Summary
Multicellular lifestyle requires cell-cell connections. In multicellular cyanobacteria, septal junctions enable molecular exchange between sister cells and are required for cellular differentiation. The structure of septal junctions is poorly understood, and it is unknown whether they are capable of controlling intercellular communication. Here, we resolved the in situ architecture of septal junctions by electron cryotomography of cryo-focused ion beam-milled cyanobacterial filaments. Septal junctions consisted of a tube traversing the septal peptidoglycan. Each tube end comprised a FraD-containing plug, which was covered by a cytoplasmic cap. Fluorescence recovery after photobleaching showed that intercellular communication was blocked upon stress. Gating was accompanied by a reversible conformational change of the septal junction cap. We provide the mechanistic framework for a cell junction that predates eukaryotic gap junctions by a billion years. The conservation of a gated dynamic mechanism across different domains of life emphasizes the importance of controlling molecular exchange in multicellular organisms.
Keywords: multicellularity, cell-cell connections, membrane trafficking, septal junctions, cyanobacteria, electron cryotomography, subtomogram averaging, fluorescence recovery after photobleaching
Graphical Abstract
Highlights
-
•
The in situ architecture of septal junctions reveals cap, plug, and tube modules
-
•
Septal junctions reversibly control cell-cell communication upon stress
-
•
FraD is a structural element of the septal junction plug module
-
•
Bacterial septal junctions are mechanistically analogous to metazoan gap junctions
The in situ architecture of cyanobacterial septal junctions reveals a gated intercellular communication channel that evolutionarily predates eukaryotic gap junctions by a billion years.
Introduction
The evolution of multicellular organisms required the invention of structures mediating intercellular molecular exchange to allow division of specialized tasks among sister cells (Brunet and King, 2017). Metazoan cells communicate via gap junctions, which are multimeric protein complexes that form two hemi-channels and can control molecular exchange by a dynamic conformational change (Hervé and Derangeon, 2013, Unwin and Zampighi, 1980). In plants, plasmodesmata generate continuity between the cytoplasm of neighboring cells. However, they are mainly composed of membranes, their structure is highly heterogeneous, and closing is possible by polysaccharide (callose) deposition on a timescale of only hours to days (Oparka et al., 1999, Sager and Lee, 2014).
Filamentous cyanobacteria are true multicellular organisms that exhibit cell-cell communication (Mullineaux et al., 2008). Under nitrogen limiting conditions, strains of the order Nostocales differentiate N2-fixing heterocysts in a semiregular pattern along the filament, which supply the neighboring vegetative cells with nitrogen-fixation products in form of glutamine and the dipeptide β-aspartyl-arginine (Burnat et al., 2014, Thomas et al., 1977). Vegetative cells, in turn, fix CO2 via oxygenic photosynthesis and provide heterocysts with sucrose as a carbon and energy source (Cumino et al., 2007, Jüttner, 1983). In addition to metabolites, signaling molecules need to be exchanged to establish the correct pattern of differentiated cells along the filament (Flores and Herrero, 2010, Flores et al., 2016, Maldener et al., 2014).
Exchanged molecules need to traverse the septum between two adjacent cells in a filament. In multicellular cyanobacteria, this septum contains one peptidoglycan (PG) disc and two cytoplasmic membranes (Hoiczyk and Baumeister, 1995, Lehner et al., 2013). The outer membrane, however, continuously surrounds the entire filament without entering the septum (Flores et al., 2006). The existence of pores in the septal PG has been known for decades (Metzner, 1955). Investigation of the septal PG of Nostoc punctiforme and Anabaena sp. PCC 7120 (hereafter Anabaena) by conventional electron microscopy (EM) methods revealed the presence of 80–150 nanopores, each ∼20 nm in diameter. These so-called nanopore arrays were shown to be required for cell-cell communication (Lehner et al., 2013, Nürnberg et al., 2015). AmiC-type cell wall-lytic amidases were suggested to drill nanopores into septal PG. Consistently, AmiC mutants were affected in cell-cell communication and cell differentiation (Berendt et al., 2012, Bornikoel et al., 2017, Lehner et al., 2011, Lehner et al., 2013).
The nanopores in the septal PG were proposed to accommodate cell-cell joining structures that traverse the septal space between neighboring cells (for reviews, see Flores et al., 2016, Flores et al., 2019). Early studies observed structures perpendicular to the cytoplasmic membranes of adjacent cells and referred to them as microplasmodesmata, in analogy to plasmodesmata in plants, suggesting cytoplasmic continuity (Giddings and Staehelin, 1978, Giddings and Staehelin, 1981, Lang and Fay, 1971). Later studies visualized channels in the septum between cells by electron tomography and suggested that they were proteinaceous (Omairi-Nasser et al., 2014, Wilk et al., 2011). The field has now settled on the widely used term “septal junctions” (SJs), describing cell-cell joining structures in multicellular cyanobacteria that might establish a direct connection between the cytoplasm of neighboring cells (Mariscal, 2014, Flores et al., 2016).
The structural components of SJs are still unknown. However, the predicted membrane proteins FraC, FraD, and SepJ (also termed FraG) are considered as possible candidates (for reviews, see Flores et al., 2016, Flores et al., 2019, Herrero et al., 2016). They were all shown to localize to the septum between vegetative cells by fusion with GFP and/or immuno-labeling (Flores et al., 2007, Merino-Puerto et al., 2010, Merino-Puerto et al., 2011). Mutants in these proteins are impaired in filament integrity and cell-cell communication, and they possess a significantly reduced number of nanopores (Bauer et al., 1995, Mariscal et al., 2011, Nayar et al., 2007, Nürnberg et al., 2015). Furthermore, AmiC1 is necessary for the correct localization of SepJ at the septum (Bornikoel et al., 2017), indicating a functional link between FraC/D, SepJ, and AmiC. More recently, the PG-binding protein SjcF1 was described as an additional factor involved in the formation of nanopores and septal junctions via the interaction of SjcF1 with FraC and SepJ. A SjcF1 mutant was also impaired in intercellular transport (Rudolf et al., 2015).
Molecular exchange through SJs occurs by diffusion (Mullineaux et al., 2008, Nieves-Morión et al., 2017), but it remains unclear whether and how SJs can control cell-cell communication under certain conditions or in stress situations (Flores et al., 2019). Single cells within a cyanobacterial filament can burst or die due to predator attack, shear force or senescence. Furthermore, the filaments fragment when the intercalated heterocysts decease (Nürnberg et al., 2015). Little is known how the organism can ensure the survival of the remaining filament in such settings. Here, we set out to uncover the architecture and mechanism of this primordial type of cell-cell junction.
Results and Discussion
In Situ Architecture of Septal Junctions Reveals Tube, Plug, and Cap Modules
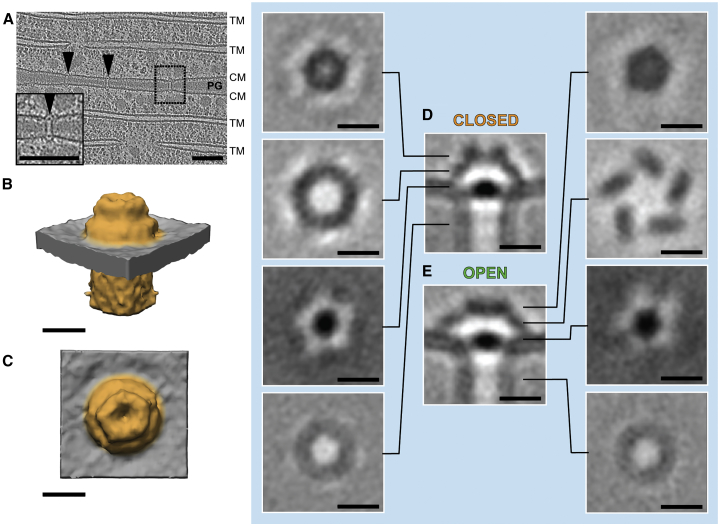
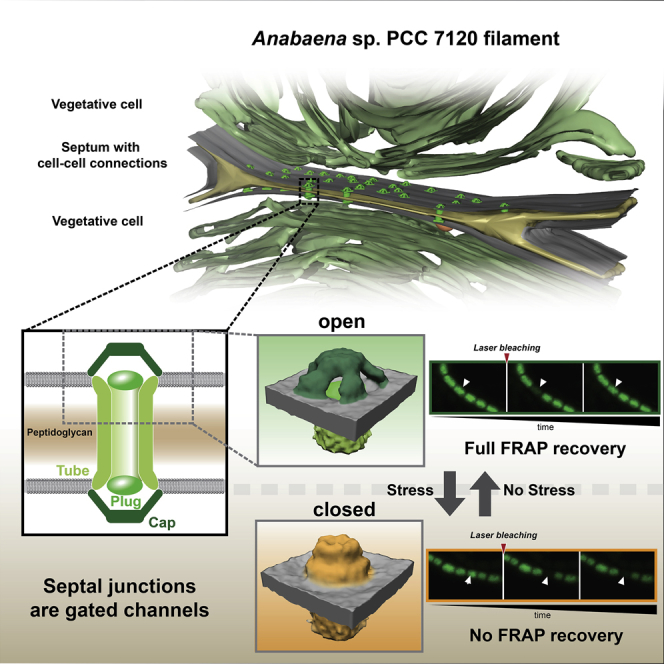
We imaged Anabaena cells by electron cryotomography (ECT) to reveal the architecture of SJs in situ and in a near-native state. To obtain a sample that was thin enough for ECT imaging, we plunge-froze cells on EM grids and prepared lamellae using cryo-focused ion beam (FIB) milling (Figure S1) (Marko et al., 2007, Medeiros et al., 2018, Rigort et al., 2010, Schaffer et al., 2017). Despite the generally relatively low throughput of the FIB milling approach, for this study we generated a comprehensive dataset of ∼480 tomograms that were recorded on an unprecedented total number of ∼120 lamellae. Tomograms of septa between vegetative cells revealed numerous putative SJs that appeared as tubular structures traversing the septum (Figures 1A and 1B; Video S1). In a 200 nm thick lamella, an average of 9.8 SJs were clearly visible (n = 22 tomograms), consistent with the reported number of ∼80 nanopores in a septum (Bornikoel et al., 2017). Structures resembling SJs were never observed in the lateral cell wall. The cross-sectional density plot of a SJ suggests that a tube structure was embedded into the septal PG (rather than the PG nanopore being empty), and the tube lumen density was relatively low compared to the PG (Figures S2A and S2B). Depending on the thickness of the septum, the length of the tube module varied between 26 and 79 nm (average 37.9 nm ± 7.1 nm, n = 208, Figure S2C), suggesting a multimeric nature of the tube.
Figure S1.
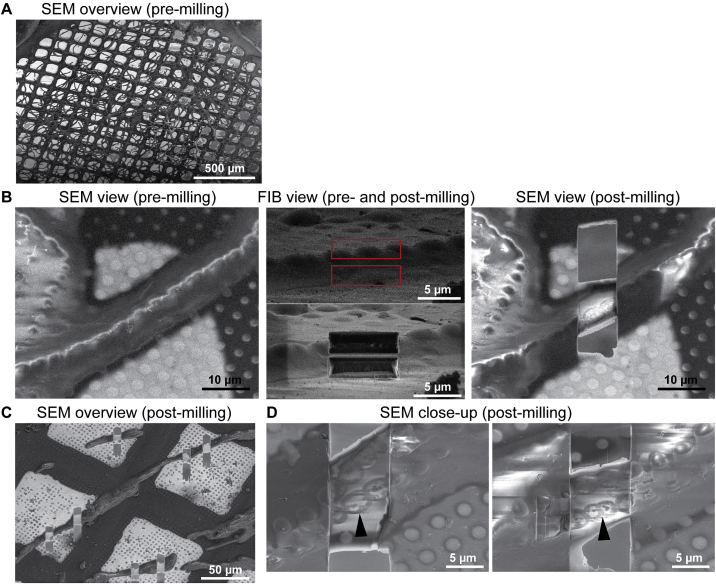
CryoFIB-Milling of Anabaena Filaments, Related to Figure 1
(A) Shown is a cryo-scanning electron microscopy (SEM) image of an EM grid with plunge-frozen Anabaena filaments.
(B) Shown is one example for the preparation of a lamella through a filament. The target was identified in SEM view (SEM view, pre-milling). The focused ion beam (FIB) was used to inspect the same filament from a shallow angle (FIB view) and to choose a milling pattern (red box, upper panel). Material was then removed using the FIB and inspected again (FIB view, lower panel). The final lamella was inspected again by SEM (SEM view, post-milling).
(C) The procedure was repeated for 9-24 lamellae. Shown is a SEM overview image of a grid area with seven lamellae.
(D) Two examples of cryoFIB-milled lamellae through Anabaena filaments. Details like cell outline (arrowheads) or thylakoid membranes are already detectable.
Figure 1.
In Situ Architecture of Septal Junctions Reveals Tube, Plug, and Cap Modules
(A and B) Cryotomograms (magnified views in boxes) of a FIB-milled Anabaena filament. The two different slices at different Z-heights show the septum between adjacent vegetative cells. Multiple SJs were seen crossing the septum (arrowheads). The SJ lengths were precisely adjusted to the septum thickness. CM, cytoplasmic membrane; OM, outer membrane; PB, phycobilisomes; PG, septal peptidoglycan; TM, thylakoid membranes. Bars, 100 nm. Shown are projections of 13.5 nm-thick slices.
(C–F) Subtomogram averaging of SJ ends revealed three structural modules: tube, cap, and plug. Shown is a 0.68 nm-thick tomographic slice through the average (C), a schematic representation of SJ modules (D; modules segmented in different shades of green), and oblique (E) and top (F) views of a surface representation (modules were segmented to match colors in D). The cap consisted of a ceiling that was held by five arches. Bars, 10 nm.
See also Figures S1 and S2 and Videos S1 and S2.
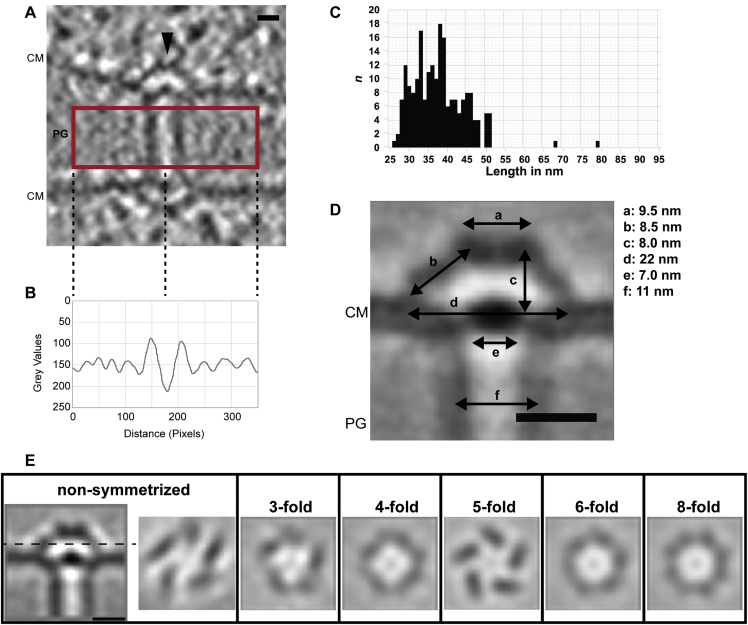
Figure S2.
Analysis of SJs in Anabaena Wild Type, Related to Figure 1
(A) Shown is a cryotomogram (9.45 nm-thick slice) of a SJ (arrowhead). The area indicated by the red box was used to calculate a density plot. CM, cytoplasmic membrane; PG, septal peptidoglycan. Bar, 10 nm.
(B) The density plot of the area indicated in (A) revealed that the SJ tube had a higher density than the surrounding PG and the tube lumen had a lower density than the PG.
(C) SJ length distribution in Anabaena PCC 7120 wild-type. SJ lengths were measured from plug to plug and their occurrence was plotted in the graph. n = 208 SJs
(D) Measurements of SJ structural features. The indicated measurements were performed using the subtomogram average of the SJ end shown in Figure 1 C/E/F. CM, cytoplasmic membrane; PG, septal peptidoglycan. Bar, 10 nm.
(E) The SJ cap module has 5-fold rotational symmetry. The cross-sectional view (position indicated in the longitudinal view by dashed line) of the non-symmetrized subtomogram average indicated a rotational 5-fold symmetry of the cap module. To further investigate this, different symmetries (indicated) were applied to the non-symmetrized subtomogram average (1.35 nm pixel size). The strongest reinforcement of densities was seen in the 5-fold symmetrized average. Bar, 10 nm.
Septal junctions are colored in lime green; thylakoid membranes in dark green; peptidoglycan in gray; outer and cytoplasmic membranes in brown; granules in light brown and yellow. Bar, 100 nm.
In addition to the tube module, the tomograms revealed a cytoplasmic cap-like structure, as well as a plug-like density in the cytoplasmic membrane (CM) (Figures 1A and 1B). Both ends of each SJ comprised cap and plug modules, without any recognizable differences between both ends. To increase contrast and resolution, we performed subtomogram averaging of 446 SJ ends (Figures 1C–1F and S2D; Video S2). The average resolved that the 11 nm-wide tube (lumen of 7 nm) made direct contact with the CM. No bilayer-like density was observed in the SJ tube wall, supporting an earlier report (Wilk et al., 2011) that suggested that the periplasm-spanning tube was assembled of proteinaceous subunits. The plug (7 nm × 2.5 nm) was sitting at the end of the tube at the level of the CM. The cap module was a 5-fold rotationally symmetric (Figure S2E) dome (8 nm height) covering the tube end. The ceiling had a diameter of 9.5 nm and was held by five arches with lengths of 8.5 nm.
Shown are longitudinal views, perpendicular views and a surface rendering. Bar, 10 nm.
Intercellular Communication Ceases upon Ionophore Treatment in a Reversible Manner
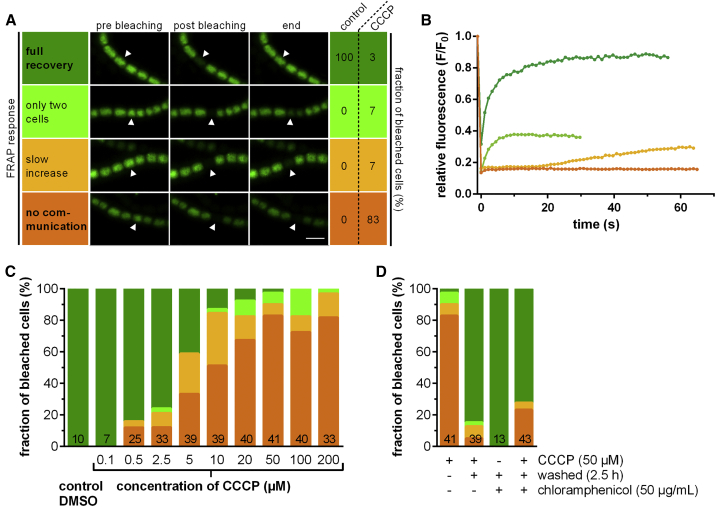
The structural complexity of the SJ ends led us to speculate that the assembly might allow the control of intercellular molecular diffusion. Cyanobacterial intercellular communication was studied previously by monitoring the exchange rate of fluorescent tracers, like calcein, 5-carboxyfluorescein diacetate and esculin by fluorescence recovery after photobleaching (FRAP) (Mullineaux et al., 2008, Nürnberg et al., 2015). We therefore monitored the molecular exchange rate of calcein by FRAP after challenging the proton motif force. We treated cells with carbonyl cyanide 3-chlorophenylhydrazone (CCCP), a protonophore that dissipates the proton gradient across membranes (Hopfer et al., 1968) and measured the FRAP response. Upon treatment with 50 μM CCCP, 83% of the analyzed cells ceased to exchange calcein, showing a “no communication” response after bleaching (Figures 2A and 2B; Video S3). This is in contrast to the control experiment (control cells were treated with DMSO to exclude effects of the solvent), where all cells displayed “full recovery” of fluorescence (Figure 2A). Seven percent of CCCP-treated cells were assigned to a “slow increase” response, because fluorescence recovery was delayed (started only 20–60 s after bleaching) and reached only <50% of the initial fluorescence (Figures 2A and 2B). In further 7% of the cells, exchange took place only with a single neighboring cell. Only 3% of CCCP-treated cells showed a normal “full recovery” FRAP response (Figures 2A and 2B). The fraction of non-communicating cells was dependent on the concentration of CCCP (Figures 2C and S3A), which had no effect below 0.5 μM. Concentrations above 50 μM did not further enhance the inhibition of molecular exchange.
Figure 2.
Intercellular Communication Ceases upon Ionophore Treatment in a Reversible Manner
(A) FRAP analysis of cells that were stained with fluorescent calcein. In the control experiment (control cells were treated with DMSO to exclude effects of the solvent), all cells showed full recovery of fluorescence after bleaching. After treatment with the ionophore CCCP (50 μM in DMSO), the bleached cells showed four different types of FRAP responses: “full recovery,” “slow increase” (delayed recovery to <50% of original fluorescence), “only two cells” (exchange of calcein only with one neighboring cell), and “no communication” (no recovery). For each FRAP response, representative images are shown at three time points (5 s before bleaching, ∼0.5 s after bleaching, 30–60 s after bleaching). Arrowheads point to the bleached cells. Anabaena was apparently able to control communication upon challenging the proton motif force, because the majority of CCCP-treated cells showed “no communication.” Bar, 5 μm.
(B) Fluorescence recovery curves corresponding to the four FRAP responses that were observed in (A) (color scheme identical to A). Time point t = 0 shows the analyzed cell directly after bleaching.
(C) Cell-cell communication after increasing the concentration of CCCP (color scheme identical to A). The effect of CCCP on cell-cell communication was concentration-dependent for CCCP concentrations between 0.5–50 μM. In the control experiment, cells were treated with 0.002% DMSO. Numbers within the bars indicate the number of analyzed cells (n) from different filaments and represent cumulated results from at least two independent cultures (except for 0.1 μM CCCP).
(D) Recovery of cell-cell communication after incubation in fresh medium lacking CCCP and in the presence of chloramphenicol (color scheme identical to A). Regaining cell-cell communication was independent of de novo protein synthesis, “+” and “−” indicate the presence and absence of CCCP, washing in fresh medium, and chloramphenicol. Numbers in bars indicate number of analyzed cells (n). Shown are cumulated results from at least two independent cultures.
Figure S3.
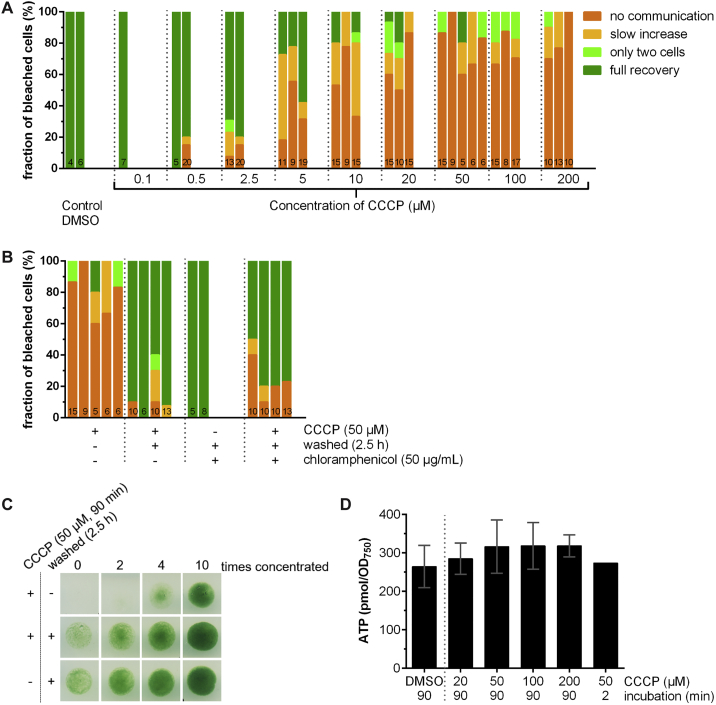
FRAP Response Distributions of Independent Cultures and Viability after CCCP Treatment, Related to Figure 2
(A and B) Shown are the distributions of FRAP responses of independent cultures (one bar represents one culture), to complement the cumulative results that are shown in Figure 2. Numbers within the bars indicate the number of analyzed cells n from different filaments. The shown graph in (A) corresponds to Figure 2C. The shown graph in (B) corresponds to Figure 2D.
(C) Cells that were washed after CCCP treatment were viable. Anabaena was grown for three days on a Bg11 agar plate, resuspended to OD750 = 1.2, split and processed by different treatments (indicated on the left, details described in STAR Methods). Subsequently, 10 μL of different concentrations of the cultures were spotted onto a solid Bg11 agar plate and incubated for two days before taking a picture of the plate (shown). No difference in viability was observed between the control (no CCCP treatment) and CCCP-treated/washed cells. All spots were grown on the same plate, white lines were introduced for a clear view.
(D) The ATP level of Anabaena cells is not affected by CCCP treatment. The ATP content of Anabaena cells was determined in cells treated with only DMSO (control to mimic the addition of calcein and CCCP), and cells treated with DMSO and different concentrations of CCCP for 90 min or 2 min as indicated in graph. The measured ATP level was normalized to OD750 = 0.6. Bars show the mean ± SD of two biological replicates with two technical replicates (one biological replicate for 2 min incubation with 50 μM CCCP). Differences between CCCP treated cells compared to DMSO control cells were not significant (Student’s t test).
Single cells were bleached after 5 s (indicated by arrows).
To test whether CCCP inhibited cell-cell communication in a reversible manner, cells were washed after a 50 μM CCCP treatment and incubated in fresh medium for 2.5 h at room temperature. Eighty-five percent of the cells resumed communication, suggesting that the inhibition of molecular exchange was indeed reversible (Figures 2D and S3B) and cells were still viable after recovering from CCCP treatment (Figure S3C). We then set out to explore whether the re-opening of SJs required the synthesis of new proteins. Hence, cells were treated with CCCP, washed, and incubated for 2.5 h in fresh medium supplemented with 50 μg/mL chloramphenicol (inhibiting protein synthesis) before monitoring the FRAP response (Figures 2D and S3B). Because 72% of the tested cells were able to restore communication (showing “full recovery” response), we concluded that the reversibility of communication was based on an opening mechanism of SJs that was independent of de novo protein synthesis. To check whether the ATP level within the cells was altered by CCCP treatment, we analyzed the ATP content of Anabaena cells after CCCP treatment. No significant differences between the control (DMSO-treated cells) and CCCP-treated cells were detected (Figure S3D), indicating that the cellular ATP level was not a signal for gating communication.
Ceased Intercellular Communication after Ionophore Treatment Coincides with a Major Structural Rearrangement of the Septal Junction Cap
To investigate whether a structural change in the macromolecular architecture of SJs was involved in the gating of cell-cell communication, we plunge froze CCCP-treated Anabaena cells and acquired tomograms of septal areas. Differences were hardly detectable in individual tomograms (n = 13 tomograms, Figure 3A). However, subtomogram averaging (n = 188 SJ ends) revealed a striking conformational change in the cap module, whereas the tube and plug modules remained unchanged (Figures 3B–3D; Video S4). Compared to the cap structure in untreated cells (Figure 3E), the individual arches were not anymore detectable, and the cap did not reveal any detectable openings (Figures 3B and S4A; Video S5). The structural rearrangement also resulted in a tightening of the cap by 6 nm and the introduction of a small cavity on the ceiling of the cap. It is possible that the closed conformation of the cap could arise from rotations of the individual arches (Figures S4B–S4E). The reversibility of SJ closing upon CCCP treatment that was seen by FRAP was confirmed by ECT imaging of cells that were CCCP-treated, washed, and incubated in fresh medium for 2.5 h before plunge freezing. The SJ architecture from these recovered cells matched the untreated (open) conformation without detectable differences (n = 8 tomograms, 66 SJ ends, Figures S4F–S4H). In order to further examine the time frame in which SJ closure was observed, we incubated Anabaena with CCCP for the shortest possible duration prior to FRAP and ECT imaging. FRAP experiments indicated that intercellular communication already ceased in <4 min after CCCP treatment (Figure S4I). ECT and subtomogram averaging (n = 5 tomograms, 28 SJ ends) revealed SJs in the closed conformation already after 45 s of CCCP treatment (Figures S4J–S4L).
Figure 3.
Ceased Intercellular Communication after Ionophore Treatment Coincides with a Major Structural Rearrangement of the Septal Junction Cap
(A) Shown is a 13.5 nm-thick slice through a cryotomogram (magnified view in box) of the septal area of a CCCP-treated Anabaena filament. SJs are indicated by arrowheads. CM, cytoplasmic membrane; PG, septal peptidoglycan; TM, thylakoid membranes. Bar, 100 nm.
(B–E) Subtomogram averaging of SJs in the CCCP-treated non-communicating “closed” state (B–D) revealed major structural rearrangements in the cap module, compared to the “open” state (E). Shown are surface representations (B and C), and longitudinal and cross-sectional slices (0.68 nm) through the averages (D and E). Sliced positions are indicated in (D) and (E). Bars, 10 nm.
Figure S4.
SJ Gating Is Fast and Reversible, Related to Figure 3
(A) Shown are Fourier Shell Correlation (FSC) curves of two half-datasets of subtomogram averages of SJs in open (green) and closed (orange) states, respectively. The estimated resolution of the averages was ∼28 Å.
(B–E) Speculative model of cap closure by arch rotations. (B) Perpendicular cross-section (0.68 nm thickness) of the subtomogram average of the SJ in the open conformation. The arches are represented by green ellipses. (C) Schematic indicating a 30° rotation of each arch around a rotation center (orange). (D) Schematic indicating the arches after the rotation indicated in (C). The result was a closed circle. (E) Overlay of the model in the closed state with a perpendicular cross-section (0.68 nm thickness) of the SJ in the closed conformation. Bars, 10 nm.
(F–H) The structural rearrangement of SJs upon ionophore treatment is reversible. Wild-type Anabaena cells were incubated for 90 mins with 50 μM CCCP. Afterward, cells were washed three times, incubated in fresh medium for 2.5 h and plunge frozen. The subtomogram average revealed SJs in their open state (F/G). Dashed line in (F) indicates the position of the cross sectional view shown in (G). The five arches of the SJ cap, a hallmark for the SJ open state, are detectable. Surface representation (H) of the SJ cap generated from the subtomogram average shown in (F/G). Bars, 10 nm.
(I–L) Intercellular communication is impaired within seconds upon CCCP treatment. Wild-type Anabaena cells were incubated with CCCP. FRAP analyses [shown in (I)] were performed either between 1.5 min and 4 min after the addition of CCCP, or more than 90 min after the addition of CCCP (data from Figure 2). Already after the short incubation time of ≤ 4 min, the majority of cells already ceased communication. Numbers within the bars indicate the number of analyzed cells n from different filaments. (J-L) show a subtomogram average (J/K) and surface representation (L) of SJs from Anabaena wild-type cells that were treated with CCCP (50 μM) for 45 s. The average [longitudinal view in (J); cross section in (K)] shows SJs in their closed state [dashed line indicates the position of the cross sectional view in (K)]. No individual arches are detectable. Bars, 10 nm.
Shown are longitudinal views, perpendicular views and a surface rendering. Bar, 10 nm.
Top view of SJs. Green, open; orange, closed. Bar, 10 nm.
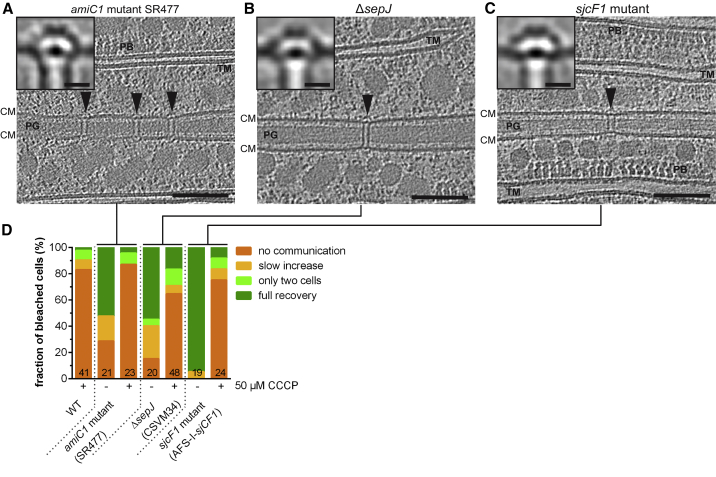
AmiC1, SepJ, and SjcF1 Mutants Are Impaired in Intercellular Communication but Nevertheless Able to Control Molecular Exchange
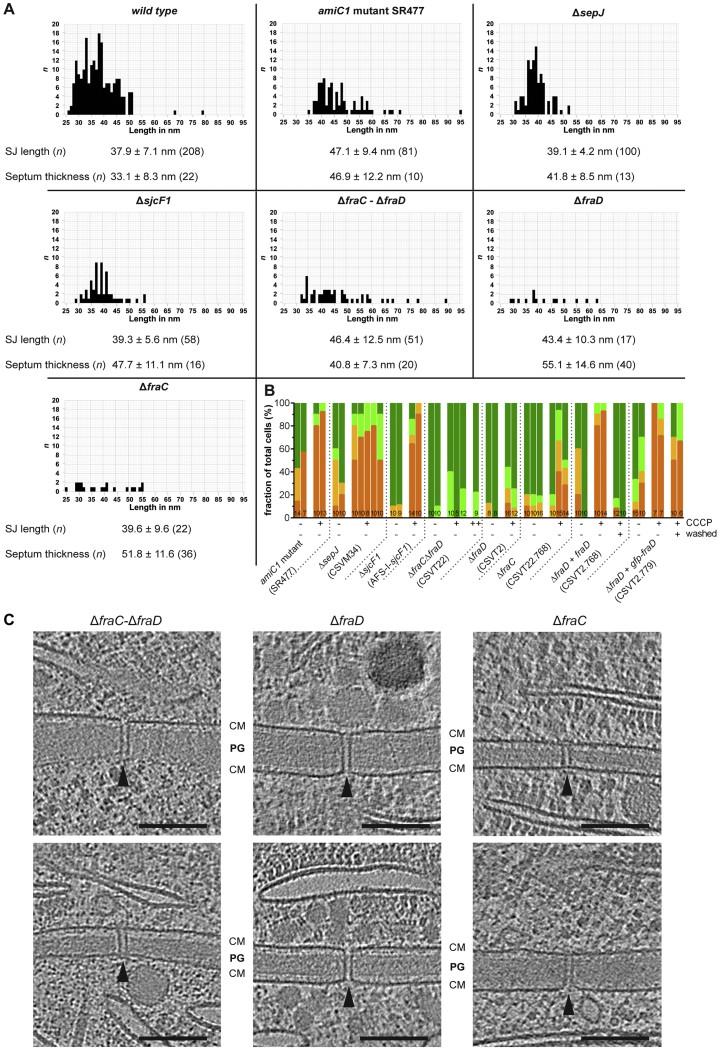
AmiC1, SepJ, and SjcF1 were proposed to play important roles in the formation of nanopores and SJs (Flores et al., 2016). We therefore analyzed the mutants’ SJ architectures (Figures 4A–4C) as well as their ability to control intercellular molecular exchange (Figure 4D). The number of SJs was significantly reduced in all tested mutants, except for ΔsjcF1, which is consistent with previous quantifications of nanopore arrays (Bornikoel et al., 2017, Nürnberg et al., 2015, Rudolf et al., 2015). The septa of all mutants were also wider, which was reflected in the increased SJ average length (Figure S5A).
Figure 4.
AmiC1, SepJ, and SjcF1 Mutants Are Impaired in Intercellular Communication but Nevertheless Able to Control Molecular Exchange
(A–C) Cryotomograms (shown are 13.5 nm-thick projections; bars, 100 nm) of different Anabaena mutants. Subtomogram averages of SJs (insets in A–C; bars, 10 nm) showed that neither the amiC1 mutant SR477 (A), nor ΔsepJ (B), nor the sjcF1 mutant (C) were missing structural modules. CM, cytoplasmic membrane; PB, phycobilisomes; PG, septal peptidoglycan; TM, thylakoid membranes.
(D) FRAP responses of the wild type and the mutants shown in (A)–(C). The amiC1 mutant SR477 and the sepJ mutant showed that compared to the wild type, a reduced fraction of cells was able to communicate already in the absence of CCCP (likely based on the lower total number of SJs). However, the open SJs of these mutants were able to close upon CCCP treatment, consistent with the unaltered SJ structure. The sjcF1 mutant was not impaired in closing its SJs upon CCCP treatment. “+” and “−” indicate the presence and absence of CCCP. Numbers within the bars indicate the number of analyzed cells (n) from different filaments. Results from at least two independent cultures were cumulated.
See also Figure S5.
Figure S5.
Details and Examples of Analyzed SJ Mutants and Their FRAP Response Distribution of Independent Cultures, Related to Figures 4 and 5
(A) SJ lengths in wild-type and mutant strains were measured from plug to plug and their frequency was plotted in the graphs. The data were also used to calculate the average SJ length. We also measured the septum thickness (as the shortest distance between the inner membranes within a tomogram of a septal region), showing an increase for all mutant strains.
(B) Shown are the distributions of FRAP responses of independent cultures (one bar represents one culture), to complement the cumulative results that are shown in Figures 4 and 5. Numbers within the bars indicate the number of analyzed cells n from different filaments. +: 50 μM CCCP; ++: 200 μM CCCP
(C) Further examples of cryotomograms showing SJs (black arrowheads) from different mutant strains. Shown are 13.5 nm thick slices. CM, cytoplasmic membrane; PG, septal peptidoglycan. Bars, 100 nm.
A subtomogram average of the amiC1 mutant SR477 (n = 6 tomograms, 156 SJ ends, Figure 4A) showed SJs in the open state and did not reveal any structural differences compared to the wild type. When we monitored intercellular molecular exchange by FRAP, we found that only 52% of SR477 cells showed “full recovery,” likely based on the low number of SJs. Upon CCCP treatment, 87% of the analyzed cells showed a “no communication” response (Figures 4D and S5B), suggesting that the low number of SJs could mostly still switch to the closed state. The amidase AmiC1 is therefore unlikely a major structural component of SJs and does not play a role in the gating mechanism, which is consistent with previous data (Bornikoel et al., 2017, Bornikoel et al., 2018, Lehner et al., 2013, Nürnberg et al., 2015).
The subtomogram average of SJs of a ΔsepJ strain (n = 6 tomograms, 62 SJ ends) revealed wild type architecture in the open state (Figure 4B). Compared to the wild type, ΔsepJ cells showed impaired intercellular communication, possibly based on the lower total number of SJs. Upon CCCP treatment, 65% of ΔsepJ cells showed a “no communication” response, which indicates that SJs could still close (Figures 4D and S5B). The presence of SJs in the ΔsepJ mutant is consistent with previous conventional EM studies that detected cell-cell connections in this mutant (Omairi-Nasser et al., 2015, Wilk et al., 2011). The overexpression of SepJ was reported to result in an increased number of nanopores (Mariscal et al., 2016). Other studies suggested a role of the divisome for the subcellular localization of SepJ (Ramos-León et al., 2015) and found that a SepJ-GFP fusion migrates with the FtsZ ring during cell division and is finally found in a central localized spot within the septum (Flores et al., 2007). Taken together, SepJ is likely not a major structural component of SJs, but rather plays a role in coordinating septum maturation and precise amidase-dependent placement of nanopores.
A subtomogram average of SJs of sjcF1 mutant cells (n = 7 tomograms, 116 SJ ends) also revealed wild type architecture in the open state (Figure 4C). 75% of the mutant cells showed a “no communication” response upon CCCP treatment, which is comparable to the wild type (Figures 4D and S5B). These findings are consistent with a previous study, suggesting that SjcF1 is not an essential component of SJs, but rather plays a regulatory role in nanopore formation (Rudolf et al., 2015).
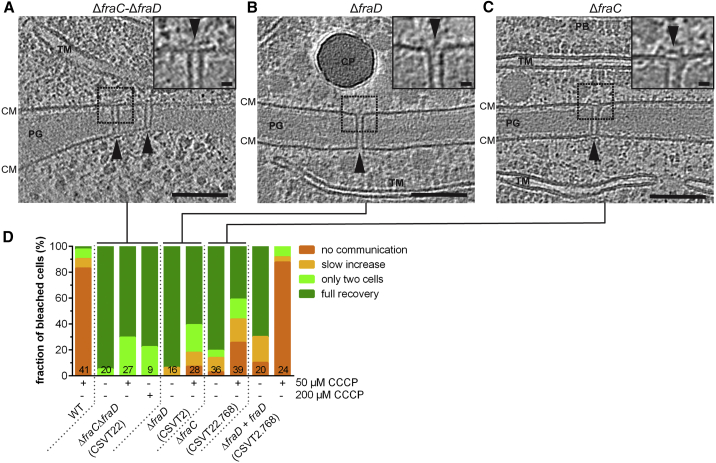
The Cap and Plug Modules Are Required to Control Intercellular Communication upon Ionophore Treatment
FraC and FraD were also reported to be related to SJs and important for filament integrity (Merino-Puerto et al., 2010, Merino-Puerto et al., 2011). We therefore analyzed the architecture of SJs in different mutants. SJs in ΔfraC-ΔfraD (n = 7 tomograms) (Figures 5A and S5C) and ΔfraD (n = 7 tomograms) (Figures 5B and S5C) mutants were missing the cap and plug modules. By contrast, the ΔfraC mutant showed a heterogeneous phenotype, exhibiting a mixture of fully assembled SJs, SJs without cap and plug, and presumably misassembled SJs (n = 11 tomograms) (Figures 5C and S5C). Importantly, none of the analyzed ΔfraC-ΔfraD cells and only 7% of the ΔfraD cells showed a “no communication” response upon CCCP treatment, even at high CCCP concentrations (Figures 5D and S5B), whereas in the ΔfraC filaments, a fraction of 26% of the cells were still able to control communication. Taken together, our data suggest that the cap and/or plug structures are required to close SJs and thereby terminate intercellular molecular diffusion. FraC might be important for correct localization and assembly of SJ components, as proposed earlier (Merino-Puerto et al., 2011). FraD, on the other hand, emerges as a possible candidate for a SJ major structural component. This is supported by the presence of five predicted transmembrane helices, as well as the localization of FraD across the entire septum in GFP-fusion and Immunogold labeling experiments (Merino-Puerto et al., 2010, Merino-Puerto et al., 2011).
Figure 5.
The Cap and Plug Modules Are Required to Control Intercellular Communication upon Ionophore Treatment
(A–C) SJs from the ΔfraC-ΔfraD (A) and ΔfraD (B) mutants were missing the cap and plug modules. SJs from ΔfraC (C) showed a mixture of fully assembled and misassembled SJs (insets show magnified views; bars, 10 nm). Shown are 13.5 nm-thick sections through cryotomograms; bars, 100 nm. CM, cytoplasmic membrane; CP, cyanophycin; PB, phycobilisomes; PG, septal peptidoglycan; TM, thylakoid membranes.
(D) FRAP experiments of the wild type and the mutants shown in (A)–(C). The CCCP-treated ΔfraD and ΔfraC single mutants showed a much smaller fraction of non-communicating cells than in the CCCP-treated wild type, indicating that the mutants were unable to gate communication. Strain CSVT2.768 is a complementation of the ΔfraD mutant and showed wild type behavior. “+” and “−” indicate the presence and absence of CCCP. Numbers within the bars indicate the number of analyzed cells (n) from different filaments. Results from at least two independent cultures were cumulated (except for ΔfraC-ΔfraD treated with 200 μM CCCP).
See also Figure S5.
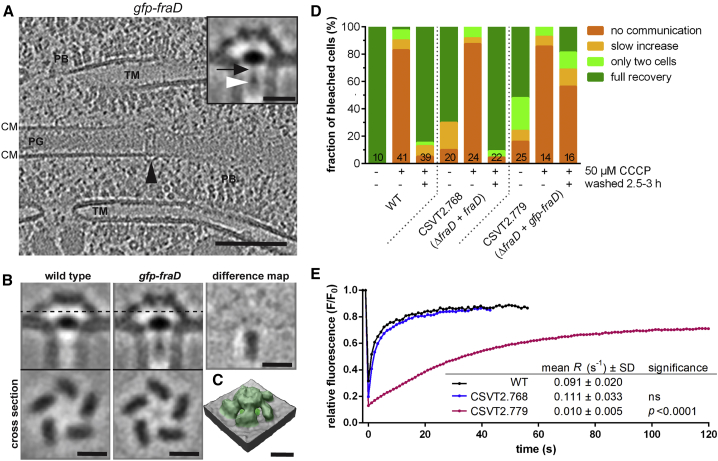
FraD Localizes to the SJ Plug
We continued to investigate whether FraD was indeed a structural component of SJs. Because there are no widely used protein localization tags suitable for ECT, other studies visualized GFP as an extra density within subtomogram averages (Chang et al., 2016, Hu et al., 2017). We therefore generated a subtomogram average of SJs in a mutant expressing an N-terminal fusion of GFP to FraD. Strikingly, the average (n = 17 tomograms, 220 SJ ends) revealed an extra density in the lumen of the SJ tube, directly adjacent to the plug (Figures 6A, S6A, and S6B; Video S6). A difference map that was generated using the wild type and GFP-FraD SJ averages identified the density in the tube lumen as the major difference and indicated an otherwise similar architecture (Figures 6B and 6C). Our data suggest that FraD localized to the SJ plug, with the N terminus likely facing the tube lumen. Without knowledge of the detailed SJ architecture, an earlier study suggested that the FraD C terminus was located in the periplasm (shown by Immunogold labeling), while the N terminus was predicted to be in the cytoplasm (because of structure predictions and because an N-terminal GFP fusion would likely not be fluorescent in the periplasm) (Merino-Puerto et al., 2011). Our data are compatible with these predictions and together with the previous study they suggest that (1) the FraD C terminus extends from the plug into the periplasm, and (2) the tube lumen has in fact cytoplasmic characteristics that allow for the maturation of the N-terminal GFP-fusion.
Figure 6.
FraD Localizes to the SJ Plug
(A) Cryotomograms of Anabaena expressing gfp-fraD showed SJs with partially filled tubes (black arrowhead). Shown is a 13.5 nm-thick slice; bar, 100 nm. Subtomogram averaging (inset; bar, 10 nm) revealed an extra density (white arrowhead) situated in the SJ lumen and connected to the SJ plug (black arrow). CM, cytoplasmic membrane; PB, phycobilisomes; PG, septal peptidoglycan; TM, thylakoid membranes.
(B) The difference map between subtomogram averages of septal junctions from Anabaena wild type and gfp-fraD expressing cells showed that the only major difference between these two structures was the extra density within the tube lumen, most likely corresponding to the GFP fusion. The cross sectional views (location indicated by dashed line) through the caps revealed that SJs from gfp-fraD expressing cells are in the open state. Bar, 10 nm.
(C) Surface representation of the average of the SJ from the gfp-fraD-expressing mutant showed that the cap was in the open state and similar to the wild type structure. Bar, 10 nm.
(D) The ΔfraD mutant CSVT2 complemented with gfp-fraD (CSVT2.779) or fraD (CSVT2.768) expressed from a plasmid was investigated by the FRAP assay under the stated conditions (“+” and “−” indicate the presence and absence of CCCP and washing in fresh medium). The gfp-fraD strain (CSVT2.779) was still able to control communication, even though reopening was less efficient. Numbers within the bars indicate the number of analyzed cells (n) from different filaments. Results from at least two independent cultures were cumulated.
(E) The fluorescence recovery rate constant R was calculated from non-treated cells, showing a “full recovery” FRAP response. (n(WT) = 10, n(CSVT2.768) =14, n(CSVT2.779) = 13). The gfp-fraD strain (CSVT2.779) showed a significantly reduced fluorescence recovery rate constant compared to wild type and the complemented ΔfraD+fraD mutant (CSVT2.768). Significance was determined using Student’s t test in comparison to the wild type. A representative FRAP curve (bleaching at t = 0) for each strain is shown.
Figure S6.
Examples of Cryotomograms Showing SJs of Anabaena Expressing gfp-fraD, Related to Figure 6
(A) Further examples of cryotomograms showing SJs (black arrowheads) from gfp-fraD expressing mutant. Shown are 13.5 nm thick slices. CM, cytoplasmic membrane; PG, septal peptidoglycan.Bars, 100 nm.
(B) Shown is a Fourier Shell Correlation (FSC) curve of two half-datasets of the subtomogram average in Figure 6A, showing SJs from gfp-fraD expressing mutant.
Bars, 100 nm for cryotomogram and 10 nm for subtomogram average.
FRAP analyses of the GFP-FraD expressing mutant revealed that this strain was still able to close SJs after CCCP treatment; however, the reopening was less efficient (Figure 6D). Interestingly, the fluorescence recovery rate constant R was significantly reduced in gfp-fraD cells compared to the wild type and the complemented ΔfraD+fraD mutant (Figure 6E), indicating a diminished diffusion rate through the SJs. This agrees with a reduced diffusion area caused by the presence of GFP (∼2.5 nm × 5 nm) within the tube lumen (7 nm).
Taken together, FraD is likely a major structural component of SJs, localized to the plug module. The analyses of mutants indicate that cap and plug form a functional unit that provides a size cutoff and gating mechanism for intercellular communication. It remains to be seen how these tasks are distributed between both modules. Our study will also serve as a framework for future efforts to identify other SJ building blocks.
SJs Close in Response to Different Stress Factors
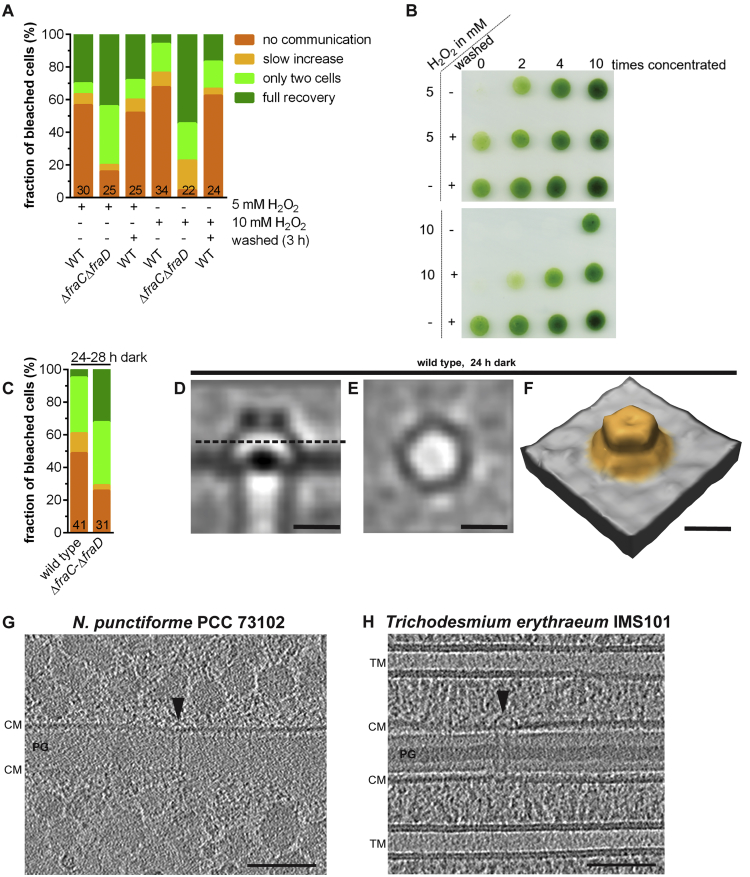
Because gating of intercellular communication is likely to be important under different environmental conditions, we analyzed other stress factors for their ability to induce SJ closure. Oxidative stress was imposed by treatment with 5 mM and 10 mM H2O2 for 3 h. It was shown that H2O2 affects the electron transport chain and therefore the activity of photosystems I and II in cyanobacteria (Samuilov et al., 2001). Similar to CCCP treatments, the fraction of cells showing the FRAP response “full recovery” dropped from 100% to 30% upon treatment with 5 mM H2O2 (Figure S7A). Cells were still viable after this treatment (Figure S7B). Nevertheless, only a small fraction of cells reopened SJs after washing (Figure S7A), indicating secondary effects of the H2O2 treatment, which is also visible in the impaired growth of H2O2-treated and washed cells (Figure S7B). Consistent with the above data is the observation that the ΔfraC-ΔfraD mutant was impaired in ceasing molecular exchange upon H2O2 treatment (Figure S7A).
Figure S7.
Intercellular Communication Was Reduced upon Different Types of Stress, and SJs in Other Cyanobacteria Share Similar Architecture, Related to Figure 7
(A and B) Treatment with H2O2 was tested as an alternative stress. Calcein-stained cells were treated with 5 mM or 10 mM H2O2, followed by washing with fresh medium and further incubation for 3 h when indicated. Molecular exchange of the treated cells was analyzed by FRAP (A). Similar to CCCP treatment, wild-type cells showed a reduced level of communication upon H2O2 treatment, while the ΔfraC-ΔfraD mutant was impaired in ceasing molecular exchange upon H2O2 treatment. Shown are cumulated results from at least two independent experiments. Numbers within the bars indicate the number of analyzed cells n from different filaments. To check the viability of wild-type cells after H2O2 treatment, 10 μL spots were placed onto a Bg11 agar plate and incubated for three days (B). Cells treated with 5 mM H2O2 and washed afterward grew comparable to the untreated control.
(C–F) Incubation in the dark was tested as an alternative stress. Cells were incubated in the dark for 28 h prior to FRAP analyses (C). FRAP indicated that a significant fraction of wild-type cells ceased to communicate upon incubation in the dark, while the ΔfraC-ΔfraD mutant was less effective in ceasing molecular exchange. Shown are cumulated results from at least two independent FRAP experiments. Numbers within the bars indicate the number of analyzed cells n from different filaments. The color code is identical to (A). For ECT imaging (D-E), cells were incubated in the dark for 24 h prior to plunge freezing. Shown is a subtomogram average of SJs from Anabaena wild-type cells. The average shows SJs in the closed state (no individual arches are detectable). The dashed line in (D) indicates the position of the cross sectional view of the cap shown in (E). A surface representation of the subtomogram average is shown in (F). Bars, 10 nm.
(G and H) SJs in other cyanobacteria share a similar architecture. We also cryoFIB-milled and imaged two further cyanobacterial representatives, revealing the presence of SJs (arrowheads) without fundamentally different SJ architecture as compared to Anabaena. Importantly, SJ-related genes (amiC, fraC/fraD, sjcF1 and the C-terminal domain of sepJ) are also present in the genomes of these cyanobacteria. CM, cytoplasmic membrane; PG, septal peptidoglycan; TM, thylakoid membrane. Bars, 100 nm.
We then tested the effect of the absence of light on gating of intercellular communication. After incubation of wild type cells for 28 h in the dark, the “full recovery” FRAP response dropped from 100% to only 5% (Figure S7C). ECT imaging after incubation in the dark for 24 h revealed SJs in the closed conformation (Figures S7D–S7F). Incubation of the ΔfraC-ΔfraD mutant in the dark resulted in a 6-fold higher number of “full recovery” FRAP response compared to the wild type (Figure S7C). The fraction of non-communicating cells in this mutant probably arose from secondary effects caused by the long period in the dark. Nevertheless, there is a clear difference in the regulation of molecular exchange between the wild type and mutant cells.
In summary, we showed that challenging the proton motif force, oxidative stress, and darkness all induce the closure of SJs and therefore allow the cell to gate molecular exchange. It is likely that gating can be induced by a wide range of environmental factors.
Conclusions
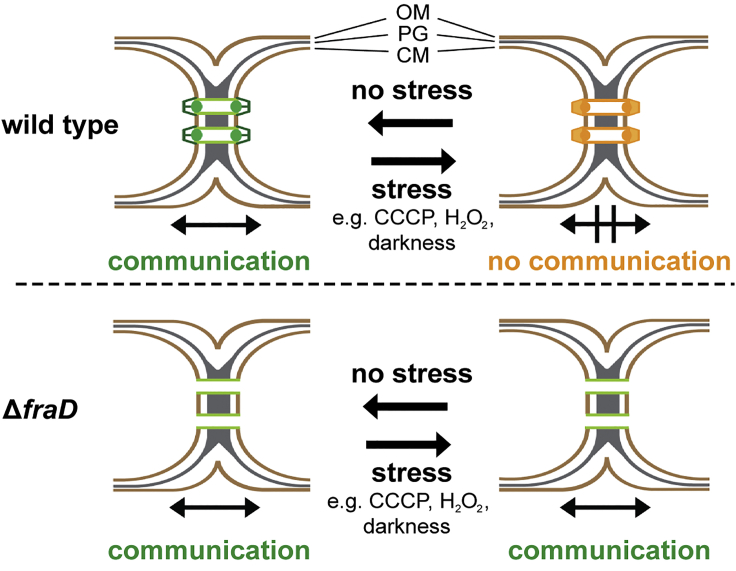
In conclusion, our data suggest that cyanobacterial SJs are dynamic, gated cell-cell connections, which reversibly block intercellular molecular diffusion along the filament upon different types of stress (Figure 7). This challenges the concept that the cyanobacterial filament is a symplast, with SJs providing cytosolic continuity between the cells—analogous to plasmodesmata. SJs rather reveal striking similarities to metazoan gap junctions, because they are both gated by a dynamic conformational change of a proteinaceous macromolecular complex. Furthermore, just like SJs, gap junction closure is triggered by disruption of the proton motif force (Hervé and Derangeon, 2013, Obaid et al., 1983, Socolar and Politoff, 1971). Interestingly, the closure of gap junctions can be only partial (Ek-Vitorin and Burt, 2013), a phenomenon that might also exist in SJs, considering the “slow increase” FRAP response (Figure 2B). Finally, gap junction and SJ closure operates on a similar timescale of only seconds (Figures S4I–S4L). Despite the functional analogy between SJs and gap junctions, the involved proteins do not have a last common ancestor, which is reflected in differences in architecture. Gap junctions are composed of two hexameric connexon hemichannels of a fixed length that bridge plasma membranes (Hervé and Derangeon, 2013, Söhl et al., 2005); in contrast to SJs, featuring a 5-fold symmetric cap, a plug module, and a tube of variable length.
Figure 7.
SJs Reversibly Gate Cell-Cell Communication by a Conformational Change
SJs (green, open; orange, closed) of Anabaena are dynamic, gated cell-cell connections, which reversibly block intercellular molecular diffusion along the filament upon different types of stress. The ΔfraD mutant was missing the cap and plug modules, consistent with the inability to close SJs upon stress. FraD was shown to localize to the plug module.
See also Figure S7.
In the bacterial domain of life, multicellularity is found in diverse phylogenetic clades. It will be exciting to investigate whether cell-cell connections are conserved among diverse bacteria. Importantly, we imaged two further cyanobacterial model organisms, Nostoc punctiforme PCC 73102 and Trichodesmium erythraeum IMS101, and revealed that they had similar SJ architectures (Figures S7G and S7H). Together with the conservation of fraC, fraD, amiC, sjcF1, and the C-terminal domain of sepJ genes in their genomes, this points toward a conserved SJ mechanism at least across diverse multicellular cyanobacteria. The branching of the cyanobacterial order Nostocales (comprising the genus Anabaena) was estimated to date back more than two billion years ago (Schirrmeister et al., 2013). Our data thus provide a mechanistic framework for an ancient cell-cell connection structure, predating metazoan gap junctions by more than a billion years. The convergent evolution of a dynamic gated mechanism in such divergent lineages emphasizes the importance of controlling molecular exchange in multicellular organisms in order to stop communication under certain metabolic conditions, or, upon predation or fragmentation. Upon stress, the closure of the septal junctions prevents leakage of cytoplasmic components into damaged cells and thereby avoids deterioration of the entire multicellular organism, which appears as a universal survival strategy across different domains of life.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and Virus Strains | ||
| Anabaena sp. PCC 7120 | Rippka et al., 1979 | PCC 7120 |
| Nostoc punctiforme PCC 73102 | Rippka et al., 1979 | PCC 73102 |
| Trichodesmium erythraeum IMS101 | Stocker Lab, ETH Zürich | IMS101 |
| E. coli NEB 10-beta (electrocompetent) | NEB | Cat#C3020K |
| E. coli HB101 | Sambrook et al., 1989 | N/A |
| E. coli J53 (RP-4) | Wolk et al., 1984 | N/A |
| Strain details | This study | see Table S1 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Calcein, AM | Invitrogen | Cat#C3099 |
| CCCP (Carbonyl cyanide 3-chlorophenylhydrazone) | Sigma-Aldrich | Cat#C2759; CAS: 555-60-2 |
| 30% hydrogen peroxide | Carl Roth | Cat#CP26; CAS: 7722-84-1 |
| Chloramphenicol | Carl Roth | Cat#3886; CAS: 56-75-7 |
| Spectinomycin dihydrochloride pentahydrate | Sigma-Aldrich | Cat#S4014; CAS: 22189-32-8 |
| Neomycin sulfate | Sigma-Aldrich | Cat#PHR1491; CAS: 1405-10-3 |
| Streptomycin sulfate | Sigma-Aldrich | Cat#S1400000; CAS: 3810-74-0 |
| Kanamycin sulfate | Carl Roth | Cat#T832; CAS: 25389-94-0 |
| Critical Commercial Assays | ||
| Monarch Plasmid Miniprep Kit | NEB | Cat#T1010S |
| Monarch DNA Gel Extraction Kit | NEB | Cat#T1020S |
| Monarch PCR & DNA Cleanup Kit | NEB | Cat#T1030S |
| ATP Determination Kit (A22066) | Invitrogen | Cat#10700345 |
| Deposited Data | ||
| Anabaena sp. PCC 7120 reference genome, CyanoBase | Kazusa DNA Research Institute (KDRI) | http://genome.microbedb.jp/cyanobase/ |
| Anabaena sp. PCC 7120 example tomogram | This study | EMDB: EMD-4949 |
| Anabaena sp. PCC 7120 CCCP treated example tomogram | This study | EMDB: EMD-4957 |
| Anabaena sp. PCC 7120 ΔSepJ example tomogram | This study | EMDB: EMD-4952 |
| Anabaena sp. PCC 7120 ΔSjcF1 example tomogram | This study | EMDB: EMD-4951 |
| Anabaena sp. PCC 7120 ΔAmiC1 example tomogram | This study | EMDB: EMD-4956 |
| Anabaena sp. PCC 7120 ΔFraD example tomogram | This study | EMDB: EMD-4953 |
| Anabaena sp. PCC 7120 ΔFraC example tomogram | This study | EMDB: EMD-4955 |
| Anabaena sp. PCC 7120 ΔFraCD example tomogram | This study | EMDB: EMD-4954 |
| Anabaena sp. PCC 7120 GFP-FraD example tomogram | This study | EMDB: EMD-4950 |
| Subtomogram average of septal junctions of Anabaena sp. PCC 7120 | This study | EMDB: EMD-4969 |
| Subtomogram average of septal junctions of Anabaena sp. PCC 7120 after 24h in the dark | This study | EMDB: EMD-4968 |
| Subtomogram average of septal junctions of Anabaena sp. PCC 7120 after CCCP treatment | This study | EMDB: EMD-4962 |
| Subtomogram average of septal junctions of Anabaena sp. PCC 7120 after washing out CCCP | This study | EMDB: EMD-4963 |
| Subtomogram average of septal junctions of Anabaena sp. PCC 7120 ΔSepJ | This study | EMDB: EMD-4965 |
| Subtomogram average of septal junctions of Anabaena sp. PCC 7120 ΔSjcF1 | This study | EMDB: EMD-4966 |
| Subtomogram average of septal junctions of Anabaena sp. PCC 7120 ΔAmiC1 | This study | EMDB: EMD-4964 |
| Subtomogram average of septal junctions of Anabaena sp. PCC 7120 FraD-GFP | This study | EMDB: EMD-4967 |
| Subtomogram average of septal junctions of Anabaena sp. PCC 7120 after 45 s CCCP treatment | This study | EMDB: EMD-4961 |
| Experimental Models: Organisms/Strains | ||
| Anabaena sp. PCC 7120 SR477 | Berendt et al., 2012 | N/A |
| Anabaena sp. PCC 7120 CSVT22 | Merino-Puerto et al., 2011 | N/A |
| Anabaena sp. PCC 7120 CSVT2 | Merino-Puerto et al., 2010 | N/A |
| Anabaena sp. PCC 7120 CSVT22 pIM496 | B. Jan, I.M., and K.F., unpublished data | N/A |
| Anabaena sp. PCC 7120 CSVM34 | Mariscal et al., 2011 | N/A |
| Anabaena sp. PCC 7120 AFS-I-sjcF1 | Rudolf et al., 2015 | N/A |
| Anabaena sp. PCC 7120 CSVT22.768 | This study | N/A |
| Anabaena sp. PCC 7120 CSVT2.768 | This study | N/A |
| Anabaena sp. PCC 7120 CSVT2.779 | This study | N/A |
| Strain details | This study | see Table S1 |
| Oligonucleotides | ||
| Primer 1998: GATATCCCGCAAGAGGCCCTTTCGTCTT CAAGAATTCTGCCGTTCCTTGTCATCTG |
This study | N/A |
| Primer 2000: ATGAGTAAAGGAGAAGAACTTTTC | This study | N/A |
| Primer 2001: ACTCCAGTGAAAAGTTCTTCTCCTTTACT CATAGACACTCAACAAAAAAGGGAAACTGTAG |
This study | N/A |
| Primer 2040: CCCTTTTTTGTTGAGTGTCTTGCTAACTC GTTAAGTTAC |
This study | N/A |
| Primer 2041: GTAACTTAACGAGTTAGCAAGACACTCAA CAAAAAAGGGAAAC |
This study | N/A |
| Primer 2042: CACTATAGGGAGACCACAACGGTTTCCCT CTACCGGGATCCTCACTGCTGCGGTGGCGCTG |
This study | N/A |
| Primer 2092: GGCATGGATGAACTATACAAGCTTAATTTA TTATTTAAAGACC |
This study | N/A |
| Primer 2093: AGCTTGTATAGTTCATCCATGCC | This study | N/A |
| Recombinant DNA | ||
| Plasmid: pRL1049 | Black and Wolk, 1994 | N/A |
| Plasmid: pIM496 | Berendt et al., 2012 | N/A |
| Plasmid: pIM768 | This study | N/A |
| Plasmid: pIM779 | This study | N/A |
| Plasmid: pIM759 | This study | N/A |
| Plasmid: pRL528 | Wolk et al., 1984 | Addgene Plasmid #58495 |
| Plasmid: pCSVT56 | Merino-Puerto et al., 2010 | N/A |
| Plasmid details | This study | see Table S1 |
| Software and Algorithms | ||
| ImageJ version 1.51j | Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA | https://imagej.nih.gov/ij |
| ZEN 2.3 (blue edition) | ZEISS | https://www.zeiss.de/mikroskopie/downloads.html?vaURL=www.zeiss.de/mikroskopie/downloads/zen.html |
| GraphPad PRISM version 6.01 for Windows | La Jolla, CA, USA | https://www.graphpad.com/ |
| ImageJ Time Series Analyzer V3 | Balaji, J., Department of Neurobiology, UCLA | https://imagej.nih.gov/ij/plugins/time-series.html |
| IMOD | Kremer et al., 1996 | http://bio3d.colorado.edu/imod/ |
| UCSF Tomography | Zheng et al., 2007 | http://msg.ucsf.edu |
| SerialEM | Mastronarde, 2005 | http://bio3d.colorado.edu/SerialEM/ |
| PEET | Nicastro et al., 2006 | http://bio3d.colorado.edu/PEET/ |
| Other | ||
| Confocal microscope | ZEISS | LSM 800 |
| Plunge freezing robot Vitrobot Mk IV | Thermo Fisher | Vitrobot Mk IV |
| Dual Beam FIB/SEM microscope Helios NanoLab 600i | Thermo Fisher | Helios Nanolab600i |
| 300kV Cryo-transmission electron microscope Titan Krios | Thermo Fisher | Titan Krios |
| Luminometer | Berthold Detection System | Sirius |
Lead Contact and Materials Availability
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Martin Pilhofer (pilhofer@biol.ethz.ch).
Experimental Model and Subject Details
Microbe strains
The bacterial strains used in this study are described in the Key Resource Table and in Table S1. All cyanobacteria strains were cultivated in liquid Bg11 media in 100 mL flasks at 28°C with constant illumination at 30-40 μE m-2 s-1, shaking at 100-120 rpm or grown on Bg11 medium solidified with 1.5% (w/v) Difco agar. Stock cultures of the wild-type strain were kept in BG110 medium, void of a nitrogen source (Rippka et al., 1979). Indicated mutant strains (see Table S1) were cultured in Bg11 media supplemented with antibiotics at the following concentrations: 50 μg mL-1 neomycin, 5 μg mL-1 spectinomycin, and 5 μg mL-1 streptomycin.
Eschericia coli (E. coli) strains were used for cloning and conjugation and were cultured in LB media at 37°C supplemented with antibiotics at concentrations of 50 μg mL-1 kanamycin, 100 μg mL-1 spectinomycin, and 25 μg mL-1 streptomycin when indicated.
Method Details
Construction of mutant strains
Plasmids (see Table S1) were introduced into Anabaena sp. strains by triparental mating (conjugation) using the E. coli strains J53 (RP-4) (Wolk et al., 1984) and HB101 (pRL528) (Wolk et al., 1984, Sambrook et al., 1989) carrying the respective cargo plasmid (Elhai and Wolk, 1988). Reference sequences were obtained from Cyanobase (http://genome.microbedb.jp/cyanobase/) and amplified performing colony PCR using Anabaena sp. PCC 7120 culture as template. PCR fragments and plasmid DNA were purified using the Monarch Kits (New England Biolabs, Frankfurt). Plasmids were inserted into E. coli cells via electroporation (1.8 kV, 25 μF, 200 Ω) and sequences were verified by sequencing (GATC biotech AG, Konstanz, Germany).
Cloning of the plasmid pIM768 was done by amplification of a 344 bp upstream region of the fraCDE operon using oligonucleotides 1998/2041. Via overlap extension PCR (OE-PCR) using oligonucleotides 1998/2042, this fragment was fused to the fraD ORF, which was amplified with oligonucleotides 2042/2040. The PCR product was fused into EcoRI/BamHI-digested pRL1049 (Black and Wolk, 1994) via Gibson Assembly cloning resulting in pIM768. The plasmid was transferred into strain CSVT22 (Merino-Puerto et al., 2011) for construction of a ΔfraC mutant and in CSVT2 (Merino-Puerto et al., 2010) for complementation of the fraD gene as expression control strain.
For construction of a GFP-FraD fusion, the fraCDE upstream region was amplified with oligonucleotides 1998/2001. Gfp-mut2 was amplified from plasmid pCSVT56 (Merino-Puerto et al., 2010) via standard PCR using oligonucleotides 2000/2093. The two PCR fragments were fused via OE-PCR with oligonucleotides 1998/2093. The fraD ORF was amplified using oligonucleotides 2092/2042 and fused via OE-PCR to the latter construct using oligonucleotides 1998/2042. The fused PCR fragment was introduced into EcoRI/BamHI-digested pRL1049 (Black and Wolk, 1994) via Gibson Assembly cloning resulting in pIM779, which was then transferred into strain CSVT2 (Merino-Puerto et al., 2010).
FRAP assays and treatment with CCCP and H2O2
Anabaena filaments were taken after growth for 3 days on Bg11 agar plates and resuspended to an optical density of OD750 = 1.2 in 500 μL liquid Bg11. Cells were washed three times with Bg11 medium and stained with the fluorescent dye calcein acetoxymethylester (1 mg/mL in DMSO) for 90 min in the dark at 28°C with gentle agitation as previously described (Merino-Puerto et al., 2011, Mullineaux et al., 2008). Thereafter, the cells were washed again three times with Bg11 medium. Either 0.25-1 μL CCCP (carbonyl cyanide 3-chlorophenylhydrazone; stock 0.1 mM, 10 mM or 100 mM in DMSO) or 1 μL DMSO as control was added to the cells, whereby the concentration of DMSO never exceeded a final concentration of 0.002%. After another 90 min of incubation with gentle shaking in the dark, the cells were spotted onto Bg11 agar and covered with a coverslip for imaging at room temperature. In order to test for reversibility of the closure of SJs after CCCP treatment, cells were washed three times with CCCP-free Bg11 medium and incubated for 2.5 h at room temperature in the light. For the H2O2 assay, cells were treated with 10 mM H2O2 instead of CCCP and incubated 3 h prior to FRAP (fluorescence recovery after photobleaching) measurements. All FRAP measurements were performed with a 63x/1.4 oil-immersion objective of a Zeiss LSM 800 confocal microscope and the ZEN 2.3 (blue edition) software as described previously (Bornikoel et al., 2017). The sample was excited using the 488 nm line of a 10 mW laser at 0,2% intensity. Chlorophyll auto-fluorescence (emission detection at 650-700 nm) and calcein fluorescence (emission detection at 400-530 nm) were imaged simultaneously using a 191 μm confocal pinhole (4.49 airy units) resulting in a point-spread of about 3 μm in the Z-direction. Further imaging settings are listed hereafter: frame size 36.2 × 36.2 μm, pixel size 0.07 μm, pixel dwell time 1.52 μs, averaging 1x line averaging. Five images of pre-bleached cells were captured before the laser intensity was increased by at least a factor of 10 for bleaching a region of interest using the ‘fast-bleach’ option. Images at 1 s intervals were taken for 30-120 s in order to record the fluorescence recovery in the bleached cell. Data were processed via ImageJ and Excel (see section QUANTIFICATION AND STATISTICAL ANALYSIS). Obtained fluorescence recovery curves were assigned into one of the four groups and percentage distribution was calculated for cumulated results from at least 2 independent experiments.
Plunge freezing of Anabaena cells
Anabaena cultures sedimented for ∼45 min and were concentrated by removing 2/3 of the medium. 3.5 μL of cell suspension was applied on glow-discharged copper or molybdenum EM grids (R2/2, Quantifoil) and automatically blotted from the back (Weiss et al., 2017) for 4-6 s and plunged into liquid ethane/propane (Tivol et al., 2008) using a Vitrobot plunge freezing robot (ThermoFisher) (Iancu et al., 2006). Frozen grids were subsequently stored in liquid nitrogen.
Cryo-Focused Ion Beam milling
CryoFIB milling was used to thin plunge frozen Anabaena filaments for subsequent ECT analysis and was done according to (Medeiros et al., 2018). Frozen grids were clipped into modified autoloader grids (provided by J. Plitzko, Max PIanck Institute of Biochemistry) (Schaffer et al., 2015), clamped into a 40° pre-tilted TEM grid holder (Leica Microsystems) and transferred from the loading station to the dual beam instrument using the VCT100 cryo-transfer system (Leica Microsystems). The holder was mounted on a custom-built cryo-stage on a Helios NanoLab600i dual beam FIB/SEM instrument (ThermoFisher). Grid quality and targeting of the cells was done by scanning EM (SEM) imaging (3-5 kV, 21 pA). After coating with Platinum precursor gas, 8-9 μm wide lamellae through Anabaena filaments were prepared in several steps using the focused ion beam. This size allowed covering roughly two septa per bacterial filament. The current of the ion beam was gradually reduced from 43 nA to 24 pA according to lamella thickness until a final lamella thickness of ∼250 nm was achieved. 9 – 24 lamellae were produced in a 12 h session and the holder was subsequently brought back to the loading station using the VCT100 transfer system. Grids were unloaded and stored under liquid nitrogen until loaded to the TEM. Lamella for the 45 s CCCP-treated Anabaena cells (Figures S4J–S4L) were prepared on a Crossbeam 550 (Zeiss), equipped with a cryo-stage (Leica Microsystems). The workflow was identical to the one described above, except that a lamella width of 11 μm and a final lamella thickness of 200 nm was targeted. For transfer of cryo-samples between the FIB/SEM microscope and the loading station, a VCT500 cryo-transfer system (Leica Microsystems) was used.
Electron Cryotomography
CryoFIB processed Anabaena filaments were examined by electron cryotomography. Images were recorded on a Titan Krios 300kV FEG transmission electron microscope (ThermoFisher) equipped with a Quantum LS imaging filter (slit width 20 eV) and K2 Summit direct electron detector (Gatan). A low magnification overview of the grid was recorded using SerialEM (Mastronarde, 2005). Tilt series were collected automatically using UCSF Tomo (Zheng et al., 2007) and covered an angular range from −60° to +60° with 2° increment with a defocus of −7 to −9 μm. The total dose of a tilt series accumulated to 120-140 e- / Å2 and the pixel size at the specimen level was 3.38 Å.
Tomogram reconstruction and subtomogram averaging
Tilt series were drift-corrected using alignframes, and CTF correction and three-dimensional reconstructions were generated using the IMOD package (Kremer et al., 1996, Mastronarde, 2008). Subtomogram averaging was done according to (Weiss et al., 2017) using PEET (Nicastro et al., 2006). Briefly, SJs were identified visually in individual tomograms and their periplasm-spanning axes were modeled with open contours in 3dmod (Mastronarde, 2008) to generate model points, the initial motive list and particle rotation axes. For the open state of the septal junctions, 446 particles were averaged with a box size of 22 × 22 × 22 pixels and a pixel size of 1.35 nm. The average indicated a 5-fold rotational symmetry. To exclude other symmetries, 3-, 4-, 5-, 6- and 8-fold rotational symmetries were imposed on the average. The averages indicated the strongest reinforcement with 5-fold symmetry. The final 5-fold symmetrized average resulted, in 1802 particles (after removal of duplicated or misaligned particles), with a box size of 44 × 44 × 44 pixels and a pixel size of 0.67 nm. A similar approach was applied to CCCP-treated SJs, resulting in 312 particles and a final average of 1471 particles after 5-fold symmetrization and the removal of duplicated particles. The box size was 44 × 44 × 44 pixels with 0.67 nm pixel size. For the subtomogram averages of SJs from Anabaena PCC7120 gfp-fraD, 220 particles were initially selected and the final subtomogram average represents 1100 particles after 5-fold symmetrization and removal of duplicate particles with a box size of 44 × 44 × 44 pixels and 0.67 nm pixel size. The difference map between subtomogram averages from wild-type and gfp-fraD was generated in Chimera (Pettersen et al., 2004) with the “vop map## substract map##” command. For subtomogram averages of SJs from Anabaena PCC 7120 wild-type (45 s CCCP treatment; 270 particles after 5-fold symmetrization), wild-type (90 min CCCP treatment, 3 times washed and 2.5 h incubation in fresh medium; 660 particles after 5-fold symmetrization), wild-type (24 h incubation in the dark; 200 particles after 5-fold symmetrization), amiC1 mutant SR477 (780 particles after 5-fold symmetrization), ΔsepJ (310 particles after 5-fold symmetrization) and ΔsjcF1 (504 particles after 5-fold symmetrization) the pixel size was 1.34 nm and box size was 26 × 26 × 26 pixels. 3D rendering, segmentations and movies were done with IMOD or Chimera. The density plot was generated with FIJI (Schindelin et al., 2012). Coloring of the different SJ modules was done with Adobe Photoshop.
ATP determination
Sample preparation for ATP determination was performed identically to CCCP-FRAP experiments. Anabaena cells grown four days on Bg11 agar were resuspended to an OD750 = 1.2 in 0.5 mL Bg11 medium. In order to mimic addition of calcein solved in DMSO, 10 μL DMSO were added to each sample (except for no treatment control) and incubated for 90 min at 28°C in the dark. After washing the cells three times with Bg11 medium, either different concentrations of CCCP or 1 μL DMSO (maximum volume added with CCCP) were added to the samples, followed by another 90 min incubation period at 28°C in the dark.
For ATP extraction, the samples were filled to 1 mL with Bg11 medium and immediately frozen in liquid nitrogen, followed by thawing at 99°C and shaking at 1400 rpm in a heating block. After three freeze/thaw cycles, the samples were centrifuged 3 min at 25,000 g and 4°C. The ATP quantification was performed using the ATP determination kit (Invitrogen) following the manufacturer’s protocol using 50 μL of the sample supernatant in a 500 μL reaction. The principle of the measurement relies on detection of bioluminescence generated by a recombinant firefly luciferase from its substrate D-luciferin and ATP. Luminescence was detected using a luminometer (Sirius Luminometer; Berthold Detection System) and ATP content was calculated using a generated ATP standard curve.
Quantification and Statistical Analysis
For detailed information for software used in this study, see the Key Resources Table. FRAP imaging was performed using the ZEN 2.3 blue edition software (ZEISS). Fluorescence intensity of a FRAP sequence was measured using the ImageJ plugin ‘Time series analyzer V3′ and normalized to the fluorescence intensity F0 prior to bleaching via the Excel software (Microsoft). The percentage distribution of bleached cells in the four groups was calculated for cumulated results from at least 2 independent experiments using GraphPad PRISM. The number of analyzed cells n is indicated within the bar graphs. All bleached cells n are from different filaments. Distribution of the groups in single FRAP experiments are shown in the Supplement and referred to in the figure legends. The fluorescence recovery rate constant of a bleached cell was calculated as previously described using the formula , with being the fluorescence of the bleached cell, the fluorescence directly after bleaching tending toward during fluorescence recovery, the fluorescence during recovery, 2R the recovery rate constant due to molecular exchange from both neighboring cells, and the time (Merino-Puerto et al., 2011, Nürnberg et al., 2015).
Data and Code Availability
Example tomograms and subtomogram averages of all Anabaena mutants described in this study were deposited in the Electron Microscopy Data Bank (accession numbers EMDB: EMD-4949–EMD-4957 for tomograms and EMDB: EMD-4961–EMD-4969 for subtomogram averages).
Acknowledgments
We thank Peter Tittmann and Christian Zaubitzer for technical support as well as ScopeM for instrument access at ETH Zürich. We thank Hannah Minas for help with preparing samples and analyzing the data. Jörn Piel and Anna Vagstaad are acknowledged for providing Anabaena and Nostoc punctiforme cultures for preliminary observations. We thank Ulrike Pfreundt and Roman Stocker for providing Trichodesmium erythraeum cultures. We thank Enrique Flores for providing plasmid pCSVT56, mutants CSVT2, CSVT22, and CSVM34, Enrico Schleiff for providing strain AFS-1-sjcF1, and Peter Wolk for pRL-plasmids. Fabian Eisenstein is acknowledged for help with movies. We thank João Medeiros, Tobias Zachs, and Andreas Schertel for help with preparing lamellae at Zeiss, Oberkochen. G.L.W. was supported by a Boehringer Ingelheim Fonds PhD fellowship. Work in Tübingen was supported by the Deutsche Forschungsgemeinschaft (SFB766). M.P. was supported by the Swiss National Science Foundation (31003A_179255), the European Research Council (679209), and the Helmut Horten Foundation.
Author Contributions
G.L.W. and A.-K.K. contributed equally. I.M., K.F., and M.P. conceptualized the study. All authors designed experiments. G.L.W. and A.-K.K. performed experiments. All authors analyzed data. All authors participated in writing the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: July 11, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.cell.2019.05.055.
Contributor Information
Iris Maldener, Email: iris.maldener@uni-tuebingen.de.
Martin Pilhofer, Email: pilhofer@biol.ethz.ch.
Supplemental Information
References
- Bauer C.C., Buikema W.J., Black K., Haselkorn R. A short-filament mutant of Anabaena sp. strain PCC 7120 that fragments in nitrogen-deficient medium. J. Bacteriol. 1995;177:1520–1526. doi: 10.1128/jb.177.6.1520-1526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendt S., Lehner J., Zhang Y.V., Rasse T.M., Forchhammer K., Maldener I. Cell wall amidase AmiC1 is required for cellular communication and heterocyst development in the cyanobacterium Anabaena PCC 7120 but not for filament integrity. J. Bacteriol. 2012;194:5218–5227. doi: 10.1128/JB.00912-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black T.A., Wolk C.P. Analysis of a Het- mutation in Anabaena sp. strain PCC 7120 implicates a secondary metabolite in the regulation of heterocyst spacing. J. Bacteriol. 1994;176:2282–2292. doi: 10.1128/jb.176.8.2282-2292.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornikoel J., Carrión A., Fan Q., Flores E., Forchhammer K., Mariscal V., Mullineaux C.W., Perez R., Silber N., Wolk C.P., Maldener I. Role of two cell wall amidases in septal junction and nanopore formation in the multicellular cyanobacterium Anabaena sp. PCC 7120. Front. Cell. Infect. Microbiol. 2017;7:386. doi: 10.3389/fcimb.2017.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornikoel J., Staiger J., Madlung J., Forchhammer K., Maldener I. LytM factor Alr3353 affects filament morphology and cell-cell communication in the multicellular cyanobacterium Anabaena sp. PCC 7120. Mol. Microbiol. 2018;108:187–203. doi: 10.1111/mmi.13929. [DOI] [PubMed] [Google Scholar]
- Brunet T., King N. The origin of animal multicellularity and cell differentiation. Dev. Cell. 2017;43:124–140. doi: 10.1016/j.devcel.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnat M., Herrero A., Flores E. Compartmentalized cyanophycin metabolism in the diazotrophic filaments of a heterocyst-forming cyanobacterium. Proc. Natl. Acad. Sci. USA. 2014;111:3823–3828. doi: 10.1073/pnas.1318564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y.-W., Rettberg L.A., Treuner-Lange A., Iwasa J., Søgaard-Andersen L., Jensen G.J. Architecture of the type IVa pilus machine. Science. 2016;351:aad2001. doi: 10.1126/science.aad2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumino A.C., Marcozzi C., Barreiro R., Salerno G.L. Carbon cycling in Anabaena sp. PCC 7120. Sucrose synthesis in the heterocysts and possible role in nitrogen fixation. Plant Physiol. 2007;143:1385–1397. doi: 10.1104/pp.106.091736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ek-Vitorin J.F., Burt J.M. Structural basis for the selective permeability of channels made of communicating junction proteins. Biochim. Biophys. Acta. 2013;1828:51–68. doi: 10.1016/j.bbamem.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhai J., Wolk C.P. Conjugal transfer of DNA to cyanobacteria. Methods Enzymol. 1988;167:747–754. doi: 10.1016/0076-6879(88)67086-8. [DOI] [PubMed] [Google Scholar]
- Flores E., Herrero A. Compartmentalized function through cell differentiation in filamentous cyanobacteria. Nat. Rev. Microbiol. 2010;8:39–50. doi: 10.1038/nrmicro2242. [DOI] [PubMed] [Google Scholar]
- Flores E., Herrero A., Wolk C.P., Maldener I. Is the periplasm continuous in filamentous multicellular cyanobacteria? Trends Microbiol. 2006;14:439–443. doi: 10.1016/j.tim.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Flores E., Pernil R., Muro-Pastor A.M., Mariscal V., Maldener I., Lechno-Yossef S., Fan Q., Wolk C.P., Herrero A. Septum-localized protein required for filament integrity and diazotrophy in the heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 2007;189:3884–3890. doi: 10.1128/JB.00085-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores E., Herrero A., Forchhammer K., Maldener I. Septal junctions in filamentous heterocyst-forming cyanobacteria. Trends Microbiol. 2016;24:79–82. doi: 10.1016/j.tim.2015.11.011. [DOI] [PubMed] [Google Scholar]
- Flores E., Nieves-Morión M., Mullineaux C.W. Cyanobacterial septal junctions: Properties and regulation. Life (Basel) 2019;9:E1. doi: 10.3390/life9010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giddings T.H., Staehelin L.A. Plasma membrane architecture of Anabaena cylindrica: Occurrence of microplasmodesmata and changes associated with heterocyst development and the cell cycle. Cytobiologie. 1978;16:235–249. [Google Scholar]
- Giddings T.H., Staehelin L.A. Observation of microplasmodesmata in both heterocyst-forming and non-heterocyst forming filamentous cyanobacteria by freeze-fracture electron microscopy. Arch. Microbiol. 1981;129:295–298. [Google Scholar]
- Herrero A., Stavans J., Flores E. The multicellular nature of filamentous heterocyst-forming cyanobacteria. FEMS Microbiol. Rev. 2016;40:831–854. doi: 10.1093/femsre/fuw029. [DOI] [PubMed] [Google Scholar]
- Hervé J.-C., Derangeon M. Gap-junction-mediated cell-to-cell communication. Cell Tissue Res. 2013;352:21–31. doi: 10.1007/s00441-012-1485-6. [DOI] [PubMed] [Google Scholar]
- Hoiczyk E., Baumeister W. Envelope structure of four gliding filamentous cyanobacteria. J. Bacteriol. 1995;177:2387–2395. doi: 10.1128/jb.177.9.2387-2395.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfer U., Lehninger A.L., Thompson T.E. Protonic conductance across phospholipid bilayer membranes induced by uncoupling agents for oxidative phosphorylation. Proc. Natl. Acad. Sci. USA. 1968;59:484–490. doi: 10.1073/pnas.59.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Lara-Tejero M., Kong Q., Galán J.E., Liu J. In situ molecular architecture of the salmonella type III secretion machine. Cell. 2017;168:1065–1074. doi: 10.1016/j.cell.2017.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iancu C.V., Tivol W.F., Schooler J.B., Dias D.P., Henderson G.P., Murphy G.E., Wright E.R., Li Z., Yu Z., Briegel A. Electron cryotomography sample preparation using the Vitrobot. Nat. Protoc. 2006;1:2813–2819. doi: 10.1038/nprot.2006.432. [DOI] [PubMed] [Google Scholar]
- Jüttner F. 14C-labeled metabolites in heterocysts and vegetative cells of Anabaena cylindrica filaments and their presumptive function as transport vehicles of organic carbon and nitrogen. J. Bacteriol. 1983;155:628–633. doi: 10.1128/jb.155.2.628-633.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer J.R., Mastronarde D.N., McIntosh J.R. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 1996;116:71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- Lang N.J., Fay P. The heterocysts of blue-green algae II. Details of ultrastructure. Proc. R. Soc. Lond. B Biol. Sci. 1971;178:193–203. doi: 10.1098/rspb.1972.0046. [DOI] [PubMed] [Google Scholar]
- Lehner J., Zhang Y., Berendt S., Rasse T.M., Forchhammer K., Maldener I. The morphogene AmiC2 is pivotal for multicellular development in the cyanobacterium Nostoc punctiforme. Mol. Microbiol. 2011;79:1655–1669. doi: 10.1111/j.1365-2958.2011.07554.x. [DOI] [PubMed] [Google Scholar]
- Lehner J., Berendt S., Dörsam B., Perez R., Forchhammer K., Maldener I. Prokaryotic multicellularity: a nanopore array for bacterial cell communication. FASEB J. 2013;27:2293–2300. doi: 10.1096/fj.12-225854. [DOI] [PubMed] [Google Scholar]
- Maldener I., Summers M.L., Sukenik A. Cellular differentiation in filamentous cyanobacteria. In: Flores E., Herrero A., editors. The Cell Biology of Cyanobacteria. Caister Academic Press; 2014. pp. 239–304. [Google Scholar]
- Mariscal V. Cell-cell joining proteins in heterocyst-forming cyanobacteria. In: Flores E., Herrero A., editors. The Cell Biology of Cyanobacteria. Caister Academic Press; 2014. pp. 293–304. [Google Scholar]
- Mariscal V., Herrero A., Nenninger A., Mullineaux C.W., Flores E. Functional dissection of the three-domain SepJ protein joining the cells in cyanobacterial trichomes. Mol. Microbiol. 2011;79:1077–1088. doi: 10.1111/j.1365-2958.2010.07508.x. [DOI] [PubMed] [Google Scholar]
- Mariscal V., Nürnberg D.J., Herrero A., Mullineaux C.W., Flores E. Overexpression of SepJ alters septal morphology and heterocyst pattern regulated by diffusible signals in Anabaena. Mol. Microbiol. 2016;101:968–981. doi: 10.1111/mmi.13436. [DOI] [PubMed] [Google Scholar]
- Marko M., Hsieh C., Schalek R., Frank J., Mannella C. Focused-ion-beam thinning of frozen-hydrated biological specimens for cryo-electron microscopy. Nat. Methods. 2007;4:215–217. doi: 10.1038/nmeth1014. [DOI] [PubMed] [Google Scholar]
- Mastronarde D.N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 2005;152:36–51. doi: 10.1016/j.jsb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Mastronarde D.N. Correction for non-perpendicularity of beam and tilt axis in tomographic reconstructions with the IMOD package. J. Microsc. 2008;230:212–217. doi: 10.1111/j.1365-2818.2008.01977.x. [DOI] [PubMed] [Google Scholar]
- Medeiros J.M., Böck D., Weiss G.L., Kooger R., Wepf R.A., Pilhofer M. Robust workflow and instrumentation for cryo-focused ion beam milling of samples for electron cryotomography. Ultramicroscopy. 2018;190:1–11. doi: 10.1016/j.ultramic.2018.04.002. [DOI] [PubMed] [Google Scholar]
- Merino-Puerto V., Mariscal V., Mullineaux C.W., Herrero A., Flores E. Fra proteins influencing filament integrity, diazotrophy and localization of septal protein SepJ in the heterocyst-forming cyanobacterium Anabaena sp. Mol. Microbiol. 2010;75:1159–1170. doi: 10.1111/j.1365-2958.2009.07031.x. [DOI] [PubMed] [Google Scholar]
- Merino-Puerto V., Schwarz H., Maldener I., Mariscal V., Mullineaux C.W., Herrero A., Flores E. FraC/FraD-dependent intercellular molecular exchange in the filaments of a heterocyst-forming cyanobacterium, Anabaena sp. Mol. Microbiol. 2011;82:87–98. doi: 10.1111/j.1365-2958.2011.07797.x. [DOI] [PubMed] [Google Scholar]
- Metzner I. [Chemistry and submicroscopic structure of cell walls, sheaths, and gelatin of Cyanophyceae. II. Cell morphology and cell physiology of Cyanophyceae] Arch. Mikrobiol. 1955;22:45–77. [PubMed] [Google Scholar]
- Mullineaux C.W., Mariscal V., Nenninger A., Khanum H., Herrero A., Flores E., Adams D.G. Mechanism of intercellular molecular exchange in heterocyst-forming cyanobacteria. EMBO J. 2008;27:1299–1308. doi: 10.1038/emboj.2008.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayar A.S., Yamaura H., Rajagopalan R., Risser D.D., Callahan S.M. FraG is necessary for filament integrity and heterocyst maturation in the cyanobacterium Anabaena sp. strain PCC 7120. Microbiology. 2007;153:601–607. doi: 10.1099/mic.0.2006/002535-0. [DOI] [PubMed] [Google Scholar]
- Nicastro D., Schwartz C., Pierson J., Gaudette R., Porter M.E., McIntosh J.R. The molecular architecture of axonemes revealed by cryoelectron tomography. Science. 2006;313:944–948. doi: 10.1126/science.1128618. [DOI] [PubMed] [Google Scholar]
- Nieves-Morión M., Mullineaux C.W., Flores E. Molecular diffusion through cyanobacterial septal junctions. MBio. 2017;8:e01756-16. doi: 10.1128/mBio.01756-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nürnberg D.J., Mariscal V., Bornikoel J., Nieves-Morión M., Krauß N., Herrero A., Maldener I., Flores E., Mullineaux C.W. Intercellular diffusion of a fluorescent sucrose analog via the septal junctions in a filamentous cyanobacterium. MBio. 2015;6:e02109. doi: 10.1128/mBio.02109-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obaid A.L., Socolar S.J., Rose B. Cell-to-cell channels with two independently regulated gates in series: analysis of junctional conductance modulation by membrane potential, calcium, and pH. J. Membr. Biol. 1983;73:69–89. doi: 10.1007/BF01870342. [DOI] [PubMed] [Google Scholar]
- Omairi-Nasser A., Haselkorn R., Austin J., 2nd Visualization of channels connecting cells in filamentous nitrogen-fixing cyanobacteria. FASEB J. 2014;28:3016–3022. doi: 10.1096/fj.14-252007. [DOI] [PubMed] [Google Scholar]
- Omairi-Nasser A., Mariscal V., Austin J.R., 2nd, Haselkorn R. Requirement of Fra proteins for communication channels between cells in the filamentous nitrogen-fixing cyanobacterium Anabaena sp. PCC 7120. Proc. Natl. Acad. Sci. USA. 2015;112:E4458–E4464. doi: 10.1073/pnas.1512232112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oparka K.J., Roberts A.G., Boevink P., Santa Cruz S., Roberts I., Pradel K.S., Imlau A., Kotlizky G., Sauer N., Epel B. Simple, but not branched, plasmodesmata allow the nonspecific trafficking of proteins in developing tobacco leaves. Cell. 1999;97:743–754. doi: 10.1016/s0092-8674(00)80786-2. [DOI] [PubMed] [Google Scholar]
- Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Ramos-León F., Mariscal V., Frías J.E., Flores E., Herrero A. Divisome-dependent subcellular localization of cell-cell joining protein SepJ in the filamentous cyanobacterium Anabaena. Mol. Microbiol. 2015;96:566–580. doi: 10.1111/mmi.12956. [DOI] [PubMed] [Google Scholar]
- Rigort A., Bäuerlein F.J.B., Leis A., Gruska M., Hoffmann C., Laugks T., Böhm U., Eibauer M., Gnaegi H., Baumeister W., Plitzko J.M. Micromachining tools and correlative approaches for cellular cryo-electron tomography. J. Struct. Biol. 2010;172:169–179. doi: 10.1016/j.jsb.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Rippka R., Deruelles J., Herdman M., Waterbury J.B., Stanier R.Y. Generic assignments, atrain histories and properties of pure cultures of cyanobacteria. Microbiology. 1979;111:1–61. [Google Scholar]
- Rudolf M., Tetik N., Ramos-León F., Flinner N., Ngo G., Stevanovic M., Burnat M., Pernil R., Flores E., Schleiff E. The peptidoglycan-binding protein Sjcf1 influences septal junction function and channel formation in the filamentous cyanobacterium Anabaena. MBio. 2015;6:e00376. doi: 10.1128/mBio.00376-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager R., Lee J.-Y. Plasmodesmata in integrated cell signalling: insights from development and environmental signals and stresses. J. Exp. Bot. 2014;65:6337–6358. doi: 10.1093/jxb/eru365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E.F., Maniatis T. Cold Spring Harbor Laboratory Press; 1989. Molecular cloning: A laboratory manual. [Google Scholar]
- Samuilov V.D., Bezryadnov D.B., Gusev M.V., Kitashov A.V., Fedorenko T.A. Hydrogen peroxide inhibits photosynthetic electron transport in cells of cyanobacteria. Biochemistry (Mosc.) 2001;66:640–645. doi: 10.1023/a:1010207314408. [DOI] [PubMed] [Google Scholar]
- Schaffer M., Engel B.D., Laugks T., Mahamid J., Plitzko J.M., Baumeister W. Cryo-focused ion beam sample preparation for imaging vitreous cells by cryo-electron tomography. Bio. Protoc. 2015;5:e1575. doi: 10.21769/bioprotoc.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer M., Mahamid J., Engel B.D., Laugks T., Baumeister W., Plitzko J.M. Optimized cryo-focused ion beam sample preparation aimed at in situ structural studies of membrane proteins. J. Struct. Biol. 2017;197:73–82. doi: 10.1016/j.jsb.2016.07.010. [DOI] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirrmeister B.E., de Vos J.M., Antonelli A., Bagheri H.C. Evolution of multicellularity coincided with increased diversification of cyanobacteria and the Great Oxidation Event. Proc. Natl. Acad. Sci. USA. 2013;110:1791–1796. doi: 10.1073/pnas.1209927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socolar S.J., Politoff A.L. Uncoupling cell junctions in a glandular epithelium by depolarizing current. Science. 1971;172:492–494. doi: 10.1126/science.172.3982.492. [DOI] [PubMed] [Google Scholar]
- Söhl G., Maxeiner S., Willecke K. Expression and functions of neuronal gap junctions. Nat. Rev. Neurosci. 2005;6:191–200. doi: 10.1038/nrn1627. [DOI] [PubMed] [Google Scholar]
- Thomas J., Meeks J.C., Wolk C.P., Shaffer P.W., Austin S.M. Formation of glutamine from [13n]ammonia, [13n]dinitrogen, and [14C]glutamate by heterocysts isolated from Anabaena cylindrica. J. Bacteriol. 1977;129:1545–1555. doi: 10.1128/jb.129.3.1545-1555.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tivol W.F., Briegel A., Jensen G.J. An improved cryogen for plunge freezing. Microsc. Microanal. 2008;14:375–379. doi: 10.1017/S1431927608080781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin P.N.T., Zampighi G. Structure of the junction between communicating cells. Nature. 1980;283:545–549. doi: 10.1038/283545a0. [DOI] [PubMed] [Google Scholar]
- Weiss G.L., Medeiros J.M., Pilhofer M. In situ imaging of bacterial secretion systems by electron cryotomography. Methods Mol. Biol. 2017;1615:353–375. doi: 10.1007/978-1-4939-7033-9_27. [DOI] [PubMed] [Google Scholar]
- Wilk L., Strauss M., Rudolf M., Nicolaisen K., Flores E., Kühlbrandt W., Schleiff E. Outer membrane continuity and septosome formation between vegetative cells in the filaments of Anabaena sp. PCC 7120. Cell. Microbiol. 2011;13:1744–1754. doi: 10.1111/j.1462-5822.2011.01655.x. [DOI] [PubMed] [Google Scholar]
- Wolk C.P., Vonshak A., Kehoe P., Elhai J. Construction of shuttle vectors capable of conjugative transfer from Escherichia coli to nitrogen-fixing filamentous cyanobacteria. Proc. Natl. Acad. Sci. USA. 1984;81:1561–1565. doi: 10.1073/pnas.81.5.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S.Q., Keszthelyi B., Branlund E., Lyle J.M., Braunfeld M.B., Sedat J.W., Agard D.A. UCSF tomography: an integrated software suite for real-time electron microscopic tomographic data collection, alignment, and reconstruction. J. Struct. Biol. 2007;157:138–147. doi: 10.1016/j.jsb.2006.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Septal junctions are colored in lime green; thylakoid membranes in dark green; peptidoglycan in gray; outer and cytoplasmic membranes in brown; granules in light brown and yellow. Bar, 100 nm.
Shown are longitudinal views, perpendicular views and a surface rendering. Bar, 10 nm.
Single cells were bleached after 5 s (indicated by arrows).
Shown are longitudinal views, perpendicular views and a surface rendering. Bar, 10 nm.
Top view of SJs. Green, open; orange, closed. Bar, 10 nm.
Bars, 100 nm for cryotomogram and 10 nm for subtomogram average.
Data Availability Statement
Example tomograms and subtomogram averages of all Anabaena mutants described in this study were deposited in the Electron Microscopy Data Bank (accession numbers EMDB: EMD-4949–EMD-4957 for tomograms and EMDB: EMD-4961–EMD-4969 for subtomogram averages).