Abstract
This review discusses various screening approaches for chronic kidney disease that are used in Europe. The criterion for defining chronic kidney disease in the various programs differs but is frequently limited to estimated glomerular filtration rate, thus offering only data on chronic kidney disease stages 3 and higher; however, screening should not be limited to measuring only estimated glomerular filtration rate but should also include a measure of microalbuminuria, because this will offer identification of chronic kidney disease stages 1 and 2. Defining these earlier stages is of importance because the risk for developing end-stage renal disease that is associated with stages 1 and 2 is nearly equal to the risk that is associated with stage 3. Moreover, the risk for cardiovascular events in stages 1 and 2 is equal to that in stage 3. Various reports argue that costs of screening programs in general practitioner or outpatient offices are high and that they are cost-effective only for preventing end-stage renal disease when they are limited to target groups, such as patients with diabetes or hypertension and elderly. The benefits of screening programs, however, should not be evaluated only with respect to the prevention of renal events but should also include the benefits of preventing cardiovascular events. The use of preselection based on either an impaired estimated glomerular filtration rate or on protein-dipstick positivity or elevated albuminuria in a morning urine void has been found effective in various European countries as an alternative for targeted screening.
The number of patients who require renal replacement therapy is increasing all around the globe (1). The rising need for dialysis and renal transplantation can only be dealt with at the expense of enormous costs. It is clear that resources to support this expansive growth are limited, both in developing and developed countries. Moreover, it has become evident that chronic kidney disease (CKD) is associated with an increased risk for cardiovascular disease (CVD) events. As a consequence, in the past decade, attention has moved from treating only advanced stages of CKD toward prevention in the earlier stages of CKD.
Because only a minority of patients who are at risk for developing ESRD are under medical attention for their renal disease, most patients’ disease is undiagnosed as yet. As a consequence, most individuals who are at risk for ESRD and the accompanying risk for CVD can receive a diagnosis only by active screening. After the call to action of the International Society of Nephrology (2), various initiatives were undertaken to set up screening programs, followed by preventive measures in case of a diagnosis of CKD. Remuzzi and Weening (2) drew attention to screening for the presence of albuminuria. They once more emphasized what was known already: One could by just looking at a patient’s urine be informed on the general well-being of that patient (Figure 1).
Figure 1.

The uroscopist. Painting by David Teniers (1610 to 1690) in the Royal Museum for Art, Brussels, Belgium. Already long ago, screening of the urine could help the village physician to monitor his patient. Although medical history has resulted in many new discoveries since then, in 2007 we still can learn much from screening of the urine.
This review focuses on screening and prevention activities for CKD in Europe. We first describe some of the running screening programs and pay attention to the various approaches to set up such activities. On the basis of these European programs, we try to reach conclusions on how best to proceed with screening and prevention campaigns.
CKD Screening Programs in Europe
Table 1 summarizes various screening programs that are running in Europe. The list is not intended to be a complete review of all studies, but it presents an overview of various strategies used. The criterion for the diagnosis of CKD in these programs is different, however, and also seems to change from originally a serum creatinine value above a certain cutoff (mostly >150 μmol/L) (4,6,12,18,19) via an estimated GFR (eGFR) <60 ml/ min (3,8,13,15) to a positive protein dipstick (7,11,16) or even the presence of microalbuminuria (9,10,14,17). The approach in which only data on eGFR are gathered allows detection of Kidney Disease Outcomes Quality Initiative (K/DOQI) stages 3, 4, and 5. Measurement of urinary albumin loss in addition to eGFR is needed to get information on stages 1 and 2. This more complete information has been gathered only in the Prevention of Renal and Vascular Endstage Disease (PREVEND) (10), North Trøndelag Health Study (HUNT) (14), and Estudio Epidemioloógico de la Insuficiencia Renal en España (EPIRCE) (17) studies.
Table 1.
Screening and prevention studies in Europea
| Country | Year | Name | Criterion | CKDb | n | Reference |
|---|---|---|---|---|---|---|
| Belgium | 1980 | BIRNH | eGFR <60 | No | 8913 | (3) |
| France | 1991 | Ile de France | SCr >200 | Yes | 2775 | (4) |
| Germany | 1984 | MONICA | eGFR<60 | No | 7534 | (5) |
| Iceland | 1967 | RHS | SCr >150 | No | 18,912 | (6) |
| Iceland | 1967 | RHS | eGFR/dipstick | No | 19,256 | (7) |
| Italy | 1983 | Gubbio | eGFR <60 | No | 4574 | (8) |
| Netherlands | 1997 | PREVEND-pre | UAC >10 | Yes | 40,856 | (9) |
| Netherlands | 1997 | PREVEND | UAE/eGFR | Yes | 3432 | (10) |
| Netherlands | 2006 | Niercheck | Dipstick positivity | Yes | 1.1 million | (11) |
| Northern Ireland | 2001 | Belfast | Scr >150 | Yes | 19,286 | (12) |
| Norway | 1995 | HUNT | eGFR <60 | No | 65,604 | (13) |
| Norway | 1995 | HUNT | eGFR/ACR | No | 65,181 | (14) |
| Norway | 1994 | Tromsø | eGFR <60 | No | 38,241 | (15) |
| Poland | 2005 | POLNEF | Dipstick positivity | Yes | 1732 | (16) |
| Spain | 2004 | EPIRCE | eGFR/ACR | Yes | 237 | (17) |
| United Kingdom | 1993 | Southampton | SCr >150 | Yes | 405,000 | (18) |
| United Kingdom | 2000 | Kent | SCr >180 | Yes | 688,193 | (19) |
ACR, albumin-creatinine ratio; BIRNH, Belgian Interuniversity Research on Nutrition and Health; CKD, chronic kidney disease; eGFR, estimated GFR as calculated from the Modification of Diet in Renal Disease (MDRD) formula and expressed in ml/min per 1.73 m2; EPIRCE, Estudio Epidemiologico de la Insuficiencia Renal en España; HUNT, North Trøndelag Health Study; MONICA, Monitoring Trends and Determinants in Cardiovascular Diseases in Augsburg; PREVEND, Prevention of Renal and Vascular End-stage Disease study; PREVEND-pre, Prevention of Renal and Vascular End-stage Disease prescreening; POLNEF, Polish Epidemiological Pilot Study in Nephrology; RHS, Reykjavik Heart Study; SCr, serum creatinine expressed in μmol/L; UAC, urinary albumin concentration; UAE, 24-h urinary albumin excretion.
Primary focus of the study was to detect CKD.
Part of the studies make use of existing screening programs, most of them initially intended to screen and treat cardiovascular risk factors (3,5– 8,13,15). In the past 10 yr, however, more and more programs were started with the primary focus to detect individuals who have or are at risk for CKD. These specific CKD screening programs used different approaches to select the screening population.
Prevalence and Incidence of CKD
The PREVEND (10), EPIRCE (17), and HUNT (14) studies offer information on the prevalence of the various stages of CKD in three different areas in Europe, although the EPIRCE study may present a less reliable reflection of the overall population because of its small sample size (Table 2). The data show that prevalence of the CKD stages is comparable in these studies, ranging from 5.1 to 7.0% for stages 1 and 2 combined, from 4.5 to 5.3% for stage 3, and much lower for stage 4, from 0.1 to 0.4%. These prevalences are nearly similar to the data obtained in the Third National Health and Nutrition Examination Survey (NHANES III) in the United States, which showed that 6.3% of the general population has stage 1 or 2 CKD, 4.3% has stage 3, 0.2% has stage 4, and 0.2% has stage 5 (21,22). This similarity in prevalence data for stages 1 through 3 is the more remarkable because inclusion criteria for age were different in the three studies, as were the serum creatinine assays and the approaches of measuring urinary albumin excretion. Standardization of serum creatinine assays has been given a lot of attention (23). Less uniformity has been obtained on the definition of abnormal albuminuria. The impact of using standardized measures is demonstrated by the difference in the prevalence of CKD stages 1 and 2 in the Reykjavik Heart Study (RHS) (7), which used a dipstick test, and in the PREVEND (10) and HUNT (14) studies, which used more accurate measurements for microalbuminuria. The first found a prevalence of only 1.6%, whereas the latter reported a prevalence of 5.1 to 6.5%.
Table 2.
Prevalence of the various stages of CKD in Europe
| Stage | (ml/min per 1.73 mGFR2) | Micro/Macroalbuminuria | Country/Study | |||
|---|---|---|---|---|---|---|
| Netherlands/PREVEND (n = 3432) | Spain/EPIRCE (n = 237) | Norway/HUNT (n = 65,181) | Iceland/RHS (n = 19,381) | |||
| 1 | >90 | Yes | 1.3 | 3.5 | 3.1 | 1.6a |
| 2 | 0 to 89 | Yes | 3.8 | 3.5 | 3.4 | |
| 3 | 30 to 59 | Yes/no | 5.3 | 5.3 | 4.5 | 7.4 |
| 4 | 15 to 29 | Yes/no | 0.1 | 0.4 | 0.2 | 0.2 |
| 5 | <15 | Yes/no | 0.1 | ? | ? | ? |
| Total | 10.6 | 12.5 | 11.2 | >9.2 | ||
The RHS study has data on dipstick positivity and does not discriminate between stages 1 and 2, whereas the other three studies use an index of microalbuminuria for both stages 1 and 2 separately.
Whereas the prevalence of the various stages of CKD in these studies is nearly comparable, the incidence of treated ESRD (CKD stage 5, requiring dialysis or transplantation) varies widely within the European countries (24), as well as between European countries and the United States (14) (Table 3). Many factors may explain these differences. In Eastern Europe, the lack of dialysis units and limited financial resources do not permit starting of renal replacement therapies for all patients with ESRD; however, in countries such as Norway and the Netherlands, no such barriers exist. Furthermore, there are no major differences between these latter countries and the United States in timing of the start of dialysis or in mortality risk, which competes with the risk for start of renal replacement therapy. Hallan et al. (14) questioned whether the difference in number of treated incident patients with ESRD between Norway and the United States is due to a difference in the prevalence of the earlier stages of CKD in those nations. They compared with that purpose the prevalence of patients with stages 3 and 4 CKD as published in the NHANES data (21) and the HUNT study with the incidence of treated ESRD in the United States and Norway. The ratio of incident white patients with ESRD per prevalence of stages 3 and 4 is approximately two- to three-fold higher in the United States than in Norway. Data from the Netherlands show a pattern comparable to that in Norway (Table 3). Given these data, Hallan et al. (14) suggested that the increased incidence of treated ESRD per CKD prevalence in the United States may result from a more rapid progression of early CKD to ESRD, rather than from a larger pool of individuals at risk. An even bigger difference in the ratio of incidence per prevalence was found in US black individuals and patients with diabetes. Finally, other factors may account for such differences, such as patient’s personal choice, physician’s decision, and other comorbidities that may influence acceptability for dialysis.
Table 3.
Prevalence and incidence of stages 3 and 4 CKD in the white participants of a US cohort (NHANES) (21), a Norwegian cohort (HUNT) (14), and a Dutch Cohort (PREVEND) (10)
| Parameter | Country/Study | ||
|---|---|---|---|
| Norway/HUNTa | Netherlands/PREVEND | United States/NHANESa | |
| Prevalence stages 3 and 4 | 4360/100,000 | 5300/100,000 | 5010/100,000 |
| Incidence ESRDb | 10.6/100,000 | 10.0/100,000 | 30.8/100,000 |
| Incidence as % of prevalence | 0.24 | 0.19 | 0.61 |
The data of the HUNT and NHANES studies were derived from reference (14). The data from that study were stratified by age, gender, and diabetes status. The mean age of the HUNT patients was 50.2 yr and of the PREVEND patients was 48.7 yr.
Data on incidence of ESRD have been derived from the respective national renal replacement registries.
These comparisons are based on the relation between two databases (CKD prevalence estimates versus ESRD registries), which does not teach us how frequently patients in the various CKD stages progress to ESRD. We therefore evaluated the number of new dialysis patients in the PREVEND study according to their original CKD stage, looking at follow-up of individual patients and taking into account all five stages of CKD (unpublished data). In a follow-up period of 7 yr, 13 patients reached ESRD. Although that number is relatively small, allowing no firm conclusions, it offers interesting data when expressing the percentage of patients of the various CKD stages who reach ESRD. Patients with stages 1 and 2 CKD reach ESRD in approximately similar frequency as patients with stage 3, with the risk for ESRD in these earlier stages being approximately 25- to 45-fold increased compared with patients without CKD (Figure 2). The risk for reaching ESRD increases strikingly in stages 4 and 5 CKD. Patients with stages 4 and 5 CKD have approximately a 100- to 1000-fold higher risk for developing ESRD compared with patients with stages 1 through 3 CKD. Furthermore, patients with CKD not only are at significant risk for ESRD but also are at much higher risk for CVD events compared with people without CKD. Although only 13 patients in the overall cohort reached ESRD, 568 patients experienced a CVD event in that follow-up period. Figure 2 shows that the risk for developing a CVD end point in stages 1 through 3 CKD is much higher than the risk for developing ESRD, but the risk increases much more steeply for renal than for CVD end points.
Figure 2.

Incidence of ESRD (Œ) and cardiovascular disease (CVD) events (f) according to baseline chronic kidney disease (CKD) stage. Data derived from the Prevention of Renal and Vascular Endstage Disease (PREVEND) study. Notice the log scale on the vertical axis.
Which Screening Approach Should Be Used?
As listed in Table 1, the European screening programs had different designs. These different designs are summarized in Figure 3. Traditionally, it has been advocated to screen specific target groups for the presence of CKD, such as patients with diabetes and hypertension and elderly. Such a targeted screening approach (see Figure 3A) will result in a higher percentage of patients with CKD and will likely be more cost-effective than screening an entire population (13,25). The benefits of targeted screening of patients with diabetes and hypertension versus screening of the general population, however, are debatable. It is dependent, among others, on the percentage of incident ESRD cases that are due to diabetes and/or hypertension in a specific country. This percentage differs from more than 70% in the US Medicare population but is limited to 40% in various European countries (26). In the United States, targeted screening for patients with diabetes and/or hypertension may be favored (27), but in Europe, targeted screening on the basis of these risk factors may be of limited benefit to decrease the overall incidence of ESRD. Furthermore, it is known from several epidemiologic studies that for every patient with known hypertension or diabetes, there is one individual in the population for whom this diagnosis is not yet made but who already can have considerable associated end-organ damage (28–30). Last, preventive measures to postpone ESRD consist of rigid BP control, preferably with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. Patients with known diabetes or hypertension frequently are already on these regimens. Consequently, the number of individuals who are identified by targeted screening and for whom the screening results in a change of medical treatment may therefore be limited, although there clearly is room for better implementation of guidelines. One finally should realize that if all patients with known diabetes or hypertension and all those aged >50 yr are to be invited to visit their general practitioner for screening for CKD, then this will affect up to 50% or more of the overall adult population (Figure 3A).
Figure 3.
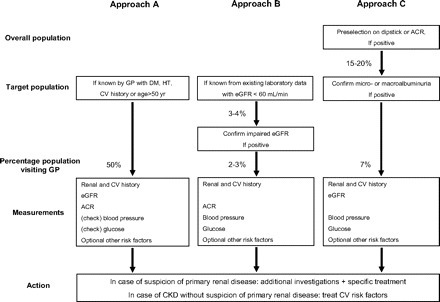
Various screening approaches for CKD applied in Europe. ACR, albumin-creatinine ratio; CV, cardiovascular; DM, diabetes; eGFR, estimated GFR; GP, general practitioner; HT, hypertension.
Another approach, applied in various projects in the United Kingdom (12,18,19), uses an existing laboratory database (Figure 3B). By selecting all patients with elevated serum creatinine or, preferably, impaired eGFR (<60 ml/min per 1.73 m2), the screening may be limited to <5% of the population. There are some disadvantages to this approach. First, one is not aware of the indication of measuring eGFR. It may just be a temporarily elevated serum creatinine as a result of an intercurrent medical problem, thus not reflecting true CKD. It is therefore wise to have a confirmation of the impaired eGFR before extensive screening is performed. Second, by screening just for eGFR, one limits further studies to relatively old people and predominantly women, because eGFR declines with age and is lower in women than in men. In that situation, one will miss individuals in the younger age categories with eGFR values that may be in the lowest range for that age and gender group but may still be >60 ml/min per 1.73 m2. Third, asymptomatic individuals probably will not be in the database and will thus not be detected. Fourth, stages 1 and 2 CKD with (relatively) normal GFR but with signs of renal damage are approximately equally as frequent as stage 3 CKD and have comparable impact on renal and cardiovascular prognosis as stage 3. Finally, preventive intervention will especially be effective when started in an early stage of the disease. When limiting screening to identify only (frequently elderly) individuals who have already (severely) impaired renal function, the benefits of early intervention for individuals with stages 1 and 2 CKD will be overlooked. In our opinion, it should be concluded that a screening program to detect CKD should also include an effort to identify individuals with early CKD by also measuring albuminuria.
A third approach is used in two different ways in the Netherlands: By preselection on an abnormal dipstick or albuminuria test (Figure 3C). In the PREVEND study, preselection was achieved on the basis of measurement of an abnormal urinary albumin concentration from a first-morning urine void (20). All inhabitants of the city of Groningen aged 28 to 75 yr were invited to send in by mail a questionnaire together with a sample of a first-morning urine void for measurement of urinary albumin concentration in a central laboratory facility. Of the 85,421 individuals, 40,856 participated (9). In the second phase of the PREVEND study, all individuals with a urinary albumin concentration >10 mg/L were invited for more accurate measurements of cardiovascular and renal risk factors and are followed in time with respect to renal and vascular survival. If one limits further screening to individuals with a urinary albumin concentration >20 mg/L, then more extensive screening could be limited to 7% of the adult population.
Preselection on an abnormal protein dipstick test was used by the Dutch Kidney Foundation (11). All adults in the Netherlands were offered the opportunity to order via internet a so-called “Kidney Check,” consisting of a set of three urinary dipstick protein tests, together with instructions on how to use these test strips. Individual dipsticks could score negative, 1+, 2+, or 3+, being >300, >1000, or >5000 mg/L, respectively. Individuals who scored ≥1+ on at least two urine samples were advised to visit their general practitioner. The general practitioners had been sent a guideline, which was developed by the Dutch Kidney Foundation together with the Dutch Society of General Practitioners, in which they were advised to confirm dipstick positivity by quantitative measurement of albuminuria in a first-morning void urine sample. In case of macroalbuminuria, they were advised to evaluate renal history and to measure BP, renal function, and plasma glucose. Altogether, 1.1 million individuals (8.7% of the target population) requested for this “Kidney Check” (11). The evaluation of the campaign showed that 19% scored weak positive (at least two of three dipsticks being 1+) and 1% scored strong positive (at least one of the dipsticks being ≥2+). In 26% of the individual with strong positive results, the general practitioner detected a new diagnosis of proteinuria, impaired renal function, diabetes, and/or hypertension. Such new diagnoses were established in 10% of the individuals with weak positive results and in only 3% of individuals who had negative results (31). Important lessons can be drawn from this audacious campaign of the Dutch Kidney Foundation. First, it seems that a large percentage of the population is interested in becoming aware of their proteinuria status. Second, an unexpectedly high percentage scored weak positive. Additional investigations proved that this most likely was because the dipstick test frequently was not read in the most optimal situation, which is in daylight. When they are read under artificial light, false-positive readings may occur frequently. Third, preselection by the “Kidney Check” results in a decrease in workload for general practitioners in a CKD screening program. To identify one individual with renal disease, diabetes, or hypertension, the number needed to screen was reduced from 30 individuals with negative results to 10 individuals with weak positive results and only four individuals with strong positive results.
Of note, the “Kidney Check” campaign was based on the strategy to identify individuals with macroalbuminuria (>200 mg/L). Individuals who are proved to have microalbuminuria at confirmation (20 to 200 mg/L) are called false positive in such a strategy. Whether this is a correct term is debatable, because it is known that microalbuminuria also entails a worse cardiovascular (32) and renal prognosis (33) when compared with normoalbuminuric individuals. The clinical utility of a dipstick proteinuria test to detect microalbuminuria has been studied by Japanese investigators. They showed that many patients with dipstick positivity seem to have microalbuminuria (34). Of the individuals who were trace, 1+, or 2+ positivity, 61, 71, and 41%, respectively, had microalbuminuria, whereas only 1, 7, and 50% had macroalbuminuria. Only the patients with 3+ positivity were mostly (91%) macroalbuminuric (Figure 4). These data show that a dipstick test might well be used as a first selection to detect individuals with (early) CKD. The actual, more extensive screening could then be limited to the individuals who have positive results on that first selection step (Figure 3C). Depending on the strictness of this preselection step, the actual screening can be limited to 20 or maybe even <10% of the population.
Figure 4.
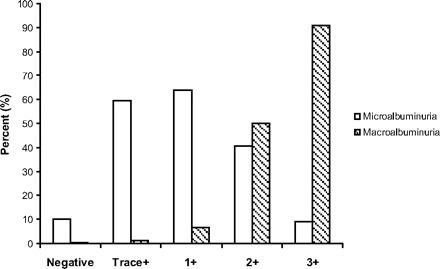
Percentage of individuals who score negative or trace, 1, 2, or 3 positive on a dipstick protein test that with closer examination seemed to have microalbuminuria or macroalbuminuria. Microalbuminuria defined as ACR 30 and 300 mg/g and macroalbuminuria as ACR 300 mg/g (33).
Costs of Screening Programs
A Markov model analysis for the costs of screening for dipstick proteinuria was performed in the United States. In that analysis, screening was to take place at the general practitioner’s office, and only costs to prevent ESRD were incorporated. Boulware et al. (25) calculated that this approach was costeffective only when screening was limited to older individuals and those with known hypertension or when it was conducted at 10-yr intervals only. These data are frequently used to oppose the benefits of screening of the overall population; however, in that analysis, the benefits of screening were assumed to be limited to the prevention of ESRD, whereas it is widely known that most of the patients with proteinuria and patients with eGFR <60 ml/min will more frequently sustain a cardiovascular event and will generally even die before they reach ESRD. Thus, an appropriate cost– benefit analysis should also take into account potential benefits with respect to preventing cardiovascular events. Atthobari et al. (35) showed that screening of an adult population for an elevated urinary albumin excretion (in that study, albuminuria >15 mg/d) and subsequent treatment of individuals with positive screening results with an angiotensin-converting enzyme inhibitor was cost-effective when calculated to prevent cardiovascular end points: 16,500 Euro per life-year gained. This figure even improved to 12,000 Euro per life-year gained when the treatment would be limited to an albuminuria level of >30 mg/d. Besides the difference in whether CVD benefits are incorporated, another difference between the approaches by Boulware et al. and Atthobari et al. is that the first studied a strategy in which screening was to take place at the general practitioners office, whereas the latter used the approach discussed in which a prescreening took place on spot morning urine samples hat were delivered by mail for measurement of urinary albumin concentration. The actual screening could then further be limited to individuals whose screening results are positive on that first urine sample (20). One could thus consider this as a variant of targeted screening, targeted not on a risk factor for CKD but on an early phase of CKD; however, it is not known whether data from this Dutch study can be extrapolated to other countries. More data are needed to substantiate which approach is most cost-effective.
This is all the more interesting because the cost-effectiveness of screening and treatment programs depends on the phase of the disease process in which an individual is identified. In a study of individuals with diabetic nephropathy, in which the benefits of early intervention were compared with late intervention, it was shown that early intervention with an angiotensin receptor blocker prevented more ESRD cases than late intervention (36). Palmer et al. (36) calculated that the earlier the individual at risk is detected and preventive treatment is started, the more cost-effective such treatment will be. The start of an angiotensin receptor blocker in individuals with incipient diabetic nephropathy was found to result in a greater cost saving than the start of this drug in case of overt diabetic nephropathy. This is further argument that screening for albuminuria, which also identifies individuals with stages 1 and 2 CKD, may be preferable over screening for only impaired eGFR.
Who Should Manage Screening and Subsequent Treatment Programs?
In Europe, approximately 10% of the general population has CKD; therefore, when we favor that it should be detected and treated to prevent progressive renal function decline, we should discuss who is able to manage that care. Traditionally, individuals with stages 4 and 5 CKD were referred to a nephrologist. Some argue that the 5% of the population with stage 3 CKD should also be seen by a nephrologist. Taking the evidence that individuals with stages 1 and 2 may be at similar risk as individuals with stage 3 for renal and cardiovascular prognosis, that conclusion could be extrapolated to individuals with stages 1 and 2 CKD. Arguments for that proposal are that generalists are not sufficiently well trained to recognize the problems of CKD (37). Moreover, if a patient is being detected by the general practitioner to have CKD, then this is not a guarantee that he or she will receive appropriate care (38). Conversely, referral of all patients with CKD is unrealistic. The best results probably can be achieved by a collaborative effort of primary and secondary care.
Lack of awareness of clinical practice guidelines and lack of clinical and administrative resources of primary care physicians have been identified as important barriers to care. Improved dissemination of existing guidelines and targeted education in conjunction with efforts to build consensus among primary care physicians and nephrologists regarding their roles in the care of patients with CKD, including the collaborative development of clinical practice guidelines, could enhance patient care (39). The recent initiative of the UK Renal National Service Framework (40) and the inclusion of CKD in the financially incentivized quality-based contract for general practices in England (41) should be considered as a great step forward in optimizing the recognition of CKD in the National Health Service in the United Kingdom. It was calculated that the costs of implementing guidelines for CKD in the United Kingdom could already be recouped by delaying dialysis requirement by 1 yr for one individual per 10,000 patients treated according to guidelines (42). As mentioned before, these figures will be even more positive when taking into account the benefits of CVD prevention.
Conclusions
Various screening programs are running in Europe. The criterion for defining CKD in the various programs differs but is frequently limited to eGFR, thus offering only data on CKD stages 3 through 5. We propose that screening should not be limited to measuring eGFR but should also include a measure of albuminuria, because this will offer the possibility also to identify individuals with stages 1 and 2 CKD. Identifying individuals with these earlier stages is of importance because evidence emerges that the renal and cardiovascular risks that are associated with stages 1 and 2 are nearly equal to those of stage 3.
The benefits of screening programs should not be evaluated only with respect to the prevention of renal events but also should include the benefits of preventing cardiovascular events. Including that latter aspect will also prove these programs to be more cost-effective. An alternative for targeted screening may be that individuals perform a dipstick test for proteinuria at home or send a urine sample by mail to a central laboratory. Individual who score positive on a dipstick test or have elevated levels of albuminuria are advised to visit their general practitioner for detailed renal and cardiovascular risk profiling and treatment.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Bello AK, Nwankwo E, El Nahas AM: Chronic kidney disease: A global challenge. Kidney Int 68[Suppl 98]: S11–S17, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Remuzzi G, Weening JJ: Albuminuria as early test for vascular disease. Lancet 365: 556–557, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Van Biessen W, de Bacquer D, Verbeke F, Delanghe E, Lameire N, Vanholder R: The glomerular filtration rate in an apparently healthy population and its relation with cardiovascular mortality during 10 years. Eur Heart J 28: 478–483, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Jungers P, Chauveau P, Descamps-Latscha B, Labrunie M, Giraud E, Man NK, Grünfeld JP, Jacobs C: Age and genderrelated incidence of chronic renal failure in a French urban area: A prospective epidemiologic study. Nephrol Dial Transplant 11: 1542–1546, 1996 [PubMed] [Google Scholar]
- 5.Meisinger C, Döring A, Löwel H, for the KORA study Group: Chronic kidney disease and risk of incident myocardial infarction and all-cause and cardiovascular disease mortality in middle-aged men and women from the general population. Eur Heart J 27: 1245–1250, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Magnason RL, Indridason OS, Sigvaldason H, Sigfusson N, Parlsson R: Prevalence and progression of CRF in Iceland: A population-based study. Am J Kidney Dis 40: 955–963, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Viktorsdottir O, Palsson R: Andresdottir MB, Aspelund T, Gudnason V, Indridason OS: Prevalence of chronic kidney disease based on estimated glomerular filtration rate and proteinuria in Icelandic adults. Nephrol Dial Transplant 20: 1799–1807, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Cirillo M, Laurenzi M, Mancini M, Zanchetti A, Lombardi C, de Santo NG: Low glomerular filtration rate in the population: Prevalence, associated disorders, and awareness. Kidney Int 70: 800–806, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Hillege HL, Janssen WM, Bak AA, Diercks GF, Grobbee DE, Crijns HJ, van Gilst WH, de Zeeuw D, de Jong PE, for the PREVEND study group: Microalbuminuria is common, also in a nondiabetic, nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J Intern Med 249: 519–526, 2001 [DOI] [PubMed] [Google Scholar]
- 10.De Zeeuw D, Hillege HL, de Jong PE: The kidney, a cardiovascular risk marker and a new target for therapy. Kidney Int 68[Suppl 98]: S25–S29, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Nierstichting: Professional: Available at: http://www.professional.nierstichting.nl/preventie/niercheck. Accessed February 8, 2008
- 12.Kee F, Reaney EA, Maxwell AP, Fogarty DG, Savage G, Patterson CC, on behalf of the TSN renal group: Late referral for assessment of renal failure. J Epidemiol Community Health 59: 386–388, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hallan SI, Dahl K, Oien CM, Grootendorst DC, Aasberg A, Holmen J, Dekker FW: Screening strategies for chronic kidney disease in the general population: Follow-up of cross sectional health survey. BMJ 333: 1047, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hallan SI, Coresh J, Astor BC, Asberg A, Powe NR, Romundstad S, Hallan H, Lydersen S, Holmen J: International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol 17: 2275–2284, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Eriksen BO, Ingrebetsen OC: The progression of chronic kidney disease: A 10-year population-based study on the effects of gender and age. Kidney Int 69: 375–382, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Krol E, Rutkowski B, Czekalski S, Sulowicz W, Wiecek A, Lizakowski S, Czarniak P, Szubert B, Karczewska-Maksymienko L, Orlikowska M, Kraszewska E, Magdon RL: Early diagnosis of renal disease: Preliminary results from the pilot study PolNef. Przegl Lek 62: 690–693, 2005 [PubMed] [Google Scholar]
- 17.Otero A, Gayoso P, Garcia F, de Francisco AL, on behalf of the EPIRCE study group: Epidemiology of chronic renal disease in the Galician population: Results of the pilot Spanish EPIRCE study. Kidney Int 68[Suppl 99]: S16–S19, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Drey N, Roderick P, Mullee M, Rogerson M: A population based study of the incidence and outcomes of diagnosed chronic kidney disease. Am J Kidney Dis 42: 677–684, 2003 [DOI] [PubMed] [Google Scholar]
- 19.John R, Webb M, Young A, Stevens PE: Unreferred chronic kidney disease: A longitudinal study. Am J Kidney Dis 43: 825–835, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Gansevoort RT, Verhave JC, Hillege HL, Burgerhof JG, Bakker SJ, de Zeeuw D, de Jong PE, for the PREVEND study group: The validity of screening based on spot morning urine samples to detect subjects with microalbuminuria in the general population. Kidney Int [Suppl 94]: S28–S35, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS: Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third NHANES. Am J Kidney Dis 41: 1–12, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, van Lente F, Levey AS: Prevalence of chronic kidney disease in the Unites States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Van Biessen W, Vanholder R, Veys N, Verbeke F, Delanghe J, de Bacquer D, Lameire N: The importance of standardization of creatinine in the implementation of guidelines and recommendations for CKD: Implications for CKD management programmes. Nephrol Dial Transplant 21: 77–83, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Lameire N, Jager K, van Biessen W, de Bacquer D, Vanholder R: Chronic kidney disease: A European perspective. Kidney Int 68[Suppl 99]: S30–S38, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Boulware LE, Jaar BG, Tarver-Carr ME, Brancati FL, Powe NR: Screening for proteinuria in US adults: A cost-effectiveness analysis. JAMA 290: 3101–3114, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Stewart JH, McCredie MR, Williams SM, Jager KJ, Trpeski L, McDonald SP: The enigma of hypertensive ESRD: Observations on incidence and trends in 18 European, Canadian, and Asian-Pacific populations. Am J Kidney Dis 48: 183–191, 2006 [DOI] [PubMed] [Google Scholar]
- 27.US Department of Health and Human Services: Healthy People 2010: Understanding and Improving Health, Washington, DC, United States Government Printing Office, 2000, pp 4.3–4.2 5 [Google Scholar]
- 28.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D: Prevalence of hypertension in the US adult population: Results from the Third National Health and Nutrition Examination Survey, 1988– 1991. Hypertension 25: 305–313, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med 157: 2413–2446, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Hajjar I, Kotchen TA: Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA 290: 199–206, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Nielen MM, Schellevis FG, Verheij RA: Evaluatie campagne “Stop beginnende nierziekte” NIVEL report, Utrecht, Netherlands, NIVEL. Available at: http://www.nivel.nl/pdf/Evaluatie-campagne-’Stop-beginnende’-2007.pdf. Accessed February 8, 2008 [Google Scholar]
- 32.Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, Gans RO, Janssen WM, Grobbee DE, de Jong PE, for the PREVEND study group: Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 106: 1777–1782, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Verhave JC, Gansevoort RT, Hillege HL, Bakker SJ, de Zeeuw D, de Jong PE, PREVEND Study Group: An elevated urinary albumin excretion predicts de novo development of renal function impairment in the general population. Kidney Int 66[Suppl 92]: S18–S21, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Konta T, Hao Z, Takasaki S, Abiko H, Takahashi T, Ichikawa K, Ikeda A, Ishikawa M, Kawata S, Kato T, Kubota I: Clinical utility of trace proteinuria for microalbuminuria screening in the general population. Clin Exp Nephrol 11: 51–55, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Atthobari J, Asselbergs FW, Boersma C, de Vries R, Hillege HL, van Gilst WH, Gansevoort RT, de Jong PE, Atthobari J, de Jong-van den Berg LT, Postma MJ, PREVEND IT Study Group.: Cost-effectiveness of screening for albuminuria and subsequent treatment with an ACE inhibitor to prevent cardiovascular events: A pharmacoeconomic analysis linked to the PREVEND and the PREVEND IT studies. Clin Ther 28: 432–444, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Palmer AJ, Annemans L, Palmer AJ, Annemans L, Roze S, Lamotte M, Lapuerta P, Chen R, Gabriel S, Carita P, Rodby RA, de Zeeuw D, Parving HH: Cost-effectiveness of early irbesartan treatment versus control or late irbesartan treatment in patients with type 2 diabetes, hypertension and renal disease. Diabetes Care 27: 1897–1903, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Frimat L, Siewe G, Loos-Ayav C, Briancon S, Kessler M, Aubrege A: Chronic kidney disease: Do generalists and nephrologists differ in their care? Nephrol Ther 2: 127–135, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Wyatt C, Konduri V, Eng J, Rohatgi R: Reporting of estimated GFR in the primary care clinic. Am J Kidney Dis 49: 634–641, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Boulware LE, Troll MU, Jaar BG, Myers DI, Powe NR: Identification and referral of patients with progressive CKD: A national study. Am J Kidney Dis 48: 192–204, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Department of Health: The National Service Framework for Renal Services. Part I and Part II. Available at: http://www.dh.gov.uk/PolicyandGuidance/healthAndSocialCareTopics/Renal/fs/en.
- 41.The Renal Association: Documents and reports. Available at: http://www.renal.org/ServiceProvision/servicefiles/IndicatorsGMSContract2006.pdf
- 42.Klebe B, Irving J, Stevens PE, O’Donoghue DJ, de Lusignan S, Cooley R, Hobbs H, Lamb EJ, John I, Middleton R, New J, Farmer CK: The cost of implementing UK guidelines for the management of chronic kidney disease. Nephrol Dial Transplant 22: 2504–2512, 2007 [DOI] [PubMed] [Google Scholar]


