Abstract
The continuous circulation of West Nile virus (WNV) in Central, South and East Europe and its recent detection in several dead birds and two horses in Germany highlights the need for information on WNV vector competence of mosquitoes from Central Europe. Therefore, three common Culex species (Culex pipiens biotype pipiens, Culex pipiens biotype molestus and Culex torrentium) from Germany were orally infected with WNV and kept at 18 °C, 21 °C, 24 °C or 27 °C for 14 or 21 days post infection (dpi). Thereafter viable WNV was present in the saliva in all tested taxa, but only at incubation temperatures of 24 °C or 27 °C and predominantly at the extended incubation period of 21 dpi. Highest transmission efficiency rates of 17 % (24 °C) and 24% (27 °C) were found for Cx. torrentium. Culex p. pipiens and Cx. p. molestus showed low transmission efficiencies with a maximum of only 3%. Consequently, temperatures above 21 °C support transmission of WNV, which matches the predominant distribution of human WNV cases around the Mediterranean Sea and in South-East Europe. Culex torrentium has been identified as a potent vector for WNV in Central and Northern Europe, which highlights the need for surveillance of mosquito-borne viruses north of the Alps.
Keywords: West Nile Virus, Culex torrentium, Arbovirus, Vector competence
1. Introduction
West Nile virus (WNV) belongs to the genus Flavivirus within the family Flaviviridae [1]. WNV is a zoonotic pathogen with an enzootic cycle between mosquitoes as vectors and birds as the primary, amplifying host. Humans, equines and other vertebrates are incidental hosts [2,3]. Human WNV infections can range from asymptomatic or mild clinical symptoms to severe outcomes due to neuroinvasive manifestations [2]. At present, there is no specific treatment or licensed vaccine for human use. WNV has a high epidemic potential as illustrated by its rapid spread after a single introduction to New York City (United States of America) in 1999 [4]. Subsequently, within the following few years, WNV rapidly spread over the North American continent, resulting in more than 20,000 human cases of neuroinvasive diseases and more than 2000 deaths [5]. During recent years, various outbreaks of WNV infections have been reported in central, southern and eastern countries of Europe resulting in several thousand human cases and dozens of fatal outcomes [6].
Mosquito species of the genus Culex have a worldwide distribution [7]. They are of medical importance as they can act as vectors for various zoonotic arboviruses from several virus families [8]. The most common Culex species in Europe are Culex torrentium Martini, 1925 and Culex pipiens s.l. L., 1758 [9]. The latter comprises two different biotypes, namely, Culex pipiens biotype pipiens (Cx. p. pipiens) and Culex pipiens biotype molestus (Cx. p. molestus). Species identification of the three taxa is challenging [10,11]. Both biotypes of Cx. pipiens s.l. cannot be identified with absolute certainty by morphological criteria and are traditionally classified by biotype-specific mating behaviours, breeding site selection and hibernation habits. Culex. p. molestus is considered stenogamous, autogenous, utilizes underground breeding sites and is non-diapausing, while Cx. p. pipiens is eurygamous, anautogenous, breeds above the ground and overwinters in diapause. The picture gets even more complex with the occurrence of the sibling species Cx. torrentium, because this species resembles Cx. p. pipiens morphologically and by its breeding ecology and both species often occur in sympatry [12,13]. Cx. torrentium males can be differentiated from Cx. pipiens s.l. by characters of the hypopygium, but a reliable morphological differentiation of females is difficult because pre-alar scales easily fall off and the use of morphometric wing characters is generally not established [9,14]. Therefore, species differentiation of the different Culex species often relies on molecular identification [10,15]. In Europe, Cx. pipiens s.l. and Cx. torrentium usually occur together, with Cx. torrentium being the dominant species in northern Europe and Cx. p. pipiens prevailing in regions south of the Alps [9,16]. In Central Europe such as Austria or Germany, both sister species can be found in sympatry [10,11,12,13].
Due to the wide distribution and high abundance of the three Culex taxa in Europe, several studies were performed to analyse their vector capacity for various arboviruses. For many years, it has been assumed, that both Cx. pipiens s.l and Cx. torrentium are primarily ornithophilic [17], hence they were not classified as important vectors for zoonotic pathogens. However, recent studies demonstrated substantial variability in host feeding patterns of Cx. p. pipiens s.l. and Cx. torrentium, comprising birds and mammals including humans [10,18,19,20]. Accordingly, the two species have to be considered as bridge vectors for the transmission of zoonotic pathogens from birds to humans. During nationwide monitoring surveys in Germany, different mosquito-borne viruses have been detected in field-collected specimens of the three Culex taxa, including Sindbis virus (SINV), Usutu virus (USUV) and Batai virus (BATV) [8]. In addition, there is growing evidence that European populations of both Cx. pipiens biotypes and their hybrids are competent vectors for WNV, USUV and SINV [18,21,22,23], but not for Zika virus [24,25] or Chikungunya virus [26]. Concerning Cx. torrentium, there is considerably less information about possible vector competences. In a single published study from Germany, it was demonstrated that Cx. torrentium is susceptible to WNV infection, but the presence of infectious virus particles in the saliva was not investigated [27]. Other studies from Sweden indicated that Cx. torrentium has a higher vector competence for SINV compared to Cx. pipiens s.l. [18,28].
The ongoing circulation of WNV in southern and eastern Europe poses a significant risk for the introduction and autochthonous human infections in central and northern Europe, in case suitable vector species and appropriate climatic conditions are present [29,30]. Transmission risk for WNV in Germany was recently demonstrated by the first detection of several WNV-positive birds in summer 2018 [31]. This highlights the need for a temperature-dependent risk assessment of the WNV vector competence for native mosquito vectors in Central Europe. Here we report on experimental WNV infection studies using Cx. p. pipiens, Cx. p. molestus and Cx. torrentium from Germany at different temperature conditions ranging from 18 °C to 27 °C. The results indicate unexpected high WNV transmission rates at 24 °C and 27 °C of 62% and 90%, respectively, but only for Cx. torrentium. For a spatial risk assessment, the results were put in relation to temperature data and the actual circulation of WNV in Europe.
2. Materials and Methods
2.1. Collection and Rearing of Mosquitoes
F0 adults of Cx. torrentium and Cx. p. pipiens were obtained from egg rafts collected in Langenlehsten, Germany (53°30′ N 10°44′ E) in 2016 and 2017. For Cx. p. molestus a labstrain was used, which was established from egg rafts collected in Heidelberg, Germany (49°11′ N 08°39′ E) in 2011. Adult mosquitoes were incubated at 26 °C, with a relative humidity of 80% and a 12:12 light:dark photoperiod. For differentiation of the three Culex taxa, DNA was extracted from 1–4 larvae (DNeasy Blood & Tissue Kit, Qiagen, Hilden, Germany) and molecular identification was performed by multiplex quantitative real-time PCR (qRT-PCR) as previously described [11,17].
2.2. Experimental Infection and Analysis
Four to 14 days-old female mosquitoes were starved 24 hours before challenged with infectious blood meals containing WNV-1 (clade 1a, strain TOS-09, Genbank HM991273/HM641225, passage 5 from Vero cells) [32] at a final concentration of 107 plaque forming units per milliliter (PFU/mL). This concentration is recommended for artificial blood meals because it corresponds to natural bird viremia [21]. Composition of blood meal was as follows: 50% expired human blood (blood preservation) from human blood bank (not suitable for humans anymore, but useful for mosquitoes), 30% fructose (8% solution), 10% filtrated bovine serum and 10% working solution of the virus stock. Artificial blood meals were provided overnight, using cotton sticks soaked in infectious blood [26]. Subsequently, mosquitoes were anesthetized with CO2 and fully engorged females were sorted into a new vial. Mosquitoes were incubated at 80% humidity at 18 °C, 21 °C, 24 °C or 27 °C, respectively. A cotton pad saturated with solution of 8% Fructose was provided during incubation time of 14 or 21 days. 10 randomly selected adult mosquitoes per species were tested by pan-Flavi-, pan-Alpha- and pan-Orthobunyavirus PCRs to test for natural virus infection [33,34,35]. All individuals revealed negative results.
Mosquitoes were analysed for infection rate (IR), transmission rate (TR) and transmission efficiency (TE) 14 and 21 days post infection (dpi) as previously described [25,36]. Infection was investigated by the analysis of extracted RNA (MagMax Pathogen RNA/DNA Kit, Thermo fisher scientific, Waltham, MA, USA) from bodies (excluding legs and wings) according to a previously described qRT-PCR protocol for WNV RNA [27]. The TR was determined by performing a salivation assay for the detection of infectious virus particles as previously described [37]. TR was defined as the number of mosquitoes with WNV-positive saliva per number of WNV-positive mosquito bodies [25,37,38]. TE was calculated as the number of specimens with WNV-positive saliva per total number of fed females [36].
2.3. Comparison of the Study Results with Previous Vector Competence Studies and the Actual Circulation of WNV in Europe
The effects of species, temperature and dpi as factors on the IR, TR or TE were tested with generalized linear models with a binomial distribution and logit link function. Where necessary, multi comparison tests between factors were applied depending on the model used with a Tukey matrix of contrast. In addition, TRs in this study were evaluated against the results of previously published studies, conducting WNV infection experiments with European Culex spp. mosquito populations. These studies were previously reviewed by Vogels et al. [21] and further updated with a recent study [39]. Furthermore, following the method of Fros et al. [40], temperature data were analyzed to put the vector competence studies in relation to the actual spatial circulation of WNV in Europe. Daily mean temperature data (European re-analysis and observations for monitoring, E-OBS v17.0, available on a 0.25° regular latitude-longitude grid) were downloaded from http://www.ecad.eu [41]. The average daily temperature conditions in July/August for each year between 2011 and 2018 were extracted for all European region on the NUTS 3-level (Nomenclature of Territorial Units for statistic, third level) and visualized in a histogram. In a next step, the number of human WNV cases reported by ECDC for the same statistical regions [6] was linked to the same underlying temperature data. Thereby, the annual averaged July/August temperature data for each reported case were visualized as an additional histogram. Following Fros et al. [40] in order to exclude imported cases, reports of single WNV cases per country and year were eliminated from the analysis. The correlation between the temperature data and human WNV cases was tested with a generalized linear model with a binomial distribution and logit link function. All data analysis and visualizations were conducted with R [42] using the packages cowplot [43], dplyr [44], ggplot2 [45], magrittr [46], maptools [47], raster [48] and multcomp [49].
3. Results
All three investigated Culex taxa were susceptible to WNV (Table 1), i.e. viral titres of mosquito bodies reached at least the detection limit of the qRT-PCR of 10,000 RNA copies per mosquito specimen. IRs were statistically significant different between the three species (likelihood-ratio test (LR)-χ2 = 0.97662, df = 2, p < 0.001). Culex torrentium had higher IRs compared to Cx. p. pipiens and Cx. p. molestus (Tukey’s post-hoc tests, p < 0.05), while no differences were found between Cx. p. pipiens and Cx. p. molestus (Tukey’s post-hoc test, p > 0.05). IRs increased with increasing temperature (LR-χ2 = 0.28262, df = 1, P < 0.01). Highest IRs of 32% were found for Cx. torrentium at an incubation temperature of 27 °C over a period of 14 days dpi, followed by Cx. p. pipiens with an IR of 23% (24 °C; 21 dpi). Lowest IRs with a maximum of only 6% were found for Cx. p. molestus (27 °C; 21 dpi). In general, IRs were considerably higher at 21 dpi compared to 14 dpi (LR-χ2 = 0.46289, df = 1, P < 0.001). The only exception was Cx. torrentium incubated at 27 °C with a slightly higher IR of 32% at 14 dpi compared to 26% at 21 dpi.
Table 1.
Infection (IR), transmission (TR) and transmission efficiency rates (TE) of three Culex species experimentally infected with West Nile virus and kept at four different temperatures, June 2016 to July 2017 (n = 788). The experimental analytical sensitivity of the qRT-PCR was analysed according the protocol of Caraguel et al. calculating the limit of detection via endpoint dilution [50]. The limit of detection was defined as 100 copies/µL, corresponding to about 10,000 copies per mosquito specimen. (NA: not analyzed; IR: number of positive saliva/positive bodies; TR: number of positive legs/positive bodies; TE: number of positive saliva per mosquito).
| 14 Days Post Infection | 21 Days Post Infection | ||||||
|---|---|---|---|---|---|---|---|
| Mosquito Taxa | T in °C | IR (%) |
TR (%) |
TE (%) |
IR (%) |
TR (%) |
TE (%) |
| Culex p. molestus | 18 | 0/29 (0.0) |
NA | NA | 1/29 (3.4) |
0/1 (0.0) |
NA |
| 24 | 0/31 (0.0) |
NA | NA | 1/31 (3.2) |
0/1 (0.0) |
NA | |
| 27 | 0/31 (0.0) |
NA | NA | 4/62 (6.4) |
1/4 (25.0) |
1/62 (1.6) |
|
| Culex p. pipiens | 18 | 1/32 (3.1) |
0/1 (0.0) |
NA | 2/33 (6.1) |
0/2 (0.0) |
NA |
| 21 | 1/30 (3.3) |
0/1 (0.0) |
NA | 3/31 (9.7) |
0/3 (0.0) |
NA | |
| 24 | 1/30 (3.3) |
0/1 (0.0) |
NA | 7/31 (22.6) |
1/7 (14.3%) |
1/31 (3.2) |
|
| 27 | 0/35 (0.0) |
NA | NA | 3/33 (9.1) |
1/3 (33.3) |
1/33 (3.0) |
|
| Culex torrentium | 18 | 2/32 (6.2) |
0/2 (0.0) |
NA | 5/33 (15.2) |
0/5 (0.0) |
NA |
| 21 | 0/31 (0.0) |
NA | NA | 4/32 (12.5) |
0/4 (0.0) |
NA | |
| 24 | 2/31 (6.4) |
0/2 (0.0) |
NA | 8/29 (27.6) |
5/8 (62.5) |
5/29 (17.2) |
|
| 27 | 11/34 (32.4) |
1/11 (9.1) |
1/34 (2.9) |
10/38 (26.3) |
9/10 (90.0) |
9/38 (23.7) |
|
All three Culex taxa tested were able to transmit infectious WNV particles, but only at elevated incubation temperatures of 24 °C or 27 °C (LR-χ2 = 4.6129, df = 1, p < 0.001). In addition, except for Cx. torrentium at 27 °C, transmission was only observed for an extended incubation period of 21 dpi compared to 14 dpi (LR-χ2 = 3.9147, df = 1, p < 0.001, Table 1). TRs differed between the three taxa (LR-χ2 = 2.4958, df = 1, p < 0.001) with higher values for Cx. torrentium compared to the other two species (Tukey’s post-hoc tests, p < 0.001). At 24 °C, TRs for Cx. p. molestus, Cx. p. pipiens and Cx. torrentium were 0%, 14% and 62%, respectively, and at 27 °C TRs were 25%, 33% and 90%, respectively. Accordingly, Cx. p. molestus and Cx. p. pipiens showed statistically lower TEs of up to a maximum of only 3%, whereas Cx. torrentium revealed significant TEs of 17% at 24°C and 24% at 27 °C (LR-χ2 = 0.65335, df = 2, p < 0.001, Tukey’s post-hoc tests, p < 0.001, Table 1).
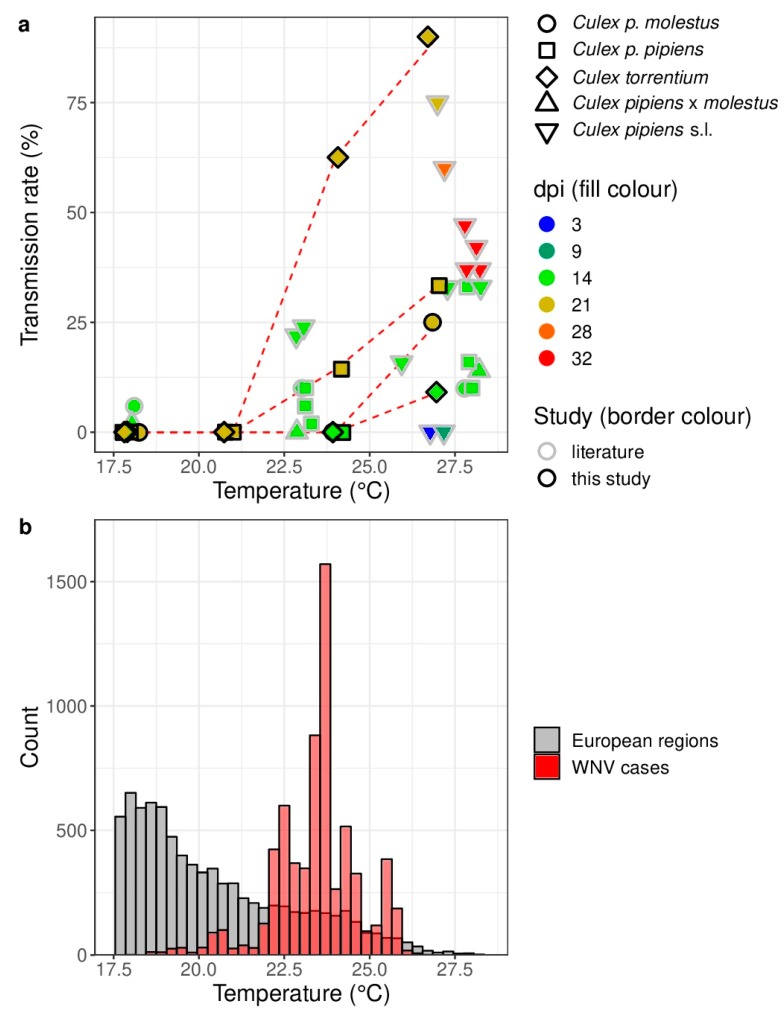
A distinct temperature dependence of WNV is in line with previous vector competence studies and the distribution of human WNV cases in Europe (Figure 1). Noticeable, transmission rates were only observed for incubation temperatures above 20 °C (Figure 1a). The probability for human WNV cases statistically significant increased with increasing temperature (LR-χ2 = 9769.4, df = 1, p < 0.001). WNV predominantly circulates in areas with average temperatures above 20 °C during July/August (2011–2018) (Figure 1b).
Figure 1.
(a) Transmission rate of Culex pipiens s.l./torrentium experimentally infected with West Nile virus at different temperatures and days post infection (dpi) in comparison to previously published vector competence studies with European populations Culex pipiens s.l [21]. A small horizontal jitter was added to the points to prevent overlapping.; (b) Histograms of the average temperatures in July/August (2011–2018) for all European NUTS 3 (Nomenclature of Territorial Units for Statistics, third level) regions (=gray) and for each detected human WNV case (=red). Count data for the European NUTS 3 regions indicate the cumulative number of times the respective average temperature in July/August was observed over all European NUTS 3 regions for each year from 2011 to 2018. Only the data for the temperatures between 17.5 °C and 28.5 °C are shown.
Similarly, count data for the human WNV cases indicate the cumulative number of times the respective average temperature in July/August was observed for each human WNV case in the respective European NUTS 3 region and year.
4. Discussion
Six European mosquito species namely Aedes albopictus, Aedes detritus, Aedes japonicus, Culex modestus, Cx. p. pipiens and Cx. p. molestus are known to be susceptible to WNV infection and able to transmit infectious WNV particles at least under experimental laboratory conditions [21,39]. The study presented here confirms vector competences for Cx. p. pipiens and Cx. p. molestus, but with moderate transmission rates of 25% and 33%, respectively, and rather low transmission efficiencies up to a maximum of 3%. In addition, we have analyzed the WNV vector competence of Cx. torrentium, a further Culex species widely distributed and abundant in Central Europe and found unexpected high WNV transmission rates of up to 90% with a calculated maximum transmission efficiency of 24%. Studies specifically addressing WNV transmission of Cx. torrentium have not been reported so far. Our findings concerning the two Cx. pipiens biotypes are in agreement with previous studies using other European populations of the two biotypes. These studies revealed TRs for WNV of up to 10% for Cx. p. molestus and of up to 30% for Cx. p. pipiens. Higher TRs of 30%–75% were also reported but in these studies Cx. pipiens s.l. mosquitoes were not further differentiated.
Questions remain concerning the mechanism responsible for the high WNV vector competence of Cx. torrentium compared to the two Culex pipiens biotypes. One thing that is not considered in this study is the number of infectious virus particles that were uptaken by each individual. The number of infectious virus particles per µL will presumably decrease as longer as the blood meal is offered and the time point when each mosquito soaked blood after starting the blood meal was not documented. However, the amount of blood which is ingested by each mosquito varies and therefore the amount of viral particles per fed mosquito varies anyway, which is why this point is not considered in this study. To our knowledge, only one vector competence study has been reported targeting Cx. torrentium specifically, indicating that the species is a potent vector of SINV. SINV is a mosquito-borne virus of the genus alphavirus, with a similar ecology as WNV, i.e. enzootic cycle with birds and spill-overs to mammalian species. Experimental infection of birds by either Cx. torrentium or Cx. pipiens s.l. revealed a noticeable higher vector competence for Cx. torrentium [18]. In addition, field studies found highest SINV infection rates for naturally infected Cx. torrentium in comparison to Cx. pipiens s.l. or Culiseta morsitans [28]. Thus, Cx. torrentium appears to be a suitable vector for at least two arthropod-borne viruses (arboviruses) from different virus families. Whether the lack of Wolbachia as recently reported for Cx. torrentium [51] is responsible for this broad vector competence and whether other viruses can be transmitted by Cx. torrentium as well, remains to be determined. Host-feeding patterns with nearly equal detections of avian and mammalian hosts [19] indicate that Cx. torrentium have to be considered as bridge vector for the transmission of zoonotic pathogens from birds to humans. Thus, the species is considered as the main vector of SINV in Northern Europe and may play a major role in WNV transmission in areas where the species is abundant and respectively favourable environmental conditions are present, in particular, elevated temperatures for an extended time period [9]. The latter may be responsible for the first emergence of WNV in Germany in 2018.
Although intensive surveillance has been conducted during previous years, no WNV circulation was detected in Germany before 2018 [52]. Since the virus is sporadically circulating in neighbouring countries (e.g. France, Czech Republic and Austria) introduction of WNV into Germany has been long-awaited [6]. Nevertheless, intensive screening of birds and mammals in Germany only identified WNV neutralizing antibodies in migratory birds, but none of the animals were positive for WNV RNA [53,54]. Likewise, intensive surveillance of mosquitoes over the last decade confirmed the circulation of various arboviruses in Germany including USUV, SINV and BATV, but did not detect WNV [8]. In contrast, in late summer 2018, several bird specimens from various parts of Germany were tested positive for WNV [31].
This first emergence of WNV in Germany during summer 2018 is clearly linked to temperature anomalies, i.e. significant positive deviation from the long-term mean temperatures, which may have shortened the extrinsic incubation period of WNV [31]. Temperature dependency of WNV replication in the vector has already been discussed [55,56]. Tropical temperatures around 27–28 °C support transmission of WNV, while moderate temperatures of 23–24 °C lead to considerably reduced TRs. In concordance with the results of Culex vector competence presented here, other studies also revealed a lack of WNV transmission at lower temperatures (≤21 °C). According to the distribution of human WNV cases in Europe between 2011 and 2018 [6,22] most cases were observed in areas around the Mediterranean Sea and south-eastern countries in Europe, comprising average temperatures between 21 °C and 26 °C in July/August. Thus, although Cx. torrentium is a highly competent vector for the transmission of WNV, the main distribution of this species lies within areas of temperate climate in Central and Northern Europe, which in general do not allow transmission of WNV north of the Alps. Temperature conditions in exceptional years, however, may allow WNV circulation even in Central Europe facilitated by the presence of Cx. torrentium. This risk may further increase with rising temperatures in the course of climate change. In conclusion, due to the continuing circulation of WNV in Europe and the prevalence of potent vectors for WNV, such as Cx. torrentium, a surveillance system that includes birds, mosquitoes and humans should be established or maintained in all European countries to enable early detection and subsequent interventions. This should include areas in Northern and Central Europe, where Cx. torrentium is the predominant Culex species [9,11]. In addition, due to the high vector competence of Cx. torrentium for WNV and SINV [18], further studies should be conducted to evaluate the species’ susceptibility to other arboviruses, such as USUV, which is presently killing thousands of birds in Central Europe [57,58].
Author Contributions
Conceptualization, S.J., A.H., R.L., J.S.-C. and E.T.; Data curation, S.J. and A.H.; Formal analysis, R.L.; Funding acquisition, J.S.-C. and E.T.; Investigation, S.J., A.H. and M.H.; Methodology, S.J., A.H., R.L., M.L. and M.H.; Project administration, S.J. and A.H.; Resources, M.B. and G.R.; Validation, S.J., A.H. and R.L.; Visualization, R.L.; Writing–original draft, S.J., A.H. and R.L.; Writing—review & editing, S.J., A.H., R.L., M.L., J.S.-C. and E.T.
Funding
This work was financially supported by the German Federal Ministry of Food and Agriculture (BMEL) through the Federal Office for Agriculture and Food (BLE) with the grant number 28-1-91.048-15 and the German Federal Ministry of Education and Research of Germany (BMBF) with the grant number 01EI1702A.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Calisher C.H., Karabatsos N., Dalrymple J.M., Shope R.E., Porterfield J.S., Westaway E.G., Brandt W.E. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J. Gen. Virol. 1989;70:37–43. doi: 10.1099/0022-1317-70-1-37. [DOI] [PubMed] [Google Scholar]
- 2.Campbell G.L., Marfin A.A., Lanciotti R.S., Gubler D.J. West Nile virus. Lancet. Infect. Dis. 2002;2:519–529. doi: 10.1016/S1473-3099(02)00368-7. [DOI] [PubMed] [Google Scholar]
- 3.Gyure K.A. West Nile virus infections. J. Neuropathol. Exp. Neurol. 2009;68:1053–1060. doi: 10.1097/NEN.0b013e3181b88114. [DOI] [PubMed] [Google Scholar]
- 4.Petersen L.R., Brault A.C., Nasci R.S. West Nile virus: review of the literature. JAMA. 2013;310:308–315. doi: 10.1001/jama.2013.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Center for Disease Control and Prevention (CDC). West Nile Virus: Final Cumulative Maps & Data for 1999–2017. [(accessed on 28 September 2018)]; Available online: https://www.cdc.gov/westnile/statsmaps/cumMapsData.html.
- 6.European Centre for Disease Prevention and Control (ECDC) No Surveillance and Disease Data for West Nile. [(accessed on 28 September 2018)]; Available online: https://ecdc.europa.eu/en/west-nile-fever/surveillance-and-disease-data.
- 7.Harbach R.E. Culex pipiens: species versus species complex—Taxonomic history and perspective. J. Am. Mosq. Control. Assoc. 2012;28:10–23. doi: 10.2987/8756-971X-28.4.10. [DOI] [PubMed] [Google Scholar]
- 8.Scheuch D.E., Schäfer M., Eiden M., Heym E.C., Ziegler U., Walther D., Schmidt-Chanasit J., Keller M., Groschup M.H., Kampen H. Detection of Usutu, Sindbis, and Batai viruses in moquitoes (Diptera: Culicidae) collected in Germany 2011–2016. Viruses. 2018;10:389. doi: 10.3390/v10070389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hesson J.C., Rettich F., Merdić E., Vignjević G., Ostman O., Schäfer M., Schaffner F., Foussadier R., Besnard G., Medlock J., et al. The arbovirus vector Culex torrentium is more prevalent than Culex pipiens in northern and central Europe. Med. Vet. Entomol. 2014;28:179–186. doi: 10.1111/mve.12024. [DOI] [PubMed] [Google Scholar]
- 10.Zittra C., Flechl E., Kothmayer M., Vitecek S., Rossiter H., Zechmeister T., Fuehrer H.P. Ecological characterization and molecular differentiation of Culex pipiens complex taxa and Culex torrentium in eastern Austria. Parasit. Vector. 2016;9:197. doi: 10.1186/s13071-016-1495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudolf M., Czajika C., Bürstler J., Melaun C., Jöst H., von Thien H., Badusche M., Becker N., Schmidt-Chanasit J., Krüger A., et al. First nationwide surveillance of Culex pipiens complex and Culex torrentium mosquitoes demonstrated the presence of Culex pipiens biotype pipiens/molestus hybrids in Germany. PLoS ONE. 2013;8:e71832. doi: 10.1371/journal.pone.0071832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lühken R., Steinke S., Leggewie M., Tannich E., Krüger A., Becker S., Kiel E. Physio-chemical characteristics of Culex pipiens sensu lato and Culex torrentium (Diptera: Culicidae) breeding sites in Germany. J. Med. Entomol. 2015;52:932–936. doi: 10.1093/jme/tjv070. [DOI] [PubMed] [Google Scholar]
- 13.Becker N., Petrić D., Zgomba M., Boase C., Madon M., Dahk C., Kaiser A. Mosquitoes and Their Control. 2nd ed. Springer; Heidelberg, Germany: 2010. p. 196. [Google Scholar]
- 14.Börstler J., Lühken R., Rudolf M., Steinke S., Melaun C., Becker S., Garms R., Krüger A. The use of morphometric wing characters to discriminate female Culex pipiens and Culex torrentium. J. Vector Ecol. 2014;39:204–212. doi: 10.1111/j.1948-7134.2014.12088.x. [DOI] [PubMed] [Google Scholar]
- 15.Vogels C.B., van de Peppe L.J., van Vliet A.J., Westenberh M., Ibañez_Justica A., Stroo A., Viesser T.M., Koenrasdt C.J. Winter activity and aboveground hybridization between the two biotypes of the West Nile virus vector Culex pipiens. Vector Borne Zoonotic Dis. 2015;15:619–626. doi: 10.1089/vbz.2015.1820. [DOI] [PubMed] [Google Scholar]
- 16.Calzolari M., Bonilauri P., Bellini R., Becker S., Dottori M. Wide recognition of Culex pipiens and lack of detection of Culex torrentium through biomolecular differentiation of mosquitoes in the Emilia-Romagna region, Northern Italy. Med. Vet. Entomol. 2016;30:435–438. doi: 10.1111/mve.12186. [DOI] [PubMed] [Google Scholar]
- 17.Hesson J.C., Schäfer M., Lundström J.O. First report on human-biting Culex pipiens in Sweden. Parasites Vectors. 2016;9:632. doi: 10.1186/s13071-016-1925-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundström J.O., Niklasson B., Francy D.B. Swedish Culex torrentium and Cx. pipiens (Diptera: Culicidae) as experimental vectors of Ockelbo virus. J. Med. Entomol. 1990;27:561–563. doi: 10.1093/jmedent/27.4.561. [DOI] [PubMed] [Google Scholar]
- 19.Börstler J., Jöst H., Garms R., Krüger A., Tannich E., Becker N., Schmidt-Chanasit J., Lühken R. Host-feeding patterns of mosquito species in Germany. Parasites Vectors. 2016;9:318. doi: 10.1186/s13071-016-1597-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osorio H.C., Ze-Ze L., Amaro F., Nunes A., Alves M.J. Sympatric occurrence of Culex pipiens (Diptera, Culicidae) biotypes pipiens, molestus and their hybrids in Portugal, Western Europe: feeding patterns and habitat determinants. Med. Vet. Entomol. 2014;28:103–109. doi: 10.1111/mve.12020. [DOI] [PubMed] [Google Scholar]
- 21.Vogels C.B.F., Göertz G.P., Pijlman G.P., Koenraadt C.J.M. Vector competence of European mosquitoes for West Nile virus. Emerg. Microbes. Infect. 2017;6:e96. doi: 10.1038/emi.2017.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romo H., Papa A., Kading R., Clark R., Delorey M., Brault A.C. Comparative vector competence of North American Culex pipiens and Culex quinquefasciatus for African and European Lineage 2 West Nile viruses. Am. J. Trop. Med. Hyg. 2018;98:1863–1869. doi: 10.4269/ajtmh.17-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camp J.V., Kolodziejek J., Nowotny N. Targeted surveillance reveals native and invasive mosquito species infected with Usutu virus. Parasites Vectors. 2019;12:46. doi: 10.1186/s13071-019-3316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boccolini D., Toma L., Di Luca M., Severini F., Romi R., Remoli M.E., Sabatucci M., Venturi G., Rezza G., Fortuna C. Experimental investigation of the susceptibility of Italian Culex pipiens mosquitoes to Zika virus infection. Eurosurveillance. 2016;21:pii30328. doi: 10.2807/1560-7917.ES.2016.21.35.30328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heitmann A., Jansen S., Lühken R., Helms M., Pluskota B., Becker N., Kuhn C., Schmidt-Chanasit J., Tannich E. Experimental transmission of Zika virus by mosquitoes from central Europe. Eurosurveillance. 2017;22:pii30437. doi: 10.2807/1560-7917.ES.2017.22.2.30437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talbalaghi A., Moutailler S., Vazeille M., Failloux A.B. Are Aedes albopictus or other mosquito species from Northern Italy competent to sustain new arboviral outbreaks? Med. Vet. Entomol. 2010;24:83–87. doi: 10.1111/j.1365-2915.2009.00853.x. [DOI] [PubMed] [Google Scholar]
- 27.Leggewie M., Badusche M., Rudolf M., Jansen S., Börstler J., Hiber K., Krüger A., Schmidt-Chansit J., Tannich E., Becker S. Culex pipiens and Culex torrentium populations from Central Europe are susceptible to West Nile virus infection. One Health. 2016;2:88–94. doi: 10.1016/j.onehlt.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hesson J.C., Verner_Carlsson J., Larsson A., Ahmed R., Lundquist A., Lundströhm J.L. Culex torrentium mosquito role as major enzootic vector defined by rate of Sindbis virus infection, Sweden, 2009. Emerg. Infect. Dis. 2015;21:875–878. doi: 10.3201/eid2105.141577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gabriel M., Emmerich P., Frank C., Fiedler M., Rashidi-Alvaijeh J., Jochhum C., Günther S., Auerhammer K., Rupprecht H.J., Blank R.T., et al. Increase in West Nile virus infections imported to Germany in 2012. J. Clin. Virol. 2013;58:587–589. doi: 10.1016/j.jcv.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 30.Calistri P., Savini L., Candeloro L., Di Sabatini D., Cito F., Bruno R., Danzetta M.L. A transitional model for the evaluation of West Nile Virus transmission in Italy. Transbound. Emerg. Dis. 2016;63:485–496. doi: 10.1111/tbed.12290. [DOI] [PubMed] [Google Scholar]
- 31.Ziegler U., Lühken R., Keller M., Cafar D., van der Grinten E., Michel F., Albrecht K., Eiden M., Rinder M., Lachmann L., et al. West Nile virus epizootic in Germany, 2018. Antivir. Res. 2019;162:39–43. doi: 10.1016/j.antiviral.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Rossini G., Carletti F., Bordi L., Cavrini F., Gaibani P., Landini M.P., Pierro A., Capobianchi M.R., Di Caro A., Sambi V. Phylogenetic analysis of West Nile Virus isolates, Italy, 2008–2009. Emerg. Infect. Dis. 2011;17:903–906. doi: 10.3201/eid1705.101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chao D.-Y., Davis. B.S., Chang G.J.J. Development of multiplex real-time reverse transcriptase PCR assays for detecting eight medically important flaviviruses in mosquitoes. J. Clin. Microbiol. 2007;45:584–589. doi: 10.1128/JCM.00842-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eshoo M.W., Whitehouse C.A., Zoll S.T., Massire C., Pennella T.T., Blyn L.B., Sampath R., Ecker J.A., Desai W., Wasieloski L.P. Direct broad-range detection of alphaviruses in mosquito extracts. Virology. 2007;368:286–295. doi: 10.1016/j.virol.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 35.Lambert A.J., Lanciotti R.S. Consensus amplification and novel multiplex sequencing method for S segment species identification of 47 viruses of the Orthobunyavirus, Phlebovirus, and Nairovirus genera of the family Bunyaviridae. J. Clin. Microbiol. 2009;47:2398–2404. doi: 10.1128/JCM.00182-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jansen S., Heitmann A., Lühken R., Jöst H., Helms M., Vapalahti O., Schmidt-Chanasit J., Tannich E. Experimental transmission of Zika virus by Aedes japonicus japonicus from Southwest Germany. Emerg. Microbes Infect. 2018;7:192. doi: 10.1038/s41426-018-0195-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heitmann A., Jansen S., Lühken R., Leggewie M., Schmidt-Chanasit J., Tannich E. Forced salivation as a method to analyze vector competence of mosquitoes. J. Vis. Exp. 2018;138:e57980. doi: 10.3791/57980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heitmann A., Jansen S., Lühken R., Helms M., Pluskota B., Becker N., Kuhn C., Schmidt-Chanasit J., Tannich E. Experimental risk assessment for chikungunya virus transmission based on vector competence, distribution and temperature suitability in Europe, 2018. Eurosurveillance. 2018;23:pii1800033. doi: 10.2807/1560-7917.ES.2018.23.29.1800033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veronesi E., Paslaru A., Silagi C., Tobler K., Glavinic U., Torgerson P., Mathis A. Experimental evaluation of infection, dissemination, and transmission rates for two West Nile virus strains in European Aedes japonicus under a fluctuating temperature regime. Parasitol. Res. 2018;117:1925–1932. doi: 10.1007/s00436-018-5886-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fros J.J., Geertsema C., Vogels C.B., Roosjen P.P., Failloux A.B., Vlak J.M., Koenradt C.J., Takken W., Pijlmann G.P. West Nile virus: high transmission rate in North-Western European mosquitoes indicates its epidemic potential and warrants increased surveillance. PLoS Negl. Trop. Dis. 2015;9:e0003956. doi: 10.1371/journal.pntd.0003956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haylock M.R., Hofstra N., Klein Tank A.M.G., Klok E.J., Jones P.D., New M. A European daily high-resolution gridded data set of surface temperature and precipitation for 1950–2006. J. Geophys. Res. Atmos. 2008;113:D20119. doi: 10.1029/2008JD010201. [DOI] [Google Scholar]
- 42.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: [(accessed on 23 May 2019)]. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ [Google Scholar]
- 43.Wilke C.O. Cowplot: Streamlined Plot theme and Plot Annotations for ‘ggplot2’. R Package Version 0.9.2. [(accessed on 23 May 2019)]; Available online: https://CRAN.R-project.org/package=cowplot.
- 44.Wickham H., Francois R., Henry L., Müller K. Dplyr: A Grammar of Data Manipulation. R Package Version 0.7.4. [(accessed on 23 May 2019)]; Available online: https://CRAN.R-project.org/package=dplyr.
- 45.Wickham H. ggplot2: Elegant Graphics for Data Analysis. 1st ed. Springer; New York, NY, USA: 2009. [Google Scholar]
- 46.Bache S.M., Wickham H. Magrittr: A Forward-Pipe Operator for R. R Package Version 1.5. [(accessed on 23 May 2019)]; Available online: https://CRAN.R-project.org/package=magrittr.
- 47.Rivand R., Lewin-Koh N. Maptools: Tools for Reading and Handling Spatial Objects. R package version 0.9–2. [(accessed on 23 May 2019)]; Available online: https://CRAN.R-project.org/package=maptools.
- 48.Hijmans R.J. Raster: Geographic Data Analysis and Modeling. R package version 2.6–7. [(accessed on 23 May 2019)]; Available online: https://CRAN.R-project.org/package=raster.
- 49.Hothorn T., Bretz F., Westfall P. Simultanous inference in general parametric models. Biom. J. 2008;50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 50.Caraguel C.G.B., Stryhn H., Gagné N., Dohoo I.R., Hammell K.L. Selection of a cutoff for real-time polymerase chain reaction results to fit a diagnostic purpose: analytical and epidemiologic approaches. J. Vet. Diagn. Investig. 2001;23:2–15. doi: 10.1177/104063871102300102. [DOI] [PubMed] [Google Scholar]
- 51.Leggewie M., Krumkamp R., Badusche M., Heitmann A., Jansen S., Schmidt-Chanasit J., Tannich E., Becker S.C. Culex torrentium mosquitoes from Germany are negative for Wolbachia. Med. Vet. Entomol. 2018;32:115–120. doi: 10.1111/mve.12270. [DOI] [PubMed] [Google Scholar]
- 52.Ziegler U., Seidowski D., Angenvoort J., Eiden M., Nowotny N., Groshup M.H. Monitoring of West Nile virus infections in Germany. Zoonoses Public Health. 2012;59(Suppl. 2):95–101. doi: 10.1111/zph.12015. [DOI] [PubMed] [Google Scholar]
- 53.Michel F., Fischer D., Eiden M., Fast C., Reuschel M., Müller R., Rinder M., Urbaniak S., Brandes F., Schwehn R., et al. West Nile Virus and Usutu virus monitoring of wild birds in Germany. Int. J. Environ. Res. Public Health. 2018;15:171. doi: 10.3390/ijerph15010171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Börstler J., Engel D., Petersen M., Poggensee C., Jansen S., Schmidt-Chanasit J., Lühken R. Surveillance of maternal antibodies against West Nile virus in chicken eggs in South-West Germany. Trop. Med. Int. Health. 2016;21:687–690. doi: 10.1111/tmi.12676. [DOI] [PubMed] [Google Scholar]
- 55.Kenney J.L., Brault A.C. The role of environmental, virological and vector interactions in dictating biological transmission of arthtopod-borne viruses by mosquitoes. Adv. Virus Res. 2014;89:39–83. doi: 10.1016/B978-0-12-800172-1.00002-1. [DOI] [PubMed] [Google Scholar]
- 56.Vogels C.B.F., Göertz G.P., Pijlman G.P., Koenradt C.J.M. Vector competence of northern and southern European Culex pipiens pipiens mosquitoes for West Nile virus across a gradient of temperatures. Med. Vet. Entomol. 2017;31:358–364. doi: 10.1111/mve.12251. [DOI] [PubMed] [Google Scholar]
- 57.Cadar D., Lühken R., van der Jeugd H., Gariglyani M., Ziegler U., Keller M., Lahoreau J., Lachmann L., Becker N., Kik M. Widespread activity of multiple lineages of Usutu virus, Western Europe, 2016. Eurosurveillance. 2017;22:pii30452. doi: 10.2807/1560-7917.ES.2017.22.4.30452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lühken R., Jöst H., Cadar D., Thomas S.M., Bosch S., Tannich E., Becker N., Ziegler U., Lachmann L., Schmidt-Chanasit J. Distribution of Usutu Virus in Germany and its effect on breeding bird populations. Emerg. Infect. Dis. 2017;23:1994–2001. doi: 10.3201/eid2312.171257. [DOI] [PMC free article] [PubMed] [Google Scholar]