Abstract
Hypertension and chronic volume overload are complications often seen in hemodialysis patients. Current hemodialysis practices adopt a standard dialysate sodium prescription that is typically higher than the plasma sodium concentration of most patients. As a general rule, hemodialysis patients have stable predialysis plasma sodium concentrations, and each patient has a fixed “osmolar set point.” Hypertonic dialysate sodium prescriptions, including sodium modeling, predispose to positive sodium balance and lead to higher blood pressure and increased interdialytic weight gain. Conversely, lowering or individualizing dialysate sodium reduces thirst, interdialytic weight gain, and blood pressure in non-hypotension prone dialysis patients. Optimization of the dialysate sodium prescription is an important step in achieving sodium balance and improving blood pressure control in hypertensive hemodialysis patients.
Hypertension (HTN) is common in patients on chronic maintenance hemodialysis (HD) and is a key mediator of cardiovascular morbidity and mortality in this population (1,2). Current HD prescribing standards use dialysate sodium concentrations that are high relative to the patient’s plasma sodium concentration, leading to less sodium loss and modest degrees of post-HD hypernatremia (3,4). The former predisposes to volume overload and HTN, whereas the latter results in increased fluid intake in response to thirst, also predisposing to chronic volume overload. We have recently demonstrated that an individualized approach to prescribing HD wherein the sodium concentration in the dialysate is adjusted to match the patient’s own plasma sodium (“dialysate sodium individualization”) results in less thirst and interdialytic weight gain (IDWG), and better blood pressure (BP) control in hypertensive patients (5). In this article, we further explore this topic, discussing the theoretical basis for this procedure and the potential benefits of an individualized dialysate sodium prescription in HD patients.
Hypertension in Hemodialysis
Cardiovascular disease is the most common cause of death in patients on chronic maintenance HD, in whom HTN is an important complicating factor (6). Hypertension is present in 50% to 90% of patients on dialysis (2). A detailed report of a contemporary cohort of 2535 HD patients in the United States revealed that, of the 2173 (86%) who had a diagnosis of HTN, BP control was inadequate in 70% despite use of antihypertensive agents by 76% of patients (7). The Dialysis Outcome and Practice Pattern Study also found a high prevalence of HTN in Europe (72.7%) and Japan (55.9%), although lower than in the United States (83.2%) (8).
The relationship between HTN and cardiovascular morbidity and mortality in dialysis patients is complex. Prospective randomized trials are conspicuously absent, and data from observational studies diverge on the impact of BP on cardiovascular endpoints. Indeed, several studies revealed an inverse relationship (i.e., the lower the BP the higher the mortality) (9–12). This controversy has led to a lack of initiative toward more aggressive management of HTN, although the recent Kidney Disease Outcomes Quality Initiative guidelines have provided opinionbased directives with relatively low targets (13) (predialysis BP goal <140/90 mmHg, postdialysis BP goal <130/80 mmHg). We and others have argued that a nihilistic approach to treatment is inappropriate (1,14–18). HTN is an important factor in the development of cardiovascular disease before the development of end-stage kidney disease. Shortly after a patient is started on dialysis (first year to several years), it continues to predict the development of left ventricular hypertrophy, de novo coronary disease, and de novo heart failure, but no longer predicts mortality (19). Current evidence suggests that this is likely a result of coexisting cardiac disease (1). It is only after several years that HTN reacquires its prognostic relevance (16,20–22); therefore, we think it is not acceptable to leave HTN uncontrolled in dialysis patients if we are to improve their long-term outcomes. Because available data indicate that most patients are already on multiple antihypertensive drugs (15), new strategies are needed to achieve better BP control.
Sodium Balance and BP in Hemodialysis
Sodium balance and extracellular volume control are at the center of BP control in HD (1,23), and it is generally agreed that establishment of an appropriate dry weight is the first and most important step in achieving normotension in dialysis patients (13,24). Sodium balance in HD is a function of intake and removal. There is good documentation that HD patients who restrict sodium intake have lower BP (16,24–27) and less left ventricular hypertrophy (16,27). In addition, dialysis modalities providing more intensive volume removal independent of total delivered dialysis dose, such as short daily HD, result in drastic improvements in measured extracellular volume expansion, BP control, and left ventricular hypertrophy (28,29). Unfortunately, achieving sodium restriction is often problematic in Western societies in which salt consumption is such an important part of daily life, and third party payers in many countries still do not cover use of daily dialysis. Therefore, alternative approaches to improved sodium balance are necessary.
The dialysate sodium prescription is an important component of sodium balance in HD patients but is underused in the management of HTN. In the anephric state, the “sodium removal arm” of sodium balance consists of removal during dialysis, which is the sum of diffusive and convective losses. The latter depends on the prescribed ultrafiltration as it represents sodium removed with ultrafiltered plasma. The former occurs across the dialyzer membrane according to the diffusion gradient between plasma and dialysate. Under current HD practices, more than 80% of sodium removal is convective and only 15% to 20% is diffusive (30).
Diffusive sodium losses were the primary modality of volume control in the early days of dialysis, when patients were dialyzed against a bath with sodium concentration of approximately 126 mEq/L (4). With the development of automated control of ultrafiltration volume, fluid management could be achieved through ultrafiltration regardless of sodium gradient, thus leading to a major advance in the way fluid control was reached in HD. In addition, the development of larger dialyzer membranes led to faster solute clearance, thus allowing targeted urea removal during a shorter dialysis session. However, shorter dialysis resulted in large osmolar shifts over short periods of time precipitating a cluster of symptoms known as the “dialysis disequilibrium syndrome” (headache, lethargy, nausea, vomiting, muscle cramps, or seizures and coma in the most severe cases), and the dialysis community shifted to the use of higher dialysate sodium concentrations in an attempt to minimize dialysis disequilibrium (4). This was done through either a constantly hypertonic dialysate (typically 143 to 145 mEq/L) or through the use of the technique of “sodium modeling,” which consists of the use of high sodium dialysate early during HD followed by lower concentrations at the end of HD allowing for some degree of equilibration between plasma and dialysate late in HD. The net result, however, regardless of approach used, is a high “time averaged” dialysate sodium concentration in the 140 to 145 mEq/L range (4,30,31). Indeed, high dialysate sodium prescriptions result in less disequilibrium symptoms; however, many patients leave the dialysis unit with relative hypernatremia, resulting in increased thirst, IDWG, and ultimately BP, as reviewed elsewhere (4,30,31). This occurs because rapid ultrafiltration with higher dialysate sodium leads to removal of fluid ingested in the interdialytic period but is not adequate to restore sodium balance, thus leading to positive sodium balance that may drive chronic HTN (Figure 1). To bring this concept to “real-life” conditions, Davenport (32) performed an audit of 7 dialysis centers (469 patients) that used different standard dialysate sodium concentrations (range, 136.8–140 mEq/L). Patients dialyzed in the centers using standard dialysate sodium concentrations of 140 mEq/L had larger IDWG, higher pre- and postdialysis systolic BP, and needed more intensive antihypertensive drug therapy. There was no difference in the frequency of episodes of intradialytic hypotension among centers.
Figure 1.
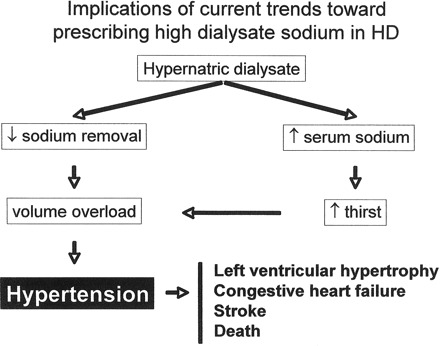
Implications of current trends toward prescribing high dialysate sodium in hemodialysis.
In a detailed analysis of the effect of sodium modeling on BP, Song et al. (33) studied 11 HD patients in a crossover study with 3 phases (phase 1, no modeling, time-averaged dialysate sodium concentration, TAC sodium, 138 mEq/L; phase 2, sodium modeling from 150 mEq/L to 138 mEq/L, dialysate TAC sodium 140 mEq/L; and phase 3, sodium modeling from 155 mEq/L to 133 mEq/L, dialysate TAC sodium 147 mEq/L). Higher dialysate TAC sodium resulted in significant, stepwise increases in thirst scores, IDWG (r = 0.823, P 0.001) and ambulatory BP during the interdialytic period (136/82 mmHg TAC sodium 138 mEq/L, 139/81 mmHg TAC sodium 140 mEq/L, 147/84 mmHg TAC sodium 147 mEq/L, P < 0.01) (33). In addition, BP effects were amplified according to the gradient between dialysate and plasma sodium. The authors showed that the difference between dialysate TAC sodium and pre-HD plasma sodium had a modest but significant correlation with interdialytic ambulatory diastolic BP (r < 0.36, P < 0.05) (33). These findings were consistent with those of other investigators who evaluated the effect of this dialysate-to-plasma sodium gradient on dialysis sodium losses (34) and IDWG (5,31,35,36).
Not only is high dialysate sodium hypertensogenic, but also the converse is true: low dialysate sodium leads to better sodium balance and BP control. Historical data indicate much better BP control when dialysate sodium concentration was much lower than presently (4). However, several other differences were operative, most importantly duration of dialysis, which is an independent determinant of BP control regardless of degree of volume control (29,37,38). Recent data from several groups have revisited the use of lower dialysate sodium in the control of HTN under modern HD techniques (Table 1). Krautzig et al. lowered dialysate sodium from 140 to 135 mEq/L (over the course of 15–20 wk) and enforced a low salt diet in 8 HD patients, an intervention that resulted in a decrease in mean arterial pressure from 108 mmHg to 98 mmHg (P < 0.02) (39). Farmer et al. decreased dialysate sodium by 5 mEq/L for 2 weeks in 10 HD patients and noted a fall in 24-h ambulatory BP from 141/83 mmHg to 133/78 mmHg (P < 0.01 for both systolic and diastolic BP) associated with a 33% decline in systemic vascular resistance (40). Similarly, Ferraboli et al. noted a fall in pre-HD BP from 150/88 to 143/82 mmHg in 14 patients whose dialysate was lowered acutely from 140 to 135 mEq/L and maintained over 2 wk (41). This maneuver was well tolerated and accompanied by a fall in thirst scores, but the BP trend was not statistically significant. Lambie et al. modified dialysate conductivity (of which sodium concentration is the principal determinant) in 16 patients, progressively trying to lower dialysate conductivity from 13.6 to 13.0 mS/cm. Using this strategy, pre-HD BP fell by 7/5 mmHg (P < 0.05 for both systolic and diastolic BP), an effect that was accompanied by more effective diffusive sodium removal (30). On the other hand, Kooman et al. were unable to replicate these BP lowering effects in 6 patients whose dialysate sodium was rapidly reduced from 140 to 136 mEq/L but no dietary control was enforced (42). Overall, these data indicate that modest reductions in dialysate sodium are well tolerated in non-hypotension prone patients and result in lower BP.
Table 1.
Clinical consequences of dialysate sodium reduction in chronic hemodialysis patients
| Reference | N | Intervention time (wk) | Standard dialysate sodium (mEq/L) | Dialysate sodium reduction (mEq/L) | Reference for sodium reduction | BP | IDWG | HD-associated symptoms or nursing interventions | Comments | |
|---|---|---|---|---|---|---|---|---|---|---|
| Krautzig (39) | 8 | NR | 140 | 5 | Random | 2 | NR | NR | Dietary sodium restriction enforced; fixed decrease in dialysate Na | |
| Farmer (40) | 10 | 2 | 138–140 | 5 | Random | 2 | NS | NS | 24 h ABPM study; fixed decrease in dialysate Na | |
| Kooman (42) | 6 | 6 | 140 | 4 | Random | NS | NS | NR | Fixed decrease in dialysate Na | |
| Ferraboli (41) | 14 | 2 | 140 | 5 | Random | 2 | NR | NR | Fixed decrease in dialysate Na | |
| De Paula (5) | 27 | 3 | 138 | 3 (mean) | Pre-HD plasma sodium | 2 2 | Improvement | Individualized adjustment in dialysate Na | ||
| Lambie (30) | 16 | NR | 136a | Up to 6a | Random | 2 2 | Limit for dialysate conductivity reduction | Progressive titration on dialysate conductivity based on tolerability | ||
| Sayarlioglu (46) | 18 | 4 | NR | Varied according to Pre-HD Na | Pre-HD plasma sodium | 2 2 | NR | Decreased inferior vena cava diameter | ||
| Thein (43) | 52 | 16 | 141 | 3 | Random | 2 2 | NS | Database analysis, not a clinical trial; hypotensionprone patients included | ||
| Selby (55) | 10 | 6 | 136a | 2–4a | None | NS | NS | NS | Only 3 patients taking antihypertensive medications; Pre-HD extracellular water decreased; progressive titration on dialysate conductivity based on tolerability | |
BP, blood pressure; IDWG, interdialytic weight gain; HD, hemodialysis; NR, not reported; 2, significant decrease; ABPM, ambulatory blood pressure monitoring; NS, not significant; Pre-HD, pre-hemodialysis.
Measurement based on conductivity (dialysate conductivity × 10).
Osmolar Set Point in Dialysis: Implications to Sodium and Volume Control
Although questioned by some (43), most of the previously published data demonstrate that HD patients have a fixed “osmolar set point” (4,31,44). A classic modeling study performed more than 25 yr ago revealed the importance of dialysate sodium concentration in relationship to the patient’s own plasma sodium in determining the amount of sodium (and volume) loading or depletion (45). Thus, if a patient is dialyzed against a high sodium dialysate concentration and develops relative hypernatremia following HD, he/she will have his/her thirst stimulated to drink enough free water to drive his/her osmolality back to the set point with little variability (coefficients of variation, 0.5%–1.9%) (5,44). We estimate that with current dialysate sodium prescriptions more than three fourths of conventional HD patients undergo dialysis with dialysate sodium concentrations that are above their “set point.” In an analysis of 100 chronic stable HD patients dialyzed against a base dialysate sodium concentration of 140 mEq/L, the average pre-HD serum sodium over the course of 12 months was 136.4 ± 0.8 mEq/L (coefficient of variation, 1.6%); only 8 patients had an average sodium ≥139 mEq/L, and 16 averaged <135 mEq/L (Gowda N, Peixoto AJ: unpublished observations). We also performed a survey of practicing nephrologists at Veterans Administration dialysis centers nationwide inquiring about dialysate sodium prescribing patterns in 2005 (Peixoto AJ: unpublished observations). We received replies from 21 of the 68 centers (31% response rate) and confirmed that the most common standard dialysate sodium concentration is 140 mEq/L. In 4 centers, the concentration was lower (135 mEq/L in one, 138 mEq/L in three), and in three it was higher than 140 mEq/L (142 mEq/L in one, 145 mEq/L in two). Therefore, standard dialysate sodium concentrations may often be hypertonic in relation to the patient’s own plasma sodium concentration and thereby may result in a net diffusion of sodium from dialysate to plasma and hamper the effectiveness of HD as a procedure to reestablish sodium balance.
Dialysate sodium individualization decreases thirst, IDWG, and BP in HD patients
The possible beneficial effect of an individualized approach to dialysate sodium prescription on thirst, IDWG, and BP was the focus of a study involving 37 non-hypotension prone HD patients in a single-blind protocol (5). Subjects underwent 9 consecutive HD sessions (3 weeks) with the dialysate sodium concentration set at 138 mEq/L (“standard sodium HD”) followed by 9 sessions wherein the dialysate sodium was set to match the patient’s average pre-HD plasma sodium measured 3 times (mid-week) during the standard sodium phase (“individualized dialysate sodium HD”). Ten of the 37 subjects had average pre-HD sodium equal to or higher than the standard dialysate. Thus, they were removed from the intervention phase, leaving 27 patients to undergo the intervention. Dry weight, dialysis prescription, and medications were not modified during the 6 weeks of the study.
As shown in Table 2, we confirmed the reproducibility of pre-HD plasma sodium regardless of period of intervention, corroborating the concept of an “osmolar set point” in this population. As expected, the post-HD plasma sodium decreased during the individualized sodium phase, which led to a significant decrease in IDWG, thirst scores, and episodes of intradialytic hypotension.
Table 2.
Plasma sodium concentration, interdialytic weight gain, thirst scores, and hypotensive episodes in the standard sodium HD and in the individualized sodium HD
| Standard sodium | Individualized sodium | P | |
|---|---|---|---|
| Pre-HD plasma sodium | 134.0 ± 1.4 | 134.0 ± 1.5 | 0.725 |
| Post-HD plasma sodium | 135.9 ± 2.0 | 133.1 ± 2.6 | <0.001 |
| IDWG (kg) | 2.91 ± 0.87 | 2.29 ± 0.87 | <0.001 |
| Interdialytic thirst, n (%) | |||
| Nil | 0 (0) | 4 (15) | 0.043 |
| Mild | 1 (4) | 17 (63) | <0.001 |
| Moderate | 11 (41) | 5 (18) | 0.07 |
| Severe | 15 (55) | 1 (4) | <0.001 |
| Hypotensive episodes, n (%) of sessions | 23 (9) | 6 (2) | <0.001 |
HD, hemodialysis; IDWG, interdialytic weight gain.
Sodium values measured by direct potentiometry (AVL 9180, AVL Medical Instruments, Schaffhausen, Switzerland). Values are in mEq/L. Adapted from reference 5.
Sodium individualization resulted in lower systolic BP in patients with uncontrolled HTN (pre-HD BP >150/85 mmHg), but not in those with controlled BP (<150/85 mmHg) (Figure 2). The ΔBP was −15.7/−6.5 mmHg in uncontrolled compared with 6.4/4.5 mmHg in controlled HTN (P = <0.001 for systolic BP and P = <0.001 for diastolic BP). The lowering effect on BP did not seem to be related only to the improvement in extracellular volume control since both controlled and uncontrolled BP patients had similar significant decreases in IDWG, and there was no difference in the achieved weight with individualized sodium prescription. We concluded that an individualized sodium dialysate based on predialysis plasma sodium levels decreases thirst, IDWG, HD-related symptoms, and pre-HD BP (in patients with uncontrolled BP at baseline).
Figure 2.
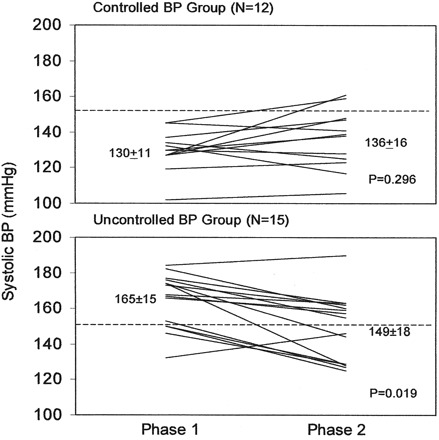
Blood pressure responses to dialysate sodium individualization according to baseline blood pressure. Phase 1, standard Na (140 mEq/L); phase 2, individualized Na. From reference 5, with permission.
In a recent study, Sayarlioglu et al. (46) used the predialysis sodium of 18 patients as a reference to set the dialysate sodium concentration. For those patients who had pre-HD sodium less than 137 mEq/L, the dialysate sodium was modified to 135 mEq/L, and for those with pre-HD sodium over 137 mEq/L, the dialysate sodium was modified to 137 mEq/L. After 8 weeks, reducing dialysate sodium resulted in a significant decrease in pre-HD SBP (151.7 ± 17.7 mmHg versus 179.7 ± 24.8 mmHg), pre-HD DBP (93.1 ± 10.5 mmHg versus 100.6 ± 12.8 mmHg), post-HD SBP (132.3 ± 16.4 mmHg versus 141.4 ± 28.8 mmHg) and IDWG (1.8 ± 0.6 kg versus 2.5 ± 1 kg). Left ventricular systolic diameter, tricuspid regurgitation, pulmonary arterial pressure, and inferior vena cava diameter also decreased with lowering dialysate sodium concentration (46). Along the same lines, Thein et al. (43) decreased the dialysate sodium prescription from 141 mEq/L to 138 mEq/L in all patients (n = 52) in their dialysis facility without changes in instructions to patients about dietary sodium. After 4 months, there was a significant decrease in predialysis SBP [161 (146–174) versus 169 (159–177) mmHg; P = 0.038] and postdialysis SBP [143 (133–158) versus 152 (146–160) mmHg; P = 0.014] in patients in the upper tertile of these dialysis parameters; and in IDWG in patients in the middle [2.3 (2.1–2.5) versus 2.5 (2.3–2.7) kg; P = 0.005] and upper tertiles [2.9 (2.6–3.4) versus 3.2 (2.9–3.6) kg; P = 0.031].
Determining the Dialysate-to-Plasma Sodium Gradient
Ionic activity, rather than total sodium concentration, determines sodium movement between plasma and dialysate (4). Direct potentiometry is currently the best method to determine the plasma and dialysate sodium concentration as it permits the determination of sodium concentration in undiluted samples and is not influenced by abnormal levels of plasma proteins and lipids (47). Furthermore, the method measures noncomplexed, free sodium concentration, which represents those sodium particles available for diffusion (47). For this reason, it is proposed that sodium levels determined by direct potentiometry be referred to as the concentration of ionized sodium rather than total sodium concentration (47). To avoid confusion in clinical interpretation, the results are usually related to flame photometry results by a conversion factor (48).
There is a difference in sodium concentration between plasma and dialysate because plasma water constitutes only 93% of total plasma, whereas it is 100% of total dialysate volume. Therefore, in isonatric dialysis, actual plasma sodium concentration is approximately 6 to 7 mEq/L higher than the dialysate. In vivo, this concentration difference is compensated by the Gibbs-Donnan effect; otherwise, the dialysate should have a concentration approximately 6 to 7 mEq/L higher to result in an isonatremic dialysis (4). The Gibbs-Donnan effect in HD occurs because plasma proteins, which are negatively charged and not diffusable through the dialysis membrane, create an electric field that attracts sodium, reducing the plasma diffusable sodium by 4% to 5% (3).
In our study (5), we measured dialysate sodium levels with direct potentiometry. Because the values in the dialysate were adjusted to the volume of plasma water by the instrument (flame photometry adjustment), we needed to adjust the dialysate sodium measurements. However, direct potentiometry is not commonly available in clinical practice because most of these devices analyze single or few samples at a time. Flame photometry and indirect potentiometry are the more widely available methods for sodium measurement in clinical laboratories. Both methods have the inconvenience of using diluted samples that are susceptible to false results depending on plasma protein and lipid concentrations. Nevertheless, both methods can provide a consistent sodium dialysate-to-plasma to gradient in most of the dialysis population and therefore are not an impediment for an individualized sodium prescription practice in HD. Therefore, in laboratories that use indirect ionometry or flame photometry, we recommend that the clinician who is interested in matching dialysate sodium to the patient’s plasma sodium level use the exact value measured in plasma as the value for the dialysate prescription, as no “extra” conversion factor is required. Because many HD patients are diabetic, the clinician must be aware that hyperglycemia causes an osmotic movement of water out of the cells, which may lead to hyponatremia by dilution.
To obtain a reliable dialysate-to-plasma sodium gradient, it is also necessary to periodically measure serum and dialysate sodium and to routinely check the accuracy of the HD machine calibration with a conductivity monitor (49).
Other Methods to Achieve Isonatremic Dialysis
Although matching the dialysate sodium concentration to the patient’s pre-HD plasma sodium can avoid an unnecessary sodium loading, this procedure may be insufficient to restore an ideal sodium balance. Water shifts from intracellular and extracellular space are not constant throughout the HD session. Furthermore, the Gibbs-Donnan effect varies with pH, which also fluctuates along the HD session (3). Therefore, the dialysate sodium necessary to achieve an isonatric dialysis can only be perfectly ascertained by measuring the dialyzable sodium concentration during the HD session (44). However, assessing sodium kinetics during a dialysis session by sequential plasma and dialysate sodium measurements is impractical (and probably unfeasible) in clinical practice.
To address this shortcoming, a device has been developed to estimate the patients’ plasma conductivity as an indicator of the ionized plasma sodium concentration (50,51). This is achieved by modifying temporarily the dialysate conductivity in response to changes in plasma water conductivity (50–52). This system was used to determine ionic mass balance using 3 dialysate sodium concentrations (144 mEq/L, 140 mEq/L, and individualized sodium setup according pre-HD plasma conductivity) (34). Throughout a 1-h period of isovolemic dialysis, ionic mass balance was significantly lower with dialysate sodium concentration of 144 mEq/L. Similar differences were observed when ultrafiltration was combined to dialysis. It was noteworthy that a net ionic influx from dialysate to the patient occurred when the dialysate to plasma sodium gradient was more than 5 mEq/L. Furthermore, ionic mass balance was significantly higher with individualized sodium dialysate compared with a fixed dialysate concentration of 140 mEq/L in patients with a predialysis sodium less than 140 mEq/L. The inverse occurred in patients with predialysis sodium equal or above 140 mEq/L. Therefore, these data confirm the relevance of the dialysate-to-plasma sodium gradient regarding the achievement of sodium balance in HD.
Although the experience with using this method to assess sodium kinetics during HD is limited, it is certainly a step toward a more effective control of sodium balance in HD (52–54). However, a recent study demonstrated no advantage of the biofeedback system over a fixed dialysate conductivity reduction in influencing BP levels, IDWG, intradialytic hypotension episodes, and dialysis tolerability (55). In other words, pushing a lower dialysate sodium concentration as tolerated may be equivalent and more practicable in a clinical practice setting.
Potential Pathophysiologic Mechanisms Underlying the Effects of Dialysate Sodium Manipulation
The data reviewed above point strongly in the direction of improved hemodynamics on a lower dialysate sodium level, especially so if individualized to the hypertensive HD patient. The more simplistic explanation for these salutary effects is that sodium balance is optimized through more diffusive loss (or less diffusive gain) of sodium during each HD session. This is certainly one, and perhaps the most important, of operating mechanisms. The relationship between sodium excess and HTN in this setting does not require further discussion. However, recent evidence has raised other possible factors, such as the effects resulting from changes in the prevailing plasma sodium concentration (56), and the possible relevance of changes in amounts of “osmotically inactive” sodium (57).
When plasma sodium is increased, however briefly, there is an associated activation of several pro-hypertensive mechanisms that are independent of an increase in volemia (58). For example, hypernatremia increases central sympathetic outflow in some animals (young Dahl salt-sensitive rats), although not in others (59–61). Increased brain sodium and osmolality are also responsible for inducing increases in angiotensin 2 levels, a factor that results in increased sympathetic outflow in several models, including renal mass reduction (56,60,62,63). It is possible that this potential relationship with mismatched dialysate sodium is responsible, at least part, for the well-documented increases in sympathetic tone in advanced kidney disease (58), which paired with the well-known vasoconstrictive and vasculotoxic effects of angiotensin 2 could contribute to the development of HTN and vascular injury. In addition, high medium sodium concentration results in hypertrophy of cardiomyocytes and vascular smooth muscle in vitro (64), changes that are associated with the long-term vascular changes in HTN. Lastly, Oberleithner et al. recently demonstrated that an increase in sodium concentration in an endothelial cell culture medium from 135 to 145 mmol/L produced significant endothelial cell stiffness in the presence of aldosterone (65). This was associated with decreased nitrite concentration in the medium, suggestive of endothelial cell dysfunction due to impaired sensitivity to sheer stress. Taken together, these data suggest that not only the unfavorable sodium overload but also the hypernatremia induced by dialysate sodium mismatching may contribute to the pathogenesis of HTN in dialysis patients.
Van Kuijk et al. studied 9 HD patients during a single HD session using dialysate sodium of 134 mEq/L or 144 mEq/L (66). There were no significant hemodynamic differences between the two dialysate sodium treatments. Interestingly, despite the absence of differences in vascular reactivity (measured with plethysmography), levels of the vasodilating prostaglandin PGE2 were significantly increased after low sodium dialysis, but not in high sodium dialysis. It is possible that after long-term exposure to lower dialysate sodium this vasodilating advantage may acquire clinical relevance.
It has long been known that ingestion of salt load does not lead to the predicted weight gain that would be expected if the entire sodium load were fully hydrated. This led to the concept that a portion of the total body sodium content is osmotically inactive (not associated with water retention) or neutral (equimolarly exchanged for potassium) (57). Animal models (deoxycorticosterone-salt hypertensive rat) suggest that this osmotically inactive sodium pool resides primarily in skin (67) and that a decrease in this pool, as induced by ovariectomy, is associated with higher BP (57). We are not aware of any data (animal of human) testing this hypothesis in chronic kidney disease, but we think that some of the effects related to a more favorable sodium balance may go beyond the simple pairing of sodium and water gain. For example, it is possible that kidney disease leads to decreased osmotically inactive sodium capacity, adding a nonrenal component to the sodium sensitivity of renal HTN. Somewhat accordingly, a study that analyzed changes in total body water resulting from lowering dialysate sodium showed a strong trend toward BP fall (6–8 mmHg for systolic and 3–5 mmHg for diastolic BP) despite minimal changes in extracellular water measured by bioimpedance (0.01 L/kg) (40). This observation raises the interesting possibility that the hemodynamic effects were partly achieved through increased capacity to store osmotically inactive sodium. It is also possible that inactive sodium storage be linked to the so-called “lag phenomenon” that is the reduction in BP in HD patients that occurs months after dry weight achievement (68). In this setting, it is feasible that a lower dialysate sodium concentration contributes to a decrease in osmotically inactive sodium stored in the interstitial matrix of blood vessels by augmenting sodium losses by diffusion (69).
Final Perspectives
Pre-HD plasma sodium concentration varies very little over time, and patients maintain the osmolar set point by means of thirst and proportional fluid ingestion. Current dialysis prescribing patterns usually underestimate the role of sodium as a parameter that is distinctive to each patient. Although most dialysis machines can deliver a dialysate with a wide range of sodium concentration the majority of dialysis centers adopt a standard dialysate sodium prescription. Recent guidelines (70) and reviews (23,24) point out the importance of sodium overload caused by hypertonic dialysate to the pathogenesis of HD related HTN and excessive IDWG (71). The use of patient’s predialysis sodium concentration as a reference to prescribe dialysate sodium seems rational. However, the safety of this procedure has to be determined with long-term observations, and this practice has not been applied to hypotension-prone HD patients, although a random reduction of 3 mEq/L in dialysate sodium was well tolerated even by patients with preexisting hypotension (43). Also, we still do not know if patients with a “high” pre-HD sodium concentration will benefit from a reduction in dialysate sodium. Nevertheless, avoiding excessive sodium loading during HD may improve BP control and reduce IDWG (72). Furthermore, sodium burden may have detrimental effects that are both dependent (73) and independent (58,74)of its effects on BP and body volume, and may have, per se, an important role in the high HD associated cardiovascular morbidity and mortality (6). Therefore, careful attention to the dialysate sodium prescription is necessary to optimize its role in assuring a favorable sodium balance in dialysis patients.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Santos SF, Peixoto AJ: Hypertension in dialysis. Curr Opin Nephrol Hypertens 14: 111–118, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Horl MP, Horl WH: Hemodialysis-associated hypertension: pathophysiology and therapy. Am J Kidney Dis 39: 227–244, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Flanigan MJ: Sodium flux and dialysate sodium in hemodialysis. Semin Dial 11: 298–304, 1998 [Google Scholar]
- 4.Flanigan MJ: Role of sodium in hemodialysis. Kidney Int Suppl 76[Suppl]: S72–S78, 2000 [DOI] [PubMed] [Google Scholar]
- 5.de Paula FM, Peixoto AJ, Pinto LV, Dorigo D, Patricio PJ, Santos SF: Clinical consequences of an individualized dialysate sodium prescription in hemodialysis patients. Kidney Int 66: 1232–1238, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Foley RN, Herzog CA, Collins AJ: Blood pressure and long-term mortality in United States hemodialysis patients: USRDS Waves 3 and 4 Study. Kidney Int 62: 1784–1790, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Agarwal R, Nissenson AR, Batlle D, Coyne DW, Trout JR, Warnock DG: Prevalence, treatment, and control of hypertension in chronic hemodialysis patients in the United States. Am J Med 115: 291–297, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Goodkin DA, Bragg-Gresham JL, Koenig KG, Wolfe RA, Akiba T, Andreucci VE, Saito A, Rayner HC, Kurokawa K, Port FK, Held PJ, Young EW: Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS). J Am Soc Nephrol 14: 3270–3277, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Port FK, Hulbert-Shearon TE, Wolfe RA, Bloembergen WE, Golper TA, Agodoa LY, Young EW: Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis 33: 507–517, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Salem MM, Bower J: Hypertension in the hemodialysis population: any relation to one-year survival? Am J Kidney Dis 28: 737–740, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Zager PG, Nikolic J, Brown RH, Campbell MA, Hunt WC, Peterson D, Van Stone J, Levey A, Meyer KB, Klag MJ, Johnson HK, Clark E, Sadler JH, Teredesai P: “U” curve association of blood pressure and mortality in hemodialysis patients. Medical Directors of Dialysis Clinic, Inc. Kidney Int 54: 561–569, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Li Z, Lacson E Jr, Lowrie EG, Ofsthun NJ, Kuhlmann MK, Lazarus JM, Levin NW: The epidemiology of systolic blood pressure and death risk in hemodialysis patients. Am J Kidney Dis 48: 606–615, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Kidney Disease Outcomes Quality Initiative: Clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis 45: 16–153, 2005 [PubMed] [Google Scholar]
- 14.Zoccali C: Arterial pressure components and cardiovascular risk in end-stage renal disease. Nephrol Dial Transplant 18: 249–252, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Agarwal R: Hypertension and survival in chronic hemodialysis patients: past lessons and future opportunities. Kidney Int 67: 1–13, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Ozkahya M, Ok E, Toz H, Asci G, Duman S, Basci A, Kose T, Dorhout Mees EJ: Long-term survival rates in haemodialysis patients treated with strict volume control. Nephrol Dial Transplant 21: 3506–3513, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Light P: Current understanding of optimal blood pressure goals in dialysis patients. Curr Hypertens Rep 8: 413–419, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Agarwal R, Foley RN: Hypertension is harmful to dialysis patients and should be controlled. Semin Dial 20: 518–522, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE: Impact of hypertension on cardiomyopathy, morbidity and mortality in end-stage renal disease. Kidney Int 49: 1379–1385, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Stidley CA, Hunt WC, Tentori F, Schmidt D, Rohrscheib M, Paine S, Bedrick EJ, Meyer KB, Johnson HK, Zager PG: Changing relationship of blood pressure with mortality over time among hemodialysis patients. J Am Soc Nephrol 17: 513–520, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Mazzuchi N, Carbonell E, Fernandez-Cean J: Importance of blood pressure control in hemodialysis patient survival. Kidney Int 58: 2147–2154, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Rajagopalan S, Dellegrottaglie S, Furniss AL, Gillespie BW, Satayathum S, Lameire N, Saito A, Akiba T, Jadoul M, Ginsberg N, Keen M, Port FK, Mukherjee D, Saran R: Peripheral arterial disease in patients with end-stage renal disease: observations from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Circulation 114: 1914–1922, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Agarwal R: Management of hypertension in hemodialysis patients. Hemodial Int 10: 241–248, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Charra B: Fluid balance, dry weight, and blood pressure in dialysis. Hemodial Int 11: 21–31, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Charra B, Bergstrom J, Scribner BH: Blood pressure control in dialysis patients: importance of the lag phenomenon. Am J Kidney Dis 32: 720–724, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Maduell F, Navarro V: Assessment of salt intake in hemodialysis. Nefrologia 21: 71–77, 2001 [PubMed] [Google Scholar]
- 27.Ozkahya M, Toz H, Qzerkan F, Duman S, Ok E, Basci A, Mees EJ: Impact of volume control on left ventricular hypertrophy in dialysis patients. J Nephrol 15: 655–660, 2002 [PubMed] [Google Scholar]
- 28.Fagugli RM, Reboldi G, Quintaliani G, Pasini P, Ciao G, Cicconi B, Pasticci F, Kaufman JM, Buoncristiani U: Short daily hemodialysis: blood pressure control and left ventricular mass reduction in hypertensive hemodialysis patients. Am J Kidney Dis 38: 371–376, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Nesrallah G, Suri R, Moist L, Kortas C, Lindsay RM: Volume control and blood pressure management in patients undergoing quotidian hemodialysis. Am J Kidney Dis 42: 13–17, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Lambie SH, Taal MW, Fluck RJ, McIntyre CW: Online conductivity monitoring: validation and usefulness in a clinical trial of reduced dialysate conductivity. ASAIO J 51: 70–76, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Levin NW, Zhu F, Keen M: Interdialytic weight gain and dry weight. Blood Purif 19: 217–221, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Davenport A: Audit of the effect of dialysate sodium concentration on inter-dialytic weight gains and blood pressure control in chronic haemodialysis patients. Nephron Clin Pract 104: 120–125, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Song JH, Lee SW, Suh CK, Kim MJ: Time-averaged concentration of dialysate sodium relates with sodium load and interdialytic weight gain during sodium-profiling hemodialysis. Am J Kidney Dis 40: 291–301, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Moret K, Hassell D, Kooman JP, van der Sande F, Gerlag PG, van den Wall Bake AW, van de Bogaart J, Leunissen KM: Ionic mass balance and blood volume preservation during a high, standard, and individualized dialysate sodium concentration. Nephrol Dial Transplant 17: 1463–1469, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Dominic SC, Ramachandran S, Somiah S, Mani K, Dominic SS: Quenching the thirst in dialysis patients. Nephron 73: 597–600, 1996 [DOI] [PubMed] [Google Scholar]
- 36.Flanigan MJ, Khairullah QT, Lim VS: Dialysate sodium delivery can alter chronic blood pressure management. Am J Kidney Dis 29: 383–391, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Luik AJ, van der Sande FM, Weideman P, Cheriex E, Kooman JP, Leunissen KM: The influence of increasing dialysis treatment time and reducing dry weight on blood pressure control in hemodialysis patients: a prospective study. Am J Nephrol 21: 471–478, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Katzarski KS, Randmaa I, Bergstrom J: Influence of hemodialysis on intravascular volume and vasoactive hormones. Clin Nephrol 52: 304–311, 1999 [PubMed] [Google Scholar]
- 39.Krautzig S, Janssen U, Koch KM, Granolleras C, Shaldon S: Dietary salt restriction and reduction of dialysate sodium to control hypertension in maintenance haemodialysis patients. Nephrol Dial Transplant 13: 552–553, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Farmer CKT, Donohoe P, Dallyn PE, Cox J, Koingswood JC, Goldsmith DJ: Low-sodium hemodialysis without fluid removal improves blood pressure control in hemodialysis patients. Nephrology 5: 237–241, 2000 [Google Scholar]
- 41.Ferraboli R, Manuel C, Abensur H, Elias R, Luders C: Reduction of sodium dialysate for hypertensive HD patients: analysis of beneficial and adverse effects [Abstract]. J Am Soc Nephrol 13 :211a, 2002 [Google Scholar]
- 42.Kooman JP, Hendriks EJ, van der Sande FM, Leunissen KM: Dialysate sodium concentration and blood pressure control in haemodialysis patients. Nephrol Dial Transplant 15: 554, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Thein H, Haloob I, Marshall MR: Associations of a facility level decrease in dialysate sodium concentration with blood pressure and interdialytic weight gain. Nephrol Dial Transplant 22: 2630–2639, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Flanigan MJ: Technology in clinical practice. ASAIO J 51: xxxii-xxxv, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Gotch FA, Lam MA, Prowitt M, Keen M: Preliminary clinical results with sodium-volume modeling of hemodialysis therapy. Proc Clin Dial Transplant Forum 10: 12–17, 1980 [PubMed] [Google Scholar]
- 46.Sayarlioglu H, Erkoc R, Tuncer M, Soyoral Y, Esen R, Gumrukcuoglu HA, Dogan E, Sayarlioglu M: Effects of low sodium dialysate in chronic hemodialysis patients: an echocardiographic study. Ren Fail 29: 143–146, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Burnett RW, Covington AK, Fogh-Andersen N, Kulpmann WR, Lewenstam A, Maas AH, Muller-Plathe O, VanKessel AL, Zijlstra WG: Use of ion-selective electrodes for bloodelectrolyte analysis. Recommendations for nomenclature, definitions and conventions. International Federation of Clinical Chemistry and Laboratory Medicine (IFCC): Scientific Division Working Group on Selective Electrodes. Clin Chem Lab Med 38: 363–370, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Burnett RW, Covington AK, Fogh-Andersen N, Kulpmann WR, Lewenstam A, Maas AH, Muller-Plathe O, Sachs C, Siggaard-Andersen O, VanKessel AL, Zijlstra WG: Recommendations for measurement of and conventions for reporting sodium and potassium by ion-selective electrodes in undiluted serum, plasma or whole blood. International Federation of Clinical Chemistry and Laboratory Medicine (IFCC): IFCC Scientific Division Working Group on Selective Electrodes. Clin Chem Lab Med 38: 1065–1071, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Misra M: The basics of hemodialysis equipment. Hemodial Int 9: 30–36, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Petitclerc T: Festschrift for Professor Claude Jacobs: recent developments in conductivity monitoring of haemodialysis session. Nephrol Dial Transplant 14: 2607–2613, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Locatelli F, Di Filippo S, Manzoni C: Relevance of the conductivity kinetic model in the control of sodium pool. Kidney Int Suppl 76[Suppl]: S89–S95, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Pozzoni P, SDIF, Pontoriero G, Locatelli F: Effectiveness of sodium and conductivity kinetic models in predicting enddialysis plasma water sodium concentration: preliminary results of a single-center experience. Hemodial Int 11: 169– 177, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Locatelli F, Covic A, Chazot C, Leunissen K, Luno J, Yaqoob M: Optimal composition of the dialysate, with emphasis on its influence on blood pressure. Nephrol Dial Transplant 19: 785–796, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Locatelli F, Buoncristiani U, Canaud B, Kohler H, Petitclerc T, Zucchelli P: Haemodialysis with on-line monitoring equipment: tools or toys? Nephrol Dial Transplant 20: 22–33, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Selby NM, Taal MW, McIntyre CW: Comparison of progressive conductivity reduction with diacontrol and standard dialysis. ASAIO J 53: 194–200, 2007 [DOI] [PubMed] [Google Scholar]
- 56.He FJ, Markandu ND, Sagnella GA, de Wardener HE, MacGregor GA: Plasma sodium: ignored and underestimated. Hypertension 45: 98–102, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Titze J, Luft FC, Bauer K, Dietsch P, Lang R, Veelken R, Wagner H, Eckardt KU, Hilgers KF: Extrarenal Na+ balance, volume, and blood pressure homeostasis in intact and ovariectomized deoxycorticosterone-acetate salt rats. Hypertension 47: 1101–1107, 2006 [DOI] [PubMed] [Google Scholar]
- 58.Kooman JP, van der Sande FM, Leunissen KM: Sodium, blood pressure and cardiovascular pathology: is it all volaemia? Nephrol Dial Transplant 19: 1046–1049, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Bealer SL: Increased dietary sodium alters neural control of blood pressure during intravenous ANG II infusion. Am J Physiol Heart Circ Physiol 284: H559–H565, 2003 [DOI] [PubMed] [Google Scholar]
- 60.Guyenet PG: The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Oparil S, Yang RH, Jin HK, Wyss JM, Chen YF: Central mechanisms of hypertension. Am J Hypertens 2: 477–485, 1989 [DOI] [PubMed] [Google Scholar]
- 62.Campese VM, Ye S, Truong RH, Gamburd M: Losartan reduces sympathetic nerve outflow from the brain of rats with chronic renal failure. J Renin Angiotensin Aldosterone Syst 1: 202–208, 2000 [DOI] [PubMed] [Google Scholar]
- 63.de Wardener HE, He FJ, MacGregor GA: Plasma sodium and hypertension. Kidney Int 66: 2454–2466, 2004 [DOI] [PubMed] [Google Scholar]
- 64.Gu JW, Anand V, Shek EW, Moore MC, Brady AL, Kelly WC, Adair TH: Sodium induces hypertrophy of cultured myocardial myoblasts and vascular smooth muscle cells. Hypertension 31: 1083–1087, 1998 [DOI] [PubMed] [Google Scholar]
- 65.Oberleithner H, Riethmuller C, Schillers H, MacGregor GA, de Wardener HE, Hausberg M: Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc Natl Acad Sci USA 104: 16281–16286, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Kuijk WH, Wirtz JJ, Grave W, de Heer F, Menheere PP, van Hooff JP, Leunissen KM: Vascular reactivity during combined ultrafiltration-haemodialysis: influence of dialysate sodium. Nephrol Dial Transplant 11: 323–328, 1996 [DOI] [PubMed] [Google Scholar]
- 67.Titze J, Lang R, Ilies C, Schwind KH, Kirsch KA, Dietsch P, Luft FC, Hilgers KF: Osmotically inactive skin Na+ storage in rats. Am J Physiol Renal Physiol 285: F1108–F1117, 2003 [DOI] [PubMed] [Google Scholar]
- 68.Shaldon S: An explanation for the “lag phenomenon” in drug-free control of hypertension by dietary salt restriction in patients with chronic kidney disease on hemodialysis. Clin Nephrol 66: 1–2, 2006 [DOI] [PubMed] [Google Scholar]
- 69.Shaldon S: Beneficial effect of strict volume control on blood pressure and mortality in patients on hemodialysis. Nat Clin Pract Nephrol 3: 130–131, 2007 [DOI] [PubMed] [Google Scholar]
- 70.Jindal K, Chan CT, Deziel C, Hirsch D, Soroka SD, Tonelli M, Culleton BF: CHAPTER 2: management of blood pressure in hemodialysis patients. J Am Soc Nephrol 17[Suppl 1]: S8–S10, 2006 [DOI] [PubMed] [Google Scholar]
- 71.Sarkar SR, Kotanko P, Levin NW: Interdialytic weight gain: implications in hemodialysis patients. Semin Dial 19: 429– 433, 2006 [DOI] [PubMed] [Google Scholar]
- 72.Wystrychowski G, Levin NW: Dry weight: sine qua non of adequate dialysis. Adv Chronic Kidney Dis 14: e10–e16, 2007 [DOI] [PubMed] [Google Scholar]
- 73.Sarkar SR, Wystrychowski G, Zhu F, Usvyat LA, Kotanko P, Levin NW: Fluid dynamics during hemodialysis in relationship to sodium gradient between dialysate and plasma. ASAIO J 53: 339–342, 2007 [DOI] [PubMed] [Google Scholar]
- 74.Ritz E: Salt: friend or foe? Nephrol Dial Transplant 21: 2052–2056, 2006 [DOI] [PubMed] [Google Scholar]


