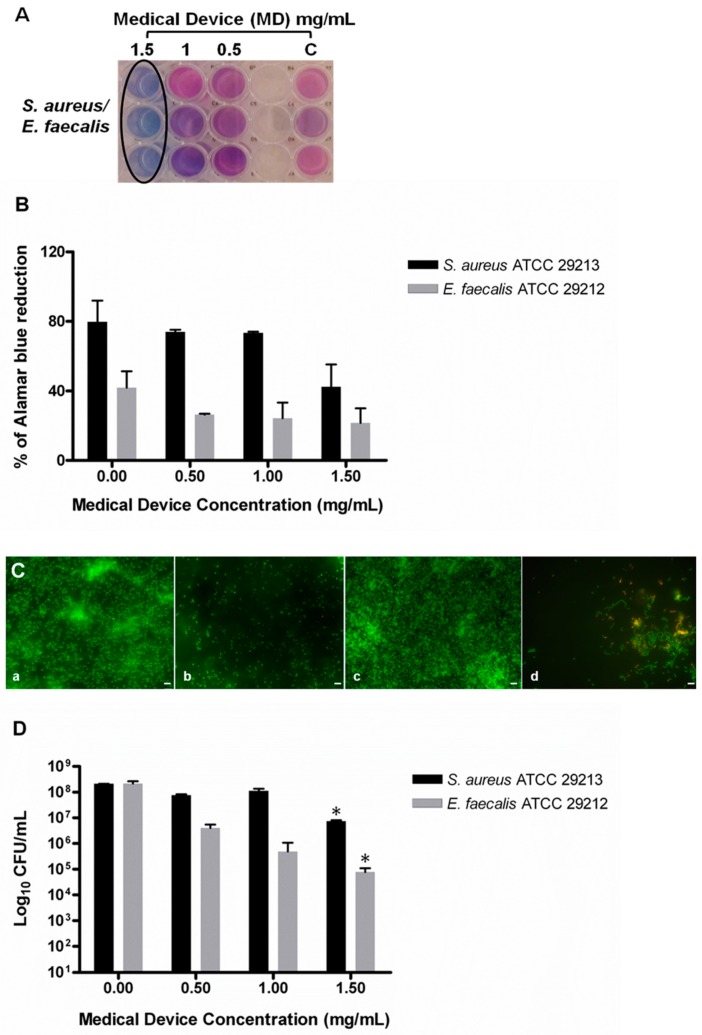
Figure 2.
Evaluation of minimum biofilm inhibitory concentration (MBIC) of the medical device (MD) versus S. aureus and E. faecalis biofilm determined by AB assay, live/dead staining, and fluorescent microscopy analysis and colony-forming unit (CFU) counts. (A) Representative image of colorimetric MIC determination using AB assay. The black circle indicates the MBIC at 1.5 mg/mL, which is the same for both S. aureus and E. faecalis. (B) The plot shows the percentage reduction of AB recorded for S. aureus and E. faecalis at different MD concentrations compared to the corresponding untreated samples (0.00), as previously described. The percentage of AB reduction was evaluated by using absorbance at 570 nm and 600 nm. (C) Representative images of live/dead staining of S. aureus and E. faecalis biofilm developed with and without the addition of MD at the inoculum. S. aureus biofilm after 24 h of incubation: (a) S. aureus treated with 1.5 mg/mL of MD at the inoculum, (b) E. faecalis biofilm after 24 h of incubation, (c) E. faecalis treated with 1 mg/mL of MD, (d) the green fluorescence indicates live cells; the samples treated with the MD did not develop a biofilm, as shown in images (b and d). bar: 5 μm. (D) Evaluation of the CFU number in the MD-treated and untreated sample. A significant reduction was detected at 1.50 mg/mL in both the microorganisms. Data are the mean of three replicates of three independent experiments; * p < 0.05 vs. the controls (0.00). C: controls or untreated samples.