Abstract
The monitoring of physiologic variables is an integral part of the diagnosis and management of the critically ill patient. Restoration of tissue perfusion and oxygen delivery is the ultimate goal for any state of circulatory collapse. Insight into a patient’s intravascular volume status and cardiac performance, particularly in the early stages of shock, can help guide management and potentially change outcome. In the past 30 years, various bedside monitoring techniques and indices have been developed in an effort to determine and optimize a patient’s cardiac performance. This article reviews the physiologic parameters that best predict intravascular volume status and volume responsiveness. We examine the controversies surrounding the pulmonary arterial catheter and describe the less invasive methods of measuring cardiac performance.
Assessing Volume Status in the Intensive Care Unit
Volume expansion, in an effort to improve cardiac output (CO) and augment tissue perfusion, is a common therapeutic goal in the hemodynamically unstable patient. Volume expansion is not without adverse effects, including pulmonary edema, worsening gas exchange, and hyperchloremic acidosis (1). It has been shown that not all patients in circulatory collapse respond to volume with improved cardiac function (1–6). Moreover, patients with capillary leak may have total body volume overload and yet still benefit from augmented intravascular volume. Discriminating between patients who will benefit from volume expansion and those for whom an inotrope or vasopressive agent will better augment perfusion can limit the adverse effects of volume overload.
Central venous pressure (CVP) and pulmonary artery occlusion pressure (PaOP; or “wedge pressure”) have traditionally been used to estimate preload and intravascular volume status. These pressure-derived preload values, or filling pressures, have been central to the management of fluid resuscitation and titration; however, numerous studies have challenged the notion that these indicators accurately predict volume status. In fact, more than a dozen studies that examined various patient populations (sepsis, perioperative cardiovascular surgery, trauma, and other critical illnesses) all failed to demonstrate a correlation among CVP/PaOP, volume status, and cardiac performance (1,3–12). The Frank-Starling curve shown in Figures 1 and 2 illustrates how filling pressures can lead to inaccurate and often misleading interpretation of intravascular volume status.
Figure 1.
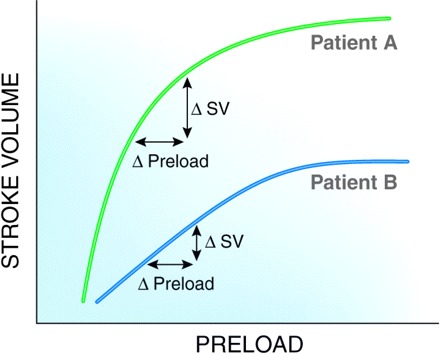
Patient A has a steeper Starling curve than patient B. Although patients A and B have the same initial preload value and although the patients have identical changes in preload (i.e., fluid bolus), patient A has a greater increase in stroke volume than patient B. Patient A is said to be “volume responsive.” SV, stroke volume.
Figure 2.
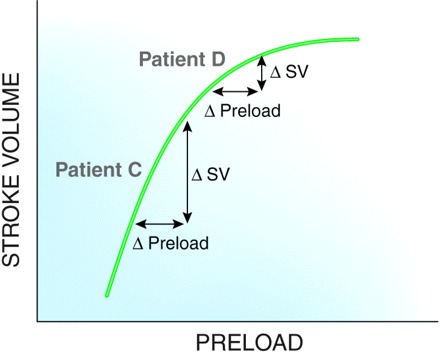
Patient C is on the steep portion of the curve. Patient D is on a flat portion. Identical changes in preload (i.e., fluid bolus) result in different stroke volumes.
Pressure-derived preload values do not identify a position of or place on the Starling curve and, therefore, poorly predict whether volume will improve hemodynamics. For example, a low CVP might prompt the clinician to give a fluid bolus; however, because of the patient’s position on the curve, volume will not result in an increase in the CO and may even result in pulmonary edema. Conversely, a patient can have “high” filling pressures yet still be on the vertical portion of the curve. Traditionally, an elevated CVP or PaOP would trigger an order to diurese, when, in actuality, the patient’s physiology would benefit from volume enhancement (1,4,8). CVP and PaOP are also poor indicators of cardiac preload because they fail to reflect cardiac volume in the setting of reduced ventricular compliance (4,13). A noncompliant, “stiff” heart may generate high filling pressures even in the setting of underfilled ventricles. The CVP or PaOP may be elevated, yet the patient’s CO would improve with volume infusion (8). Finally, external forces, including positive end expiratory pressure, abdominal pressures, and vascular compliance, alter the relationship between filling pressures and end diastolic volume (14).
In a study by Osman et al. (8), CVP, PaOP, and cardiac index (CI) were measured before and after a challenge of 6% hydroxyethyl starch infusion. Volume responsiveness was defined as an increase in CI by ≥15%. Preinfusion values of CVP were not statistically different between responders and nonresponders (Figure 3). The preinfusion values of PaOP, although statistically different between the two groups, had a great deal of overlap; therefore, no threshold value to predict volume responsiveness could be defined (Figure 4) (8). Even when combining low CVP and low PaOP values, the filling pressures still failed to predict whether volume would enhance CO (8).
Figure 3.
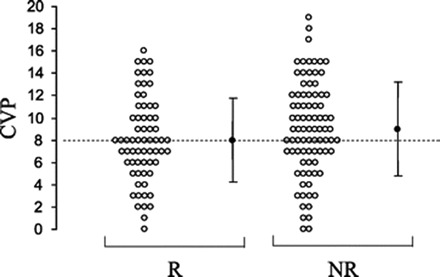
Preinfusion CVP (○, individual values; ●, mean values) of responders (R) and nonresponders (NR). Reprinted from Osman et al. (8), with permission.
Figure 4.
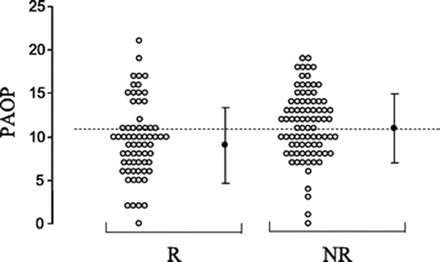
Preinfusion PaOP (○, individual values; ●, mean values) of responders (R) and nonresponders (NR). Reprinted from Osman et al. (8), with permission.
When examining the patterns shown in Figures 3 and 4, there is certainly a trend suggesting that patients with low filling pressures correspond to a response of fluid challenge. In fact, if one were to take a large population of patients and gather an aggregate of filling pressures, the low CVP and PaOP would likely predict volume responsiveness among this large group; however, when caring for the individual patient, these filling pressures do not predict with enough accuracy or reliability that a low value translates to volume responsiveness.
Not only are the absolute values of CVP and PaOP poor surrogates for volume responsiveness, but also several studies have shown that changes in CVP and PaOP after a fluid challenge do not correlate with changes in CO (9,13,15,16). Kumar et al. (13) demonstrated this concept definitively in a study of healthy volunteers. In this study, cardiac contractility failed to respond in any consistent or predictable manner when compared with the changes in CVP or PaOP after a 3-L saline infusion. The authors concluded that filling pressures, both absolute values and trends after volume infusion, failed to predict volume responsiveness in this population.
In the landmark study of early goal-directed therapy by Rivers et al. (17), CVP of 8 to 12 mmHg was one of the resus-citation end points. The American College of Critical Care Medicine guidelines for hemodynamic support of patients with sepsis also use CVP and PaOP to define resuscitation goals (6), yet the predefined increase in CI in the study by Osman et al. (8) was seen after fluid bolus in only 46% of patients whose CVP was <12 mmHg and 54% of patients whose PaOP was <12 mmHg. These findings are congruous with the results of many other studies and strongly question the accuracy and utility of CVP and PaOP as predictors of preload and targets of volume therapy (3,5,13,18,19).
Although CVP and PaOP may predict intravascular volume overload in certain clinical situations and PaOP is used in the definition of acute respiratory distress syndrome (ARDS), these values are just as likely to be misleading. A clinical example is the patient with bacterial pneumonia who is hypotensive and hyperlactemic and requires high levels of positive end expiratory pressure to promote gas exchange. Although this patient’s PaOP may be 19 mmHg (a level defined as consistent with cardiogenic pulmonary edema), diuresis would only worsen tissue perfusion (20). The clinician should use caution when making interventions on the basis of a single parameter whose reliability is of great uncertainty.
Additional parameters that have proved to be moderately useful in quantifying intravascular volume status include left ventricular end diastolic volume and area determined by echocardiogram. Although these cardiac dimensions are more direct assessments of intravascular volume, they still have limited ability to predict whether changes in preload will affect hemodynamics (1). Cardiomyopathy, valvular disease, and echocardiogram variability adversely affect the reliability of these measurements.
In contrast to PaOP, CVP, and cardiac dimensions, the so called “static” markers, “dynamic” markers are those that use variations in either stroke volume or arterial pressure because the physiologic effects of respiratory variation more reliably predict volume responsiveness. In patients who are on positive pressure mechanical ventilation, inspiration causes a reduction in right ventricular preload as a result of compression of the vena cava, whereas right ventricular afterload is increased because of increased alveolar pressures. The result is a reduction in right ventricular ejection during inspiration. Because of the transit time of blood flow from the right to the left side of the heart (approximately 2 s), a fall in stroke volume and BP is seen during expiration (Figure 5). Because the hemodynamic effects that are induced by mechanical ventilation are exaggerated in the hypovolemic patient, the greater the variation, the more likely the patient’s hemodynamics will improve with volume. These dynamic markers can be seen at the bedside as variations in arterial pressure, pulse pressure, or stroke volume (21,22). A graphic depiction and definitions of these dynamic markers are shown in Figure 5 and Table 1 (21).
Figure 5.
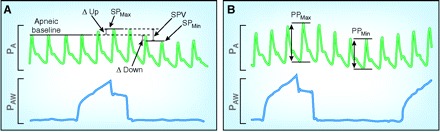
Dynamic markers. (A) Systolic pressure (SP) variation and decrease in arterial pressure during expiration (∆Down). (B) Pulse pressure (PP) variation. Further definitions provided in Table 1.
Table 1.
The dynamic markersa
| Dynamic Marker | Definition |
|---|---|
| SPV | The difference between the maximum systolic pressure and minimal systolic pressure after a breath |
| ΔDown | The decrease in arterial pressure during expiration |
| PPV | The maximum minus the minimum of the pulse pressure divided by the average of the two over a mechanical breath |
| SVV | The percentage change between the maximum stroke volume and minimum stroke volume over a designated interval |
PPV, pulse pressure variation.
Perel et al. (23) performed one of the first studies that examined systolic pressure variation as a predictor of volume responsiveness in a dog model of hemorrhagic shock. Variation in systolic pressure correlated with degree of hemorrhage better than CVP, heart rate, or mean arterial pressure. Tavernier et al. (5) examined dynamic and static variables including systolic pressure variation, decrease in arterial pressure during expiration, PAOP, and left ventricular end diastolic area index in patients with sepsis. Hemodynamic values were recorded before and after a volume challenge. Responders were predefined as having an increase in CI by 15%. The decrease in arterial pressure during expiration was the best predictor of improved cardiac function with volume (receiver operating characteristic 0.97) compared with PaOP (receiver operating characteristic 0.67). Marx et al. (19) compared stroke volume variation (SVV) with PaOP and CVP as a predictor of volume responsiveness in patients with sepsis. SVV predicted the cardiovascular response to fluid, whereas the filling pressures were no better than chance in anticipating hemodynamic change (10). With the use of a similar study design, dynamic markers have been tested among various patient populations, including neurosurgical patients (22), coronary artery bypass graft patients (18,24), and patients with acute lung injury (25). To our knowledge, all but one study have shown that the dynamic markers predict with reasonable accuracy whether a volume infusion will result in improved cardiac function (5,10,18,24,25).
There are some limitations to the use of dynamic markers. For example, dynamic parameters are affected by varying tidal volumes and therefore require a well-sedated, mechanically ventilated patient to ensure accuracy. Arrhythmias, particularly atrial fibrillation, will also affect the precision of SVV analyses. Finally, alterations in myocardial contractility as a result of titration of inotropic or vasopressive agents can affect the accuracy of these dynamic markers (4,18,19).
It should also be emphasized that the dynamic markers do not provide information of absolute intravascular volume status (i.e., a low SVV does not suggest volume overload) but, rather, predict whether a patient will benefit from volume infusion. Although dynamic measures are more accurate at predicting volume responsiveness, as a practical matter, a clinician will often try a volume challenge in a patient who is hypotensive or oliguric. If volume produces no effect on CO, then an inotrope may be started. Because BP is often being modified with vasopressors and oliguria may be slow to resolve, this underscores the need for bedside monitoring of CO, as we will discuss in the next section.
Hemodynamic Devices: The Pulmonary Artery Catheter
Developed in the 1970s, the pulmonary artery catheter (PAC) provided bedside hemodynamic parameters unlike other devices before its time. The PAC provides measurements including CVP, right atrial and right ventricular pressures, pulmonary artery pressures, PaOP, mixed venous saturation (SvO2), and CO. Systemic and pulmonary vascular resistances are calculated from these values (26).
The value of the PAC has been a subject of debate in the past two decades. Proponents of its use argue that the PAC provides data necessary for the appropriate diagnoses and treatment of cardiovascular failure. The PAC is an accurate technique for obtaining CO, and it continues to be the gold standard for CO measurement at the bedside. In addition, pulmonary pressures obtained from the PAC can be used to evaluate and modify pharmacologic treatment for pulmonary hypertension. Last, the SvO2 reflects the overall balance among oxygen supply, demand, and use by the body’s tissues and is used, along with lactate levels, to guide resuscitation.
Despite the real-time, hemodynamic parameters provided by the PAC, there are still no data supporting its use in the care of the critically ill patient. Moreover, there are other means of obtaining similar data yet in a less invasive manner. For example, unlike SvO2, the central venous saturation (ScvO2) is drawn from the superior vena cava via a central catheter and does not require a PAC. The ScvO2 has gained increasing acceptance since its use in the early goal-directed therapy algorithm by Rivers et al. (17) and can be measured in a continuous manner. Although ScvO2 values are higher than SvO2, the gradient is consistent and therefore ScvO2 is a reliable surrogate for SvO2 (27). (On average, ScvO2 is approximately 5% higher than SvO2. This difference, however, can vary among individual patients [27].) Finally, less invasive devices that provide hemodynamic parameters similar to that of the PAC are now available. These less invasive devices as well as data on the usefulness of the PAC are described in the sections to follow.
As with any invasive device, complication rates are an important consideration in a benefit-to-risk analysis. The process of obtaining initial venous access with a central venous catheter and PAC are similar; therefore, complication rates at the stage of catheter insertion are comparable. (The risk for mechanical complications from catheter insertion can be diminished by the use of ultrasound guidance. When compared with the standard landmark technique, live ultrasound guidance has been shown to decrease arterial puncture and other complications [28].) The PAC carries additional risks given its advancement and residence in the right heart and pulmonary vasculature. Significant dysrhythmias, heart block, pulmonary artery rupture, knotting, and increased risks for infection over a simple central venous catheter are examples of these potential adverse events (29,30).
The hemodynamic parameters and adverse effects of the PAC and central venous catheter are compared in Table 2 (30).
Table 2.
A comparison of the pulmonary artery catheter and the central venous cathetera
| Parameter | PAC | Central Venous Catheter | Relative Accuracy of Each Parameter |
|---|---|---|---|
| Obtainable measurements | |||
| SvO2 | + | − | ++ |
| CO | + | − | ++ |
| intrapulmonary vascular pressures (pulmonary artery systolic and pulmonary artery diastolic) | + | − | ++ |
| preload variable, PaOP | + | − | − |
| preload variable, CVP | + | + | − |
| ScvO2 | + | + | + |
| continuous ScvO2b | + | + | + |
| Adverse effects | Incidence (%) | ||
| thrombosisb | + | + | 2 to 67 |
| pneumothorax | + | + | 0.3 to 3.0 |
| arterial puncture | + | + | 1.1 to 1.3 |
| hematoma or hemorrhage | + | + | 1 to 5 |
| vascular infection | + | + | 0.7 to 11.4 |
| arrhythmias | + | + | |
| minor dysrhythmias | + | + | 4.7 to 68.9 |
| severe dysrhythmias | + | − | 0.3 to 62.7 |
| right bundle brand block | + | − | 0.1 to 4.3 |
| complete heart block | + | − | 0.05 to 5.00 |
| catheter fragment or knots | + | − | <1 |
| pulmonary infarct | + | − | <1 |
| pulmonary rupture | + | − | 0.1 to 1.5 |
| Can be used outside the ICU (regular medical or surgical floor) | − | + | |
| Costc | ++ | + |
CO, cardiac output; CVP, central venous pressure; ICU, intensive care unit; PAC, pulmonary artery catheter; PaOP, pulmonary artery occlusion pressure; ScvO2, central venous saturation; SvO2, mixed venous oxygen saturation
Incidence varies depending on location (femoral versus subclavian) and duration of the catheter. Many thromboses are discovered on postmortem evaluation and are not clinically relevant (29).
A meta-analysis of five trials demonstrated that costs were higher for patients who were treated using PAC compared with those without PAC. One study showed that the cost per quality-adjusted life year gained from withdrawing a PAC was $5672(31,32).
Recent evidence demonstrates that the PAC does not improve outcome in critical care patients (33). A multicenter, prospective trial by Connors et al. (34) compared a population of patients with and without PAC. Patients in the PAC group had a higher mortality rate and increased use of resources. This study has been criticized for its nonrandomization and lack of algorithms used to guide treatment in response to derived PAC data, although a lack of standard algorithms probably reflects common practice. In addition, the statistical analysis has been scrutinized, particularly the use of control subjects who were case-matched with controversial propensity scores (26).
Sandham et al. (35) performed the first randomized, controlled trial that used goal-oriented therapy to evaluate PAC use in high-risk surgical patients. The study included predefined physiologic goals and treatment protocols to optimize hemodynamics. Results revealed that the use of the PAC did not alter hospital mortality rates or survival at 6 and 12 months. A similar study by Richard et al. (36) examined the early use of the PAC and its effect on outcome in patients with septic shock and ARDS. Again, the authors failed to show a mortality difference. In addition, organ system failure, renal support, or vasoactive agents were also not altered by use of the PAC. Finally, a recent study from the ARDS clinical trials network, part of the Fluid and Catheter Treatment Trial (FACTT), compared the use of the PaOP with CVP in the management of acute lung injury (37). BP and urinary output were additional variables that guided management in both groups. A predefined protocol was established for all variables and interventions. The authors observed that 60-day mortality rates, renal replacement therapy, and days spent in the intensive care unit (ICU) did not differ significantly between the two groups (37). One criticism of this study is that the efficacy of the PAC was based on interventions and outcomes from high and low levels of PaOP and CVP. As discussed in detail already, both of these parameters predict preload responsiveness poorly (37).
Less Invasive Hemodynamic Devices
Many other, less invasive monitoring devices have been developed to measure cardiac performance and guide hemodynamic therapy. Echocardiography can be used to assess the pericardium, valves, and global and regional left ventricular function. Because the echocardiogram is not performed continuously (other than in the operating room), it remains predominately a diagnostic tool rather than a monitor of therapy in the ICU (38). The esophageal Doppler uses an esophageal probe that continuously measures the velocity of blood flow in the descending aorta (26,38,39). CO is extrapolated from the concept that flow in a cylinder is equal to the cross-sectional area of the cylinder (πr2) times the velocity of flow in the cylinder. This technique operates on the assumption that the aorta is a static cylinder and flow is always laminar (39). A study by Monnet et al. (40) demonstrated that measured versus estimated aortic area was significantly different after fluid infusion. The accuracy of the esophageal Doppler also depends on positioning of the probe. The probe must be within 20° of axial view and therefore requires that the patient be immobile and sedated. High interobserver variability also alters the precision of this device. Despite its drawbacks, when compared with thermodilution under controlled conditions, the esophageal Doppler correlates well (39,41).
Using the Fick principle, a carbon dioxide rebreathing technique can be used to calculate CO. Carbon dioxide is substituted for oxygen into the Fick equation, and a rebreathing technique is used to estimate mixed venous CO2. The advantage of the CO2 rebreathing technique, or “indirect Fick method,” is that it does not require invasive catheterization. This technique has several limitations. In critically ill patients, CO2 production is often altered by changes in metabolism. This method is also less accurate in patients with abnormal gas exchange. V/Q mismatching can affect exhaled CO2, which can be erroneously reflected as a change in CO (30).
The thoracic electrical bioimpedance (TEB) is another device used to estimate cardiac function in the ICU. Eight electrodes are placed on the patient’s neck and thorax and then connected to the TEB device. Alternating current is transmitted through the chest via the eight electrodes. The bioimpedance is the electrical resistance of the thorax to this current. The bioimpedance is indirectly proportional to the amount of thoracic fluid. The CO is calculated using the impedance of the thoracic aorta, which varies with blood flow (39). The advantage of the TEB device is that it is completely noninvasive, requiring no venipuncture. TEB CO estimate loses accuracy when thoracic fluid (pulmonary edema, pleural effusions, and chest wall edema) is increased (39). In a meta-analysis of 154 studies that compared TEB with the Fick method, thermodilution, dye dilution, and the echocardiogram, TEB did not correlate well. The authors concluded that TEB is useful to monitor trends but not consistent enough to provide single diagnoses (39). Our own experience with using the TEB echoes the findings of the metaanalyses, and we no longer use this technique.
CO can also be obtained by the transpulmonary thermodilution technique, a process similar to that of the PAC. A cold injectate is administered intravenously. A thermistor-tipped arterial catheter calculates the downstream temperature change. Mathematical analyses of the change in temperature over time estimate the CO. The thermodilution curve allows for the calculation of other variables, including global end diastolic volume and extravascular lung water (26,39,42). Studies that have compared transpulmonary technique with pulmonary artery thermodilution and Fick have been encouraging (39).
Lithium dilution uses lithium chloride to estimate CO. Lithium-selective electrodes are positioned in an arterial pressure catheter. Lithium is injected into a venous catheter, and blood is subsequently drawn from the arterial line that contains the lithium sensor. A dilutional curve is then used to calculate CO.
The formula for calculating the CO depends on accurate sodium and hemoglobin concentrations (39,43). The lithium technique compares well with the thermodilution technique. It does not require central access, but it does require arterial access and multiple blood draws and cannot be used continuously (43).
Pulse contour analysis represents a continuous CO measurement using the arterial waveform. The technique is based on the principle that the contour of the arterial waveform, the area under the systolic portion, is proportional to stroke volume and the mechanical properties of the artery. The stroke volume is calculated by the integral change from end diastole to end systole over time (39,43). SVV is also displayed continuously and is derived from the difference between the minimum and maximum stroke volume divided by the mean of the maximum and minimum stroke volume. There are three different pulse contour devices on the market. The first is the PICCO (Pulsion Medical Systems, Munich, Germany). This device must first be calibrated with the pulmonary thermodilution technique. It has been shown to correlate well with thermodilution in various patient populations, using different arteries and after changes in preload (42,44,45). The PulsCO (LiDCO Limited, Cambridge, UK) is the second of the three pulse contour devices. Like the PICCO, it requires precalibration but uses a lithium indicator technique. Recalibration is required every 8 hours to maintain accuracy (43). The PulsCO beat-to-beat stoke volume analysis has compared well with lithium dilution techniques in postoperative patients (43). The Flotrac (Edwards Lifescience LLC, Irvine, CA) calculates stroke volume without the need for external calibration. The algorithm used for calculating stroke volume takes into account the pulse pressure, the arterial compliance (estimated by age, gender, and body surface area), and the conformation of the arterial waveform. In one study that compared the intermittent pulmonary artery thermodilution technique with the FloTrac device among patients who were undergoing coronary artery bypass grafting and valvular repair, the FloTrac device showed a moderate agreement (46). All three pulse contour analysis devices offer a reliable alternative approach to the invasive PAC in estimating CO. The continuous display of cardiac function and SVV allows for the evaluation and response to instantaneous changes in CO that can be missed by intermittent monitoring. SVV is inaccurate in the presence of atrial arrhythmias and frequent premature ventricular contractions. Our own experience with the FloTrac has been encouraging. It has proved to be a safe and easy tool to help guide resuscitative efforts and optimize hemodynamics. Tables 3 and 4 summarize the less invasive devices.
Table 3.
Features of minimally invasive CO techniques and devices
| Technique/Device | Invasiveness | Estimate of Cardiac Preload | Limitations/Considerations |
|---|---|---|---|
| Echocardiogram | Noninvasive | Yes | Not continuous |
| Trained interpreter required | |||
| Esophageal Doppler flow | Esophageal probe required | Yes | Patient needs to be immobile |
| Probe must be in proper position | |||
| Specialized training required | |||
| Partial CO2 rebreathing technique | Noninvasive | No | Affected by V/Q mismatch |
| Affected by changes in metabolism | |||
| TEB | Noninvasive | No | Decreased accuracy with edema and cardiac arrhythmias |
| Only modest correlation with thermodilution | |||
| Transpulmonary thermodilution | Venous and arterial access | Yes (ITBV) | Good correlation with pulmonary artery thermodilution |
| Lithium dilution | Venous and arterial access | No | Calculation of CO depends on accurate sodium and hemoglobin concentration |
| Multiple blood draws | |||
| Potential for lithium adverse effects | |||
| Pulse contour analyses | Arterial catheter One technique | Yes | See Table 4 |
| (PICCO) requires central access |
TEB, thoracic electrical bioimpedance; ITBV, intrathoracic blood volume.
Table 4.
Pulse contour analysisa
| Device | Required Access | Precalibration Technique | Additional Feature or Drawback |
|---|---|---|---|
| PICCO (Pulsion Medical System, Munich, Germany) | Central venous catheter and arterial line | Transpulmonary thermodilution | Able to calculate ETLV |
| PulseCO (LiDCOLimited, Cambridge, UK) | Peripheral access and arterial line | Lithium indicator dilution | Cannot be used in patients on lithium therapy |
| FloTrac (Edwards Lifescience LLC, Irvine, CA) | Arterial line only | None | More studies to confirm its accuracy are required |
ETLV, extravascular lung volume.
Conclusions
Monitoring physiologic variables and optimizing organ perfusion are fundamental to the care of the critical care patient. For years, the PAC and the conventional preload variables (CVP and PaOP) have been central to the treatment of the hemodynamically unstable patient. It has become increasingly apparent that the traditional markers do not reliably reflect preload, and the utility of the PAC in the ICU remains controversial. Modern technology has led to the growth and development of newer, less invasive means of assessing hemodynamic instability. An understanding of these emerging devices will assist the clinician in making more accurate assessments of volume status while optimizing cardiovascular function.
After reviewing the various physiologic markers and monitoring devices, it would seem logical that we might provide an algorithm for the treatment of the hemodynamically unstable patient. The protocol used in the study by Rivers et al. (17) emphasized early recognition and goal-directed care and can provide a stepping stone for treatment of these patients; however, hemodynamic optimization of a critically ill patient is a complex and multifaceted process. It is our opinion that an adequate and appropriate resuscitation requires that the physician be at the bedside, assessing real-time hemodynamic changes with each intervention. The physician must have a concrete understanding of physiologic principles coupled with an appreciation for an individual patient’s response to each intervention as well as his or her unique disease process. Moreover, as was illustrated in this review, understanding the limitations of each hemodynamic variable and device is essential. Although no single recipe or algorithm will suffice to meet every need, it is clear that hemodynamic monitoring, coupled with close clinical attention, can reduce complications and significantly enhance the effectiveness of resuscitation through metabolic and hemodynamic goal-directed therapy.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Gunn SR, Pinsky MR: Implications of arterial pressure variation in patients in the intensive care unit. Curr Opin Crit Care 7: 212–217, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Feissel M, Michard F, Mangin I, Ruyer O, Faller JP, Teboul JL: Respiratory changes in aortic blood velocity as an indicator of fluid responsiveness in ventilated patients with septic shock. Chest 119: 867–873, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Michard F, Boussat S, Chemla D, Anguel N, Mercat A, Lecarpentier Y, Richard C, Pinsky MR, Teboul JL: Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med 162: 134–138, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Michard F, Teboul JL: Predicting fluid responsiveness in ICU patients: A critical analysis of the evidence. Chest 121: 2000–2008, 2002. [DOI] [PubMed]
- 5.Tavernier B, Makhotine O, Lebuffe G, Dupont J, Scherpereel P: Systolic pressure variation as a guide to fluid therapy in patients with sepsis-induced hypotension. Anesthesiology 89: 1313–1321, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Hollenberg SM, Ahrens TS, Annane D, Astiz ME, Chalfin DB, Dasta JF, Heard SO, Martin C, Napolitano LM, Susla GM, Totaro R, Vincent JL, Zanotti-Cavazzoni S: Practice parameters for hemodynamic support of sepsis in adult patients: 2004 update. Crit Care Med 32: 1928–1948, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Bendjelid K, Romand JA: Fluid responsiveness in mechanically ventilated patients: A review of indices used in intensive care. Intensive Care Med 29: 352–360, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Osman D, Ridel C, Ray P, Monnet X, Anguel N, Richard C, Teboul JL: Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit Care Med 35: 64–68, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Diebel L, Wilson RF, Heins J, Larky H, Warsow K, Wilson S: End-diastolic volume versus pulmonary artery wedge pressure in evaluating cardiac preload in trauma patients. J Trauma 37: 950–955, 1994 [DOI] [PubMed] [Google Scholar]
- 10.Calvin JE, Driedger AA, Sibbald WJ: The hemodynamic effect of rapid fluid infusion in critically ill patients. Surgery 90: 61–76, 1981 [PubMed] [Google Scholar]
- 11.Schneider AJ, Teule GJ, Groeneveld AB, Nauta J, Heidendal GA, Thijs LG: Biventricular performance during volume loading in patients with early septic shock, with emphasis on the right ventricle: A combined hemodynamic and radionuclide study. Am Heart J 116: 103–112, 1988 [DOI] [PubMed] [Google Scholar]
- 12.Reuse C, Vincent JL, Pinsky MR: Measurements of right ventricular volumes during fluid challenge. Chest 98: 1450– 1454, 1990 [DOI] [PubMed] [Google Scholar]
- 13.Kumar A, Anel R, Bunnell E, Habet K, Zanotti S, Marshall S, Neumann A, Ali A, Cheang M, Kavinsky C, Parrillo JE: Pulmonary artery occlusion pressure and central venous pressure fail to predict ventricular filling volume, cardiac performance, or the response to volume infusion in normal subjects. Crit Care Med 32: 691–699, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Cheatham ML: The holy grail of shock resuscitation. Crit Care Med 33: 2691–2692, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Sakka SG, Reinhart K, Meier-Hellmann A: Comparison of pulmonary artery and arterial thermodilution cardiac output in critically ill patients. Intensive Care Med 25: 843–846, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Buhre W, Weyland A, Schorn B, Scholz M, Kazmaier S, Hoeft A, Sonntag H: Changes in central venous pressure and pulmonary capillary wedge pressure do not indicate changes in right and left heart volume in patients undergoing coronary artery bypass surgery. Eur J Anaesthesiol 16: 11–17, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M: Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 345: 1368–1377, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Hofer CK, Muller SM, Furrer L, Klaghofer R, Genoni M, Zollinger A: Stroke volume and pulse pressure variation for prediction of fluid responsiveness in patients undergoing off-pump coronary artery bypass grafting. Chest 128: 848–854, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Marx G, Cope T, McCrossan L, Swaraj S, Cowan C, Mostafa SM, Wenstone R, Leuwer M: Assessing fluid responsiveness by stroke volume variation in mechanically ventilated patients with severe sepsis. Eur J Anaesthesiol 21: 132–138, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Binanay C, Califf RM, Hasselblad V, O’Connor CM, Shah MR, Sopko G, Stevenson LW, Francis GS, Leier CV, Miller LW: Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: The ESCAPE trial. JAMA 294: 1625–1633, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Michard F: Changes in arterial pressure during mechanical ventilation. Anesthesiology 103: 419–428, quiz 449–415, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Berkenstadt H, Margalit N, Hadani M, Friedman Z, Segal E, Villa Y, Perel A: Stroke volume variation as a predictor of fluid responsiveness in patients undergoing brain surgery. Anesth Analg 92: 984–989, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Perel A, Pizov R, Cotev S: Systolic blood pressure variation is a sensitive indicator of hypovolemia in ventilated dogs subjected to graded hemorrhage. Anesthesiology 67: 498– 502, 1987 [DOI] [PubMed] [Google Scholar]
- 24.Wiesenack C, Prasser C, Rodig G, Keyl C: Stroke volume variation as an indicator of fluid responsiveness using pulse contour analysis in mechanically ventilated patients. Anesth Analg 96: 1254–1257, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Michard F, Chemla D, Richard C, Wysocki M, Pinsky MR, Lecarpentier Y, Teboul JL: Clinical use of respiratory changes in arterial pulse pressure to monitor the hemodynamic effects of peep. Am J Respir Crit Care Med 159: 935–939, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Boldt J: Clinical review: Hemodynamic monitoring in the intensive care unit. Crit Care 6: 52–59, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chawla LS, Zia H, Gutierrez G, Katz NM, Seneff MG, Shah M: Lack of equivalence between central and mixed venous oxygen saturation. Chest 126: 1891–1896, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Randolph AG, Cook DJ, Gonzales CA, Pribble CG: Ultrasound guidance for placement of central venous catheters: A meta-analysis of the literature. Crit Care Med 24: 2053– 2058, 1996 [DOI] [PubMed] [Google Scholar]
- 29.Bowdle TA: Complications of invasive monitoring. Anesthesiol Clin North Am 20: 571–588, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Vender JS, Franklin M: Hemodynamic assessment of the critically ill patient. Int Anesthesiol Clin 42: 31–58, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Harvey S, Young D, Brampton W, Cooper AB, Doig G, Sibbald W, Rowan K: Pulmonary artery catheters for adult patients in intensive care. Cochrane Database Syst Rev 3: CD003408, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Fontes M, Barash PG: Pulmonary artery catheter under the microscope. Crit Care Med 28: 891–892, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Pinsky MR, Vincent JL: Let us use the pulmonary artery catheter correctly and only when we need it. Crit Care Med 33: 1119–1122, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Connors AF Jr, Speroff T, Dawson NV, Thomas C, Harrell FE Jr, Wagner D, Desbiens N, Goldman L, Wu AW, Califf RM, Fulkerson WJ Jr, Vidaillet H, Broste S, Bellamy P, Lynn J, Knaus WA: The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT investigators. JAMA 276: 889–897, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Sandham JD, Hull RD, Brant RF, Knox L, Pineo GF, Doig CJ, Laporta DP, Viner S, Passerini L, Devitt H, Kirby A, Jacka M: A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med 348: 5–14, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Richard C, Warszawski J, Anguel N, Deye N, Combes A, Barnoud D, Boulain T, Lefort Y, Fartoukh M, Baud F, Boyer A, Brochard L, Teboul JL: Early use of the pulmonary artery catheter and outcomes in patients with shock and acute respiratory distress syndrome: A randomized controlled trial. JAMA 290: 2713–2720, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Wheeler AP, Bernard GR, Thompson BT, Schoenfeld D, Wiedemann HP, deBoisblanc B, Connors AF Jr, Hite RD, Harabin AL: Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med 354: 2213–2224, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Polanco PM, Pinsky MR: Practical issues of hemodynamic monitoring at the bedside. Surg Clin North Am 86: 1431– 1456, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Chaney JC, Derdak S: Minimally invasive hemodynamic monitoring for the intensivist: Current and emerging technology. Crit Care Med 30: 2338–2345, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Monnet X, Chemla D, Osman D, Anguel N, Richard C, Pinsky MR, Teboul JL: Measuring aortic diameter improves accuracy of esophageal Doppler in assessing fluid responsiveness. Crit Care Med 35: 477–482, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Dark PM, Singer M: The validity of trans-esophageal Doppler ultrasonography as a measure of cardiac output in critically ill adults. Intensive Care Med 30: 2060–2066, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Felbinger TW, Reuter DA, Eltzschig HK, Bayerlein J, Goetz AE: Cardiac index measurements during rapid preload changes: A comparison of pulmonary artery thermodilution with arterial pulse contour analysis. J Clin Anesth 17: 241–248, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Pittman J, Bar-Yosef S, SumPing J, Sherwood M, Mark J: Continuous cardiac output monitoring with pulse contour analysis: A comparison with lithium indicator dilution cardiac output measurement. Crit Care Med 33: 2015–2021, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Felbinger TW, Reuter DA, Eltzschig HK, Moerstedt K, Goedje O, Goetz AE: Comparison of pulmonary arterial thermodilution and arterial pulse contour analysis: Evaluation of a new algorithm. J Clin Anesth 14: 296–301, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Goedje O, Hoeke K, Lichtwarck-Aschoff M, Faltchauser A, Lamm P, Reichart B: Continuous cardiac output by femoral arterial thermodilution calibrated pulse contour analysis: Comparison with pulmonary arterial thermodilution. Crit Care Med 27: 2407–2412, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Mayer J, Boldt J, Schollhorn T, Rohm KD, Mengistu AM, Suttner S: Semi-invasive monitoring of cardiac output by a new device using arterial pressure waveform analysis: A comparison with intermittent pulmonary artery thermodilution in patients undergoing cardiac surgery. Br J Anaesth 98: 176–182, 2007 [DOI] [PubMed] [Google Scholar]


