is only 0.5% using the lignin as the raw material. Stefanidis et al. [78] studied the pyrolysis products using cellulose, hemicellulose, and lignin as the raw material. It indicates that the main liquefaction products of cellulose are sugars, levoglucosan, and low concentrations of phenols, ketones, aldehydes, and alcohols, the main liquefaction products of hemicellulose are ketones, phenols, acids, and aldehydes, and the main liquefaction products of lignin are complex phenols with high molecular weights. Moreover, ash also has a significant effect on the proportion and distribution of fast pyrolysis products [79]. High content of ash component leads to a low yield of bio-oil and high proportions of biochar and gas-phase substance [80]. Especially, Na and K could reduce the yield of liquid-phase products, and S and P could also reduce the yield of bio-oil resulting in the higher biochar formation [80,81]. The content of moisture is another critical factor in the fast pyrolysis process [82]. The production and collection of raw material affects the content of water [83]. The water in bio-oil is the result of the absorbed water in raw materials and dehydration reactions during the pyrolysis process. Generally, the maximum moisture content is 10% in the raw material to minimize the water content in the bio-oil [17].
(2) Reaction Temperature
The reaction temperature is the key factor in the fast pyrolysis process. It could provide the necessary energy for the decomposition of biomass bonds [84]. The decomposition efficiency increases with reaction temperature increase, because the high temperature could provide the high energy to break the biomass bonds [85]. Numerous studies have shown that the high yield of bio-oil generally ranges from 450 °C to 500 °C [86,87,88], while the temperature changes with the raw material or the other variables. Although the high temperature may cause a positive effect on the yield of bio-oil, the extremely high temperature often has the negative impact on the yield, which may occur the secondary cracking of the volatiles [89]. Table 2 lists the suitable temperatures for different biomasses. Besides the yield, the temperature also has a significant effect on the quality of bio-oil. At low temperatures, the main components in bio-oil are alkenes, alkanes, long-chain fatty acids and esters, aliphatic nitriles, and amides, while the components obtained at higher temperature change to short aliphatic carbons and lower-molecular-weight compounds of alcohols, ketones with lower H/C ratio [90,91].
Table 2.
Suitable temperatures for different biomasses.
| Raw Material | Temperature | Yield | Ref. |
|---|---|---|---|
| Rice husk | 450 | 70 wt. % | [91] |
| Plam | 500 | 72.4 | [92] |
| Neem deoiled cake | 400 | 40.2 | [93] |
| Cynara cardunculus L. | 400 | 46.23 | [94] |
| Olive bagasse | 600 | 46.3 | [95] |
| Sugarcane bagasse | 475 | 56 | [96] |
| Cassava rhizome | 472 | 63.23 | [97] |
| Cassava stalk | 472 | 61.39 | [97] |
| Jatropha seed shell cake | 470 | 48 | [98] |
| Poplar | 455 | 69 | [99] |
| Pistachio shell | 550 | 20.5 | [100] |
| Bamboo sawdust | 510 | 61 | [101] |
(3) Residence Time
A large amount of vapor would be released during the fast pyrolysis process. After the reaction, the vapor should be removed rapidly from the reactor. If the vapor were not removed in the minimum time, the secondary reactions would occur [74,102], such as thermal cracking, repolymerization, and recondensation of the biochar residue. The secondary reactions would cause a decrease in the yield of bio-oil. In order to purge the pyrolysis vapor, N2 is the most popular gas due to being inert, cheap, and readily available. In the process, a higher inert gas flow should be introduced to reduce vapor residence time [103]. Several studies analyzed the effect of inert gas flow on the yield of bio-oil show that the increase in the flow of inert gas causes an improvement in the bio-oil yield (Table 3). However, a very high gas flow would lead to a lower yield of bio-oil, because the uncompleted condensation of vapors and biomass are swept out of the reactor, resulting in a low yield of bio-oil and high yield of the gas-phase products.
Table 3.
Suitable residence time for different biomasses.
| Raw Material | Gas Flow Rate/Time | Yield of Bio-Oil | Ref |
|---|---|---|---|
| Safflower seed | 100 cm, 3/min | 67 wt. % | [104] |
| Palm kernel shell | 1 L/min | 50 wt. % | [7] |
| 1.5 L/min | 53 wt. % | ||
| 2 L/min | 57 wt. % | ||
| Sewage sludge | 300 mL/min | 45.3 wt. % | [6] |
| Rice husk | 3 L/min | 45 wt. % | [102] |
| 4 L/min | 47.5 wt. % | ||
| 5 L/min | 49 wt. % | ||
| Cassava stalk | 0.1 L/min | 48 wt. % | [105] |
| 0.5 L/min | 51 wt. % | ||
| 1.5 L/min | 53 wt. % | ||
| 3 L/min | 52 wt. % | ||
| Sugarcane bagasse | 5 s (8 L/min) | 56+1.3 wt. % | [106] |
| 10 s (4 L/min) | 52+2.5 wt. % | ||
| 20 s (2 L/min) | 47.5+28 wt. % | ||
| Jatropha cake | 1.25 m, 3/h | 37.78 wt. % | [107] |
| 1.75 m, 3/h | 64.25 wt. % | ||
| 2.4 m, 3/h | 30.5 wt. % | ||
| Babool seeds | 100 cm, 3/min | 44 wt. % | [86] |
| 400 cm, 3/min | 30 wt. % | ||
| Rice husk | 0.255 m/s | 19.5 wt. % | [108] |
| 0.340 m/s | 20.5 wt. % | ||
| 0.425 m/s | 17.9 wt. % | ||
| Euphorbia rigida | 400 cm, 3/min | 31.5 wt. % | [109] |
| Sunflower pressed bagasse | 200 cm, 3/min | 45.7 wt. % | |
| Hazelnut shells | 100 cm, 3/min | 23.1 wt. % |
(4) Particle Size
As a poor conductor of heat, of biomass, it is difficult to transfer heat into biomass in the pyrolysis process. Hence the yield of bio-oil is dependent on the particle size, and decreasing the particle size is a vital task in minimizing the heat transfer problem [74]. Shen et al. [110] reported that the yield of bio-oil with a 0.3 mm particle size increased by 12–14% compared to that with a 1.5 mm particle size. Garg et al. [86] reported that the yield of bio-oil increases when the particle size of a babool seed decreases in the fixed bed reactor, and the raw material with less than 0.4 mm leads to a maximum yield of bio-oil (32%). Kang et al. [111] observed similar results. However, there are some negative opinions in the effect of particle size. Encinar et al. [94] reported the fast pyrolysis of grape bagasse, Cynara cardunculus L. and soybean cake in the fixed bed reactor. The result indicates that it has less effect on the yield of bio-oil when the particle size of the raw material is up to 2 mm. Abnisa et al. [112] believed that the yield of bio-oil is independent of the particle size, and they observed the opposite effect in palm shell pyrolysis. The result indicates that the yield of bio-oil increases to 69.6 wt. % when the particle size increase from 0.5 mm to 2 mm. Generally, it is known that the smaller particle size has the faster and uniform heating resulting in the higher yield. However, the fine raw material would reduce the yield of bio-oil as the uncompleted fine biomass would be removed out of the reactor by inert gas [106].
(5) Other Factors
Reaction time refers to the time sustained in the reactor for the raw material at the specific pyrolysis temperature. In order to obtain the high yield of bio-oil, a sufficient reaction time is required during the pyrolysis process. If the reaction time is too long, the secondary reactions would be occurred for the pyrolysis vapors, e.g., carbonization, gasification, or thermal cracking, resulting in a lower yield of bio-oils [113]. Moreover, this parameter is vital to the reactor design. The reaction time for some biomasses is listed in Table 4. Feed rate is another factor in the fast pyrolysis process that affects the product distribution and quality. The formation of gas and organic vapor occurs under the low feeding rate and fast heat transfer, which contributes to faster liquefaction [7]. However, as the yield of the gas-phase products with low feed rate is lower than that with fast feed rate, the higher residence time is required, which leads to an increase in the yield of biochar due to the long interaction between vapor and biochar. Furthermore, the long residence time could also cause secondary reactions [114].
Table 4.
Suitable reaction time for different biomasses.
| Raw Material | Reaction Time/min | Yield of Bio-Oil | Ref |
|---|---|---|---|
| Rice husk | 1 | 36 wt. % | [5] |
| 2 | 41 wt. % | ||
| 4 | 40 wt. % | ||
| 8 | 39 wt. % | ||
| Rice straw | 1 | 9 wt. % | [115] |
| 2 | 10 wt. % | ||
| 4 | 9.5 wt. % | ||
| 8 | 8 wt. % | ||
| Bagasse | 1 | 7 wt. % | |
| 2 | 16 wt. % | ||
| 4 | 11 wt. % | ||
| 8 | 10 wt. % | ||
| Coconut shell | 1 | 5 wt. % | |
| 2 | 13 wt. % | ||
| 4 | 7.5 wt. % | ||
| 8 | 11 wt. % | ||
| Pistachio shell | 10 | 52.96 wt. % | [116] |
| 20 | 53.08 wt. % | ||
| 50 | 50.13 wt. % | ||
| Physic nut | 15 | 27 wt. % | [117] |
| 240 | 22.5 wt. % | ||
| 30 | 28 wt. % | [118] | |
| 60 | 46 wt. % | ||
| 90 | 45.5 wt. % | ||
| 120 | 45 wt. % | ||
| 150 | 45.8 wt. % | ||
| Cassava stalk | 60 | 52 wt. % | [4] |
| 180 | 39.5 wt. % | ||
| Cassava rhizome | 60 | 50 wt. % | |
| 180 | 42 wt. % |
Reactor Types
(1) Bubbling fluidized bed reactor
As is well known, the bubbling fluidized bed reactor has become popular in the petrochemical industry for decades [119]. The reactor design could provide a high heat transfer rate and uniform bed temperature in the fast pyrolysis process of biomass. Generally, the residence time in the freeboard section above the bed ranges from 0.5 to 2.0 s dependent on the size of the bed fluidizing media [120]. The reaction temperature is usually set to 500–550 °C resulting in the highest yield of bio-oil, while the temperature and residence time could be operated at a low level in the larger systems. The parameters are also influenced by the type of raw material. During the reaction, the yield of bio-oil would be oxidized using the flue gases as the direct heating media. The small particle size (2–3 mm) is required to reach the good heat transfer. Self-cleaning is an advantage of this reactor with a relatively narrow particle size distribution, as the biochar (by-product) would be carried out with the gas and vapor. If the biomass particle size is too large, the biochar could not be entrained out of the reactor and then the lower density of biochar would stick on the top of the bed [121]. Figure 6 shows the schematic of a typical bubbling fluidized bed [122,123]. The biochar on the top of the bed would act as the role of the catalyst to converse the vapors when they pass through the biochar, resulting in the lower yield of bio-oil. If the size of the raw material is too fine, it must be introduced lower in the bed to prevent the fine feed from entraining out of the bed before complete pyrolysis. Hence, a mean should be designed for skimming and discharging biochar from the top of the bed. If it is not to be designed, the raw material should carefully grind to obtain a narrow particle size distribution leading to a high cost. Otherwise, heat applied to the reactor in different ways should be also designed to improve the flexibility of the reactor.
Figure 6.
Schematic for the bubbling fluidized bed.
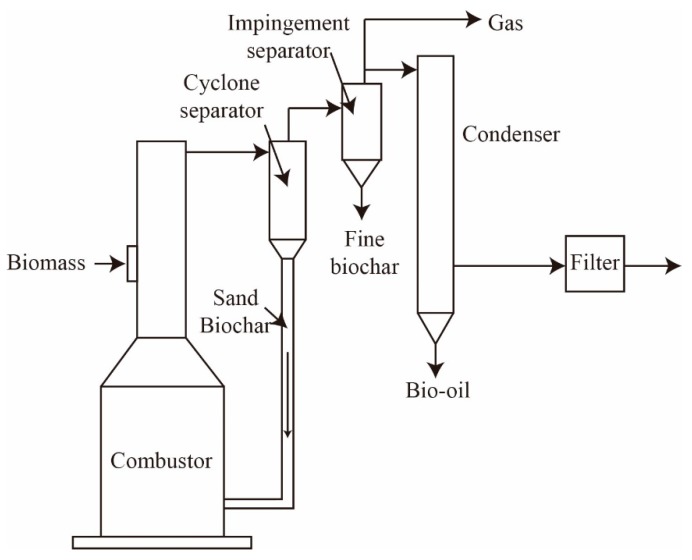
(2) Circulating fluidized bed
Circulating fluidized bed technology has been extensively applied to biomass pyrolysis. The advantage of the reactor is high heat transfer rates and short vapor residence times [124]. Figure 7 shows the schematic of this circulating fluidized bed system [125,126]. In the design, large quantities of sand should be moved into the reactor resulting in the high challenge of the operability. The hot sand could be circulated between combustor and pyrolyzer units. The most important difference is the supplying heat method for various developed system designs. The first generation contains the simple units, e.g., single indirectly heated reactor, cyclone, and standpipe configuration. In this system, the particle size of the raw material should be smaller than that in bubbling beds, and the residence time would only have 0.5–1.0 s (s) in the high heat transfer pyrolysis zone [127]. For the large particle size of the raw material, there is not enough time to transfer the heat. Especially, biochar is formed on the surface of biomass, which plays a vital role to prevent further penetration of heat. The incomplete pyrolyzed larger particles would be burned in the char combustor with a low yield of bio-oil. Hence, the suitable particle size is 1–2 mm [128].
Figure 7.
Schematic for the circulating fluidized bed.
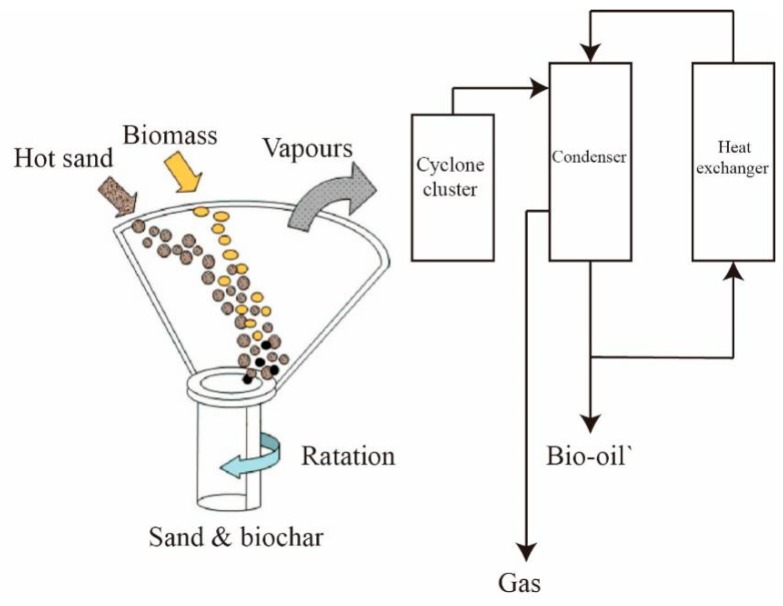
(3) Rotating cone reactor
The rotating cone reactor has been in development since the early 1990s, and the processing capacity has been improved to 200 kg/h. The schematic for the rotating cone reactor [129] is shown in Figure 8. Similar to the circulated fluidized bed, the pyrolysis reaction executes with the mixture of hot sand and biomass. While, the primary distinction between them is that the carrier gas is replaced by the rotary cone to form a centrifugal force. Firstly, the raw material and sand are introduced to the base of the rotating cone, and then the mixture is moved to the lip of the cone under a centrifugal force. Finally, the mixture spills over the lip of the cone, and the pyrolysis vapor would be introduced to the condenser. The sand would be re-heated after introducing biochar and sand into the combustor at the base of the cone. The literature demonstrates that the yield of bio-oil would achieve to a 70% yield and there are two advantages in this design. The first is that the bio-oil would recover easier without the carrier gas, and the second is that the wear problems are reduced because the transport dynamics of sand and biomass are not as aggressive as in the circulating fluid bed. However, the disadvantages are a complex integrated process and scale up issue [130].
Figure 8.
Schematic for the rotating cone reactor.
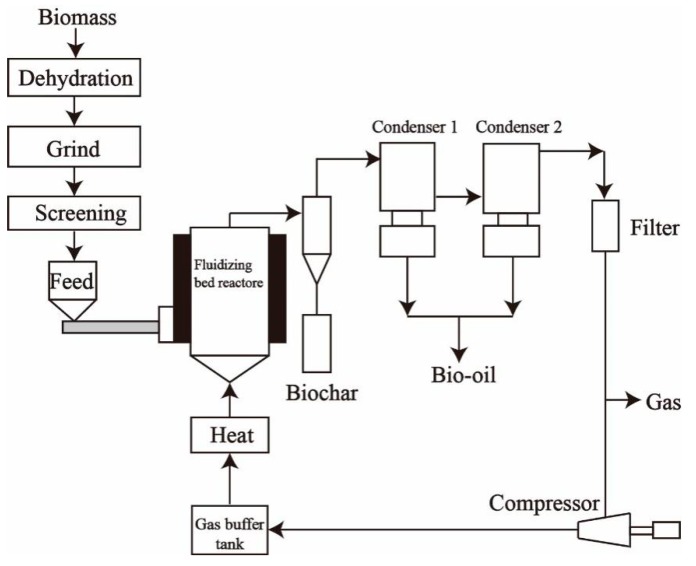
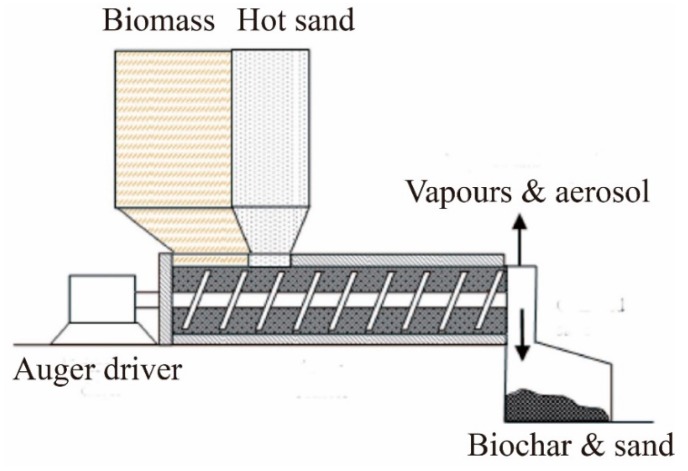
(4) Auger reactor
The important advantage of an Auger reactor has been identified for its potential to reduce operating costs [131]. However, this reactor is not suitable for large-scale pyrolysis [132]. Figure 9 shows a schematic for the Auger reactor. In the reactor, the Auger system plays the role of the commingler for the hot sand and raw material. Generally, the design contains hot, heating, and circulation systems. Once the raw material is introduced into the reactor, it would direct contact with a bulk solid heat transfer medium. The materials of the heat transfer medium are usually sand or a steel shot, which is heated independently before sending into the reactor. According to the gravimetric basis, it is suggested that the feed rate of the heat transfer medium is 20 times to that of the raw material. It is effective when the biomass and heat transfer medium are combined with two intermeshing, co-rotating augers quickly in a shallow bed during the pyrolysis reactions. This is an unintelligible mechanical mixing process. Finally, the vapors and aerosols are introduced out of the different ports respectively, and biochar would be transported through a long section and stored in a canister with the heat transfer medium [133,134].
Figure 9.
Schematic for the Auger reactor.
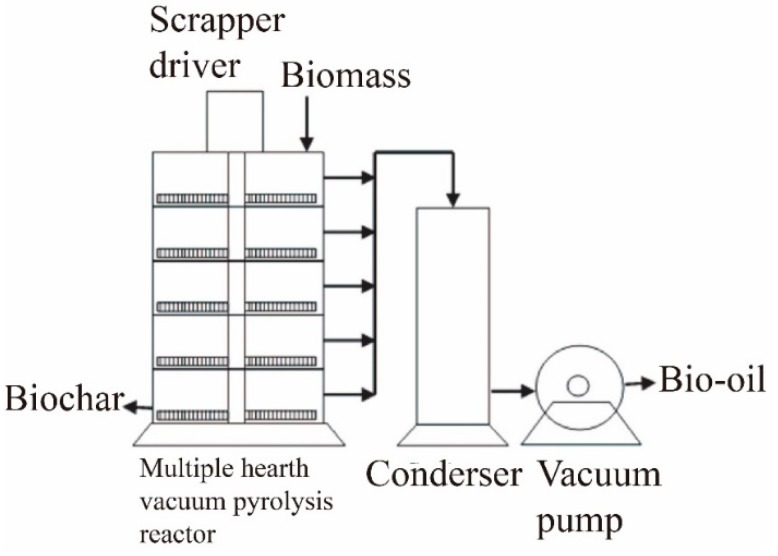
(5) Vacuum pyrolysis
The vacuum pyrolysis process has been developed by Canada. In fact, vacuum pyrolysis belongs to the slow pyrolysis process. The low heat transfer rate and short vapor residence time would produce the bio-oil product [135]. The yield of bio-oil is only half of that obtained by the fluid bed technologies [136]. Figure 10 shows the schematic design of vacuum pyrolysis. In this design, the raw material is moved by gravity and rotating scrappers through multiple hearth pyrolyzers, and the reaction temperature changes from 200 °C to 400 °C. Furthermore, a larger particle size and a little carrier gas are required. The raw material would be carried into the high temperature vacuum chamber using a moving metal belt. The biomass on the belt is stirred by the mechanical agitator periodically. All of this mechanical transport is being done at 500 °C. The special devices of feeding and discharging are required to achieve a good seal at all times. However, the high investment and maintenance cost limit its development [137,138].
Figure 10.
Schematic for the vacuum pyrolysis reactor.
3.2.2. Hydrothermal Liquefaction
Hydrothermal Processing
According to the literature, the content of water in the biomass raw material is usually more than 40% for pyrolysis liquefaction [139]. Hence, it is usually required to pre-treat the biomass to make it suitable for pyrolysis application. The reported pre-treatments are atmospheric drying, solar drying, and evaporation [140,141]. Few studies suggest that the water could be mechanically dehydrated via atmospheric drying. Although the cost of the solar drying method is low, it requires a long dehydrated time to lower the water content [142,143]. The presence of water in the raw material could consume more energy to evaporate it, which limits the operability and economy of this technology [140,144]. Compared to the pyrolysis liquefaction, hydrothermal liquefaction of biomass is one of the effective methods to treat the biomass with high water content [54,144]. This liquefaction of biomass is not affected by the level of water content and the types of biomass with high conversion and relatively pure products [145]. The suitable properties for liquefaction of biomass are demonstrated [146], including a high density, good heat, mass transfer capability, fast decomposition, and extraction under hydrothermal conditions. This is an environment friendly technology, and the heteroatom in biomass could be converted into undesired by-products. Biomass and O element would be rapidly oxidized to form CO2 or H2O [142]. N element in biomass is mainly converted to N2 or N2O. S, Cl, and P elements are mainly oxidized to their inorganic acids, which would destroy the vessel lining [147]. Hence, extreme operating conditions, corrosion, and scaling are major limitations of hydrothermal liquefaction.
Operating Parameters
(1) Temperature
It is known that the yield of bio-oil and the biomass fragmentations would be increased with the temperature increase [145,148]. The depolymerization of biomass would occur extensively when the reaction temperature is higher than the activation energies for the bond cessation. However, the high temperature increases not only the concentration of free radicals, but also the probability of repolymerization of fragmented species [140,149]. The suitable reaction temperature for bio-oil production is the competitive result of hydrolysis, fragmentation, and repolymerization. At the initial stage, depolymerization of biomass is the dominant reaction, and the biochar would be formed via repolymerization at the later stage [150]. Hence, the higher yield of bio-oil would be obtained at the intermediate temperature. As the properties of water would change rapidly under a super-critical condition, it is a huge challenge to select the optimum temperature [151]. The yield of bio-oil is influenced by the reaction temperature sequentially. The reaction temperatures for bio-oil production via hydrothermal liquefaction of some biomass are listed in Table 5. Generally, when the temperature is lower than 374 °C, the yield of bio-oil increases with the reaction temperature. While, the gas-phase products would increase when the temperature is higher than 374 °C. Furthermore, it is not suitable to produce bio-oil at a high temperature considering the operational cost and bio-oil yield. A large amount of gas-phase products would be formed via secondary decompositions and Bourdard gas reactions, and formation of biochar would be produced due to the recombination of a high concentration of free radical [152,153]. In addition, the yield of bio-oil would be suppressed by the incomplete decomposition of components when the temperature is lower than 280 °C. Hence, the effective temperature range would be located from 300 °C to 350 °C for hydrothermal liquefaction of biomass.
Table 5.
Reaction temperatures via hydrothermal liquefaction for some biomasses.
(2) Pressure
In general, pressure is another key factor for the hydrothermal liquefaction of biomass. The single-phase solvent would be maintained under the high pressure, and the large heat is required to maintain the energy of the reaction system for the two-phase system [158]. When the pressure of the system is higher than the critical pressure of the solvent, the favorable reaction pathway would be enhanced for the production of liquid fuels. On the other hand, the solvent density would be improved under the high pressure, and the molecules of biomass components could be penetrated by the high density solvent, which enhances the pyrolysis and extraction. While, there is little influence on the yield of bio-oil when the reaction pressure overcomes the supercritical conditions for liquefaction [153,154].
(3) Residence time
The residence time is also the key factor for the hydrothermal liquefaction of biomass, and the products components and conversion of biomass would be defined by the residence time [157]. In the supercritical conditions, the reaction rate of hydrolysis and decomposition is rapid, so the short reaction time is contribution to the decomposition of the raw material [159]. However, the reaction time is required to optimize the destruction of the organic compounds in different biomasses [160]. Different researches have studied the function of reaction time on the yield of bio-oil. Boocock et al. [161] reported that the yield of bio-oil decreases for a long residence time except for a very high mass ratio of biomass to water. Generally, the breakage of C–C bands leads to the depolymerization that biomass depends upon. However, the cage effect for C–C bands would inhibit C–C bonds breakage under the supercritical pressures, resulting in a low fragmentation.
(4) Other parameters
Firstly, the higher heating rate would promote the fragmentation of biomass and inhibit the formation of biochar, while there is a low effect on the product distributions [162]. The reason for the phenomenon is that the fragmented species would be dissolved and stabilized in the supercritical conditions [156,162]. Secondly, the particle size of raw material also affects the yield of bio-oil. In order to achieve the high-efficiency hydrothermal liquefaction, reducing the particle size of raw material would increase its accessibility [156]. While, the treatment would improve the consumption of energy resulting in the increase of cost. Hence, the hydrothermal liquefaction should be executed with an optimum particle size at a low grinding cost. In fact, it has a low effect in hydrothermal liquefaction for the particle size of biomass [163]. The sub/supercritical water acts as not only a heat transfer medium but also an extractant, and it could overcome the heat transfer limitations. Therefore, the particle size of the biomass is less important, it is not needed for excessive size reduction of the raw material. Thirdly, there is the potential effect of the solvent type and density on the yield of bio-oil in hydrothermal liquefaction. The common solvents in the reported researches are water, methyl alcohol, ethyl alcohol, and other organic solvents [148]. For solvent density, it is a key factor for the mass ratio of solvent to biomass. The high mass ratio of solvent to biomass contributes to the high yield of bio-oil and gas products, which is because the products would be exacted by the solvent medium [162]. Wang et al. [164] reported that the high mass ratio of solvent to biomass could increase the amount of bio-oil, meanwhile there is a decrease in the yields of residues and gas-phase products. It indicates that the solvent could enhance the stability and solubility of fragmented components. When the mass ratio of biomass to solvent is high, the relative interactions between biomass components and the solvent become weak, leading to a low dissolution capacity of biomass components.
3.2.3. Upgrading of Bio-Oil
Bio-Oil Characteristics
Bio-oil is the dark brown and viscous liquid with a distinctive odor [66,92]. Bio-oil is a complex mixture of several hundreds of chemical composition, and affected by the type of raw material, thermochemical process, and operating parameters [91,141]. The main components in bio-oil are acids, alcohols, aldehydes, esters, ketones, phenols, and guaiacol [55,165]. The undesirable properties of bio-oil are caused by these compounds. The physical characteristics of liquefaction-derived bio-oil [59,166,167,168] are listed in Table 6. As shown in the table, the concentrations of water and the O element in bio-oil are higher than that in heavy petroleum fuel oil, resulting in a lower heating value of bio-oil (about 35 MJ/kg). In addition, the concentration of the N element in bio-oil is also higher than that in heavy petroleum fuel oil. The pH value of bio-oil ranges from 3.5–4.2 leading to a high corrosivity. For instance, the acidity and corrosiveness is the result of a considerable amount of fatty acids. Moreover, the side reactions would proceed during the storage period, e.g., polymerization and evaporation would cause a slow increase in viscosity [90,169]. Besides, aldehydes are the most unstable component in bio-oil. The above undesired characteristics of bio-oil have limited its application as a transportation fuel. Hence, it is necessary to upgrade the quality of bio-oil.
Table 6.
Physical characteristics of liquefaction-derived bio-oil and heavy petroleum fuel oil.
| Properties | Bio-Oil | Heavy Petroleum Fuel Oil |
|---|---|---|
| pH | 3.8–4.0 | - |
| Acid value (mgKOH/g) | 1.8 | - |
| Density (g/cm3) | 1150–1200 at 40 °C | 940 |
| Viscosity (cP) | 650 at 40 °C | 180 at 40 °C |
| HHV (MJ/kg) | 28.42 | 40 |
| C (wt. %) | 66 | 85 |
| H (wt. %) | 11 | 11 |
| O (wt. %) | 12 | 1.0 |
| N (wt. %) | 9 | 0.3 |
| S (wt. %) | 1 | - |
| Water content (wt. %) | 13–12 | 0.1 |
| Ash content (wt. %) | 0.4–0.7 | 0.1 |
Bio-Oil Upgrading
Reported by the literature, there are physical and chemical methods to upgrade bio-oil, including extraction, solvent addition, emulsification, esterification/alcoholysis, supercritical fluids (SCFs), hydrotreating, catalytic cracking, and steam reforming. Table 7 lists the process conditions, advantages, and disadvantages for each method [58,59,60,61,62,63].
Table 7.
Brief description of bio-oil upgrading techniques.
| Upgrading Techniques | Process Conditions | Pros. | Cons. |
|---|---|---|---|
| Extraction | Mild conditions, solvents | Extracts valuable chemicals from bio-oil | Low cost separation and refining techniques are still needed |
| Solvent addition | Mild conditions, polar solvents | Simple | No chemical reaction to convert or remove undesired compound within bio-oil |
| Emulsification | Mild conditions, surfactant | Simple | High energy consumption, no chemical reaction to convert or remove undesired |
| Esterification/alcoholysis | Mild conditions, alcohol | Relatively simple, mild conditions, low cost of alcohol if methanol is used | Not effective to remove nitrogen-containing compounds |
| Supercritical fluids (SCFs) | Relatively high pressure and temperature, organic solvents | Effective to increase HHV and reduce viscosity | Needs high pressure equipment, some solvents are expensive |
| Hydrotreating | Relatively high pressure and temperature, catalysts | Removes N, O, and S as NH3, H2O, and H2S, and increase HHV, commercialized already | Needs high pressure equipment, high cocking and catalyst deactivation |
| Catalytic cracking | Relatively high temperature, atmospheric pressure, catalysts, | Produces large amounts of light products | Needs high pressure equipment, catalyst deactivation |
| Steam reforming | High temperature, catalyst | Produces H2 as a clean energy resource | Needs high temperature equipment |
(1) Extraction
As listed above, there are a large number of chemicals in bio-oil, and some of the chemicals have industrial applications, e.g., phenol for resin industry, organic acids, and alkanes [170]. Therefore, the chemicals extracted from bio-oil are effective in increasing the value of bio-oil. Similar to petrochemical plants, extraction from bio-oil is the separation technology [171]. The technology includes absorption, distillation, and fractionation. The solvent for adsorption is acetone, and the fractionation is divided into phase separation and aqueous extraction [172]. In Eboibi et al.’s work [173], they reported that there is a decrease in the concentration of the O element, and an increase in HHV. In order for commercialization, the low-cost separation and refining techniques must be developed [174].
(2) Solvent addition
Using polar solvents to reduce viscosity of bio-oils has been popularized for several decades, and the common polar solvents include ethyl acetate, acetone, methanol, and ethanol [175,176]. After polar solvents addition, the heating value increases as the solvents has the higher heating value than bio-oil [177]. On the other hand, the viscosity of bio-oil would be reduced because there is a physical dilution and chemical reaction between solvent and bio-oil components.
(3) Emulsification
Emulsification with other chemicals is another method for bio-oil upgrading. While, it is difficult to mix bio-oil derived from biomass and fuels derived from petroleum, so the surfactants are commonly used [178]. The ignition property of bio-oil emulsions is excellent, while higher corrosion is observed in engine applications. Emulsification is a short-term pathway to upgrade bio-oil without a chemical reaction [59]. Although, this pathway could improve the ignition characteristics, there is no significant improvement in heating value, corrosiveness, and cetane number [179]. Emulsification is a relatively simple technique to upgrade bio-oil with diesel or bio-diesel, however the addition of surfactants and the high energy consumption result in a high cost [180].
(4) Esterification/alcoholysis
In order to obtain bio-diesel, esterification (also named alcoholysis) of free fatty acids to alkyl ester is popular [181]. Acid catalyst plays an important role in the process for the reaction between fatty acids and alcohol to produce alkyl ester or bio-diesel under atmospheric pressure [182]. Due to the low cost, the most common alcohol is methanol for esterification. The reaction is usually carried out at a lower temperature (<60 °C), which is lower than the boiling point of methanol. The esterification reaction could also be performed under super-critical conditions. The super-critical upgrading of bio-oil is more effective than sub-critical upgrading. However, there is plenty of water formation in the reaction as the by-product. Esterification is a reversible reaction, and water could be separated and removed to increase the yield after reactive distillation. Although the catalysts for esterification could be divided into homogeneous and heterogeneous, the easier separation from the products have been preferred to apply in bio-oil upgrading, and the popular catalysts are HZSM-5 and aluminum silicate. Besides fatty acids, there is also the esterification reaction for aldehydes to produce acetal. Some characteristics of bio-oil have been improved after esterification and acetalization, such as viscosity, density, aging rate, acidity, oxygen content, and water content, leading to a high heating value. However, there is no literature that reported significant nitrogen removing. In brief, the advantages of esterification are simplicity, low temperature, pressure, and cost of alcohols, which seems to be one of the promising techniques for upgrading bio-oil [183].
(5) Supercritical fluids (SCFs)
A supercritical fluid is a new method for upgrading bio-oil and has attracted a lot of attention. Beyond the critical point of the solvent, the solvent would maintain a fluid at a high temperature and pressure [184], which refers to the super-critical fluid, and it is difficult to distinguish the liquid phase and gas phase [185]. The material could be dissolved in the super-critical fluid, while it could also diffuse through solids like a gas [186]. It is a promising alternative to organic solvents. In the supercritical fluid, the ability of undissolved materials could be improved [187]. The unique properties of the technology are liquid-like density, gas-like diffusivity and viscosity, and faster rates of mass and heat transfer. According to the literature [188,189,190,191], some solvents have been studied to upgrade bio-oil, including ethanol, methanol, and water. It is similar to esterification under super-critical conditions using alcohol as a solvent and acid catalyst as a catalyst [192]. Xu et al. reported that the properties of bio-oil would be improved using supercritical 1-butanol on Ru/C catalyst [193]. Duan et al. [194] reported the upgrading of bio-oil at high H2 pressure under a supercritical condition with a Pt/C catalyst. After treatment, the higher heating value of bio-oil increases, and acid number, O and N contents, and viscosity decrease.
(6) Hydrotreating
It is generally recognized that the higher content of hydrogen in the fuel product leads to a better quality [184]. Hydrotreating of bio-oil is an established process to reduce the concentrations of O, S, and N, and this technology would be executed with a catalytic reaction under high pressure hydrogen (10–20 MPa) at moderate temperatures (250–450 °C) [195]. O element is removed in the form of water, N element and S element are also removed in the form of NH3 and H2S respectively [196]. The primary methods for hydrotreating technology are hydrogenation and hydrodeoxygenation [65].
For hydrogenation, it aims to improve the quality and stability of bio-oil via reducing the contents of reactive compounds, e.g., organic acids and aldehydes [197,198]. The traditional technology for hydrogenation of bio-oil is single, which is carried out at specific conditions, e.g., high hydrogen pressure, high temperature, and suitable catalysts [14,199]. The catalysts used in the hydrogenation of bio-oil are usually Al2O3-based catalysts and the Ru/SBA-15 catalyst. After upgrading, all of the pH value, water content, and H element concentration increase, while the viscosity of bio-oil decreases to some extent [194,200]. Currently, the new hydrogenation method names one-step hydrogenation-esterification (OHE). In OHE, acids and aldehydes could be converted to stable and combustible substances, and the common catalysts are bifunctional, e.g., Pd-Al-SBA-15 catalyst [201,202]. The bifunctional catalysts could offer the abilities to upgrade bio-oil via hydrogenation and esterification. Yu et al. [203] reported that 5 wt. % Pd@Al2(SiO3)3 exhibits a catalytic performance, and it could convert the unstable components in bio-oil to esters or alcohols via the effective OHE reaction. Tang et al. [204] also reported similar results. Besides, the catalytic performance for bifunctional catalyst has been improved via some treatments, and the OHE method is much better than the traditional method [205].
For hydrodeoxygenation (HDO), it is a variant of hydrogenation upgrading, and the O element in many kinds of oxygenated chemical groups could be removed under high hydrogen pressure, e.g., acids, aldehydes, esters, ketones, and phenols [169,206]. The common catalysts in previous literature for HDO focused on Ni-Mo or Co-Mo sulfide/supported hydrotreating catalysts (Table S1). Wang et al. [207] reported that the mesoporous Pt/ZSM-5 exhibits a more excellent performance than Pt/ZSM-5 and Pt/Al2O3. However, there are typical disadvantages for the HDO, including by-product formation, catalyst deactivation, and a high cost for noble metal. Hence, the novel and economical catalysts should be developed that contains a high oxygen content. In China, the largest scale reactor is a 500 mL autoclave reactor with a 10 mm diameter and 420 mm length. Recently, the amorphous catalysts have attracted the attentions as the excellent hydrodeoxygenation activity and selectivity. Amorphous Co-W-B catalyst shows an excellent catalytic hydrodeoxygenation ability [208,209]. Simple preparation, high stability and activity, and a low cost make the amorphous catalyst as the potential candidate for HDO.
(7) Catalytic cracking
Catalytic cracking could be divided into two pathways to improve the quality of bio-oil: One is traditional catalytic cracking and another one is the combination of catalytic pyrolysis and catalytic cracking [63]. The traditional catalytic cracking means that bio-oil suffers a thermal conversion treatment at a higher temperature with high pressure hydrogen flow using the proper catalysts in a tubular fixed bed reactor. There are solid (coke), liquid, and combustible gases products after the catalytic cracking process [210]. The liquid products could be divided into the organic phase and aqueous phase. The common catalyst in this reaction is HZSM-5 [63], while the bottleneck for sustainable application of catalysts is the coke deposition of this catalyst [211]. The combination of catalytic pyrolysis and catalytic cracking is the integration of catalytic pyrolysis and catalytic cracking. The reactor design is divided into a traditional pyrolysis reactor and a decomposition of gaseous intermediate apparatus. According to the literature, the length is 1.0 m with an inner diameter of 20 mm for the biggest reactor design using a 316 stainless tube [72]. The combined technology has the superiority of improving the yield of bio-oil and the quality of fuel. Complementally, hydro-cracking is the less popular catalytic cracking process with an external hydrogen source. The reaction temperature is usually greater than 350 °C, and the reaction pressure exceeds 2000 psi. During the reaction, the complex organic substances are cracked into simpler molecules. Currently, the C–C bands are broken by hydrogen [212]. Moreover, it is popular for the combination of hydrotreating and hydro-cracking for upgrading bio-oil. In the combination, the hydrotreating of bio-oil is carried out firstly and then heavy components in the bio-oil are broken into light components after the hydro-cracking process [213,214]. Although hydro-cracking is an effective method to break heavy components in bio-oil, the high reaction temperature and hydrogen pressure are required resulting in a high cost [215].
(8) Steam reforming
Steam reforming refers to a technology to produce synthesis gas from fossil fuels, and the target products are hydrogen and carbon monoxide [216]. During the reaction, the liquid fuel is usually reacted with a high temperature steam. The steam reforming of bio-oil is the extension of the steam reforming of fossil fuels, and the target products are also syngases [217]. The technology is investigated in a fixed bed reactor and luidized bed reactor [218]. The reaction temperature, steam-to-carbon ratio, and catalyst-to-feed ratio play important roles in steam reforming of bio-oil, and the most common catalysts in steam reforming of bio-oil are Ni-based catalysts [219,220]. Similar to hydrotreating, the bottleneck for the sustainable application of catalysts is also the coke deposition of these catalysts [221].
4. Conclusion and Recommendations for Future Work
4.1. Conclusion
Fossil fuels may run out in several decades, and the renewable fuels (nuclear energy, solar energy, wind energy, and biomass energy) should be further developed. Biomass has the potential to be used on a large scale. The liquefaction of biomass is an important technology to converse the biomass into valuable biofuel. Direct liquefaction of biomass is promising technology to converse the biomass into biofuels, and the main methods are hydrolysis fermentation and thermodynamic liquefaction. Bio-oil could be upgraded by physicochemical methods.
4.2. Recommendations for Future Work
(1) As we know that the components of biomass are very complex, and the types of biomass are also various. These would be the key factors to affect the liquefaction process. Thus the liquefaction method should be selected based on the type of biomass.
(2) Considering the indirect liquefaction, conversion CO2/CO and H2 to ethyl alcohol is a potential technology for biomass utilization. In this process, the catalysts should be developed to achieve efficient conversion.
(3) For the hydrolysis-fermentation liquefaction, this is a traditional technique. While the primary issue is the long reaction period. The more efficient engineering bacteria should be explored based on genetic technology.
(4) The liquid fuel (bio-oil) obtained from fast pyrolysis and hydrothermal liquefaction is the potential mixture to replace fossil fuels. However, the high concentrations of water and the O element in bio-oil limited its application. Further work should focus on the hydrotreating upgrading.
Acknowledgments
The authors appreciate the financial support and thank the editor and reviewers for their very useful suggestions and comments.
Supplementary Materials
The following are available online, Table S1: Catalysts for hydrodeoxygenation.
Author Contributions
Conceptualization, L.L. and M.J.; methodology, S.Z.; software, X.Y.; validation, L.L. and M.J.; formal analysis, H.Z.; investigation, S.Z., X.Y. and K.Z.; resources, C.C. and K.Z.; data curation, C.C. and K.Z.; writing—original draft preparation, S.Z.; writing—review and editing, L.L. and M.J.; visualization, L.L. and C.C.; supervision, L.L. and M.J.; project administration, K.Z.; funding acquisition, L.L. and M.J.
Funding
This study was funded by the National Natural Science Foundation of China (51708301), National Natural Science Foundation of China (21878163), Young Elite Scientists Sponsorship Program by Tianjin (TJSQNTJ-2018-06), Natural Science Foundation of Tianjin, China (17JCZDJC39500), 2017 Science and Technology Demonstration Project of Industrial Integration and Development, Tianjin (17ZXYENC00100).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Zhao N., Li B. The effect of sodium chloride on the pyrolysis of rice husk. Appl. Energy. 2016;178:346–352. doi: 10.1016/j.apenergy.2016.06.082. [DOI] [Google Scholar]
- 2.Bridgwater A.V. Renewable fuels and chemicals by thermal processing of biomass. Chem. Eng. J. 2003;91:87–102. doi: 10.1016/S1385-8947(02)00142-0. [DOI] [Google Scholar]
- 3.Demirbaş A. Biomass resource facilities and biomass conversion processing for fuels and chemicals. Energy Convers. Manag. 2001;42:1357–1378. doi: 10.1016/S0196-8904(00)00137-0. [DOI] [Google Scholar]
- 4.Mohan D., Pittman C. Activated carbons and low cost adsorbents for remediation of tri- and hexavalent chromium from water. J. Hazard. Mater. 2006;137:762–811. doi: 10.1016/j.jhazmat.2006.06.060. [DOI] [PubMed] [Google Scholar]
- 5.Liu L., Yang X., Hou Q. Corn stalk conversion into 5-hydroxymethylfurfural by modified biochar catalysis in a multi-functional solvent. J. Clean. Prod. 2018;187:380–389. doi: 10.1016/j.jclepro.2018.03.234. [DOI] [Google Scholar]
- 6.Zhang S.Q., Yang X., Liu L. Adsorption Behavior of Selective Recognition Functionalized Biochar to Cd(II) in Wastewater. Materials. 2018;11:299. doi: 10.3390/ma11020299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asadullah M., Ab Rasid N.S., Kadir S.A.S.A., Azdarpour A. Production and detailed characterization of bio-oil from fast pyrolysis of palm kernel shell. Biomass Bioenergy. 2013;59:316–324. doi: 10.1016/j.biombioe.2013.08.037. [DOI] [Google Scholar]
- 8.Gnansounou E., Dauriat A. Ethanol fuel from biomass: A review. J. Sci. Ind. Res. 2005;64:809–821. [Google Scholar]
- 9.Singh R., Shukla A., Tiwari S., Srivastava M. A review on delignification of lignocellulosic biomass for enhancement of ethanol production potential. Renew. Sustain. Energy Rev. 2014;32:713–728. doi: 10.1016/j.rser.2014.01.051. [DOI] [Google Scholar]
- 10.Spivey J.J., Egbebi A. Heterogeneous catalytic synthesis of ethanol from biomass-derived syngas. Chem. Soc. Rev. 2007;36:1514–1528. doi: 10.1039/b414039g. [DOI] [PubMed] [Google Scholar]
- 11.Park B.-G. A Hybrid Adsorbent-Membrane Reactor (HAMR) System for Hydrogen Production. Korean J. Chem. Eng. 2004;21:782–792. doi: 10.1007/BF02705521. [DOI] [Google Scholar]
- 12.Apanel G., Johnson E. Direct methanol fuel cells—Ready to go commercial? Fuel Cells Bull. 2004;2004:12–17. doi: 10.1016/S1464-2859(04)00410-9. [DOI] [Google Scholar]
- 13.Deluga G.A., Salge J.R., Schmidt L.D., Verykios X.E. Renewable hydrogen from ethanol by autothermal reforming. Science. 2004;303:993–997. doi: 10.1126/science.1093045. [DOI] [PubMed] [Google Scholar]
- 14.Inui T., Yamamoto T., Inoue M. Highly effective synthesis of ethanol by CO2-hydrogenation on well balanced multi-functional FT-type composite catalysts. Appl. Catal. A Gen. 1999;186:395–406. [Google Scholar]
- 15.Mullins D.R. Adsorption of CO and C2H4 on Rh-loaded thin-film dysprosium oxide. Surf. Sci. 2006;600:2718–2725. doi: 10.1016/j.susc.2006.04.037. [DOI] [Google Scholar]
- 16.Shi B., O’Brien R.J., Bao S. Mechanism of the Isomerization of 1-Alkene during Iron-Catalyzed Fischer–Tropsch Synthesis. J. Catal. 2001;199:202–208. doi: 10.1006/jcat.2001.3175. [DOI] [Google Scholar]
- 17.Bridgwater A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy. 2012;38:68–94. doi: 10.1016/j.biombioe.2011.01.048. [DOI] [Google Scholar]
- 18.South C.R., Hogsett D.A., Lynd L.R. Continuous fermentation of cellulosic biomass to ethanol. Appl. Biochem. Biotechnol. 1993;39–40:587–600. doi: 10.1007/BF02919020. [DOI] [Google Scholar]
- 19.Chen Y. Development and application of co-culture for ethanol production by co-fermentation of glucose and xylose: A systematic review. J. Ind. Microbiol. Biotechnol. 2011;38:581–597. doi: 10.1007/s10295-010-0894-3. [DOI] [PubMed] [Google Scholar]
- 20.Dionisi D., Anderson J.A., Aulenta F. The potential of microbial processes for lignocellulosic biomass conversion to ethanol: A review. J. Chem. Technol. Biotechnol. 2015;90:366–383. doi: 10.1002/jctb.4544. [DOI] [Google Scholar]
- 21.Galbe M., Zacchi G. Pretreatment: The key to efficient utilization of lignocellulosic materials. Biomass Bioenergy. 2012;46:70–78. doi: 10.1016/j.biombioe.2012.03.026. [DOI] [Google Scholar]
- 22.Zhu J.Y., Pan X.J. Woody biomass pretreatment for cellulosic ethanol production: Technology and energy consumption evaluation☆. Bioresour. Technol. 2010;101:4992–5002. doi: 10.1016/j.biortech.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Lin Y., Tanaka S. Ethanol fermentation from biomass resources: Current state and prospects. Appl. Microbiol. Biotechnol. 2006;69:627–642. doi: 10.1007/s00253-005-0229-x. [DOI] [PubMed] [Google Scholar]
- 24.Sun Y., Cheng J. Hydrolysis of Lignocellulosic Materials for Ethanol Production: A Review. Volume 83. Elsevier Ltd.; Oxford, UK: 2002. pp. 1–11. [DOI] [PubMed] [Google Scholar]
- 25.Hsu T., Guo G., Chen W. Effect of dilute acid pretreatment of rice straw on structural properties and enzymatic hydrolysis. Bioresour. Technol. 2010;101:4907–4913. doi: 10.1016/j.biortech.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L., Li D., Wang L. Effect of steam explosion on biodegradation of lignin in wheat straw. Bioresour. Technol. 2008;99:8512–8515. doi: 10.1016/j.biortech.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 27.Cara C., Ruiz E., Ballesteros I. Enhanced enzymatic hydrolysis of olive tree wood by steam explosion and alkaline peroxide delignification. Process Biochem. 2006;41:423–429. doi: 10.1016/j.procbio.2005.07.007. [DOI] [Google Scholar]
- 28.Okano K., Kitagawa M., Sasaki Y. Conversion of Japanese red cedar (Cryptomeria japonica) into a feed for ruminants by white-rot basidiomycetes. Anim. Feed Sci. Technol. 2005;120:235–243. doi: 10.1016/j.anifeedsci.2005.02.023. [DOI] [Google Scholar]
- 29.Zhu J.Y., Pan X., Zalesny R.S. Pretreatment of woody biomass for biofuel production: Energy efficiency, technologies, and recalcitrance. Appl. Microbiol. Biotechnol. 2010;87:847–857. doi: 10.1007/s00253-010-2654-8. [DOI] [PubMed] [Google Scholar]
- 30.Zhu J.Y., Wang G.S., Pan X.J. Specific surface to evaluate the efficiencies of milling and pretreatment of wood for enzymatic saccharification. Chem. Eng. Sci. 2009;64:474–485. doi: 10.1016/j.ces.2008.09.026. [DOI] [Google Scholar]
- 31.Patel M., Kumar A. Production of renewable diesel through the hydroprocessing of lignocellulosic biomass-derived bio-oil: A review. Renew. Sustain. Energy Rev. 2016;58:1293–1307. doi: 10.1016/j.rser.2015.12.146. [DOI] [Google Scholar]
- 32.Ohgren K., Galbe M., Zacchi G. Optimization of steam pretreatment of SO2-impregnated corn stover for fuel ethanol production. Appl. Biochem. Biotechnol. 2005;121–124:1055–1067. doi: 10.1385/ABAB:124:1-3:1055. [DOI] [PubMed] [Google Scholar]
- 33.Zheng M., Li X., Li L. Enhancing anaerobic biogasification of corn stover through wet state NaOH pretreatment. Bioresour. Technol. 2009;100:5140–5145. doi: 10.1016/j.biortech.2009.05.045. [DOI] [PubMed] [Google Scholar]
- 34.Pan X., Xie D., Yu R.W. The bioconversion of mountain pine beetle-killed lodgepole pine to fuel ethanol using the organosolv process. Biotechnol. Bioeng. 2008;101:39–48. doi: 10.1002/bit.21883. [DOI] [PubMed] [Google Scholar]
- 35.Mosier N. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005;96:673–686. doi: 10.1016/j.biortech.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 36.Klinke H.B., Ahring B.K., Schmidt A.S. Characterization of degradation products from alkaline wet oxidation of wheat straw. Bioresour Technol. 2002;82:15–26. doi: 10.1016/S0960-8524(01)00152-3. [DOI] [PubMed] [Google Scholar]
- 37.Mosier N.S., Hendrickson R., Brewer M. Industrial scale-up of pH-controlled liquid hot water pretreatment of corn fiber for fuel ethanol production. Appl. Biochem. Biotechnol. 2005;125:77–97. doi: 10.1385/ABAB:125:2:077. [DOI] [PubMed] [Google Scholar]
- 38.van Walsum G.P., Allen S.G., Spencer M.J. Conversion of lignocellulosics pretreated with liquid hot water to ethanol. Appl. Biochem. Biotechnol. 1996;57–58:157–170. doi: 10.1007/BF02941696. [DOI] [Google Scholar]
- 39.Imman S., Laosiripojana N., Champreda V. Effects of Liquid Hot Water Pretreatment on Enzymatic Hydrolysis and Physicochemical Changes of Corncobs. Appl. Biochem. Biotechnol. 2018;184:432–443. doi: 10.1007/s12010-017-2541-1. [DOI] [PubMed] [Google Scholar]
- 40.Marcotullio G., Krisanti E., Giuntoli J. Selective production of hemicellulose-derived carbohydrates from wheat straw using dilute HCl or FeCl3 solutions under mild conditions. X-ray and thermo-gravimetric analysis of the solid residues. Bioresour. Technol. 2011;102:5917–5923. doi: 10.1016/j.biortech.2011.02.092. [DOI] [PubMed] [Google Scholar]
- 41.Geddes C.C., Peterson J.J., Roslander C. Optimizing the saccharification of sugar cane bagasse using dilute phosphoric acid followed by fungal cellulases. Bioresour. Technol. 2010;101:1851–1857. doi: 10.1016/j.biortech.2009.09.070. [DOI] [PubMed] [Google Scholar]
- 42.Zhang R., Lu X., Liu Y. Kinetic Study of Dilute Nitric acid Treatment of Corn Stover at Relatively High Temperature. Chem. Eng. Technol. 2011;34:409–414. doi: 10.1002/ceat.201000258. [DOI] [Google Scholar]
- 43.Zhao Y., Yu Z., Li J. Dilute Acid Pretreatment, Enzymatic Saccharification and Fermentation of Elephantgrass and Sudangrass to Ethanol. International Conference on Biomass Energy; Guangzhou, China: 2008. pp. 803–806. [Google Scholar]
- 44.Saha B.C., Cotta M.A. Lime pretreatment, enzymatic saccharification and fermentation of rice hulls to ethanol. Biomass Bioenergy. 2008;32:971–977. doi: 10.1016/j.biombioe.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 45.Wyman C.E., Dale B.E., Elander R.T. Comparative sugar recovery data from laboratory scale application of leading pretreatment technologies to corn stover. Bioresour. Technol. 2005;96:2026–2032. doi: 10.1016/j.biortech.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 46.Zhu J.Y., Pan X.J., Wang G.S. Sulfite pretreatment (SPORL) for robust enzymatic saccharification of spruce and red pine. Bioresour. Technol. 2009;100:2411–2418. doi: 10.1016/j.biortech.2008.10.057. [DOI] [PubMed] [Google Scholar]
- 47.Tabka M.G., Herpoël-Gimbert I., Monod F. Enzymatic saccharification of wheat straw for bioethanol production by a combined cellulase xylanase and feruloyl esterase treatment. Enzyme Microb. Technol. 2006;39:897–902. doi: 10.1016/j.enzmictec.2006.01.021. [DOI] [Google Scholar]
- 48.Ohgren K., Bura R., Saddler J. Effect of hemicellulose and lignin removal on enzymatic hydrolysis of steam pretreated corn stover. Bioresour. Technol. 2007;98:2503–2510. doi: 10.1016/j.biortech.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Monavari S., Galbe M., Zacchi G. Impact of impregnation time and chip size on sugar yield in pretreatment of softwood for ethanol production. Bioresour. Technol. 2009;100:6312–6316. doi: 10.1016/j.biortech.2009.06.097. [DOI] [PubMed] [Google Scholar]
- 50.Ewanick S.M., Bura R., Saddler J.N. Acid-catalyzed steam pretreatment of lodgepole pine and subsequent enzymatic hydrolysis and fermentation to ethanol. Biotechnol. Bioeng. 2007;98:737–746. doi: 10.1002/bit.21436. [DOI] [PubMed] [Google Scholar]
- 51.Holtzapple M.T., Jun J.H. The Ammonia Freeze Explosion (AFEX) process. Appl. Biochem. Biotechnol. 1991;28–29:59–74. doi: 10.1007/BF02922589. [DOI] [Google Scholar]
- 52.Teymouri F., Laureano-Perez L., Alizadeh H. Optimization of the ammonia fiber explosion (AFEX) treatment parameters for enzymatic hydrolysis of corn stover. Bioresour. Technol. 2005;96:2014–2018. doi: 10.1016/j.biortech.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y.H., Ding S.Y., Mielenz J.R. Fractionating recalcitrant lignocellulose at modest reaction conditions. Biotechnol. Bioeng. 2007;97:214–223. doi: 10.1002/bit.21386. [DOI] [PubMed] [Google Scholar]
- 54.Knez Ž., Markočič E., Hrnčič M.K. High pressure water reforming of biomass for energy and chemicals: A short review. J. Supercrit. Fluids. 2015;96:46–52. doi: 10.1016/j.supflu.2014.06.008. [DOI] [Google Scholar]
- 55.Lian X., Xue Y., Zhao Z. Progress on upgrading methods of bio-oil: A review. Int. J. Energy Res. 2017;41:1798–1816. doi: 10.1002/er.3726. [DOI] [Google Scholar]
- 56.Li X., Xuan K., Zhu Y. A mechanistic study on the decomposition of a model bio-oil compound for hydrogen production over a stepped Ni surface: Formic acid. Appl. Surf. Sci. 2018;452:87–95. doi: 10.1016/j.apsusc.2018.05.049. [DOI] [Google Scholar]
- 57.Pourzolfaghar H., Abnisa F., Wan Daud W.M.A. Atmospheric hydrodeoxygenation of bio-oil oxygenated model compounds: A review. J. Anal. Appl. Pyrolysis. 2018;133:117–127. doi: 10.1016/j.jaap.2018.04.013. [DOI] [Google Scholar]
- 58.Ross A.B., Biller P., Kubacki M.L. Hydrothermal processing of microalgae using alkali and organic acids. Fuel. 2010;89:2234–2243. doi: 10.1016/j.fuel.2010.01.025. [DOI] [Google Scholar]
- 59.Jiang X., Ellis N. Upgrading Bio-oil through Emulsification with Biodiesel: Thermal Stability. Energy Fuels. 2010;24:2699–2706. doi: 10.1021/ef901517k. [DOI] [Google Scholar]
- 60.Oasmaa A., Kuoppala E., Selin J. Fast pyrolysis of forestry residue and pine. 4. improvement of the product quality by solvent addition. Energy Fuels. 2004;18:1578–1583. doi: 10.1021/ef040038n. [DOI] [Google Scholar]
- 61.Toor S., Rosendahl L., Rudolf A. Hydrothermal liquefaction of biomass: a review of subcritical water technologies. Energy. 2011;36:2328–2342. doi: 10.1016/j.energy.2011.03.013. [DOI] [Google Scholar]
- 62.Nava R., Pawelec B., Castaño P. Upgrading of bio-liquids on different mesoporous silica-supported CoMo catalysts. Appl. Catal. B: Environ. 2009;92:154–167. doi: 10.1016/j.apcatb.2009.07.014. [DOI] [Google Scholar]
- 63.Guo X., Zheng Y., Zhang B. Analysis of coke precursor on catalyst and study on regeneration of catalyst in upgrading of bio-oil. Biomass Bioenergy. 2009;33:1469–1473. doi: 10.1016/j.biombioe.2009.07.002. [DOI] [Google Scholar]
- 64.Vagia E.C., Lemonidou A.A. Hydrogen production via steam reforming of bio-oil components over calcium aluminate supported nickel and noble metal catalysts. Appl. Catal. A Gen. 2008;351:111–121. doi: 10.1016/j.apcata.2008.09.007. [DOI] [Google Scholar]
- 65.Saber M., Nakhshiniev B., Yoshikawa K. A review of production and upgrading of algal bio-oil. Renew. Sustain. Energy Rev. 2016;58:918–930. doi: 10.1016/j.rser.2015.12.342. [DOI] [Google Scholar]
- 66.Zhu X., Lobban L., Mallinson R.G. Bifunctional transalkylation and hydrodeoxygenation of anisole over a Pt/HBeta catalyst. J. Catal. 2011;281:21–29. doi: 10.1016/j.jcat.2011.03.030. [DOI] [Google Scholar]
- 67.Laird D.A., Brown R.C., Amonette J.E. Review of the pyrolysis platform for coproducing bio-oil and biochar. Biofuels Bioprod. Biorefin. 2009;3:547–562. doi: 10.1002/bbb.169. [DOI] [Google Scholar]
- 68.Zhang H., Xiao R., Huang H. Comparison of non-catalytic and catalytic fast pyrolysis of corncob in a fluidized bed reactor. Bioresour. Technol. 2009;100:1428–1434. doi: 10.1016/j.biortech.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 69.Zhang H., Xiao R., Wang D. Catalytic Fast Pyrolysis of Biomass in a Fluidized Bed with Fresh and Spent Fluidized Catalytic Cracking (FCC) Catalysts. Energy Fuels. 2009;23:6199–6206. doi: 10.1021/ef900720m. [DOI] [Google Scholar]
- 70.Isahak W.N.R.W., Hisham M.W.M., Yarmo M.A. A review on bio-oil production from biomass by using pyrolysis method. Renew. Sustain. Energy Rev. 2012;16:5910–5923. doi: 10.1016/j.rser.2012.05.039. [DOI] [Google Scholar]
- 71.Guedes R.E., Luna A.S., Torres A.R. Operating parameters for bio-oil production in biomass pyrolysis: A review. J. Anal. Appl. Pyrolysis. 2018;129:134–149. doi: 10.1016/j.jaap.2017.11.019. [DOI] [Google Scholar]
- 72.Zhang L., Liu R., Yin R. Upgrading of bio-oil from biomass fast pyrolysis in China: A review. Renew. Sustain. Energy Rev. 2013;24:66–72. doi: 10.1016/j.rser.2013.03.027. [DOI] [Google Scholar]
- 73.Zhang S., Yang X., Zheng K. In-situ hydrogenation of furfural conversion to furfuryl alcohol via aqueous-phase reforming of methanol. Appl. Catal. A Gen. 2019;581:103–110. doi: 10.1016/j.apcata.2019.05.031. [DOI] [Google Scholar]
- 74.Akhtar J., Saidina Amin N. A review on operating parameters for optimum liquid oil yield in biomass pyrolysis. Renew. Sustain. Energy Rev. 2012;16:5101–5109. doi: 10.1016/j.rser.2012.05.033. [DOI] [Google Scholar]
- 75.Qu T., Guo W., Shen L. Experimental Study of Biomass Pyrolysis Based on Three Major Components: Hemicellulose, Cellulose, and Lignin. Ind. Eng. Chem. Res. 2011;50:10424–10433. doi: 10.1021/ie1025453. [DOI] [Google Scholar]
- 76.Lyu H., Tang J., Huang Y. Removal of hexavalent chromium from aqueous solutions by a novel biochar supported nanoscale iron sulfide composite. Chem. Eng. J. 2017;322:516–524. doi: 10.1016/j.cej.2017.04.058. [DOI] [Google Scholar]
- 77.Quan C., Gao N., Song Q. Pyrolysis of biomass components in a TGA and a fixed-bed reactor: Thermochemical behaviors, kinetics, and product characterization. J. Anal. Appl. Pyrolysis. 2016;121:84–92. doi: 10.1016/j.jaap.2016.07.005. [DOI] [Google Scholar]
- 78.Stefanidis S.D., Kalogiannis K.G., Iliopoulou E.F. A study of lignocellulosic biomass pyrolysis via the pyrolysis of cellulose, hemicellulose and lignin. J. Anal. Appl. Pyrolysis. 2014;105:143–150. doi: 10.1016/j.jaap.2013.10.013. [DOI] [Google Scholar]
- 79.Venderbosch R.H., Prins W. Fast pyrolysis technology development. Biofuels Bioprod. Biorefin. 2010;4:178–208. doi: 10.1002/bbb.205. [DOI] [Google Scholar]
- 80.Lee Y., Park J., Ryu C. Comparison of biochar properties from biomass residues produced by slow pyrolysis at 500 °C. Bioresour. Technol. 2013;148:196–201. doi: 10.1016/j.biortech.2013.08.135. [DOI] [PubMed] [Google Scholar]
- 81.Fu P., Hu S., Xiang J. FTIR study of pyrolysis products evolving from typical agricultural residues. J. Anal. Appl. Pyrolysis. 2010;88:117–123. doi: 10.1016/j.jaap.2010.03.004. [DOI] [Google Scholar]
- 82.Lu Q., Yang X., Zhu X. Analysis on chemical and physical properties of bio-oil pyrolyzed from rice husk. J. Anal. Appl. Pyrolysis. 2008;82:191–198. doi: 10.1016/j.jaap.2008.03.003. [DOI] [Google Scholar]
- 83.Liu L., Zhang S., Yang X. Cellulose isolation from corn stalk treated by alkaline biochars in solvent system. BioResources. 2018;13:691–703. doi: 10.15376/biores.13.1.691-703. [DOI] [Google Scholar]
- 84.Angın D. Effect of pyrolysis temperature and heating rate on biochar obtained from pyrolysis of safflower seed press cake. Bioresour. Technol. 2013;128:593–597. doi: 10.1016/j.biortech.2012.10.150. [DOI] [PubMed] [Google Scholar]
- 85.Tsai W., Lee M., Chang Y. Fast pyrolysis of rice husk: Product yields and compositions. Bioresour. Technol. 2007;98:22–28. doi: 10.1016/j.biortech.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 86.Garg R., Anand N., Kumar D. Pyrolysis of babool seeds (Acacia nilotica) in a fixed bed reactor and bio-oil characterization. Renew. Energy. 2016;96:167–171. doi: 10.1016/j.renene.2016.04.059. [DOI] [Google Scholar]
- 87.Asadullah M., Rahman M.A., Ali M.M. Production of bio-oil from fixed bed pyrolysis of bagasse. Fuel. 2007;86:2514–2520. doi: 10.1016/j.fuel.2007.02.007. [DOI] [Google Scholar]
- 88.Zheng J., Yi W., Wang N. Bio-oil production from cotton stalk. Energy Convers. Manag. 2008;49:1724–1730. doi: 10.1016/j.enconman.2007.11.005. [DOI] [Google Scholar]
- 89.Zheng J., Zhu X., Guo Q. Thermal conversion of rice husks and sawdust to liquid fuel. Waste Manag. 2006;26:1430–1435. doi: 10.1016/j.wasman.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 90.Huang X., Cao J., Shi P. Influences of pyrolysis conditions in the production and chemical composition of the bio-oils from fast pyrolysis of sewage sludge. J. Anal. Appl. Pyrolysis. 2014;110:353–362. doi: 10.1016/j.jaap.2014.10.003. [DOI] [Google Scholar]
- 91.Alvarez J., Lopez G., Amutio M., Bilbao J., Olazar M. Bio-oil production from rice husk fast pyrolysis in a conical spouted bed reactor. Fuel. 2014;128:162–169. doi: 10.1016/j.fuel.2014.02.074. [DOI] [Google Scholar]
- 92.Abdullah N., Gerhauser H. Bio-oil derived from empty fruit bunches. Fuel. 2008;87:2606–2613. doi: 10.1016/j.fuel.2008.02.011. [DOI] [Google Scholar]
- 93.Volli V., Singh R.K. Production of bio-oil from de-oiled cakes by thermal pyrolysis. Fuel. 2012;96:579–585. doi: 10.1016/j.fuel.2012.01.016. [DOI] [Google Scholar]
- 94.Encinar J.M., González J.F., González J. Fixed-bed pyrolysis of Cynara cardunculus L. Product yields and compositions. Fuel Process. Technol. 2000;68:209–222. doi: 10.1016/S0378-3820(00)00125-9. [DOI] [Google Scholar]
- 95.Encinar J.M., Beltrán F.J., Ramiro A. Pyrolysisr/gasification of agricultural residues by carbon dioxide in the presence of different additives: Influence of variables. Fuel Process. Technol. 1998;55:219–233. doi: 10.1016/S0378-3820(98)00052-6. [DOI] [Google Scholar]
- 96.Islam M.R., Parveen M., Haniu H. Properties of sugarcane waste-derived bio-oils obtained by fixed-bed fire-tube heating pyrolysis. Bioresour. Technol. 2010;101:4162–4168. doi: 10.1016/j.biortech.2009.12.137. [DOI] [PubMed] [Google Scholar]
- 97.Pattiya A., Suttibak S. Production of bio-oil via fast pyrolysis of agricultural residues from cassava plantations in a fluidised-bed reactor with a hot vapour filtration unit. J. Anal. Appl. Pyrolysis. 2012;95:227–235. doi: 10.1016/j.jaap.2012.02.010. [DOI] [Google Scholar]
- 98.Kim S.W., Koo B.S., Ryu J.W. Bio-oil from the pyrolysis of palm and Jatropha wastes in a fluidized bed. Fuel Process. Technol. 2013;108:118–124. doi: 10.1016/j.fuproc.2012.05.002. [DOI] [Google Scholar]
- 99.Makibar J., Fernandez-Akarregi A.R., Amutio M. Performance of a conical spouted bed pilot plant for bio-oil production by poplar flash pyrolysis. Fuel Process. Technol. 2015;137:283–289. doi: 10.1016/j.fuproc.2015.03.011. [DOI] [Google Scholar]
- 100.Apaydin-Varol E., Pütün E., Pütün A.E. Slow pyrolysis of pistachio shell. Fuel. 2007;86:1892–1899. doi: 10.1016/j.fuel.2006.11.041. [DOI] [Google Scholar]
- 101.Jung S., Kang B., Kim J. Production of bio-oil from rice straw and bamboo sawdust under various reaction conditions in a fast pyrolysis plant equipped with a fluidized bed and a char separation system. J. Anal. Appl. Pyrolysis. 2008;82:240–247. doi: 10.1016/j.jaap.2008.04.001. [DOI] [Google Scholar]
- 102.Heo H.S., Park H.J., Dong J. Fast pyrolysis of rice husk under different reaction conditions. J. Ind. Eng. Chem. 2010;16:27–31. doi: 10.1016/j.jiec.2010.01.026. [DOI] [Google Scholar]
- 103.Tripathi M., Sahu J.N., Ganesan P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016;55:467–481. doi: 10.1016/j.rser.2015.10.122. [DOI] [Google Scholar]
- 104.Onay O. Influence of pyrolysis temperature and heating rate on the production of bio-oil and char from safflower seed by pyrolysis, using a well-swept fixed-bed reactor. Fuel Process. Technol. 2007;88:523–531. doi: 10.1016/j.fuproc.2007.01.001. [DOI] [Google Scholar]
- 105.Pattiya A., Sukkasi S., Goodwin V. Fast pyrolysis of sugarcane and cassava residues in a free-fall reactor. Energy. 2012;44:1067–1077. doi: 10.1016/j.energy.2012.04.035. [DOI] [Google Scholar]
- 106.Hou Q.D., Ju M.T., Li W.Z., Liu L., Chen Y., Yang Q. Pretreatment of lignocellulosic biomass with ionic liquids and ionic liquid-based solvent systems. Molecules. 2017;22:490. doi: 10.3390/molecules22030490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Raja S.A., Kennedy Z.R., Pillai B.C. Flash pyrolysis of jatropha oil cake in electrically heated fluidized bed reactor. Energy. 2010;35:2819–2823. doi: 10.1016/j.energy.2010.03.011. [DOI] [Google Scholar]
- 108.Huang A., Hsu C., Hou B. Production and separation of rice husk pyrolysis bio-oils from a fractional distillation column connected fluidized bed reactor. Powder Technol. 2018;323:588–593. doi: 10.1016/j.powtec.2016.03.052. [DOI] [Google Scholar]
- 109.Pütün A.E., Özcan A., Gerçel H.F., Putun E. Production of biocrudes from biomass in a fixed-bed tubular reactor: Product yields and compositions. Fuel. 2001;80:1371–1378. doi: 10.1016/S0016-2361(01)00021-7. [DOI] [Google Scholar]
- 110.Shen J., Wang X., Garcia-Perez M. Effects of particle size on the fast pyrolysis of oil mallee woody biomass. Fuel. 2009;88:1810–1817. doi: 10.1016/j.fuel.2009.05.001. [DOI] [Google Scholar]
- 111.Kang B., Lee K.H., Park H.J. Fast pyrolysis of radiata pine in a bench scale plant with a fluidized bed: Influence of a char separation system and reaction conditions on the production of bio-oil. J. Anal. Appl. Pyrolysis. 2006;76:32–37. doi: 10.1016/j.jaap.2005.06.012. [DOI] [Google Scholar]
- 112.Abnisa F., Daud W.M.A.W., Husin W.N.W. Utilization possibilities of palm shell as a source of biomass energy in Malaysia by producing bio-oil in pyrolysis process. Biomass Bioenergy. 2011;35:1863–1872. doi: 10.1016/j.biombioe.2011.01.033. [DOI] [Google Scholar]
- 113.Bartoli M., Rosi L., Giovannelli A. Production of bio-oils and bio-char from Arundo donax through microwave assisted pyrolysis in a multimode batch reactor. J. Anal. Appl. Pyrolysis. 2016;122:479–489. doi: 10.1016/j.jaap.2016.10.016. [DOI] [Google Scholar]
- 114.Wu S., Chang C., Chang Y. Comparison of oil-tea shell and Douglas-fir sawdust for the production of bio-oils and chars in a fluidized-bed fast pyrolysis system. Fuel. 2016;175:57–63. doi: 10.1016/j.fuel.2016.02.008. [DOI] [Google Scholar]
- 115.Tsai W.T., Lee M.K., Chang Y.M. Fast pyrolysis of rice straw, sugarcane bagasse and coconut shell in an induction-heating reactor. J. Anal. Appl. Pyrolysis. 2006;76:230–237. doi: 10.1016/j.jaap.2005.11.007. [DOI] [Google Scholar]
- 116.Açıkalın K., Karaca F., Bolat E. Pyrolysis of pistachio shell: Effects of pyrolysis conditions and analysis of products. Fuel. 2012;95:169–177. doi: 10.1016/j.fuel.2011.09.037. [DOI] [Google Scholar]
- 117.Sricharoenchaikul V., Pechyen C., Aht-Ong D. Preparation and Characterization of Activated Carbon from the Pyrolysis of Physic Nut (Jatropha curcas L.) Waste†. Energy Fuels. 2008;22:31–37. doi: 10.1021/ef700285u. [DOI] [Google Scholar]
- 118.Zhang S., Yang X., Liu L., Zheng K., Ju M.T., Liu J.P. Bisphenol S adsorption behavior on ferralsol and biochar modified soil with dissolved organic matter. Int. J. Env. Res. Pub. He. 2019;16:764. doi: 10.3390/ijerph16050764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rüdisüli M., Schildhauer T.J., Biollaz S.M.A. Scale-up of bubbling fluidized bed reactors—A review. Powder Technol. 2012;217:21–38. doi: 10.1016/j.powtec.2011.10.004. [DOI] [Google Scholar]
- 120.Gerber S., Behrendt F., Oevermann M. An Eulerian modeling approach of wood gasification in a bubbling fluidized bed reactor using char as bed material. Fuel. 2010;89:2903–2917. doi: 10.1016/j.fuel.2010.03.034. [DOI] [Google Scholar]
- 121.Nemtsov D.A., Zabaniotou A. Mathematical modelling and simulation approaches of agricultural residues air gasification in a bubbling fluidized bed reactor. Chem. Eng. J. 2008;143:10–31. doi: 10.1016/j.cej.2008.01.023. [DOI] [Google Scholar]
- 122.Mastellone M.L., Zaccariello L., Arena U. Co-gasification of coal, plastic waste and wood in a bubbling fluidized bed reactor. Fuel. 2010;89:2991–3000. doi: 10.1016/j.fuel.2010.05.019. [DOI] [Google Scholar]
- 123.Ly H.V., Kim S., Woo H.C. Fast pyrolysis of macroalga Saccharina japonica in a bubbling fluidized-bed reactor for bio-oil production. Energy. 2015;93:1436–1446. doi: 10.1016/j.energy.2015.10.011. [DOI] [Google Scholar]
- 124.Li X.T., Grace J.R., Lim C.J. Biomass gasification in a circulating fluidized bed. Biomass Bioenergy. 2004;26:171–193. doi: 10.1016/S0961-9534(03)00084-9. [DOI] [Google Scholar]
- 125.Lappas A.A., Samolada M.C., Iatridis D.K. Biomass pyrolysis in a circulating fluid bed reactor for the production of fuels and chemicals. Fuel. 2002;81:2087–2095. doi: 10.1016/S0016-2361(02)00195-3. [DOI] [Google Scholar]
- 126.Drift A.V.D., Doorn J.V., Vermeulen J.W. Ten residual biomass fuels for circulating fluidized-bed gasification. Biomass Bioenergy. 2001;20:45–56. doi: 10.1016/S0961-9534(00)00045-3. [DOI] [Google Scholar]
- 127.Kersten SR A., Prins W., van der Drift B. Principles of a novel multistage circulating fluidized bed reactor for biomass gasification. Chem. Eng. Sci. 2003;58:725–731. doi: 10.1016/S0009-2509(02)00601-2. [DOI] [Google Scholar]
- 128.Youssef M.A., Wahid S.S., Mohamed M.A. Experimental study on Egyptian biomass combustion in circulating fluidized bed. Appl. Energy. 2009;86:2644–2650. doi: 10.1016/j.apenergy.2009.04.021. [DOI] [Google Scholar]
- 129.Janse A.M.C., de Jong X.A., Prins W. Heat transfer coefficients in the rotating cone reactor. Powder Technol. 1999;106:168–175. doi: 10.1016/S0032-5910(99)00076-5. [DOI] [Google Scholar]
- 130.Janse A.M.C., Biesheuvel P.M., Prins W., van Swaaij W.P.M. A novel interconnected fluidised bed for the combined flash pyrolysis of biomass and combustion of char. Chem. Eng. J. 2000;76:77–86. doi: 10.1016/S1385-8947(99)00128-X. [DOI] [Google Scholar]
- 131.Puy N., Murillo R., Navarro M.V. Valorisation of forestry waste by pyrolysis in an auger reactor. Waste Manag. 2011;31:1339–1349. doi: 10.1016/j.wasman.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 132.Bhattacharya P., Steele P.H., Hassan E.B.M. Wood/plastic copyrolysis in an auger reactor: Chemical and physical analysis of the products. Fuel. 2009;88:1251–1260. doi: 10.1016/j.fuel.2009.01.009. [DOI] [Google Scholar]
- 133.Veses A., Aznar M., Martínez I. Catalytic pyrolysis of wood biomass in an auger reactor using calcium-based catalysts. Bioresour. Technol. 2014;162:250–258. doi: 10.1016/j.biortech.2014.03.146. [DOI] [PubMed] [Google Scholar]
- 134.Li B., Lv W., Zhang Q. Pyrolysis and catalytic upgrading of pine wood in a combination of auger reactor and fixed bed. Fuel. 2014;129:61–67. doi: 10.1016/j.fuel.2014.03.043. [DOI] [Google Scholar]
- 135.Garcìa-Pèrez M., Chaala A., Roy C. Vacuum pyrolysis of sugarcane bagasse. J. Anal. Appl. Pyrolysis. 2002;65:111–136. doi: 10.1016/S0165-2370(01)00184-X. [DOI] [Google Scholar]
- 136.Roy C., Chaala A., Darmstadt H. The vacuum pyrolysis of used tires: End-uses for oil and carbon black products. J. Anal. Appl. Pyrolysis. 1999;51:201–221. doi: 10.1016/S0165-2370(99)00017-0. [DOI] [Google Scholar]
- 137.Britt P.F., Buchanan A.C., Cooney M.J. Flash Vacuum Pyrolysis of Methoxy-Substituted Lignin Model Compounds. J. Organic Chem. 2000;65:1376–1389. doi: 10.1021/jo991479k. [DOI] [PubMed] [Google Scholar]
- 138.Garcìa-Pérez M., Chaala A., Pakdel H. Vacuum pyrolysis of softwood and hardwood biomass. J. Anal. Appl. Pyrolysis. 2007;78:104–116. doi: 10.1016/j.jaap.2006.05.003. [DOI] [Google Scholar]
- 139.Tekin K., Karagöz S., Bektaş S. A review of hydrothermal biomass processing. Renew. Sustain. Energy Rev. 2014;40:673–687. doi: 10.1016/j.rser.2014.07.216. [DOI] [Google Scholar]
- 140.Akhtar J., Amin N.A.S. A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2011;15:1615–1624. doi: 10.1016/j.rser.2010.11.054. [DOI] [Google Scholar]
- 141.Chan Y.H., Yusup S., Quitain A.T. Bio-oil production from oil palm biomass via subcritical and supercritical hydrothermal liquefaction. J. Supercrit. Fluids. 2014;95:407–412. doi: 10.1016/j.supflu.2014.10.014. [DOI] [Google Scholar]
- 142.Xue Y., Chen H., Zhao W. A review on the operating conditions of producing bio-oil from hydrothermal liquefaction of biomass. Int. J. Energy Res. 2016;40:865–877. doi: 10.1002/er.3473. [DOI] [Google Scholar]
- 143.Zhang S., Yang X., Ju M. Mercury adsorption to aged biochar and its management in China. Environ. Sci. Pollut. Res. 2019;26:4867–4877. doi: 10.1007/s11356-018-3945-3. [DOI] [PubMed] [Google Scholar]
- 144.Chan Y.H., Yusup S., Quitain A.T. Effect of process parameters on hydrothermal liquefaction of oil palm biomass for bio-oil production and its life cycle assessment. Energy Convers. Manag. 2015;104:180–188. doi: 10.1016/j.enconman.2015.03.075. [DOI] [Google Scholar]
- 145.Elliott D.C., Biller P., Ross A.B. Hydrothermal liquefaction of biomass: Developments from batch to continuous process. Bioresour. Technol. 2015;178:147–156. doi: 10.1016/j.biortech.2014.09.132. [DOI] [PubMed] [Google Scholar]
- 146.Pham M., Schideman L., Scott J. Chemical and Biological Characterization of Wastewater Generated from Hydrothermal Liquefaction of Spirulina. Environ. Sci. Technol. 2013;47:2131–2138. doi: 10.1021/es304532c. [DOI] [PubMed] [Google Scholar]
- 147.Kang S., Yu J. Hydrophobic organic compounds from hydrothermal liquefaction of bacterial biomass. Biomass Bioenergy. 2015;74:92–95. doi: 10.1016/j.biombioe.2015.01.008. [DOI] [Google Scholar]
- 148.Karagoz S., Bhaskar T., Muto A. Hydrothermal upgrading of biomass: Effect of KCO concentration and biomass/water ratio on products distribution. Bioresour. Technol. 2006;97:90–98. doi: 10.1016/j.biortech.2005.02.051. [DOI] [PubMed] [Google Scholar]
- 149.Xu C., Etcheverry T. Hydro-liquefaction of woody biomass in sub- and super-critical ethanol with iron-based catalysts. Fuel. 2008;87:335–345. doi: 10.1016/j.fuel.2007.05.013. [DOI] [Google Scholar]
- 150.Yu Y., Lou X., Wu H. Some Recent Advances in Hydrolysis of Biomass in Hot-Compressed Water and Its Comparisons with Other Hydrolysis Methods. Energy Fuels. 2008;22:46–60. doi: 10.1021/ef700292p. [DOI] [Google Scholar]
- 151.Sugano M., Takagi H., Hirano K. Hydrothermal liquefaction of plantation biomass with two kinds of wastewater from paper industry. J. Mater. Sci. 2008;43:2476–2486. doi: 10.1007/s10853-007-2106-8. [DOI] [Google Scholar]
- 152.Zhang L., Champagne P., Charles Xu C. Bio-crude production from secondary pulp/paper-mill sludge and waste newspaper via co-liquefaction in hot-compressed water. Energy. 2011;36:2142–2150. doi: 10.1016/j.energy.2010.05.029. [DOI] [Google Scholar]
- 153.Xiu S., Shahbazi A., Shirley V. Hydrothermal pyrolysis of swine manure to bio-oil: Effects of operating parameters on products yield and characterization of bio-oil. J. Anal. Appl. Pyrolysis. 2010;88:73–79. doi: 10.1016/j.jaap.2010.02.011. [DOI] [Google Scholar]
- 154.Zhou D., Zhang L., Zhang S. Hydrothermal Liquefaction of Macroalgae Enteromorpha prolifera to Bio-oil. Energy Fuels. 2010;24:4054–4061. doi: 10.1021/ef100151h. [DOI] [Google Scholar]
- 155.Yin S., Dolan R., Harris M. Subcritical hydrothermal liquefaction of cattle manure to bio-oil: Effects of conversion parameters on bio-oil yield and characterization of bio-oil. Bioresour. Technol. 2010;101:3657–3664. doi: 10.1016/j.biortech.2009.12.058. [DOI] [PubMed] [Google Scholar]
- 156.Zhang B., von Keitz M., Valentas K. Thermochemical liquefaction of high-diversity grassland perennials. J. Anal. Appl. Pyrolysis. 2009;84:18–24. doi: 10.1016/j.jaap.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 157.Qu Y., Wei X., Zhong C. Experimental study on the direct liquefaction of Cunninghamia lanceolata in water. Energy. 2003;28:597–606. doi: 10.1016/S0360-5442(02)00178-0. [DOI] [Google Scholar]
- 158.Kersten S.R.A., Potic B., Prins W. Gasification of Model Compounds and Wood in Hot Compressed Water. Ind. Eng. Chem. Res. 2006;45:4169–4177. doi: 10.1021/ie0509490. [DOI] [Google Scholar]
- 159.Xu C., Lancaster J. Conversion of secondary pulp/paper sludge powder to liquid oil products for energy recovery by direct liquefaction in hot-compressed water. Water Res. 2008;42:1571–1582. doi: 10.1016/j.watres.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 160.Zhang R., Zhang H., Su X. Investigation on degradation of polyethylene to oil in a continuous supercritical water reactor. J. Fuel Chem. Technol. 2007;35:487–491. doi: 10.1016/S1872-5813(07)60030-9. [DOI] [Google Scholar]
- 161.Boocock D.G.B., Sherman K.M. Further aspects of powdered poplar wood liquefaction by aqueous pyrolysis. Can. Soc. Chem. Eng. 1985;63:627–633. doi: 10.1002/cjce.5450630415. [DOI] [Google Scholar]
- 162.Demirbas A. Effect of initial moisture content on the yields of oily products from pyrolysis of biomass. J. Anal. Appl. Pyrolysis. 2004;71:803–815. doi: 10.1016/j.jaap.2003.10.008. [DOI] [Google Scholar]
- 163.Mani S., Tabil L.G., Sokhansanj S. Grinding performance and physical properties of wheat and barley straws, corn stover and switchgrass. Biomass Bioenergy. 2004;27:339–352. doi: 10.1016/j.biombioe.2004.03.007. [DOI] [Google Scholar]
- 164.Wang C., Pan J., Li J., Yang Z. Comparative studies of products produced from four different biomass samples via deoxy-liquefaction. Bioresour. Technol. 2008;99:2778–2786. doi: 10.1016/j.biortech.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 165.Xu Y., Li Y., Wang C. In-situ hydrogenation of model compounds and raw bio-oil over Ni/CMK-3 catalyst. Fuel Process. Technol. 2017;161:226–231. doi: 10.1016/j.fuproc.2016.08.018. [DOI] [Google Scholar]
- 166.Fisk C.A., Morgan T., Ji Y. Bio-oil upgrading over platinum catalysts using in situ generated hydrogen. Appl. Catal. A Gen. 2009;358:150–156. doi: 10.1016/j.apcata.2009.02.006. [DOI] [Google Scholar]
- 167.Feng J., Yang Z., Hse C., Su Q., Wang K., Jiang J., Xu J. In situ catalytic hydrogenation of model compounds and biomass-derived phenolic compounds for bio-oil upgrading. Renew. Energy. 2017;105:140–148. doi: 10.1016/j.renene.2016.12.054. [DOI] [Google Scholar]
- 168.Liu L., Ju M.T., Li W.Z., Liu Y.L. Influence of solid alkali application on corn stalk dissolution and degradation in solvent systems. Polym. Degrad. Stabil. 2015;120:98–106. doi: 10.1016/j.polymdegradstab.2015.06.010. [DOI] [Google Scholar]
- 169.Li X., Chen G., Liu C., Ma W.C., Yan B.B., Zhang J.G. Hydrodeoxygenation of lignin-derived bio-oil using molecular sieves supported metal catalysts: A critical review. Renew. Sustain. Energy Rev. 2017;71:296–308. doi: 10.1016/j.rser.2016.12.057. [DOI] [Google Scholar]
- 170.Cao J., Zhao X., Morishita K., Li L.Y., Xiao X.B., Obala R., Wei X.Y., Takarda T. Triacetonamine formation in a bio-oil from fast pyrolysis of sewage sludge using acetone as the absorption solvent. Bioresour. Technol. 2010;101:4242–4245. doi: 10.1016/j.biortech.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 171.Wang S., Gu Y., Liu Q. Separation of bio-oil by molecular distillation. Fuel Process. Technol. 2009;90:738–745. doi: 10.1016/j.fuproc.2009.02.005. [DOI] [Google Scholar]
- 172.Elkasabi Y., Mullen C.A., Boateng A.A. Distillation and Isolation of Commodity Chemicals from Bio-Oil Made by Tail-Gas Reactive Pyrolysis. ACS Sustain. Chem. Eng. 2014;2:2042–2052. doi: 10.1021/sc5002879. [DOI] [Google Scholar]
- 173.Eboibi B.E., Lewis D.M., Ashman P.J., Chinnasamy S. Hydrothermal liquefaction of microalgae for biocrude production: Improving the biocrude properties with vacuum distillation. Bioresour. Technol. 2014;174:212–221. doi: 10.1016/j.biortech.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 174.Schulzke T., Conrad S., Westermeyer J. Fractionation of flash pyrolysis condensates by staged condensation. Biomass Bioenergy. 2016;95:287–295. doi: 10.1016/j.biombioe.2016.05.022. [DOI] [Google Scholar]
- 175.Liu R., Fei W., Shen C. Influence of acetone addition on the physicochemical properties of bio-oils. J. Energy Inst. 2014;87:127–133. doi: 10.1016/j.joei.2013.08.001. [DOI] [Google Scholar]
- 176.Pidtasang B., Udomsap P., Sukkasi S., Chollacoop N., Pattiya A. Influence of alcohol addition on properties of bio-oil produced from fast pyrolysis of eucalyptus bark in a free-fall reactor. J. Ind. Eng. Chem. 2013;19:1851–1857. doi: 10.1016/j.jiec.2013.02.031. [DOI] [Google Scholar]
- 177.Lu Q., Li W., Zhu X. Overview of fuel properties of biomass fast pyrolysis oils. Energy Convers. Manag. 2009;50:1376–1383. doi: 10.1016/j.enconman.2009.01.001. [DOI] [Google Scholar]
- 178.Xu Y., Wang Q., Hu X., Liu X., Zhu X.F. Characterization of the lubricity of bio-oil/diesel fuel blends by high frequency reciprocating test rig. Energy. 2010;35:283–287. doi: 10.1016/j.energy.2009.09.020. [DOI] [Google Scholar]
- 179.Jiang X., Zhong Z., Ellis N., Wang Q. Aging and Thermal Stability of the Mixed Product of the Ether-Soluble Fraction of Bio-Oil and Bio-Diesel. Chem. Eng. Technol. 2011;34:727–736. doi: 10.1002/ceat.201000441. [DOI] [Google Scholar]
- 180.Jiang X., Ellis N. Upgrading Bio-oil through Emulsification with Biodiesel: Mixture Production. Energy Fuels. 2010;24:1358–1364. doi: 10.1021/ef9010669. [DOI] [Google Scholar]
- 181.Xu J., Jiang J., Dai W., Zhang T.J., Xu Y. Bio-Oil Upgrading by Means of Ozone Oxidation and Esterification to Remove Water and to Improve Fuel Characteristics. Energy Fuels. 2011;25:1798–1801. doi: 10.1021/ef101726g. [DOI] [Google Scholar]
- 182.Xu Y., Wang T., Ma L., Zhang Q., Liang W. Upgrading of the liquid fuel from fast pyrolysis of biomass over MoNi/γ-Al2O3 catalysts. Appl. Energy. 2010;87:2886–2891. doi: 10.1016/j.apenergy.2009.10.028. [DOI] [Google Scholar]
- 183.Song M., Zhong Z., Dai J. Different solid acid catalysts influence on properties and chemical composition change of upgrading bio-oil. J. Anal. Appl. Pyrolysis. 2010;89:166–170. doi: 10.1016/j.jaap.2010.07.007. [DOI] [Google Scholar]
- 184.Xiu S., Shahbazi A. Bio-oil production and upgrading research: A review. Renew. Sustain. Energy Rev. 2012;16:4406–4414. doi: 10.1016/j.rser.2012.04.028. [DOI] [Google Scholar]
- 185.Zhang Q., Chang J., Wang T., Xu Y. Review of biomass pyrolysis oil properties and upgrading research. Energy Convers. Manag. 2007;48:87–92. doi: 10.1016/j.enconman.2006.05.010. [DOI] [Google Scholar]
- 186.Peng J., Chen P., Lou H., Zheng X. Upgrading of Bio-oil over Aluminum Silicate in Supercritical Ethanol. Energy Fuels. 2008;22:3489–3492. doi: 10.1021/ef8001789. [DOI] [Google Scholar]
- 187.Tang Z., Zhang Y., Guo Q. Catalytic Hydrocracking of Pyrolytic Lignin to Liquid Fuel in Supercritical Ethanol. Ind. Eng. Chem. Res. 2010;49:2040–2046. doi: 10.1021/ie9015842. [DOI] [Google Scholar]
- 188.Zhang J., Luo Z., Dang Q. Upgrading of Bio-oil over Bifunctional Catalysts in Supercritical Monoalcohols. Energy Fuels. 2011;26:2990–2995. doi: 10.1021/ef201934a. [DOI] [Google Scholar]
- 189.Cui H., Ma C., Li Z., Yi W. Effect of the reactive compounds in bio-oils on esterification of the contained carboxylic acids in supercritical methanol. J. Fuel Chem. Technol. 2011;39:347–354. doi: 10.1016/S1872-5813(11)60025-X. [DOI] [Google Scholar]
- 190.Li W., Pan C., Sheng L. Upgrading of high-boiling fraction of bio-oil in supercritical methanol. Bioresour. Technol. 2011;102:9223–9228. doi: 10.1016/j.biortech.2011.07.071. [DOI] [PubMed] [Google Scholar]
- 191.Cui H., Wang J., Wei S. Upgrading bio-oil by esterification under supercritical CO2 conditions. J. Fuel Chem. Technol. 2010;38:673–678. doi: 10.1016/S1872-5813(11)60003-0. [DOI] [Google Scholar]
- 192.Wang J., Chang J., Fan J. Upgrading of Bio-oil by Catalytic Esterification and Determination of Acid Number for Evaluating Esterification Degree. Energy Fuels. 2010;24:3251–3255. doi: 10.1021/ef1000634. [DOI] [Google Scholar]
- 193.Xu X., Zhang C., Zhai Y. Upgrading of Bio-Oil Using Supercritical 1-Butanol over a Ru/C Heterogeneous Catalyst: Role of the Solvent. Energy Fuels. 2014;28:4611–4621. doi: 10.1021/ef500968a. [DOI] [Google Scholar]
- 194.Duan P., Savage P.E. Upgrading of crude algal bio-oil in supercritical water. Bioresour. Technol. 2011;102:1899–1906. doi: 10.1016/j.biortech.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 195.George W.H., Sara I., Avelino C. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006;106:4044–4098. doi: 10.1021/cr068360d. [DOI] [PubMed] [Google Scholar]
- 196.Mortensen P.M., Grunwaldt J.D., Jensen P.A., Knudsen K.G., Jensen A.D. A review of catalytic upgrading of bio-oil to engine fuels. Appl. Catal. A Gen. 2011;407:1–19. doi: 10.1016/j.apcata.2011.08.046. [DOI] [Google Scholar]
- 197.Li H., Luo H., Zhuang L., Dai W.L., Qiao M.H. Liquid phase hydrogenation of furfural to furfuryl alcohol over the Fe-promoted Ni-B amorphous alloy catalysts. J. Mol. Catal. A-Chem. 2003;203:267–275. doi: 10.1016/S1381-1169(03)00368-6. [DOI] [Google Scholar]
- 198.Huang Y., Xia S., Ma P. Effect of zeolite solid acids on the in situ hydrogenation of bio-derived phenol. Catal. Commun. 2017;89:111–116. doi: 10.1016/j.catcom.2016.11.002. [DOI] [Google Scholar]
- 199.Wang F., Zhang Z. Catalytic Transfer Hydrogenation of Furfural into Furfuryl Alcohol over Magnetic γ-Fe2O3@HAP Catalyst. ACS Sustain. Chem. Eng. 2016;5:942–947. doi: 10.1021/acssuschemeng.6b02272. [DOI] [Google Scholar]
- 200.Zhang X., Wang T., Ma L., Zhang Q., Jiang T. Hydrotreatment of bio-oil over Ni-based catalyst. Bioresour. Technol. 2013;127:306–311. doi: 10.1016/j.biortech.2012.07.119. [DOI] [PubMed] [Google Scholar]
- 201.Tang Y., Yu W., Mo L., Lou H., Zheng X.M. One-Step Hydrogenation−Esterification of Aldehyde and Acid to Ester over Bifunctional Pt Catalysts: A Model Reaction as Novel Route for Catalytic Upgrading of Fast Pyrolysis Bio-Oil. Energy Fuels. 2008;22:3484–3488. doi: 10.1021/ef800148q. [DOI] [Google Scholar]
- 202.Yu W., Tang Y., Mo L., Chen P., Lou H., Zheng X.M. Bifunctional Pd/Al-SBA-15 catalyzed one-step hydrogenation–esterification of furfural and acetic acid: A model reaction for catalytic upgrading of bio-oil. Catal. Commun. 2011;13:35–39. doi: 10.1016/j.catcom.2011.06.004. [DOI] [Google Scholar]
- 203.Yu W., Tang Y., Mo L., Chen P., Lou H., Zheng X.M. One-step hydrogenation–esterification of furfural and acetic acid over bifunctional Pd catalysts for bio-oil upgrading. Bioresour. Technol. 2011;102:8241–8246. doi: 10.1016/j.biortech.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 204.Tang Y., Miao S., Shanks B.H., Zheng X.M. Bifunctional mesoporous organic–inorganic hybrid silica for combined one-step hydrogenation/esterification. Appl. Catal. A Gen. 2010;375:310–317. doi: 10.1016/j.apcata.2010.01.015. [DOI] [Google Scholar]
- 205.Tang Y., Miao S., Pham H.N., Datye A. Enhancement of Pt catalytic activity in the hydrogenation of aldehydes. Appl. Catal. A Gen. 2011;406:81–88. doi: 10.1016/j.apcata.2011.08.011. [DOI] [Google Scholar]
- 206.Jeon S., Park Y.M., Park J., Saravanan K., Jeong H.K., Bae J.W. Synergistic effects of Nb2O5 promoter on Ru/Al2O3 for an aqueous-phase hydrodeoxygenation of glycerol to hydrocarbons. Appl. Catal. A Gen. 2018;551:49–62. doi: 10.1016/j.apcata.2017.12.006. [DOI] [Google Scholar]
- 207.Wang Y., Fang Y., He T., Hu H.Q., Wu J.H. Hydrodeoxygenation of dibenzofuran over noble metal supported on mesoporous zeolite. Catal. Commun. 2011;12:1201–1205. doi: 10.1016/j.catcom.2011.04.010. [DOI] [Google Scholar]
- 208.Wang W., Yang Y., Luo H., Hu T., Liu W.Y. Amorphous Co–Mo–B catalyst with high activity for the hydrodeoxygenation of bio-oil. Catal. Commun. 2011;12:436–440. doi: 10.1016/j.catcom.2010.11.001. [DOI] [Google Scholar]
- 209.Wang W., Yang Y., Luo H., Hu T., Liu W.Y. Preparation and hydrodeoxygenation properties of Co–Mo–O–B amorphous catalyst. React. Kinet. Mech. Catal. 2011;102:207–217. doi: 10.1007/s11144-010-0253-4. [DOI] [Google Scholar]
- 210.Wang S., Guo Z., Cai Q., Guo L. Catalytic conversion of carboxylic acids in bio-oil for liquid hydrocarbons production. Biomass Bioenergy. 2012;45:138–143. doi: 10.1016/j.biombioe.2012.05.023. [DOI] [Google Scholar]
- 211.Xu X., Jiang E., Wang M., Li B. Rich hydrogen production from crude gas secondary catalytic cracking over Fe/γ-Al2O3. Renew. Energy. 2012;39:126–131. [Google Scholar]
- 212.Butler E., Devlin G., Meier D., Mcdonnel K. A review of recent laboratory research and commercial developments in fast pyrolysis and upgrading. Renew. Sustain. Energy Rev. 2011;15:4171–4186. doi: 10.1016/j.rser.2011.07.035. [DOI] [Google Scholar]
- 213.Hu M., Laghari M., Cui B., Xiao B., Zhang B.P., Guo D.B. Catalytic cracking of biomass tar over char supported nickel catalyst. Energy. 2018;145:228–237. doi: 10.1016/j.energy.2017.12.096. [DOI] [Google Scholar]
- 214.Lu Q., Guo H., Zhou M., Cui M., Dong C., Yang Y.P. Selective preparation of monocyclic aromatic hydrocarbons from catalytic cracking of biomass fast pyrolysis vapors over Mo2N/HZSM-5 catalyst. Fuel Process. Technol. 2018;173:134–142. doi: 10.1016/j.fuproc.2018.01.017. [DOI] [Google Scholar]
- 215.Lu Q., Guo H., Zhou M., Cui M., Dong C., Yang Y.P. Monocyclic aromatic hydrocarbons production from catalytic cracking of pine wood-derived pyrolytic vapors over Ce-Mo2N/HZSM-5 catalyst. Sci. Total Environ. 2018;634:141–149. doi: 10.1016/j.scitotenv.2018.03.351. [DOI] [PubMed] [Google Scholar]
- 216.Wu C., Huang Q., Sui M., Yan Y., Wang F. Hydrogen production via catalytic steam reforming of fast pyrolysis bio-oil in a two-stage fixed bed reactor system. Fuel Process. Technol. 2008;89:1306–1316. doi: 10.1016/j.fuproc.2008.05.018. [DOI] [Google Scholar]
- 217.Lan P., Xu Q., Zhou M., Lan L., Zhang S., Yan Y. Catalytic Steam Reforming of Fast Pyrolysis Bio-Oil in Fixed Bed and Fluidized Bed Reactors. Chem. Eng. Technol. 2010;33:2021–2028. doi: 10.1002/ceat.201000169. [DOI] [Google Scholar]
- 218.Wang Z., Pan Y., Dong T., Zhu X.F., Kan T., Yuan L.X., Torimoto Y., Sadakata M., Li Q.X. Production of hydrogen from catalytic steam reforming of bio-oil using C12A7-O-based catalysts. Appl. Catal. A Gen. 2007;320:24–34. doi: 10.1016/j.apcata.2006.12.003. [DOI] [Google Scholar]
- 219.Zhang Y., Li W., Zhang S., Xu Q., Yan Y. Steam Reforming of Bio-Oil for Hydrogen Production: Effect of Ni-Co Bimetallic Catalysts. Chem. Eng. Technol. 2012;35:302–308. doi: 10.1002/ceat.201100301. [DOI] [Google Scholar]
- 220.Kan T., Xiong J., Li X. High efficient production of hydrogen from crude bio-oil via an integrative process between gasification and current-enhanced catalytic steam reforming. Int. J. Hydrogen Energy. 2010;35:518–532. doi: 10.1016/j.ijhydene.2009.11.010. [DOI] [Google Scholar]
- 221.Chen T., Wu C., Liu R. Steam reforming of bio-oil from rice husks fast pyrolysis for hydrogen production. Bioresour. Technol. 2011;102:9236–9240. doi: 10.1016/j.biortech.2011.07.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.