The goal of a medical screening program is to recognize a disease in its preclinical phase so that intervention can occur at earlier stages, hopefully leading to better outcomes. Screening programs may also promote public awareness and education, encourage physician adherence to clinical practice guidelines (1,2), and serve as medical outreach to underserved populations (3). Such programs have benefits, risks, and costs. For a screening program to be beneficial, it should fulfill several criteria, as outlined by Wilson and Jungner (4) in 1968 in Principles and Practice of Screening for Disease (Table 1). In 1978, Rose and Barker (5), in a classic article on screening, stated that, to establish that a screening program was useful, health care providers needed to answer three important questions: “Does earlier treatment improve the prognosis?” “How valid and repeatable is the screening test?” and, “What is the yield of the screening service?” Today, these three questions remain fundamental to the success of any screening program.
Table 1.
Wilson-Jungner criteria for appraising the validity of a screening program
| 1. The condition sought should be an important health problem. |
| 2. There should be an accepted treatment for patients with recognized disease. |
| 3. Facilities for diagnosis and treatment should be available. |
| 4. There should be a detectable preclinical stage. |
| 5. There should be a suitable test. |
| 6. The test should be acceptable to the population. |
| 7. The natural history of the condition should be well understood. |
| 8. A policy on whom to treat as patients should be developed and agreed on. |
| 9. The cost of case-finding should be balanced with medical care availability. |
| 10. Screening should be a continuous process and not a one-time project. |
Screening programs became widespread after 1945 with the earliest modern and mass screening programs focusing on diabetes and cervical cancer (6). It is interesting that one of the first interventions that qualified as a “screening program” was implemented in 1917 by the US Army, in an attempt to prevent young men with psychologic disorders to join the military. The aim of this program was to examine large numbers of recruits in an efficient way with a low chance for error. Later, in 1951, the US Commission of Chronic Illness characterized screening as “the presumptive identification of unrecognized disease or defect by the application of tests, examinations, or other procedures which can be applied rapidly. A screening test is not intended to be diagnostic. Persons with positive or suspicious findings must be referred to their physicians for diagnosis and necessary treatment” (7). Benefits of widespread screening programs, such as the decline in syphilis incidence and the decrease in mortality as a result of uterine cancer, have been observed throughout history, yet screening is still considered controversial in other clinical areas, such as mass screening for HIV (8).
An evidence-based treatment regimen for patients with recognized chronic kidney disease (CKD) already exists and serves as a prerequisite for CKD screening programs (Table 1, criterion 2). Screening for CKD occurs in many contexts, including during routine care (9), within high-risk populations (1,2), and in the general population (10,11). Population-based screening must ensure follow-up and successful referral to care of test-positive individuals for appropriate diagnosis, treatment, and counseling. In addition, because screening in these different contexts entails different risks and benefits, informed patient choice is essential to a successful screening program. To provide a better understanding of these and other issues involved in early identification of kidney diseases, we review the principles of screening and diagnostic testing as they apply to CKD in the adult population in North America.
CKD as a Public Health Problem in Need of Early Identification and Delay in Progression
CKD represents a significant public health problem, with nearly 20 million people in the United States having kidney damage or reduced kidney function (Table 1, criterion 1) (12). More than 400,000 people currently receive some form of renal replacement therapy, and this number is expected to reach 2.2 million by the year 2030 (13). Furthermore, CKD is now recognized as a cardiovascular risk factor, according to a recent consensus of the American Heart Association, and the vast majority of patients with CKD die before any renal replacement therapy is needed (14,15). Early treatment in the course of the disease, aimed at slowing the progression of CKD or reducing adverse cardiovascular outcomes by the use of angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB), for example, could have a positive economic impact on the staggering cost of CKD and related hospitalizations (16–18) (Table 1, criterion 7).
Unfortunately, CKD awareness is low in the US population (19). Using the Third National Health and Nutrition Survey (NHANES III; conducted from 1988 to 1994), Coresh et al. (19) reported that, among all individuals with moderately decreased GFR (stage 3 CKD, estimated GFR [eGFR] 30 to 59 ml/min per 1.73 m2), awareness was only 8.2%. Awareness was higher among patients with lower kidney function and higher albuminuria levels. Also, chart review of patients suggests that CKD is often undiagnosed, and associated complications (e.g., anemia) often remain untreated (20,21). These findings further highlight the need to improve early detection and management of CKD in the US population. In an effort to achieve these goals, the Kidney Disease Outcomes Quality Initiative (KDOQI) of the National Kidney Foundation has developed and published screening recommendations for individuals who are at risk for CKD (22), which were introduced after more broadly disseminated guidelines on screening for diabetic nephropathy (23) and hypertension (24), possibly explaining the low awareness rates for CKD relative to diabetes and hypertension.
Methodologic Considerations for Early Kidney Disease Detection
Validity of Screening Methods
Early disease detection methods can be applied in the general population or in medical settings. A successful detection method—that is, a test that can separate the part of the population that has the disease from the part that does not have the disease—is considered to be a test with high validity (Table 2 for screening glossary of terms). There are two important components in assessing the validity of any disease detection method: Sensitivity and specificity. Sensitivity of a test is the ability of that test to identify correctly those who actually have the disease. Specificity is the ability of the test to identify correctly those who do not have the disease. Another important issue to consider in screening evaluation is reliability, which can be defined as the consistency of the results across repeated tests under the same conditions. Reliability is usually assessed using correlation measures, such as the κ statistic. A κ statistic >0.75 usually signifies excellent agreement beyond that expected by chance alone, whereas a κ <0.40 signifies poor agreement. A κ between 0.40 and 0.75 signifies intermediate to good agreement. Validity is measured by comparison of the test of interest with a “gold standard,” a test that detects true cases 100% of the time.
Table 2.
Glossary of screening termsa
| Concept | Definition |
|---|---|
| Reliability | Consistency of the results if the disease detection method were repeated more than once under the same conditions |
| Validity | The ability of the disease detection method to separate those who have the disease from those who do not |
| Sensitivity | The ability of the disease detection method to identify correctly those who have the disease as positive |
| Specificity | The ability of the disease detection method to identify correctly those who do not have the disease as negative |
| PPV | The probability of having the disease, given that the person tested positive |
| NPV | The probability of not having the disease, given that the person tested negative |
| Bias | A systematic error in the design, conduct, or analysis of a study that results in a wrong estimate of a disease detection method’s ability to identify disease or nondisease |
| Selection bias | A systematic error resulting from a differential selection of those who are screened and those who are not |
| Information bias | A systematic error resulting from flawed collection of the data or imperfect definition of study variables |
NPV, negative predictive value; PPV, positive predictive value.
For assessment of validity, sensitivity and specificity of a detection method should be calculated. Tests results reported as continuous values need specific cutoffs to decide whether an individual has the disease; therefore, sensitivity and specificity calculations are more challenging than if the results were dichotomous. For example, estimation of GFR is a continuous test because it is derived from serum creatinine, a continuous variable. Such test results need to be transformed to a dichotomous variable so that cutoffs for the best diagnostic values (balancing sensitivity and specificity) can be defined. An eGFR cutoff that is too high compromises specificity and often yields many false-positive results, leading to overestimation of the prevalence of CKD and unnecessary further testing at increased cost and possibly even with adverse psychological consequences. A low eGFR cutoff compromises sensitivity and gives a larger number of false-negative results, which would underestimate the true prevalence of CKD, with too many patients not appropriately recognized as being at probable increased risk for progression to ESRD and treated accordingly (25).
The process of early disease detection can be performed using only one test or multiple tests on the population of interest, which would improve validity. Two or more tests can be applied sequentially or simultaneously. The sequential approach often includes using a less expensive and less invasive test at first (e.g., urine albumin–specific dipstick); those who screen positive in that first test are subsequently retested using a different confirmatory method (e.g., a quantitative measurement such as urine albumin-to-creatinine ratio). This second test in certain situations could be more invasive and more expensive but usually has a higher sensitivity and specificity. The second test is done in an attempt to identify those with false-positive results on the first test and correctly labeling them as not having the disease, thereby improving the validity of the screening strategy. The simultaneous testing approach (e.g., measurements of both urinary albumin excretion and serum creatinine level to estimate GFR) is more common in clinical settings. Testing positive on either test is considered positive in this approach, yet a person has to have negative results on both tests to be considered negative. The choice of which approach is used usually depends on the objectives of the screening program. Sequential testing usually improves specificity of screening, but it may result in loss of sensitivity. The simultaneous testing approach usually improves sensitivity, but it may result in loss of specificity.
Whereas sensitivity and specificity (i.e., validity) describe a test’s ability to identify the probability of disease given that an individual tests positive or negative, a more frequently considered question in clinical practice is what proportion of individuals with a positive test truly have the disease. The ability of a positive test to predict disease given a positive result is referred to as the positive predictive value (PPV). Similarly, the ability of a negative test to rule out disease given a negative result is referred to as the negative predictive value (NPV) of the test. PPV and NPV are strongly related to the prevalence of the disease of interest. PPV can be defined as the number of true positives divided by everyone who tested positive; this suggests that increasing the prevalence of the disease in a population will result in a higher PPV. Thus, a more beneficial and successful screening program would be one targeted at highrisk groups in which prevalence of the disease of interest is high, such as screening for CKD in patients with diabetes or hypertension. Recently, Hallan et al. (10) nicely illustrated this issue in their 8-yr follow-up of a cross-sectional study (The Nord-Trøndelag Health Study [HUNT II Study]) in which they screened 65,604 adults in the general population of Norway. Defining CKD as an eGFR <60 ml/min per 1.73 m2, if the whole population were screened, then one would need to screen 20.6 individuals to identify one CKD case; whereas if screening were restricted to patients with diabetes, hypertension, or age >55 yr, then one would identify 93.2% of patients with CKD and would need only 8.7 individuals to identify one CKD case. Of note, the predictive value also depends on the specificity of the test used for screening. This relationship is significant only when the prevalence is small, where a small increase in specificity can greatly improve predictive value.
Bias in Screening Methods
Bias remains a major issue in all types of epidemiologic study designs and should always be considered in the interpretation of results. Bias is defined as “any systematic error in the design, conduct, or analysis of a study that results in a mistake estimate of an exposure’s effect on the risk of disease” (26). Although bias may not affect the assessment of an individual patient who tests positive and is adequately treated in the clinical setting, it should always be considered in the assessment of the value of a screening program for the overall population, because it can spuriously boost the effects of screening. Three potential types of bias should be considered in screening: Selection bias, the most important, but also lead-time bias, and overdiagnosis bias.
Selection bias usually occurs when individuals are more (or less) likely to be included in a study sample according to a specific characteristic. In implementing a screening program, the main types of selection biases include volunteer bias and length-bias sampling. Most screening programs screen those who volunteer to be tested instead of randomly choosing who gets to participate in the screening process. In CKD screening of a general population, those who volunteer to be screened might have a strong family history of kidney disease; in this situation, results of the screening will show a higher prevalence of CKD, whereas in reality, increased prevalence is observed because those who were screened were at higher risk for having the disease of interest. Selection bias might also operate in the opposite direction, whereby the more educated and healthconscious (and likely healthier) individuals of the population might volunteer for screening, resulting in screening’s detecting a low prevalence of disease in this population, a consequence of screening “healthier” individuals. The other common type of selection bias, length-biased sampling, is directly related to the type of disease being screened. Patients with CKD can have different patterns of CKD progression; some might have a short natural history, usually with a short preclinical phase and a short clinical phase before ESRD (e.g., rapidly progressive glomerulonephritis), whereas others, the majority of patients with CKD, might have a longer natural history of their disease process, with a longer preclinical as well as a longer clinical phase (e.g., diabetic nephropathy). Those with a longer natural history of kidney disease are more likely to be picked up at the screening because their preclinical phase (the phase during which screening is most beneficial) is longer. Because these cases have longer preclinical phases, they are more likely also to have longer clinical phases and, thus, presumably, a better renal survival after being diagnosed. If no intervention is available, then the longer renal survival will not be due to early detection because of the screening process but rather because of the longer natural history of the underlying disease.
Lead-time bias (Figure 1) results in a problem similar to that due to length-biased sampling. Lead-time is the time between early diagnosis and the usual time of diagnosis. Bias in this case occurs when diagnosis is made earlier than when symptoms occur, which increases the time of being diagnosed but does not increase renal survival or improve health.
Figure 1.
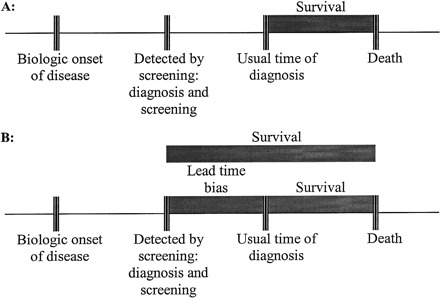
Lead-time bias. (A) The usual time of diagnosis and the length of survival after diagnosis. (B) Earlier diagnosis increases observed survival time, but death is not actually delayed. Including the survival times between the periods of early diagnosis as a result of screening and the usual time of diagnosis, when comparing them with observed survival times in those who are not screened, results in lead-time bias.
The third common type of bias in any screening program is overdiagnosis bias. This occurs because some programs might overdiagnose individuals as a result of high enthusiasm about the program. This might result in overreading of the results and possibly in false-positive results; if results in this situation were compared with another population that did not go through the screening program, then it might seem that there was a higher prevalence in the population screened, but the difference would be due only to a greater number of false-positive results.
Screening for CKD among Adults
Definition and Tests for CKD Screening
Despite the publication of several practice guidelines in recent years (22,27), the effectiveness of early detection of CKD among adults in the general US population has not yet been studied using randomized clinical trials. The KDOQI guidelines defined the five stages of CKD (Table 3), on the basis of renal damage as manifested by the presence of abnormal urinary albumin excretion (albuminuria) and the level of kidney function measured by GFR (22). CKD is a mostly asymptomatic condition in its earlier stages, with a preclinical phase that may not be detectable by usual testing (28), which makes definition of CKD and choice of screening tests a more difficult issue (criterion 4).
Table 3.
Stages of chronic kidney disease (22)
| Stage | Definition | Estimated GFR (ml/min per 1.73 m2) |
|---|---|---|
| 1 | Kidney damage with normal or increased GFR | ≥90 |
| 2 | Kidney damage with mild decreased GFR | 60 to 89 |
| 3 | Moderately decreased GFR | 30 to 59 |
| 4 | Severely decreased GFR | 15 to 29 |
| 5 | Kidney failure | <15 (or dialysis/transplantation) |
The measurement of albuminuria provides a sensitive marker of CKD from very early to more advanced stages of the disease process. A 24-h urine collection for the diagnosis of albuminuria remains the gold standard; however, because of the inconvenience and errors associated with a timed urine sample, a spot urine sample using either albumin-specific dipstick or albumin-to-creatinine ratio is now an accepted screening method (22,29). Houlihan et al. (30) assessed the characteristics of the albumin-to-creatinine ratio as a screening test and found sensitivities >90% for both men and women, with excellent accuracy reflected by area under the receiver operator characteristics curve. Recently, both the Prevention of Renal and Vascular Endstage Disease (PREVEND) study group in Europe (29) and Jafar et al. (31) in an Indo-Asian population assessed the validity of urinary albumin concentration and the albumin-to-creatinine ratio to detect individuals with microalbuminuria in subsequent 24-h urine samples. Both studies found the diagnostic performance of albumin concentration and albumin-to-creatinine ratio to be similar and acceptable with area under the receiver operator characteristics curve of 0.92 and 0.93, respectively, in the PREVEND study group versus 0.87 and 0.88 in the study by Jafar et al. In the most recent study by Jafar et al. (31), urine albumin concentration (cutoff value 2 mg/dl) had a sensitivity of 69% and a specificity of 94% in men and a sensitivity of 37% and a specificity of 97% in women. For the albumin-to-creatinine ratio (cutoff value 30 mg/g), sensitivity and specificity were 60 and 97%, respectively, in men and 46 and 95%, respectively, in women. Importantly, the relatively low sensitivity of these urinary screening methods may lead to an unacceptably high false-negative test result. For improvement of the sensitivity, a lower cutoff value for albumin concentration of 10 or 15 mg/dl has been proposed for mass screening (32).
The other option to screen for CKD is to measure GFR independent of the albuminuria status. Two common gold standard methods, plasma clearance of inulin and iothalamate infusions, available to measure GFR more accurately, are not appropriate for mass screening because they are expensive and take a few hours to complete. In addition, because reduced GFR is part of the definition of CKD, validity of screening by this method could not be assessed; however, equations that estimate GFR and creatinine clearance from serum creatinine have been tested in several studies and are now recommended (22). The most commonly used equations in adults are the Cockroft-Gault (33) and the Modification of Diet in Renal Disease (MDRD) (34). These equations have limitations, particularly because of the variability of the creatinine assay itself and standardization of the results over time for the same person (35). The National Kidney Disease Education Program has begun a creatinine standardization program to improve and standardize serum creatinine results generated by clinical laboratories for use in estimating equations (36,37). Several studies have assessed the reliability of such tests in different populations and have shown overall that the MDRD equation tended to underestimate true GFR, particularly in healthy individuals, whereas the Cockroft-Gault equation tended to overestimate true GFR, particularly in patients with CKD (38–40). It should also be noted that even the gold standard methods of GFR measurement have intraindividual variation across laboratories and time as reported by Coresh et al. (35). In their study, test-to-test variability was inversely correlated with eGFR, particularly when renal function was >60 ml/min per 1.73 m2; thus, GFR estimation at multiple time points, as recommended for proteinuria, may be warranted in a screening program.
Previous CKD Screening Efforts
In 2000, the National Kidney Foundation kicked off the Kidney Early Evaluation Program (KEEP 2.0), as a result of a pilot study carried out in 1997. KEEP, a screening program, was limited to people who were at high risk for CKD, such as a personal history of diabetes or hypertension or a first-degree relative with diabetes, hypertension, or kidney disease (1,2). Participants provided demographic information as well as height and weight. The KEEP screening process offered, at no cost, several tests, including BP check, plasma glucose level, serum creatinine, hemoglobin, and urine tests for microalbuminuria, pyuria, and hematuria; on-site consultation with a physician, referrals, and further follow-up for those with abnormal test results were also provided, satisfying Wilson and Jungner (4) criteria 2 through 6, 8, and 10 (Table 1). Between August 2000 and December 2001, 31 National Kidney Foundation affiliates conducted 135 screening programs in 33 states, enrolling 6071 participants. These KEEP participants were predominantly black (43%) and female (68%), with a mean age of 52 yr (range 18 to 101). Of the participants, 1778 (29%) tested positive for microalbuminuria and 898 (16%) had an eGFR <60 ml/min per 1.73 m2. Importantly, only 156 (3%) of participants reported a history of CKD at screening (1,2). This first targeted community screening program in the United States was particularly effective in identifying people with CKD, but there was no systematic confirmation of CKD diagnosis or comparison with a control group that received no screening.
To date, few evidence-based effective screening strategies have been confirmed in the United States; however, Hallan et al. (10) recently reported and compared different screening strategies for detecting patients with CKD in the general population of adults ≥20 yr of age in Nord-Trøndelag County, Norway. The authors used a cross-sectional health survey with 8 yr of follow-up (the HUNT II study). Among the 65,604 adults screened, the prevalence of CKD, defined as an eGFR <60 ml/min per 1.73 m2 (using the abbreviated MDRD formula), was 4.7%. Limiting screening to adults with diabetes, hypertension, or age >55 yr identified 93.2% (95% confidence interval 92.4 to 94.0%) of the CKD cases (10). Of note, this strategy remains unconfirmed in the US population, because prevalence of other known risk factors such as obesity and African descent might be different (41,42).
Also recently, Bang et al. (11) developed a prediction model for CKD using the cross-sectional population-based survey Screening for Occult Renal Disease (SCORED) of the National Health and Nutrition Examination Surveys (NHANES) conducted in 1999 to 2000 and 2001 to 2002. The authors analyzed data on 8530 adults and similarly defined CKD as an eGFR <60 ml/min per 1.73 m2 (using the abbreviated MDRD formula). The prevalence of CKD in this population was 5.4% (weighted proportion). In their multivariate model, nine variables were associated with CKD: Age, gender, hypertension, diabetes, peripheral vascular disease, history of cardiovascular disease, congestive heart failure, proteinuria, and anemia. Subsequently, the authors estimated the patient-specific probability of having CKD by developing a numeric scoring system: Assigning 1 point for each variable, except age (2 points for age 50 to 59, 3 points for age 60 to 69, and 4 points for age 70 or older). A score of at least 4 was used as the cutoff point for screening. This model yielded a sensitivity of 92% with an NPV of 99% but specificity and PPV of only 68 and 18%, respectively (11). This low specificity and PPV might lead to unnecessary anxiety and testing of the people screened and workload for the health care system. Validation of the SCORED model in a communitybased screening program is warranted before recommendations can be made.
Cost-Effectiveness of Screening for CKD
Cost-effectiveness analysis is a research method that allows for the comparison of the costs and benefits of different screening programs. In cost-effectiveness analysis, costs are expressed in monetary terms, but benefits are expressed in clinical terms such as lives saved or years of life saved. In cost-utility analysis, a variant of cost-effectiveness analysis, benefits are usually reported in quality-adjusted life years (QALY) saved. This unit of measurement accounts for the quality of those years of life resulting from the use of one screening program versus another or versus no screening. Even though an initial screening test itself might not be expensive, the total cost of such a test must include the cost of further testing (e.g., imaging studies, kidney biopsy) as well as the complications that are rarely associated with these more or less invasive tests. Finally, the balance of cost-effectiveness must include the nonfinancial costs incurred to the patient, such as inconvenience, anxiety, and emotional distress.
Observational study designs (e.g., case-control studies) (43) and experimental designs (e.g., randomized, controlled trials) (44) have been used to evaluate screening programs. Such studies are usually difficult to organize and implement. The Health Insurance Plan (HIP) of New York trial of screening for breast cancer using mammography represents an excellent model of screening program evaluation using a randomized clinical trial (44). Deciding who should be screened is one of the most important questions for nearly all screening programs; it might be cost-effective to screen only high-risk groups or the whole population in a different situation. For example, studies of cost-effectiveness have found that “routine, one-time rapid HIV testing for all adult patients is cost-effective even when the prevalence of undiagnosed HIV infection is as low as 0.2%” (45). Conclusions concerning cost-effectiveness have differed by disease.
The choice of a cost-effectiveness threshold depends on several factors, such as the goal of the program; how the decision maker values health, money, and risk; and, more important, the available resources. Thus, the search for a single cost-effectiveness threshold is not likely to be successful. Most decision makers in the United States will conclude that interventions that cost <$50,000 to $60,000 per QALY saved are reasonably efficient (46). A clear example is screening for hypertension, which costs $27,519 per life-year saved in 40-yr-old men (47,48). For interventions that cost $60,000 to approximately >$175,000 per QALY, some decision makers may find the interventions sufficiently cost-effective; however, only a minority of decision makers will conclude that interventions that cost $175,000 per QALY are cost-effective (46). Finally, cost-effectiveness analysis is a useful tool to help us understand what we get in return for the money that we spend on health care but is often not the sole determinant of whether screening should be performed.
In the case of renal disease, early identification of CKD and appropriate treatment with an ACEI or an ARB can help decrease both progression of CKD to ESRD and cardiovascular-related morbidity and mortality (16,49–55). Furthermore, for patients with diabetes, screening for proteinuria has been shown to be cost-effective (56,57); however, screening for CKD in a nondiabetic population remains debatable, because very few studies have addressed its cost-effectiveness (58–60), and these studies have screened for CKD mainly by assessing proteinuria. In their cost-effectiveness population-based study using a Markov decision analytic model, Boulware et al. (58) showed that annual screening for proteinuria by dipstick, followed by treatment with an ACEI or an ARB for those who tested positive, was cost-effective only in high-risk groups (older people and people with hypertension) or performed at an infrequent interval of 10 yr (Table 1, criterion 9). For people with hypertension, the cost-effectiveness ratio was highly favorable, improving with increasing age: From $26,320 per QALY saved if screening started at age 30 yr to $15,484 per QALY saved if screening started at age 70 yr. Annual screening for proteinuria for the majority of the US population (without hypertension or diabetes) was not cost-effective, except when screening started at age 60 yr ($53,372 per QALY saved) (58).
Two other studies outside the U.S. have addressed the costeffectiveness of screening for CKD. Craig et al. (60) conducted a feasibility study among the general population of Australians ≥50 yr of age using a single urine dipstick test for proteinuria, followed by a 24-h urine test for protein and initiation of ACEI in appropriate individuals. The authors estimated that 20,000 people would need to be screened to prevent one case of ESRD. To achieve this, 1000 people would need to have a 24-h urine test for protein (700 of which would have false-positive results) and 100 people would need to be treated with ACEI for 2 to 3 yr (60). More recently, in the Netherlands, Atthobari et al. (59) reported on the cost-effectiveness of screening for albuminuria with subsequent treatment with fosinopril to prevent cardiovascular events using data from a single-center, double-blind, randomized, placebo-controlled trial within the larger observational study Prevention of Renal and Vascular Endstage Disease (PREVEND). Among 864 people, cardiovascular events occurred in 45 (5.2%), and people who received fosinopril had a 40% lower incidence of cardiovascular events compared with people who received placebo. The cost-effectiveness of screening for albuminuria in the general Dutch population was determined to be 16,700 Euro per life-year gained (59).
Conclusions
In an attempt to decrease the societal burden of kidney disease and reduce the high morbidity and mortality associated with CKD, detection of CKD, particularly at early stages, is essential because therapeutic interventions are likely to be effective if they are implemented early in the course of the disease process. We have shown that the majority of the Wilson and Jungner criteria (4) could be satisfied for CKD screening, and evidence is mounting that a population-based screening program should be implemented in certain high-risk groups (e.g., older patients, patients with hypertension) in addition to patients with diabetes. In addition, a high-risk population may be more motivated to participate in a screening program and be more likely to follow recommendations if their screening results are positive. Further studies of the benefits, risks, and costs of screening for CKD including randomized, controlled trials are still needed in the general US population before final recommendations can be made.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Ohmit SE, Flack JM, Peters RM, Brown WW, Grimm R: Longitudinal Study of the National Kidney Foundation’s (NKF) Kidney Early Evaluation Program (KEEP). J Am Soc Nephrol 14[Suppl]: S117–S121, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Brown WW, Peters RM, Ohmit SE, Keane WF, Collins A, Chen SC, King K, Klag MJ, Molony DA, Flack JM: Early detection of kidney disease in community settings: the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 42: 22–35, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Wald NJ: The definition of screening. J Med Screen 8: 1, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Wilson JM, Jungner G: Principles and Practice of Screening for Disease, Geneva, World Health Organization, 1968. (Public Health Papers, No. 34) [Google Scholar]
- 5.Rose G, Barker D: Epidemiology for the uninitiated: Screening. BMJ 2: 1417–1418, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morabia A, Zhang FF: History of medical screening: From concepts to action. Postgrad Med J 80: 463–469, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Commission on Chronic Illness: Prevention of chronic illness. In: Chronic Illness in the United States, Cambridge, MA, Harvard University Press, 1957, p 45 [Google Scholar]
- 8.Branson BM: To screen or not to screen: Is that really the question? Ann Intern Med 145: 857–859, 2006 [DOI] [PubMed] [Google Scholar]
- 9.McClellan WM, Ramirez SP, Jurkovitz C: Screening for chronic kidney disease: Unresolved issues. J Am Soc Nephrol 14[Suppl]: S81–S87, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Hallan SI, Dahl K, Oien CM, Grootendorst DC, Aasberg A, Holmen J, Dekker FW: Screening strategies for chronic kidney disease in the general population: Follow-up of cross sectional health survey. BMJ 333: 1047, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bang H, Vupputuri S, Shoham DA, Klemmer PJ, Falk RJ, Mazumdar M, Gipson D, Colindres RE, Kshirsagar AV: SCreening for Occult REnal Disease (SCORED): A simple prediction model for chronic kidney disease. Arch Intern Med 167: 374–381, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS: Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 41: 1–12, 2003 [DOI] [PubMed] [Google Scholar]
- 13.US Renal Data System: USRDS 2005 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2005 [Google Scholar]
- 14.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW: Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296– 1305, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Jafar TH, Stark PC, Schmid CH, Landa M, Maschio G, de Jong PE, de Zeeuw D, Shahinfar S, Toto R, Levey AS, for the AIPRD Study Group: Progression of chronic kidney disease: The role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition—A patient-level meta-analysis. Ann Intern Med 139: 244–252, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Trivedi HS, Pang MM, Campbell A, Saab P: Slowing the progression of chronic renal failure: Economic benefits and patients’ perspectives. Am J Kidney Dis 39: 721–729, 2002 [DOI] [PubMed] [Google Scholar]
- 18.St Peter WL, Khan S, Ebben JP, Pereira BJ, Collins AJ: Chronic kidney disease: The distribution of health care dollars. Kidney Int 66: 313–321, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Coresh J, Byrd-Holt D, Astor BC, Briggs JP, Eggers PW, Lacher DA, Hostetter TH: Chronic kidney disease awareness, prevalence, and trends among US adults, 1999 to 2000. J Am Soc Nephrol 16: 180–188, 2005 [DOI] [PubMed] [Google Scholar]
- 20.McClellan WM, Knight DF, Karp H, Brown WW: Early detection and treatment of renal disease in hospitalized diabetic and hypertensive patients: Important differences between practice and published guidelines. Am J Kidney Dis 29: 368–375, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Kausz AT, Khan SS, Abichandani RE, Kazmi WH, Obrador GT, Ruthazer RO, Pereira BJ: Management of patients with chronic renal insufficiency in the northeastern united states. J Am Soc Nephrol 12: 1501–1507, 2001 [DOI] [PubMed] [Google Scholar]
- 22.National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis S1–S266, 2002 [PubMed] [Google Scholar]
- 23.Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH, Steffes MW; American Diabetes Association: Nephropathy in diabetes. Diabetes Care 27[Suppl 1]: S79–S83, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Chobanian AV, Bakris G, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ, National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, National High Blood Pressure Education Program Coordinating Committee: The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA 289: 2560–2572, 2003. [published erratum appears in JAMA 290: 197, 2003] [DOI] [PubMed] [Google Scholar]
- 25.Couchoud C, Pozet N, Labeeuw M, Pouteil-Noble C: Screening early renal failure: Cut-off values for serum creatinine as an indicator of renal impairment. Kidney Int 55: 1878–1884, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Schlesselman JJ: Case-Control Studies: Design, Conduct, and Analysis, New York, Oxford University Press, 1982 [Google Scholar]
- 27.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, de Zeeuw D, Hostetter TH, Lameire N, Eknoyan G: Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 67: 2089–2100, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Shlipak MG, Katz R, Sarnak MJ, Fried LF, Newman AB, Stehman-Breen C, Seliger SL, Kestenbaum B, Psaty B, Tracy RP, Siscovick DS: Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med 145: 237–246, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Gansevoort RT, Verhave JC, Hillege HL, Burgerhof JG, Bakker SJ, de Zeeuw D, de Jong PE: The validity of screening based on spot morning urine samples to detect subjects with microalbuminuria in the general population. Kidney Int Suppl 94: S28–S35, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Houlihan CA, Tsalamandris C, Akdeniz A, Jerums G: Albumin to creatinine ratio: A screening test with limitations. Am J Kidney Dis 39: 1183–11899, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Jafar TH, Chaturvedi N, Hatcher J, Levey AS: Use of albumin creatinine ratio and urine albumin concentration as a screening test for albuminuria in an Indo-Asian population. Nephrol Dial Transplant 22: 2194–2200, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Gansevoort RT, Lambers H, Witte EC: Methodology of screening for albuminuria. Nephrol Dial Transplant 22: 2109–2111, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Cockcroft DW, Gault MH: Prediction of creatinine clearance from serum creatinine. Nephron 16: 31–41, 1976 [DOI] [PubMed] [Google Scholar]
- 34.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Coresh J, Astor BC, McQuillan G, Kusek J, Greene T, Van Lente F, Levey AS: Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis 39: 920–929, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Myers GL, Miller WG, Coresh J, Fleming J, Greenberg N, Greene T, Hostetter T, Levey AS, Panteghini M, Welch M, Eckfeldt JH, for the National Kidney Disease Education Program Laboratory Working Group: Recommendations for improving serum creatinine measurement: A report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem 52: 5–18, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F, for Chronic Kidney Disease Epidemiology Collaboration: Expressing the Modification of Diet in Renal Disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 53: 766–772, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Poggio ED, Wang X, Greene T, Van Lente F, Hall PM: Performance of the Modification of Diet in Renal Disease and Cockcroft-Gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol 16: 459–466, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Froissart M, Rossert J, Jacquot C, Paillard M, Houillier P: Predictive performance of the Modification of Diet in Renal Disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol 16: 763–773, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG: Using serum creatinine to estimate glomerular filtration rate: Accuracy in good health and in chronic kidney disease. Ann Intern Med 141: 929–937, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS: Body mass index and risk for end-stage renal disease. Ann Intern Med 144: 21–28, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J: End-stage renal disease in African-American and white men: 16-Year MRFIT findings. JAMA 277: 1293–1298, 1997 [PubMed] [Google Scholar]
- 43.Hosek RS, Flanders WD, Sasco AJ: Bias in case-control studies of screening effectiveness. Am J Epidemiol 143: 193– 201, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Shapiro S, Venet W, Strax P, Venet L, Roeser R: Selection, follow-up, and analysis in the Health Insurance Plan Study: A randomized trial with breast cancer screening. Natl Cancer Inst Monogr 67: 65–74, 1985 [PubMed] [Google Scholar]
- 45.Paltiel AD, Walensky RP, Schackman BR, Seage GR III, Mercincavage LM, Weinstein MC, Freedberg KA: Expanded HIV screening in the United States: Effect on clinical outcomes, HIV transmission, and costs. Ann Intern Med 145: 797–806, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Garber AM, Phelps CE: Economic foundations of cost-effectiveness analysis. J Health Econ 16: 1–31, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Littenberg B, Garber AM, Sox HC: Screening for hypertension. Ann Intern Med 112: 192–202, 1990 [DOI] [PubMed] [Google Scholar]
- 48.Littenberg B: A practice guideline revisited: Screening for hypertension. Ann Intern Med 122: 937–939, 1995 [DOI] [PubMed] [Google Scholar]
- 49.Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S, the HOPE Investigators : Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: The HOPE randomized trial. Ann Intern Med 134: 629–636, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Lindholm LH, Ibsen H, Dahlof B, Devereux RB, Beevers G, de Faire U, Fyhrquist F, Julius S, Kjeldsen SE, Kristiansson K, Lederballe-Pedersen O, Nieminen MS, Omvik P, Oparil S, Wedel H, Aurup P, Edelman J, Snapinn S: Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): A randomised trial against atenolol. Lancet 359: 1004–1010, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Agodoa LY, Appel L, Bakris GL, Beck G, Bourgoignie J, Briggs JP, Charleston J, Cheek D, Cleveland W, Douglas JG, Douglas M, Dowie D, Faulkner M, Gabriel A, Gassman J, Greene T, Hall Y, Hebert L, Hiremath L, Jamerson K, Johnson CJ, Kopple J, Kusek J, Lash J, Lea J, Lewis JB, Lipkowitz M, Massry S, Middleton J, Miller ER III, Norris K, O’Connor D, Ojo A, Phillips RA, Pogue V, Rahman M, Randall OS, Rostand S, Schulman G, Smith W, Thornley-Brown D, Tisher CC, Toto RD, Wright JT Jr, Xu S, the African American Study of Kidney Disease and Hypertension Study Group: Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: A randomized controlled trial. JAMA 285: 2719–2728, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I, the Collaborative Study Group: Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851– 860, 2001 [DOI] [PubMed] [Google Scholar]
- 53.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD, the Collaborative Study Group: The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med 329: 1456–1462, 1993 [DOI] [PubMed] [Google Scholar]
- 54.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S, the RENAAL Study Investigators: Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Palmer AJ, Annemans L, Roze S, Lamotte M, Lapuerta P, Chen R, Gabriel S, Carita P, Rodby RA, de Zeeuw D, Parving HH: Cost-effectiveness of early irbesartan treatment versus control (standard antihypertensive medications excluding ACE inhibitors, other angiotensin-2 receptor antagonists, and dihydropyridine calcium channel blockers) or late irbesartan treatment in patients with type 2 diabetes, hypertension, and renal disease. Diabetes Care 27: 1897–1903, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Golan L, Birkmeyer JD, Welch HG: The cost-effectiveness of treating all patients with type 2 diabetes with angiotensin-converting enzyme inhibitors. Ann Intern Med 131: 660–667, 1999 [DOI] [PubMed] [Google Scholar]
- 57.Kiberd BA, Jindal KK: Screening to prevent renal failure in insulin dependent diabetic patients: An economic evaluation. BMJ 311: 1595–1599, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boulware LE, Jaar BG, Tarver-Carr ME, Brancati FL, Powe NR: Screening for proteinuria in US adults: A cost-effectiveness analysis. JAMA 290: 3101–3114, 2003 [DOI] [PubMed] [Google Scholar]
- 59.Atthobari J, Asselbergs FW, Boersma C, de Vries R, Hillege HL, van Gilst WH, Gansevoort RT, de Jong PE, de Jong-van den Berg L, Postma MJ: Cost-effectiveness of screening for albuminuria with subsequent fosinopril treatment to prevent cardiovascular events: A pharmacoeconomic analysis linked to the Prevention of Renal and Vascular Endstage Disease (PREVEND) study and the Prevention of Renal and Vascular Endstage Disease Intervention Trial (PREVEND IT). Clin Ther 28: 432–444, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Craig JC, Barratt A, Cumming R, Irwig L, Salkeld G: Feasibility study of the early detection and treatment of renal disease by mass screening. Intern Med J 32: 6–14, 2002 [PubMed] [Google Scholar]


