Abstract
Iron dyshomeostasis is a feature of Alzheimer’s disease (AD). The impact of iron on AD is attributed to its interactions with the central proteins of AD pathology (amyloid precursor protein and tau) and/or through the iron-mediated generation of prooxidant molecules (e.g., hydroxyl radicals). However, the source of iron accumulation in pathologically relevant regions of the brain and its contribution to AD remains unclear. One likely contributor to iron accumulation is the age-associated increase in tissue-resident senescent cells that drive inflammation and contribute to various pathologies associated with advanced age. Iron accumulation predisposes ageing tissue to oxidative stress that can lead to cellular dysfunction and to iron-dependent cell death modalities (e.g., ferroptosis). Further, elevated brain iron is associated with the progression of AD and cognitive decline. Elevated brain iron presents a feature of AD that may be modified pharmacologically to mitigate the effects of age/senescence-associated iron dyshomeostasis and improve disease outcome.
Keywords: Alzheimer’s disease, iron homeostasis, ferroptosis, senescence, chelators
1. Introduction
Alzheimer’s disease (AD) is the most common type of dementia. Pathological hallmarks of AD are the accumulation of extracellular amyloid plaques seeded by aggregated amyloid beta peptide (Aβ) and intracellular neurofibrillary tangles (NFTs) composed of hyper-phosphorylated microtubule-associated protein tau. The accumulation of Aβ is considered a toxic component of pathology and has been a primary target of clinical strategies [1]. However, strategies that have focused on reducing Aβ burden, including those that have demonstrated the lowering of plaque burden to normal levels, have not been successful in slowing cognitive decline in AD patients [1,2,3]. A careful re-examination of the factors that may lead to AD and contribute to cognitive decline may facilitate the formulation of new therapeutic strategies to prevent or arrest disease processes. Homeostatic regulation of iron is one such pathway amenable to therapeutic targeting, and it has been observed to be perturbed in several neurodegenerative disorders in addition to AD [4].
Iron is essential for life processes and cellular functions. These include essential “housekeeping” functions such as cellular respiration, DNA synthesis, and cell division, as well as specialized cellular functions such as oxygen transport and neurotransmission [4,5,6]. The ability of iron to cycle through its oxidation states is fundamental to its biological utility but can lead to oxidative damage of biomolecules resulting in cellular dysfunction [4,5,6]. This has led to the evolution of tightly regulated homeostatic mechanisms to ensure iron availability and mitigate toxicity [4,5,6]. However, the brain accumulates iron with age and several neurodegenerative conditions are associated with increased iron levels in affected regions of the brain [4,5].
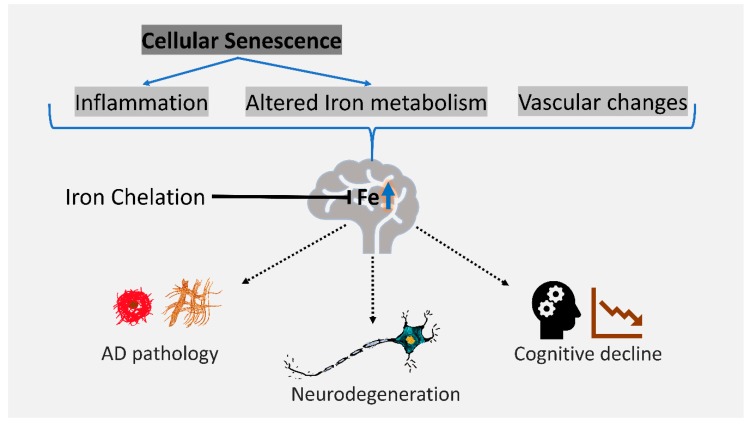
The cause of age-associated iron accumulation in brain regions relevant to AD and its impact on disease are relevant questions to determine the utility of brain iron redistribution as a therapeutic strategy for AD. In this review we explore the contribution of iron to AD and describe the potential contribution of the proinflammatory senescence program to brain iron accumulation (Figure 1). Notably, while iron accumulation in AD may not be sufficiently high to result in iron toxicity [6], iron dyshomeostasis and elevated iron predisposes and enhances the susceptibility of brain tissue to oxidative dysfunction (e.g., lower glutathione, increased lipid peroxidation, and increased reactive oxygen species) and accelerates cell death modalities such as ferroptosis (reviewed in [4]). Finally, we describe the therapeutic opportunities that may be explored to alleviate AD through pharmacological chelation of iron in the brain.
Figure 1.
Cellular senescence is a potential contributor to the age-associated accumulation of brain iron. Factors that influence brain iron with age include inflammation, altered vasculature, and altered metabolism. Elevated brain iron is associated with Alzheimer’s disease (AD) pathology, cognitive decline, and may lead to neuron loss via iron-dependent oxidative cell death such as ferroptosis. Iron chelation may mitigate some of these effects and alleviate AD progression.
2. Iron Dyshomeostasis is Associated with AD
In the brain, iron accumulation is observed in regions affected by AD such as the parietal cortex, motor cortex, and hippocampus [7,8,9,10,11,12,13,14,15]. The intensity of iron accumulation, observed by histology, in the frontal cortex is different between the subtypes of AD. This can be used to distinguish between sporadic (late onset) and familial (early onset) AD [16] and reflects disease severity [17,18]. Overall, patients with familial AD are affected more in magnetic resonance imaging (MRI) scores compared to patients with sporadic AD, which may reflect higher iron accumulation in familial AD [16]. Further, the forms of iron observed in AD patients vary in their magnetic moment (measured via superconducting quantum interference device magnetometry) when compared with those from age- and gender-matched controls [19]. Magnetic moment is the property of a substance that determines the torque it experiences when influenced by an external magnetic field. Thus, change in magnetic moment reflects a change in the molecular state of iron and indicates possible dysregulation of iron homeostasis [19]. For example, a higher magnetic moment in AD brain tissue vs. control tissue in the absence of changes in the concentration of magnetite (an oxide of iron that can be magnetized) may indicate the larger size of magnetite particles in AD brains. This in turn suggests a dysfunction of iron storage in ferritin (iron storage protein) and/or accumulation of Aβ in AD tissue [19]. Further, iron levels measured post-mortem are elevated in the inferior temporal cortex only in patients diagnosed with AD during their lives, with AD pathology confirmed post-mortem [20].
Iron is associated with the pathological lesions of AD [17,21,22,23,24,25,26,27,28,29]. Some studies implicate iron as a direct contributor to AD pathology by promoting the aggregation and oligomerization of Aβ peptides [17,26,28,29,30,31,32,33]. Levels of iron and ferritin (iron storage protein) in brain tissue are associated with the amount of amyloid deposition [17,34,35]. Iron is accumulated in amyloid plaques as a mineralized magnetite species in mouse and human models of amyloid deposition [26,27,28,29]. Aβ may enhance iron mineralization as Aβ peptides can lead to the production of iron-mineral nanoparticles in vitro [36]. It is hypothesized that the oxidative damage associated with the aggregation of Aβ is due to redox active metals (e.g., iron and copper) to which it binds, leading to the production of oxidants such as hydrogen peroxide [37,38].
Iron may impact the production of Aβ by enhancing the translation and amyloidogenic processing of amyloid precursor protein (APP) [39,40,41,42,43,44,45]. APP, when processed through a non-amyloidogenic pathway, is cleaved by α-secretase followed by cleavage by γ-secretase. Iron availability may perturb this process through the aberrant binding of iron response proteins to putative iron response elements on APP mRNA [43,44,45]. Additionally, iron can mediate tau phosphorylation and aggregation [46,47,48]. These events can be mitigated by the chelation of iron [49]. Tau accumulation in NFTs is associated with an induction of heme oxygenase-1 which can exacerbate oxidative stress through the release of iron by the breakdown of heme [45,50,51,52].
Brain iron and ferritin are associated with cognitive loss in AD. Iron was strongly associated with the rate of cognitive decline 12 years prior to death in the subjects from the Memory and Ageing Project (n = 209) [20]. While the direct measurement of brain iron is challenging, ferritin levels in cerebrospinal fluid can be used as a proximate reporter of brain iron load. While brain iron levels are reflected in CSF (cerebrospinal fluid) ferritin, they may also be impacted by the inflammation status of the brain. Regardless, CSF ferritin can predict cognitive decline and the transition from mild cognitive impairment to AD [53]. Ferritin in the CSF predicts the rate of decline in brain metabolism (proximate indicator of neurodegeneration), as measured by fluorodeoxyglucose positron emission tomography (FDG-PET) in subjects with high amyloid pathology (high CSF t-tau/Aβ42 ratio) but not in subjects with low amyloid pathology [54]. Another longitudinal study conducted over six years determined that elevated magnetic susceptibility in the hippocampus, determined by an MRI technique called quantitative susceptibility mapping (QSM), is a strong predictor for an accelerated rate of cognitive decline in amyloid positive subjects [55]. QSM may also be used to longitudinally and non-invasively monitor amyloid accumulation and iron deposition, and may serve as a diagnostic aid for AD [35]. Taken together, these studies suggest that iron is important for cognitive deterioration when there is underlying pathology.
3. Cellular Senescence is Associated with AD and Iron Dyshomeostasis
Cellular senescence is a proinflammatory cell fate associated with several age-related disorders, including AD [56,57,58]. The senescence phenotype is classically described in cultured cells undergoing terminal replicative arrest [59] that display enlarged cell morphology and characteristic alterations of their chromatin, secretory profile (senescence-associated secretory phenotype (SASP)), and cell cycle regulatory proteins (cyclins and cyclin-dependent kinases) [60]. Senescence in culture is typically induced through sub-culturing to replicative exhaustion, inducing DNA damage (through oxidative stress, ionizing radiation, or pharmacological agents), or aberrant oncogenic activation (e.g., overexpression of HrasV12) [60]. The occurrence of senescent cells in tissue is now widely accepted [61,62,63,64,65] and several functions have been ascribed to them, including involvement in wound healing [66,67], tissue repair [68], and embryonic development (developmental senescence) [69,70]. Despite these beneficial roles, the persistent accumulation of senescence in tissue with age is associated with age-associated pathologies and functional decline. The clearance of senescent cells from tissue in mice can alleviate pathologies related to ageing [56,57,58,71,72,73,74,75,76,77,78,79,80,81]. While the overall burden of senescent cells in ageing tissue is low, these cells persistently propagate degenerative and proinflammatory conditions in their microenvironment [56,57,58,71,72,73,74,75,76,77,78,79,80,81,82,83]. In the brain the senescence program can be triggered in astrocytes and microglia, with recent evidence suggesting that even neurons may display a senescent signature despite being post-mitotic. A more flexible definition of senescence based on the outcomes of senescent cell phenotypes (e.g., chronic inflammation) may be relevant to explain age-related pathologies in vivo. As the molecular changes associated with normal ageing that promote AD are yet to be fully defined, the impact of cellular senescence on age-related neuroinflammation, decline in cognition, and AD is currently unknown [75].
Evidence for the induction of senescence in cells of the brain and its links with neurodegenerative disorders is steadily increasing. In cell culture, astrocytes exposed to hydrogen peroxide (oxidative stress) or irradiation display markers of senescence, such as senescence-associated βgalactosidase staining (SAβgal) (visualized by enzymatic activity assay for lysosomal βgalactosidase at pH 6.0) and elevated expression levels of p16INK4A and p21 (measured via quantitative PCR for the increase in transcript levels or Western blotting to reflect changes in protein abundance), and develop a proinflammatory secretory phenotype similar to the SASP observed in senescent fibroblasts [84,85,86]. Similarly, irradiated neuronal cells show features of senescence (SAβgal positivity) and become susceptible to the senolytic pharmacological cocktail of dasatinib (kinase inhibitor anti-cancer drug) and quercetin (plant-derived flavonoid) [58]. In human brain tissue an increase in the burden of p16INK4A-expressing astrocytes is observed with age [87]. Cellular senescence in neuronal progenitor cells is implicated in the reduced remyelination observed in progressive multiple sclerosis [88]. In mouse models, senescence in the brain has been observed in response to physiological (e.g., obesity-induced [89]) and physical (e.g., traumatic brain injury following controlled cortical impact [90]) stressors.
In the context of AD, astrocytes expressing p16INK4A are enriched in the frontal cortex of AD patients compared to age-matched non-AD adults [87]. Further, oligodendrocyte progenitor cells displaying a senescent phenotype (high p21 expression) are associated with amyloid plaques in the brains of human AD patients [58]. Recently, studies using mouse models that overexpress mutant tau and phenocopy aspects of AD have indicated a strong link between the induction of cellular senescence and the appearance of AD pathology [56,57]. In particular, the ablation of senescent cells from the tissue of these mice led to a reduction in the phosphorylation and aggregation of tau [56,57]. In another mouse model of AD (APP/PS1), senolytic treatment, using a combination of dasatinib and quercetin that reduced the burden of senescent cells associated with amyloid plaque, lowered Aβ load, reduced neuroinflammation, and reduced cognitive defects [58]. Interestingly, Aβ can induce senescence in cultured oligodendrocyte progenitor cells [58] and drive SASP in cultured epithelial cells and fibroblasts via CD36 [91]. This may indicate that AD pathologies can further sustain/enhance tissue-resident senescence burden. Taken together, these studies suggest that senescence induction occurs in the brain and is associated with AD.
While the clearance of senescent cells in tissues of mice has now been demonstrated to mitigate age-associated pathologies, including AD, the impact of removing them in tissues of longer-living mammals, including humans, is yet to be determined. A small (n = 14) first in-human clinical trial of the senolytic cocktail of dasatinib and quercetin in idiopathic pulmonary fibrosis (IPF), a fatal cellular senescence-associated disease [76], provided no conclusive evidence of senescence clearance or reduction in SASP despite achieving its primary endpoints (retention and completion rate; both 100%) and some benefit in the physical function of patients [92]. Considering the known functional benefits of senescence in wound healing and tissue repair, as well as other potential benefits that may be currently unknown, the life-long clearance of senescent cells in humans may be deleterious. Further, therapeutic strategies based on the complete clearance of senescent cells may be difficult to administer in disorders like AD where the disease develops over several decades. An alternative strategy is to target specific phenotypic features of senescent cells that are relevant in certain disease settings. Iron accumulation is one such feature that is observed in senescent cells and is of relevance in AD [82].
Senescent cells in vitro display aberrant iron homeostasis [82], and there are some indications that their abundance influences iron levels in ageing tissue [82,93,94]. Senescent cells display elevated iron and a concomitant increase in ferritin and markers of oxidative stress in vitro [82,93,94,95]. Iron is known to promote the induction of senescence in cultured microglia [96]. Iron chelators such as deferoxamine and deferiprone can reduce and prevent the accumulation of iron and ferritin observed in cellular senescence in vitro [82]. The chelation of iron in Caenorhabditis elegans, a model organism of ageing that displays iron dysfunction in senescent intestinal cells [97], leads to a reduction in iron-dependent oxidation and cell death [98]. In the context of brain tissue, SASP may drive ferritin expression in neurons and glia as an acute phase response which may enhance their susceptibility to the iron-mediated cell death process, ferroptosis. The effects of iron chelation on senescence-associated iron accumulation and its impact on SASP or ferroptotic vulnerability are yet to be explored in vivo and present a therapeutic opportunity to treat AD (Figure 1).
4. Iron as a Therapeutic Target in AD
Iron neurochemistry as a modifiable feature to treat AD has generated renewed interest since clinical trials for iron chelators have shown promise recently in other neurodegenerative disorders such as Parkinson’s disease (PD) and motor neuron disease (MND) [6,99,100,101,102,103,104]. The efforts towards the “iron hypothesis” of AD have also been bolstered by the unravelling of complex molecular crosstalk between iron regulatory proteins and the suspected players of AD pathology.
Historically, the first study that explored iron chelation against AD was published in 1991, which tested the effectiveness of intramuscular application of the iron chelator deferoxamine in 48 patients over two years [105]. The study showed that low-dose administration of this iron chelator slowed the clinical progression of dementia associated with AD compared with controls. A decade later, a pilot phase 2 clinical trial in patients with moderately severe AD using clioquinol (PBT1), a drug inhibiting zinc and copper ions from binding to Aβ, was conducted on 36 patients [106]. A positive clinical effect, corresponding to a reduction in the rate of cognitive decline, was seen in the more severely affected patients. Moreover, a biological effect corresponding to a decline in plasma Aβ42 levels was observed. Currently, the safety and efficacy of deferiprone, an iron chelator that passes the blood–brain barrier, is under evaluation in a phase 2 randomized placebo-controlled clinical trial in participants with prodromal AD and mild AD (NCT03234686).
5. Conclusions
Despite the ever-increasing socioeconomic burden of AD, there is frustratingly no disease-modifying treatment for this affliction. The historic and emerging evidence that iron contributes to the clinical progression of AD should not be ignored as a potential avenue for therapy development. The reported benefits for iron chelation in other neurodegenerative diseases such as PD and MND should open the possibility of pharmacological manipulation of brain iron as an alternative therapeutic approach for AD. The perturbed iron homeostasis observed in senescent cells presents a possible cell-specific target of iron chelation therapy which may enhance the efficacy of approaches aimed at lowering age/pathology-related iron accumulation. Further, the strategy to modify specific phenotypic features (e.g., reducing iron accumulation using chelators such as deferiprone) of senescent cells that contribute to pathology may be of benefit in the human context where the complete ablation of senescent cells from tissue may be difficult or even deleterious due to a loss of beneficial effects associated with senescence (e.g., tissue repair and wound healing). The consequence of cellular senescence on the iron homeostasis of the brain requires further characterization. This may open additional avenues for the development of new classes of drugs that may provide benefits for AD patients and provide strategies for halting their decline and delaying the onset of neurodegeneration.
Acknowledgments
The Florey Institute of Neuroscience and Mental Health acknowledges support from the Victorian Government, particularly funding from the Operational Infrastructure Support Grant.
Author Contributions
S.M. produced the original draft and figure. A.A.B., S.A. and A.I.B. reviewed and edited the manuscript and provided critical feedback.
Funding
Supported by funds from the Australian National Health and Medical Research Council (NHMRC: GNT1159403). Support from the Alzheimer’s Association (AARFD-16-442821 to AAB) is gratefully acknowledged. A.I.B. is supported by funds from the National Health and Medical Research Council of Australia (GNT1103703, GNT1101533).
Conflicts of Interest
A.I.B. is a shareholder in Prana Biotechnology Ltd., Cogstate Ltd., Brighton Biotech LLC, Grunbiotics Pty Ltd., Eucalyptus Pty Ltd., and Mesoblast Ltd. He is a paid consultant for, and has a profit share interest in, Collaborative Medicinal Development Pty Ltd.
References
- 1.Nikseresht S., Bush A.I., Ayton S. Treating Alzheimer’s disease by targeting iron. Br. J. Pharmacol. 2019 doi: 10.1111/bph.14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alborzinia H., Ignashkova T.I., Dejure F.R., Gendarme M., Theobald J., Wölfl S., Lindemann R.K., Reiling J.H. Golgi stress mediates redox imbalance and ferroptosis in human cells. Commun. Biol. 2018;1:210. doi: 10.1038/s42003-018-0212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biogen and Eisai to Discontinue Phase 3 Engage and Emerge Trials of Aducanumab in Alzheimer’s Disease. [(accessed on 24 April 2019)]; Available online: http://investors.Biogen.Com/news-releases/news-release-details/biogen-and-eisai-discontinue-phase-3-engage-and-emerge-trials.
- 4.Masaldan S., Bush A.I., Devos D., Rolland A.S., Moreau C. Striking while the iron is hot: Iron metabolism and ferroptosis in neurodegeneration. Free Radic. Biol. Med. 2018;133:221–233. doi: 10.1016/j.freeradbiomed.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 5.Belaidi A.A., Bush A.I. Iron neurochemistry in Alzheimer’s disease and Parkinson’s disease: Targets for therapeutics. J. Neurochem. 2016;139(Suppl. 1):179–197. doi: 10.1111/jnc.13425. [DOI] [PubMed] [Google Scholar]
- 6.Eid R., Arab N.T.T., Greenwood M.T. Iron mediated toxicity and programmed cell death: A review and a re-examination of existing paradigms. Biochim. Biophys. Acta. 2017;1864:399–430. doi: 10.1016/j.bbamcr.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Bartzokis G., Sultzer D., Mintz J., Holt L.E., Marx P., Phelan C.K., Marder S.R. In vivo evaluation of brain iron in Alzheimer’s disease and normal subjects using MRI. Biol. Psychiatry. 1994;35:480–487. doi: 10.1016/0006-3223(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 8.Bartzokis G., Tishler T.A. MRI evaluation of basal ganglia ferritin iron and neurotoxicity in Alzheimer’s and Huntingon’s disease. Cell. Mol. Biol. 2000;46:821–833. [PubMed] [Google Scholar]
- 9.Ding B., Chen K.M., Ling H.W., Sun F., Li X., Wan T., Chai W.M., Zhang H., Zhan Y., Guan Y.J. Correlation of iron in the hippocampus with MMSE in patients with Alzheimer’s disease. J. Magn. Reson. Imaging. 2009;29:793–798. doi: 10.1002/jmri.21730. [DOI] [PubMed] [Google Scholar]
- 10.Pfefferbaum A., Adalsteinsson E., Rohlfing T., Sullivan E.V. MRI estimates of brain iron concentration in normal aging: Comparison of field-dependent (FDRI) and phase (SWI) methods. NeuroImage. 2009;47:493–500. doi: 10.1016/j.neuroimage.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bilgic B., Pfefferbaum A., Rohlfing T., Sullivan E.V., Adalsteinsson E. MRI estimates of brain iron concentration in normal aging using quantitative susceptibility mapping. NeuroImage. 2012;59:2625–2635. doi: 10.1016/j.neuroimage.2011.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo Z., Zhuang X., Kumar D., Wu X., Yue C., Han C., Lv J. The correlation of hippocampal T2-mapping with neuropsychology test in patients with Alzheimer’s disease. PLoS ONE. 2013;8:e76203. doi: 10.1371/journal.pone.0076203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langkammer C., Ropele S., Pirpamer L., Fazekas F., Schmidt R. MRI for iron mapping in Alzheimer’s disease. Neuro Degener. Dis. 2014;13:189–191. doi: 10.1159/000353756. [DOI] [PubMed] [Google Scholar]
- 14.Tao Y., Wang Y., Rogers J.T., Wang F. Perturbed iron distribution in Alzheimer’s disease serum, cerebrospinal fluid, and selected brain regions: A systematic review and meta-analysis. J. Alzheimer’s Dis. 2014;42:679–690. doi: 10.3233/JAD-140396. [DOI] [PubMed] [Google Scholar]
- 15.Ghadery C., Pirpamer L., Hofer E., Langkammer C., Petrovic K., Loitfelder M., Schwingenschuh P., Seiler S., Duering M., Jouvent E., et al. R2* mapping for brain iron: Associations with cognition in normal aging. Neurobiol. Aging. 2015;36:925–932. doi: 10.1016/j.neurobiolaging.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Bulk M., Abdelmoula W.M., Nabuurs R.J.A., van der Graaf L.M., Mulders C.W.H., Mulder A.A., Jost C.R., Koster A.J., van Buchem M.A., Natte R., et al. Postmortem MRI and histology demonstrate differential iron accumulation and cortical myelin organization in early- and late-onset Alzheimer’s disease. Neurobiol. Aging. 2018;62:231–242. doi: 10.1016/j.neurobiolaging.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Bulk M., Kenkhuis B., van der Graaf L.M., Goeman J.J., Natte R., van der Weerd L. Postmortem t2*- weighted MRI imaging of cortical iron reflects severity of Alzheimer’s disease. J. Alzheimer’s Dis. 2018;65:1125–1137. doi: 10.3233/JAD-180317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Duijn S., Bulk M., van Duinen S.G., Nabuurs R.J.A., van Buchem M.A., van der Weerd L., Natte R. Cortical iron reflects severity of Alzheimer’s disease. J. Alzheimer’s Dis. 2017;60:1533–1545. doi: 10.3233/JAD-161143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bulk M., van der Weerd L., Breimer W., Lebedev N., Webb A., Goeman J.J., Ward R.J., Huber M., Oosterkamp T.H., Bossoni L. Quantitative comparison of different iron forms in the temporal cortex of Alzheimer patients and control subjects. Sci. Rep. 2018;8:6898. doi: 10.1038/s41598-018-25021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayton S., Wang Y., Diouf I., Schneider J.A., Brockman J., Morris M.C., Bush A.I. Brain iron is associated with accelerated cognitive decline in people with Alzheimer pathology. Mol. Psychiatry. 2019;24 doi: 10.1038/s41380-019-0375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodman L. Alzheimer’s disease: A clinico-pathologic analysis of twenty-three cases with a theory on pathogenesis. J. Nerv. Ment. Dis. 1953;118:97–130. doi: 10.1097/00005053-195308000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Smith M.A., Harris P.L., Sayre L.M., Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc. Natl. Acad. Sci. USA. 1997;94:9866–9868. doi: 10.1073/pnas.94.18.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith C.D., Chebrolu H., Wekstein D.R., Schmitt F.A., Jicha G.A., Cooper G., Markesbery W.R. Brain structural alterations before mild cognitive impairment. Neurology. 2007;68:1268–1273. doi: 10.1212/01.wnl.0000259542.54830.34. [DOI] [PubMed] [Google Scholar]
- 24.Lovell M.A., Robertson J.D., Teesdale W.J., Campbell J.L., Markesbery W.R. Copper, iron and zinc in Alzheimer’s disease senile plaques. J. Neurol. Sci. 1998;158:47–52. doi: 10.1016/S0022-510X(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 25.Connor J.R., Snyder B.S., Beard J.L., Fine R.E., Mufson E.J. Regional distribution of iron and iron-regulatory proteins in the brain in aging and Alzheimer’s disease. J. Neurosci. Res. 1992;31:327–335. doi: 10.1002/jnr.490310214. [DOI] [PubMed] [Google Scholar]
- 26.Meadowcroft M.D., Peters D.G., Dewal R.P., Connor J.R., Yang Q.X. The effect of iron in MRI and transverse relaxation of amyloid-beta plaques in Alzheimer’s disease. NMR Biomed. 2015;28:297–305. doi: 10.1002/nbm.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Everett J., Collingwood J.F., Tjendana-Tjhin V., Brooks J., Lermyte F., Plascencia-Villa G., Hands-Portman I., Dobson J., Perry G., Telling N.D. Nanoscale synchrotron x-ray speciation of iron and calcium compounds in amyloid plaque cores from Alzheimer’s disease subjects. Nanoscale. 2018;10:11782–11796. doi: 10.1039/C7NR06794A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plascencia-Villa G., Ponce A., Collingwood J.F., Arellano-Jimenez M.J., Zhu X., Rogers J.T., Betancourt I., Jose-Yacaman M., Perry G. High-resolution analytical imaging and electron holography of magnetite particles in amyloid cores of Alzheimer’s disease. Sci. Rep. 2016;6:24873. doi: 10.1038/srep24873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Telling N.D., Everett J., Collingwood J.F., Dobson J., van der Laan G., Gallagher J.J., Wang J., Hitchcock A.P. Iron biochemistry is correlated with amyloid plaque morphology in an established mouse model of Alzheimer’s disease. Cell Chem. Biol. 2017;24:1205–1215. doi: 10.1016/j.chembiol.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Mantyh P.W., Ghilardi J.R., Rogers S., DeMaster E., Allen C.J., Stimson E.R., Maggio J.E. Aluminum, iron, and zinc ions promote aggregation of physiological concentrations of beta-amyloid peptide. J. Neurochem. 1993;61:1171–1174. doi: 10.1111/j.1471-4159.1993.tb03639.x. [DOI] [PubMed] [Google Scholar]
- 31.Schubert D., Chevion M. The role of iron in beta amyloid toxicity. Biochem. Biophys. Res. Commun. 1995;216:702–707. doi: 10.1006/bbrc.1995.2678. [DOI] [PubMed] [Google Scholar]
- 32.Huang X., Atwood C.S., Moir R.D., Hartshorn M.A., Tanzi R.E., Bush A.I. Trace metal contamination initiates the apparent auto-aggregation, amyloidosis, and oligomerization of Alzheimer’s Abeta peptides. J. Biol. Inorg. Chem. 2004;9:954–960. doi: 10.1007/s00775-004-0602-8. [DOI] [PubMed] [Google Scholar]
- 33.Liu B., Moloney A., Meehan S., Morris K., Thomas S.E., Serpell L.C., Hider R., Marciniak S.J., Lomas D.A., Crowther D.C. Iron promotes the toxicity of amyloid beta peptide by impeding its ordered aggregation. J. Biol. Chem. 2011;286:4248–4256. doi: 10.1074/jbc.M110.158980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwiatek-Majkusiak J., Dickson D.W., Tacik P., Aoki N., Tomasiuk R., Koziorowski D., Friedman A. Relationships between typical histopathological hallmarks and the ferritin in the hippocampus from patients with Alzheimer’s disease. Acta Neurobiol. Exp. 2015;75:391–398. [PubMed] [Google Scholar]
- 35.Gong N.J., Dibb R., Bulk M., van der Weerd L., Liu C. Imaging beta amyloid aggregation and iron accumulation in Alzheimer’s disease using quantitative susceptibility mapping MRI. NeuroImage. 2019;191:176–185. doi: 10.1016/j.neuroimage.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 36.Tahirbegi I.B., Pardo W.A., Alvira M., Mir M., Samitier J. Amyloid Abeta 42, a promoter of magnetite nanoparticle formation in Alzheimer’s disease. Nanotechnology. 2016;27:465102. doi: 10.1088/0957-4484/27/46/465102. [DOI] [PubMed] [Google Scholar]
- 37.Huang X., Cuajungco M.P., Atwood C.S., Hartshorn M.A., Tyndall J.D., Hanson G.R., Stokes K.C., Leopold M., Multhaup G., Goldstein L.E., et al. Cu(ii) potentiation of Alzheimer Abeta neurotoxicity. Correlation with cell-free hydrogen peroxide production and metal reduction. J. Biol. Chem. 1999;274:37111–37116. doi: 10.1074/jbc.274.52.37111. [DOI] [PubMed] [Google Scholar]
- 38.Jomova K., Vondrakova D., Lawson M., Valko M. Metals, oxidative stress and neurodegenerative disorders. Mol. Cell. Biochem. 2010;345:91–104. doi: 10.1007/s11010-010-0563-x. [DOI] [PubMed] [Google Scholar]
- 39.Rogers J.T., Leiter L.M., McPhee J., Cahill C.M., Zhan S.S., Potter H., Nilsson L.N. Translation of the Alzheimer amyloid precursor protein mRNA is up-regulated by interleukin-1 through 5′-untranslated region sequences. J. Biol. Chem. 1999;274:6421–6431. doi: 10.1074/jbc.274.10.6421. [DOI] [PubMed] [Google Scholar]
- 40.Rogers J.T., Randall J.D., Cahill C.M., Eder P.S., Huang X., Gunshin H., Leiter L., McPhee J., Sarang S.S., Utsuki T., et al. An iron-responsive element type ii in the 5′-untranslated region of the Alzheimer’s amyloid precursor protein transcript. J. Biol. Chem. 2002;277:45518–45528. doi: 10.1074/jbc.M207435200. [DOI] [PubMed] [Google Scholar]
- 41.Caldwell J.H., Klevanski M., Saar M., Muller U.C. Roles of the amyloid precursor protein family in the peripheral nervous system. Mech. Dev. 2013;130:433–446. doi: 10.1016/j.mod.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Huang Y.A., Zhou B., Wernig M., Sudhof T.C. Apoe2, apoe3, and apoe4 differentially stimulate app transcription and abeta secretion. Cell. 2017;168:427–441. doi: 10.1016/j.cell.2016.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bodovitz S., Falduto M.T., Frail D.E., Klein W.L. Iron levels modulate alpha-secretase cleavage of amyloid precursor protein. J. Neurochem. 1995;64:307–315. doi: 10.1046/j.1471-4159.1995.64010307.x. [DOI] [PubMed] [Google Scholar]
- 44.Silvestri L., Camaschella C. A potential pathogenetic role of iron in Alzheimer’s disease. J. Cell. Mol. Med. 2008;12:1548–1550. doi: 10.1111/j.1582-4934.2008.00356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ward R.J., Zucca F.A., Duyn J.H., Crichton R.R., Zecca L. The role of iron in brain ageing and neurodegenerative disorders. The Lancet. Neurology. 2014;13:1045–1060. doi: 10.1016/S1474-4422(14)70117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamamoto A., Shin R.W., Hasegawa K., Naiki H., Sato H., Yoshimasu F., Kitamoto T. Iron (iii) induces aggregation of hyperphosphorylated tau and its reduction to iron (ii) reverses the aggregation: Implications in the formation of neurofibrillary tangles of Alzheimer’s disease. J. Neurochem. 2002;82:1137–1147. doi: 10.1046/j.1471-4159.2002.t01-1-01061.x. [DOI] [PubMed] [Google Scholar]
- 47.Lovell M.A., Xiong S., Xie C., Davies P., Markesbery W.R. Induction of hyperphosphorylated tau in primary rat cortical neuron cultures mediated by oxidative stress and glycogen synthase kinase-3. J. Alzheimer’s Dis. 2004;6:659–671. doi: 10.3233/JAD-2004-6610. discussion 673–681. [DOI] [PubMed] [Google Scholar]
- 48.Chan A., Shea T.B. Dietary and genetically-induced oxidative stress alter tau phosphorylation: Influence of folate and apolipoprotein e deficiency. J. Alzheimer’s Dis. 2006;9:399–405. doi: 10.3233/JAD-2006-9405. [DOI] [PubMed] [Google Scholar]
- 49.Amit T., Avramovich-Tirosh Y., Youdim M.B., Mandel S. Targeting multiple Alzheimer’s disease etiologies with multimodal neuroprotective and neurorestorative iron chelators. FASEB J. 2008;22:1296–1305. doi: 10.1096/fj.07-8627rev. [DOI] [PubMed] [Google Scholar]
- 50.Wang D., Hui Y., Peng Y., Tang L., Jin J., He R., Li Y., Zhang S., Li L., Zhou Y., et al. Overexpression of heme oxygenase 1 causes cognitive decline and affects pathways for tauopathy in mice. J. Alzheimer’s Dis. 2015;43:519–534. doi: 10.3233/JAD-140567. [DOI] [PubMed] [Google Scholar]
- 51.Perry G., Nunomura A., Hirai K., Zhu X., Perez M., Avila J., Castellani R.J., Atwood C.S., Aliev G., Sayre L.M., et al. Is oxidative damage the fundamental pathogenic mechanism of Alzheimer’s and other neurodegenerative diseases? Free Radic. Biol. Med. 2002;33:1475–1479. doi: 10.1016/S0891-5849(02)01113-9. [DOI] [PubMed] [Google Scholar]
- 52.Schipper H.M., Bennett D.A., Liberman A., Bienias J.L., Schneider J.A., Kelly J., Arvanitakis Z. Glial heme oxygenase-1 expression in Alzheimer disease and mild cognitive impairment. Neurobiol. Aging. 2006;27:252–261. doi: 10.1016/j.neurobiolaging.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 53.Ayton S., Faux N.G., Bush A.I., Weiner M.W., Aisen P., Petersen R., Jack Jr C.R., Jagust W., Trojanowki J.Q., Toga A.W. Ferritin levels in the cerebrospinal fluid predict Alzheimer’s disease outcomes and are regulated by apoe. Nat. Commun. 2015;6:6760. doi: 10.1038/ncomms7760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diouf I., Fazlollahi A., Bush A.I., Ayton S., Alzheimer’s disease Neuroimaging I. Cerebrospinal fluid ferritin levels predict brain hypometabolism in people with underlying beta-amyloid pathology. Neurobiol. Dis. 2019;124:335–339. doi: 10.1016/j.nbd.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 55.Ayton S., Fazlollahi A., Bourgeat P., Raniga P., Ng A., Lim Y.Y., Diouf I., Farquharson S., Fripp J., Ames D. Cerebral quantitative susceptibility mapping predicts amyloid-β-related cognitive decline. Brain. 2017;140:2112–2119. doi: 10.1093/brain/awx137. [DOI] [PubMed] [Google Scholar]
- 56.Bussian T.J., Aziz A., Meyer C.F., Swenson B.L., van Deursen J.M., Baker D.J. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature. 2018;562:578–582. doi: 10.1038/s41586-018-0543-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Musi N., Valentine J.M., Sickora K.R., Baeuerle E., Thompson C.S., Shen Q., Orr M.E. Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell. 2018;17:e12840. doi: 10.1111/acel.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang P., Kishimoto Y., Grammatikakis I., Gottimukkala K., Cutler R.G., Zhang S., Abdelmohsen K., Bohr V.A., Misra Sen J., Gorospe M., et al. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat. Neurosci. 2019;22:719–728. doi: 10.1038/s41593-019-0372-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 60.Van Deursen J.M. The role of senescent cells in ageing. Nature. 2014;509:439–446. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dimri G.P., Lee X., Basile G., Acosta M., Scott G., Roskelley C., Medrano E.E., Linskens M., Rubelj I., Pereira-Smith O., et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choi J., Shendrik I., Peacocke M., Peehl D., Buttyan R., Ikeguchi E.F., Katz A.E., Benson M.C. Expression of senescence-associated beta-galactosidase in enlarged prostates from men with benign prostatic hyperplasia. Urology. 2000;56:160–166. doi: 10.1016/S0090-4295(00)00538-0. [DOI] [PubMed] [Google Scholar]
- 63.Te Poele R.H., Okorokov A.L., Jardine L., Cummings J., Joel S.P. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res. 2002;62:1876–1883. [PubMed] [Google Scholar]
- 64.Vasile E., Tomita Y., Brown L.F., Kocher O., Dvorak H.F. Differential expression of thymosin beta-10 by early passage and senescent vascular endothelium is modulated by VPF/VEGF: Evidence for senescent endothelial cells in vivo at sites of atherosclerosis. FASEB J. 2001;15:458–466. doi: 10.1096/fj.00-0051com. [DOI] [PubMed] [Google Scholar]
- 65.Minamino T., Miyauchi H., Yoshida T., Ishida Y., Yoshida H., Komuro I. Endothelial cell senescence in human atherosclerosis. Circulation. 2002;105:1541. doi: 10.1161/01.CIR.0000013836.85741.17. [DOI] [PubMed] [Google Scholar]
- 66.Jun J.I., Lau L.F. The matricellular protein ccn1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat. Cell Biol. 2010;12:676–685. doi: 10.1038/ncb2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Demaria M., Ohtani N., Youssef S.A., Rodier F., Toussaint W., Mitchell J.R., Laberge R.M., Vijg J., Van Steeg H., Dolle M.E., et al. An essential role for senescent cells in optimal wound healing through secretion of pdgf-aa. Dev. Cell. 2014;31:722–733. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krizhanovsky V., Yon M., Dickins R.A., Hearn S., Simon J., Miething C., Yee H., Zender L., Lowe S.W. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rajagopalan S., Long E.O. Cellular senescence induced by cd158d reprograms natural killer cells to promote vascular remodeling. Proc. Natl. Acad. Sci. USA. 2012;109:20596–20601. doi: 10.1073/pnas.1208248109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Storer M., Mas A., Robert-Moreno A., Pecoraro M., Ortells M.C., Di Giacomo V., Yosef R., Pilpel N., Krizhanovsky V., Sharpe J., et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell. 2013;155:1119–1130. doi: 10.1016/j.cell.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 71.Baker D.J., Childs B.G., Durik M., Wijers M.E., Sieben C.J., Zhong J., Saltness R.A., Jeganathan K.B., Verzosa G.C., Pezeshki A., et al. Naturally occurring p16(ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530:184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baker D.J., Wijshake T., Tchkonia T., LeBrasseur N.K., Childs B.G., van de Sluis B., Kirkland J.L., van Deursen J.M. Clearance of p16ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yousefzadeh M.J., Zhu Y., McGowan S.J., Angelini L., Fuhrmann-Stroissnigg H., Xu M., Ling Y.Y., Melos K.I., Pirtskhalava T., Inman C.L., et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine. 2018;36:18–28. doi: 10.1016/j.ebiom.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baar M.P., Brandt R.M.C., Putavet D.A., Klein J.D.D., Derks K.W.J., Bourgeois B.R.M., Stryeck S., Rijksen Y., van Willigenburg H., Feijtel D.A., et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell. 2017;169:132–147. doi: 10.1016/j.cell.2017.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baker D.J., Petersen R.C. Cellular senescence in brain aging and neurodegenerative diseases: Evidence and perspectives. J. Clin. Investig. 2018;128:1208–1216. doi: 10.1172/JCI95145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schafer M.J., White T.A., Iijima K., Haak A.J., Ligresti G., Atkinson E.J., Oberg A.L., Birch J., Salmonowicz H., Zhu Y., et al. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 2017;8:14532. doi: 10.1038/ncomms14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ogrodnik M., Miwa S., Tchkonia T., Tiniakos D., Wilson C.L., Lahat A., Day C.P., Burt A., Palmer A., Anstee Q.M., et al. Cellular senescence drives age-dependent hepatic steatosis. Nat. Commun. 2017;8:15691. doi: 10.1038/ncomms15691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chang J., Wang Y., Shao L., Laberge R.M., Demaria M., Campisi J., Janakiraman K., Sharpless N.E., Ding S., Feng W., et al. Clearance of senescent cells by abt263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 2016;22:78–83. doi: 10.1038/nm.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roos C.M., Zhang B., Palmer A.K., Ogrodnik M.B., Pirtskhalava T., Thalji N.M., Hagler M., Jurk D., Smith L.A., Casaclang-Verzosa G., et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016;15:973–977. doi: 10.1111/acel.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu Y., Tchkonia T., Fuhrmann-Stroissnigg H., Dai H.M., Ling Y.Y., Stout M.B., Pirtskhalava T., Giorgadze N., Johnson K.O., Giles C.B., et al. Identification of a novel senolytic agent, navitoclax, targeting the bcl-2 family of anti-apoptotic factors. Aging Cell. 2016;15:428–435. doi: 10.1111/acel.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu Y., Tchkonia T., Pirtskhalava T., Gower A.C., Ding H., Giorgadze N., Palmer A.K., Ikeno Y., Hubbard G.B., Lenburg M., et al. The achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell. 2015;14:644–658. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Masaldan S., Clatworthy S.A.S., Gamell C., Meggyesy P.M., Rigopoulos A.T., Haupt S., Haupt Y., Denoyer D., Adlard P.A., Bush A.I., et al. Iron accumulation in senescent cells is coupled with impaired ferritinophagy and inhibition of ferroptosis. Redox Biol. 2018;14:100–115. doi: 10.1016/j.redox.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saint-Germain E., Mignacca L., Vernier M., Bobbala D., Ilangumaran S., Ferbeyre G. Socs1 regulates senescence and ferroptosis by modulating the expression of p53 target genes. Aging. 2017;9:2137–2162. doi: 10.18632/aging.101306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zou Y., Zhang N., Ellerby L.M., Davalos A.R., Zeng X., Campisi J., Desprez P.Y. Responses of human embryonic stem cells and their differentiated progeny to ionizing radiation. Biochem. Biophys. Res. Commun. 2012;426:100–105. doi: 10.1016/j.bbrc.2012.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pertusa M., Garcia-Matas S., Rodriguez-Farre E., Sanfeliu C., Cristofol R. Astrocytes aged in vitro show a decreased neuroprotective capacity. J. Neurochem. 2007;101:794–805. doi: 10.1111/j.1471-4159.2006.04369.x. [DOI] [PubMed] [Google Scholar]
- 86.Bitto A., Sell C., Crowe E., Lorenzini A., Malaguti M., Hrelia S., Torres C. Stress-induced senescence in human and rodent astrocytes. Exp. Cell Res. 2010;316:2961–2968. doi: 10.1016/j.yexcr.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 87.Bhat R., Crowe E.P., Bitto A., Moh M., Katsetos C.D., Garcia F.U., Johnson F.B., Trojanowski J.Q., Sell C., Torres C. Astrocyte senescence as a component of Alzheimer’s disease. PLoS ONE. 2012;7:e45069. doi: 10.1371/journal.pone.0045069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nicaise A.M., Wagstaff L.J., Willis C.M., Paisie C., Chandok H., Robson P., Fossati V., Williams A., Crocker S.J. Cellular senescence in progenitor cells contributes to diminished remyelination potential in progressive multiple sclerosis. Proc. Natl. Acad. Sci. USA. 2019;116:9030–9039. doi: 10.1073/pnas.1818348116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ogrodnik M., Zhu Y., Langhi L.G.P., Tchkonia T., Kruger P., Fielder E., Victorelli S., Ruswhandi R.A., Giorgadze N., Pirtskhalava T., et al. Obesity-induced cellular senescence drives anxiety and impairs neurogenesis. Cell Metab. 2019;29:1061–1077. doi: 10.1016/j.cmet.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tominaga T., Shimada R., Okada Y., Kawamata T., Kibayashi K. Senescence-associated-beta-galactosidase staining following traumatic brain injury in the mouse cerebrum. PLoS ONE. 2019;14:e0213673. doi: 10.1371/journal.pone.0213673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chong M., Yin T., Chen R., Xiang H., Yuan L., Ding Y., Pan C.C., Tang Z., Alexander P.B., Li Q.J. CD36 initiates the secretory phenotype during the establishment of cellular senescence. EMBO Rep. 2018;19:e45274. doi: 10.15252/embr.201745274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Justice J.N., Nambiar A.M., Tchkonia T., LeBrasseur N.K., Pascual R., Hashmi S.K., Prata L., Masternak M.M., Kritchevsky S.B., Musi N., et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–563. doi: 10.1016/j.ebiom.2018.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Killilea D.W., Wong S.L., Cahaya H.S., Atamna H., Ames B.N. Iron accumulation during cellular senescence. Ann. N. Y. Acad. Sci. 2004;1019:365–367. doi: 10.1196/annals.1297.063. [DOI] [PubMed] [Google Scholar]
- 94.Ott C., Konig J., Hohn A., Jung T., Grune T. Reduced autophagy leads to an impaired ferritin turnover in senescent fibroblasts. Free Radic. Biol. Med. 2016;101:325–333. doi: 10.1016/j.freeradbiomed.2016.10.492. [DOI] [PubMed] [Google Scholar]
- 95.Masaldan S., Clatworthy S.A.S., Gamell C., Smith Z.M., Francis P.S., Denoyer D., Meggyesy P.M., Fontaine S., Cater M.A. Copper accumulation in senescent cells: Interplay between copper transporters and impaired autophagy. Redox Biol. 2018;16:322–331. doi: 10.1016/j.redox.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Angelova D.M., Brown D.R. Altered processing of beta-amyloid in sh-sy5y cells induced by model senescent microglia. ACS Chem. Neurosci. 2018;9:3137–3152. doi: 10.1021/acschemneuro.8b00334. [DOI] [PubMed] [Google Scholar]
- 97.James S.A., Roberts B.R., Hare D.J., de Jonge M.D., Birchall I.E., Jenkins N.L., Cherny R.A., Bush A.I., McColl G. Direct in vivo imaging of ferrous iron dyshomeostasis in ageing Caenorhabditis elegans. Chem. Sci. 2015;6:2952–2962. doi: 10.1039/C5SC00233H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jenkins N.L., James S.A., Salim A., Sumardy F., Speed T.P., Conrad M., Richardson D.R., Bush A.I., McColl G. Ferrous-glutathione coupling mediates ferroptosis and frailty in Caenorhabditis elegans. bioRxiv. 2019 doi: 10.1101/594408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martin-Bastida A., Ward R.J., Newbould R., Piccini P., Sharp D., Kabba C., Patel M.C., Spino M., Connelly J., Tricta F., et al. Brain iron chelation by deferiprone in a phase 2 randomised double-blinded placebo controlled clinical trial in Parkinson’s disease. Sci. Rep. 2017;7:1398. doi: 10.1038/s41598-017-01402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moreau C., Danel V., Devedjian J.C., Grolez G., Timmerman K., Laloux C., Petrault M., Gouel F., Jonneaux A., Dutheil M., et al. Could conservative iron chelation lead to neuroprotection in amyotrophic lateral sclerosis? Antioxid. Redox Signal. 2018;29:742–748. doi: 10.1089/ars.2017.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Devos D., Moreau C., Devedjian J.C., Kluza J., Petrault M., Laloux C., Jonneaux A., Ryckewaert G., Garcon G., Rouaix N., et al. Targeting chelatable iron as a therapeutic modality in Parkinson’s disease. Antioxid. Redox Signal. 2014;21:195–210. doi: 10.1089/ars.2013.5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morris G.P., Clark I.A., Vissel B. Inconsistencies and controversies surrounding the amyloid hypothesis of Alzheimer’s disease. Acta Neuropathol. Commun. 2014;2:135. doi: 10.1186/s40478-014-0135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ayton S., Lei P., Bush A.I. Metallostasis in Alzheimer’s disease. Free Radic Biol. Med. 2013;62:76–89. doi: 10.1016/j.freeradbiomed.2012.10.558. [DOI] [PubMed] [Google Scholar]
- 104.Bush A.I., Tanzi R.E. Therapeutics for Alzheimer’s disease based on the metal hypothesis. Neurotherapeutics. 2008;5:421–432. doi: 10.1016/j.nurt.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Crapper McLachlan D.R., Dalton A.J., Kruck T.P., Bell M.Y., Smith W.L., Kalow W., Andrews D.F. Intramuscular desferrioxamine in patients with Alzheimer’s disease. Lancet. 1991;337:1304–1308. doi: 10.1016/0140-6736(91)92978-B. [DOI] [PubMed] [Google Scholar]
- 106.Ritchie C.W., Bush A.I., Mackinnon A., Macfarlane S., Mastwyk M., MacGregor L., Kiers L., Cherny R., Li Q.X., Tammer A., et al. Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Abeta amyloid deposition and toxicity in Alzheimer disease: A pilot phase 2 clinical trial. Arch. Neurol. 2003;60:1685–1691. doi: 10.1001/archneur.60.12.1685. [DOI] [PubMed] [Google Scholar]