Abstract
With the aim of understanding the mechanisms involved in the regurgitation behavior of tephritid flies, we performed a structural study of the digestive system of the economically important fruit-fly pest, Anastrepha ludens (Loew) using optical, scanning electronic microscopy (SEM) and transmission electron microscopy (TEM), plus a feeding assay. Most structures studied are similar to those previously reported in other adult dipterans, but, importantly, we found sexual differences in some structures that apparently affect regurgitation. We report for the first time sexual differences in the crop duct nerve and large numbers of dense core vesicles within the nerve bundle. Male nerve bundles are bigger and have more secretory vesicles than female ones. The close proximity to the muscles of both the crop lobes and duct suggest that these vesicles (i.e., possibly neurosecretions) might help modulate the muscles regulating regurgitation. The salivary glands are connected to the crop via tracheae, however, SEM/TEM studies failed to find any direct structural connection. Results of the feeding assay indicate that, independently of food type (sucrose or protein) and age, males regurgitate significantly more than females. Regurgitation behavior may also play an important role in capturing bacteria in the environment, and possibly help adults eliminate ingested toxicants such as insecticides. Our findings shed light on an interesting phenomenon that has important practical implications.
Keywords: feeding behavior, digestive-tract structure, diverticulated crop, nerve bundle, Mexican fruit fly
The efficacy of pest control techniques using toxic baits, attractants, mating disruption, and others based on behavior, hinges on a deep understanding of the behavior of the particular pest species (Rodriguez-Saona and Stelinski 2009, Onstad 2014). Insect behavior is regulated by a complex interplay of external and internal inputs, guided by complex structures and neuronal pathways, which are not completely understood or have so far been poorly studied (O’Leary and Marder 2014, Dus et al. 2015, Schlegel et al. 2016). Various chemicals, such as neuropeptides, can modulate how these pathways function (Gorissen et al. 2006, Audsley and Weaver 2009, Audsley et al. 2015).
Anastrepha ludens (Loew), the Mexican fruit fly, is one of the most important pests attacking fruit such as mangos, oranges, and grapefruits. At present, it is distributed from Mexico (in the recent past it was also found in Southern USA) to Costa Rica and it is considered one of the most threatening quarantine pests in South America, S. Europe, Africa, and Asia (Birke et al. 2013, Aluja et al. 2014). As has been the case with other pests, in our quest to move as fast as possible in controlling them we have neglected studying their basic internal morphology and the study of organs and structures from which metabolites and nerves that modulate behavior are produced. With this in mind, here we aimed at better understanding the structure of the digestive tract of A. ludens adults, to help us gain insights into the complex behavior exhibited by these insects known as ‘regurgitation and bubbling’ (Aluja et al. 2000).
The current knowledge about Dipteran feeding behavior is still based on the classical studies of Dethier (1976) and some recent findings on Drosophila species (Pool and Scott 2014, Sun et al. 2014, Eriksson et al. 2017). With the incredible recent technological advances in microscopy, a new effort at dissecting the digestive tract of fruit flies is warranted as our ability to control this pest via environmentally friendly mechanisms still needs much refinement.
The recent explosion on the study and impact of the microbiota on almost every aspect of the biology and behavior (Dillon and Dillon 2004, Buchon et al. 2013, Engel and Moran 2013, Ben-Yosef et al. 2015, Clark et al. 2015, Hammer and Bowers 2015) requires a complete picture of the entire gut, as that is where most microorganisms live and exert their critical influence on the wellbeing of the host. Without a doubt, much research lies ahead in this exciting area of host plant–herbivorous insect (pest) relationships (Douglas 2013), mass rearing for the use of the Sterile Insect Technique (Kyritsis et al. 2017), as well as environmentally friendly pest control (Kumar 2012), but successful control greatly depends on the microbiota and a much deeper understanding of its role in pest survival. Excellent examples of the kind of progress recently achieved in this area, are the studies about one of the most serious worldwide pests, Drosophila suzukii (Matsumura), by Chandler et al. (2014) and (Bing et al. 2018). In all future studies between fly vectors and microbes, closer attention must be given the role of the foregut.
Regurgitation and bubbling behavior in the Anastrepha genus was first described by Aluja et al. (1989) but controlling mechanisms are still poorly understood. It has been fairly well studied in the temperate Rhagoletis pomonella (Walsh) (Hendrichs et al. 1992). Its exact role is still being investigated, but Hendrichs et al. (1992) indicated that it was a mechanism to evaporate excess water from liquid food. More recently, Gomes et al. (2018), working with blowfly (Chrysomya megacephala [Fabricius]), suggested that bubbling serves as a thermoregulatory mechanism. Importantly, in other insect groups, regurgitation may also be a symptomatic response to poisoning or could even be a mechanism for getting rid of toxicants (Lang 1969, Ferguson and Metcalf 1985, Michaelides and Wright 1997). It can also play a critical role in behavioral interactions between insects and plants when regurgitants function as allomones (Cammaerts 1991, 1995), kairomones (Mattiacci et al. 1995), resources in nutrient dynamics and communication in many social insects (Suarez and Thorne 2000), or as plant growth promotors (reward feedback) (Dyer et al. 1995), or for defense (Sivinski 1980, Peterson et al. 1987). Another proposed function for regurgitation is briefly discussed in the review by Stoffolano and Haselton (2013). If a fly transfers a large volume of liquid into the crop, flight efficiency is compromised. In an examination of the crops of Phormia regina Meigen captured in the field, none of them were filled to capacity (Stoffolano, unpublished data). Thus, to obtain maximum flight efficiency, adult flies have to get rid of excess water in the crop meal. Whether male A. ludens fly more than females could also play a role in their higher regurgitation rates. Another possibility relating to regurgitation/bubbling may be its role in capturing bacteria (i.e., also concentrating them in the digestive tract) and other microorganisms from the environment.
In the few existing studies on the digestive tract of fruit fly pests (Lee et al. 1998, Caetano et al. 2006), little attention was given to the foregut, which includes the crop organ and associated structures. Research emphasis was originally given to the dorsal esophageal bulb or dorsal diverticulum (Ratner and Stoffolano 1982, Estes et al. 2009) because of its importance in housing essential symbiotic bacteria that are often passed onto the host fruit during oviposition (Solferini 1990, Mazzon et al. 2011, Ben-Yosef et al. 2015, Ventura et al. 2018). Research was also directed at learning more about the involvement of the crop in producing sexual pheromones used as lekking markers in some species (Nation 1974, 1981; Sivinski et al. 1994; Walse et al. 2008). The process of bubbling or regurgitation was also studied from a morphological perspective, invariably finding that the crop was implicated as the organ from which the regurgitant, or bubble, was produced (Hendrichs et al. 1992). More recently, the crop organ literature related to dipterans has been reviewed and its involvement in many aspects of a fly’s behavior demonstrated (Stoffolano and Haselton 2013).
To study in depth the structures associated with the crop, and elucidate how they may regulate or be involved in regurgitation, here, we investigated the adult digestive tract of A. ludens, placing particular emphasis in sexual dimorphisms as we predicted that males should regurgitate more than females, since they produce and release the sexual pheromone that modulates sexual interactions in this.
Materials and Methods
Experimental Insects
Anastrepha ludens adults were obtained from a colony kept in the Red de Manejo Biorracional de Plagas y Vectores (RMBPV) at the Clúster Científico y Tecnológico BioMimic, Instituto de Ecología, A.C. (INECOL) in Coatepec/Xalapa, Veracruz, Mexico. The rearing process of this colony is described by Aluja et al. (2009). Newly emerged, adult flies were placed in Plexiglas cages (30 × 30 × 30 cm) and nourished, ad libitum, with an artificial diet containing a mix of hydrolyzed protein: sugar (3:1) and water offered in a plastic container through a soaked cotton until their use for dissections. These flies were used for both microscopy studies and behavioral tests. Microscopy studies were conducted in the Microscopy Unit of the Red de Estudios Moleculares Avanzados (REMAV) while the behavioral experiments were in laboratories of the RMBPV, under controlled conditions at 27 ± 1°C, 70 ± 5 % of R.H. and photoperiod of L12:D12 h.
Microscopy Bioassays
Seven-day-old flies were fed with a mixture of sucrose and protein (3:1) and dissected before they had regurgitated for light microscopy (LM) and SEM studies. In the case of TEM studies, flies were starved for 24 h before being dissected.
Light Microscope (LM) Analysis
The samples were visualized using a Nikon SMZ1500 stereomicroscope and Digital Sight DS 2Mv within the Nis-elements (AR) image analyzing software (Nikon Corporation Copyright 1991–2006).
Transmission Electron Microscopy (TEM) Assay
Crops of five females and five males were fixed for 8 h in 4% paraformaldehyde and 5% glutaraldehyde in 0.1 M in sodium cacodylate at pH 7.2 (Karnovsky 1965), rinsed overnight in the same buffer, postfixed in 1% OsO4 for 1 h at 4°C, and then dehydrated using a graded ethanol series (30–100%) (10 min for each solution). Specimens were then embedded in LR-White resin. The resin was polymerized at 55°C overnight before thin sections were cut with the aid of an ultramicrotome (Leica EMUC7). Ultrathin sections of 70 nm were collected on copper grids, stained with uranyl acetate and lead citrate, and examined with a JEM-1400 PLUS transmission electron microscope (JEOL).
Scanning Electron Microscopy (SEM) Assay
Crops of five adult females and five males were dissected under a Stereomicroscope with Sorenson’s phosphate buffer (pH 7.2). Crops were then fixed in 2.5% glutaraldehyde buffered with Sorenson’s phosphate for 12 h at 4°C, rinsed twice in the same buffer for 5 min, dehydrated in a graded ethanol series (30–100%) for 30 min at each concentration, dried in a Quorum K850 critical point drying with CO2 and attached to aluminum stubs using a carbon adhesive prior to coating with gold in a sputtering Quorum Q150 RS (Bozzola and Russell 1992). The preparations were studied and photographed with a FEI Quanta 250 FEG scanning electron microscope.
Behavioral Assay—Effect of Age, Sex, and Type of Food on Regurgitation
One-, three-, five-, and seven-day-old flies of both sexes, previously starved for 24 h, were individually placed inside Petri dishes (10 cm diameter). Base and lid of the Petri dishes were covered with medium-pore white filter paper. The lid of a 1.5 ml plastic vial (Eppendorf) was used as the container for 10 µl of either a sucrose solution (0.5 M = 0.17 g/ml) or a hydrolyzed protein solution (0.17 g/ml). To facilitate the observation of regurgitated drops on the filter paper (Fig. 1a and b), both food solutions contained vegetable green dye 50 µl/ml (McCormick Food coloring). A lid with one of the food types was placed in the center of each Petri dish (Fig. 1b). Flies were then allowed to feed and regurgitate (Fig. 1c) on the filter paper inside each Petri dish for 3 h. After this time, all were removed from Petri dishes and the number of regurgitated droplets recorded. Flies that did not ingest the colored food were not considered for data collection (easily identified as their abdomens were not clearly green). Ten flies (replicates) were tested for each treatment (combination of fly age [1, 3, 5, and 7 days] × sex [female and male] × food type [sucrose or protein]), yielding a total of 160 flies.
Fig. 1.
(a) Anastrepha ludens male inside a Petri dish showing the numerous regurgitated droplets; (b) A. ludens male regurgitating in filter paper after ingestion of colored food; (c) A. ludens female with green abdomen and droplet in proboscis after ingestion of colored food.
Statistical Analyses
We used the R software (R Core team 2017) for statistical analyses. Of the three explanatory variables, fly age was considered a numeric discrete variable, whereas sex (female and male) and food type (sugar and protein) were deemed categorical. We used a generalized linear model (GLM) with a negative binomial error distribution and log link function to model the number of droplets flies regurgitated as a function of age, sex, food type, and their double interactions (i.e., age: sex, age: food type, and sex: food type). The log link function ensures positive fitted values, and the negative binomial distribution provides a better fit than a Poisson model for overdispersed count data and allows estimation of Akaike’s Information Criteria (AIC) values useful for model selection (Buckley 2015, Zuur and Ieno 2016).
The maximal model containing main and interactive effects was fitted, and then, nonsignificant terms were deleted starting with the double interaction terms and ranking them by order of least significance (critical level of α = 0.05). Each time a term was removed, we used a likelihood ratio test via the analysis of variance command in R, to assess the significance of changes in explanatory power between the candidate models and compared its AIC values (Crawley 2013). The goal was to determine a minimal adequate model (Crawley 2013). After obtaining a model fit, the assumptions of normality and homoscedasticity were checked graphically with the model residuals.
Results
Structure of Digestive/Foregut Endocrine System
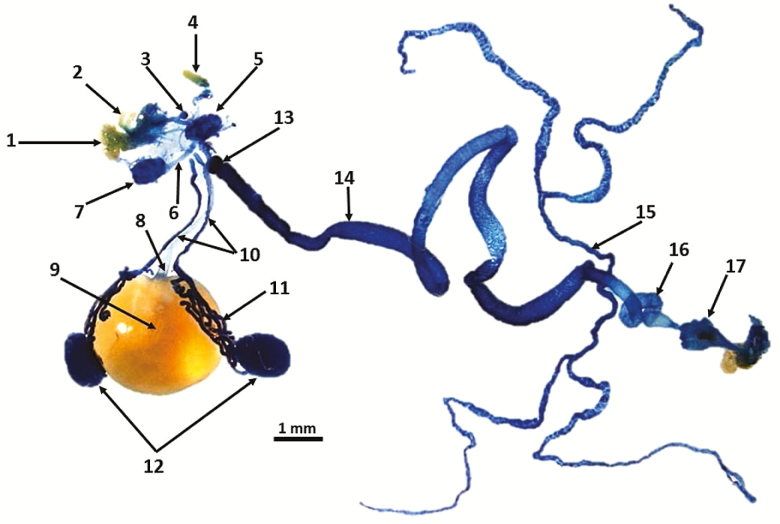
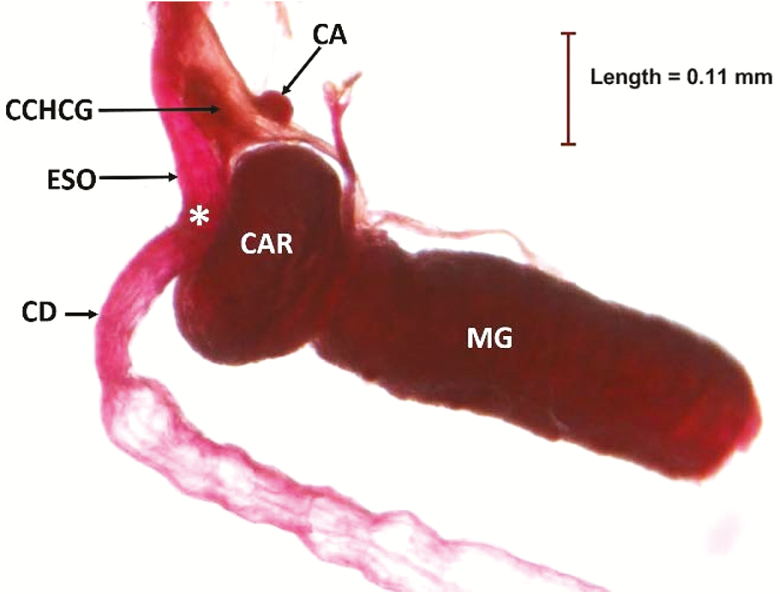
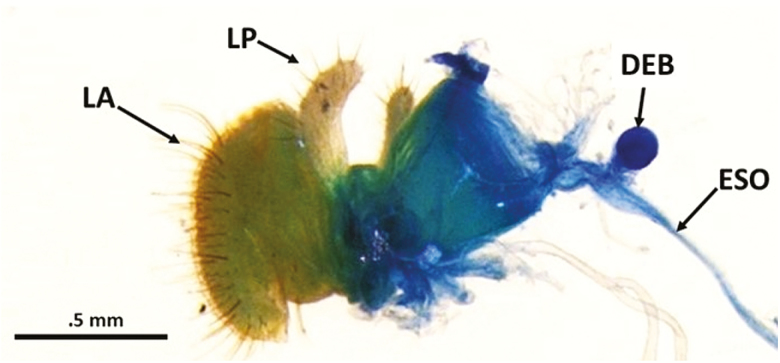
Dissection of the entire digestive system (gut) clearly shows the position of the crop lobes and crop duct with respect to the salivary gland system (Fig. 2). The crop duct originates at the posterior part of the foregut and descends ventrally at the junction just prior to the proventriculus, and terminates in a sac-like structure known as the crop lobes. The dissection also provides an overall map of how the crop position relates to the other structures of the digestive system (Fig. 2). Other structures, especially the endocrine glands present in the dissection are labeled in Fig. 3, which is an enlargement of the functional area between the esophagus and crop ducts as they descend ventrally. The relationship between the corpus allatum and corpus cardiacum-hypocerebral ganglion are also shown in Fig. 3. A dissected segment depicting the labellum and its relationship to the dorsal esophageal bulb and esophagus is presented in Fig. 4.
Fig. 2.
Digestive tract of a male A. ludens with emphasis on foregut structures. 1. Labellum (LA), 2. Labial palps (LP), 3. Dorsal esophageal bulb (DEB), 4. Antenna (AN), 5. Brain (B), 6. Ventral nerve cord (VNC), 7. Thoracicoabdominal ganglion (TAG), 8. Crop duct (CD), 9. Crop filled with a sugar and protein solution, 10. Salivary gland ducts (SGD), 11. Salivary glands (SG), 12. Terminal ball of convoluted salivary gland tubules, 13. Proventriculus/cardia (CAR), 14. Midgut (MG), 15. Malpighian tubules (MT), 16. Hindgut (HG), 17. Rectum with papillae.
Fig. 3.
Enlargement of the foregut/midgut area of male A. ludens. Corpus allatum (CA), corpus cardiacum hypocerebral ganglion (CCHCG), esophagus (ESO), crop duct (CD), *=junction of esophagus and crop duct, cardia or proventriculus (CAR), midgut (MG).
Fig. 4.
Dissected mouthpart region showing the labellum (LA), the labial palps (LP), esophagus (ESO), and the circular dorsal esophageal bulb (DEB).
Crop and Salivary Glands
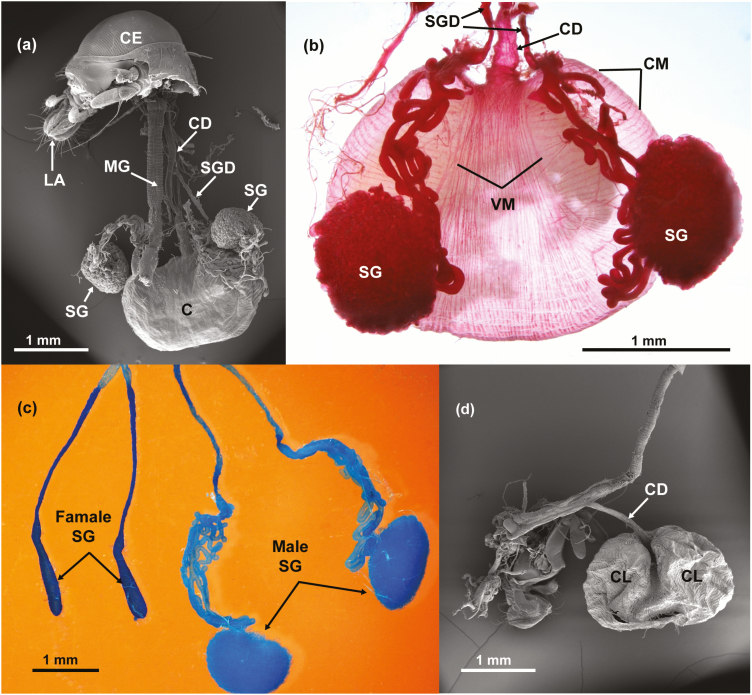
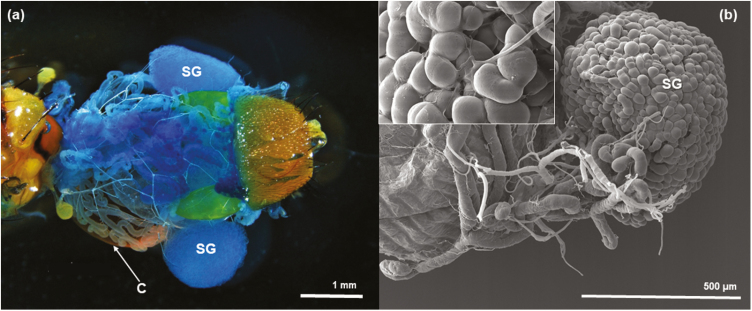
A SEM image of most of the male’s digestive system reveals the general relationship of the crop to the midgut and the salivary glands, which terminate in convoluted, winding tubules that end as two ‘balls’ in the terminal part of the salivary glands (Fig. 5a). In the LM, Fig. 5b, obtained from a fresh dissection, a better picture emerges indicating how the crop and the salivary glands appear to be connected to the crop lobes. However, although salivary glands are surrounding the crop sac (Figs. 5b and 6a), we failed to find a connection between them. Many tracheae cover both crop lobes and salivary glands and tracheal tips are apparently attached to both structures (Fig. 6b).
Fig. 5.
(a) SEM of male A. ludens showing the position of the various organs. Compound eye (CE), labellum (LA), midgut (MG), crop duct (CD), salivary gland duct (SGD), crop (C) and the convoluted tubules forming two balls= salivary glands (SG); (b) LM dissection of male system showing the crop duct (CD), salivary gland ducts (SGD), a series of circular muscle (CM), series of dorsal ventral muscles (VM) and the two convoluted tubules forming two balls=salivary glands (SG); (c) LM dissection of female and male salivary glands (SG); (d) SEM of empty crop showing the two crop lobes (CL) and the crop duct (CD).
Fig. 6.
(a) LM dissection of dorsal view of a male, showing parts of a full crop (C) and salivary glands (SG) on each side of the crop lobes; (b) SEM of salivary gland next to the crop lobe with some of the tracheae that usually wrap it loose and an enlargement of the salivary gland with a trachea penetrating between the convoluted tubules of the ball of the salivary gland (see left top inset).
Also, two sets of external muscles on the crop lobes become apparent (e.g., a circular set and a dorsal-ventral set). The large size of the ‘ball-shaped’ terminals of the male salivary glands in contrast to the size of a full crop is apparent. Salivary glands are notably larger in males than in females (Fig. 5c). Interestingly, the crop looks bi-lobed when it is not completely full (Fig. 5d), but is a globose structure when it is completely filled with food or water (yellow ‘ball’ in Figs. 2 and 5b).
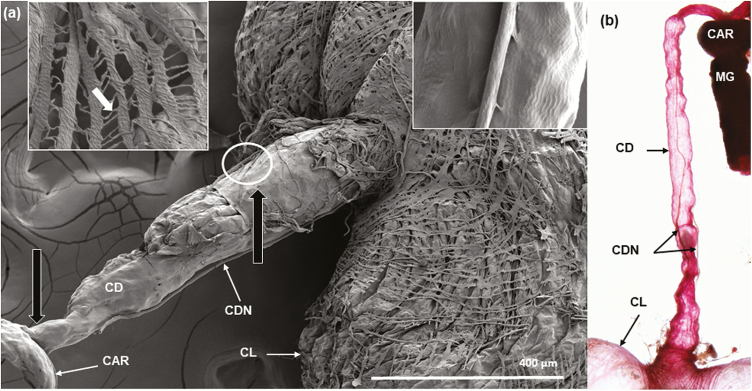
A SEM image of the crop shows the crop duct connecting the esophagus just anterior to the proventriculus with the crop lobes, which are covered with numerous intertwining series of muscles (Fig. 7a). An enlargement of this muscle layer shows that these muscle bands are connected by intercellular cytoplasmic bridges (top left inset, Fig. 7a). By carefully looking at the lower lobe where the crop duct joins the lobes, it is possible to see that the layer of muscles is arranged in concentric rows at the junction of the crop lobes and the crop duct (Fig. 7a). In a fresh dissection stained with acid fuchsin, it is possible to clearly see the two crop nerve bundles (Fig. 7b) and a nerve connecting the two crop duct nerves (Fig. 8).
Fig. 7.
(a) Composite SEM of the crop and associated structures. The crop duct (CD) is shown as a tubular structure connecting the crop lobes (CL) to the esophagus just prior to the proventriculus/cardia (CAR). The muscles covering the crop lobe look as net-like ribbons. An enlargement of the lobe area (box in upper left corner) shows that these muscles are connected by intercellular cytoplasmic bridges (white arrow pointing to one bridge). The two black unlabeled arrows are positional arrows that will be used in other figures and show the regions near where the sections were made. The white circle shows little connectors of the nerves to the duct and, the box in upper right corner shows a magnification on these little conector; (b) Fresh dissection stained with acid fuchsin showing crop duct (CD), two crop duct nerves bundles (CDN), cardia (CAR), midgut (MG) and part of the crop lobes (CL).
Fig. 8.
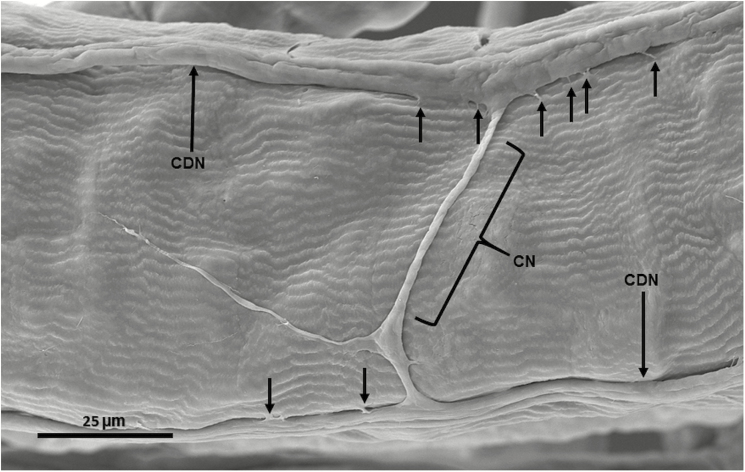
SEM of crop duct of male A. ludens showing lack of muscles on the duct, two crop duct nerves (CDN) (the small vertical arrows show the connectors of the nerves to the duct) and a connecting nerve (CN) between the two crop duct nerves.
Crop Duct and Crop Nerve Bundle
The size of the crop duct nerve bundle is visually narrower in diameter (Fig. 7a) when measured closer to the proventriculus in comparison to where it joins the crop sac (Fig. 7a and b). A SEM image of the exterior of the crop duct (Fig. 7a) reveals that the external surface of the crop duct is not covered by muscles, as are the crop lobes. Also evident in Fig. 7a and b, is a pair of crop nerve bundles (called the stomodeal nerve by Caetano et al. 2006), which are present on each side of the crop duct and connect the crop lobes with the corpus cardiacum. The white encircled area indicates that the crop duct nerve bundles are attached to the crop duct at several points along its length via some sort of connector (Fig. 7a, see top right inset); the latter is shown more clearly in Fig. 8.
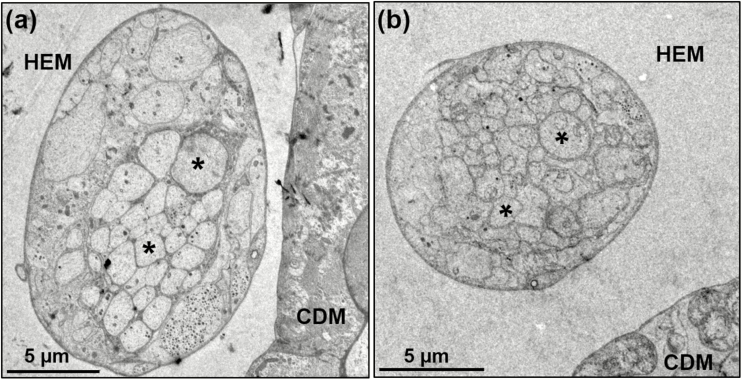
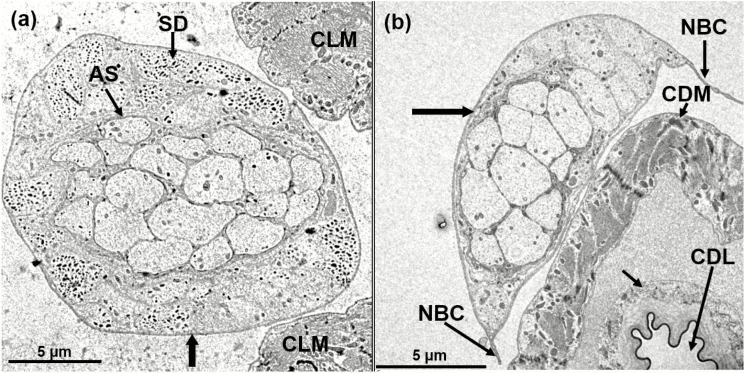
Cross-sections taken with TEM from the regions closest to the junction of the proventriculus, and proximal to the crop sac, show that the crop duct nerve bundle has different sizes and shapes in females and males. In both regions, male crop duct nerves are bigger than in females (Table 1; Figs. 9 and 10). At this level, one can still see several axons, the hemolymph and its close proximity to the crop duct muscles. A little anterior to the lobes (see Fig. 7a and the longblack, unlabeled arrow) the crop duct is wider than at the anterior end. In this region, the crop nerve bundle is also larger in diameter (Table 1) and shows that the basal lamina of both the crop duct nerve and the crop duct are very close to one another, and possibly touching in some areas (Fig. 10b). Figure 10b reveals the nerve bundle connector and, just above an axon showing dense core, dense opaque droplets are recognizable. The dark, dense epicuticle of the lining of the crop duct is evident and just to the right is the lumen. An endocuticular layer is evident and is surrounded by the epithelial cell layer. The next layer is that of the crop duct muscles. A cross-section of the crop duct nerve bundle (unlabeled, black vertical arrow in Fig. 10a) of a male, shows the adjacent muscles of the crop lobe. Clearly, 19 axons coming from the corpus cardiacum are surrounded by a neuronal sheath. Also, evident are several separate units containing dense/opaque secretory droplets. Another cross-section of the crop duct nerve bundle (unlabeled horizontal black arrow, Fig. 10b) of a female reveals the crop duct and adjacent crop duct nerve bundle (Fig. 10b).
Table 1.
Size (diameter) by sex and location of axons of the crop duct´s nerve
| Location | Big axons diameter (µm) | Small axons diameter (µm) | ||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| Distal to crop sac | 20.26 × 13.03 | 12.03 × 10.71 | 19.71 × 13.03 | 8.46 × 6.33 |
| Proximal to crop sac | 22.97 × 13.41 | 11.38 × 9.17 | 20.19 16.19 | 10.67 × 5.42 |
Fig. 9.
TEM of cross-section of crop duct nerve from the region near the proventriculus/cardia (unlabeled black arrow to the left in Fig. 7a) showing small and big axons (asterisks), the crop duct muscles (CDM) and hemolymph area (HEM). Sexual dimorphism of crop duct nerve is evident, (a) Male; (b) Female.
Fig. 10.
TEM of cross-section of crop duct nerve from the region near to crop sac (unlabeled vertical, black arrow to the right in Fig. 7). (a) Male crop duct nerve showing 19 axons in the center, which are surrounded by a neuronal sheath (AS), adjacent muscles of the crop lobe (CLM), and separate units containing dense/opaque secretory droplets (SD), magnification 500×; (b) Female crop duct nerve showing 13 axons, the crop duct muscles (CDM), the crop duct lumen (CDL) which is encased by a dense epicuticular material; the unlabeled, smaller arrow points to the crop duct lumen unit, which is composed of the endocuticular layer and outside of it is a layer of epithelial cells. The crop duct unit is surrounded by an unknown fluid, which may be hemolymph and this in turn is surrounded by the crop duct muscles (CDM). As shown in Figs. 7 and 8, it is obvious that the crop duct nerve bundle is attached to the crop duct by a connector (NBC), magnification 600×.
The crop duct lumen is evident and is encased in a dense epicuticular material followed by a lighter layer of endocuticle. The unlabeled smaller arrow points to the crop duct lumen unit, which is composed of the endocuticular layer, and outside of it, is a layer of epithelial cells. The crop duct unit is surrounded by an unknown fluid, possibly hemolymph, which in turn is surrounded by the crop duct muscles. As shown in Figs. 7, 8, and 10, it is obvious that the crop duct nerve bundle is attached to the crop duct by connectors. The crop lobes of A. ludens are similar to that of other flies examined by having a complex external set of numerous ribbon-like muscles that are connected by intercellular cytoplasmic bridges that cover the crop lobes (Fig. 7a). The crop duct, however, lacks external muscles covering its outside surface, but instead is covered by a smooth surface (Figs. 7a and 8). The only other structures associated with the crop duct are the pair of crop duct nerve bundles that go from the corpus allatum to the surface of the crop lobes. These nerve bundles are evident in both SEM and fresh dissections (compare Fig. 7a and b).
By comparing Fig. 10a and b, related to male and female structures, respectively, it becomes apparent that there are many more secretory droplets/packages in the male (Fig. 10a) than in the female (Fig. 10b). A cross-section (Fig. 11a) shows the lumen of the crop and its epicuticular and endocuticular layers. Also present within the muscle layer of the duct, are numerous mitochondria. In the lower left section of Fig. 11b, and in close proximity to the crop duct muscles, a cell is highlighted containing numerous dense droplets and an arrow which might indicate the direction of the droplets to the surface of the muscles of the crop duct.
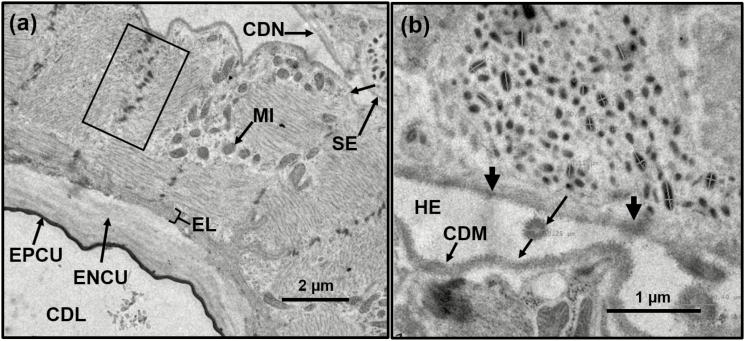
Fig. 11.
TEM of cross-section of crop duct. (a) Area showing the lumen of the crop and its epicuticular (EPCU), endocuticular (ENCU), and epithelial layers (EL). The rectangle shows the Z-bands or discs of the muscle layer of the duct, which contains numerous mitochondria (MI). At the top right is the crop duct nerve bundle (CDN) containing secretory material (SE) and an unlabeled arrow showing the direction the droplets take to potentially interact with the muscles of the crop duct muscles; (b) Enlargement of a section of the crop duct nerve bundle showing one of the secretory cells and dark imaging of its basal lamina in two areas (two unlabeled arrows at the basal lamina). Between the two unlabeled arrows is a released secretory droplet (0.25 µm). A larger secretory droplet is seen below, to the right and is 0.40 µm. Both droplets may be destined for the muscles of the crop duct (CDM).
Dense Core Droplets Associated with the Crop Nerve Bundle
At the top right of Fig. 11a, one can visualize the crop duct nerve bundle containing numerous secretory droplets. Some of these are circular in cross-section while others are oval. An enlargement of a section of the crop duct nerve bundle clearly shows a secretory cell (Fig. 11b). Between the two unlabeled arrows one can visualize what is believed to be a released secretory droplet (0.25 µm). A larger secretory droplet is seen below and to the right and is 0.40 µm in diameter. Measurements of the mean width and length of these droplets are 0.10 µm for width (n = 8) and 0.18 µm for length (n = 11), respectively. In this region, the crop nerve bundle is larger in diameter (Table 1) and reveals that the basal lamina of both the crop duct nerve and the crop duct are very close to one another, if not actually touching in some areas. The top of Fig. 10b shows the nerve bundle connector of the nerve bundle having dense droplets. In the same Fig. 10b, the dark, dense epicuticle of the lining of the crop duct is evident and separates the crop duct muscle from the crop duct lumen. An endocuticular layer is evident and is surrounded by the epithelial cell layers. The next layer is that of the crop duct muscles. Other cross-sections through the crop duct nerve bundle show numerous axons surrounded by an axonal sheath (Fig. 10a). In addition, we were surprised to find, surrounding the axonal sheath of a male adult, numerous cells that contained dense core droplets (Fig. 10a). This unit is in close proximity of the muscles of the crop lobe. Notably, when a similar section was obtained from a female, the large number of cells containing dense core droplets was not observed (Fig. 10b). In that same section, the nerve bundle connectors were observed connecting the bundle to the crop duct. The crop duct lumen is surrounded by a sheath. The crop duct muscle surrounding the duct is separated from the lumen area itself by an amorphous material that might be hemolymph (Fig. 10b). When a cross-section was made of the duct, more details became evident (Fig. 11a). The crop lumen is surrounded by both the epi- and endocuticle with an epithelial layer surrounding both. The crop duct muscles contain Z-bands, which appear to be in register and they contained numerous mitochondria (Fig. 11a). In close proximity to the duct muscle is a crop duct nerve bundle housing numerous dense core droplets. An enlargement of one of the cells contained within the nerve bundle is shown in Fig. 11b and reveals numerous dense core droplets that appear to be releasing their contents onto the crop duct muscle where it is in close proximity to the bundle. Examination of the basal lamina of the nerve bundle shows what appear to be areas where the dense core droplets are released into the hemolymph and where they could come into contact with the crop duct muscle. Within the hemolymph are what appear to be two droplets that were released and have increased in size (Fig. 11b). Importantly, the crop duct/nerve bundle association may somehow be involved in the bubbling or regurgitation of crop contents.
Bubbling/Regurgitation
Having a crop containing a sugar solution and other substances (i.e., sexual pheromone only in males), flies will regurgitate numerous droplets, which were deposited on the floor surface of the Petri dish they were housed in, and are arranged in a continuous trail (Fig. 1a and b). Bubbling is really regurgitation of material from the diverticulated crop. Both males and females produce lines of regurgitated droplets, which they deposit on the surface they are walking on.
Behavioral Assay
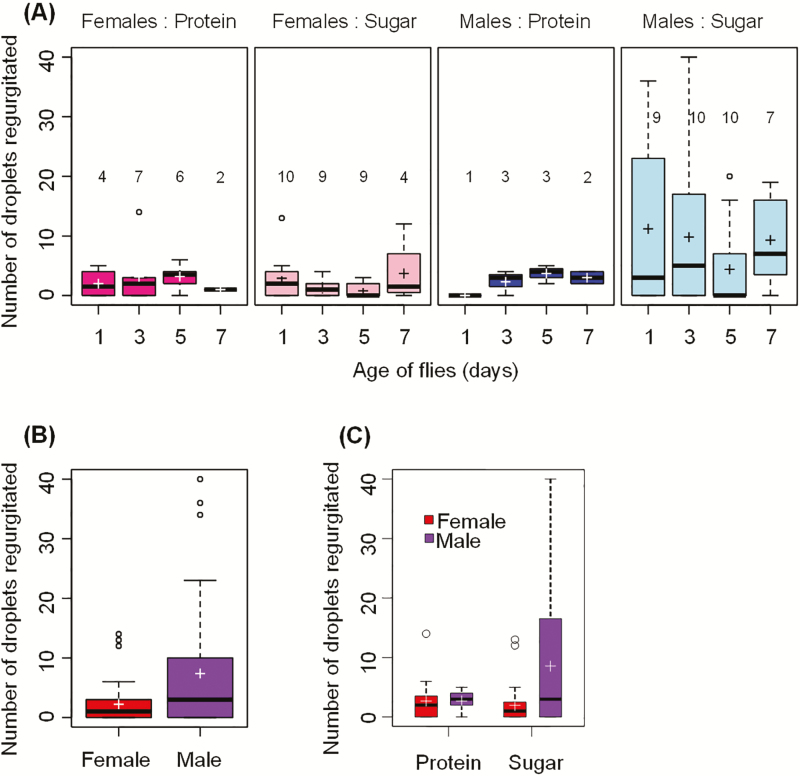
The number of droplets adult flies regurgitated ranged from 0 to 40, and the relationship between the age of flies and the number of droplets regurgitated was not statistically significant (deviance = 3.29, P = 0.3482, Fig. 12a). The minimal adequate model fitted to the data only included Sex (deviance = 14.26, P < 0.0001) as a significant predictor of regurgitation. Regardless of food type and age of flies, males regurgitated significantly more droplets than females (z = 3.7, P < 0.001; Fig. 12b). The model also included Food (deviance = 0.1535, P = 0.6952) and Food:Sex (deviance = 3.6994, P = 0.0544) as nonsignificant predictors because the model with the Food:Sex interaction had a smaller AIC value (466.1) than the model without this interaction (468.0). Males fed on the sugar solution regurgitated more droplets than males fed on the protein solution or females fed on protein or sugar solutions (Fig. 12c). The residual analyses indicated no violation of model assumptions.
Fig. 12.
(a) Boxplots of the number of droplets regurgitated by A. ludens females and males aged 1–7 d fed on protein or sugar solutions; (b) Boxplot of the number of droplets regurgitated by A. ludens females and males regardless of their age and the type of food they consumed; (c) Boxplot of the number of droplets regurgitated as a function of the type of food consumed and the sex of flies. The boxes extend from the 25% to the 75% quartile, and the horizontal line in each box indicates the median. Whiskers indicate the minimum and maximum values; if there are outliers (circles beyond whiskers), the whiskers indicate 1.5 times the size of the hinge, which is the 75% minus 25% quartiles. The cross indicates the mean. The numbers above each box in (A) indicate the number of flies that ate (out of 10 flies) at each age in a 3-h period.
Discussion
General Digestive Tract Structure
The main focus of this study was to examine more closely structures found in the foregut (Figs. 2–5), as the mid- and hindgut in fruit flies have already been fairly well described (Caetano et al. 2006, Lemaitre and Miguel-Aliaga 2013). The digestive tracts shown in Figs. 2 and 5a for male A. ludens are very similar to those reported for Anastrepha fraterculus (Wiedemann) and Ceratitis capitata (Wiedemann) by Caetano et al. (2006) and for P. regina by Stoffolano et al. (2010). However, our study on A. ludens, flushed out to date unknown differences on the size of the crop duct nerves between the sexes (Fig. 9; Table 1), and also considerable differences in the numbers of axons inside the male crop duct nerve, possibly related to the control of regurgitation, which we also found to be more intense in males (Fig. 12).
Diverticulated Crop
A general description (Caetano et al. 2006) of the crop lobes in A. fraterculus and C. capitata indicated that they are very similar to those previously reported for P. regina (Stoffolano et al. 2010) and, the ones observed in this study (Fig. 5) for A. ludens. The only difference in the Caetano et al. (2006) report and, the Stoffolano et al. (2010) study, is in naming the intercellular cytoplasmic bridges. We believe that they mistakenly called them longitudinal muscle fibers in their ‘Figure 3b’.
The crop is an extremely important structure because it is the region of the gut where food is initially processed. Not only is the crop important as a food storage organ in the Diptera, but this structure has been shown to be the site where horizontal transmission of the genes involved in microbial resistance takes place (Petridis et al. 2006). Recently, it has also been shown to be the site housing bacteria capable of degrading pesticides (de Almeida et al. 2017).
Crop Duct
Few studies have been specifically conducted on the crop duct of dipterans. Knight (1962), working with P. regina reported mainly on the reversal of fluids based on crop duct muscles prior to food entering the midgut through the cardia valve or sphincter. Thomson and Holling (1976), also using P. regina adults, produced a model of peristalsis that is based on the crop duct musculature. Thomson (1975) and, Thomson and Holling (1976) mapped the various sphincters (valves) and pumps of P. regina and attributed crop duct peristalsis or waves of movement during the filling and emptying of the crop lobes to volume size of the bolus and thickness of the duct muscles at the sites of sphincters. At that time, no internal structure of the crop had been reported, rendering our study here, a valuable addition to this type of literature.
Here, we provide SEM evidence (Figs. 7 and 8) supporting previous unpublished reports that the crop duct nerve bundles are attached to the crop duct via the crop duct nerve connectors and explains why previous attempts to free them from the crop turned out difficult or unsuccessful. Caetano et al. (2006) working with A. fraterculus describe (their Figure 1b) the same connecting system between the crop duct nerve and the crop duct, although they failed to mention it in their publication. Caetano et al. (2006) also show, as is the case in this study, that the surface of the crop duct does not contain a series of muscle-coverings on the hemolymph side. The surface of the crop duct, however, lacks any external muscles (they are found covering the lobes). In addition to our report, other studies (Lee et al. 1998, Stoffolano et al. 2010) have shown that the inner lining of the crop lobe lumen, as well as those of the crop duct, include an opaque epicuticular layer, a thicker endocuticular layer, both of which are produced and are surrounded by the cuticular epithelium. It is known that the dipteran crop is impermeable to leaking substances from the lumen into the hemolymph and/or taking on substances into its lumen from the hemolymph. We dwell on this later when discussing the crop lobes.
Caetano et al. (2006) note that the crop duct was innervated by the stomodeal nerve and documented the latter by identifying connectors between the crop duct and the crop nerve bundle. Our study, however, especially Fig. 6, does not support this statement, but instead, we believe these structures are connectors helping to keep the nerve bundle attached to the crop duct throughout its length between the corpus cardiacum and lobes.
Our study reveals that the function (i.e., contraction) of the crop duct muscles may be influenced by the dense core droplets found inside the crop duct nerve bundle (Figs. 9 and 10). These secretions may be released at the appropriate time and could affect the adjacent portions of the crop duct muscles. Also, Cognigni et al. (2011) show, through staining with antibody anti-fasciclin III, that neurites project through the circular muscles towards the epithelium of the crop duct. Thus, both this study and the one by Cognigni et al. (2011)suggest that the crop duct is modulated by factors other than muscle diameter as proposed by Thomson (1975). Importantly, we report for the first time the presence of a nerve connector (Fig. 8), which connects both nerve bundles and suggests there may be some type of communication between the two parallel bundles located on each side of the crop duct.
Crop Nerve Bundle and Neurosecretion
Based on our TEM studies, we suggest that the dense core vesicles observed in the crop nerve bundle are neurosecretions. When Copenhaver (2007) wrote his seminal paper on gut innervation in insects, he did not know about the existence of innervation in the dipteran crop (PFC, personal correspondence). In his correspondence, he notes that there are remarkable variations that have evolved in different species. Here, we document that such ‘variations’ are related to the innervation involved in regulating and modulating the dipteran crop.
Our finding related to axons in the crop nerve bundle containing dense core vesicles was unexpected. Even more surprising to us, was finding them only in males, but not females (Figs. 9 and 10). This is very interesting because males are the ones that have the large ball-like salivary gland structures associated with the crop (Fig. 5) and males are the only ones that produce the lekking pheromone. To conclusively answer this, more TEM sections need to be studied in the female, and/or various antibodies to some of the common insect peptides need to be tested on both sexes (D.R. Nässel, personal communication). The general cross-sectional shape of the dense core droplets is circular in shape, but sometimes we observed oval shaped droplets as well (details in Fig. 11). Similar shaped secretions, oval in section, were reported by Juberthie-Jupeau (1983) for Schizophyllum sabulosum (L.). The close proximity of these dense core vesicles to the muscles of the crop lobes (Fig. 11), and muscles of the crop duct, suggest that they are somehow involved in modulating contractions in these muscles.
Salivary Glands
Nation (1974, 1981) noted that the salivary glands in Anastrepha suspensa (Loew), A. ludens, and other eight species of tephritids (six Anastrepha spp. and two Ceratitis spp.) were sexually dimorphic. The salivary glands in those species terminate in blind, convoluted diverticula that normally are held together by tracheae, but when the tracheae are broken, the salivary gland becomes longer, but still terminates in a ball-like structure. Here, we were able to confirm the same sex differences in salivary glands reported by Nation (1974, 1981) (Fig. 5c).
Crop Duct/Crop/Salivary Gland Interface
Our TEM results failed to show any structural connection between the salivary glands and the crop duct or crop lobes. These findings lead us to question the reports of Lu and Teal (2001) and Walse et al. (2008) that there is a direct exchange of pheromone components from the salivary glands into the crop lumen in the species studied. Rather, we propose that the components present in the salivary glands, and those within the crop lumen and/or its regurgitated droplet, are first produced in the salivary gland, then deposited onto a surface, and then reingested and deposited in the crop. Also, it is difficult to imagine the crop tissue being involved in pheromone production. Instead, we believe that because the crop is intimately connected to the salivary glands, Lu and Teal (2001) did not totally separate the latter from the former, thus reaching inexact conclusions.
In another study, Walse et al. (2008) report that the oral secretion from the crop of A. ludens males only produce the pheromone when an abiotic chemical reaction occurs outside the fly and the release of the volatile from this droplet is influenced by both humidity and temperature. If this is so, we suggest that when placing droplets onto a leaf, there must be substantial amounts of water or another liquid from the salivary glands to support their finding as humidity is critical. Our findings here, help explain why fruit flies, especially those of the genus Anastrepha, regurgitate more compared to other dipterans (mean of 7.4 droplets after each food ingestion in our study which is similar to that observed in Musca domestica, Musca autumnalis, Phormia regina, and Protophormia terraenovae, noting the that the latter species do so more slowly (around seven spots/h, El-Bassiony et al. 2016). In Anastrepha striata Schiner, a species that exhibist trophalaxis, the mean number of droplets was as high as 23.5 per regurgitation bout (Aluja et al. 1993). We believe that flies procucing and regurgitating a lot of droplets and then reingesting them, are not only getting rid of excess water, but are also concentrating food, as it is shown in Fig. 5b, where it is possible to observe solid food inside the full crop. Alternatively, they may be partially synthesizing the oral pheromones by exposing them to the external environment as proposed by Walse et al. (2008). An additional and plausible, nonexclusive option, is that they may be releasing a growth media for bacterial growth/farming or for capturing/gleaning bacteria from the environment.
In Lu and Teal (2001), that same paper, the authors mistakenly noted that crop development changes with the development of the ability of males to produce the pheromone. Rather than the crop changing, the authors must have meant that the development of the salivary glands changed.
Considering that during courtship, the males of A. ludens and other species of Anastrepha, expand (exhibiting apparent ‘pumping movements’) the proboscis as they do during regurgitation (Aluja et al. 2000), we also suggest that this behavior may have the purpose of introducing air into the salivary glands so that the latter can be expanded as much as possible to push out the salivary glands (Fig. 6a) and look larger (a potential signal of male prowess). That is, this enlargement may be a signal females use to gauge the health and strength of potential partners.
Regurgitation
Caetano et al. (2006) were correct when they stated that the muscles of the crop lobes of A. fraterculus and C. capitata may be important in postfeeding bubbling and in the process of regurgitation where droplets from the crop are deposited on a substrate and reingested. The process of regurgitation in flies may serve several functions, the original one being the removal of excess water from the diet (Aluja et al. 1989, Hendrichs et al. 1992). In the case of Anastrepha adults, regurgitation is so widespread that the behavior must have other functions than just water removal. Why do members of this genus regurgitate so much more when compared to other flies (El-Bassiony et al. 2016)? The answer might be that in this group of fruit flies, and other fruit flies that produce a pheromone in their salivary glands, the regurgitating behavior might be involved in pheromone production and deposition, enhancing or facilitating behavioral meeting sites or leks. Consistent with this, here we found that A. ludens males have much bigger salivary glands and regurgitate significantly more than females (Figs. 5c and 12b), probably as a strategy to evaporate water and concentrate pheromone components in the regurgitated droplets. Our study did not find a relationship between the age of flies and regurgitation (Fig. 12a). However, it would be important to examine this relationship further, to determine whether older and sexually mature males regurgitate more than sexually immature males. Lu and Teal (2001) stated that “Unfortunately, information regarding the significance of regurgitation of oral secretions on leaves is lacking.” These drops of oral secretions, deposited with frequency during sexual signaling, are similar in color to the liquid contents of the crop and development of the crop of male flies is strongly correlated with the ability of males to attract females (Nation 1974).
Our TEM study looking for any possible physical connection between the crop and the salivary glands failed to show any structural evidence that materials from the salivary glands pass directly to the crop lumen/tissue. In fact, all TEM sections (Fig. 11a) show that as conventional knowledge has proven, and as briefly mentioned above, that the crop duct and lobes have a cuticular lining based on its embryonic origin, and that its impermeability has been known for a long time. Thus, we question that any substances can pass through the cuticular layer of the crop. We therefore suggest that during their dissections (Lu and Teal 2001), salivary gland tissue remained attached to the crop via tracheal connections, and thus, these authors mistakenly interpreted that the crop tissue produced these chemicals. Instead, we believe that since pheromone components produced in the salivary glands are the same as those found in the liquid droplet when the males regurgitate, these components are released and then transported into the crop when the fly consumes any food components.
In sum, we suggest that the greater rate of regurgitation and deposition of ‘trap lines’ (i.e., a series of meticulously deposited droplets in a line or spiral; Fig. 1a and b), in some fruit fly species, may be associated with production of oral pheromones. In addition to getting rid of excess water from the crop droplets, and possibly toxicants, reingestion is one way to concentrate the pheromone within the crop and also a way to introduce air to expand the crop and push out the salivary glands during courtship, thus making the male appear larger. The crop lobe system and digestive tract in A. ludens appears similar to other dipterans. Based on TEM and SEM, the terminal ball of the salivary glands does not appear to be able to pass pheromonal components that directly end up in the crop lumen, but rather they are secreted by the salivary glands and sucked up into the crop. Finding dense core droplets (i.e., believed to be neurosecretions) within the crop nerve bundle, suggests a previously unidentified way in which the crop muscles might be modulated. However, more information on the mechanism(s) regulating crop regurgitation within this important group of fruit fly pests needs to be accrued before definitive conclusions can be reached. If indeed it is proven that regurgitation may also be associated with possibly eliminating toxicants ingested by the insect, our findings can lead the way to better understanding why fruit fly chemical control is not always as efficient as expected.
Dedication
This paper is dedicated to Peter Teal who passed away at the age of 62 on 11 February 2015, and whose research on natural product chemistry of fruit flies will be seriously missed.
Acknowledgments
This research is based upon work supported by the National Institute of Food and Agriculture (NIFA), the U.S. Department of Agriculture (USDA), the Massachusetts Agricultural Experiment Station and the Stockbridge School of Agriculture at the University of Massachusetts, Amherst, under project number MAS00448 to J.G.S. and was conducted when J.G.S. was on sabbatical in the Instituto de Ecologia, A.C. - INECOL. In Mexico, the research was supported by the Consejo Nacional Consultivo Fitosanitario (CONACOFI), Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación (SAGARPA) and the Consejo Nacional de Ciencia y Tecnología (CONACYT), as well as INECOL. We gratefully acknowledge critical reviews by two anonymous referees and additional suggestions by the journal editor who helped us substantially improve the quality of this manuscript. We also thank Adriana Santos-Ramiro and Enedina Cruz-Hernández (INECOL) for technical assistance in the behavioral assay. The contents of this work are the responsibility of only the authors and do not necessarily represent the official views of the USDA or NIFA. Thanks are due to Drs. P.F. Copenhaver and D.R. Nässel for their insightful comments on the enteric/stomatogastric nervous system (i.e., here the crop nerve bundle) and the dense core droplets, respectively.
References Cited
- de Almeida L. G., Moraes L. A., Trigo J. R., Omoto C., and Cônsoli F. L.. . 2017. The gut microbiota of insecticide-resistant insects houses insecticide-degrading bacteria: a potential source for biotechnological exploitation. PLoS One 12: e0174754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aluja M., Cabrera M., Guillén J., Celedonio H., and Ayora F.. . 1989. Behaviour of Anastrepha ludens, A. obliqua and A. serpentina (Diptera: Tephritidae) on a wild mango tree (Mangifera indica) harbouring three McPhail traps. Insect Sci. Appl. 10: 309–318. [Google Scholar]
- Aluja M., Jácome I., Birke A., Lozada N., and Quintero G.. . 1993. Basic patterns of behavior in wild Anastrepha striata (Diptera: Tephritidae) flies under field-cage conditions. Ann. Entomol. Soc. Am. 86: 776–793. [Google Scholar]
- Aluja M., Piñero J., Jácome I., Díaz-Fleischer F., and Sivinski J.. . 2000. Behavior of flies in the genus Anastrepha (Trypetinae: Toxotrypanini), pp.375–406. InAluja M. and Norrbom A.. (eds.),Fruit flies (Tephritidae): phylogeny and evolution of behavior. CRC Press, Boca Raton, FL. [Google Scholar]
- Aluja M., Ordano M., Teal P. E., Sivinski J., García-Medel D., and Anzures-Dadda A.. . 2009. Larval feeding substrate and species significantly influence the effect of a juvenile hormone analog on sexual development/performance in four tropical tephritid flies. J. Insect Physiol. 55: 231–242. [DOI] [PubMed] [Google Scholar]
- Aluja M., Birke A., Ceymann M., Guillén L., Arrigoni E., Baumgartner D., Pascacio-Villafán C., and Samietz J.. . 2014. Agroecosystem resilience to an invasive insect species that could expand its geographical range in response to global climate change. Agri. Ecosyst. Environ. 186: 54–63. [Google Scholar]
- Audsley N., and Weaver R. J.. . 2009. Neuropeptides associated with the regulation of feeding in insects. Gen. Comp. Endocrinol. 162: 93–104. [DOI] [PubMed] [Google Scholar]
- Audsley N., Down R. E., and Isaac R. E.. . 2015. Genomic and peptidomic analyses of the neuropeptides from the emerging pest, Drosophila suzukii. Peptides. 68: 33–42. [DOI] [PubMed] [Google Scholar]
- Ben-Yosef M., Pasternak Z., Jurkevitch E., and Yuval B.. . 2015. Symbiotic bacteria enable olive fly larvae to overcome host defences. R. Soc. Open Sci. 2: 150–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing X., Gerlac J., Loeb G., and Buchon N.. . 2018. Nutrient-dependent impact of microbes on Drosophila suzukii development. mBio. 9: e02199-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birke A., Guillén L. D., Midgarden D., and Aluja M.. . 2013. Fruit flies, Anastrepha ludens (Loew), A. obliqua (Macquart) and A. grandis (Macquart) (Diptera: Tephritidae): three pestiferous tropical fruit flies that could potentially expand their range to temperate areas, pp. 192–213. InPeña J. E. (ed), Potential invasive pests of agricultural crops. Editorial CABI, Florida. [Google Scholar]
- Bozzola J. J., and Russell L. D.. . 1992. Electron microscopy: principles and techniques for biologists. Jones and Bartlett Publishers, Inc, USA. [Google Scholar]
- Buchon N., Broderick N. A., and Lemaitre B.. . 2013. Gut homeostasis in a microbial world: insights from Drosophila melanogaster. Nat. Rev. Microbiol. 11: 615–626. [DOI] [PubMed] [Google Scholar]
- Buckley Y. M. 2015. Generalized linear models, pp. 131–147. InFox G. A., Negrete-Yankelevich S. and Sosa V. J. (eds.), Ecological statistics: contemporary theory and application. Oxford University Press, Oxford, UK. [Google Scholar]
- Caetano F. H., Solferini V. N., Barros de Britto F., Silva Lins D., Aluani T., Garcia de Brito V., and Zara F. J.. . 2006. Ultra morphology of the digestive system of Anastrepha fraterculus and Ceratitis capitata (Diptera Tephritidae). Braz. J. Morphol. Sci. 23: 455–462. [Google Scholar]
- Cammaerts R. 1991. Behavioural interactions between the ant Lasius-flavus formicidae and the myrmecophilous beetle Claviger testaceus Pselaphidae. II. Frequency duration and sequence of the workers behaviour. Bull. Ann. Soc. R. Belge. Entomol. 127: 271–307. [Google Scholar]
- Cammaerts R. 1995. Regurgitation behaviour of the Lasius flavus worker (Formicidae) towards the myrmecophilous beetle Claviger testaceus (Pselaphidae) and other recipients. Behav. Processes. 34: 241–264. [DOI] [PubMed] [Google Scholar]
- Chandler J. A., James P. M., Jospin G., and Lang J. M.. . 2014. The bacterial communities of Drosophila suzukii collected from undamaged cherries. PeerJ 2: e474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. I., Salazar A., Yamada R., Fitz-Gibbon S., Morselli M., Alcaraz J., Rana A., Rera M., Pellegrini M., Ja W. W., . et al. 2015. Distinct shifts in microbiota composition during Drosophila aging impair intestinal function and drive mortality. Cell Rep. 12: 1656–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cognigni P., Bailey A. P., and Miguel-Aliaga I.. . 2011. Enteric neurons and systemic signals couple nutritional and reproductive status with intestinal homeostasis. Cell Metab. 13: 92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copenhaver P. F. 2007. How to innervate a simple gut: familiar themes and unique aspects in the formation of the insect enteric nervous system. Dev. Dyn 236: 1841–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley M. J. 2013. The R book. 2nd edition John Wiley & Sons, West Sussex, UK. [Google Scholar]
- Dethier V. G. 1976. The Hungry Fly - A physiological study of the behavior associated with feeding. Harvard Univ. Press, Cambridge, MA. [Google Scholar]
- Dillon R. J., and Dillon V. M.. . 2004. The gut bacteria of insects: nonpathogenic interactions. Annu. Rev. Entomol. 49: 71–92. [DOI] [PubMed] [Google Scholar]
- Douglas A. E. 2013. Microbial brokers of insect-plant interactions revisited. J. Chem. Ecol. 39: 952–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dus M., Lai J. S., Gunapala K. M., Min S., Tayler T. D., Hergarden A. C., Geraud E., Joseph C. M., and Suh G. S.. . 2015. Nutrient sensor in the brain directs the action of the brain-gut axis in Drosophila. Neuron. 87: 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer M. I., Moon A. M., Brown M. R., and Crossley D. A. Jr. 1995. Grasshopper crop and midgut extract effects on plants: an example of reward feedback. Proc. Natl. Acad. Sci. USA 92: 5475–5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Bassiony G., Lotfy M., and Stoffolano J. G. Jr. 2016. Comparison of sucrose intake and production of elimination spots among adult Musca domestica, Musca autumnalis, Phormia regina and Protophormia terraenovae. APJTB. 6: 640–645. [Google Scholar]
- Engel P., and Moran N. A.. . 2013. The gut microbiota of insects - diversity in structure and function. FEMS Microbiol. Rev. 37: 699–735. [DOI] [PubMed] [Google Scholar]
- Eriksson A., Raczkowska M., Navawongse R., Choudhury D., Stewart J. C., Tang Y. L., Wang Z., and Claridge-Chang A.. . 2017. Neuromodulatory circuit effects on Drosophila feeding behaviour and metabolism. Sci. Rep. 7: 8839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes A. M., Hearn D. J., Bronstein J. L., and Pierson E. A.. . 2009. The olive fly endosymbiont, “Candidatus Erwinia dacicola,” switches from an intracellular existence to an extracellular existence during host insect development. Appl. Environ. Microbiol. 75: 7097–7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson J. E., and Metcalf R. L.. . 1985. Cucurbitacins: plant-derived defense compounds for diabroticites (Coleoptera: Chrysomelidae). J. Chem. Ecol. 11: 311–318. [DOI] [PubMed] [Google Scholar]
- Gomes G., Köberle R., Von Zuben C. J., and Andrade D. V.. . 2018. Droplet bubbling evaporatively cools a blowfly. Sci. Rep. 8: 5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorissen M., Flik G., and Huising M.. . 2006. Peptides and proteins regulating food intake: a comparative view. Anim. Biol. 56: 447–473. [Google Scholar]
- Hammer T. J., and Bowers M. D.. . 2015. Gut microbes may facilitate insect herbivory of chemically defended plants. Oecologia. 179: 1–14. [DOI] [PubMed] [Google Scholar]
- Hendrichs J., Cooley S. S., and Prokopy R. J.. . 1992. Post feeding behaviour in fluid-feeding Diptera: concentration of crop contents by oral evaporation of excess water. Physiol. Entomol. 17: 153–61. [Google Scholar]
- Juberthie-Jupeau L. 1983. Neurosecretory systems and neurohemal organs of myriapoda, pp. 204–278. InGupta A. P. (ed.), Neurohemal organs of arthropods – Their development, evolution, structures, and functions. Charles C. Thomas Pub Ltd, Springfield, IL. [Google Scholar]
- Karnovsky M. J. 1965. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron-microscopy. J. Cell Biol. 27: 137–138A. [Google Scholar]
- Knight S. M. R. 1962. Rhythmic activities of the alimentary canal of the black blowfly, Phormia regina. Ann. Entomol. Soc. Am. 55: 380–382. [Google Scholar]
- Kumar S. 2012. Biopesticides: a need for food and environmental safety. J. Biofertil. Biopestici. 3: e107. [Google Scholar]
- Kyritsis G. A., Augustinos A. A., Cáceres C., and Bourtzis K.. . 2017. Medfly gut microbiota and enhancement of the sterile insect technique: similarities and differences of Klebsiella oxytoca and enterobacter sp. AA26 probiotics during the larval and adult stages of the VIENNA 8D53+ genetic sexing strain. Front. Microbiol. 8: 2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang J. M. 1969. Effects of regurgitation and reflex bleeding on mortality in western budworm (Choristoneura occidentalis) treated with lannate. Entomol. Exp. Appl. 12: 288–296. [Google Scholar]
- Lee W. Y., Chen M. E., and Lin T. L.. . 1998. Morphology and ultrastructure of the alimentary canal of oriental fruit fly Bactrocera dorsalis (Hendel) (Diptera: Tephritidae) (I): the structure of the foregut and cardia. Zool. Stud. 37: 95–101. [Google Scholar]
- Lemaitre B., and Miguel-Aliaga I.. . 2013. The digestive tract of Drosophila melanogaster. Annu. Rev. Genet. 47: 377–404. [DOI] [PubMed] [Google Scholar]
- Lu F., and Teal P. E.. . 2001. Sex pheromone components in oral secretions and crop of male Caribbean fruit flies, Anastrepha suspensa (Loew). Arch. Insect Biochem. Physiol. 48: 144–154. [DOI] [PubMed] [Google Scholar]
- Mattiacci L., Dicke M., and Posthumus M. A.. . 1995. beta-Glucosidase: an elicitor of herbivore-induced plant odor that attracts host-searching parasitic wasps. Proc. Natl. Acad. Sci. USA 92: 2036–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzon L., Martinez I.‑Sanudo C. Savio M. Simonato, and Squartini A.. . 2011. Stammerula and other symbiotic bacteria within the fruit flies inhabiting Asteraceae flowerheads, pp. 90–111. InBourtziscrc K. (ed.), Manipulative tenants. Bacteria associated with arthropods. CRC Press. [Google Scholar]
- Michaelides P. K., and Wright D. J.. . 1997. Insecticide penetration and symptomology studies on larvae of Diabrotica undecimpunctata howardi (Barber). Pestic. Sci. 49: 953–361. [Google Scholar]
- Nation J. L. 1974. The structure and development of two sex specific glands in male Caribbean fruit flies. Ann. Entomol. Soc. Am. 67: 731–734. [Google Scholar]
- Nation J. L. 1981. Sex-specific glands in tephritid fruit flies of the genera Anastrepha, Ceratitis, Dacus and Rhagoletis (Diptera: Tephritidae). J. Insect Morphol. and Embryol. 10: 121–129. [Google Scholar]
- O’Leary T., and Marder E.. . 2014. Mapping neural activation onto behavior in an entire animal. Science. 344: 372–373. [DOI] [PubMed] [Google Scholar]
- Onstad D. W. 2014. Major issues in insect resistance management, pp. 1–23. InOnstad D. W. (ed.), Insect resistance management - biology, economics, and prediction, 2nd ed Academic Press, New York, NY. [Google Scholar]
- Peterson S. C., Johnson N. D., and Leguyader J. L.. . 1987. Defensive regurgitation of allelochemicals derived from host cyanogenesis by eastern tent caterpillars. Ecology 68: 1268–1272. [Google Scholar]
- Petridis M., Bagdasarian M., Waldor M. K., and Walker E.. . 2006. Horizontal transfer of Shiga toxin and antibiotic resistance genes among Escherichia coli strains in house fly (Diptera: Muscidae) gut. J. Med. Entomol. 43: 288–295. [DOI] [PubMed] [Google Scholar]
- Pool A. H., and Scott K.. . 2014. Feeding regulation in Drosophila. Curr. Opin. Neurobiol. 29: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team 2017. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: https://www.R-project.org/ [Google Scholar]
- Ratner S. S., and Stoffolano J. G. Jr. 1982. Development of the esophageal bulb of the apple maggot, Rhagoletis pomonella (Diptera:Tephritidae): morphological, histological, and histochemical study. Ann. Entomol. Soc. Am. 75: 555–562. [Google Scholar]
- Rodriguez-Saona C. R., and Stelinski L. L.. . 2009. Behavior modifying Strategies in IPM: theory and Practice. InPeshin R., and Dhawan A. K. (eds.), Integrated pest management: innovation-development process. C. Springer Science+Business Media BV, Dordrecht, Netherlands. [Google Scholar]
- Schlegel P., Texada M. J., Miroschnikow A., Schoofs A., Hückesfeld S., Peters M., Schneider-Mizell C. M., Lacin H., Li F., Fetter R. D., . et al. 2016. Synaptic transmission parallels neuromodulation in a central food-intake circuit. eLife. 5: e16799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivinski J. 1980. The effects of mating on predation in the stick insect Diapheromera veliei Phasmatodea Heteronemiidae. Ann. Entomol. Soc. Am. 73: 553–556. [Google Scholar]
- Sivinski J., Epsky N., and Heath R.. . 1994. Pheromone deposition on leaf territories by male Caribbean fruit flies, Anastrepha suspensa (Loew) (Diptera: Tephritidae). J. Insect Behav. 7: 43–51. [Google Scholar]
- Solferini V. N. 1990. Interaracoes entre bacterias e Anastrepha (Diptera: Tephritidae). Ph.D. thesis. Universty of Sao Paulo, Sao Paulo, Brasil. [Google Scholar]
- Stoffolano J. G., Jr 2019. Fly foregut and transmission of microbes. Adv. Insect Physiol. 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffolano J. G. Jr, and Haselton A. T.. . 2013. The adult Dipteran crop: a unique and overlooked organ. Annu. Rev. Entomol. 58: 205–225. [DOI] [PubMed] [Google Scholar]
- Stoffolano J. G. Jr, Guerra L., Carcupino M., Gambellini G., and Fausto A. M.. . 2010. The diverticulated crop of adult Phormia regina. Arthropod Struct. Dev. 39: 251–260. [DOI] [PubMed] [Google Scholar]
- Suarez M. E., and Thorne B. L.. . 2000. Rate, amount, and distribution pattern of alimentary fluid transfer via trophallaxis in three species of termites (Isoptera: Rhinotermitidae, Termopsidae). Ann. Entomol. Soc. Am. 93: 145–155. [Google Scholar]
- Sun F., Wang Y., Zhou Y., Van Swinderen B., Gong Z., and Liu L.. . 2014. Identification of neurons responsible for feeding behavior in the Drosophila brain. Sci. China. Life Sci. 57: 391–402. [DOI] [PubMed] [Google Scholar]
- Thomson A. J. 1975. Synchronization of function in the foregut of the blowfly Phormia regina (Diptera: Calliphoridae) during the crop-emptying process. Can. Entomol. 107: 1193–1198. [Google Scholar]
- Thomson A. J., and Holling C. S.. . 1976. A model of foregut activity in the blowfly Phormia regina Meigen. II. Peristalsis in the crop duct during the crop-emptying process. Can. J. Zool. 54: 172–179. [DOI] [PubMed] [Google Scholar]
- Ventura C., Briones-Roblero C. I., Hernández E., Rivera-Orduña F. N., and Zúñiga G.. . 2018. Comparative analysis of the gut bacterial community of four Anastrepha fruit flies (Diptera: Tephritidae) based on pyrosequencing. Curr. Microbiol. 75: 966–976. [DOI] [PubMed] [Google Scholar]
- Walse S. S., Alborn H. T., and Teal P. E. A.. . 2008. Environmentally regulated abiotic release of volatile pheromones from the sugar-based oral secretions of Carib flies, Green Chem. Lett. and Rev. 1: 205–217. [Google Scholar]
- Zuur A. F., and Ieno E. N.. . 2016. A protocol for conducting and presenting results of regression‐type analyses. Methods Ecol. Evol. 7: 636–645. [Google Scholar]